Abstract
Background and Aims Extreme climatic events such as severe droughts are expected to increase with climate change and to limit grassland perennity. The present study aimed to characterize the adaptive responses by which temperate herbaceous grassland species resist, survive and recover from a severe drought and to explore the relationships between plant resource use and drought resistance strategies.
Methods Monocultures of six native perennial species from upland grasslands and one Mediterranean drought-resistant cultivar were compared under semi-controlled and non-limiting rooting depth conditions. Above- and below-ground traits were measured under irrigation in spring and during drought in summer (50 d of withholding water) in order to characterize resource use and drought resistance strategies. Plants were then rehydrated and assessed for survival (after 15 d) and recovery (after 1 year).
Key Results Dehydration avoidance through water uptake was associated with species that had deep roots (>1·2 m) and high root mass (>4 kg m−3). Cell membrane stability ensuring dehydration tolerance of roots and meristems was positively correlated with fructan content and negatively correlated with sucrose content. Species that survived and recovered best combined high resource acquisition in spring (leaf elongation rate >9 mm d−1 and rooting depth >1·2 m) with both high dehydration avoidance and tolerance strategies.
Conclusions Most of the native forage species, dominant in upland grassland, were able to survive and recover from extreme drought, but with various time lags. Overall the results suggest that the wide range of interspecific functional strategies for coping with drought may enhance the resilience of upland grassland plant communities under extreme drought events.
Keywords: Dehydration avoidance, dehydration tolerance, drought survival, forbs, fructans, functional traits, grasses, membrane stability, resilience index, root system, sucrose, upland grassland
INTRODUCTION
In Europe, grasslands cover 22 % of the EU-25 land area (FAOSTAT data, http://faostat.fao.org) and provide various ecosystemic services such as forage production, carbon storage, soil protection and biodiversity preservation (Millenium Ecosystem Assessment, 2005). Climate change will probably impact this ecosystem through shifts in grassland composition and production potential (Grime et al., 2000; Gilgen and Buchmann, 2009; Craine et al., 2011; Zwicke et al., 2013). In temperate areas, more extreme rainfall regimes are expected to increase the duration and severity of drought (Knapp et al., 2008; Seneviratne et al., 2012). In this context, more knowledge is required to understand the mechanisms of resistance, survival under severe drought and recovery of herbaceous species after drought (Chaves et al., 2003; Volaire et al., 2014).
There is evidence for a persistence–productivity trade-off, species with a high maximum growth rate having a higher mortality under restricted resources (Sibly and Calow, 1989; Wright et al., 2010). In the case of drought tolerance, evidence for this trade-off has been described for woody plants (Reich, 2014), but the relationships between plant growth rate under optimal conditions and subsequent performance under severe drought has hardly been explored in herbaceous species (Pérez-Ramos et al., 2013). Perennial herbaceous species are interesting plant models, since they can resist and survive severe drought through adaptive strategies contributing to dehydration avoidance and dehydration tolerance (Ludlow, 1989; Blum, 1996). Under moderate drought, dehydration avoidance ensures the maintenance of plant tissue hydration and osmotic potential by maximizing water uptake and minimizing water losses (Ludlow, 1989; Volaire et al., 2009). Under severe drought, once complete leaf senescence is reached, dehydration tolerance ensures plant survival by maintaining cell integrity in meristematic tissues through cell membrane stabilization and accumulation of water-soluble carbohydrates (WSCs) and dehydrins (Volaire et al., 1998a, b; Verslues et al., 2006). Grassland C3 Poaceae and Asteraceae species of temperate areas accumulate large amounts of reserve carbohydrates mainly as fructans in leaf meristems, and contain only a low level of starch (Chatterton et al., 1989; Pollock and Cairns, 1991; Janeček et al., 2011; Jensen et al., 2014). Furthermore, it has been shown that fructan accumulation during drought improves plant survival after drought (Volaire et al., 1998a; Clark et al., 2004). Fructans in particular were shown to stabilize membranes in vitro by interaction with lipids under stress (Vereyken et al., 2001, 2003; Hincha et al., 2002, 2007) and because they may act as antioxidants (Peshev et al., 2013). These compounds play an indirect role in drought survival when they are hydrolysed to fuel growth after rehydration (Volaire et al., 1998b; Thomas and James, 1999; Amiard et al., 2003).
Under progressive soil drying, higher biomass allocation to roots (Poorter et al., 2012b) and an extensive root system have been commonly observed in drought-resistant species (Comas et al., 2013), since deep roots can take up water from moister soil layers. Under severe soil drought conditions, root mortality increases due to tissue dehydration (Eissenstat and Yanai, 1997; Facette et al., 1999). However, it has been shown in Lolium perenne that some roots can survive very dry conditions (soil water content less than –10 MPa), especially root apices, and that production of lateral roots from existing roots is observed after soil rewetting (Jupp and Newman, 1987). Thus, maintaining live roots in severe soil drying conditions should enhance plant recovery (Weaver and Zink, 1946; Huang et al., 1997). However, despite their crucial role in water uptake under drought, below-ground plant traits remain poorly quantified compared with leaf traits (Reich, 2014). The combination of these plant responses depends on species and genotypes, and can be investigated by assessing leaf and root traits (Pérez-Ramos et al., 2013) associated with water status and WSC metabolism (Volaire, 2008).
The present study aimed (1) to characterize the adaptive responses by which temperate grassland species resist, survive and recover from a severe drought and (2) to explore the relationships between plant resource use and drought resistance strategies. To this end, we compared six dominant perennial herbaceous species (five grasses and one forb) originating from upland temperate grasslands (Louault et al., 2005), according to their resistance to and recovery from summer extreme drought (Zwicke et al., 2013), with a Mediterranean grass cultivar used as a reference for grass species for its high drought survival. These species were grown in semi-controlled and non-limiting rooting depth conditions to analyse leaf and root traits. Optimum resource use strategies were identified under irrigation in spring, dehydration avoidance strategy was assessed under moderate drought (20 d of withholding water) and dehydration tolerance was estimated under severe drought (50 d). Plant survival and resilience indices (according to Van Ruijven and Berendse, 2010) were measured 2 weeks and 1 year, respectively, after rehydration. We tested the following hypotheses: (1) the drought survival of temperate species mainly depends on dehydration avoidance through water acquisition strategies; (2) accumulation of WSCs, especially fructans and sucrose, in surviving organs including not only leaf meristems, but also all root types and root meristem parts (apex), is associated with dehydration tolerance; and (3) there is a trade-off between high resource use under non-limiting conditions and high ability to survive and recover from severe drought.
MATERIALS AND METHODS
Experimental set-up and conditions
The experiment was conducted outdoors at Clermont-Ferrand (45°47′N, 03°05′E, 350 m a.s.l.), under a semi-continental climate (mean annual temperature, 12·4 °C; mean annual precipitation, 579 mm). An automatic weather station recorded air temperature, global radiation, wind speed and vapour pressure deficit, allowing the calculation of potential evapotranspiration (PET; Supplementary Data Table S1).
In summer 2010, granitic brown soil (12 % clay, 17 % loam, 59 % sand, 13 % organic matter), extracted from an upland grassland (45°43′N, 03°01′E, 850 m a.s.l.), was sieved at 7 mm and left to dry in the air. Plastic sleeves were placed inside tubes (PVC, 150 cm deep, 10 cm diameter, 1·2 mm thick; PUM Plastiques, France, n = 105) for easy sampling of the whole root system. The tubes were filled with the soil mixed with slow-release fertilizer (3·5 kg m−3, NPK 14-7-14, Multicote 12, Haifa, Israel). In September 2010, seeds of five native grass species: Dactylis glomerata (Dg), Festuca arundinacea (Fa), Poa pratensis (Pp), Poa trivialis (Pt) and Trisetum flavescens (Tf), one native forb species Taraxacum officinale (To) from an upland grassland (45°43′N, 3°01′E, 880 m a.s.l., Pontes et al., 2007) and one Mediterranean cultivar of D. glomerata ‘Medly’ (RAGT, France) (Md) were sown to ensure a homogeneous plant cover in the following spring before the drought treatment onset (about 1800 plants m−2). The Mediterranean cultivar was used as a control for high drought survival (Volaire and Lelièvre, 2001). All the tubes were placed outside in soil trenches (150 cm deep) during the winter and kept well watered with rainfall and additional watering.
In spring 2011, a drip irrigation system was set up [day of year (DOY) 78] to maintain soil water content (SWC) near field capacity. In addition, three volumetric SWC (m3 m−3) probes (ECHO-5, Decagon, USA) were inserted horizontally at depths of 10, 40 and 100 cm on three tubes per species, and connected to a datalogger (EM50, Decagon, USA). From May to November (DOY 132–326), SWC was measured every 15 min (Fig. 1). Three tubes per species were also installed on a weighing scale (60 × 60 cm, Arpege Master K, type N PAC + SAT MB, France) to record by gravimetry plant evapotranspiration, a proxy of plant water use (g kg−1), as bare soil represented <1 % of the tube surface area (Fig. 6). To limit soil warming due to light radiation, the tubes were insulated with a polystyrene (50 mm thick; Styrodur®, BASF, France) home-made box (three tubes together).
Fig. 1.
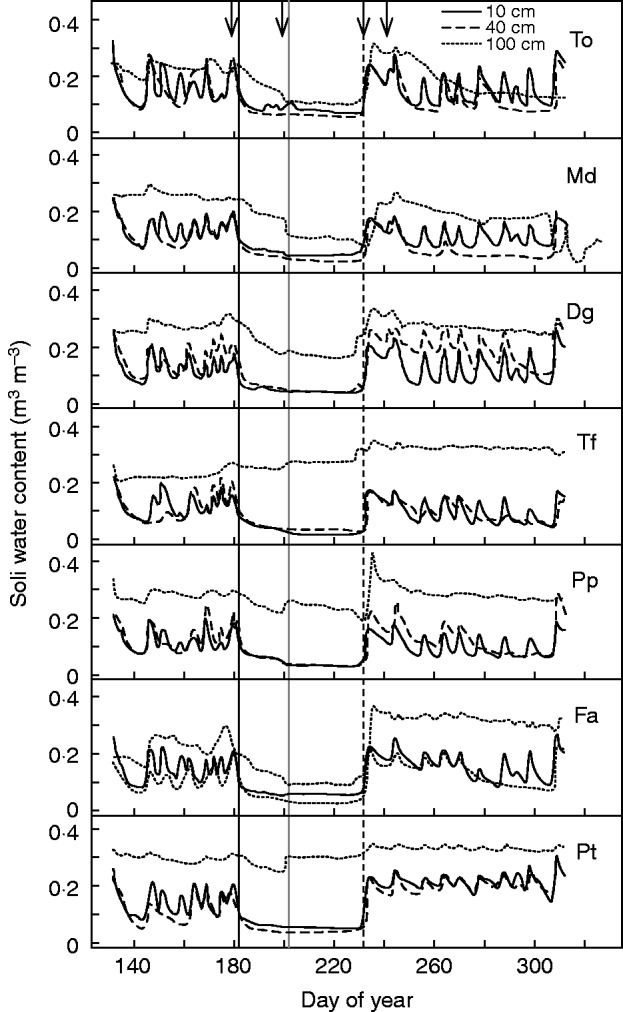
Time course of soil volumetric water content (m3 m−3) for Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt) of the drought treatment successively under irrigation before drought, summer drought and autumn recovery, at 10, 40 and 100 cm below the soil surface (as indicated in the key). Vertical lines represent the last watering (black solid line), end of leaf growth (grey solid line) and rehydration (black dashed line), delimiting periods of moderate drought (DOY 182–201) and severe drought (DOY 202–231), respectively. Arrows mark the four sampling dates (DOY 180, 200, 235 and 242). The soil water content of the control treatment is not shown.
Fig. 6.
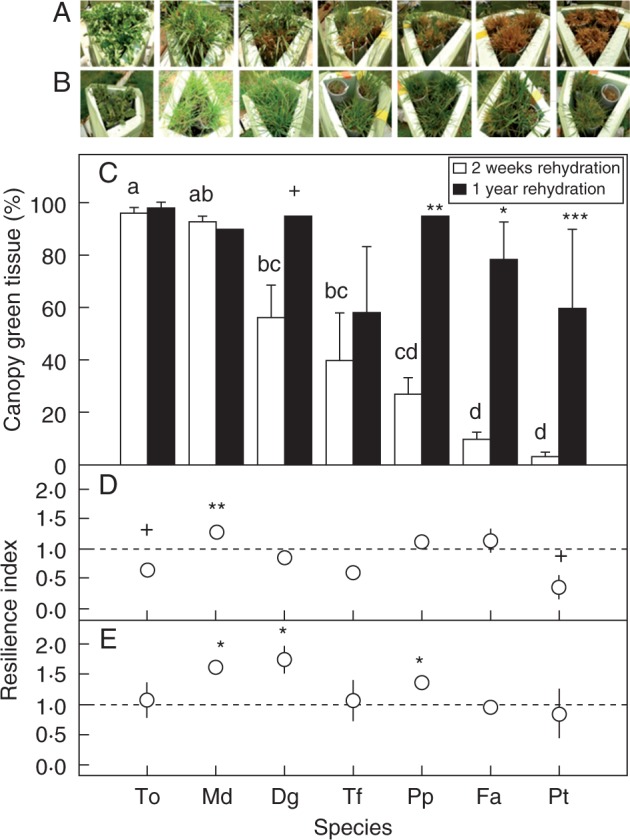
Images of Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt) 2 weeks after rehydration (A) and 1 year after rehydration on drought plants (B). (C) Canopy greenness of the tissue (%) 2 weeks after rehydration and 1 year after rehydration, and (D) resilience index of spring forage production and (E) standing root mass of the seven species. Mean values ± s.e. are shown (n = 3). Significant effects of periods on canopy green tissue, spring forage production and standing root mass for each species are indicated: +P < 0·1; *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001. In (C), different letters correspond to among-species differences for canopy green tissue measured 2 weeks after rehydration.
The drought treatment (50 d of withholding water) was induced from DOY 182 by stopping irrigation and intercepting all precipitations with a transparent polycarbonate shelter [12·5 × 10·8 m, 6·2 m high, 90 % transmitted photosynthetically active radiation (PAR), Batiroc, France]. This shelter was automatically controlled by a rain sensor. The drought treatment consisted first of 20 d of gradual soil drying until cessation of leaf growth (moderate drought, DOY 182–201) and then 30 d at a SWC <0·1 m3 m−3 (severe drought, DOY 202–231) (Fig. 1). This treatment was applied on 12 tubes per species. Three tubes per species were maintained at field capacity during the growing season for the control treatment. From DOY 232, the tubes of the drought treatment were rehydrated and maintained at field capacity until the growing season ended (DOY 306). All the tubes then received local precipitation until the end of the experiment in June 2012 (DOY 174). In total, 21 and 84 tubes were used for control and drought treatments, respectively.
During the experiment, plants were cut to a height of 5 cm, six times in 2011 (DOY 97, 116, 136, 165, 230 and 271) and then twice until spring 2012 (DOY 78 and 151) to simulate frequent mowing.
Leaf and root traits
Leaf length (mm) of the youngest leaf was measured on 36 plants per species (12 tubes per species) every 2–3 d from DOY 146 to 193 (moderate drought conditions) to calculate the leaf elongation rate (mm d−1; Carrère et al., 1997). During more severe drought conditions (DOY 193–222), leaf senescence (0–100 %) was visually assessed every 2–5 d.
Three tubes per species were harvested four times, corresponding to the end of the watering period (DOY 180, date 1), the end of moderate drought after the cessation of leaf growth (DOY 200, date 2), the end of severe drought (DOY 231, date 3) and the control treatment (DOY 242, date 4), in which plants were maintained at field capacity.
For each sampling date, nine mature leaves per species were collected and rehydrated to measure leaf relative water content (%) according to Volaire et al. (1998b). Specific leaf area (SLA; m2 kg−1) and leaf dry matter content (LDMC; g g−1) were measured at sampling date 1. Leaf meristems (enclosed leaf bases) were sampled after removal of mature leaves (Lattanzi et al., 2004), and fresh and dry (oven-dried at 60 °C for 48 h) weighed to determine their water content (%). For P. trivialis and T. flavescens, stolons (storage organs) were also collected for subsequent WSC measurements. The whole intact root system was extracted from each tube and carefully washed with tap water. Maximum rooting depth was measured (cm), then the deepest root apical zone (15 cm length, called root apices) and other roots including all root types were collected according to five soil layers (0–15, 15–30, 30–40, 40–90 and 90–150 cm; hereafter called roots). For P. pratensis and T. officinale, rhizomes and tap root (storage organs) were separated from fine roots. Root dry matter content (RDMC; g g−1) and total root mass (kg m−3) were calculated. The 95 % rooting depth (cm), i.e. the depth including 95 % of the root biomass, was calculated according to Schenk and Jackson (2002). Finally, the initial root–shoot ratio (R:S) was calculated at sampling date 1 as the ratio of total root mass (g) to standing shoot mass harvested at soil level (g).
Cell membrane stability
At the end of severe drought (dates 3 and 4), three sub-samples of leaf meristems, roots from each soil layer, root apices and storage organs were harvested to measure cell membrane stability (%), according to Volaire (1995) and Charrier and Améglio (2011). The cell membrane stability of each root layer was averaged.
Water-soluble carbohydrate analysis
At each sampling date, fresh sub-samples of leaf meristems, roots per soil layer, root apices and storage organs (rhizomes, stolons and tap roots) were quickly fresh-weighed, dropped into liquid nitrogen and stored at –80 °C before freeze-drying (–100 °C for 48 h). Fine powder samples (30–50 mg) were extracted in 80 % ethanol, and purified in mini-columns (Mobicols from MoBITec, Göttingen, Germany) with ion-exchange resins (see Amiard et al., 2003 for details). Water-soluble carbohydrates (mg g−1) were analysed by high-performance liquid chromatography (HPLC) on a cation exchange column (Sugar-PAK, 300 × 6·5 mm, Millipore Waters, Milford, MA, USA) eluted at 0·5 mL min−1 and 85 °C with 0·1 mm Ca-EDTA in water and quantified using a refractive index detector (2410 Differential Refractometer, Millipore Waters). External standards used to quantify carbohydrates were glucose, fructose, sucrose and Cichorium intybus inulin (Sigma-Aldrich, MO, USA). The HPLC system enabled the quantification of fructans with a low degree of polymerization (DP 3–4) and fructans with a high degree of polymerization (DP ≥5). The mean WSC content of the whole root system was calculated from the WSC content (mg g−1 d. wt) and the dry mass of each root layer. In addition, the amount of WSC (mg plant−1) in roots was calculated by multiplying the dry mass of roots (g plant−1) by the WSC content (mg g−1 d. wt) measured after severe drought.
Plant survival, recovery and resilience indices
Two weeks after rehydration (DOY 246) and in the following spring 2012 (DOY 158), the percentage of green tissue in aerial tissues was visually assessed on three tubes per species to determine plant survival and 1 year recovery, respectively.
In spring 2011 (DOY 97, 116, 136 and 165) and spring 2012 (DOY 78 and 151) above-ground biomass was oven-dried (60 °C for 48 h) and weighed to determine pre- and post-drought forage production. In addition, three tubes per species were harvested in spring 2011 (date 1) and in spring 2012 (DOY 172) to assess pre- and post-drought standing root mass (kg m−3). Resilience indices of spring forage production and spring standing root mass were calculated as the ratio of post-drought (2012) to pre-drought (2011) above-ground biomass and standing root mass, respectively (Van Ruijven and Berendse, 2010).
Statistical analyses
Statistical analyses were carried out with R software (R Core Team, 2012). Data were transformed when necessary (arcsine-square root or square root) before analysis to conform to the assumptions of normality (Shapiro–Wilk test) and homogeneity of variances (Fligner Killeen test). For each sampling date, single-factor (species and treatment) analysis of variance (ANOVA) and the post-hoc Tukey test were performed with the ‘multcomp’ package (Hothorn and Bretz, 2009). The SWC was estimated at the end of leaf growth and for 50 % of leaf senescence from linear and sigmoid regressions between the leaf elongation rate with SWC and leaf senescence with SWC, respectively. In addition, a Student t-test was used to compare means of two variables (green tissue percentage and resilience indices) for each species.
Pearson’s coefficients of correlation among plant traits were calculated with the ‘Hmisc’ package (Harrel and Dupont, 2007). Plant functional strategies for resource acquisition, dehydration avoidance and dehydration tolerance were analysed with matrices crossing traits and species replicates, using three principal component analyses (PCAs) with the ‘ade4’ package (Dray and Dufour, 2007). The first PCA tested seven traits measured before drought. The second PCA tested six traits measured during the moderate drought. The third PCA tested ten traits measured during moderate or severe drought. For each PCA, plant survival 2 weeks after rehydration, 1 year recovery of greenness, and resilience indices of spring forage production and of standing root mass were added as supplementary variables. Multiple regressions with backward selection then identified the traits most closely correlated with plant performance.
RESULTS
Soil volumetric water content
Before drought, SWC in shallow soil layers (depths of 10 and 40 cm) fluctuated according to irrigation or rainfall events and plant transpiration, averaging 0·139 m3 m−3 across species. At a depth of 100 cm, SWC varied less and averaged 0·250 m3 m−3 (Fig. 1). One week after the last irrigation, SWC in shallow soil layers rapidly decreased and stabilized at 0·045 m3 m−3. At 100 cm depth and during the moderate drought, SWC declined to either 0·102 m3 m−3 (F. arundinacea, T. officinale and D. glomerata ‘Medly’) or 0·279 m3 m−3 (T. flavescens, P. pratensis and P. trivialis), and remained stable during the severe drought. After rehydration (DOY 232–246), SWC in shallow and deep layers reached 0·174 and 0·295 m3 m−3, respectively.
Plant traits before drought
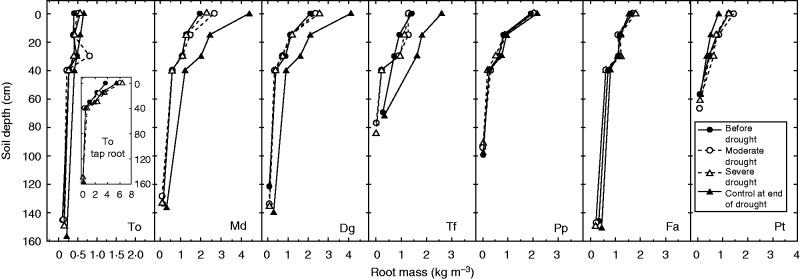
Traits differed among species (Table 1). The SLA ranged between 49·5 (P. trivialis) and 22·8 (P. pratensis) m2 kg−1. Dactylis glomerata, D. glomerata ‘Medly’ and T. officinale had the highest elongation rate (>9 mm d−1), while T. flavescens, F. arundinacea, P. trivialis and P. pratensis had the lowest (<7 mm d−1). Taraxacum officinale had significantly higher tap root mass (7·5 kg m−3) than grasses (P < 0·001). Within grasses, F. arundinacea, D. glomerata and D. glomerata ‘Medly’ had the highest root mass and P. trivialis the lowest. Higher values of maximum rooting depth and 95 % rooting depth were observed for T. officinale, F. arundinacea, D. glomerata and D. glomerata ‘Medly’, whereas P. trivialis had the shortest root system (P < 0·001, Fig. 2). The R:S differed significantly among species, especially between the forb and grasses. Within grasses, D. glomerata ‘Medly’ showed a higher R:S (0·49) than P. trivialis (0·29). Finally, water use was significantly higher for T. officinale and F. arundinacea (>120 g kg−1, P < 0·001) than for species with shallower root systems, such as T. flavescens, P. pratensis and P. trivialis. The RDMC ranged between 0·101 (T. officinale fine roots) and 0·228 g g−1 (P. pratensis), D. glomerata showing significantly lower values (0·189 g g−1) than D. glomerata ‘Medly’ (0·211 g g−1, P < 0·01).
Table 1.
Plant traits measured under irrigation before drought (DOY 78–181) and at the end of moderate (DOY 182–201) and severe (DOY 202–231) drought
| Period | Traits | Totap root | To | Md | Dg | Tf | Pp | Fa | Pt |
|---|---|---|---|---|---|---|---|---|---|
| Before drought | LDMC | 168c | 259ab | 242ab | 91d | 311a | 223bc | 267ab | |
| SLA | 29·2bc | 24·9bc | 27·3bc | 33·5b | 22·8c | 24·5c | 49·5a | ||
| LER | 9·4abc | 10·3ab | 11·8a | 6·7c | 4·6c | 6·6bc | 3·3c | ||
| LRWC1 | 96ab | 98a | 97ab | 96ab | 95ab | 96ab | 88b | ||
| MWC1 | 80a | 77ab | 80a | 75ab | 74ab | 80a | 69b | ||
| RM1 | 7·5 | 1·7c | 4·9a | 4·6ab | 3·3b | 3·6bc | 5·0a | 2·6c | |
| RDMC1 | 0·257 | 0·101d | 0·211ab | 0·189c | 0·195bc | 0·228a | 0·206bc | 0·204bc | |
| Dmax1 | 146a | 134a | 122ab | 70cd | 100bc | 146a | 57d | ||
| D951 | 47 | 98a | 82ab | 71bc | 62bc | 60bc | 106a | 48c | |
| R:S1 | 2·21a | 0·49b | 0·34bc | 0·34bc | 0·46bc | 0·43bc | 0·29c | ||
| WU1 | 125a | 106ab | 111ab | 78b | 75b | 133a | 82b | ||
| Moderate drought | LRWC2 | 69a | 53ab | 39ab | 30b | 29b | 33b | 27b | |
| MWC2 | 63·0a | 47·8bc | 46·4bc | 40·5cd | 38·0d | 49·6b | 38·3 d | ||
| LS2 | 20·8a | 6·7a | 19·2a | 20·8a | 12·5a | 23·3a | 54·2b | ||
| RM2 | 11·5 | 2·1d | 5·9a | 4·7ab | 3·7cd | 4·0bc | 4·9ab | 3·0cd | |
| RDMC2 | 0·340 | 0·154c | 0·305a | 0·278ab | 0·243b | 0·286ab | 0·306a | 0·302ab | |
| Dmax2 | 145a | 129ab | 134a | 78c | 95bc | 147a | 67c | ||
| D952 | 53 | 91a | 74bc | 67c | 58c | 67c | 98ab | 49c | |
| WU2 | 103 | 75 | 76 | 46 | 47 | 70 | 37 | ||
| SWCel | 0·122 | 0·100 | 0·110 | 0·071 | 0·065 | 0·099 | 0·062 | ||
| Severe drought | LRWC3 | 16 | 28 | 19 | 11 | 16 | – | – | |
| MWC3 | 43·8a | 46·2a | 35·5ab | 28·5ab | 26·3ab | 22·2ab | 14·6b | ||
| LS3 | 90bc | 81c | 96ab | 97ab | 90bc | 100a | 100a | ||
| RM3 | 13·0 | 1·8c | 5·3a | 4·9a | 3·6b | 3·7b | 5·0a | 2·8bc | |
| RDMC3 | 0·431 | 0·237b | 0·343a | 0·339a | 0·266ab | 0·291ab | 0·326a | 0·371a | |
| Dmax3 | 149a | 133a | 136a | 85b | 91b | 150a | 61b | ||
| D953 | 57 | 113a | 78bc | 67cd | 61cd | 53d | 93ab | 56cd | |
| WU3 | 70 | 28 | 36 | 20 | 40 | 20 | 19 | ||
| SWCs | 0·085 | 0·052 | 0·092 | 0·029 | 0·018 | 0·053 | 0·061 |
Mean values are shown (n = 3). Letters show significant differences among the seven species, except for tap root of To, according to one-way ANOVA and Tukey tests (P < 0·05).
Dmax, maximum rooting depth (cm); D95, 95 % rooting depth (cm); LER, leaf elongation rate (mm d−1); LDMC, leaf dry matter content (g g−1); LRWC, leaf relative water content (%); LS, leaf senescence (%); MWC, leaf meristem water content (%); RM, total root mass (kg m−3); RDMC, root dry matter content (g g−1); R:S, root–shoot ratio; SWCel, soil water content at the end of leaf growth (m3 m−3); SLA, specific leaf area (m2 kg−1); SWCs, soil water content at 50 % of leaf senescence (m3 m−3); WU, water use (g kg−1). Dg, Dactylis glomerata; Fa, Festuca arundinacea; Md, Dactylis glomerata ‘Medly’; Pp, Poa pratensis; Pt, Poa trivialis; Tf, Trisetum flavescens; To, Taraxacum officinale; Totap root, tap root of T. officinale.
Subscripts (1, 2, 3) of traits when present correspond to sampling dates.
Fig. 2.
Root mass (kg m−3) profile in the soil of Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt) measured before drought treatment (black circles), during moderate drought (white circles) and severe drought (white triangles), and in the control plants at the end of drought (black triangles). For T. officinale, the insert corresponds to the tap root mass profile. Mean values are shown ± s.e. unless hidden by symbols, n = 3.
For all species and all analysed tissues, fructans with a high DP were the dominant sugar compounds (73·2 ± 1·6 %) of total WSCs, followed by sucrose (17·8 ± 1·2 %), fructans with a low DP (DP 3–4 fructans, 7·1 ± 0·4 %) and monosaccharides (glucose and fructose, 4·4 ± 0·6 %). The present study focuses on the dominant carbohydrates (high DP fructans and sucrose). Results for the other sugars (DP 3–4 fructans and monosaccharides) are presented as Supplementary Data Figs. S1 and S2. Before drought, high DP fructan contents in leaf meristems and root apices were not significantly different among species (Fig. 3). In contrast, species were discriminated by high DP fructan and sucrose contents in roots (P < 0·001) to form three groups: (1) T. officinale and T. flavescens; (2) P. pratensis; and (3) D. glomerata, D. glomerata ‘Medly’, F. arundinacea and P. trivialis. Three species (T. officinale, P. pratensis and P. trivialis) accumulated fructans and sucrose in storage organs.
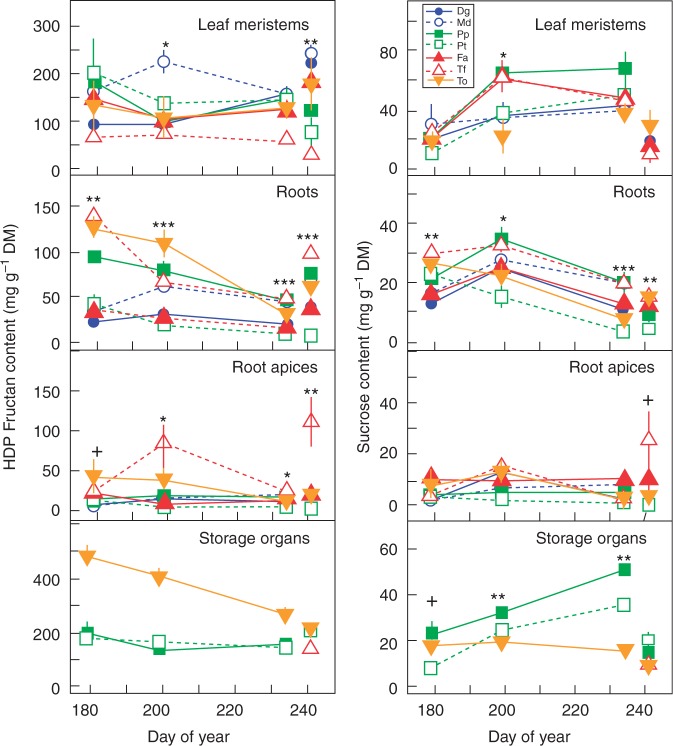
Fig. 3.
Fructans with degree of polymerization ≥5 (HDP fructans) and sucrose contents in leaf meristems, roots and root apices of Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavesce (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt), and in storage organs of To (tap root), Tf (stolon), Pp (rhizome) and Pt (stolon) during the experimental drought (DOY 182–231) and in the control plants (DOY 242). DM, dry mass. Mean values are shown ± s.e. (n = 3). Symbols indicate significant differences among species after one-way ANOVA: +P ≤ 0·1; *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
Plant responses to moderate drought
For all the species, the leaf elongation rate rapidly declined and elongation stopped 10–12 d after the last irrigation when SWC at leaf growth cessation and leaf senescence reached on average 0·090 m3 m−3 and 23 %, respectively (Table 1). Leaf relative water content, meristem water content and water use decreased (P < 0·001), and RDMC and leaf senescence increased (P < 0·001) in comparison with irrigated treatment, whereas root mass, maximum rooting depth and 95 % rooting depth did not change significantly. However, all trait responses to moderate drought differed among species (Table 2). The forb species (T. officinale) showed higher values of leaf relative water content and meristem water content, and lower values of RDMC than the grass species. Within grasses, leaf relative water content and meristem water content values were >33 and 46 %, respectively, for D. glomerata, F. arundinacea and D. glomerata ‘Medly’ and lower for the remaining species (Table 1). Leaf senescence was the highest for P. trivialis. The RDMC of T. flavescens was significantly lower than that of D. glomerata ‘Medly’ and F. arundinacea. For root mass, maximum rooting depth and 95 % rooting depth, among-species differences followed the same pattern as that observed before drought (Fig. 2). Water use of T. officinale was about twice higher than that of T. flavescens, P. pratensis and P. trivialis (Table 1).
Table 2.
Effects of moderate drought (date 1 vs. date 2) and severe drought (date 2 vs. date 3) on plant traits
| Trait | Moderate drought |
Severe drought |
||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | SD | Sp | SD × Sp | d.f. | SD | Sp | SD × Sp | |
| LRWC | 22 | 25·2*** | 6·1*** | 7·8*** | 17 | 26·1*** | 2·1 | 1·3 |
| MWC | 27 | 811·1*** | 19·9*** | 4·4** | 26 | 47·4*** | 8·4*** | 2·1+ |
| LS | 49 | 198·7*** | 11·7*** | 0·8 | 70 | 660·2*** | 16·0*** | 2·4* |
| RM | 32 | 5·6* | 32·1*** | 0·7 | 28 | 1·0 | 30·1*** | 0·4 |
| RDMC | 28 | 242·1*** | 51·3*** | 2·9* | 28 | 46·6*** | 21·2*** | 0·6 |
| Dmax | 28 | 0·6 | 47·4*** | 0·5 | 28 | 0·13 | 44·2*** | 0·2 |
| D95 | 28 | 0·6 | 9·9*** | 0·3 | 28 | 0·3 | 10·1*** | 0·9 |
| WU | 21 | 62·4*** | 13·4*** | 1·3 | 14 | 49·0*** | 10·4*** | 1·7 |
| WSCm | 26 | 0·1 | 2·7* | 0·5 | 26 | 0·1 | 2·1+ | 1·0 |
| HDPm | 26 | 1·4 | 4·7** | 1·3 | 26 | 0·4 | 5·3** | 1·7 |
| DP3-4m | 26 | 5·7* | 6·1*** | 1·6 | 26 | 0·4 | 4·7** | 0·2 |
| SOm | 26 | 27·9*** | 2·3+ | 1·5 | 26 | 0·13 | 3·5* | 0·8 |
| GOm | 26 | 8·0** | 6·0*** | 1·0 | 26 | 48·4*** | 1·8 | 2·5+ |
| FOm | 26 | 2·3 | 10·3*** | 1·4 | 26 | 0·2 | 22·5*** | 1·5 |
| WSCr | 27 | 1·7 | 37·7*** | 6·2*** | 28 | 120·5*** | 41·2*** | 3·6** |
| HDPr | 27 | 8·3** | 51·7*** | 6·1*** | 28 | 96·6*** | 56·4*** | 3·7** |
| DP3-4r | 27 | 30·0*** | 22·3*** | 2·1+ | 28 | 47·3*** | 18·1*** | 0·9 |
| SOr | 27 | 11·0** | 5·2** | 4·2** | 28 | 75·1*** | 10·9*** | 0·5 |
| GOr | 27 | 67·1*** | 13·3*** | 1·2 | 28 | 98·3*** | 17·6*** | 2·4+ |
| FOr | 27 | 0·2 | 68·1*** | 0·8 | 28 | 4·0+ | 19·1*** | 1·1 |
| WSCa | 22 | 0·8 | 7·9*** | 3·5* | 24 | 6·1* | 10·8*** | 2·2+ |
| HDPa | 22 | 0·1 | 10·6*** | 4·2** | 24 | 2·3 | 14·3*** | 2·4+ |
| DP3-4a | 22 | 0·5 | 8·9*** | 2·4+ | 24 | 4·8* | 11·8*** | 3·2* |
| SOa | 22 | 9·3** | 3·8** | 2·8* | 24 | 14·8*** | 6·5*** | 2·3+ |
| GOa | 22 | 8·2** | 7·9*** | 4·3** | 24 | 32·4*** | 13·2*** | 2·5+ |
| FOa | 22 | 0·3 | 4·9** | 0·8 | 24 | 9·1** | 11·3*** | 1·4 |
| WSCo | 11 | 2·3 | 35·4*** | 0·6 | 14 | 10·3** | 24·4*** | 5·0* |
| HDPo | 11 | 4·0+ | 34·7*** | 0·4 | 14 | 13·1** | 26·4*** | 4·2* |
| DP3-4o | 11 | 4·8+ | 562·8*** | 0·2 | 14 | 0·2 | 121·3*** | 0·8 |
| SOo | 11 | 21·2*** | 8·9** | 4·3* | 16 | 4·7* | 15·0*** | 4·0* |
| GOo | 11 | 35·4*** | 7·9** | 1·8 | 14 | 43·1*** | 18·9*** | 3·0+ |
| FOo | 11 | 0·1 | 18·1*** | 4·0* | 14 | 0·1 | 18·1*** | 4·0* |
Degree of freedom (d.f.) and F-values are shown.
SD, sampling date; Sp, species.
Dmax, maximum rooting depth (cm); D95, 95% rooting depth (cm); FO, fructose content in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); GO, glucose content in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); HDP, content of fructans with degree of polymerization ≥5 in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); DP3-4, content of fructans with degree of polymerization 3 and 4 in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); LRWC, leaf relative water content (%); LS, leaf senescence (%); MWC, water content of leaf meristems (%); RDMC, root dry matter content (g g−1); RM, total root mass (kg m−3); SO, sucrose content in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); WSC, water-soluble carbohydrate content in leaf meristems (m), roots (r), root apices (a) and storage organs (o) (mg g−1 DM); WU, water use during moderate drought (g kg−1).
Symbols indicate a significant effect after one-way ANOVA: +P ≤ 0·1; *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
Across species, high DP fructan contents did not change in response to the moderate drought in leaf meristems and in root apices, whereas sucrose contents increased in all organs, especially in leaf meristems (+177 %, P < 0·001) and root apices (+72 %, P < 0·01) (Fig. 3; Table 2). The pattern of high DP fructans in roots (excluding tap roots and apices) and the pattern of sucrose in leaf meristems, roots and root apices differed among species. For T. officinale, high DP fructan and sucrose contents in roots and tap root were maintained, whereas significant increases in high DP fructan (+61 %) and sucrose (+73 %) contents were observed in roots of D. glomerata ‘Medly’ (P < 0·001). Sucrose content in leaf meristems remained stable for D. glomerata ‘Medly’ and D. glomerata, but strongly increased in T. flavescens, P. pratensis, F. arundinacea and P. trivialis (+200 % on average, P < 0·01). An accumulation of sucrose was also observed in roots of D. glomerata and F. arundinacea (+92 % and +56 %, respectively, P < 0·01), and in root apices of D. glomerata (+362 %, P < 0·05). Conversely, for T. flavescens and P. trivialis, high DP fructan content in roots decreased (–52 % on average, P < 0·01), whereas sucrose content in roots and root apices remained stable. Also, sucrose content increased in stolons of P. trivialis (+219 %, P < 0·001) but did not change significantly in rhizomes of P. pratensis. The content of DP 3–4 fructans decreased in root apices (–71 %, Supplementary Data Fig. S1).
At the end of moderate drought, high DP fructan content in leaf meristems was more than twice as high in D. glomerata ‘Medly’ as in the upland species, whereas sucrose content was much higher in T. flavescens, P. pratensis and F. arundinacea than in T. officinale, D. glomerata ‘Medly’ and D. glomerata (Fig. 3). In roots, high DP fructan content of T. officinale, P. pratensis, T. flavescens and D. glomerata ‘Medly’ was higher than that of D. glomerata, F. arundinacea and P. trivialis. Roots of P. pratensis showed the highest sucrose content, 130 % higher than in roots of P. trivialis. Trisetum flavescens had eight times higher high DP fructan content in root apices than D. glomerata, F. arundinacea and P. trivialis. Regarding the storage organs, the tap root of T. officinale had the highest high DP fructan content, whereas the sucrose content was higher in the rhizomes of P. pratensis.
Plant responses to severe drought
For all the species, leaf senescence increased, reaching on average 93 % (P < 0·001, Table 1). Leaf relative water content of the remaining living leaves and meristem water content declined on average to 18 and 31 %, respectively (P < 0·001), and water use was half that under the moderate drought (P < 0·001). The RDMC increased significantly (P < 0·001), whereas root mass and maximum rooting depth were unchanged between dates 2 and 3. The contents of high DP fructan and sucrose in leaf meristems were not affected by the severe drought (Table 2). However, in roots, the high DP fructan content significantly decreased by 49 % on average for T. officinale, D. glomerata ‘Medly’ and P. pratensis (P < 0·01), and the sucrose content also declined for T. officinale, D. glomerata ‘Medly’, D. glomerata, F. arundinacea and P. pratensis (P < 0·01, Fig. 3). Production of stolons appeared in T. flavescens during the severe drought, and this organ accumulated high DP fructan and sucrose contents similar to those of P. trivialis.
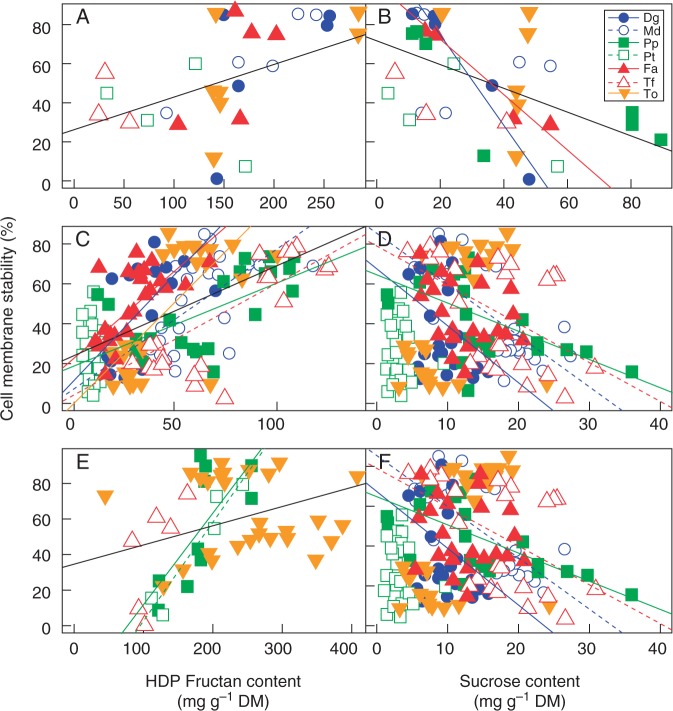
Cell membrane stability significantly declined in response to the drought (P < 0·01), except in leaf meristems of D. glomerata ‘Medly’ and T. flavescens, in root apices of D. glomerata ‘Medly’ and P. trivialis, and in stolons of T. flavescens (Fig. 4). Considering both treatments and all the species together, cell membrane stability of leaf meristems, roots and storage organs was positively correlated with the high DP fructan content of the corresponding tissues (r2 = 0·16, P < 0·01; r2 = 0·31, P < 0·001; r2 = 0·10, P < 0·01, respectively), measured after severe drought in stressed and control plants (Fig. 5A, C, E). In contrast, cell membrane stability was negatively correlated with sucrose content (r2 = 0·24, P < 0·01 in leaf meristems and r2 = 0·24, P < 0·01 in storage organs). At species level, correlation between cell membrane stability and high DP fructans could not be found in leaf meristems, but in roots cell membrane stability and high DP fructan content were positively linked (r2 > 0·39; P < 0·001) for each species except P. trivialis (Fig. 5C). Cell membrane stability was negatively correlated with sucrose content in leaf meristems of F. arundinacea and T. officinale, in roots of D. glomerata, D. glomerata ‘Medly’, P. pratensis and T. flavescens, and in the storage organs of the four species considered (Fig. 5B, D, F). Cell membrane stability was not correlated with carbohydrate contents in root apices (data not shown).
Fig. 4.
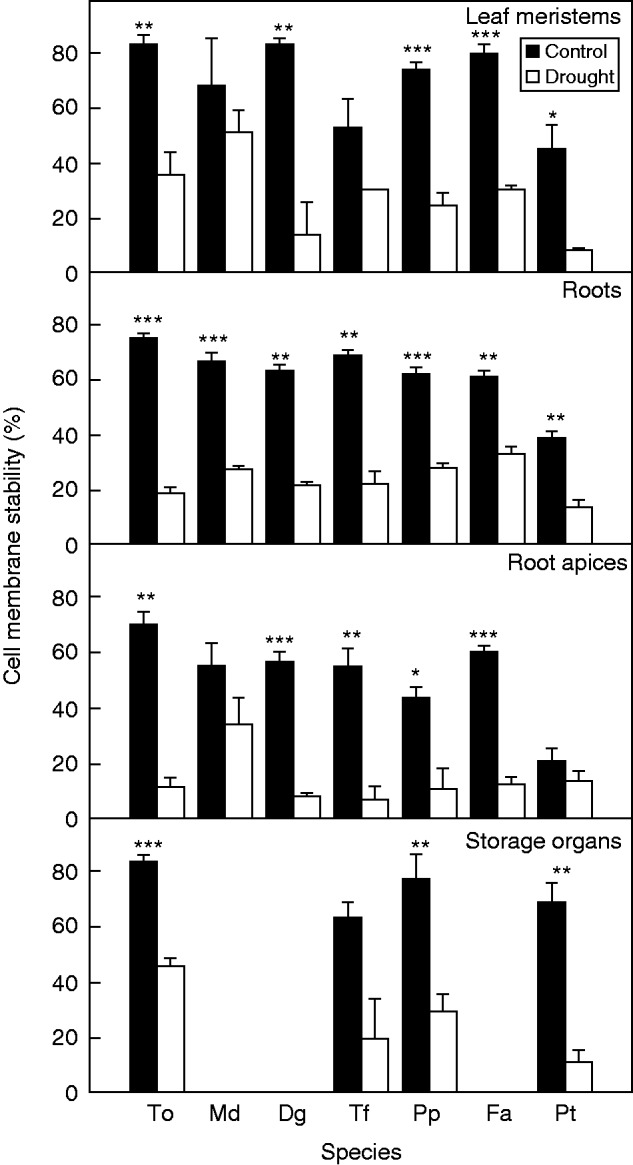
Cell membrane stability measured in leaf meristems, roots and root apices of Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt), and in storage organs (D) of To (tap root), Tf (stolon), Pp (rhizome) and Pt (stolon) measured at the end of severe drought in control and drought plants. Mean values ± s.e. are shown (n = 3). Symbols indicate a significant effect of drought treatment on plant organs for each species: *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001.
Fig. 5.
Correlations between cell membrane stability and fructans with degree of polymerization ≥5 (HDP fructans) (A, C, E) and sucrose contents (B, D, F) measured in leaf meristems (A, B), in roots (C, D) of Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt), and in storage organs (E, F) of To (tap root), Tf (stolon), Pp (rhizome) and Pt (stolon) measured at the end of the severe drought in control and drought plants. Lines represent the significant linear regressions (P < 0·05) for all species (black line) and for each species as indicated in the key in (B). DM, dry mass.
Plant survival and 1 year recovery after drought
All the species were able to regrow 2 weeks after rehydration, but the plant survival differed significantly among species (P < 0·001, Fig. 6A, B). Thus, three groups of species were identified: (1) high survival for T. officinale and D. glomerata ‘Medly’ (green tissue >90 %); (2) intermediate survival for D. glomerata, T. flavescens and P. pratensis (50–70 %); and (3) low survival for F. arundinacea and P. trivialis (<20 %). However, greenness measured 1 year after drought application was not significantly different among species, but high standard error values were noted for T. flavescens, F. arundinacea and P. trivialis (Fig. 6B).
The resilience index of forage production, calculated as the ratio of spring forage production measured 1 year after drought to spring forage production measured before drought, was the highest for D. glomerata ‘Medly’ (P < 0·01), and large among-species differences were observed (P < 0·001, Fig. 6C). Dactylis glomerata ‘Medly’, P. pratensis and F. arundinacea showed a higher resilience index than T. officinale, T. flavescens and P. trivialis. The root resilience index, estimated as the ratio of spring standing root mass measured 1 year after drought and spring standing root mass measured before drought, was close to one, except for D. glomerata ‘Medly’ and D. glomerata (P < 0·05), which showed the highest values (Fig. 6D).
Plant strategies during drought and after rehydration
Pearson’s correlation coefficients (Table 3) showed positive and significant correlations between plant survival and some of the traits measured before drought (leaf elongation rate, root mass and maximum rooting depth), during moderate drought (high DP fructan content in roots, leaf relative water content, root mass, SWC at leaf growth cessation and water use) and during severe drought (meristem water content, cell membrane stability of leaf meristems and sucrose content in roots). Plant survival was negatively correlated with RDMC, sucrose content in leaf meristems measured at leaf growth cessation, and leaf senescence at the end of drought. The resilience index of spring forage production was positively correlated with maximum rooting depth, 95 % rooting depth and RDMC measured before drought, and also with cell membrane stability of roots, amount of carbohydrates in roots and sucrose content in the deepest root apices measured after severe drought. The resilience index of spring forage production was also negatively correlated with SLA and SWC at 50 % of leaf senescence. However, only a positive correlation with SWC at leaf growth cessation was observed for the 1 year recovery of greenness and the root resilience index, which were correlated with each other and not with plant survival.
Table 3.
Pearson’s correlation coefficients among plant survival, plant recovery, plant resilience and plant traits measured under irrigation (DOY 78–181), moderate drought (DOY 182–201) and severe drought (DOY 202–231) treatments
| SURV | RECOV | RI | RRI | |
|---|---|---|---|---|
| LER | 0·68 | 0·35 | 0·26 | 0·32 |
| SLA | –0·38 | –0·38 | –0·76 | –0·38 |
| RM1 | 0·62 | 0·31 | 0·05 | –0·14 |
| Dmax1 | 0·53 | 0·41 | 0·48 | 0·18 |
| D951 | 0·00 | 0·01 | 0·47 | 0·01 |
| RDMC1 | –0·42 | –0·10 | 0·48 | 0·08 |
| LDMC | –0·19 | 0·29 | 0·35 | 0·26 |
| WU1 | 0·32 | 0·23 | 0·29 | 0·05 |
| LRWC2 | 0·67 | 0·39 | 0·10 | 0·31 |
| RDMC2 | –0·51 | –0·27 | 0·34 | 0·14 |
| RM2 | 0·65 | 0·35 | –0·08 | –0·02 |
| WU2 | 0·72 | 0·43 | 0·23 | 0·20 |
| HDPm2 | 0·28 | 0·07 | 0·21 | 0·10 |
| SOm2 | –0·50 | –0·22 | 0·23 | –0·14 |
| HDPr2 | 0·60 | 0·26 | 0·10 | 0·02 |
| SOr2 | 0·26 | 0·06 | 0·32 | 0·15 |
| HDPa2 | 0·09 | –0·02 | –0·13 | –0·05 |
| SOa2 | 0·30 | 0·22 | 0·03 | 0·01 |
| SWCel | 0·45 | 0·46 | 0·14 | 0·49 |
| LS3 | –0·70 | –0·24 | –0·32 | –0·25 |
| SWCs | –0·42 | –0·22 | –0·72 | –0·25 |
| MWC3 | 0·76 | 0·34 | 0·31 | 0·21 |
| CMSm | 0·51 | 0·09 | 0·37 | 0·09 |
| CMSr | 0·06 | –0·09 | 0·50 | 0·09 |
| CMSa | 0·36 | 0·17 | 0·39 | 0·38 |
| HDPm3 | 0·07 | 0·39 | 0·40 | 0·42 |
| SOm3 | –0·35 | 0·08 | 0·05 | 0·21 |
| HDPr3 | 0·29 | 0·13 | 0·32 | 0·20 |
| SOr3 | 0·56 | 0·25 | 0·25 | 0·08 |
| HDPa3 | 0·04 | –0·05 | 0·47 | 0·21 |
| SOa3 | –0·10 | –0·11 | 0·65 | 0·04 |
| QWSCr | 0·30 | 0·14 | 0·61 | 0·36 |
| SURV | 1 | 0·28 | 0·12 | 0·24 |
| RECOV | 1 | 0·40 | 0·66 | |
| RI | 1 | 0·55 | ||
| RRI | 1 |
Significant correlations are shown in bold (P < 0·05) and underlined text (P < 0·001).
Dmax, maximum rooting depth (cm); D95, 95 % rooting depth (cm); HDP, fructan DP ≥5 content in leaf meristems (m), roots (r) and root apices (a) (mg g−1 DM); LDMC, leaf dry matter content (g g−1); LER, leaf elongation rate (mm d−1); LRWC, leaf relative water content (%); LS, leaf senescence (%); MWC, leaf meristem water content (%); QWSCr, amount of WSC in roots (mg), RDMC, root dry matter content (g g−1); RECOV, 1 year recovery of aerial green tissue (%); RI, resilience index of spring forage production; RM, total root mass (kg m−3); RRI, resilience index of standing root mass; SO, sucrose content in leaf meristems (m), roots (r) and root apices (a) (mg g−1 DM); SLA, specific leaf area (m2 kg−1); SURV, plant survival rate 2 weeks after rehydration (%); SWCel, soil water content at the end of leaf growth (m3 m−3); SWCs, soil water content at 50 % of leaf senescence (m3 m−3); WU, water use (g kg−1).
Subscripts (1, 2, 3) of traits when present correspond to sampling dates.
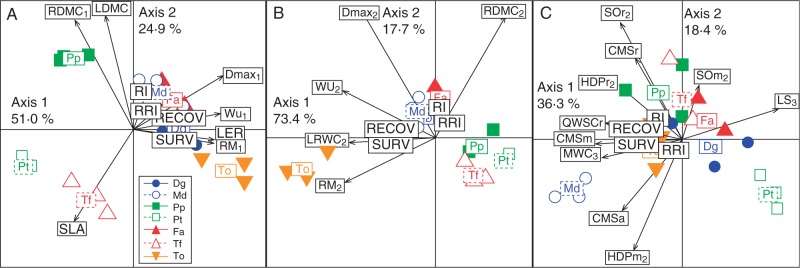
The first two axes of the PCA performed with traits related to resource acquisition before drought (Fig. 7A), dehydration avoidance (Fig. 7B) and dehydration tolerance during drought treatment (Fig. 7C) accounted for 75·9, 91·1 and 54·7 % of the total variance observed among species, respectively. For each PCA, plant survival was closely correlated with the first axis: 0·65, –0·74 and –0·73, respectively. The resilience index of spring forage production was correlated with the second axis of the PCA related to resource acquisition (0·74) and dehydration avoidance (0·66). However, 1 year recovery of greenness and the resilience index of spring standing root mass were not explained by these syndromes of traits. The multiple regressions performed with these traits showed that the contribution to plant survival differed according to the strategies with the following decreasing ranking: dehydration tolerance strategy (r2 = 0·76, P < 0·001), dehydration avoidance (r2 = 0·54, P < 0·001) and resource acquisition before drought (r2 = 0·44, P < 0·001). Conversely, the resilience index of spring forage production was mostly associated with the resource acquisition strategy (r2 = 0·68, P < 0·001).
Fig. 7.
Analyses of principal components combining traits related to strategies of resource acquisition (A), dehydration avoidance (B) and dehydration tolerance (C), measured on Taraxacum officinale (To), Dactylis glomerata ‘Medly’ (Md), Dactylis glomerata (Dg), Trisetum flavescens (Tf), Poa pratensis (Pp), Festuca arundinacea (Fa) and Poa trivialis (Pt) under field capacity (date 1), during moderate drought (date 2), during severe drought (date 3) and after rehydration. CMSa, CMSm, CMSr, cell membrane stability in root apices, leaf meristems and roots; Dmax, maximum rooting depth; HDPm, HDPr, content of fructans with degree of polymerization ≥5 in leaf meristems and roots; LDMC, leaf dry matter content; LER, leaf elongation rate; LRWC, leaf relative water content; LS, leaf senescence; MWC, leaf meristem water content; RDMC, root dry matter content; RECOV, 1 year recovery of aerial green tissue; RI, resilience index of spring forage production; RM, root mass; RRI, resilience index of standing root mass; SLA, specific leaf area; SOm, SOr, sucrose content in leaf meristems and roots; SURV, plant survival 2 weeks after rehydration; WU, water use. Subscripts (1, 2, 3) of traits when present correspond to sampling dates.
The first axis of the PCA, related to resource acquisition (Fig. 7A) and drought avoidance (Fig. 7B), showed a main contrast among the species with small root systems (P. trivialis, P. pratensis and T. flavescens) and those with deep root systens (D. glomerata, F. arundinacea, D. glomerata ‘Medly’ and T. officinale) (Fig. 7A, B; P < 0·001). Species were also segregated by the first axis of the PCA related to dehydration avoidance, with a main contrast between the forb (T. officinale) and the grass species (Fig. 7B; P < 0·001), while the first axis of the PCA related to tolerance strategy mostly opposed the upland species from the Mediterranean cultivar of D. glomerata (Fig. 7C; P < 0·001).
When considering only the six species from the upland grassland, the multiple regressions show a modified contribution of traits of both dehydration avoidance (maximum rooting depth, root mass, leaf relative water content, RDMC and water use during moderate drought, r2 = 0·65, P < 0·01), and dehydration tolerance after backward selection (high DP fructan content in roots, sucrose content in leaf meristems measured after moderate drought, leaf meristem water content and cell membrane stability in leaf meristems after severe drought, r2 = 0·67, P < 0·001) on drought survival.
DISCUSSION
Temperate and Mediterranean species display different strategies for dehydration avoidance
Against our first hypothesis, the results show a similar contribution of dehydration avoidance and tolerance to drought survival in species from temperate upland grassland. Dehydration avoidance is achieved through a combination of responses favouring maximized water uptake and minimized water loss under drought (Ludlow, 1989). The changes in leaf and meristem water content (Table 1) are indicators of the cellular adjustments (accumulation of solutes and/or cell wall hardening) made by the plant to achieve a low water potential while avoiding water loss (Verslues et al., 2006). In our study, leaf relative water content at moderate drought was correlated with root mass, water use and plant survival (Fig. 7B; Table 3), which confirms that maximizing water uptake during drought is an important mechanism in maintaining the hydration of lamina (Passioura, 1981; Chaves and Pereira, 1992; Volaire et al., 2009; Lelièvre et al., 2011). Although, the pot diameter may have impacted the lateral distribution of the roots (Poorter et al., 2012a), the length of the tubes (1·5 m) allowed a good estimation of root depth potential for the seven species. As previously shown for other species (Jackson et al., 2000; Pinheiro et al., 2005; Volaire, 2008), water uptake is mainly associated with initial root mass and maximum rooting depth (Fig. 7A). The species with higher root mass and maximum rooting depth, such as T. officinale, D. glomerata ‘Medly’, D. glomerata and F. arundinacea, had a higher water use before and during moderate drought (Tables 1 and 3; Fig. 7B). In arid and semi-arid environments, relatively small proportions of roots in deeper, moister soil layers may suffice to sustain water absorption of plants (Ehleringer and Dawson, 1992). The maintenance of water uptake by roots during a drought period is mainly driven by hydraulic continuity between soil, roots and leaves, which all depend on plant transpiration, hydraulic properties of roots (Passioura, 1988) and also on root morphology and anatomy (North and Nobel, 1991; Rieger and Litvin, 1999; Huang and Eissenstat, 2000; Steudle, 2000; Hernández et al., 2010). Among-species differences observed on leaf relative water content, meristem water content and RDMC (Table 1) suggest differences in water acquisition strategy, and therefore in the ability to maintain water uptake, explained by rooting depth (Fig. 7A, B; Table 3) and differences in root diameter and tissue density (Picon-Cochard et al., 2012). Overall, root lifespan maintenance during drought could be related to root hydration maintenance by the hydraulic lift mechanism (Bauerle et al., 2008), cell osmotic increase in cortex cells (Sharps and Davies, 1979) and suberization of the endoderm protecting xylem cells against dehydration, which could explain at least part of the increase in RDMC and decrease in plant water content (Table 1). The species able to maintain a root production during moderate drought, such as T. officinale and D. glomerata ‘Medly’ (Table 1; Fig. 2), had higher drought survival rates (Fig. 6B). Root growth maintenance under moderate water stress was also identified as an important mechanism to avoid dehydration by maximizing water uptake through an increase in root absorption area (Passioura, 1981, 1988; Vartanian, 1981; Pérez-Ramos et al., 2013).
Other mechanisms, such as modifications in water use efficiency and regulation of stomatal conductance, are involved in dehydration avoidance (Jones et al., 1981; Chaves and Pereira, 1992; Golluscio and Oesterheld, 2007). In addition, when stress intensifies, increased leaf senescence contributes to the minimization of water loss by reducing leaf area and evaporation (Ludlow, 1989; Gepstein, 2004). In this study, leaf senescence, which was inversely correlated with plant survival, was not adaptive but rather an indicator of drought vulnerability (Fig. 7C; Table 3). Conversely, gradual foliage senescence was positively correlated with plant survival in a range of Mediterranean grasses (Volaire et al., 1998b; Volaire and Lelièvre, 2001; Pérez-Ramos et al., 2013). This suggests differences in plant strategies for dehydration avoidance between temperate and Mediterranean herbaceous plants, which can exhibit summer dormancy with induced foliage senescence, as a specific strategy to survive extreme drought (Volaire and Norton, 2006).
Water-soluble carbohydrate metabolism is involved in dehydration avoidance and in dehydration tolerance
This study shows that plant survival is associated with high leaf meristem hydration and cell membrane stability under severe drought (Table 3; Fig. 4), which confirms that the ability of plant to protect leaf meristems is a key mechanism of dehydration resistance (including both avoidance and tolerance strategies) to ensure plant survival (Volaire et al., 1998b; Volaire and Lelièvre, 2001). Higher dehydration avoidance and dehydration tolerance were both previously attributed to WSCs, through their involvement in osmotic adjustment and cell membrane stabilization (Thomas, 1991; Volaire et al., 1998b; Livingston et al., 2009). Interestingly, among non-specific storage organs, it was in the leaf meristems of all the species that total WSC content was highest at the end of severe drought, but it was not discriminating among species (Supplementary Data Fig. S1). However, each carbohydrate was modulated differently by drought according to the species considered (Fig. 3; Figs S1 and S2). This indicates that carbohydrate composition rather than carbohydrate content is involved in drought resistance mechanisms, as already suggested (Ingram and Bartels, 1996).
When the photosynthetic machinery is impaired by prolonged drought (Chaves and Pereira, 1992), carbohydrate contents are modified, which may act as metabolic signals to promote leaf senescence and reserve mobilization (McDowell, 2011; Zeppel et al., 2011; Sala et al., 2012). In the sensitive species, T. flavescens, P. pratensis, F. arundinacea and P. trivialis (Fig. 6), sucrose was accumulated in leaf meristems during the moderate drought, and high DP fructan content declined significantly in the most sensitive ones, F. arundinacea and P. trivialis (Fig. 3). Accumulation of sucrose might result from sucrose synthesis enhancement and/or from fructan degradation. Although increasing sucrose synthesis could be stimulated by drought (Ingram and Bartels, 1996), more studies on enzyme activities are needed to elucidate the role of fructan hydrolysis in sucrose accumulation under drought. Hydrolysis of the polymers and concomitant synthesis of sucrose could be the mechanism whereby plant tissues survive water deficits (Spollen and Nelson, 1994; Xue et al., 2008; Saeedipour and Moradi, 2011). As sucrose is known to play an important role in osmotic adjustment during drought (Jones et al., 1981; Thomas, 1991; Volaire et al., 1998a, b), these results suggest that sucrose is involved in the dehydration avoidance strategy for the most sensitive populations. Depending on species and duration of drought, fructans are either accumulated (Volaire and Lelièvre, 1997; Volaire et al., 1998b; De Roover et al., 2000), modified in chain length (Thomas, 1991; Volaire et al., 1998b; Thomas and James, 1999) or reduced (Thomas, 1991; Spollen and Nelson, 1994; Clark et al., 2004). In this study, maintenance of high DP fructan content in leaf meristems during drought (Fig. 3) could contribute to higher survival in the more tolerant species, T. officinale, D. glomerata ‘Medly’ and D. glomerata (Fig. 6), as already reported for the Mediterranean cultivar (Volaire and Thomas, 1995; Volaire et al., 1998b; Volaire, 2008).
Despite a decline in cell membrane stability, this comparative study clearly reveals a positive correlation between high DP fructan content and cell membrane stability in roots for all the species, except for the most sensitive one, P. trivialis (Fig. 5). This can be attributed to the role of fructans in cell membrane protection (Valluru and Van den Ende, 2008; Livingston et al., 2009). While several studies have demonstrated this role through in vitro approaches (Vereyken et al., 2001; Hincha et al., 2002, 2007), only one study on D. glomerata supported it in planta (Volaire et al., 1998b). The present data corroborate the protective role of fructans in D. glomerata and support it in a broader panel of temperate species, including a forb and several grass species. In leaves of resurrection plants which do not accumulate fructans, sucrose is the main carbohydrate contributing to drought tolerance by membrane and protein stabilization (Ingram and Bartels, 1996; Scott, 2000). In fructan-accumulating plants, fructans might play this role. In addition, we found that the cell membrane stability of roots was not significantly correlated to plant survival (Table 3). This suggests that dehydration tolerance of the root system was not enough to ensure plant survival, underlining the role of leaf meristems in re-growth capacity after rehydration in species from upland grasslands and especially in the Mediterranean cultivar.
The combination of resource acquisition, dehydration avoidance and tolerance strategies enhances drought survival and recovery of temperate species
As in Ludlow (1989), the present results show that species were able to combine strategies for resource acquisition and strategies for drought resistance (Fig. 7; Table 3). The highest drought survival and resilience of spring forage production was observed for the fast-growing species with a higher leaf elongation rate and deeper roots, such as T. officinale, D. glomerata ‘Medly’ and D. glomerata (Tables 1 and 3; Figs 6 and 7). A higher leaf elongation rate or SLA can confer a competitive advantage for maintaining photosynthesis and root growth, although the resulting greater leaf area may be less efficient in avoiding dehydration (Pérez-Ramos et al., 2013). As the leaf elongation rate and water use were positively correlated (Fig. 7A; Table 3), our results confirm that differences among species in dehydration avoidance strategy are closely associated with the strategy to acquire water depending on root mass and depth (Chaves, 2002).
However, a dehydration avoidance strategy mainly based on maximizing water acquisition was not efficient enough to ensure plant survival and fast recovery in the case of F. arundinacea. This species had a deep root system and a high root mass, but was unable to maintain leaf and meristem water content, and therefore had a low survival rate (Table 1; Fig. 6B). This could be explained by higher leaf transpiration and lower water use efficiency in comparison with T. officinale, D. glomerata ‘Medly’ and D. glomerata (Brock and Galen, 2005; Milbau et al., 2005). However, at a longer time scale, F. arundinacea was able to recover to the level of D. glomerata ‘Medly’, D. glomerata and T. officinale. In addition, species that were least efficient in avoiding dehydration, but which had storage organs, such as T. flavescens and P. pratensis, had higher drought survival than F. arundinacea (Fig. 5B), probably due to the amount of carbohydrate reserves in their storage organs (Fig. 3; Supplementary Data Fig. S1). These results therefore confirm that storage organs can contribute to short-term plant re-growth (Klimešová and Klimeš, 2007; Carter et al., 2012). However, at a longer time scale, P. pratensis recovered better than T. flavescens and was able to extend its root system (root resilience index >1; Fig. 6D). Higher ion solutes, WSC contents in roots, and root development after drought treatment (Jiang and Huang, 2000) may explain the better recovery capacity of P. pratensis than of T. flavescens and P. trivialis (Fig. 6).
All the species showed dehydration avoidance and tolerance mechanisms involving sucrose accumulation and/or fructan maintenance in plant organs. Also, differences between carbohydrate dynamics in response to drought were as high among the grasses as between the grasses and the forb (T. officinale) (Fig. 3; Supplementary Data Figs S1 and S2). However, we do not know if other forb species commonly present in upland grassland such as Achillea millefolium, Plantago lanceolata or Cerastium sp. will behave as T. officinale. We have shown that the presence of storage organs having a high WSC content is a key clonal trait to resist and recover from extreme drought. However, to compare drought strategies of forbs with those of grasses on a level playing field, it will be necessary in the future to study more forb species. The Mediterranean cultivar of D. glomerata survived better than the ecotype of cocksfoot of upland origin, probably because of the higher initial fructan level in its leaf meristems, confirming previous results for this cultivar (Volaire, 1995; Volaire and Lelièvre, 2001). However, the recovery of D. glomerata and its Mediterranean cultivar 1 year after drought was similar, emphasizing the capacity for resilience of the native species after a severe drought.
This study on forage grassland species showed diversity in plant strategies to survive and recover after severe drought, as observed by Craine et al. (2012) or Pérez Ramos et al. (2013). Against our third hypothesis, no trade-off was found between high resource acquisition under non-limiting conditions and drought survival within the plant material tested originating from upland areas subjected to infrequent severe drought. This performance results mainly from dehydration avoidance and tolerance strategies associated with a strong allocation of carbon to the root system ensured by an efficient carbon acquisition at the whole-plant level. It is also noteworthy that plant survival observed 2 weeks after rehydration was not a good indicator of 1 year recovery. This emphasizes the need to assess recovery after drought at a longer time scale and to take into consideration storage organs as key organs for resilience capacity of species. Our results also suggest that most of the native forage species studied are able to survive and recover from extreme drought, but with various time lags. Overall these results suggest that the wide range of interspecific functional strategies under drought may enhance the resilience of upland grassland plant communities under extreme drought events.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: climatic conditions during the experiment. Figure S1: total water-soluble carbohydrate and fructan contents DP3-4 in leaf meristems, roots and root apices in control and droughted plants. Figure S2: glucose and fructose contents in leaf meristems, roots and root apices in control and droughted plants.
ACKNOWLEDGEMENTS
We thank Boris Adam, Caroline Bernard, Robert Falcimagne, Patrick Pichon, Alexandre Salcedo, Christophe Serre, Lionel Thiery and Patrice Chaleil for their help with the experimental set-up, data collection and site management. This work was supported by the INRA project Climagie (ACCAF Métaprogramme: Adaptation to Climate Change of Agriculture and Forest), Auvergne Région and European funding for Regional Development (‘L’Europe s’engage en Auvergne’) through a doctoral fellowship awarded to M.Z., and a Marie Louise FURNESTIN-FAURE Scholarship 2011 (association of French women graduates).
LITTERATURE CITED
- Amiard V, Morvan-Bertrand A, Billard JP, Huault C, Keller F, Prud’homme MP. 2003. Fructans, but not the sucrosyl-galactosides, raffinose and loliose, are affected by drought stress in perennial ryegrass. Plant Physiology 132: 2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. 2008. Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell and Environment 31: 177–186. [DOI] [PubMed] [Google Scholar]
- Blum A. 1996. Crop responses to drought and the interpretation of adaptation. Plant Growth Regulation 20: 135–148. [Google Scholar]
- Brock MT, Galen C. 2005. Drought tolerance in the alpine dandelion, Taraxacum ceratophorum (Asteraceae), its exotic congenert T. officinale, and interspecific hybrids under natural and experimental conditions. American Journal of Botany 92: 1311–1321. [DOI] [PubMed] [Google Scholar]
- Carrère P, Louault F, Soussana JF. 1997. Tissue turnover within grass–clover mixed swards grazed by sheep. Methodology for calculating growth, senescence and intake fluxes. Journal of Applied Ecology 34: 333–348. [Google Scholar]
- Carter DL, VanderWeide BL, Blair JM. 2012. Drought-mediated stem and below-ground bud dynamics in restored grasslands. Applied Vegetation Science 15: 470–478. [Google Scholar]
- Charrier G, Améglio T. 2011. The timing of leaf fall affects cold acclimation by interactions with air temperature through water and carbohydrate contents. Environmental and Experimental Botany 72: 351–357. [Google Scholar]
- Chatterton NJ, Harrison P, Bennett JH, Asay KH. 1989. Carbohydrate partitioning in 185 accessions of Gramineae grown under warm and cool temperatures. Journal of Plant Physiology 134: 169–179. [Google Scholar]
- Chaves MM. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Pereira JS. 1992. Water stress, CO2 and climate change. Journal of Experimental Botany 43: 1131–1139. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought – from genes to the whole plant. Functional Plant Biology 30: 239–264. [DOI] [PubMed] [Google Scholar]
- Clark GT, Zuther E, Outred HA, McManus MT, Heyer AG. 2004. Tissue-specific changes in remobilisation of fructan in the xerophytic tussock species Festuca novae-zelandiae in response to a water deficit. Functional Plant Biology 31: 377–389. [DOI] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA. 2013. Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine JM, Nippert J, Towne E, et al. 2011. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia 165: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Craine JM, Ocheltree TW, Nippert JB, et al. 2012. Global diversity of drought tolerance and grassland climate-change resilience. Nature Climate Change 3: 63–67. [Google Scholar]
- De Roover J, Vandenbranden K, Van Laere A, Van den Ende W. 2000. Drought induces fructan synthesis and 1-SST (sucrose:sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 210: 808–814. [DOI] [PubMed] [Google Scholar]
- Dray S, Dufour AB. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20. [Google Scholar]
- Ehleringer J, Dawson T. 1992. Water uptake by plants: perspectives from stable isotope composition. Plant, Cell and Environment 15: 1073–1082. [Google Scholar]
- Eissenstat DM, Yanai R. 1997. The ecology of root lifespan. Advances in Ecological Research 27: 1–60. [Google Scholar]
- Facette MR, McCully ME, Canny MJ. 1999. Responses of maize roots to drying – limits of viability. Plant, Cell and Environment 22: 1559–1568. [Google Scholar]
- Gepstein S. 2004. Leaf senescence – not just a ‘wear and tear’ phenomenon. Genome Biology 5: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgen AK, Buchmann N. 2009. Response of temperate grasslands at different altitudes to simulated summer drought differed but scaled with annual precipitation. Biogeosciences 6: 2525–2539. [Google Scholar]
- Golluscio RA, Oesterheld M. 2007. Water-use efficiency of twenty-five co-existing Patagonian species growing under different soil water availability. Oecologia 154: 207–217. [DOI] [PubMed] [Google Scholar]
- Grime JP, Brown VK, Thompson K, et al. 2000. The response of two contrasting limestone grasslands to simulated climate change. Science 289: 762–765. [DOI] [PubMed] [Google Scholar]
- Harrel JFE, Dupont MC. 2007. The Hmisc Package . [Google Scholar]
- Hernàndez EI, Vilagrosa A, Pausas JG, Bellot J. 2010. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecology 207: 233–244. [Google Scholar]
- Hincha DK, Zuther E, Hellwege EM, Heyer AG. 2002. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 12: 103–110. [DOI] [PubMed] [Google Scholar]
- Hincha DK, Livingston DP, Premakumar R, et al. 2007. Fructans from oat and rye: composition and effects on membrane stability during drying. Biochimica and Biophysica Acta 1768: 1611–1619. [DOI] [PubMed] [Google Scholar]
- Hothorn AT, Bretz F. 2009. The multcomp Package. [Google Scholar]
- Huang B, Eissenstat DM. 2000. Linking hydraulic conductivity to anatomy in plants that vary in specific root length. Journal of the American Society for Horticultural Science 125: 260–264. [Google Scholar]
- Huang B, Duncan RR, Carrow RN. 1997. Drought-resistance mechanisms of seven warm-season turfgrasses under surface soil drying: II. Root aspects. Crop Science 37: 1863–1869. [Google Scholar]
- Ingram J, Bartels D. 1996. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 377–403. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Sperry JS, Dawson TE. 2000. Root water uptake and transport: using physiological predictions in global predictions. Trends in Plant Science 5: 482–488. [DOI] [PubMed] [Google Scholar]
- Janeček Š, Lanta V, Klimešová J, Doležal J. 2011. Effect of abandonment and plant classification on carbohydrate reserves of meadow plants. Plant Biology 13: 243–251. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Harrison P, Chatterton NJ, Bushman BS, Creech JE. 2014. Seasonal trends in non structural carbohydrates in cool- and warm-season grasses. Crop Science 54: 2328–2340. [Google Scholar]
- Jiang Y, Huang B. 2000. Effects of drought or heat stress alone and in combination on Kentucky bluegrass. Crop Science 40: 1358–1362. [Google Scholar]
- Jones MM, Turner NC, Osmond CB. 1981. Mechanisms of drought resistance. In: Paleg LG, Aspinall D, eds. The physiology and biochemistry of drought resistance in plants. Sydney, Australia: Academic Press, 15–37. [Google Scholar]
- Jupp AP, Newman EI. 1987. Morphological and anatomical effects of severe drought on the roots of Lolium perenne L. New Phytologist 105: 393–402. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Klimeš L. 2007. Bud banks and their role in vegetative regeneration – a literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology, Evolution and Systematics 8: 115–129. [Google Scholar]
- Knapp AK, Beier C, Briske DD, et al. 2008. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58: 811–821. [Google Scholar]
- Lattanzi FA, Schnyder H, Thornton B. 2004. Defoliation effects on carbon and nitrogen substrate import and tissue-bound efflux in leaf growth zones of grasses. Plant, Cell and Environment 27: 347–356. [Google Scholar]
- Lelièvre F, Seddaiu G, Ledda L, Porqueddu C, Volaire F. 2011. Water-use efficiency and drought survival in Mediterranean perennial forage grasses. Field Crops Research 121: 333–342. [Google Scholar]
- Livingston DP, Hincha DK, Heyer AG. 2009. Fructan and its relationship to abiotic stress tolerance in plants. Cellular and Molecular Life Science 66: 2007–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louault F, Pillar VD, Aufrere J, Garnier E, Soussana JF. 2005. Plant traits and functional types in responses to reduced disturbance in semi-natural grassland. Journal of Vegetation Science 16: 151–160. [Google Scholar]
- Ludlow MM. 1989. Strategies of response to water stress. In: Kreeb K, Richter H, Hinckley T, eds. Structural and functional responses to environmental stresses. The Hague, The Netherlands: SPB Academic Publishers, 269–281. [Google Scholar]
- McDowell NG. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology 155: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenium Ecosystem Assessment. 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press. [Google Scholar]
- Milbau A, Scheerlinck L, Reheul D, De Cauwer B, Nijs I. 2005. Ecophysiological and morphological parameters related to survival in grass species exposed to an extreme climatic event. Physiologia Plantarum 125: 500–512. [Google Scholar]
- North G, Nobel P. 1991. Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave deserti (Agavaceae). American Journal of Botany 78: 906–915. [Google Scholar]
- Passioura JB. 1981. Water collection by roots. In: Paleg LG, Aspinall D, eds. The physiology and biochemistry of drought resistance in plants. Sidney, Australia: Academic Press, 39–53. [Google Scholar]
- Passioura JB. 1988. Water transport in and to roots. Annual Review of Plant Physiology and Plant Molecular Biology 39: 245–265. [Google Scholar]
- Pérez-Ramos IM, Volaire F, Fattet M, Blanchard A, Roumet C. 2013. Tradeoffs between functional strategies for resource-use and drought-survival in Mediterranean rangeland species. Environmental and Experimental Botany 87: 126–136. [Google Scholar]
- Peshev D, Vergauwen R, Moglia A, Hideg E, Van den Ende W. 2013. Towards understanding vacuolar antioxidant mechanisms: a role for fructans? Journal of Experimental Botany 64: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picon-Cochard C, Pilon R, Tarroux E, Pagès L, Robertson J, Dawson L. 2012. Effect of species, root branching order and season on the root traits of 13 perennial grass species. Plant and Soil 353: 47–57. [Google Scholar]
- Pinheiro HA, DaMatta FM, Chaves ARM, Loureiro ME, Ducatti C. 2005. Drought tolerance is associated with rooting depth and stomatal control of water-use in clones of Coffea canephora. Annals of Botany 96: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CJ, Cairns AJ. 1991. Fructan metabolism in grasses and cereals. Annual Review of Plant Physiology and Plant Molecular Biology 42: 77–101. [Google Scholar]
- Pontes LDS, Carrère P, Andueza D, Louault F, Soussana JF. 2007. Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: responses to cutting frequency and N supply. Grass and Forage Science 62: 485–496. [Google Scholar]
- Poorter H, Bühler J, Van Dusschoten D, Climent J, Postma JA. 2012a. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology 39: 839–850. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012b. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Rieger M, Litvin P. 1999. Root system hydraulic conductivity in species with contrasting root anatomy. Journal of Experimental Botany 50: 201–209. [Google Scholar]
- Saeedipour S, Moradi F. 2011. Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: impact of invertase activity on carbon metabolism during kernel development. Journal of Agricultural Science 3: 32–44. [Google Scholar]
- Sala A, Woodruff DR, Meinzer FC. 2012. Carbon dynamics in trees: feast or famine? Tree Physiology 32: 764–775. [DOI] [PubMed] [Google Scholar]
- Schenk HJ, Jackson RB. 2002. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. Journal of Ecology 90: 480–494. [Google Scholar]
- Scott P. 2000. Resurrection plants and the secrets of eternal leaf. Annals of Botany 85: 159–166. [Google Scholar]
- Seneviratne SI, Nicholls N, Easterling D. 2012. Changes in climate extremes and their impacts on the natural physical environment. Managing the risks of extreme events and disasters to advance climate change adaptation. In: Field CB, Barros V, Stocker TF, Dahe Q, eds. Cambridge, UK and New York, USA: Cambridge University Press; 109–230. [Google Scholar]
- Sharps RE, Davies WJ. 1979. Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 147: 43–49. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Calow P. 1989. A life-cycle theory of responses to stress. Biological Journal of the Linnean Society 37: 101–107. [Google Scholar]
- Spollen WG, Nelson CJ. 1994. Response of fructan to water-deficit in growing leaves of Tall Fescue. Plant Physiology 106: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51: 1531–1542. [DOI] [PubMed] [Google Scholar]
- Thomas H. 1991. Accumulation and consumption of solutes in swards of Lolium perenne during drought and after rewatering. New Phytologist 118: 35–48. [Google Scholar]
- Thomas H, James A. 1999. Partitioning of sugars in Lolium perenne (perennial ryegrass) during drought and on rewatering . New Phytologist 142: 295–305. [Google Scholar]
- Valluru R, Van den Ende W. 2008. Plant fructans in stress environments: emerging concepts and future prospects. Journal of Experimental Botany 59: 2905–2916. [DOI] [PubMed] [Google Scholar]
- Van Ruijven J, Berendse F. 2010. Diversity enhances community recovery, but not resistance, after drought. Journal of Ecology 98: 81–86. [Google Scholar]
- Vartanian N. 1981. Some aspects of structural and functional modifications induced by drought in root systems. Plant and Soil 63: 83–92. [Google Scholar]
- Vereyken IJ, Chupin V, Demel RA, Smeekens SC, de Kruijff B. 2001. Fructans insert between the headgroups of phospholipids. Biochimica et Biophysica Acta 1510: 307–320. [DOI] [PubMed] [Google Scholar]
- Vereyken IJ, Chupin V, Hoekstra FA, Smeekens SCM, de Kruijff B. 2003. The effect of fructan on membrane lipid organization and dynamics in the dry state. Biophysical Journal 84: 3759–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. 2006. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. The Plant Journal 45: 523–539. [DOI] [PubMed] [Google Scholar]
- Volaire F. 1995. Growth, carbohydrate reserves and drought survival strategies of contrasting Dactylis glomerata populations in a Mediterranean environment. Journal of Applied Ecology 32: 56–66. [Google Scholar]
- Volaire F. 2008. Plant traits and functional types to characterise drought survival of pluri-specific perennial herbaceous swards in Mediterranean areas. European Journal of Agronomy 29: 116–124. [Google Scholar]
- Volaire F, Lelièvre F. 1997. Production, persistence, and water-soluble carbohydrate accumulation in 21 contrasting populations of Dactylis glomerata L. subjected to severe drought in the south of France. Australian Journal of Agricultural Research 48: 933–944. [Google Scholar]
- Volaire F, Lelièvre F. 2001. Drought survival in Dactylis glomerata and Festuca arundinacea under similar rooting conditions in tubes. Plant and Soil 229: 225–234. [Google Scholar]
- Volaire F, Norton M. 2006. Summer dormancy in perennial temperate grasses. Annals of Botany 98: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Thomas H. 1995. Effects of drought on water relations, mineral utpake, water-soluble carbohydrate accumulation and survival of two contrasting populations of cocksfoot (Dactylis glomerata L.). Annals of Botany 75: 513–524. [Google Scholar]
- Volaire F, Thomas H, Bertagne N, Bourgeois E, Gautier MF, Lelièvre F. 1998a. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought II. Water status, solute accumulation, abscisic acid concentration and accumulation of dehydrin transcripts in bases of immature leaves. New Phytologist 140: 451–460. [DOI] [PubMed] [Google Scholar]
- Volaire F, Thomas H, Lelièvre F. 1998b. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought I. Growth, death, water relations and solute content in herbage and stubble. New Phytologist 140: 439–449. [DOI] [PubMed] [Google Scholar]
- Volaire F, Norton MR, Lelièvre F. 2009. Summer drought survival strategies and sustainability of perennial temperate forage grasses in Mediterranean areas. Crop Science 49: 2386–2392. [Google Scholar]
- Volaire F, Barkaoui K, Norton M. 2014. Designing resilient and sustainable grasslands for a drier future: adaptive strategies, functional traits and biotic interactions. European Journal of Agronomy 52: 81–89. [Google Scholar]
- Weaver J, Zink E. 1946. Length of life of roots of ten species of perennial range and pasture grasses. Plant Physiology 45: 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SJ, Kitajima K, Kraft NJB, et al. 2010. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91: 3664–3674. [DOI] [PubMed] [Google Scholar]
- Xue G-P, McIntyre CL, Glassop D, Shorter R. 2008. Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Molecular Biology 67: 197–214. [DOI] [PubMed] [Google Scholar]
- Zeppel MJB, Adams HD, Anderegg WRL. 2011. Mechanistic causes of tree drought mortality: recent results, unresolved questions and future research needs. New Phytologist 192: 800–803. [DOI] [PubMed] [Google Scholar]
- Zwicke M, Alessio GA, Thiery L, et al. 2013. Lasting effects of climate disturbance on perennial grassland above-ground biomass production under two cutting frequencies. Global Change Biology 19: 3435–3448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.