Abstract
Background and Aims Climate change is advancing the leaf-out times of many plant species and mostly extending the growing season in temperate ecosystems. Laboratory experiments using twig cuttings from woody plant species present an affordable, easily replicated approach to investigate the relative importance of factors such as winter chilling, photoperiod, spring warming and frost tolerance on the leafing-out times of plant communities. This Viewpoint article demonstrates how the results of these experiments deepen our understanding beyond what is possible via analyses of remote sensing and field observation data, and can be used to improve climate change forecasts of shifts in phenology, ecosystem processes and ecological interactions.
Scope The twig method involves cutting dormant twigs from trees, shrubs and vines on a single date or at intervals over the course of the winter and early spring, placing them in containers of water in controlled environments, and regularly recording leaf-out, flowering or other phenomena. Prior to or following leaf-out or flowering, twigs may be assigned to treatment groups for experiments involving temperature, photoperiod, frost, humidity and more. Recent studies using these methods have shown that winter chilling requirements and spring warming strongly affect leaf-out and flowering times of temperate trees and shrubs, whereas photoperiod requirements are less important than previously thought for most species. Invasive plant species have weaker winter chilling requirements than native species in temperate ecosystems, and species that leaf-out early in the season have greater frost tolerance than later leafing species.
Conclusions This methodology could be extended to investigate additional drivers of leaf-out phenology, leaf senescence in the autumn, and other phenomena, and could be a useful tool for education and outreach. Additional ecosystems, such as boreal, southern hemisphere and sub-tropical forests, could also be investigated using dormant twigs to determine the drivers of leaf-out times and how these ecosystems will be affected by climate change.
Keywords: Dormant twigs, woody plants, phenology, leaf-out, flowering time, phenology, climate change, winter chilling, photoperiod, humidity, frost tolerance, trees, shrubs, invasive species
INTRODUCTION
Climate change is already shifting the spring leaf-out and flowering times of trees, shrubs and vines in temperate ecosystems, with major implications for ecosystem processes and interactions among species (Rosenzweig et al., 2008; Richardson et al., 2010; Walther, 2010). Spring warming is one of the major drivers of leaf-out in temperate plants (Polgar and Primack 2011), and warming conditions and earlier leaf-out are lengthening the temperate growing season, shifting patterns of carbon dioxide absorption and biomass accumulation (Richardson et al., 2010, 2013; Migliavacca et al., 2012), and altering the competitive interactions of tree and shrub species (Willis et al., 2008; Cleland et al., 2012; Fridley, 2012). Phenological shifts and associated ecosystem alterations will progress in coming decades as climate conditions continue to change (Kirtmann et al., 2013).
Currently, observed changes in leaf-out and their associated drivers are primarily studied via remote sensing and field observations, whereas field experiments have aimed at process understanding. These methods have provided many insights into the phenology and ecology of woody plants (Fitter and Fitter, 2002; Menzel et al., 2006; Ibáñez et al., 2010; Richardson et al., 2010), but they also have several key limitations. Remote sensing data often fail to parse out variation at the species level. Field observations of leaf-out times can provide species-level information, but they lack predictive power for no-analogue climates. More importantly, using only observational methods of leaf-out times, it is difficult to quantify independently the influence of key environmental drivers – such as winter chilling requirements, spring warming and photoperiod (Chuine et al., 2010; Körner and Basler, 2010) – and to investigate systematically the role of rare and unpredictable events, such as late spring frosts, droughts and insect outbreaks. Field experiments allow researchers to disentangle and systematically investigate drivers of leaf phenology, but experiments on communities of woody plant species growing in the wild can be methodologically challenging and expensive.
Increasingly, scientists are conducting experiments on dormant twigs of woody plants as a proxy for wild plants in the field. Experiments using dormant twigs in the lab provide an alternative method for quantifying the environmental factors that affect leaf-out times, flowering times and other spring phenomena. This method is providing new insights into many of the processes that affect plant responses to climate change, such as winter chilling requirements, photoperiod requirements, spring warming requirements, humidity requirements and frost tolerance (Miller-Rushing and Primack, 2008; Basler and Körner, 2012; Lenz et al., 2013; Basler and Körner, 2014; Dantec et al., 2014; Laube et al., 2014a, b; Polgar et al., 2014; Vitasse and Basler, 2014). The use of dormant twigs provides unique opportunities for climate change and ecological research, and will probably expand in coming years. In this Viewpoint article, we review the ways in which twig experiments are presently being used, examine some benefits and challenges of this approach, and suggest some additional approaches that could provide new insights.
THE TWIG METHOD: HISTORY AND PRACTICE
The twig method is not new – people have long cut twigs from woody plants and exposed them to warm temperatures to force leaf-out or flowering. For example, it was a folk Roman Catholic custom in Central Europe to cut dormant cherry twigs on 4 December (Saint Barbara’s day), take them home and watch them flower at Christmas (Gulevich, 2003). Today, the same principle of using twigs or cuttings is employed in horticulture (Balandier et al., 1993; Ruiz et al., 2007; Campoy et al., 2012; Sønsteby and Heide, 2014) and ecology (Heide, 1993a; Miller-Rushing and Primack, 2008; Basler and Körner, 2012, 2014; Laube et al., 2014a; Polgar et al., 2014) to determine winter chilling requirements, obtain predictions for budburst and flowering dates or otherwise investigate twig and bud physiology or behaviour.
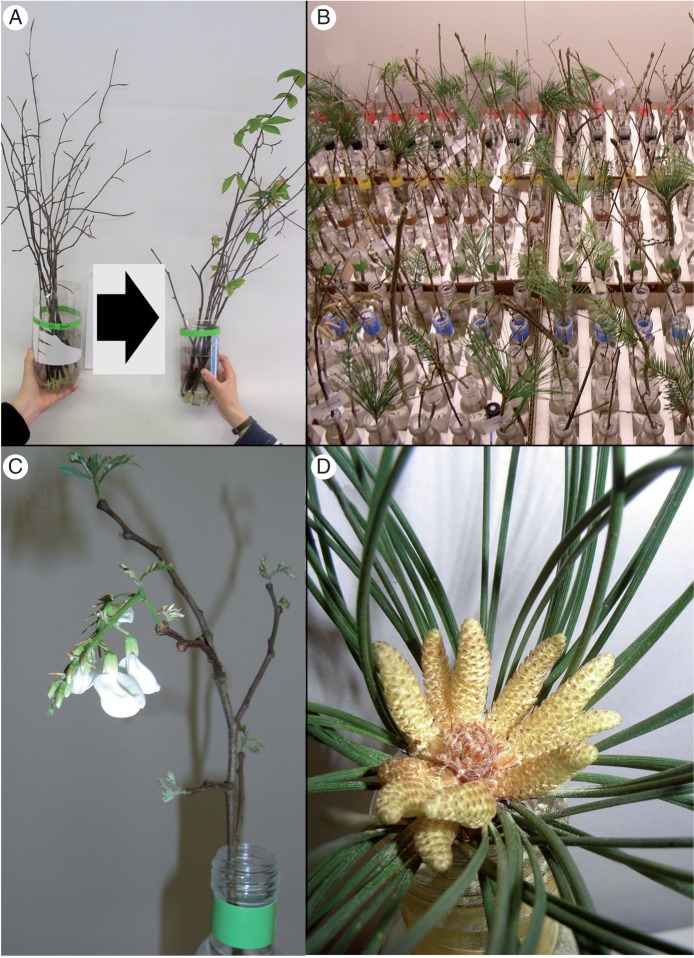
Methods can vary depending on the species, purpose of the study and facilities available. Twig cuttings of variable length (usually 10–30 cm) are brought indoors and put into tap water (Balser and Körner, 2012; Laube et al., 2014a; Polgar et al., 2014) or distilled water (Heide, 1993a), at times with the addition of nutrients or sucrose (Ruiz et al., 2007; Campoy et al., 2012; Sønsteby and Heide, 2014). Often disinfectants are used to clean the cut ends of the twigs (Basler and Körner, 2012, 2014; Laube et al., 2014a). These twigs are placed in controlled conditions, such as laboratory conditions or growth chambers with artificial lights, and monitored until they leaf-out or reach the stage of interest (Fig. 1).
Fig. 1.
Examples of research projects using twig experiments; all photographs are of twigs sampled during winter and then monitored for leaf-out and flowering. (A) Beech (Fagus grandifolia) twigs kept in ambient (left) and extended (right) daylength (demonstrating that twigs can be used to study the influence of photoperiod on leaf-out). (B) A diversity of twigs exposed to a common set of conditions to investigate species differences in winter chilling requirements. (C) Flowering twig of locust (Robinia pseudoacacia) (flowering in the lab during a leaf-out study, demonstrating that twigs can be used to study flowering). (D) Pollen release in a twig of an evergreen mountain pine (Pinus nigra) (demonstrating the ability to study reproductive phenology and pollen amount or allergen content with twigs). (Photographs by J. Laube and R. Primack.)
Recent studies have found that cut twigs develop similarly to those remaining on donor trees in the wild when exposed to similar conditions (Laube et al., 2014a; Polgar et al., 2014; Sønsteby and Heide, 2014; Vitasse and Basler, 2014), supporting the use of cut twigs as proxies for the behaviour of woody plants in the field. It remains unclear how differences in twig preparation and treatments influence experimental results. However, one-node cuttings develop differently from longer twigs and whole plants, and their use is discouraged (Sønsteby and Heide, 2014). Methods that result in fungal growth, embolisms or otherwise alter water movement in the plants can also affect results. However, when done carefully, the results from twig experiments seem broadly robust; they reflect observations in the wild and can aid predictions of phenological and other behaviours in future climate conditions (Laube et al., 2014a; Polgar et al., 2014). For instance, models used to estimate the fulfilment of chilling requirements of fruit and nut trees are based in part on data from controlled twig experiments, and are used to predict future impacts of climate change (Luedeling, 2012).
PRIMARY ENVIRONMENTAL DRIVERS OF LEAF-OUT PHENOLOGY
Twig experiments are well suited to investigate environmental factors that influence the leaf-out times of woody plants. Leaf-out times are widely considered to be controlled by a combination of winter chilling, photoperiod and spring warming (Murray et al., 1989; Heide, 1993a; Chuine et al., 2010; Körner and Basler, 2010; Hänninen and Tanino, 2011; Polgar and Primack, 2011; Way and Montgomery, 2014). However, so far the evidence for this is based on a limited number of experiments and species (e.g. Murray et al., 1989; Heide, 1993a, b; Caffarra and Donnelly, 2011; Taeger et al., 2015), combined with analyses of long-term observations (e.g. Menzel et al., 2006; Ibáñez et al., 2010; Yu et al., 2010) and phenological models describing leaf-out (Chuine, 2000; Morin et al., 2009; Blümel and Chmielewski, 2012; Olsson and Jönsson, 2014). Additionally, the magnitude and nature of phenological responses to environmental conditions, such as warming spring temperatures and reduced winter chilling, are known to vary substantially from species to species, and we currently lack information on the responses of most species.
Three recent experiments (Basler and Körner, 2012; Laube et al., 2014a; Polgar et al., 2014) highlight the potential value of twig experiments for leaf-out studies; they used dormant twigs, clipped from wild temperate woody plants during the winter and warmed in laboratory conditions, to investigate species-specific leaf-out requirements. These studies investigated dozens of tree, shrub and vine species, and found that the extent of spring warming strongly affected leaf-out phenology in all species studied. In contrast, the influence of winter chilling was highly variable among species, and photoperiod affected only a limited number of species. These three experiments were successful at isolating the roles of various factors and comparing large numbers of species in a manner previous studies had not accomplished.
Specifically, these three studies demonstrated that 34 out of the 36 species tested in Germany had winter chilling requirements (Laube et al., 2014a), as did the majority of species tested in North America (Polgar et al., 2014). Photoperiod influenced leaf-out times for five of 14 native species in Europe (Basler and Körner, 2012) and one of 17 species in North America (Polgar et al., 2014) (Fig. 1A). In Germany, an experiment of both winter chilling and photoperiod revealed that 12 of 36 species responded to photoperiod; however, even in those 12 species photoperiod had only a minor effect on leaf-out time, and was not significant once winter chilling requirements had been met (Laube et al., 2014a) (Fig. 1B). Although the twig method has only been used in a handful of studies investigating the ecological drivers of leaf-out times, it has already proven to be a simple and effective approach for disentangling multiple drivers and studying many species simultaneously.
Despite these successful experiments, investigators should be concerned about how the results of twig experiments are affected by variations in the experimental treatments. Each of four recent experimental studies that investigated the primary drivers of leaf-out phenology (Basler and Körner, 2012; Dantec et al., 2014; Laube et al., 2014a; Polgar et al., 2014) used different combinations of daylength and temperature, different observation frequency and different methods of calculating growing degree days (Table 1). Direct comparison of the results is not possible for most pairs of studies due to the use of different species. However, the studies by Basler and Körner (2012) and Laube et al. (2014a) had 11 species in common and a highly significant positive correlation in the growing degree days of spring warming required for leaf-out among the species (Spearman rank correlation rho = 0·72). At the species level, both studies found that Larix decidua had the lowest requirement for growing degree days, and that Fraxinus excelsior had the highest requirement. This comparison of two studies in which twigs were exposed to different temperature and photoperiod conditions suggests that the use of twigs is fairly robust to variations in the experimental treatment.
Table 1.
Experimental procedures of four recent studies using dormant twigs from woody plants to investigate leaf-out requirements (Basler and Körner, 2012; Dantec et al., 2014; Laube et al., 2014a; Polgar et al., 2014)
| Study | Light treatments (daylength) | Temperature treatments (night/day) | Observation frequency | Development stage | Base temperature used for GDD calculation | Chilling days prior to experiment | Species groups used |
|---|---|---|---|---|---|---|---|
| Polgar | Constant: 14 h | Constant: 22/22 °C | 1 per week | Leaf-out | 5 °C | 114 | Shrubs, trees and vines |
| Dantec | Constant: 16 h | Constant: 25/25 °C | 3 per week | Budburst | 5 and 10 °C | 100 | Trees |
| Basler | Increasing: 9·5 to 12 h, and 11 to 14 h | Increasing: 5 to 10 °C; night/day difference 5 °C | 3 per week | Budburst | 0 °C | 92–119 | Trees |
| Laube | Constant: 8, 12 and 16 h | Increasing: 7 to 27·5 °C; night/day difference 5 °C | 3 per week | Budburst | 0 °C | 33–110 | Shrubs and trees |
The studies differed in the number of chilling days experienced by twigs prior to collection, exposed twigs to different light and temperature treatments in the laboratory, had different frequencies of observations, monitored different stages of leaf-out and used different formulas to calculate the growing degrees (GDD) required in order for twigs to leaf out.
INVESTIGATING FLOWERING TIMES
Apart from leaf-out studies, twig experiments have also been used to investigate the environmental drivers of flowering times (and pollen release in the case of gymnosperms) in temperate trees. For example, studies have used twigs to investigate flowering times in birches (Miller-Rushing and Primack, 2008) and the early stages of flower bud development of apricots (Ruiz et al., 2007; Campoy et al., 2012). The same factors that affect the breaking of dormancy of leaf-out similarly affect the breaking of dormancy of flower buds (Campoy et al., 2011). Therefore, twig experiments can be used to study the role of temperature, light, frost and other factors in influencing flowering times of woody plants (Fig. 1C, D). This has special economic and cultural significance for ornamental plants, such as the blossoming of Japanese cherry trees, and for fruit trees in which earlier flowering results in earlier fruit set.
For species that are major contributors to pollen allergies, such as pines, oaks, poplars, hazels and birches, twig experiments can be used to investigate the climatic factors that contribute to the date of pollen release. For example, a study using dormant birch twigs showed that species release pollen earlier in warmer temperatures (Miller-Rushing and Primack, 2008). The study also showed that species vary in their responses – species with higher water content in the catkins release pollen later in the spring. Such studies on pollen release from catkins of spring-flowering, wind-pollinated tree species have practical value in predicting the allergy season, with significant public health consequences. Seasonal timing, population differences or other environmental factors, such as ozone, that influence major pollen allergens (Beck et al., 2013), could be readily studied with twig experiments.
HUMIDITY AND OTHER ENVIRONMENTAL DRIVERS
Winter chilling, photoperiod and spring warming are not the only factors that control leaf-out phenology. In a recent study (Laube et al., 2014b), twigs were used to investigate the importance of air humidity in the leaf-out process. The study showed that high air humidity accelerates leaf-out phenology, suggesting that twigs and buds may take up moisture from the air during early spring development.
The study highlights the lack of physiological understanding of the spring leaf-out process of trees. While much of the current knowledge is based on correlative studies, the findings of the twig humidity experiment conducted by Laube et al. (2014b) illustrate a weakness in resolving confounding environmental triggers, since absolute humidity is often positively correlated with temperature, i.e. warm air holds more moisture than cold air. Thus, earlier leaf-out times of woody plants in warmer conditions may actually be due, at least in part, to higher humidity rather than higher temperatures. Twig experiments present the opportunity to explore the role of other complex factors on leaf-out phenology, such as nutrient availability, temperature variability and the role of daytime vs. night-time temperatures.
DIFFERENCES AMONG SPECIES ACCORDING TO ECOLOGICAL STRATEGY
Researchers using twig experiments have found that different groups of woody species, such as trees and shrubs, pioneer and climax species, and native and non-native invasive species can differ in their phenological responses to changes in climate (Basler and Körner, 2012; Laube et al., 2014a; Polgar et al., 2014). Polgar et al. (2014) found that across 43 species from a single community in North America, non-native invasive shrub species had lower winter chilling requirements and faster leaf-out than did native shrub and tree species (Fig. 2). In a study of 36 species from a single site in Germany, both pioneer and non-native invasive shrub and tree species showed lower chilling requirements and faster leaf-out than did native climax tree species (Laube et al., 2014a). A study in Switzerland (Basler and Körner, 2012) suggests that photoperiod effects, although minor, affect plants differently based on their ecological strategies; for 14 tree species in Switzerland in one chilling treatment, climax species responded more strongly to photoperiod than did pioneer species. The fact that these studies differed in methodology (Table 1) and species, but were each able to detect an ecological pattern, suggests that it would be worthwhile to expand on these studies using a wider range of species and ecosystems.
Fig. 2.
Twig experiments allow species differences in spring development to be easily communicated. In this case, twig experiments revealed that non-native invasive shrubs (right) on average leaf out earlier and have a lower winter chilling requirement than native trees (left), with native shrubs (middle) intermediate. (Photograph by A. Gallinat and R. Primack.)
SEPARATING GENETIC AND ENVIRONMENTAL FACTORS
Twigs of different populations or provenances, as well as from across a species’ range, have been examined under controlled conditions, with individuals from different elevations showing different photoperiod requirements (Basler and Körner, 2012) and different thermal requirements (Dantec et al., 2014) for leaf-out. Thus, twig experiments can also be used to determine genetic differences in response to environmental drivers, i.e. genetic variation within and between populations or provenances. Twigs can also be used in reciprocal transplant experiments. Traditional reciprocal transplant experiments are used to investigate genetic and environmental contributions to observed population differences. These experiments are conducted by transplanting individual plants among different sites or gardens, which can limit the number of species and replicates in each study. However, the twig method could be used to test the same hypotheses as traditional reciprocal transplant experiments, by collecting twigs of the same species from multiple locations and exposing them to reciprocal field sites or to environmental treatments in the laboratory. For instance, twigs from different populations could be sampled and brought to different sites along an elevation gradient to study leaf-out times and other phenological characteristics. This would be both faster and less expensive than establishing long-term experimental gardens along altitudinal gradients. In such reciprocal transplant experiments, researchers should consider the clonal nature of the species under investigation, especially for shrub species, and the possibility of residual environmental effects of the source population that take more than one season to subside.
INVESTIGATING FROST TOLERANCE
Twig experiments, field experiments and observational studies show that leaf-out and flowering are advancing with warmer spring temperatures, and that this phenomenon can increase the risk of frost damage to woody plants if a late frost occurs (Inouye, 2008; Augspurger, 2013; Vitasse et al., 2014a). Frost damage to young leaves and flowers can affect both wild plants in natural habitats and economically important plants, such as fruit trees (Palonen and Buszard, 1997). Twig experiments offer a method for examining potential drivers of frost tolerance, and variation in frost tolerance among multiple species in the laboratory.
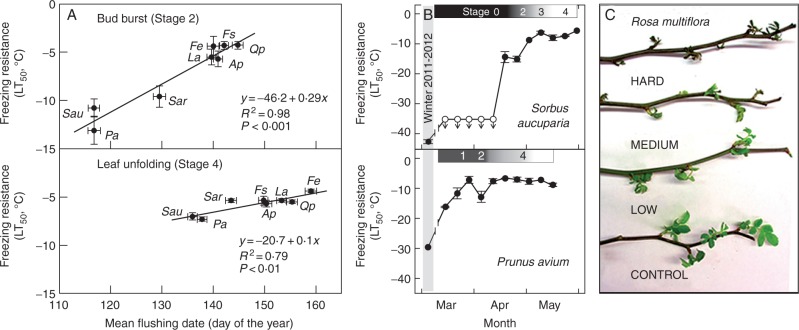
Studies have indicated that frost tolerance is genetically conserved and is frequently the limiting factor in the species ranges of fruit trees (Palonen and Buszard, 1997). Still, relatively little is known about the relative frost tolerance of young leaves in wild woody species. In a recent study, Lenz et al. (2013) used twigs to test the hypothesis that the earliest tree species to leaf-out in the spring are more frost tolerant than species that leaf-out later. They demonstrated that for eight deciduous tree species, the earliest species to leaf-out are the most frost tolerant (Lenz et al., 2013) (Fig. 3A). They also demonstrated that dormant twigs were far more frost tolerant than twigs undergoing leaf-out (Fig. 3B), and that variation in the age of the plants did not have significant effects on the degree of frost tolerance (Vitasse et al., 2014b). The study by Lenz et al. (2013) also suggests that the visual inspection of frost damage is as effective as more elaborate biochemical and anatomical tests (Fig. 3C), a result which greatly simplifies the experimental procedures needed to conduct frost studies (Lenz et al., 2013).
Fig. 3.
Freezing resistance during spring development using twigs. (A) Twig experiments show that tree species with later dates of budburst and leaf-out dates have lower frost tolerance (adapted from Lenz et al., 2013). Ap, Acer pseudoplatanus; Fe, Fraxinus excelsior; Fs, Fagus sylvatica; La, Laburnum alpinum; Pa, Prunus avium; Qp, Quercus petraea; Sar, Sorbus aria; Sau, Sorbus aucuparia; y-axis: lethal temperatures (at which 50 % of the samples died). (B) Twig experiments are used to show a decrease in frost tolerance with successive stages of phenological development (adapted from Lenz et al., 2013). Stage: phenological development from 0 (no development) to 4 (leaf unfolding); y-axis: lethal temperatures (at which 50 % of the samples died). (C) Use of twigs to assess frost tolerance in multi-flora rose (Rosa multiflora) using exposure to different frost temperatures: hard (–5 °C), medium (–3 °C), light (–1 °C) and control (5 °C). The amount of frost damage increases with lower temperatures. (Photograph by A. Gallinat.)
Twig experiments can be used to advance our understanding of the drivers of frost tolerance in wild plants and to identify vulnerable species. For example, lower winter chilling requirements of invasive species, demonstrated by previous twig experiments (Laube et al. 2014a; Polgar et al., 2014), suggest that invasive species are at greater risk of encountering late frosts. Thus, information on the frost tolerance of invasive species is important to predict whether frost may limit their ability to invade an area or whether their frost tolerance could facilitate spread. Researchers can also use twig experiments to explore the molecular and anatomical mechanisms of frost tolerance, identify how frost tolerance is contributing to current range shifts, and examine interactions with other factors, such as exposure to sun and wind.
Twig experiments to determine the frost tolerance of flowers are particularly appropriate for investigations of early-flowering species grown for commercial fruit production, such as apples, pears, peaches, cherries and blueberries, and ornamental species grown for early flowering, such as forsythias, magnolias and redbuds. Commercial fruiting species are often selected for early flowering times in order to have earlier fruits for selling. However, it is currently unknown whether the selection for earlier flowering makes species more or less vulnerable to late frosts. While early-flowering species are more likely to be exposed to frost, Reig et al. (2013) used the twig method on flowering peach stems to demonstrate that the earliest flowering cultivars are the most frost tolerant. The flowering times and frost tolerance of other species can be studied by monitoring flowering times of twigs in the lab and subjecting these flowering twigs to frost tolerance experiments similar to those which have been carried out on young leaves.
ADVANTAGES AND DISADVANTAGES OF TWIG EXPERIMENTS
Twig experiments have the advantages of being simple and highly repeatable relative to experiments carried out under field conditions. Twigs can be brought into laboratory conditions where light, temperature and other environmental variables can be controlled, and unpredictable field conditions can be avoided. Several lines of evidence suggest that dormant twigs used in laboratory and field experiments are effective model systems for investigating the responses of adult plants (Laube et al., 2014a; Polgar et al., 2014; Sønsteby and Heide, 2014; Vitasse and Basler, 2014). In addition, while seedlings and young plants may be easier to manipulate than adult plants, they often exhibit different phenological patterns (Augspurger, 2008; Vitasse, 2013; Vitasse et al., 2014b), and so twigs taken from adult plants offer an easily manipulated alternative that is more reflective of adult plant phenology.
Because twigs can be gathered in large numbers, and their maintenance requires limited space, it is easy to have many samples or replicates and carry out frequent observations. It can be difficult and expensive to set up experiments with large numbers of rooted tree seedlings, and, as previously stated, they can show unwanted ontogenetic effects. Daily observations of large numbers of plants in the wild are often restricted by logistics.
Moreover, the development of standardized protocols using twigs is relatively easy, such as using a fixed set of temperatures, frost, light and humidity conditions, which could facilitate extensive international collaborations. As mentioned above, even when twigs are exposed to different sets of laboratory conditions, the key differences among species in their winter chilling requirements are still apparent. Because of their relative ease, twig experiments could promote phenological research in understudied ecosystems, such as southern hemisphere temperate forests, tropical deciduous and semi-deciduous forests, boreal forests and dry shrublands.
There are also disadvantages to using twigs in the laboratory. For instance, a certain small percentage of plant species do not leaf-out well from twigs, perhaps due to damage to the xylem or phloem systems, especially when having long vessels, or increased chance of fungal infections and other disease affecting the developing tissues. A frequent problem in these experiments is fungal moulds growing on the stems in water; these fungi can be reduced and removed by weekly scrubbing, re-cutting the bottom of each stem, changing the water in the containers and through the use of chemical solutions. Insect outbreaks, from occasional dormant or developing insects brought in from the field on the twigs, can damage the tender young leaves and bud tissues. Also, sometimes the twigs and buds simply dry out and fail to leaf-out. However, our experience is that only a small number of species (<10 %), (e.g. Buddleja davidii, Lonicera subsessilis and Euonymus sieboldianus) are not appropriate for twig experiments. Further work is needed to determine if the success of using twigs can be increased by techniques to prevent embolisms (air bubbles) and blockages in the xylem, such as re-cutting twigs under water. Further research could also investigate whether problematic species share certain wood anatomical traits (such as ring porous stem anatomy or twig diameter), phylogenetic affinities or growth habits (such as the shrubs mentioned above).
Additionally, using twigs in laboratory conditions does not replicate factors affecting the root system, including soil conditions and snow cover (e.g. Inouye, 2008; Cornelius et al., 2013); nor does it always replicate the fluctuating levels of temperature and light, and other aspects of microsite conditions in the field, depending on the sophistication of the laboratory conditions. Frost tolerance experiments in particular might be affected by environmental conditions, particularly if light levels are too low for adequate photosynthesis and sugar production prior to freezing (Gusta and Wisniewski, 2013). High quality growth chambers can more accurately mimic field conditions of spring temperature, frost, humidity and the precise qualities of light, but on the other hand they can be costly to buy and maintain. However, simpler alternatives exist or can be built, and may be adequate for many studies. For example, various published winter chilling experiments have used both growth chambers and ordinary laboratory conditions with no substantial difference in the results (Laube et al. 2014a; Polgar et al., 2014), or have used a wide range of growing conditions already available for other purposes (Miller-Rushing and Primack, 2008). For frost tolerance experiments, one approach is to use fairly expensive scientific, programmable freezers or growth chambers, but it is also possible to achieve a suitable range of temperatures by modifying and re-programming commercial refrigerators and freezers (Lenz et al., 2013). In many temperate and boreal systems, snow cover often protects low-lying shrubs from frost damage and desiccation since it affects soil temperature and the depth of soil freezing. Combining frost tolerance experiments in the lab with field experiments that manipulate snow cover and depth for wild shrubs could be an effective approach in future climate change research.
Researchers using twig experiments need to be vigilant in detecting artefacts caused by the experimental process itself, and to be aware that the rates of response in the lab might be different from those in the field. In recent warming studies, for example, plants have been shown to have lower responses to experimental temperature variation than are indicated by long-term observations of wild plant populations (Wolkovich et al., 2012).
CONCLUSIONS
Because of their numerous advantages, twig experiments can continue to advance our understanding of phenology in important ways.
Additional factors that may influence phenology
Many factors, such as nutrient status (Jochner et al., 2013), precipitation (Fu et al., 2014) and light quality (Linkosalo and Lechowicz, 2006), could influence spring leaf-out phenology, but their effects are understudied. Chief among these factors may be the influence of phylogeny, i.e. do closely related species tend to behave similarly? A few recent studies involving numerous species have found phylogenetic patterns in leaf-out and flowering times (Fridley, 2012; Davies et al., 2013; Panchen et al., 2014). Twig experiments could be used to extend these studies to determine if closely related species tend to share similar characteristics, such as photoperiod and winter chilling requirements.
Interactions
The flexibility and large sample sizes possible in twig experiments make them particularly attractive for studies investigating interactions among factors that influence phenology and among plant traits. For example, twig experiments that couple measurements of moisture content during dormancy and spring leaf-out with experimental frost treatments could deepen our knowledge of how tissue moisture, phenological development and frost tolerance interact. As another example, ecological chilling experiments in which twigs were collected from the wild on different dates have not separated out temperature and photoperiod factors; twigs cut earlier in winter had experienced both less chilling and shorter daylength than twigs cut in late winter with both more chilling and longer daylength. However, further improvements are easily possible with a combination of controlled dormancy treatments, as already used in the horticultural field, and controlled forcing and photoperiod conditions.
Test model assumptions, parameters and thresholds
Experiments in which twigs are exposed to a range of environmental conditions, especially factorial experiments, could significantly improve current models of leaf-out responses to climate change. Specifically, the somewhat arbitrary temperature thresholds for chilling or forcing and photoperiod requirements derived from model optimization routines (Kramer 1994; Hänninen, 1995; Richardson et al., 2006; Linkosalo et al., 2008; Blümel and Chmielewski, 2012; Migliavacca et al., 2012; Olsson and Jönsson, 2014) need thorough experimental testing for individual species. It is quite likely that many individual species have winter chilling requirements that are somewhat different from the standard models developed for a small number of fruit species, or show differing temperature thresholds and photoperiod requirements for the start of physiological processes.
Other spring phenomena
The use of twig experiments could provide a method for examining other spring phenomena, such as the increasing photosynthetic activity of young leaves as they expand in size and mature, the effects of plant nutrient status and light levels on the physiological ecology of the leaves, the timing of production of secondary plant compounds within the leaf tissue, the related timing of herbivore damage on leaf tissue, and the shifting balance of plant hormones during development. For investigations of herbivore damage, leaves of different ages could be exposed to herbivores for short intervals of time to determine the effects of plant secondary compounds and leaf toughness on insect growth and feeding preference.
Further possibilities
In addition, the use of twigs might have applications for investigating the effects of climate change on plants in other seasons of the year. In autumn especially, twig cuttings with a reduced number of leaves could potentially be used to investigate the effects of frost, temperature and photoperiod on leaf senescence and dormancy induction. Twigs collected in the winter could be exposed to brief warm periods, simulating the effects of unseasonable warm periods, then returned to the cold temperatures of winter, to investigate how more variable climatic conditions, or extreme events (Jentsch et al., 2009), will affect the phenological development. However, it remains to be seen if such experiments will work in practice.
Lastly, twig experiments could be incorporated into environmental education, school science lessons and science fair projects related to climate change and plant science. Such experiments are valuable because twigs will leaf-out earlier or later in the range of temperatures and light environments found in school buildings and homes during the winter and early spring, demonstrating principles of species variability and the effects of environmental drivers on seasonal plant processes.
Dormant twigs provide a valuable and underutilized approach for investigating a wide range of topics relevant to climate change biology, ecosystem ecology, phenology research and plant physiological ecology. The most obvious next step is to expand the use of twig experiments to investigate how different facets of climate change impact the ecophysiology and leaf dynamics for a wide diversity of trees, shrubs and vine species, and across a range of ecosystems. In addition, experiments on frost tolerance and humidity requirements can be used to predict which species may benefit from a changing climate and which species may decline and become locally extinct. Information from these experiments can then be used to improve climate change forecasts of shifts in phenology, ecosystem processes and ecological interactions.
ACKNOWLEDGEMENTS
We thank Abe Miller-Rushing, Libby Ellwood and two reviewers for comments on the manuscript. Funds to support this project came from a Humboldt Research Award (to R.B.P.), a National Science Foundation Graduate Research Fellowship (grant no. DGE-1247312 to A.S.G.) and the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC (grant agreement no. 282250) to J.L. and A.M.
LITERATURE CITED
- Augspurger CK. 2008. Early spring leaf out enhances growth and survival of saplings in a temperate deciduous forest. Oecologia 156: 281–286. [DOI] [PubMed] [Google Scholar]
- Augspurger CK. 2013. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology 94: 41–50. [DOI] [PubMed] [Google Scholar]
- Balandier P, Gendraud M, Rageau R, Bonhomme M, Richard JP, Parisot E. 1993. Bud break delay on single node cuttings and bud capacity for nucleotide accumulation as parameters for endodormancy and paradormancy in peachtrees in a tropical climate. Scientia Horticulturae 55: 249–261. [Google Scholar]
- Basler D, Körner C. 2012. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agricultural and Forest Meteorology 165: 73–81. [Google Scholar]
- Basler D, Körner C. 2014. Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiology 34: 377–388. [DOI] [PubMed] [Google Scholar]
- Beck I, Jochner S, Gilles S, et al. 2013. High environmental ozone levels lead to enhanced allergenicity of birch pollen. PLoS One 8: e80147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümel K, Chmielewski F-M. 2012. Shortcomings of classical phenological forcing models and a way to overcome them. Agricultural and Forest Meteorology 164: 10–19. [Google Scholar]
- Caffarra A, Donnelly A. 2011. The ecological significance of phenology in four different tree species: effects of light and temperature on bud burst. International Journal of Biometeorology 55: 711–721. [DOI] [PubMed] [Google Scholar]
- Campoy JA, Ruiz D, Egea J. 2011. Dormancy in temperate fruit trees in a global warming context: a review. Scientia Horticulturae 130: 357–372. [Google Scholar]
- Campoy JA, Ruiz D, Allderman L, Cook N, Egea J. 2012. The fulfilment of chilling requirements and the adaptation of apricot (Prunus armeniaca L.) in warm winter climates: an approach in Murcia (Spain) and the Western Cape (South Africa). European Journal of Agronomy 37: 43–55. [Google Scholar]
- Chuine I. 2000. A unified model for budburst of trees. Journal of Theoretical Biology 207: 337–347. [DOI] [PubMed] [Google Scholar]
- Chuine I, Morin I, Bugmann H. 2010. Warming, photoperiods, and tree phenology. Science 329: 277–278. [DOI] [PubMed] [Google Scholar]
- Cleland EE, Allen JM, Dunne JA, et al. 2012. Phenological tracking enables positive species resposnes to climate change. Ecology 93: 1765–1771. [DOI] [PubMed] [Google Scholar]
- Cornelius C, Leingaertner A, Hoiss B, Krauss J, Steffan-Dewenter I, Menzel A. 2013. Phenological response of grassland species to manipulative snowmelt and drought along an altitudinal gradient. Journal of Experimental Botany 64: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantec CF, Vitasse Y, Bonhomme M, Louvet J-M, Kremer A, Delzon S. 2014. Chilling and heat requirements for leaf unfolding in European beech and sessile oak populations at the southern limit of their distribution range. International Journal of Biometeorology 58: 1853–1864. [DOI] [PubMed] [Google Scholar]
- Davies T, Wolkovich EM, Kraft NJ, et al. 2013. Phylogenetic conservatism in plant phenology. Journal of Ecology 101: 1520–1530. [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Fridley JD. 2012. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485: 359–362. [DOI] [PubMed] [Google Scholar]
- Fu YH, Piao S, Zhao H, et al. 2014. Unexpected role of winter precipitation in determining heat requirement for spring vegetation green-up at northern middle and high latitudes. Global Change Biology 20: 3743–3755. [DOI] [PubMed] [Google Scholar]
- Gulevich T. 2003. Encyclopedia of Christmas and New Year’s celebrations, 2nd edn. Detroit: Omnigraphics. [Google Scholar]
- Gusta LV, Wisniewski M. 2013. Understanding plant cold hardiness: an opinion. Physiologia Plantarum 147: 4–14. [DOI] [PubMed] [Google Scholar]
- Hänninen H. 1995. Effects of climatic change on trees from cool and temperate regions: an ecophysiological approach to modeling of bud burst phenology. Canadian Journal of Botany 73: 183–199. [Google Scholar]
- Hänninen H, Tanino K. 2011. Tree seasonality in a warming climate. Trends in Plant Science 16: 412–416. [DOI] [PubMed] [Google Scholar]
- Heide OM. 1993a. Daylength and thermal time responses of budburst during dormancy release in some northern deciduous trees. Physiologia Plantarum 88: 531–540. [DOI] [PubMed] [Google Scholar]
- Heide OM. 1993b. Dormancy release in beech buds (Fagus sylvatica) requires both chilling and long days. Physiologia Plantarum 89: 187–191. [Google Scholar]
- Ibáñez I, Primack RB, Miller-Rushing AJ, et al. 2010. Forecasting phenology under global warming. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3247–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89: 353–362. [DOI] [PubMed] [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. 2009. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology 15: 837–849. [Google Scholar]
- Jochner S, Höfler J, Beck I, et al. 2013. Nutrient status: a missing factor in phenological and pollen research? Journal of Experimental Botany 64: 2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtman B, Power SB, Adedoyin JA, et al. 2013. Near-term climate change: projections and predictability. In: Stocker TF, Qin D, Plattner G-K, et al. eds. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, USA: Cambridge University Press. [Google Scholar]
- Körner C, Basler D. 2010. Phenology under global warming. Science 327: 1461–1462. [DOI] [PubMed] [Google Scholar]
- Kramer K. 1994. Selecting a model to predict the onset of growth of Fagus sylvatica . Journal of Applied Ecology 31: 172–181. [Google Scholar]
- Laube J, Sparks TH, Estrella N, Höfler J, Ankerst DP, Menzel A. 2014a. Chilling outweighs photoperiod in preventing precocious spring development. Global Change Biology 20: 170–182. [DOI] [PubMed] [Google Scholar]
- Laube J, Sparks TH, Estrella N, Menzel A. 2014b. Does humidity trigger tree phenology? Proposal for an air humidity based framework for bud development in spring. New Phytologist 202: 350–355. [DOI] [PubMed] [Google Scholar]
- Lenz A, Hoch G, Vitasse Y, Körner C. 2013. European deciduous trees exhibit similar safety margins against damage by spring freeze events along elevational gradients. New Phytologist 200: 1166–1175. [DOI] [PubMed] [Google Scholar]
- Linkosalo T, Lechowicz M. 2006. Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula). Tree Physiology 26: 1249–1256. [DOI] [PubMed] [Google Scholar]
- Linkosalo T, Lappalainen HK, Hari P. 2008. A comparison of phenological models of leaf bud burst and flowering of boreal trees using independent observations. Tree Physiology 28: 1873–1882. [DOI] [PubMed] [Google Scholar]
- Luedeling E. 2012. Climate change impacts on winter chill for temperate fruit and nut production: a review. Scientia Horticulturae 144: 218–229. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Migliavacca M, Sonnentag O, Keenan TF, Cascatti A, O’Keefe J, Richardson AD. 2012. On the uncertainty of phenological responses to climate change and its implication for terrestrial biosphere models. Biogeosciences Discussions 9: 879–926. [Google Scholar]
- Miller-Rushing AJ, Primack RB. 2008. A comparison of the impacts of winter temperatures on two birch (Betula) species. Tree Physiology 28:659–664. [DOI] [PubMed] [Google Scholar]
- Morin X, Lechowicz MJ, Augspurger C, O’Keefe J, Viner D, Chuine I. 2009. Leaf phenology in 22 North American tree species during the 21st century. Global Change Biology 15: 961–975. [Google Scholar]
- Murray MB, Cannell MGR, Smith RI. 1989. Date of budburst of 15 tree species in Britain following climatic warming. Journal of Applied Ecology 26: 693–700. [Google Scholar]
- Olsson C, Jönsson AM. 2014. Process-based models not always better than empirical models for simulating budburst of Norway spruce and birch in Europe. Global Change Biology 20: 3492–3507. [DOI] [PubMed] [Google Scholar]
- Palonen P, Buszard D. 1997. Current state of cold hardiness research on fruit crops. Canadian Journal of Plant Science 77: 399–420. [Google Scholar]
- Panchen ZA, Primack RB, Nordt B, et al. 2014. Leaf out times of temperate woody plants are related to phylogeny, deciduousness, growth habit, and wood anatomy. New Phytologist 203: 1208–1219. [DOI] [PubMed] [Google Scholar]
- Polgar CA, Primack RB. 2011. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist 191: 926–941. [DOI] [PubMed] [Google Scholar]
- Polgar C, Gallinat A, Primack RB. 2014. Drivers of leaf-out phenology and their implications for species invasions: insights from Thoreau’s Concord. New Phytologist 202: 106–115. [DOI] [PubMed] [Google Scholar]
- Reig G, Iglesias I, Miranda C, Gatius F, Alegre S. 2013. How does simulated frost treatment affect peach [Prunus persica (L.)] flowers of different cultivars from worldwide breeding programmes? Scientia Horticulturae 160: 70–77. [Google Scholar]
- Richardson AD, Schenck Bailey A, Denny EG, Martin CW, O’Keefe J. 2006. Phenology of a northern hardwood forest canopy. Global Change Biology 12: 1174–1188. [Google Scholar]
- Richardson AD, Black TA, Ciais P, et al. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3227–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Keenan TF, Migliavacca M, Ryu Y, Sonnentag O, Toomey M. 2013. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agricultural and Forest Meteorology 169: 156–173. [Google Scholar]
- Rosenzweig C, Karoly D, Vicarelli M, et al. 2008. Attributing physical and biological impacts to anthropogenic climate change. Nature 453: 353–357. [DOI] [PubMed] [Google Scholar]
- Ruiz D, Campoy JA, Egea J. 2007. Chilling and heat requirements of apricot cultivars for flowering. Environmental and Experimental Botany 61: 254–263. [Google Scholar]
- Sønsteby A, Heide OM. 2014. Chilling requirements of contrasting black currant (Ribes nigrum L.) cultivars and the induction of secondary bud dormancy. Scientia Horticulturae 179: 256–265. [Google Scholar]
- Taeger S, Sparks TH, Menzel A. 2015. Effects of temperature and drought manipulations on seedlings of Scots pine provenances. Plant Biology 17: 361–372. [DOI] [PubMed] [Google Scholar]
- Vitasse Y. 2013. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytologist 198: 149–155. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Basler D. 2014. Is the use of cuttings a good proxy to explore phenological responses of temperate forests in warming and photoperiod experiments? Tree Physiology 34: 174–183. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Lenz A, Körner C. 2014a. The interaction between freezing tolerance and phenology in temperate deciduous trees. Frontiers in Plant Science 5: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitasse Y, Lenz A, Hoch G, Körner C. 2014b. Earlier leaf-out rather than difference in freezing resistance puts juvenile trees at greater risk of damage than adult trees. Journal of Ecology 102: 981–988. [Google Scholar]
- Walther GR. 2010. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Montgomery RA. 2014. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant, Cell and Environment (in press). [DOI] [PubMed] [Google Scholar]
- Willis CG, Ruhfel B, Primack RB, Miller-Rushing A, Davis CC. 2008. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proceedings of the National Academy of Sciences, USA 105: 17029–17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkovich EM, Cook B, Allen J, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485: 494–497. [DOI] [PubMed] [Google Scholar]
- Yu H, Luedeling E, Xu J. 2010. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proceedings of the National Academy of Sciences, USA 107: 22151–22156. [DOI] [PMC free article] [PubMed] [Google Scholar]