Abstract
Background and Aims Autumn leaf senescence marks the end of the growing season in temperate ecosystems. Its timing influences a number of ecosystem processes, including carbon, water and nutrient cycling. Climate change is altering leaf senescence phenology and, as those changes continue, it will affect individual woody plants, species and ecosystems. In contrast to spring leaf out times, however, leaf senescence times remain relatively understudied. Variation in the phenology of leaf senescence among species and locations is still poorly understood.
Methods Leaf senescence phenology of 1360 deciduous plant species at six temperate botanical gardens in Asia, North America and Europe was recorded in 2012 and 2013. This large data set was used to explore ecological and phylogenetic factors associated with variation in leaf senescence.
Key Results Leaf senescence dates among species varied by 3 months on average across the six locations. Plant species tended to undergo leaf senescence in the same order in the autumns of both years at each location, but the order of senescence was only weakly correlated across sites. Leaf senescence times were not related to spring leaf out times, were not evolutionarily conserved and were only minimally influenced by growth habit, wood anatomy and percentage colour change or leaf drop. These weak patterns of leaf senescence timing contrast with much stronger leaf out patterns from a previous study.
Conclusions The results suggest that, in contrast to the broader temperature effects that determine leaf out times, leaf senescence times are probably determined by a larger or different suite of local environmental effects, including temperature, soil moisture, frost and wind. Determining the importance of these factors for a wide range of species represents the next challenge for understanding how climate change is affecting the end of the growing season and associated ecosystem processes.
Keywords: Leaf senescence, phenology, growing season, phylogeny, botanical gardens, climate change, deciduous woody plants, trees, shrubs, vines
INTRODUCTION
Research on phenological responses to climate change has concentrated on the spring season, while the autumn has been comparatively neglected (Walther, 2010; Vitasse et al., 2011; Gallinat et al., 2015). Nonetheless, the phenology of autumn leaf senescence is important to ecosystem function and species performance (Lim et al., 2007). The time from leaf out to leaf senescence defines the length of photosynthetic activity and affects water, carbon and nutrient cycling and hence annual net ecosystem production in temperate ecosystems (Wu et al., 2013). Leaf senescence also represents the loss of a food source to insects and herbivores. At the community level, a warmer autumn may provide an opportunity for some species, such as non-native shrubs, to keep their leaves longer than native shrubs, so gaining a competitive advantage through added carbon uptake and growth (Fridley, 2012). At the ecosystem level, the extension of the growing season could result in higher plant productivity, greater wood growth and increased carbon dioxide uptake (Menzel and Fabian, 1999; Kramer et al., 2000; Richardson et al., 2010; Wu et al., 2013), while greater respiration could reduce or negate some of these effects (Piao et al., 2008). Through its effect on water cycling and surface albedo, leaf senescence phenology (and growing season length) can also affect climate and climate models (Piao et al., 2007).
Given the major role of autumn leaf senescence phenology in temperate and boreal forest ecology, it is important to understand the factors that influence variation in leaf senescence across space and time; these factors could include phylogeny, location, spring leaf out phenology, growth habit or wood anatomy, all of which are known to be related to general plant phenology (Polgar and Primack, 2011; Panchen et al., 2014). Unfortunately, our understanding of leaf senescence phenology is primarily limited to a few species and locations (Kikuzawa, 1983; Lee et al., 2003; Leuzinger et al., 2005; Richardson et al., 2006; Friedman et al., 2011; Fridley, 2012) and large-scale observational studies remain rare (Menzel and Fabian, 1999; Gordo and Sanz, 2009; Ibanez et al., 2010). In addition, satellite-derived data for the end of the growing season cover large areas but do not allow researchers to consistently distinguish among species.
Another challenge for quantifying variation in leaf senescence phenology is the lack of a standard definition of leaf senescence across species. Researchers have typically constructed their own definitions of leaf senescence, to suit their particular species and research site (Gallinat et al., 2015). Such definitions often involve some percentage of leaf drop or leaf colour change (IPGE, 1960; Jolly et al., 2005; Estrella and Menzel, 2006; Ibanez et al., 2010). The inconsistency in definitions, however, limits comparisons across studies, species and locations.
Here we report the results of a study of autumn leaf senescence of 1360 deciduous woody species at six botanical gardens and arboreta in Asia, Europe and North America to characterize variation among species, years and locations. At each location and for each species, we used a single consistent definition of leaf senescence for individual plants at the canopy level. This study, which builds on an earlier study of leaf out times for these same species (Panchen et al., 2014), allows us to address the following questions. (1) What is the range of variation in leaf senescence phenology among species, years and locations? (2) Do species senesce their leaves in a consistent sequence from year to year and across locations? (3) Is leaf senescence phylogenetically conserved and are certain genera or families of plants more likely to senesce early or late in the autumn? (4) Do spring leaf out times, growth habit (tree, shrub or vine), wood anatomy or proportion of leaf colour to leaf drop explain variation in leaf senescence phenology?
MATERIALS AND METHODS
Field work
We monitored leaf senescence of 1360 deciduous woody species at six botanical gardens and arboreta in Asia, North America and Europe in 2012 and at four of these botanical gardens and arboreta in 2013 (Table 1). Not every species was monitored at every site (see Supplementary Data Table S1). Only one plant per species was monitored at a site, as our goal was to obtain data on as many species as possible. We avoided using horticultural varieties that had been selected for leaf colour change in the summer and autumn.
Table 1.
Mean leaf senescence date, standard deviation (s.d.), number of species (n), start and finish of leaf senescence and range of leaf senescence in days for 2012 and 2013 leaf senescence dates monitored at the Arnold Arboretum, Boston, MA, USA; Botanic Garden and Botanical Museum Berlin-Dahlem, Berlin, Germany (Berlin BG); Garden in the Woods, Framingham, MA, USA; Ottawa Arboretum, Ottawa, Canada; Beijing Botanical Garden, Beijing, China (Beijing BG); and Morton Arboretum, Lisle, IL, USA with their respective latitude, longitude, altitude (Alt.) and mean annual temperature
| Year | Latitude, longitude | Alt. (m) | Annual temp (°C) | Mean date | s.d. | n | Start date | Finish date | Range (d) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Arnold Arboretum | 2012 | 42°18′N, 71°07′W | 22 | 10·7 | 20 Oct. 12 | 21 | 895 | 29 Jul. 12 | 15 Dec. 12 | 139 |
| 2013 | 19 Oct. 13 | 19 | 831 | 28 Aug. 13 | 12 Dec. 13 | 106 | ||||
| Garden in the Woods | 2012 | 42°20′N, 71°25′W | 57 | 10·7 | 18 Oct. 12 | 23 | 123 | 3 Sep. 12 | 13 Dec. 12 | 101 |
| Berlin BG | 2012 | 52°27′N, 13°17′E | 57 | 9·2 | 15 Oct. 12 | 18 | 880 | 5 Sep. 12 | 11 Dec. 12 | 97 |
| 2013 | 14 Oct. 13 | 19 | 943 | 16 Sep. 13 | 19 Jan. 14 | 125 | ||||
| Ottawa Arboretum | 2012 | 45°23′N, 75°42′W | 80 | 5·8 | 19 Oct. 12 | 12 | 143 | 27 Sep. 12 | 9 Nov. 12 | 43 |
| 2013 | 23 Oct. 13 | 17 | 150 | 27 Sep. 13 | 18 Nov. 13 | 52 | ||||
| Beijing BG | 2012 | 39°59′N, 116°12′E | 74 | 11·9 | 26 Oct. 12 | 14 | 109 | 31 Aug. 12 | 2 Dec. 12 | 93 |
| 2013 | 2 Nov. 13 | 30 | 144 | 10 Sep. 13 | 10 Jan. 14 | 144 | ||||
| Morton Arboretum | 2012 | 41°49′N, 88°03′W | 223 | 10·0 | 5 Oct. 12 | 9 | 147 | 24 Sep. 12 | 2 Nov.12 | 39 |
We defined leaf senescence as the date on which at least half of the leaves on an individual plant had changed colour (were no longer green) and/or had dropped from the plant. This definition aims to capture the date at which an individual plant has reached an estimated loss of 50 % of the photosynthetic capacity of its entire canopy and can be applied to all deciduous species in our study. For example, the plant could be considered to have senesced in any of these scenarios: 50 % or more of its leaves had fallen off and all remaining leaves were still green; 20 % of the leaves had fallen off and 30 % of the leaves had changed colour; 50 % of its leaves had changed colour, even if all of the leaves were still present. At the Arnold Arboretum, we verified the reliability of visual determinations of leaf senescence in Viburnum species by measuring the chlorophyll content in the field using the atLEAF (FT Green LLC, Wilmington, DE, USA) and SPAD-502 (Soil Plant Analysis Development, Minolta Camera Co., Ltd, Tokyo, Japan) chlorometers, as well as in the lab using spectrophotometry. We confirmed that if a leaf is yellow, orange or very light green, it has lost virtually all of its chlorophyll content and hence lost the associated photosynthetic capacity (Gallinat et al., 2013), as has also been observed for crop species (Zhu et al., 2012).
For the same, single individual of each species at each site, we recorded the leaf senescence date, the percentage of leaves dropped, the percentage of leaves that had changed colour and the colour of the leaves. We measured only one individual of each species at each site to maximize the number of species we could monitor, as we had done in our previous study (Panchen et al., 2014). The timing of leaf senescence can vary by several days to more than a week among individuals within a species even at a single location (Lee et al., 2003; Fracheboud et al., 2009), so results for individual species in our study should be treated with care. Also, the few species showing very early leaf senescence dates in July or August at the Arnold Arboretum could have been reacting to drought stress or insect damage.
We monitored the species at each garden at 7- to 14-d intervals over an approx. 3-month period. This resulted in monitoring each garden about 8–10 times during the leaf senescence period, which is approximately the same number of sampling times as the previous leaf out study (Panchen et al., 2014). Due to the large number of species, it was not possible to monitor the plants at more frequent intervals. For each species, we recorded only a single date for leaf senescence: we did not record the dates on which less than 50 % of the leaves had undergone leaf senescence. Similarly, once a species had been recorded as having reached 50 % leaf senescence, we stopped monitoring it.
Our monitoring method has a certain degree of subjectivity, which is common to all observational studies of leaf senescence where observers must measure the proportion of coloured and dropped leaves. We reduced the subjectivity as much as possible by having representatives of the six botanical gardens agree to the protocol before the field season, having just one or relatively few people carry out the monitoring at each garden and having the observers at a garden practise evaluating plants together to ensure that their observations would be comparable.
Analysis of range of variation
We began our analysis by calculating descriptive statistics (mean, s.d., start, finish and range) at each site to characterize the range of variation in leaf senescence phenology across species.
Phylogenetic analysis
We tested for a phylogenetic signal in the leaf senescence dates at each site using Pagel’s λ (Pagel, 1999) and Blomberg’s K (Blomberg et al., 2003) using the ‘phylosig’ function in the package ‘phytools’ version 0.2-1 in R version 2.15.1 (R Foundation, Vienna, Austria) (Revell, 2012). Both Pagel’s λ and Blomberg’s K are continuous indices that indicate the phylogenetic relatedness of an observed trait across a phylogeny assuming Brownian motion. A Pagel’s λ of 0 or a Blomberg’s K of 0 both indicate no phylogenetic signal. A Pagel’s λ of 1 indicates a complete phylogenetic signal while a Blomberg’s K of 1 indicates a phylogenetic signal and greater than 1 indicates a strong phylogenetic signal (Pagel, 1999; Blomberg et al., 2003; Ackerly, 2009; CaraDonna and Inouye, 2015). We used the same methodology and phylogenies used in a previous study on leaf out times (Panchen et al., 2014). The purpose of this test is to determine if evolutionary relationships among species explain any of the variation in leaf senescence dates, indicating that the character is evolutionarily conserved. If a phylogenetic signal is seen then the statistical analyses should control for the phylogeny. The phylogenetic signal for leaf senescence timing was either not significant or very weak (Table 2) so we did not control for the phylogeny in our further statistical analyses.
Table 2.
The phylogenetic signal (Pagel’s λ with associated P value and Blomberg’s K with associated P value) in the 2012 and 2013 leaf senescence dates at the Arnold Arboretum (AA), Garden in the Woods (GitW), Berlin Botanic Garden (Berlin), Ottawa Arboretum (Ottawa), Beijing Botanical Garden (Beijing) and Morton Arboretum (Morton) for the PHYLOMATIC tree, a composite phylogenetic tree of all woody species in our study and the high-resolution PHLAWD phylogeny, a subset of the woody species included in the study
| λ | P | K | P | |
|---|---|---|---|---|
| PHYLOMATIC tree | ||||
| AA 2012 | 0·2290 | 0 | 0·3062 | 0·569 |
| AA 2013 | 0·3133 | 0 | 0·3085 | 0·593 |
| GitW 2012 | 0 | 1 | 0·5313 | 0·17 |
| Berlin 2012 | 0·2305 | 0 | 0·2973 | 0·735 |
| Berlin 2013 | 0·2522 | 0 | 0·3379 | 0·453 |
| Ottawa 2012 | 0·291 | 0·0002 | 0·5771 | 0·286 |
| Ottawa 2013 | 0·3414 | 0 | 0·5221 | 0·335 |
| Beijing 2012 | 0 | 1 | 0·348 | 0·835 |
| Beijing 2013 | 0·3915 | 0 | 0·9588 | 0·106 |
| Morton 2012 | 0·4588 | 0·0003 | 0·7027 | 0·052 |
| PHLAWD phylogeny | ||||
| AA 2012 | 0·2837 | 0 | 0·0165 | 0·106 |
| AA 2013 | 0·4291 | 0 | 0·0179 | 0·091 |
| GitW 2012 | 0 | 1 | 0·026 | 0·868 |
| Berlin 2012 | 0·4291 | 0·0004 | 0·0149 | 0·493 |
| Berlin 2013 | 0·5047 | 0·0004 | 0·0121 | 0·807 |
| Ottawa 2012 | 0 | 1 | 0·0451 | 0·396 |
| Ottawa 2013 | 0 | 1 | 0·0311 | 0·566 |
| Beijing 2012 | 0 | 1 | 0·1198 | 0·237 |
| Beijing 2013 | 0 | 1 | 0·1095 | 0·254 |
| Morton 2012 | 0·294 | 0·0219 | 0·0434 | 0·184 |
Sequence of leaf senescence analysis
To test whether species (as represented by a single individual at each garden) senesce leaves in a consistent sequence from year to year, we used analysis of covariance (ANCOVA), with the 2013 leaf senescence date as the continuous response variable, the 2012 leaf senescence date as a continuous predictor variable and site as a categorical predictor variable. To test whether species senesce leaves in a consistent sequence across sites we used ANCOVA, with the leaf senescence date at the Arnold Arboretum as the continuous response variable, leaf senescence date at each of the other sites separately as continuous predictor variables and year as a categorical predictor variable. The Arnold Arboretum was used as the standard because this site had the most species monitored in 2012 (see Supplementary Data Table S1). We also tested whether species senesce leaves in a consistent sequence within one site, specifically between two distinct areas within the Berlin Botanic Garden. We used ANCOVA with leaf senescence date at one area as the continuous response variable, leaf senescence date at the other area as the continuous predictor variable and area as a categorical predictor; in this case one area had more widely spaced woody plants than the other area.
We compared the order of leaf senescence between years and sites at the genus and family levels. We calculated the average leaf senescence date for each genus with three or more species and for each family with five or more species. We ran ANCOVA models at the genus and at the family levels with the 2013 leaf senescence date as the continuous response variable, the 2012 leaf senescence date as a continuous predictor variable and site as a categorical predictor variable. We also ran ANCOVA models at the genus and at the family levels with leaf senescence dates at the Arnold Arboretum as the continuous response variable, leaf senescence dates at each of the other sites separately as the continuous predictor variable and year as the categorical predictor variable.
Analysis of other factors that might influence leaf senescence phenology
We tested for the influence of four factors on the phenology of leaf senescence: date of spring leaf out, growth habit, wood anatomy and leaf colour versus leaf drop.
To test the effect of leaf out dates on leaf senescence dates, we used ANCOVA with the 2012 leaf senescence date as the continuous response variable, the 2012 leaf out date (Panchen et al., 2014) as a continuous predictor variable and site as a categorical predictor variable.
We used the growth habit (tree, shrub or vine) classification for each species from Panchen et al. (2014). We used the Welch difference of means test with α = 0·05 to determine if there were significant differences in leaf senescence dates for different growth habits at each site in 2012 and in 2013. The Welch test accounted for our unequal sample sizes by assuming unequal variance between samples.
We used the wood anatomy (diffuse, ring or semi-ring porous) classification for each species from Panchen et al. (2014). We used the Welch difference of means test with α = 0·05 to determine if there were significant differences in leaf senescence dates for different wood anatomy classifications at each site in 2012 and in 2013.
To determine if there is a consistent pattern whereby certain species tended to undergo leaf senescence by changing colour or by dropping leaves, we calculated a leaf colour index. The colour index for each species in a year is the per cent colour change divided by the sum of the per cent colour change and the per cent leaf drop. A species with a colour index value of 1 underwent leaf senescence exclusively through leaf colour change. A species with a colour index value of 0 underwent leaf senescence exclusively through leaf drop. To test whether the colour index was consistent from year to year, we ran an ANCOVA model for leaf colour index with the 2013 colour index as the continuous response variable, 2012 colour index as the continuous predictor and site as a categorical predictor. We used standard least squares modelling to determine how much of the variation in leaf senescence dates was explained by percentage leaf drop and percentage leaf colour. We used JMP11 (SAS Institute, Cary, NC, USA) for all non-phylogenetic statistical analyses.
RESULTS
Range of variation
A substantial amount of variation exists in leaf senescence dates among species within a site (Table 1). At the Arnold Arboretum, Berlin Botanic Garden, Garden in the Woods and Beijing Botanical Garden, there are more than 3 months between the dates on which the first and last species senesced. At the Arnold Arboretum in 2012, species varied by 139 d or more than 4 months in leaf senescence dates, with the first species undergoing leaf senescence at the end of July and the last species senescing in the middle of December. The leaf senescence period was distinctly briefer in Ottawa, the coldest site with a smaller taxonomic diversity (see Supplementary Data Tables S2 and S3), beginning in late September and ending in mid-November in both years.
There are large differences in the leaf senescence dates of different genera and families (see Supplementary Data Tables S2 and S3). For example, at the Arnold Arboretum in 2012, the genera Aronia and Gleditsia had particularly early leaf senescence dates at the end of September while the genera Rosa, Forsythia and Neillia were the last to senesce in the second week of November. There was a 1- to 3-month difference in leaf senescence dates of the early genera compared with the later genera. At the family level, early senescence dates at the Berlin Botanic Garden in 2012 were Araliaceae, Grossulariace and Vitaceae at the end of September and late senescence dates in the deciduous species of Pinaceae, Rutaceae and Ulmaceae at the end of October. There were some families and genera with a wide range in leaf senescence dates. Rosaceae at the Berlin Botanic Garden provides one example where species of Chaenomeles, Rosa and Cotoneaster were among the first to senesce their leaves and some species of Rosa and Cotoneaster were also among the last.
Phylogenetic analysis
The phylogenetic signal in leaf senescence dates at each site was generally low, with Pagel’s λ ranging from 0 to 0·5 and Blomberg’s K not being significant, suggesting that timing of leaf senescence at each site is not phylogenetically conserved (Table 2). This indicates that evolutionary relationships are not a significant factor in determining leaf senescence patterns, and hence phylogeny was not controlled for in the statistical analyses. Notably, in the previous leaf out study, phylogenetic signal was highly significant (Panchen et al., 2014).
Sequence of leaf senescence
At the Arnold Arboretum, Berlin Botanic Garden, Ottawa Arboretum and Beijing Botanical Garden, the association between leaf senescence dates in 2012 and 2013 in the ANCOVA model was moderate but significant (R2 = 0·43, n = 1802, P < 0·0001), indicating that species that senesce earlier in one year tend to do so in the following year but more than half of the variation remains unexplained (Fig. 1). The association of leaf senescence dates between the Arnold Arboretum and four of the other five sites in the ANCOVA models was weak but significant, with R2 values at or below 0·17, suggesting that species are not undergoing leaf senescence in the same sequence at different sites (Table 3, Fig. 2). The association of leaf senescence dates between the Arnold Arboretum and the Beijing Botanical Garden was not significant. The association between the two distinct areas at Berlin Botanic Garden in the ANCOVA model was very weak but significant (R2 = 0·083, N = 168, P = 0·0026). In the previous study, the associations of leaf out times at the Arnold Arboretum with other gardens were much stronger and highly significant, with R2 values of 0·32–0·72 (Panchen et al., 2014).
Fig. 1.
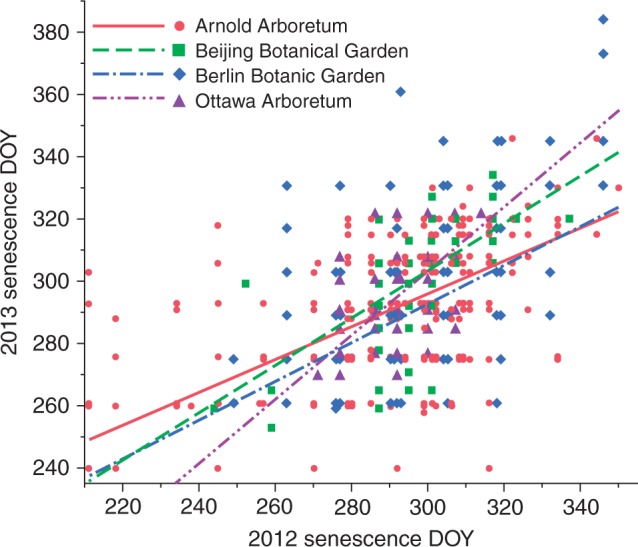
Association between leaf senescence day of year (DOY) in 2012 and 2013 at the Arnold Arboretum, Beijing Botanical Garden, Berlin Botanic Garden and Ottawa Arboretum (R2 = 0·43, n = 1802, P < 0·0001), showing the sequence of leaf senescence of species between years at each site. Each point on the graph represents an individual species at a site.
Table 3.
ANCOVA results for species leaf senescence dates, comparing the dates at the Arnold Arboretum with the dates at Berlin Botanic Garden (Berlin BG), Garden in the Woods, Ottawa Arboretum, Beijing Botanical Garden (Beijing BG) and Morton Arboretum with year (2012 and 2013) as a categorical predictor variable
| Arnold Arboretum with: | R2 | n | P |
|---|---|---|---|
| Garden in the Woods | 0·17 | 90 | <0·0001 |
| Berlin BG | 0·04 | 1134 | <0·0001 |
| Ottawa Arboretum | 0·10 | 237 | <0·0001 |
| Morton Arboretum | 0·06 | 121 | 0·0076 |
| Bejing BG | 0·03 | 166 | 0·1458 |
Fig. 2.
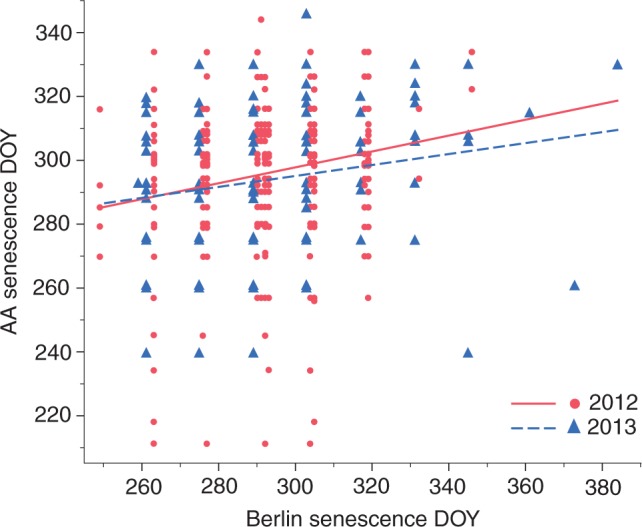
Association between leaf senescence day of year (DOY) at Arnold Arboretum (AA) and the Berlin Botanic Garden (Berlin) for 2012 and 2013 (R2 = 0·04, n = 1134, P < 0·0001), illustrating the sequence of leaf senescence of species between sites. Each point on the graph represents an individual species in a particular year.
The ANCOVA model for mean leaf senescence of genera between years was significant (R2 = 0·58, n = 165, P < 0·0001); that is, certain genera tend to undergo leaf senescence earlier and others later in a consistent pattern. Leaf senescence dates at the genus level at the Arnold Arboretum were moderately to weakly associated with leaf senescence dates at the genus level at four of the other five sites with R2 values of 0·013–0·57 but not the Beijing Botanical Garden, indicating varying levels of consistency across sites (Table 4). The strongest genus-level senescence sequence association was between the Arnold Arboretum and Garden in the Woods, both of which are located in Massachusetts, USA. Using these mean genus-level values, the dates of leaf senescence had a stronger association between successive years at the same site than between sites. Note that although the same genera are being compared between sites, the actual species monitored were often different between sites, potentially weakening the power of the model. In the earlier leaf out study, there was a much higher degree of association in the order of leaf out between botanical gardens at the genus level; for example, at the genus level, the association between the Arnold Arboretum and the Berlin Botanical Garden had an R2 of 0·68 for leaf out but an R2 of only 0·13 for leaf senescence (Panchen et al., 2014).
Table 4.
ANCOVA results for leaf senescence dates at the genus level, comparing the dates at the Arnold Arboretum with the dates at Berlin Botanic Garden (Berlin BG), Garden in the Woods, Ottawa Arboretum, Beijing Botanical Garden (Beijing BG) and Morton Arboretum with year (2012 and 2013) as a categorical predictor variable
| Arnold Arboretum with: | R2 | n | P |
|---|---|---|---|
| Garden in the Woods | 0·57 | 10 | 0·0114 |
| Berlin BG | 0·13 | 127 | 0·0008 |
| Ottawa Arboretum | 0·32 | 33 | 0·0104 |
| Morton Arboretum | 0·29 | 17 | 0·0253 |
| Bejing BG | 0·05 | 21 | 0·8422 |
The ANCOVA model indicates there is a moderately strong association in mean leaf senescence dates between years at the family level (R2 = 0·64, n = 80, P < 0·0001). There was, however, no association of leaf senescence dates at the family level between sites, suggesting that the order of senescence of families differs by location. Again it should be noted that although the same families are being compared between sites, the actual species being monitored were often different between sites. This is in major contrast to the leaf out study, in which there was a highly significant association at the family level between the Arnold Arboretum and the Berlin Botanical Garden, with an R2 of 0·83 (Panchen et al., 2014).
Analysis of other factors that influence leaf senescence phenology
Leaf out time
There was a very weak but significant association between leaf out dates and leaf senescence dates in the ANCOVA model (R2 = 0·02, n = 1672, P < 0·0001). That is, species leaf out times in the spring are not predictive of when species are likely to undergo leaf senescence in the autumn.
Growth habit
At the Arnold Arboretum, in both 2012 and 2013, trees senesced their leaves significantly earlier than shrubs and vines, by about 1–2 weeks [P < 0·0001; 2012: n (shrub, tree, vine) = 423, 427, 45; 2013: n (shrub, tree, vine) = 386, 403, 42]. At the Berlin Botanic Garden in 2013, trees senesced their leaves significantly earlier than vines by an average of 5 d [P = 0·0267, n (trees, vines) = 414, 54] but there were no significant differences among trees, shrubs and vines in 2012. However, at the Garden in the Woods, Ottawa Arboretum, Beijing Botanical Garden and Morton Arboretum in 2012 and 2013, there were no significant differences in leaf senescence dates among trees, shrubs and vines. In the leaf out study, there were large and highly significant differences among shrubs, trees and vines; in particular, shrubs leafed out an average of 10 d earlier than trees (Panchen et al., 2014).
Wood anatomy
At the Arnold Arboretum and Berlin Botanic Garden in 2013, diffuse porous woody plants senesced their leaves significantly earlier than ring porous and semi-ring porous woody plants by an average of 3–5 d [Arnold Arboretum: P = 0·013, n (diffuse, ring, semi-ring porous) = 340, 141, 94; Berlin Botanic Garden: P = 0·031, n (diffuse, ring, semi-ring porous) = 377, 157, 133]. However, at the Garden in the Woods, Ottawa Arboretum, Beijing Botanical Garden and Morton Arboretum in 2013, there were no significant differences in leaf senescence dates among diffuse porous, ring porous and semi-ring porous woody plants. No site in 2012 showed any differences in leaf senescence pattern with wood anatomy. In the leaf out study, there was a much stronger association: diffuse and semi-ring porous species leafed out an average of 9–12 d earlier than ring porous species (Panchen et al., 2014).
Colour index
The association in the ANCOVA model of the leaf colour index between years was weak but significant (R2 = 0·11, n = 1615, P < 0·0001), indicating that while there was a slight tendency for species to have a similar colour index in successive years, very little of the variation was explained by this tendency. The least squares model indicated a very weak but significant association between leaf senescence date and percentage leaf colour and percentage leaf drop in both 2012 and 2013 except at the Beijing Botanical Garden, indicating that percentage leaf drop and percentage leaf colour explain only a very small portion of the variance in leaf senescence (Table 5); that is, there is little tendency for species that undergo leaf senescence through leaf colour change to start this process earlier or later than species that undergo leaf senescence mainly by dropping their leaves.
Table 5.
Least squares model indicating how much variation in leaf senescence dates is explained by percentage leaf drop and percentage leaf colour at the Arnold Arboretum (AA), Garden in the Woods (GitW), Berlin Botanic Garden (Berlin), Ottawa Arboretum (Ottawa), Beijing Botanical Garden (Beijing) and Morton Arboretum (Morton) in 2012 and 2013
| R2 | n | P | F | d.f. | Intercept estimate | % leaf drop estimate | % leaf drop P | % leaf colour estimate | % leaf colour P | |
|---|---|---|---|---|---|---|---|---|---|---|
| AA 2012 | 0·05 | 893 | <0·0001 | 22·27 | 892 | 282·84 | 0·096 | 0·0332 | 0·279 | <0·0001 |
| AA 2013 | 0·06 | 831 | <0·0001 | 25·49 | 830 | 282·05 | 0·080 | 0·0364 | 0·248 | <0·0001 |
| GitW 2012 | 0·23 | 123 | <0·0001 | 18·33 | 122 | 242·18 | 0·629 | <0·0001 | 0·663 | <0·0001 |
| Berlin 2012 | 0·06 | 880 | <0·0001 | 29·42 | 879 | 275·36 | 0·222 | <0·0001 | 0·134 | 0·0005 |
| Berlin 2013 | 0·01 | 937 | <0·0001 | 62·23 | 936 | 285·99 | 0·123 | <0·0001 | -0·112 | 0·0022 |
| Ottawa 2012 | 0·11 | 143 | 0·0004 | 8·23 | 142 | 281·09 | 0·214 | <0·0001 | 0·139 | 0·0032 |
| Ottawa 2013 | 0·41 | 150 | <0·0001 | 51·48 | 149 | 271·47 | 0·426 | <0·0001 | 0·230 | 0·0001 |
| Beijing 2012 | 0·06 | 105 | 0·0495 | 3·10 | 102 | 290·03 | 0·202 | 0·0283 | 0·090 | 0·2868 |
| Beijing 2013 | 0·01 | 140 | 0·5829 | 0·54 | 137 | 292·26 | 0·160 | 0·3094 | 0·133 | 0·4432 |
| Morton 2012 | 0·05 | 134 | 0·0401 | 3·30 | 133 | 274·97 | 0·062 | 0·0326 | 0·036 | 0·2216 |
DISCUSSION
This study examined the leaf senescence times of 1360 woody plant species at six botanical gardens over two years. The main conclusion of our study is that there is an enormous amount of variation in the timing of leaf senescence among species; at any one botanical garden, species’ senescence dates vary by several months. Some species undergo leaf senescence as early as August and September, while others undergo senescence in October, November or even December. Interspecific differences in leaf senescence dates could strongly affect changes in growing season length, local micro-climate, community composition, and the feeding behaviour and movement patterns of insects, birds and other animals. Interspecific variation in leaf out phenology, although less strong, could have similar effects (Morisette et al., 2008; Fridley, 2012; Panchen et al., 2014).
A second major conclusion of our study is that autumn leaf senescence phenology appears to be more variable and less predictable than spring leaf out phenology in the temperate species we examined (Panchen et al., 2014). This contrast is not simply due to a difference in monitoring intensity, as plants in both seasons were monitored about the same number of times over the leafing out and leaf senescence season. What could explain the difference between spring and autumn leaf phenology? Most probably, spring leaf out is determined almost exclusively by temperatures, through winter chilling requirements and spring forcing requirements, while autumn leaf senescence is determined by interactions among multiple factors, possibly including summer and autumn temperatures, photoperiod, North Atlantic Oscillation Index, soil moisture, frost events, wind, disease, pest attacks, leaf out and other microsite factors (Menzel, 2003; Jolly et al., 2005; Leuzinger et al., 2005; Fracheboud et al., 2009; Fu et al., 2014). This larger number of important drivers makes the variation in leaf senescence dates more difficult to explain with environmental models than leaf out times. Moreover, based on the weaker association in senescence dates between years and among sites, it appears that species are highly variable in how their leaf senescence phenology responds to these factors. There is also the possibility of genetic variation within species in their reaction to this environmental variation. This is suggested by the weak association in leaf senescence times for the same species monitored at two distinct areas at the Berlin Botanic Garden. Here we examine more closely some of the factors that we explored and the implications of our findings and areas for further research.
Sequence of leaf senescence
We found that leaf senescence phenology is only moderately consistent from year to year within sites and relatively inconsistent across sites. That is, species differ substantially in how their leaf senescence phenology responds to changes in environmental conditions from year to year or from site to site. This pattern is in contrast to spring leaf out phenology, which is strongly correlated from year to year and across sites (Panchen et al., 2014). Leaf out phenology is mostly driven by spring temperature cues and species tend to respond similarly to changes in temperature (Menzel et al., 2006; Polgar and Primack, 2011; Rollinson and Margot, 2012).
Many environmental factors may influence leaf senescence timing at particular sites, including microsite characteristics such as soil moisture and temperature, soil type, shading, slope and the presence of disease or pests (Risley, 1993; Leuzinger et al., 2005; Lim et al., 2007; Richardson et al., 2010; Wu et al., 2013). At the Berlin Botanic Garden and Ottawa Arboretum, we observed that trees senesce first on their most exposed side where there was strong sun and warming during the day, followed by low temperatures at night. In one notable example of a temperature relationship, at the Berlin Botanic Garden in 2013, after the first night in early October with especially low temperatures, about half of the species reached senescence by the next monitoring date, indicating that perhaps a temperature threshold can trigger and accelerate the rate of leaf senescence for many species (Fracheboud et al., 2009). We also observed that shaded plants tended to senesce their leaves later than less shaded plants of the same species. The associations of senescence timing between botanical gardens would be expected to be even weaker where species are exposed to different climates, soils and microsite conditions. Similarly the interactions of soil factors, disease, insect damage and weather will vary between years, weakening the associations between years even at the same sites.
We can infer from the weak relationship of leaf senescence phenology across sites at different latitudes that photoperiod probably does not play a major role in the timing of leaf senescence for most species (Chmielewski and Rötzer, 2001; Delpierre et al., 2009). If photoperiod were a major factor across species, then we would expect species to undergo a regular sequence of leaf senescence at different latitudes, just shifted by some number of days. This result is similar to recent experimental studies that have shown that photoperiod affects the leaf out times of only a small number of species (Laube et al., 2014; Polgar et al., 2014). Some previous studies of single species or small groups of species under experimental conditions have suggested that photoperiod might play a role in leaf senescence (Schwabe, 1970; Fracheboud et al., 2009; Friedman et al., 2011), but we do not find general support for this in our study.
We observed that species with late senescence dates are often species that tend to be semi-evergreen and from a more southern origin than where the species was monitored. Hence another promising line of analysis would be to examine how the variation in leaf senescence times observed in this study is affected by geographical origin of these species or their cold hardiness zones.
Phylogenetic and taxonomic variation
Our phylogenetic analysis suggests that the timing of leaf senescence is not phylogenetically conserved. This means that the patterns of leaf senescence timing are not strongly related to the evolutionary relatedness of species. Species and their senescence dates can therefore be largely considered as independent data points in the data analysis.
However, different genera and families can vary by a month or more in when they undergo leaf senescence, with certain genera undergoing leaf senescence early and others undergoing leaf senescence much later. These patterns are seen both in successive years and to a lesser extent between sites. This may indicate that there could be a small number of clades or traditional taxonomic groups that senesce their leaves earlier or later than would be expected by chance. Alternatively, it is possible that botanical gardens tend to plant related species in the same section of a garden, where they share microsite conditions that might drive associations among leaf senescence times in our data set. For example, at the Arnold Arboretum, the Lonicera collection is mainly on a flat, moist area under the shade of large trees, in contrast to the Corylopsis collection, which is growing in full sunlight near the top of a hill.
Leaf out phenology
Although a study of two species showed evidence that there is a relationship between leafing out and senescence date (Fu et al., 2014), this is not true for the large number of species that we investigated in the present study; that is, dates of leaf out are not correlated with dates of leaf senescence. This suggests that forecasts of future changes in growing season length and associated effects on climate and ecosystem processes will be particularly complex. Spring and autumn phenology are driven by different factors and species vary substantially in their responses to environmental conditions (Steltzer and Post, 2009).
Habit and wood anatomy
Our results suggest that trees may senesce their leaves before shrubs or vines. However, this effect is seen only at two gardens (Arnold Arboretum and Berlin Botanic Garden), those with the largest selection of species and fairly even numbers of trees and shrubs. In spring, shrubs generally leaf out earlier than trees (Panchen et al., 2014). By leafing out earlier and undergoing leaf senescence later, understorey species can achieve longer growing seasons than canopy trees and can maximize photosynthetic activity by leafing out before tree canopies close and by retaining leaves after the canopy trees have dropped their leaves (Seiwa, 1999; Augspurger and Bartlett, 2003; Fridley, 2012).
There is some suggestion that species with diffuse porous stem anatomy may senescence their leaves before species with ring porous and semi-ring porous stem anatomy. However, this pattern is only seen in one year and not at all of the gardens. This is contrary to our prediction that species with diffuse porous stem anatomy would undergo leaf senescence later due to their smaller vessel element size and greater resistance to embolisms associated with frost events (Essiamah and Eschrich, 1986; Michelot et al., 2012).
Leaf colour
At the onset of this study, we expected that each species would tend to undergo leaf senescence in a characteristic way every year, with certain species tending to drop their leaves and others tending to undergo leaf colour change while the leaves remained on the plants. We used a colour index as an indicator to investigate if such a pattern exists. However, the relationship of the colour index between successive years was very weak and the percentage of leaf colour or leaf drop at leaf senescence explained very little of the variation in leaf senescence dates. Species can vary greatly between years in whether they senescence their leaves primarily by colour change or leaf drop. The timing of leaf colour and leaf drop may be related to the variable date on which the autumn temperature rapidly drops and the variable date of first frost (Estrella and Menzel, 2006; Delpierre et al., 2009).
It is possible that any pattern that did exist was weakened by our frequency of sampling. There were times when the leaves on a tree rapidly changed colour and then, a few days later, the leaves were mostly blown off on a windy day. If such a tree had been sampled on one day, it would have been recorded as having primarily coloured leaves, but after a few days, it would have been recorded as having mostly dropped its leaves. More frequent sampling of individual plants could reveal insights missed in our study, particularly the process of leaf colour change and its interaction with leaf drop. However, our intensity of sampling of leaf senescence dates was similar to what we had used previously in our leaf out study at these same gardens (Panchen et al., 2014)
Field monitoring techniques
Our study used a simple index of leaf senescence dates based on a 50 % threshold of the leaves across the whole plant having undergone a combination of leaf drop and colour change. This method has the advantage of simplicity of use and allowed a large number of species at widely distributed sites to be quickly categorized under one definition, but at the expense of understanding the complexity of change over time. Even with this relatively simple definition, field measurements require subjective judgements of a process that can take several days or weeks. In contrast, leaf out times are easy to identify in the field and the process of leaf out happens quite rapidly once it starts (Polgar and Primack, 2011; Basler and Körner, 2014).
Conclusion and recommendations for future research
This study represents an early step in understanding the variation in phenology and mode of leaf senescence across a large group of species common in temperate and boreal ecosystems. Understanding this variation is critical to inform climate models and forecasts of future changes in ecosystems as climate and other environmental conditions change.
Much additional work on this topic is needed. Experiments and further observational studies are necessary to determine what environmental factors, including microsite effects and woody plant traits, affect leaf senescence phenology for whole communities of woody plant species. Similar studies are needed to understand variation within species in leaf senescence times at specific sites and across the range of the species. In particular, factorial experiments may elucidate the combined effects of multiple environmental cues on leaf senescence times. Comparable studies on leaf out times have begun to determine the relative importance of winter chilling, spring warming and photoperiod for whole communities of species (Laube et al., 2014; Polgar et al., 2014).
Further studies could also continue to test the utility of our method for measuring leaf senescence in the field. A lack of reliable and replicable field methods currently limits our understanding of autumn leaf senescence, which in turn limits our ability to accurately anticipate many of the consequences of changes in climate and other environmental conditions.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: leaf senescence dates for all species monitored at the six arboreta, with associated family, growth habit and wood anatomy. Table S2: mean genus-level leaf senescence day of year, number of species per genus and standard deviation at the six arboreta. Table S3: mean family-level leaf senescence day of year, number of species per family and standard deviation at the six arboreta. Fig. S1: leaf senescence of Morus sp. at Arnold Arboretum on 22 November 2011. Fig. S2: leaf senescence of Orixa sp. at Arnold Arboretum on 12 November 2011. Fig. S3: leaf senescence of Prunus sargentii at Arnold Arboretum on 22 October 2011.
ACKNOWLEDGEMENTS
We thank Ulrike Lohmann, Botanic Garden and Botanical Museum Berlin-Dahlem, and David Carter, Forest Ecology Lab, Morton Arboretum, for assistance with monitoring leaf senescence times. We also thank Abe Miller-Rushing and Julia Laube for their comments on earlier drafts of the manuscript and Amy Iler and Jason Fridley for providing thoughtful and detailed reviews. We thank the botanical gardens for permission to carry out leaf senescence monitoring. This work was supported by a National Science Foundation Graduate Research Fellowship (grant number DGE-1247312 to A.G.) and Humboldt Research Award (R.P.).
LITERATURE CITED
- Ackerly D. 2009. Conservatism and diversification of plant functional traits: evolutionary rates versus phylogenetic signal. Proceedings of the National Academy of Sciences of the USA 106: 19699–19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augspurger CK, Bartlett EA. 2003. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiology 23: 517–525. [DOI] [PubMed] [Google Scholar]
- Basler D, Körner C. 2014. Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiology 34: 377–388. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T, Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- CaraDonna PJ, Inouye DW. 2015. Phenological responses to climate change do not exhibit phylogenetic signal in a subalpine plant community. Ecology, in press, http://dx.doi.org/10.1890/14-1536.1. [DOI] [PubMed] [Google Scholar]
- Chmielewski FM, Rötzer T. 2001. Response of tree phenology to climate change across Europe. Agricultural and Forest Meteorology 108: 101–112. [Google Scholar]
- Delpierre N, Dufrêne E, Soudani K, et al. 2009. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agricultural and Forest Meteorology 149: 938–948. [Google Scholar]
- Essiamah S, Eschrich W. 1986. Water uptake in deciduous trees during winter and the role of conducting tissue in spring reactivation. International Association of Wood Anatomists Bulletin 7: 31–38. [Google Scholar]
- Estrella N, Menzel A. 2006. Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Climate Research 32: 253–267. [Google Scholar]
- Fracheboud Y, Luquez V, Björkén L, Sjödin A, Tuominen H, Jansson S. 2009. The control of autumn senescence in European aspen. Plant Physiology 149: 1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridley JD. 2012. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485: 359–362. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Roelle JE, Cade BS. 2011. Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. International Journal of Biometeorology 55: 775–787. [DOI] [PubMed] [Google Scholar]
- Fu YSH, Campioli M, Vitasse Y, et al. 2014. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proceedings of the National Academy of Sciences of the United States of America 111: 7355–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat AS, Garrison LM, Primack RB. 2013. Comparison of SPAD-502 and atLEAF chlorophyll meters for measuring leaf senescence in woody species. http://primacklab.blogspot.com/2013/12/using-handheld-chlorophyll-meters-to.html [accessed 17 December 2014]. [Google Scholar]
- Gallinat AS, Primack RB, Wagner DL. 2015. Autumn, the neglected season in climate change research. Trends in Ecology and Evolution. doi:10.1016/j.tree.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Gordo O, Sanz JJ. 2009. Long-term temporal changes of plant phenology in the Western Mediterranean. Global Change Biology 15: 1930–1948. [Google Scholar]
- Ibanez I, Primack RB, Miller-Rushing AJ, et al. 2010. Forecasting phenology under global warming. Philosophical Transactions of the Royal Society B-Biological Sciences 365: 3247–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPGE. 1960. Phenological observation guide of the International Phenological Gardens. International Phenological Gardens of Europe, Berlin, Germany. http://www.agrar.hu-berlin.de/fakultaet-en/departments/dntw-en/agrarmet-en/phaenologie/ipg/IPG_ObsGuide.pdf/view [accessed 9 October 2014]. [Google Scholar]
- Jolly WM, Nemani R, Running SW. 2005. A generalized, bioclimatic index to predict foliar phenology in response to climate. Global Change Biology 11: 619–632. [Google Scholar]
- Kikuzawa K. 1983. Leaf survival of woody plants in deciduous broad-leaved forests. 1. Tall trees. Canadian Journal of Botany 61: 2133–2139. [Google Scholar]
- Kramer K, Leinonen I, Loustau D. 2000. The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: an overview. International Journal of Biometeorology 44: 67–75. [DOI] [PubMed] [Google Scholar]
- Laube J, Sparks TH, Estrella N, et al. 2014. Chilling outweighs photoperiod in preventing precocious spring development. Global Change Biology 20: 170–182. [DOI] [PubMed] [Google Scholar]
- Lee DW, O’Keefe J, Holbrook NM, Feild TS. 2003. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research 18: 677–694. [Google Scholar]
- Leuzinger S, Zotz G, Asshoff R, Korner C. 2005. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiology 25: 641–650. [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Gil Nam H. 2007. Leaf senescence. Annual Review of Plant Biology 58: 115–136. [DOI] [PubMed] [Google Scholar]
- Menzel A. 2003. Plant phenological anomalies in Germany and their relation to air temperature and NAO. Climatic Change 57: 243–263. [Google Scholar]
- Menzel A, Fabian P. 1999. Growing season extended in Europe. Nature 397: 659. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Michelot A, Simard S, Rathgeber C, et al. 2012. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petrea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiology 32: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Morisette JT, Richardson AD, Knapp AK. 2008. Tracking the rhythm of the seasons in the face of global change: phenological research in the 21st century. Frontiers in Ecology and the Environment 7: 253–260. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Panchen ZA, Primack RB, Nordt B, et al. 2014. Leaf out times of temperate woody plants are related to phylogeny, deciduousness, growth habit and wood anatomy. New Phytologist 203: 1208–1219. [DOI] [PubMed] [Google Scholar]
- Piao S, Friedlingstein P, Ciais P, et al. 2007. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Global Biogeochemical Cycles 21: GB3018 doi: 10.1029/2006GB002888. [Google Scholar]
- Piao SL, Ciais P, Friedlingstein P, et al. 2008. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451: 49–52. [DOI] [PubMed] [Google Scholar]
- Polgar CA, Primack RB. 2011. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist 191: 926–941. [DOI] [PubMed] [Google Scholar]
- Polgar CA, Gallinat A, Primack RB. 2014. Drivers of leaf-out phenology and their implications for species invasions: insights from Thoreau’s Concord. New Phytologist 202: 106–115. [DOI] [PubMed] [Google Scholar]
- Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology. Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Richardson AD, Bailey AS, Denny EG, et al. 2006. Phenology of a northern hardwood forest canopy. Global Change Biology 12: 1174–1188. [Google Scholar]
- Richardson AD, Black TA, Ciais ND, et al. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philosophical Transactions of the Royal Society B-Biological Sciences 365: 3227–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley LS. 1993. Effect of simulated insect herbivore damage on survival of tree leaves. Environmental Entomology 22: 57–61. [Google Scholar]
- Rollinson CR, Margot WK. 2012. Experimental warming alters spring phenology of certain plant functional groups in an early successional forest community. Global Change Biology 18: 1108–1116. [DOI] [PubMed] [Google Scholar]
- Schwabe WW. 1970. The control of leaf senescence in Kleinia articulata by photoperiod. Annals of Botany 34: 43–55. [Google Scholar]
- Seiwa K. 1999. Changes in leaf phenology are dependent on tree height in Acer mono, a deciduous broad-leaved tree. Annals of Botany 83: 355–361. [Google Scholar]
- Steltzer H, Post E. 2009. Seasons and life cycles. Science 324: 886–887. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, François C, Delpierre N, et al. 2011. Assessing the effects of climate change on the phenology of European temperate trees. Agricultural and Forest Meteorology 151: 969–980. [Google Scholar]
- Walther GR. 2010. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Gough CM, Chen JM, Gonsamo A. 2013. Evidence of autumn phenology control on annual net ecosystem productivity in two temperate deciduous forests. Ecological Engineering 60: 88–95. [Google Scholar]
- Zhu Z, Tremblay N, Liang Y. 2012. Comparing SPAD and atLEAF values for chlorophyll assessment in crop species. Canadian Journal of Soil Science 92: 645–648. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


