Abstract
Background and Aims Much evidence suggests that plant communities on infertile soils are relatively insensitive to increased water deficit caused by increasing temperature and/or decreasing precipitation. However, a multi-decadal study of community change in the western USA does not support this conclusion. This paper tests explanations related to macroclimatic differences, overstorey effects on microclimate, variation in soil texture and plant functional traits.
Methods A re-analysis was undertaken of the changes in the multi-decadal study, which concerned forest understorey communities on infertile (serpentine) and fertile soils in an aridifying climate (southern Oregan) from 1949–1951 to 2007–2008. Macroclimatic variables, overstorey cover and soil texture were used as new covariates. As an alternative measure of climate-related change, the community mean value of specific leaf area was used, a functional trait measuring drought tolerance. We investigated whether these revised analyses supported the prediction of lesser sensitivity to climate change in understorey communities on infertile serpentine soils.
Key Results Overstorey cover, but not macroclimate or soil texture, was a significant covariate of community change over time. It strongly buffered understorey temperatures, was correlated with less change and averaged >50 % lower on serpentine soils, thereby counteracting the lower climate sensitivity of understorey herbs on these soils. Community mean specific leaf area showed the predicted pattern of less change over time in serpentine than non-serpentine communities.
Conclusions Based on the current balance of evidence, plant communities on infertile serpentine soils are less sensitive to changes in the climatic water balance than communities on more fertile soils. However, this advantage may in some cases be lessened by their sparser overstorey cover.
Keywords: Plant community change, climate change, climate resistance, climate resilience, soil fertility, stress tolerance, plant functional traits, serpentine soil, specific leaf area, biogeographical affinity, topographic affinity, Klamath–Siskiyou
INTRODUCTION
Faced with the onslaught of evidence that global warming is causing range changes, altered phenology and disrupted interspecific interactions (Parmesan, 2006; Rosenzweig et al., 2008), ecologists sometimes seek to identify broad patterns such as the average pace of distributional shifts (e.g. Parmesan and Yohe, 2003); in other cases, they focus attention on particular species or communities that appear to be at greatest risk, such as those in the alpine zone (e.g. Pauli et al., 2007). Relatively little attention is drawn to species or communities facing lower than average risk, even though identifying these helps to complete our picture of ecological resistance and resilience in the face of global warming (e.g. Burkett et al., 2005). In one outstanding exception, Grime et al. (2000, 2008) showed that a native grassland on infertile limestone soils changed very little in response to a prolonged application of warming, drought and watering treatments that had caused dramatic compositional changes in other plant communities. These authors largely attributed the relative climate resistance of infertile limestone grasslands to a suite of plant functional traits associated with low relative growth rate and high tolerance for resource scarcity. [See also Damschen et al. (2012) for related evidence.]
Our research in recent years has focused on testing ‘Grime’s hypothesis’ in native-dominated plant communities on infertile soils in the California Floristic Province. In this semi-arid region, climatic warming is expected to drive increased seasonal water deficits that will dominate the future impacts of climate change on natural vegetation (Cornwell et al., 2012; Hannah et al., 2012; Thorne et al., 2012), making water availability a natural focus. Our work has compared responses to variable water availability in typical vegetation with that found on serpentine or ultramafic soils. Serpentine soils are high in Mg and low in Ca and primary nutrients; they are found throughout the world, and are particularly floristically rich in tropical and mediterranean climates (Brooks, 1987). Plant ecology and evolution on these soils, including the roles of nutrient scarcity, water balance and competition, have been especially well studied in California (Harrison and Rajakaruna, 2011). Prior to our recent work, several lines of evidence hinted at unusually low sensitivity to climatic variation in Californian serpentine plants. Vegetation on serpentine barrens looked scarcely different in photos taken several decades apart (Kruckeberg, 1984). Plant community turnover on a coastal-to-interior climatic gradient was much lower on serpentine than on non-serpentine soils (Harrison, 1999). Post-Pleistocene forest change, documented by pollen in lake sediments, was lower on serpentine than on granitic substrates (Briles et al., 2011).
Since 2010, our work in serpentine plant communities has added considerably more evidence supporting both lower climate sensitivity on infertile soils and the causal role of plant functional traits. In two multi-year experiments, the effects of watering on grassland biomass and/or composition were lower on serpentine soils (Eskelinen and Harrison, 2013; Fernandez-Going and Harrison, 2013). In a 10-year observational study, grasslands on serpentine soils fluctuated less in response to annual variability in rainfall than those on non-serpentine soils, and functional traits were important predictors of this difference (Fernandez-Going et al., 2012). In a geographical study across a 1200-km, 10-fold precipitation gradient, serpentine plant communities had consistently ‘stress-tolerant’ functional trait composition, while communities on more fertile soils varied strongly from ‘stress tolerant’ at the dry southern end to ‘stress intolerant’ at the wet northern end (Fernandez-Going et al., 2013). Finally, in a 15-year observational analysis, grassland species diversity has declined and composition has changed to more ‘stress tolerant’ as the climate has become more arid, but less so on serpentine than on non-serpentine soils (S. Harrison, E. Gornish and S. Copeland, unpubl. data).
Being able to conclude that Grime’s hypothesis is true in Californian serpentine plants would represent a small but important step toward understanding ecological resistance and resilience in the face of climate change. One piece of evidence that contradicts this conclusion, however, is the only existing study examining multi-decadal, climate-driven changes in communities on different soils (Damschen et al., 2010). In that study, we resurveyed 108 forest plots in the Siskiyou Mountains (southern Oregon, USA; climatically part of the California Floristic Province) that were first surveyed by Robert H. Whittaker in 1949–1951 (Whittaker, 1960). Understorey herb communities in 2007–2008 differed from those in 1949–1951 in two ways. First, they had become compositionally more similar to communities in warmer, more south-facing topographical positions on the same soils; second, they contained lower proportions of species with northern biogeographical affinities (henceforth we call these the ‘topographic’ and ‘biogeographical’ changes, respectively). These changes were consistent with the 2 °C higher temperatures and unchanged mean precipitation of the region, leading to increased climatic water deficit, which are expected to cause relative declines in stress-intolerant species. Contrary to Grime’s hypothesis, however, these changes were seen approximately equally in communities on serpentine and non-serpentine soils (Damschen et al., 2010).
Because understanding community variation in vulnerability to climate change is critically important, and because multi-decadal observations of change are among the best sources of evidence (Parmesan and Yohe, 2003; Parmesan, 2006; Rosenzweig et al., 2008), we sought to reconcile the apparent conflict between the Oregon study and other evidence. We considered four possibilities that arose from our prior knowledge of the Oregon study system.
Macroclimate.
In Oregon, the serpentine sites lie 20–45 km west (coastward) of the non-serpentine sites. Differences in present-day macroclimate (and, by implication, differences in how the macroclimate has changed since 1950) could have counteracted and obscured real differences in the climate sensitivity of understorey herb communities on the two soils. We would not see this effect in our Californian studies, where serpentine and non-serpentine sites were geographically well interspersed.
Overstorey.
In Oregon, our serpentine sites had less tree and shrub cover than our non-serpentine sites. Overstorey shading is known to buffer forest understorey herbs against climate change by modulating temperature extremes (De Frenne et al., 2013), and differences in shading could have counteracted differences between herb communities on the two soils. We would not see this effect in our Californian studies because these largely took place in open grasslands.
Soil texture.
Water availability to plants is less variable over time in coarser- than finer-textured soils (Sala et al., 1988), and some evidence suggests that soil texture correlates with community responsiveness to changes in rainfall (B. Fernandez-Going and A. Eskelinen, unpubl. data). If serpentine soils are especially coarse in our Californian but not Oregonian sites, this could explain why serpentine communities appeared less responsive only in California.
Functional traits as superior indicators of community change.
Growing evidence supports plant functional traits as indicators of climatic effects (e.g. Cornelissen et al., 2003; Westoby and Wright, 2006). In particular, species with low specific leaf area (SLA = leaf area/dry mass) have high water use efficiency and low relative growth rates. Thus, decreases in community mean values of SLA indicate compositional shifts toward more drought-tolerant species (e.g. Harrison et al., 2010; Sandel et al., 2010; Soudzilovskaia et al., 2013). Because this functional trait is so directly linked to the physiology of drought tolerance, if we use it as our metric of climatically driven community change, we may find a predicted difference (i.e. lower change on serpentine soils) that we were unable to detect with our other two metrics.
MATERIALS AND METHODS
Study system and prior results
Our study (Damschen et al., 2010) took place in the Klamath–Siskiyou Mountains (California and Oregon, USA), a well-known centre of plant diversity and endemism (Whittaker, 1960; Coleman and Kruckeberg, 1999). Dominant vegetation types include mixed evergreen forest on non-serpentine substrates, and open woodland on ultramafic (serpentine, peridotite) substrates, with variable amounts of shrub cover and herb understorey in both cases.
In 2007–2008, we resampled as closely as possible 55 serpentine and 53 non-serpentine sites at similar elevations that had first been sampled by ecologist Robert H. Whittaker in 1949–1951 (Whittaker, 1960). We located sites using the information left by Whittaker, namely the road or trail along which they were found and their elevation (to 30·5 m), slope (to 5°) and aspect (to 15°). At each site, Whittaker and we estimated the percentage cover of each tree, shrub and herb species using 100 points (i.e. the corners of 25, 1-m2 quadrats) along a 50 -m transect. Whittaker found 117 herb species and we found 122; sites varied considerably in herb species composition both within and between soils.
In our analyses, we focused on understorey herbs because we expected them to have the greatest potential for change. The climate had warmed by 2 °C and precipitation had not changed since 1949–1951, so we expected compositional differences consistent with increased water deficit. No individual species were common enough to use as indicators, and the elevational precision of the data was insufficient to detect upward shifts. We devised two community-level tests for changes consistent with warming.
In the topographic test, we ordinated the community data from sites on each soil using non-metric dimensional scaling (NMDS), and identified the ordination axis associated with the variation in species composition from cool north-facing to warm south-facing aspects (this so-called topographic moisture gradient was a central element in Whittaker’s sampling design). As predicted, communities had shifted over time along this axis, so that the species composition of each site in 2007–2008, as compared with the same site in 1949–1951, was slightly more similar to the species composition of a warm south-facing site at the same elevation and on the same soil. This shift occurred equally on the two soils (Table 1).
Table 1.
Physical variables and community change on serpentine and non-serpentine study sites
| Serpentine |
Non-serpentine |
||||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | P | |
| Number of sites | 55 | 53 | |||
| Elevation (m) | 726 | 226 | 804 | 163 | 0·89 |
| Latitude (°N) | 40·62 | 7·69 | 42·13 | 0·01 | <0·001 |
| Longitude (°W) | 119·29 | 23·36 | 123·45 | 0·03 | <0·001 |
| Mean annual precipitation (cm) | 97·70 | 11·52 | 97·72 | 9·45 | 0·995 |
| Precipitation seasonality | 1488·50 | 137·59 | 1057·72 | 50·89 | <0·001 |
| Mean annual temp. (°C) | 9·77 | 1·15 | 9·77 | 0·95 | 0·995 |
| Maximum annual temp. (°C) | 27·15 | 1·97 | 29·18 | 1·43 | <0·001 |
| Temperature seasonality | 512·69 | 30·34 | 574·48 | 10·61 | <0·001 |
| Overstorey sover (%) | 87·1 | 48·0 | 194·8 | 42·8 | <0·001 |
| Mean July ground temp. (°C) | 22·4 | 2·1 | 18·9 | 0·9 | <0·001 |
| Maximum July ground temp. (°C) | 50·3 | 3·8 | 45·3 | 4·4 | <0·001 |
| Soil texture (% sand) | 52·2 | 10·0 | 58·5 | 9·5 | 0·002 |
Macroclimatic values came from BIOCLIM (http://www.worldclim.org/bioclim), while ground (=understorey) temperatures were measured in the field in July 2014. P-values are for individual t-tests comparing sites on the two soils (values in bold are significant).
In the biogeographical test, we categorized herbs as belonging to families or genera of ‘northerly’ (Arcto-Tertiary) or ‘other’ (Madro-Tertiary, Californian, desert, cosmopolitan, unknown) origin, using the monograph of Raven and Axelrod (1978). As predicted, the proportional cover of species of ‘northerly’ origin had declined from 1949–1951 to 2007–2008. The change was again not significantly different on the two soils.
For more details of the study site, methods and earlier results, see Damschen et al. (2010).
New data collection
To examine macroclimate as a covariate, we extracted bioclimatic variables for the latitude and longitude of each site from Hijmans et al. (2005). These values are derived from miscellaneous sources in 1960–1990 and spatially interpolated to 0·5′ precision. We considered mean, maximum and seasonal variability of annual temperature, and mean and seasonal variability of annual precipitation (Hijmans et al., 2005).
To examine overstorey as a modifier of the microclimate, we calculated overstorey cover for each site by summing the cover values for each tree and shrub species as measured by Damschen et al. (2010). We measured the summer microclimate of each site by deploying Thermochron I-Buttons (Maxim Integrated, San Jose, CA, USA) to one randomly chosen location at each site from 30 June to 31 July 2014. These recorded temperatures every 2 h for 30 d, from which we calculated mean and maximum July understorey temperatures.
To examine soil texture, we collected soil samples from 5–20 cm depth at each site in June 2014. These were analysed for percentage sand (as well as silt and clay) by A&L Western Laboratories (Modesto, CA, USA).
To consider community mean SLA as an alternative metric of change, we collected 1–2 leaves from each of five mature and non-senesced individuals of each herb species. Leaves were photographed while fresh and their areas were measured from these photos. Leaves were then dried for 72 h at 60 °C and weighed, and SLA was calculated as area/dry mass (mm2 g−1). We summed the product of each species’ SLA and its percentage cover to obtain an abundance-weighted mean SLA for each site at each time period (1949–1951 or 2007–2008). We use this metric to determine if drought-intolerant (high SLA) species have declined over time relative to other species; we have no data bearing on the question of intraspecific trait changes, which would be expected (if anything) to reinforce the patterns caused by changes in species abundance.
Statistical analyses
For each newly collected variable described above (macroclimate, overstorey, microclimate, soil texture and SLA), we compared serpentine and non-serpentine sites using t-tests.
We next investigated whether macroclimate, overstorey or soil texture affected community change in ways that could alter our inferences about soil-related differences. For each variable that differed significantly between soil types (serpentine and non-serpentine), we used two general linear models with soil type, the variable and their interaction as predictors, and community change measured by either the topographic or the biogeographical variable (described above) as the response.
To use SLA to test for differences between soils in the extent to which communities shifted in a drought-tolerant direction, we took the difference between each site’s abundance-weighted mean SLA in 1949–1951 and in 2007–2008, and used a t-test to see if this change differed between the two soils.
RESULTS
Serpentine sites were significantly north and west of non-serpentine sites. In terms of macroclimate, mean annual temperature and precipitation did not differ between soils, but serpentine sites had lower seasonal variability in temperature and higher seasonal variability in precipitation. Serpentine sites averaged >50 % lower in overstorey cover, 3·5 °C higher in field-measured mean July understorey temperatures and 10 % less coarse in soil texture than non-serpentine sites (Table 1).
The macroclimate hypothesis was not supported. No macroclimatic variables that differed between the two soils, or latitude or longitude, had significant main or interactive effects on either the topographic measure of community change (P = 0·33–0·90) or the biogeographical measure of community change (P = 0·47–0·81).
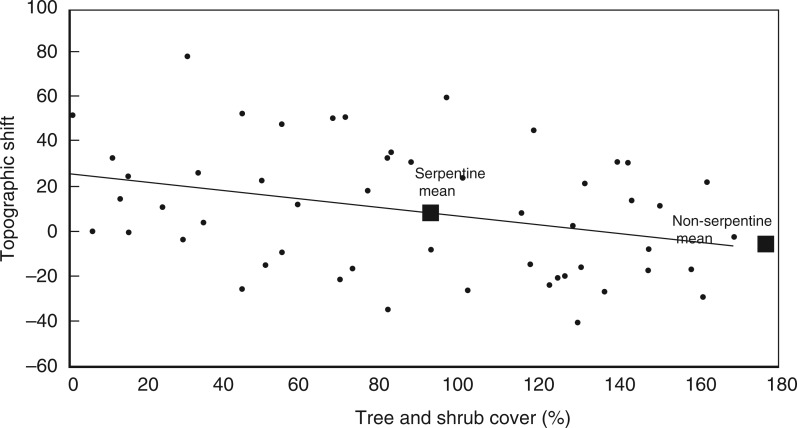
In support of our overstorey hypothesis, higher tree and shrub cover was associated with significantly lower mean (r = −0·73, P < 0·001) and maximum (r = −0·23, P < 0·001) understorey temperatures as measured in July 2014. Overstorey cover was negatively related to the biogeographical measure of community change, and interacted with soil to affect the topographic measure of community change (Table 2, Fig. 1). Overstorey cover was negatively related to the topographic measure of change on serpentine soils (r = −0·19, P = 0·018), but had no effect on non-serpentine soils (r = 0·06, P = 0·51). Using the regression relationship observed on serpentine soils, a serpentine community would change much less if it had the same overstorey cover as the average non-serpentine community (Fig. 1).
Table 2.
Soil type, tree cover and their interaction as predictors of (a) topographic change (= change in ordination axis 1 scores), (b) biogeographical change (= change in percentage northerly-origin species); no other physical variables considered in similar models had significant main or interactive effects as predictors of community change (see text)
| SS | d.f. | MS | F | P | |
|---|---|---|---|---|---|
| (a) Change in ordination Axis 1 score (R2 = 0·070, n = 97) | |||||
| Soil | 1264 | 1 | 1264 | 1·896 | 0·172 |
| Tree cover | 883 | 1 | 0883 | 1·324 | 0·263 |
| Interaction | 3051 | 1 | 3051 | 4·574 | 0·035 |
| Error | 62031 | 93 | 667 | ||
| (b) Change in percentage northerly-origin species (R2 = 0·065, n = 108) | |||||
| Soil | <0·001 | 1 | <0·001 | 0·001 | 0·975 |
| Tree cover | 0·504 | 1 | 0·504 | 5·057 | 0·027 |
| Interaction | 0·017 | 1 | 0·017 | 0·168 | 0·682 |
| Error | 10·365 | 104 | 0·100 | ||
Fig. 1.
Relationship of overstorey cover to the topographic measure of community change over time on serpentine soils. Units on the y-axis are dimensionless ordination scores.
In a model including soil type, overstorey cover and their interaction, mean understorey temperature remained different between the two soils (P < 0·001) as well as being influenced by overstorey cover (P < 0·001) and the interaction (P = 0·008).
The soil texture hypothesis was not supported. Soil coarseness, measured as percentage sand, had no main or interactive effects on either the topographic measure of change (texture P = 0·77, texture–soil interaction P = 0·43) or the biogeographical measure of community change (texture P = 0·20, texture–soil interaction P = 0·21).
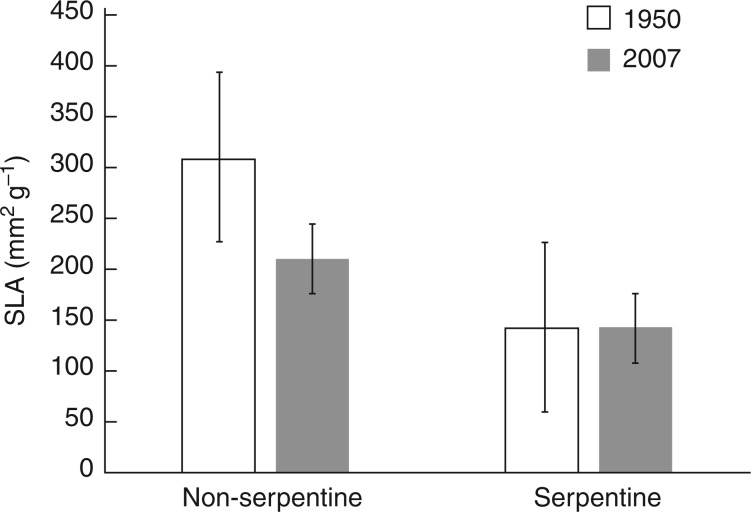
As expected, community mean SLA was considerably lower on serpentine than on non-serpentine soils in both time periods (1949–1951 and 2007–2008), suggesting that serpentine species are more stress-tolerant on average. Also as expected, community mean SLA declined between these time periods on non-serpentine soils only (Fig. 2), indicating that on these soils, there was a differential loss of drought-intolerant (high SLA) species.
Fig. 2.
Community mean values of specific leaf area (SLA) on serpentine and non-serpentine soils at two time periods. The change in community mean SLA between time periods was significantly higher on non-serpentine soils (t-test, P < 0·001).
DISCUSSION
Historical resampling studies have provided some of the most compelling evidence for climate-related changes in species distributions and phenology (reviewed by Parmesan and Yohe, 2003; Parmesan, 2006). Compared with experiments and other approaches, resurvey studies yield direct indications of future change and avoid many well-known artefacts. Yet historical resampling studies are not without complexities and surprises. One prominent problem is disentangling the contribution of climate from those of other factors that may cause change (Rosenzweig et al., 2008). Many other problems lie in relocating sites and reinterpreting old data. Yet other issues may arise from sampling designs that were determined by other researchers decades ago with different questions in mind. In our case, the existence of climate-driven changes in Whittaker’s sites was supported by multiple lines of evidence, but the more subtle question of whether these changes varied between sites on different soils was harder to answer, especially when these sites were not well interspersed geographically. Faced with conflict between one historical resampling study and many other sources of evidence, then, we believed it was reasonable to revisit the historical study and look for additional insights.
Using as our measure of change the mean value of SLA, a key climate-related functional trait, we found a result that supported ‘Grime’s hypothesis’ and helped to reconcile our historical study with other evidence: initially higher prevalence, followed by greater decline, of drought-intolerant species on fertile soils. A growing number of climate change studies are using SLA and other functional traits as both predictors and indicators of change (e.g. Harrison et al., 2010; Sandel et al., 2010; Soudzilovskaia et al., 2013). Traits show great promise as a currency of plant community change, because they are easily measured by highly repeatable means (e.g. Garnier et al., 2001; Cornelissen et al., 2003) and make it possible to compare responses among communities with few or no species in common (e.g. Wright et al., 2004; Westoby et al., 2006). The topographic and biogeographical tests we devised to analyse change in our diverse Oregonian communities are supported in principle by a handful of other studies (e.g. Valiente-Banuet et al., 2006; Debinski et al., 2013). However, they also have some practical disadvantages; biogeographical designations may be somewhat subjective or simply unavailable, and ordination scores are not very transparent. Regardless, our results are a reminder that community change is multidimensional and it may be advisable to compare the results of different metrics.
Using overstorey cover as a covariate, we found that well-shaded understorey communities changed less over the six decades of climatic warming than did more open understories. This result agrees well with recent work suggesting that forest canopies buffer understorey herbs against climatic ‘thermophilization’ (De Frenne et al., 2013). Our results suggest that for herb communities on serpentine soils, the benefit of more stress-tolerant functional traits is counterbalanced by the disadvantage of less shading. Clearly, any generalizations about the relative vulnerability of plant communities on infertile soils to climate change (e.g. Grime et al., 2000, 2008; Damschen et al., 2012) need to take into account whether soil infertility is associated with lower overstorey cover.
We conclude that the balance of evidence supports what we have called Grime’s hypothesis, linking soil infertility to a stress-tolerant functional trait syndrome that tends to confer unusually high resistance of plant species and communities to climate change. We qualify this by saying that our evidence only applies to communities that are becoming effectively drier because of declining precipitation and/or increasing temperatures leading to greater water deficits. Such aridification is both observed and projected in California, much of the western US, and other arid and semi-arid climates (Seager et al., 2007; Thorne et al., 2012). We also caution that less sensitive does not mean insensitive, and that the future survival of endemic-rich communities on infertile soils could be undermined by nutrient deposition, stress-tolerant invasive species and habitat loss (Damschen et al., 2012).
ACKNOWLEDGEMENTS
For field and laboratory assistance we thank MackNeal Byers, Roger Stephens and Stefan Groszev. For access to the study sites we thank the Rogue–Siskiyou National Forest and Sis-Q Camp. For providing us a base of operations we thank Jim Gurley and Kathy Mechling. This study was supported by NSF grant 1439246 to S. Harrison and A. Latimer.
LITERATURE CITED
- Briles CE, Whitlock C, Skinner CN, Mohr J. 2011. Holocene forest development and maintenance on different substrates in the Klamath Mountains, northern California, USA. Ecology 92: 590–601. [DOI] [PubMed] [Google Scholar]
- Brooks RR. 1987. Serpentine and its vegetation . Portland, OR: Dioscorides Press. [Google Scholar]
- Burkett VR, Wilcox DA, Stottlemyer R, et al. 2005. Nonlinear dynamics in ecosystem response to climatic change: case studies and policy implications. Ecological Complexity 2: 357–394. [Google Scholar]
- Coleman RG, Kruckeberg AR. 1999. Geology and plant life of the Klamath-Siskiyou mountain region. Natural Areas Journal 19: 320–340. [Google Scholar]
- Cornelissen JH, Lavorel CS, Garnier E, et al. 2003. A handbook for protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- Cornwell WK, Stuart S, Ramirez A, Dolanc CR, Thorne JH, Ackerly DD. 2012. Climate change impacts on California vegetation: physiology, life history, and ecosystem change. California Energy Commission Report CEC-500-2012-023. [Google Scholar]
- Damschen EI, Harrison SP, Grace JB. 2010. Climate change effects on an endemic-rich edaphic flora: resurveying Robert H. Whittaker’s Siskiyou sites (Oregon, USA). Ecology 91: 3609–3619. [DOI] [PubMed] [Google Scholar]
- Damschen EI, Harrison S, Ackerly DD, Fernandez-Going BM, Anacker BL. 2012. Endemic plant communities on special soils: early victims or hardy survivors of climate change? Journal of Ecology 100: 1122–1130. [Google Scholar]
- Debinski DM, Carruthers JC, Cook D, Crowley J, Wickham H. 2013. Gradient-based habitat affinities predict species vulnerability to drought. Ecology 94: 1036–1045. [DOI] [PubMed] [Google Scholar]
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, et al. 2013. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences USA 110: 18561–18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen AM, Harrison S. 2013. Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition. Ecology 95: 682–692. [DOI] [PubMed] [Google Scholar]
- Fernandez-Going BM, Harrison S. 2013. Effects of experimental water addition depend on grassland community characteristics. Plant Ecology 214: 777–796. [Google Scholar]
- Fernandez-Going BM, Anacker BL, Harrison S. 2012. Temporal variability in California grasslands: soil type and species functional traits mediate response to precipitation. Ecology 93: 2104–2114. [DOI] [PubMed] [Google Scholar]
- Fernandez-Going BM, Anacker BL, Harrison S, Safford HD. 2013. Climate interacts with soil to produce beta diversity in the Californian flora. Ecology 94: 2007–2018. [DOI] [PubMed] [Google Scholar]
- Garnier E, Laurent G, Bellmann A, et al. 2001. Consistency of species ranking based on functional leaf traits. New Phytologist 152: 69–83. [DOI] [PubMed] [Google Scholar]
- Grime JP, Brown VK, Thompson K, et al. 2000. The response of two contrasting limestone grasslands to simulated climate change. Science 289: 762–765. [DOI] [PubMed] [Google Scholar]
- Grime JP, Fridley JD, Askew AP, Thompson K, Hodgson JG, Bennett CR. 2008. Long-term resistance to simulated climate change in an infertile grassland. Proceedings of the National Academy of Sciences of the United States of America 105: 10028–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L, Shaw MR, Roehrdanz P, Ikegami M, Soong O, Thorne J. 2012. Consequences of climate change for native plants and conservation. California Energy Commission Report CEC-500-2012-024. [Google Scholar]
- Harrison S. 1999. Local and regional diversity in a patchy landscape: native, alien and endemic herbs on serpentine soils. Ecology 80: 70–80. [Google Scholar]
- Harrison S, Rajakaruna N. 2011. Serpentine: evolution and ecology of a model system. Berkeley, CA: University of California Press. [Google Scholar]
- Harrison S, Damschen EI, Grace JB. 2010. Ecological contingency in the effects of climatic warming on forest herb communities. Proceedings of the National Academy of Sciences USA 107: 19362–19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RI, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Kruckeberg AR. 1984. California serpentines: flora, vegetation, geology, soils, and management problems . Berkeley, CA: University of California Press. [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics 37: 637–669. [Google Scholar]
- Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- Rosenzweig C, Karoly D, Vicarelli M, et al. 2008. Attributing physical and biological impacts to anthropogenic climate change. Nature 453: 353–357. [DOI] [PubMed] [Google Scholar]
- Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13: 147–156. [Google Scholar]
- Raven P, Axelrod D. 1978. Origin and relationships of the California flora. Berkeley, CA: University of California Publications in Botany. [Google Scholar]
- Sala OE, Parton WJ, Joyce LA, Lauenroth WJ. 1988. Primary production of the central grassland region of the United States. Ecology 69: 40–45. [Google Scholar]
- Sandel B, Goldstein LJ, Kraft NJ, et al. 2010. Contrasting trait responses in plant communities to experimental and geographic variation in precipitation. New Phytologist 188: 565–575. [DOI] [PubMed] [Google Scholar]
- Seager R, Ting M, Held I, et al. 2007. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316: 1181–1184. [DOI] [PubMed] [Google Scholar]
- Soudzilovskaia NA, Elumeeva TG, Onipchenko VG, et al. 2013. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proceedings of the National Academy of Sciences USA 110: 18180–18184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JH, Boynton R, Flint L, Flint A, N’goc Le T. 2012. Development and application of downscaled hydroloclimatic predictor variables for use in climate vulnerability and assessment studies. California Energy Commission Report CEC-500-2012-010. [Google Scholar]
- Valiente-Banuet A, Rumebe AV, Verdu M, Callaway RM. 2006. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proceedings of the National Academy of Sciences USA 103: 16812–16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westoby M, Wright IJ. 2006. Land-plant ecology on the basis of functional traits. Trends in Ecology & Evolution 21: 261–268. [DOI] [PubMed] [Google Scholar]
- Whittaker R. 1960. Vegetation of the Siskiyou mountains, Oregon and California. Ecological Monographs 30: 279–338. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]