Abstract
Background and Aims Benefits to crop productivity arising from increasing CO2 fertilization may be offset by detrimental effects of global climate change, such as an increasing frequency of drought. Phosphorus (P) nutrition plays an important role in crop responses to water stress, but how elevated CO2 (eCO2) and P nutrition interact, especially in legumes, is unclear. This study aimed to elucidate whether P supply improves plant drought tolerance under eCO2.
Methods A soil-column experiment was conducted in a free air CO2 enrichment (SoilFACE) system. Field pea (Pisum sativum) was grown in a P-deficient vertisol, supplied with 15 mg P kg−1 (deficient) or 60 mg P kg−1 (adequate for crop growth) and exposed to ambient CO2 (aCO2; 380–400 ppm) or eCO2 (550–580 ppm). Drought treatments commenced at flowering. Measurements were taken of soil and leaf water content, photosynthesis, stomatal conductance, total soluble sugars and inorganic P content (Pi).
Key Results Water-use efficiency was greatest under eCO2 when the plants were supplied with adequate P compared with other treatments irrespective of drought treatment. Elevated CO2 decreased stomatal conductance and transpiration rate, and increased the concentration of soluble sugars and relative water contents in leaves. Adequate P supply increased concentrations of soluble sugars and Pi in drought-stressed plants. Adequate P supply but not eCO2 increased root length distribution in deeper soil layers.
Conclusions Phosphorus application and eCO2 interactively enhanced periodic drought tolerance in field pea as a result of decreased stomatal conductance, deeper rooting and high Pi availability for carbon assimilation in leaves.
Keywords: Climate change, crop nutrition, drought tolerance, free air CO2 enrichment, FACE, P nutrition, pea, Pisum sativum, root length distribution, stomatal conductance, water-use efficiency
INTRODUCTION
In the scenario of climate change, drought may become more frequent, intensive and erratic in some regions (Robredo et al., 2007; Allen et al., 2010). In Western Australia, for example, rainfall has decreased by 15–20 % compared with the 1970s (Petrone et al., 2010), which may be partly driven by anthropogenic climate change (Cai et al., 2005; van Ommen and Morgan, 2010). A consequence of this reduced rainfall will be reduced growth and yield of many dryland crops (Araus et al., 2002; Volaire, 2003).
As a fact of an increasing atmospheric CO2 concentration during climate change (Calzadilla et al., 2013; Wheeler and von Braun, 2013), elevated CO2 (eCO2) has been reported to be able to mitigate the impact of drought stress in many legume species. In soybean, for example, eCO2 enhanced drought tolerance by lowering stomatal conductance and maintaining photosynthesis at the seed-filling stage (Li et al., 2013). In alfalfa, eCO2 improved water relations, and thereby enhanced photosynthetic rate and yield by alleviating drought stress (Erice et al., 2006). The increased tolerance to drought under eCO2 was attributed to changes in concentrations and/or composition of soluble carbohydrates in leaves that mediate osmotic adjustments and plant water potential (ψw) (Tyree and Alexander, 1993; Seneweera et al., 2001; Allen et al., 2011). This physiological strategy enables plants to reduce stomatal and canopy conductance, and lower soil water consumption.
Applying phosphorus (P) to P-deficient soils reportedly stimulates growth responses to eCO2, particularly for legumes (Edwards et al., 2005; Jin et al., 2012, 2013), and plays an important role in drought tolerance (Graciano et al., 2005). The question arises as to whether P application could improve drought tolerance under eCO2. Increasing P supply has been shown to improve the tolerance of white clover and soybean to dry soil conditions (Singh et al., 2000; Jin et al., 2006). The reasons for this improved tolerance include increasing root hydraulic conductivity, maintaining leaf water potential (Radin and Eidenbock, 1984; Singh et al., 1997) and increasing root access to more soil water in deep soil layers (Jin et al., 2005). Increasing P application is also likely to enhance the synthesis of the osmotically active carbohydrates in the leaf cells responsible for maintenance of leaf water potential under drought conditions because inorganic P (Pi) plays a key role in translocation of triose sugars out of chloroplasts (Abel et al., 2002; Rychter and Rao, 2005; Lambers et al., 2006). These assumptions on how P supply mediates the effect of eCO2 on drought tolerance, however, need to be experimentally tested.
This study aimed to elucidate whether increasing P supply in P-limiting soil and/or eCO2 would affect drought tolerance of the legume species field pea via changes in water-use efficiency, leaf water relations and altered root growth in soil profiles. We hypothesized that increasing P supply would enhance the tolerance of field pea to soil water stress (drought) and this tolerance would be greater under eCO2 than under ambient CO2 (aCO2), due to stimulations in root growth, increasing carbohydrate synthesis and maintenance of a higher relative water content (RWC). Furthermore, eCO2 would enhance drought tolerance by reducing stomatal conductance and maintaining leaf water status. We anticipate that optimizing P application may become one strategy that helps in minimizing the impact of water stress in future eCO2 climates.
MATERIALS AND METHODS
Experimental design and plant growth
The experiment had a split-plot design with CO2 as the main plot, and P application and drought as sub-plot treatments. Ambient CO2 (380–400 ppm) and eCO2 (550–580 ppm) levels were achieved using the free air CO2 enrichment (SoilFACE) facility in Horsham, Victoria, Australia (36° 42′S, 142° 11′E) (Mollah et al., 2011). There were four FACE rings (four replicates) for each CO2 concentration. Phosphorus was applied as KH2PO4 at two rates: 15 (P15) and 60 mg P kg−1 (P60) mixed evenly throughout the soil. These P application rates were designed to provide deficient and adequate P nutrition, respectively, of the field pea. Field pea (Pisum sativum ‘OzP0601’) was used as the test species. Two soil water treatments, well-watered and periodic drought, commenced at the initial flowering stage (lasting for 21 d). The P and water treatments were replicated in all of eCO2 and aCO2 plots.
Soil was collected near the SoilFACE site (36° 42′S, 142° 11′E). The soil type used is known as Vertisol (FAO–UNESCO, 1976). Relevant soil properties are as follows: organic C of 7·8 mg g−1 (Rayment and Higginson, 1992), 2 m KCl-extractable N of 4·2 mg kg−1 and NH4-N of 1·0 mg kg−1, total P of 114 mg kg−1, Colwell P of 5 mg kg−1 (Colwell, 1963) and a pH (1:5 in 0·01 m CaCl2) of 7·7. This Colwell P level is considered to be severely deficient for the growth of crops (Richardson et al., 2009). After air-drying and sieving through a 4 mm sieve, the soil was mixed with siliceous sand (w/w = 1:1) to facilitate root washing at harvest. Each column (60 cm long, 15 cm in diameter) contained 13 kg of experimental soil in total, and the soil was mixed with the following basal nutrients (mg kg−1): K2SO4, 147; MgSO4·7H2O, 122; CaCl2, 186; CuSO4·5H2O, 6; ZnSO4·7H2O, 8; MnSO4·5H2O, 6; FeCl3, 0·6; CoCl2, 0·4; NaMoO4·2H2O, 0·4; and NaB4O7, 1·6, and the required amount of P for each treatment.
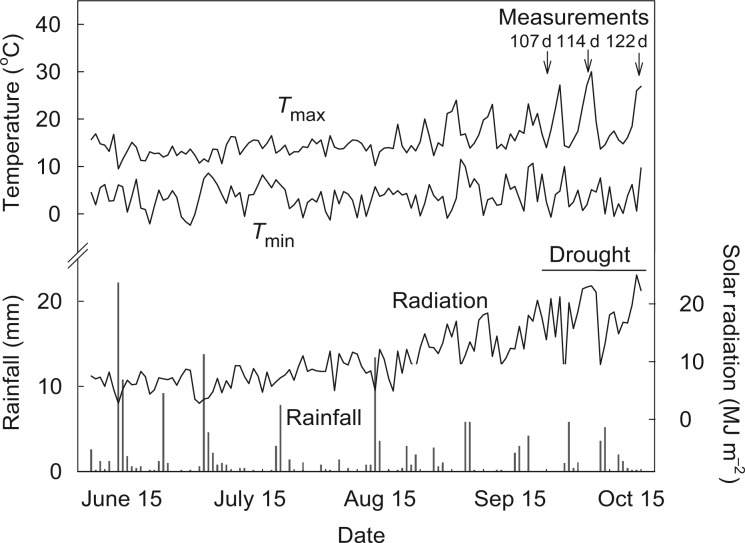
Eight uniform germinated seeds were sown in each column and inoculated with rhizobia (Group E® Rhizobium leguminosarum for field pea) on 15 June 2012. All columns were allocated into underground bunkers that have been built in the SoilFACE. The seedlings were thinned to four plants per column 3 weeks after sowing (at the V1 stage). Temperatures, radiation and rainfall during the experimental period are shown in Fig. 1. These meteorological data were obtained from Horsham Airport, located 6·6 km away from the SoilFACE site. Drought stress was imposed at the initial flowering stage (102 d after sowing). During the drought treatment, all columns were covered just above the soil surface to exclude rainfall. Watering was withheld in the drought treatments until the soil reached 43 % of field water capacity (FWC), the permanent wilting point (which occurred after 3 weeks). The well-watered treatments were maintained at 80 % of FWC by weighing and watering every 2 d throughout the experimental period. The total amount of water applied for each treatment was recorded.
Fig. 1.
Daily rainfall, solar radiation, and minimal (Tmin) and maximal (Tmax) temperatures during the experimental period from 15 June to 15 October 2012 near the experimental site. Three rounds of measurement on water status were at the initial-phase drought (63–70 % FWC) (Day 107 after sowing), mid-phase drought (52–57 % FWC) (Day 114) and final-phase drought (43–46 % FWC) (Day 122), respectively.
Measurements
Soil water content was recorded every 2 or 3 d during the period of drought treatment both by weighing the columns (to measure water loss) and via a Theta probe (ML2X, DELTA-T DEVICES, Cambridge, UK). Parameters on water status were determined when the soil water content in the drought treatment had dropped to 66 % (63–70 %, n = 16) (initial-phase drought, Day 107 after sowing), 55 % (52–57 %) (mid-phase drought, Day 114) and 45 % (43–46 %) of FWC (final-phase drought, Day 122) as showed in Fig. 1. Using a portable photosynthesis system (Li-Cor, Lincoln, NE, USA), stomatal conductance (gs), transpiration rate (E) and photosynthetic rate (Pn) were measured on the second or third youngest fully expanded leaves through the drought treatment period. Measurements were taken between 0900 and 1200 h on days with full sunlight and a temperature of 22–25 °C. Measurements were performed in duplicate on two plants in each column and from replicate to replicate across the treatments. This procedure was performed throughout the three phases during the period of drought treatment. The conditions inside the leaf chamber such as photosynthetically active radiation and reference CO2 concentration were hold constant across all samples. Instantaneous transpiration efficiency (ITE) was calculated by dividing Pn by E (Robredo et al., 2007).
Immediately after measuring photosynthesis, the same leaves in each replicate were then sampled and used for measurement of RWC, total soluble sugars (TSS) and Pi. The samples for TSS and Pi measurements were weighed, frozen in liquid nitrogen and stored in a −80 °C freezer for later measurements. For RWC analysis (Conroy et al., 1988), the fresh leaves were weighed and floated on distilled water for 4 h at 25 °C under full sunlight. The turgid weight of these leaves was then recorded, and the dry weight was determined after drying at 70 °C for 72 h. The RWC was then calculated using the following formula:
Samples for TSS analysis were crushed in 95 % (v/v) ethanol. The insoluble fraction of the extract was washed twice with 70 % ethanol, followed by 10 min of centrifugation at 3500 g to collect soluble fractions. An aliquot of 0·1 mL of combined supernatants was reacted with 3 mL of freshly prepared anthrone [150 mg of anthrone + 100 mL of 72 % (w/w) H2SO4] and put in a boiling water bath for 10 min. After cooling, the absorbance at 625 nm was determined with a spectrophotometer (Irigoyen et al., 1992).
For the measurement of leaf Pi concentration, frozen leaves were ground in distilled water, before centrifuging at 5000 g for 10 min. The supernatant was boiled at 100 °C for 7 min, and filtered through a 0·45 µm filter to remove debris (Mimura et al., 1996). The concentrations of Pi in the extract were colorimetrically measured using malachite green (Motomizu et al., 1980).
At the final harvest (123 d after sowing), shoots were removed at ground level, washed with 0·1 m HCl and then rinsed twice in deionized water to remove any adhering dust. Each soil column was opened vertically and was separated into three soil layers, i.e. 0–20, 20–40 and 40–60 cm. Roots in each layer were recovered by carefully sieving with a 2 mm sieve. The root system was rinsed with tap water, and then soaked in 0·01 m CaCl2 solution for 5 min to desorb nutrients on the root surface. Root length was determined using the WinRhizo Pro version 2003b program (Régent Instruments Inc., Québec, Canada).
Roots and shoots were dried at 70 °C for 72 h, weighed and then ground. Sub-samples of shoots and roots were digested with a mixture of nitric and perchloric acid (4:1) (Yuen and Pollard, 1954), and the concentrations of P in the digests were colorimetrically measured using malachite green.
The stress tolerance index (STI) was calculated as
where DWw and DWs were the dry weights under well-watered and drought conditions, respectively, and was the mean dry weight under well-watered conditions (Fernandez, 1992).
Plant water-use efficiency (WUE) was estimated as total dry weight divided by water use, where water use equals the amounts of rain plus water added to a column plus the difference in total column water mass between the beginning and end of the experiment (Jones et al., 2005).
Statistical analysis
All data were analysed using GenStat 10. Analysis of variance (ANOVA) tests were undertaken and least significant difference (LSD) calculated to assess the differences between treatment means (Steel and Torrie, 1980). The data for leaf water status, plant biomass, root morphology, concentrations of sugars and Pi in leaves, plant P concentrations and total P uptake were statistically analysed by factorial ANOVA to determine the effects of P, CO2 and drought (42–45 % of FWC), and their interactions.
RESULTS
Plant growth
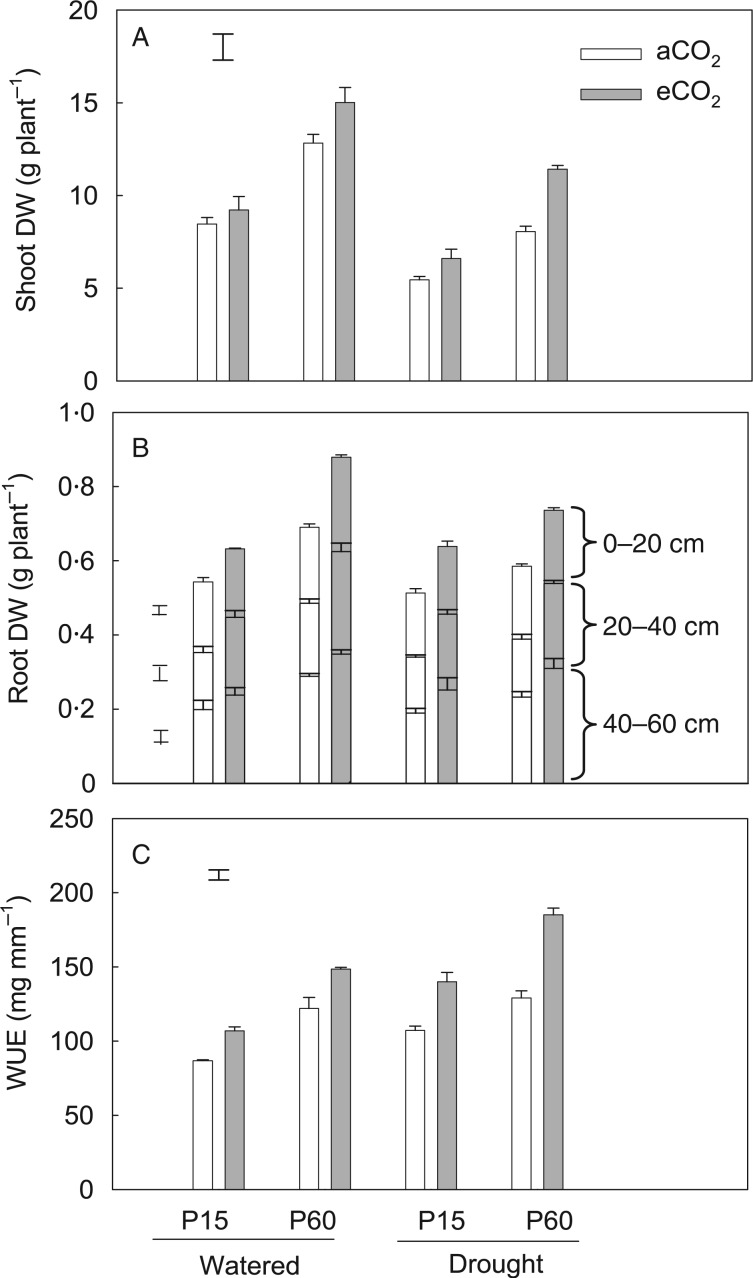
Drought markedly decreased shoot dry weight (by 31 %), but the drought-stressed plants exhibited the same response to CO2 and P application of shoot dry weight as did well-watered plants, resulting in an insignificant CO2 × P × drought interaction (P > 0·05) (Table 1). However, under drought conditions, eCO2 and adequate supply of P resulted in significantly greater shoot dry weight (11·5 g plant−1) than aCO2 + P15. Both P application and eCO2 significantly increased shoot dry weight (Tables 1 and 2). Increasing the P application rate from 15 to 60 mg kg−1 increased shoot dry weight from 8·5 to 12·8 g plant−1 (51 % increase) under aCO2 (Fig. 2A).When the plants were grown under eCO2, there was a further 9 and 17 % increase in shoot dry weight under P15 and P60, respectively, leading to a significant CO2 × P interaction (P < 0·05) (Table 1). Similarly, eCO2 and increasing P application increased leaf area by 21 and 61 %, respectively, while drought decreased leaf area by 36 % (Table 2).
Table 1.
Significant levels of main effects and interactions of CO2, P and drought
| Factors | CO2 | P | Drought | CO2 × P | CO2 × drought | P × drought |
|---|---|---|---|---|---|---|
| Shoot dry weight | *** | *** | *** | * | n.s. | n.s. |
| Root dry weight | *** | *** | * | n.s. | n.s. | ** |
| Water-use efficiency | *** | *** | *** | * | ** | n.s. |
| Stress tolerance index | *** | *** | – | ** | – | – |
| Nodule number | *** | *** | *** | ** | n.s. | * |
| Nodule dry weight | *** | *** | *** | * | n.s. | n.s. |
| N concentration | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| N uptake | *** | *** | *** | *** | n.s. | n.s. |
| Shoot P concentration | n.s. | *** | *** | n.s. | n.s. | * |
| Root P concentration | ** | *** | ** | n.s. | n.s. | n.s. |
| P uptake | *** | *** | *** | *** | n.s. | n.s. |
| Stomatal conductance | *** | *** | *** | n.s. | *** | *** |
| Transpiration efficiency | *** | *** | n.s. | n.s. | * | ** |
| Relative water content | ** | n.s. | *** | n.s. | *** | n.s. |
| Total soluble sugars | *** | * | *** | n.s. | *** | n.s. |
| Leaf Pi | n.s. | ** | *** | n.s. | n.s. | n.s. |
The CO2 × P × drought interaction was not significant for any of the measurements.
*, **, *** and n.s. indicate P < 0.05, P < 0.01, P < 0.001 and P > 0.05, respectively.
Table 2.
Average responses (%) to main treatments relative to the corresponding controls at the final measurements/harvest
| Factors | eCO2 | P60 | Drought |
|---|---|---|---|
| Shoot dry weight | 22 | 59 | −31 |
| Leaf area | 21 | 61 | −36 |
| Root dry weight | 26 | 26 | −9 |
| Water-use efficiency | 30 | 33 | 21 |
| Nodule number | 39 | 133 | −48 |
| Nodule dry weight | 27 | 106 | −39 |
| N concentration | 4 | 2 | 2 |
| N uptake | 21 | 70 | −13 |
| Shoot P concentration | −3 | 61 | 16 |
| Root P concentration | −6 | 40 | 7 |
| P uptake | 22 | 145 | −17 |
| Stomatal conductance | −26 | −20 | −87 |
| Transpiration efficiency | 34 | 32 | 5 |
| Relative water content | 5 | −2 | −21 |
| Total soluble sugars | 5 | 20 | 60 |
| Leaf Pi | −6 | 27 | 36 |
Fig. 2.
The effects of CO2, P and water regime on dry weight (DW) of shoots (A) and roots in the 0–20, 20–40 and 40–60 cm soil layers (B), and water-use efficiency (WUE) (C) of field pea. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol soil supplied with 15 (P15) or 60 mg P kg−1 (P15) soil, and drought-stressed plants had water withheld until the soil reached the permanent wilting point in the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the the LSD (P = 0.05).
Similar to the effects on shoot dry weight, eCO2 and P60 also increased root dry weight compared with their respective controls under both well-watered and drought conditions, but no significant CO2 × P × drought interaction was observed (P > 0·05). Elevated CO2 increased root dry weight by an average of 26 % (Table 2). Increasing P supply also increased root dry weight by an average of 26 %; this increase was most pronounced in deeper soil layers (45 %) than in the topsoil (10 % increase) (Fig. 2B; Table 2).
Water-use efficiency and stress tolerance index
On average, P60 and eCO2 increased WUE by 33 and 30 %, respectively (Table 2). Elevated CO2 increased WUE by 33 % at P60 supply, whereas the response to eCO2 was only 26 % when P was deficient (Fig. 2C). This contributed to a significant CO2 × P interaction (P < 0·05) (Table 1).
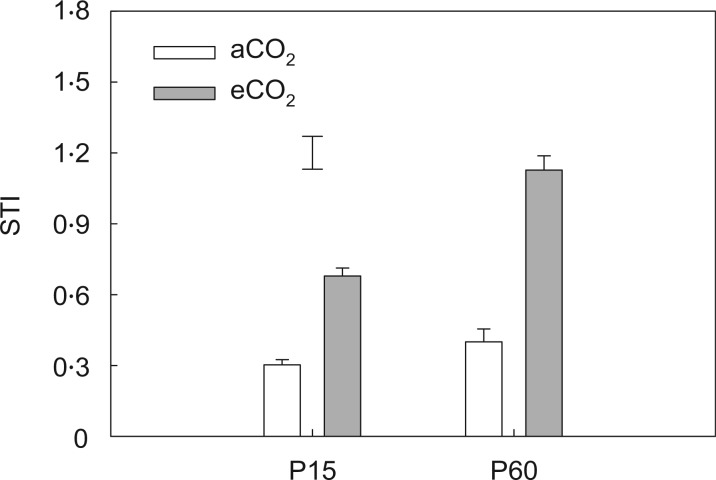
Alleviating P deficiency increased the STI by 123 %. This trend was greater when plants were grown under eCO2, resulting in a 175 % increase (Fig. 3). A significant CO2 × P interaction effect (P < 0·01) was observed for the STI (Table 1).
Fig. 3.
The effects of CO2 and P water regime on the stress tolerance index (STI) of field pea. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol soil supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld until the soil reached the permanent wilting point in the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the the LSD (P = 0.05).
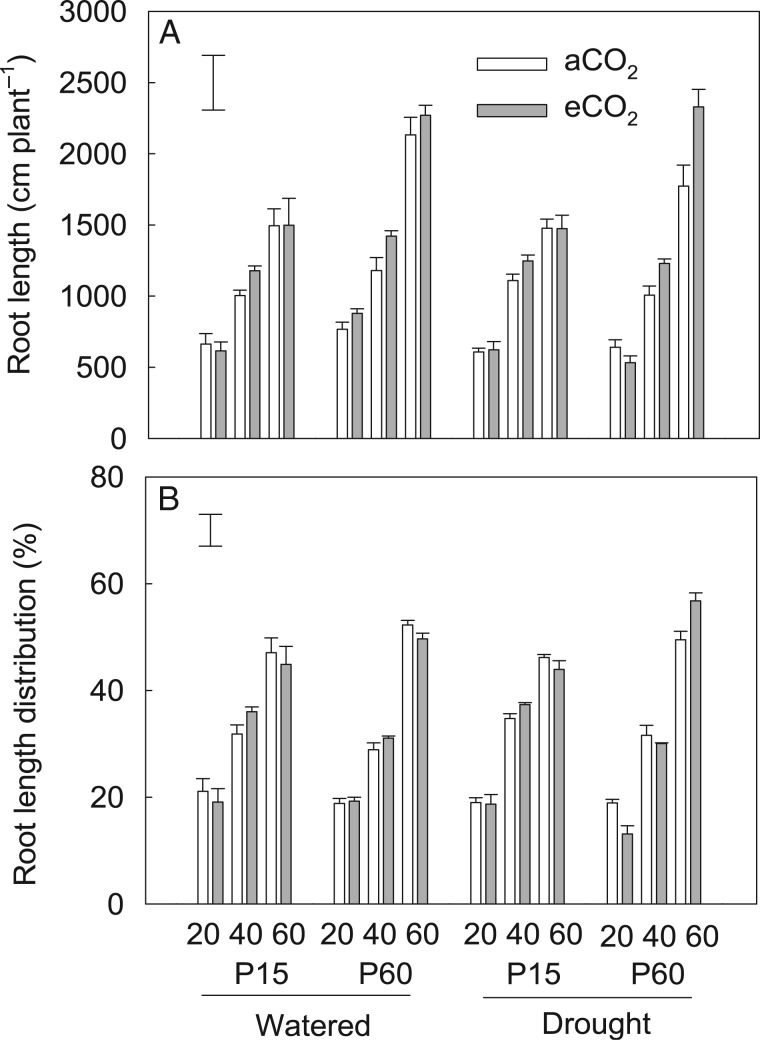
Spatial root length in soil profiles
Drought decreased overall root length, but did not interact with P supply or CO2 treatment. Although there was no significant effect of P × CO2 interaction on root length, each treatment alone had a significant effect. Plants had greater root length in soil profiles supplied with P60 than in those supplied with P15 (Fig. 4A). Compared with P15, P60 resulted in a 43 % increase in the root length in the bottom soil layer, but only a 17 % increase in the top soil layer. Elevated CO2 significantly increased root length regardless of P and drought treatments. Compared with P15, P60 significantly increased the distribution of root length in the bottom soil layer, resulting in an increase from 47 % at P15 to 53 % at P60 (Fig. 4B). However, eCO2 and drought did not affect the root length distribution within the soil profile.
Fig. 4.
The effects of CO2, P and water regime on root length (A) and root length distribution of field pea in the 0–20 cm (20), 20–40 cm (40) and 40–60 cm (60) soil layers (B). Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld until the soil reached the permanent wilting point in the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the the LSD (P = 0.05).
Nodulation and N uptake
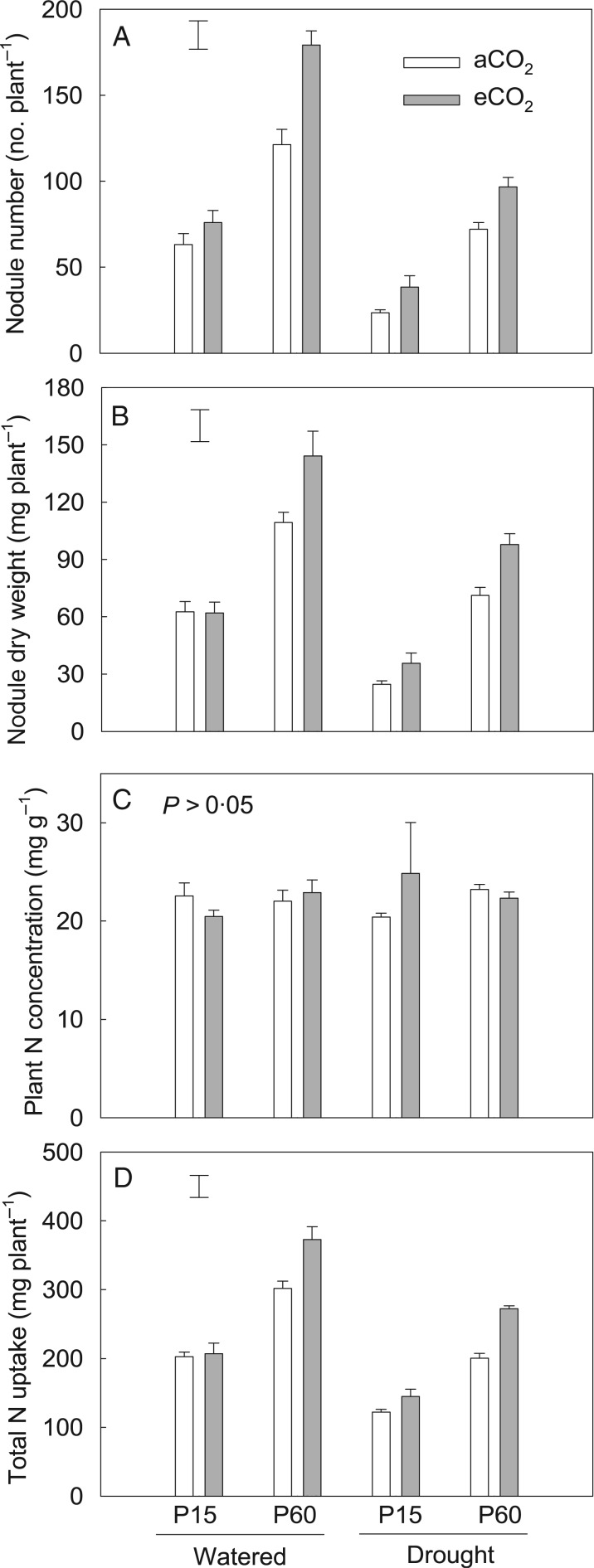
On average, drought decreased the nodule number by 48 % irrespective of P or CO2 treatments. Plants formed 133 % more nodules at P60 than at P15 (Table 2). The effect of CO2 concentration on nodulation depended on P supply; eCO2 increased nodule number by 21 % at P15 but increased it by 48 % at adequate P supply compared with aCO2 (Fig. 5A; Table 1). Nodule dry weight exhibited the same trend as nodule number (Fig. 5B; Table 2).
Fig. 5.
The effects of CO2, P and water regime on root nodule number (A), nodule dry weight (B), plant N concentration (C) and total N uptake (d) of field pea. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld until the soil reached the permanent wilting point in the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the the LSD (P = 0.05).
The concentration of N in shoot was not affected by P or CO2 treatment and reached 23 mg g−1 (Fig. 5C) which is in the adequate range (Reuter and Robinson, 1997; Deibert and Utter, 2004). Both P supply and eCO2 increased total N uptake by an average of 70 and 21 %, respectively (Table 2). Elevated CO2 resulted in greater plant N content when P supply was adequate than when it was deficient (Fig. 5D) (P < 0·01) (Table 1).
Plant P status
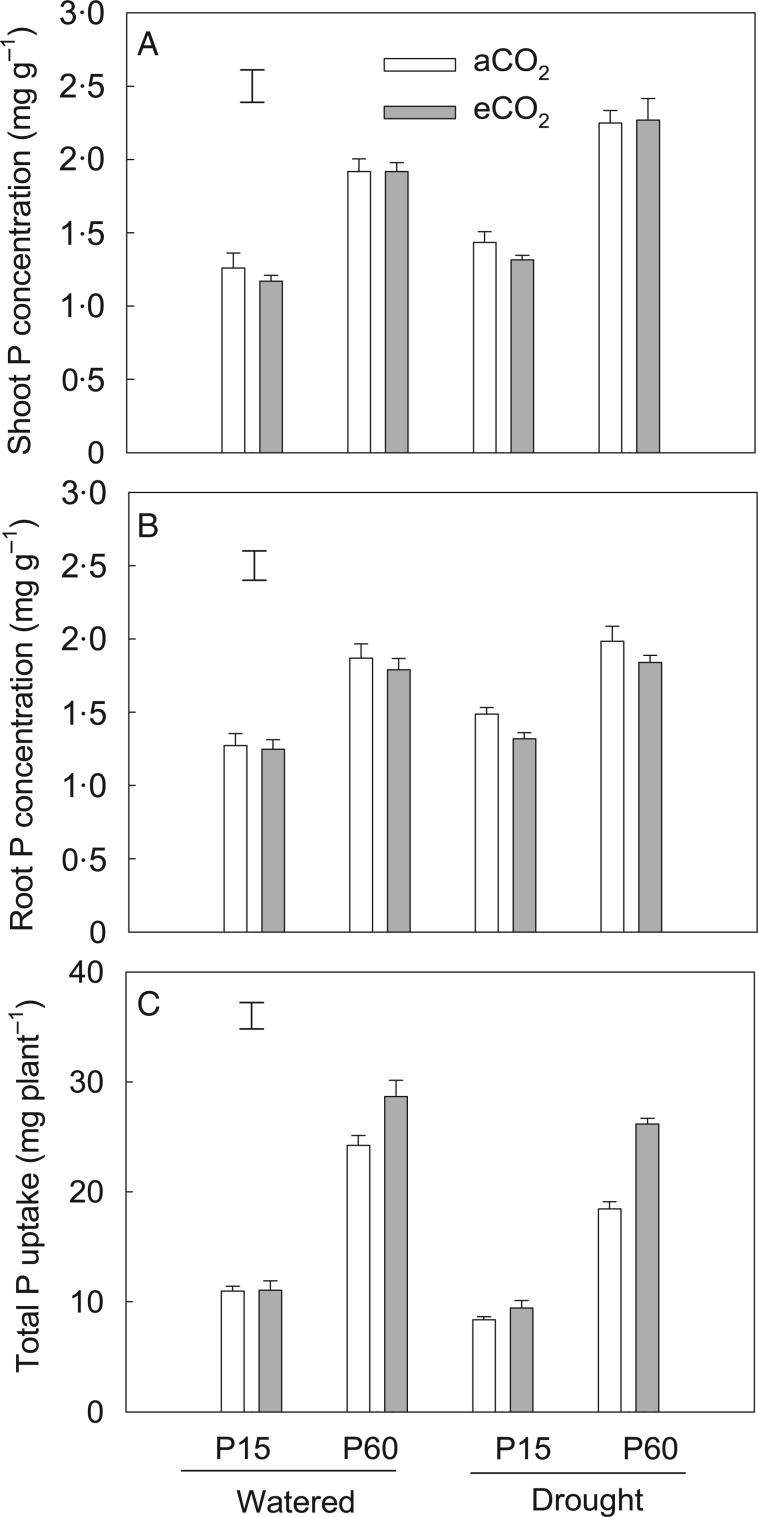
Drought increased P concentration in shoot by 16 % and in roots by 7 %, but decreased total P uptake by 17 % across P and CO2 treatments compared with well-watered plants (Fig. 6; Table 2). Irrespective of drought treatment, increasing P application significantly increased P concentrations in shoot and roots, and also increased total P uptake (Table 2). However, eCO2 did not affect P concentrations in either shoot or roots under either P or drought treatments (Fig. 6; Table 1). Elevated CO2 increased total P uptake by 22 %, and this increase was greater at P60 than at P15. Thus, a significant CO2 × P interaction effect (P < 0·001) on total P uptake was observed (Table 1).
Fig. 6.
The effects of CO2, P and water regime on P concentration in shoot (A) and roots (B), and total P uptake (C) of field pea. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld until the soil reached the permanent wilting point in the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the the LSD (P = 0.05).
Water relations
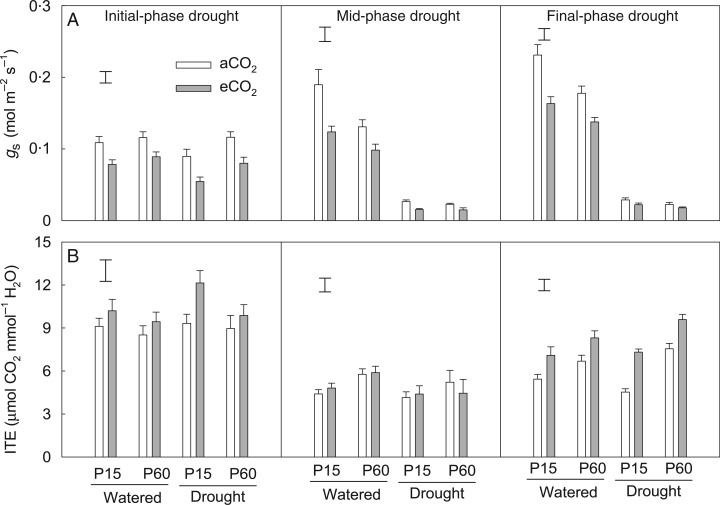
Both eCO2 and supplying an adequate level of P decreased stomatal conductance by 26 and 20 %, respectively, compared with their respective controls (Fig. 7A; Table 2), but no CO2 × P interaction was observed (Table 1). Compared with well-watered treatments, drought decreased stomatal conductance by 9 % during the initial drought phase and by 83–87 % during the mid and final phases. The effect of eCO2 or increasing P application on stomatal conductance was less in drought than in well-watered plants (Table 1), resulting in a significant CO2 × drought or P × drought interaction (P < 0·001).
Fig. 7.
The effects of CO2, P and water regime on stomatal conductance (gs) (A) and instantaneous transpiration efficiency (ITE) (B) of field pea at the flowering stage. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld to generate 63–70 % of field water capacity (FWC) (initial-phase drought at Day 107), 52–57 % of FWC (mid-phase drought at Day 114) and 43–46 % of FWC (final-phase drought at Day 122) during the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the LSD (P = 0.05).
Elevated CO2 significantly increased the ITE across P and drought treatments (Table 2). Increasing P application also increased the ITE at the mid and final phase of drought, with a rise of 28 % on average. Drought did not affect the ITE until the final drought phase (Fig. 7B). The ITE at P60 was higher during the final phase of drought than the respective well-watered control, whereas no difference was observed at P15 (Table 1). Interestingly, the ITE of plants supplied with P60 under eCO2 reached 9·6 µmol CO2 mmol−1 H2O during the final drought phase, which was the highest among all the treatments.
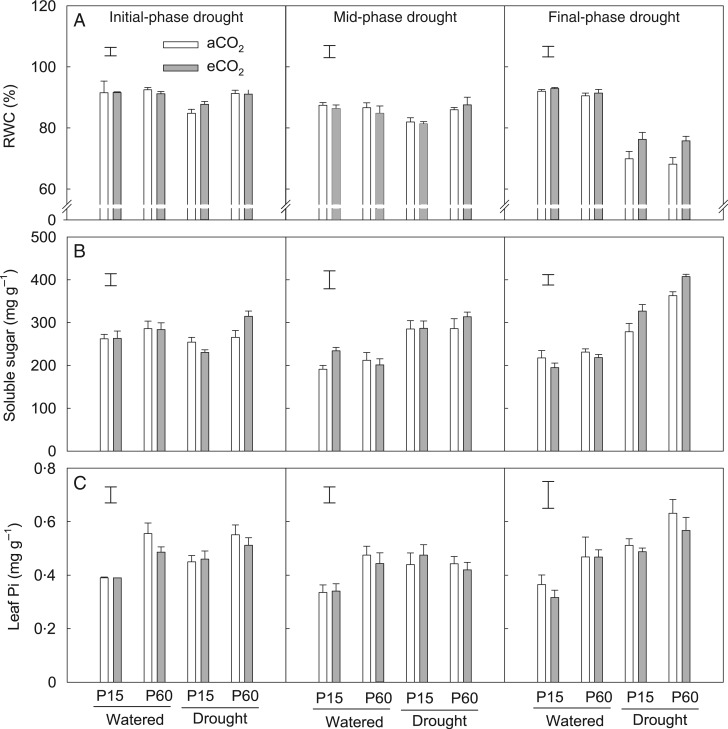
The initial- and mid-phase drought only had a significant effect on RWC when soil P supply was low, resulting in a 6 % decrease compared with the respective well-watered control (Fig. 8A). Compared with the mid-phase drought, the final phase of drought treatment led to a greater decrease in RWC regardless of P treatment. However, this drought-induced decrease in RWC at the final phase was less severe under eCO2 than that under aCO2, resulting in a significant CO2 × drought interaction (P < 0·001) (Table 1). There was no CO2 × P × drought interaction observed (P > 0·05).
Fig. 8.
The effects of CO2, P and water regime on relative water content of leaf (RWC) (A), and concentrations of total soluble sugars (B) and inorganic P (Pi) (C) in leaves of field pea at the flowering stage. Plants were exposed to ambient (aCO2) or elevated CO2 (eCO2) treatments for 123 d in a P-deficient vertisol supplied with 15 (P15) or 60 mg P kg−1 (P60) soil, and drought-stressed plants had water withheld to generate 63–70 % of field water capacity (FWC) (initial-phase drought at Day 107), 52–57 % of FWC (mid-phase drought at Day 114) and 43–46 % of FWC (final-phase drought at Day 122) during the last 3 weeks of the experiment. Columns are means of four replicates ± s.e. The vertical bars indicate the LSD (P = 0.05).
The imposition of drought treatments initially (phase 1) had no effect on TSS concentration except for the treatment with P60 + eCO2, where it significantly increased. As the drought continued, however (mid and final phases), the TSS concentration increased significantly, with this increase being greater under P60 and eCO2 than under other treatments (Fig. 8B; Tables 1 and 2).
Leaf Pi concentration was higher under P60 than under P15, but was not affected by eCO2 (Fig. 8C; Table 2). Drought at the initial and mid phases increased leaf Pi only when P supply was low, but, as the drought treatment continued (final phase), leaf Pi was significantly greater compared with their respective well-watered controls regardless of P supply. At the final phase of drought, the leaf Pi concentration was 27 % higher at P60 than that at P15. Leaf Pi concentration was positively correlated with TSS under drought conditions (r = 0·84, P < 0·05).
DISCUSSION
This study demonstrated for the first time that eCO2 and P application interactively improved drought tolerance of field pea. This is best exemplified by the finding that the eCO2-induced increase in the STI was greater in plants grown with a sufficient P supply than in those with an inadequate P supply (Fig. 3). Furthermore, the same trend was found for WUE, where eCO2 coupled with sufficient P supply resulted in the highest WUE when drought stress was imposed (Fig. 2). Consequently, in future eCO2 environments, increasing P supply may help to reduce the impact of drought on plant growth, while drought is predicted to occur more frequently in some environments (Allen et al., 2010). Previous studies have shown that changes in either CO2 or P can alter drought tolerance. For example, soybean, alfalfa and barley can utilize water more efficiently and are more tolerant to drought under eCO2 than under a CO2 (Erice et al., 2006; Robredo et al., 2007; Li et al., 2013). Similarly, alleviating P deficiency can also reduce water stress in white clover, soybean and cotton (Radin, 1984; Singh et al., 1997; Jin et al., 2005). Shen et al. (2013) also stated that appropriately manipulating P supply can enhance plant growth, nutrient uptake and the ability to resist various stresses, including water deficit.
The greater drought tolerance exhibited by field pea under eCO2 in this current experiment appears to be due to decreased stomatal conductance and associated reductions in water loss via transpiration which consequently increased instantaneous transpiration efficiency under drought stress (Fig. 7). The net effect was to increase RWC in the canopy of field pea (Fig. 8A). Many studies also found that the enhanced tolerance of plants to drought under eCO2 is consistent with a lower stomatal conductance and lower transpiration rate (Bunce, 1998; Morgan et al., 2004; Robredo et al., 2007). This reduction of stomatal conductance was the consequence of partial closure of the stomata, which was probably attributable to increased intercellular CO2 concentration (Ci) under eCO2 (Robredo et al., 2007). In this present study, even though the leaf area was greater under eCO2 than aCO2 (data not shown), the greater stomatal closure and the resulting lower transpiration rate led to a greater conservation of soil water as observed in other FACE studies (Manderscheid et al., 2014), and subsequently greater plant adaptability to soil water deficit.
Furthermore, in dry soils, eCO2 favoured an accumulation of soluble sugars in leaf cells (Fig. 8B), which in turn contributed to the flux of water into the leaf cells to maintain cell volume during drought (Seneweera et al., 2001; Sperdouli and Moustakas, 2012). The increased ITE under eCO2 (Fig. 7B) can explain the higher sugar accumulation in drought-stressed plants, indicating that the increased Ci by eCO2 enabled plants to assimilate more C during photosynthesis whilst using less water and thus minimizing drought-induced stress. It has also been suggested that the greater availability of sugars under eCO2 lowers osmotic potential at full turgor, allowing osmotic adjustment (Wullschleger and Norby, 2001) and thereby maintaining a high ψw (Tyree and Alexander, 1993) and RWC (Fig. 8A).
The beneficial effect of increasing P application on improving the tolerance of field pea to drought stress was greater at eCO2 than at aCO2. Increasing P application improved the water status during severe drought, resulting from the significant increase of TSS (Fig. 8B). This change appeared to result from the higher concentrations of leaf Pi that were recorded under sufficient P supply facilitating the accumulation of soluble sugars in leaves under eCO2 in the final phases of drought (Fig. 8C). Since low soil moisture inhibits P diffusion in soil through increasing the tortuosity (Barber and Wiley, 1995) as well as in plant tissues, the high leaf Pi would help to maintain energy-metabolic processes whilet the plant experienced temporary water stress (Peuke and Rennenberg, 2004). The significant relationship between Pi and TSS under drought conditions (r = 0·84, P < 0·05) indicates that the high Pi facilitated the translocation of triose sugars from the chloroplast, thereby enhancing the sugar status of plant tissue (Abel et al., 2002; Rychter and Rao, 2005; Lambers et al., 2006). Increasing P application in eCO2 environments is likely to enhance this positive effect further. Thus, large numbers of osmotically active molecules were synthesized (Wahid and Close, 2007; Farooq et al., 2009), which improved osmotic adjustment and maintained turgor under drought stress (Graciano et al., 2005). Therefore, the reduction of stomatal conductance and the increase of ITE under sufficient P supply and eCO2 (Fig. 7B) slowed water depletion and enhanced WUE.
The improved drought tolerance under eCO2 at adequate P supply was partly attributable to increased rooting depth. Although eCO2 did not alter the root distribution in soil profiles, it significantly increased root biomass (Fig. 2B) and length (Fig. 4A). Increasing P application led to both an overall increase in root growth and a greater proportion of these roots being distributed in deep soil layers, so that eCO2 combined with an adequate P supply resulted in the highest root length being recorded in the deepest soil layer (Fig. 4). This would possibly be attributable to higher P availability and/or greater P diffusion in the deep soil layer, which favoured the deep rooting, when the topsoil was dried out under the drought conditions. Consequently, deep root systems are potentially able to obtain greater amounts of soil water (Singh and Sale, 1998; Duursma et al., 2011), and improve drought adaptation (Zhou et al., 2008; Vadez et al., 2012; Kong et al., 2013). An additional contributor to the drought adaptation is the potential increases of root hydraulic conductance with higher P application (Al-Karaki et al., 1995; Singh et al., 2000). Alternatively, the enhanced water-extracting capability of deep roots is likely to maintain photosynthetic function when soils are dry. Significant relationships of deep rooting to ITE (r = 0·87, P < 0·01) and WUE (r = 0·74, P < 0·05) observed in this study also support this view.
This study showed that field pea plants supplied with adequate P exhibited decreased stomatal conductance compared with P-deficient plants (Fig. 7A). Shubhra et al. (2004) also found that P supply increased ψw in cluster bean under both water-sufficient and deficit conditions. Because xylem sap pH and abscisic acid have been considered as signals to modulate the stomatal behaviour in response to water deficit (Rodrigues et al., 2008), it is speculated that these signals may be enhanced by P supply under drought conditions, and thus lead to the reduction of stomatal conductance. However, this assumption needs further investigation. In contrast, many previous studies reported that P addition did not affect stomatal conductance, osmotic potential or transpiration rate in plants (Nelsen and Safir, 1982; Graciano et al., 2005). The discrepancy between the findings of the presnt study and those of these other published studies may be explained first by interspecific variation in sensitivity of stomatal conductance to P application. For example, P addition did not alter stomatal conductance in Eucalyptus but significantly increased stomatal conductance of common bean (Phaseolus vulgaris L.) (Graciano et al., 2005; dos Santos et al., 2006). Secondly, the response to P supply may differ with the duration of the experiment. Most of the previous studies on P-induced drought tolerance were conducted for periods of <70 d (Nelsen and Safir, 1982; Fitter, 1988; Graciano et al., 2005), while the present study lasted until the pod setting stage (116 d post-emergence). Physiology-related water relations can change markedly at different growth stages (Jin et al., 2005).
Although the original level of available N in this vertisol soil was low for plant growth, the field pea did not exhibit N limitation in this experiment, with plant N concentrations being above the deficient level (Reuter and Robinson, 1997; Deibert and Utter, 2004). An obvious reason for this is that the inoculated plant offset this limitation by fixing N2, as observed in a previous study (Jin et al., 2012). Thus, the low availability of mineral N in soil is unlikely to have restricted the plant response to eCO2, P and drought treatment in this study. However, it is worth noting that increasing P application increased nodulation and subsequent N uptake in the low-N vertisol, especially under eCO2 (Fig. 5; Table 1), which in turn may favour plant photosynthesis and stress tolerance (Jin et al., 2012; Devi and Sinclair, 2013).
Conclusions
Elevated CO2 increased the STI of field pea, especially when an adequate level of P was supplied to the plant, via decreased stomatal conductance, increased concentration of soluble sugars and maintenance of higher RWCs of leaves under drought stress. Increasing P supply under eCO2 increased root growth in the deep soil layer. The increased leaf Pi under sufficient P supply is likely to facilitate further the accumulation of soluble sugars in leaves under eCO2 and drought stress. These results imply that pulse crops supplied with sufficient levels of P may better withstand periodic drought stress in future eCO2 environments.
ACKNOWLEDGEMENTS
This research was supported by an Australian Research Council Linkage Project (LP100200757), and utilized the SoilFACE facility of the Department of Environment and Primary Industries, Victoria at Horsham. We thank Dr Saman Seneweera (The University of Melbourne) for valuable discussions during this experiment.
LITERATURE CITED
- Abel S, Ticconi CA, Delatorre CA. 2002. Phosphate sensing in higher plants. Physiologia Plantarum 115: 1–8. [DOI] [PubMed] [Google Scholar]
- Al-Karaki GN, Clark RB, Sullivan CY. 1995. Effects of phosphorus and water-stress levels on growth and phosphorus uptake of bean and sorghum cultivars. Journal of Plant Nutrition 18: 563–578. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Allen LHJ, Kakani VG, Vu JCV, Boote KJ. 2011. Elevated CO2 increases water use efficiency by sustaining photosynthesis of water-limited maize and sorghum. Journal of Plant Physiology 168: 1909–1918. [DOI] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. 2002. Plant breeding and drought in C3 cereals: what should we breed for? Annals of Botany 89: 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SA, Wiley J. 1995. Soil nutrient bioavailability: a mechanistic approach, 2nd edn New York: Wiley. [Google Scholar]
- Bunce JA. 1998. Effects of humidity on short-term responses of stomatal conductance to an increase in carbon dioxide concentration. Plant, Cell and Environment 21: 115–120. [Google Scholar]
- Cai WJ, Shi G, Li Y. 2005. Multidecadal fluctuations of winter rainfall over southwest Western Australia simulated in the CSIRO Mark 3 coupled model. Geophysical Research Letters 32: L12701. [Google Scholar]
- Calzadilla A, Rehdanz K, Betts R, Falloon P, Wiltshire A, Tol RSJ. 2013. Climate change impacts on global agriculture. Climatic Change 120: 357–374. [Google Scholar]
- Colwell JD. 1963. The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry 3: 190–198. [Google Scholar]
- Conroy JP, Virgona JM, Smillie RM, Barlow EW. 1988. Influence of drought acclimation and CO2 enrichment on osmotic adjustment and chlorophyll-A fluorescence of sunflower during drought. Plant Physiology 86: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibert EJ, Utter RA. 2004. Field pea growth and nutrient uptake: response to tillage systems and nitrogen fertilizer application. Communications in Soil Science and Plant Analysis 35: 1141–1165. [Google Scholar]
- Devi MJ, Sinclair TR. 2013. Nitrogen fixation drought tolerance of the slow-wilting soybean PI 471938. Crop Science 53: 2072–2078. [Google Scholar]
- Duursma RA, Barton CVM, Eamus D, et al. 2011. Rooting depth explains CO2 × drought interaction in Eucalyptus saligna. Tree Physiology 31: 922–931. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, McCaffery S, Evans JR. 2005. Phosphorus status determines biomass response to elevated CO2 in a legume:C4 grass community. Global Change Biology 11: 1968–1981. [Google Scholar]
- Erice G, Irigoye JJ, Pérez P, Martínez-Carrasco R, Sánchez-Díaz M. 2006. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiologia Plantarum 126: 458–468. [Google Scholar]
- FAO–UNESCO. 1976. Soil map of the world, 1:5 000 000, vol. X, Australia: UNESCO, Paris [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185–212. [Google Scholar]
- Fernandez GCJ. 1992. Effective selection criteria for assessing stress tolerance. In: Kuo CG, ed. Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress. Tainan, Taiwan. [Google Scholar]
- Fitter AH. 1988. Water relations of red-clover Trifolium-pratense L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. Journal of Experimental Botany 39: 595–603. [Google Scholar]
- Graciano C, Guiamet JJ, Goya JF. 2005. Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptus grandis seedlings. Forest Ecology and Management 212: 40–49. [Google Scholar]
- Irigoyen JJ, Emerich DW, Sanchezdiaz M. 1992. Water-stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Meidicago sativa) plants. Physiologia Plantarum 84: 55–60. [Google Scholar]
- Jin J, Wang GH, Liu XB, Pan XW, Herbert SJ. 2005. Phosphorus application affects the soybean root response to water deficit at the initial flowering and full pod stages. Soil Science and Plant Nutrition 51: 953–960. [Google Scholar]
- Jin J, Wang GH, Liu XB, Pan XW, Herbert SJ, Tang C. 2006. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. Journal of Plant Nutrition 29: 1433–1449. [Google Scholar]
- Jin J, Tang C, Armstrong R, Sale P. 2012. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient Vertisol. Plant and Soil 358: 86–99. [Google Scholar]
- Jin J, Tang C, Armstrong R, Butterly C, Sale P. 2013. Elevated CO2 temporally enhances phosphorus immobilization in the rhizosphere of wheat and chickpea. Plant and Soil 368: 315–328. [Google Scholar]
- Jones CA, Jacobsen JS, Wraith JM. 2005. Response of malt barley to phosphorus fertilization under drought conditions. Journal of Plant Nutrition 28: 1605–1617. [Google Scholar]
- Kong L, Si J, Sun M, Feng B, Zhang B, Li S, Wang Z, Wang F. 2013. Deep roots are pivotal for regulating post-anthesis leaf senescence in wheat (Triticum aestivum L.). Journal of Agronomy and Crop Science 199: 209–216. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu H, Qiao Y, et al. 2013. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max (L.) Merr.) under drought stress. Agricultural Water Management 129: 105–112. [Google Scholar]
- Manderscheid R, Erbs M, Weigel HJ. 2014. Interactive effects of free-air CO2 enrichment and drought stress on maize growth. European Journal of Agronomy 52: 11–21. [Google Scholar]
- Mimura T, Sakano K, Shimmen T. 1996. Studies on the distribution, re-translocation and homeostasis of inorganic phosphate in barley leaves. Plant, Cell and Environment 19: 311–320. [Google Scholar]
- Mollah M, Parington D, Fitzgerald G. 2011. Understand distribution of carbon dioxide to interpret crop growth data: Australian grains free-air carbon dioxide enrichment experiment. Crop and Pasture Science 62: 883–891. [Google Scholar]
- Morgan JA, Pataki DE, Körner C, et al. 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140: 11–25. [DOI] [PubMed] [Google Scholar]
- Motomizu S, Wakimoto T, Toei K. 1980. Spectrophotometric determination of phosphate in river waters with molybdite and malachite green. Analyst 108: 361–367. [DOI] [PubMed] [Google Scholar]
- Nelsen CE, Safir GR. 1982. Increased drought tolerance of mycorrhizal onion plants caused by improved phosphorus nutrition. Planta 154: 407–413. [DOI] [PubMed] [Google Scholar]
- van Ommen TD, Morgan V. 2010. Snowfall increase in coastal East Antarctica linked with southwest Western Australian drought. Nature Geoscience 3: 267–272. [Google Scholar]
- Petrone KC, Hughes JD, Van Niel TG, Silberstein RP. 2010. Streamflow decline in southwestern Australia, 1950−2008. Geophysical Research Letters 37: 1–7. [Google Scholar]
- Peuke A, Rennenberg H. 2004. Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): phosphorus most affected by drought. Trees-Structure and Function 18: 639–648. [Google Scholar]
- Radin JW. 1984. Stomatal response to water-stress and to abscisic acid in phosphorus deficient cotton plants. Plant Physiology 76: 392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW, Eidenbock MP. 1984. Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton plants. Plant Physiology 75: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment GE, Higginson FR. 1992. Australian laboratory handbook of soil and water chemical methods. Melbourne: Inkata Press. [Google Scholar]
- Reuter DJ, Robinson JB. 1997. Plant analysis: an interpretation manual, 2nd edn Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. 2009. Plant mechanisms to optimise access to soil phosphorus. Crop and Pasture Science 60: 124–143. [Google Scholar]
- Robredo A, Perez-Lopez U, de la Maza HS, et al. 2007. Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environmental and Experimental Botany 59: 252–263. [Google Scholar]
- Rodrigues ML, Santos TP, Rodrigues AP, et al. 2008. Hydraulic and chemical signalling in the regulation of stomatal conductance and plant water use in field grapevines growing under deficit irrigation. Functional Plant Biology 35: 565–579. [DOI] [PubMed] [Google Scholar]
- Rychter AM, Rao IM. 2005. Role of phosphorus in photosynthetic carbon metabolism. In: Pessarakli M, ed. Handbook of photosynthesis. Tucson, AZ: Taylor & Francis Group, LLC, 123–148. [Google Scholar]
- dos Santos MG, Ribeiro RV, de Oliveira RF, Machado EC, Pimentel C. 2006. The role of inorganic phosphate on photosynthesis recovery of common bean after a mild water deficit. Plant Science 170: 659–664. [Google Scholar]
- Seneweera S, Ghannoum O, Conroy JP. 2001. Root and shoot factors contribute to the effect of drought on photosynthesis and growth of the C4 grass Panicum coloratum at elevated CO2 partial pressures. Australian Journal of Plant Physiology 28: 451–460. [Google Scholar]
- Shen JB, Li CJ, Mi GH, et al. 2013. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. Journal of Experimental Botany. 64: 1181–1192. [DOI] [PubMed] [Google Scholar]
- Shubhra, Dayal J, Goswami CL, Munjal R. 2004. Influence of phosphorus application on water relations, biochemical parameters and gum content in cluster bean under water deficit. Biologia Plantarum 48: 445–448. [Google Scholar]
- Singh DK, Sale PWG. 1998. Phosphorus supply and the growth of frequently defoliated white clover (Trifolium repens L.) in dry soil. Plant and Soil 205: 155–162. [Google Scholar]
- Singh DJK, Sale PWG, McKenzie BM. 1997. Water relations of white clover (Trifolium repens L.) in a drying soil, as a function of phosphorus supply and defoliation frequency. Australian Journal of Agricultural Research 48: 675–681. [Google Scholar]
- Singh DK, Sale PWG, Pallaghy CK, McKenzie BM. 2000. Phosphorus concentrations in the leaves of defoliated white clover affect abscisic acid formation and transpiration in drying soil. New Phytologist 146: 249–259. [DOI] [PubMed] [Google Scholar]
- Sperdouli I, Moustakas M. 2012. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. Journal of Plant Physiology 169: 577–585. [DOI] [PubMed] [Google Scholar]
- Steel RG, Torrie JH. 1980. Principles and procedures of statistics: a biometrical approach, 2nd edn New York: McGraw-Hill. [Google Scholar]
- Tyree MT, Alexander JD. 1993. Plant water relations and the effects of elevated CO2 – a review and suggestions for future research. Vegetation 104: 47–62. [Google Scholar]
- Vadez V, Soltani A, Sinclair TR. 2012. Modelling possible benefits of root related traits to enhance terminal drought adaptation of chickpea. Field Crops Research 137: 108–115. [Google Scholar]
- Volaire F. 2003. Seedling survival under drought differs between an annual (Hordeum vulgare) and a perennial grass (Dactylis glomerata). New Phytologist 160: 501–510. [DOI] [PubMed] [Google Scholar]
- Wahid A, Close TJ. 2007. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biologia Plantarum 51: 104–109. [Google Scholar]
- Wheeler T, von Braun J. 2013. Climate change impacts on global food security. Science 341: 508–513. [DOI] [PubMed] [Google Scholar]
- Wullschleger SD, Norby RJ. 2001. Sap velocity and canopy transpiration in a sweetgum stand exposed to free-air CO2 enrichment (FACE). New Phytologist 150: 489–498. [Google Scholar]
- Yuen SH, Pollard AG. 1954. Determination of nitrogen in agricultural materials by the Nessler reagent. II. Micro-determination of plant tissue and soil extracts. Journal of the Science of Food and Agriculture 5: 364–369. [Google Scholar]
- Zhou SL, Wu YC, Wang ZM, Lu LQ, Wang RZ. 2008. The nitrate leached below maize root zone is available for deep-rooted wheat in winter wheat-summer maize rotation in the North China Plain. Environmental Pollution 152: 723–730. [DOI] [PubMed] [Google Scholar]