Abstract
Background and Aims Asymmetric warming is one of the distinguishing features of global climate change, in which winter and night-time temperatures are predicted to increase more than summer and diurnal temperatures. Winter warming weakens vernalization and hence decreases the potential to flower for some perennial herbs, and night warming can reduce carbohydrate concentrations in storage organs. This study therefore hypothesized that asymmetric warming should act to reduce flower number and nectar production per flower in a perennial herb, Saussurea nigrescens, a key nectar plant for pollinators in Tibetan alpine meadows.
Methods A long-term (6 years) warming experiment was conducted using open-top chambers placed in a natural meadow and manipulated to achieve asymmetric increases in temperature, as follows: a mean annual increase of 0·7 and 2·7 °C during the growing and non-growing seasons, respectively, combined with an increase of 1·6 and 2·8 °C in the daytime and night-time, respectively, from June to August. Measurements were taken of nectar volume and concentration (sucrose content), and also of leaf non-structural carbohydrate content and plant morphology.
Key Results Six years of experimental warming resulted in reductions in nectar volume per floret (64·7 % of control), floret number per capitulum (8·7 %) and capitulum number per plant (32·5 %), whereas nectar concentration remained unchanged. Depletion of leaf non-structural carbohydrates was significantly higher in the warmed than in the ambient condition. Overall plant density was also reduced by warming, which, when combined with reductions in flower development and nectar volumes, led to a reduction of ∼90 % in nectar production per unit area.
Conclusions The negative effect of asymmetric warming on nectar yields in S. nigrescens may be explained by a concomitant depletion of leaf non-structural carbohydrates. The results thus highlight a novel aspect of how climate change might affect plant–pollinator interactions and plant reproduction via induction of allocation shifts for plants growing in communities subject to asymmetric warming.
Keywords: Global climate change, asymmetric warming, nectar rewards, nectar yield, alpine meadow, Saussurea nigrescens, Asteraceae, plant–pollinator interactions, non-structural carbohydrates
INTRODUCTION
By virtue of their close mutualistic association, pollinator population density and activity are largely dependent upon floral resource availability and quality (Hanley et al., 2008, 2014; Brandenburg et al., 2012; Scaven and Rafferty, 2013). For example, pollinators typically show fidelity towards plants producing more and higher quality nectar (Lake and Hughes, 1999; Mitchell et al., 2004), such that nectar production directly affects pollinator activity (Klinkhamer and de Jong, 1990; Kudo and Harder, 2005) and pollinator community structure (Potts et al., 2004). Similarly, any decrease in floral quantity and quality often leads to a reduction of pollinator abundance and activity (Larsson and Franzen, 2007; Wallisdevries et al., 2012), which has the potential to disrupt mutualistic plant–pollinator relationships (Roulston and Goodell, 2011; Rafferty and Ives, 2012). Indeed, according to a USDA report (USDA, 2013), world-wide nectar production has declined yearly. Although this phenomenon is often attributed to the decline of bee colonies and the disruption of plant–animal interactions due to climate change (Memmott et al., 2007), it is not clear whether the decline in world-wide nectar production is also attributable to the decrease in nectar production resulting from global warming.
Indeed, many factors, including habitat loss, invasive species, pathogens and pesticide usage, currently threaten pollinator communities globally (Fortuna and Bascompte, 2006; Alston et al., 2007; Cox-Foster et al., 2007; Stout and Morales, 2009).The effect of climate change on plant–pollinator interactions is another factor, albeit one that remains relatively unknown. In recent decades, studies have shown that changes in precipitation, temperature, CO2 concentration and nitrogen deposition can alter plant phenology, flower abundance and floral quality, which can further change flower attractiveness and nutritional rewards to pollinators and hence affect plant–pollinator interactions (Burkle and Alarcón, 2011; Hoover et al., 2012). For example, elevated temperature can increase floral nectar sugar concentration (Singh, 2013), and thus directly enhance the abundance and diversity of pollinators (Scaven and Rafferty, 2013; Singh, 2013). Similarly, high plant nectar yields often occur in years with high precipitation (Petanidou and Smets, 1996, 1999; Lloyd et al., 2002), thereby providing greater rewards to pollinators (Hoover et al., 2012). In contrast, few studies have focused on the effect of asymmetric warming on flowering potential and floral nectar production, which can significantly affect plant–pollinator mutualisms. Meteorological records and climate model projections have shown that temperatures have increased more in the winter than in the summer and that temperatures have increased more during the night-time than during the daytime, particularly at high latitudes and altitudes (Bonsal et al., 2001; Shabbar and Bonsal, 2003; Solomon et al., 2007; Xia et al., 2014). Since many temperate plants require vernalization and must experience a period of low winter temperature to initiate flower production (Chouard, 1960), winter warming is likely to reduce flowering potential (Hennessy and Clayton-Greene, 1995; Cook et al., 2012; Liu et al., 2012). Indeed, warming disrupts vernalization and results in reduced flower and seed production (Saure, 1985; Hennessy and Clayton-Greene, 1995; Liu et al., 2012) and delayed flowering (Warner and Erwin, 2006; Liu et al., 2012). In addition, night-time warming may reduce nectar yield by increasing plant respiration and causing a draw-down on non-structural carbohydrate reserves (Wan et al., 2009) that would otherwise be allocated to nectar production (Davis et al., 1998). It is therefore surprising that comparatively few studies have examined whether asymmetric warming significantly affects nectar production (Scaven and Rafferty, 2013; but see Jakobsen and Kristjánsson, 1994).
The extensive alpine meadows of the Tibetan Plateau provide ideal sites to explore asymmetric warming effects on nectar production. The Tibetan Plateau has experienced significant warming over the past 50 years, the average air temperature having increased 0·21 °C per decade (Stocker et al., 2013; You et al., 2013), with likely significant implications for the ecology of the high-altitude alpine meadows that typify the region. The aim of this study was to simulate asymmetric warming and quantify its impact on nectar yields of an ecologically representative insect-pollinated plant species and, by so doing, to gain a better understanding of the effects of global climate change on an important plant–insect mutualistic relationship.
MATERIALS AND METHODS
Study site and natural history
This study was conducted at Hongyuan Alpine Meadow Ecosystem Research Station (Chinese Academy of Sciences), located in the eastern Qinghai Tibetan Plateau, Sichuan province (32 ° 48′ N, 102 ° 33′ E; 3500 m asl). The climate is cold, continental, and characterized by a short and cool spring, summer and autumn and a long cold winter. According to the data collected at the Hongyuan County Climate Station (located 5 km from the study site and at the same altitude) during 1961–2012, the annual mean temperature was 0·95 °C, with maximum and minimum monthly means of 10·2 and –5·3 °C in July and January, respectively. Data collected during the same time period show that mean annual temperature increased by 0·29 °C per decade, with temperature increases in the non-growing season (October to April) and growing season (May to September) of 0·48 °C and 0·22 °C per decade, and increases in night-time and daytime temperature (as indicated by daily minimum and maximum temperatures, respectively) of 0·50 °C and 0·29 °C per decade, respectively. Annual mean precipitation is 744 mm, of which 80 % occurs between May and August (Wu et al., 2011), and soils are typified by a high organic content (250 g kg–1) and low total N (8 g kg–1) and P (5 mg kg–1) (Liu et al., 2012).
The vegetation is dominated by grasses, such as Kobresia uncinoides and Kobresia pygmaea, and forbs, such as Saussurea nigrescens, Potentilla anserina, Polygonum macrophyllum and Anemone trullifolia var. linearis (Li et al., 2011). Our study species, S. nigrescens (Asteraceae) is common throughout the study site and more generally in alpine meadows in China at altitudes ranging from 2900 to 4300 m. Plants are 15–40 cm high, bearing two to five capitula, each of which is composed of 20–55 flowers with an annular bowl-shaped nectary at the distal end of the ovary between the ovary and anthers. Saussurea nigrescens is a monoclinous and dichogamous species with male anthers reaching maturity earlier than stigmas, flowering from late July to August and fruiting in September before senescence. In addition to being used by native pollinators such as Bombus filchnerae, Bombus humilis and Bombus supremus (Macior et al., 2001), S. nigrescens is the most important forage plant for honeybees in the region.
Warming experiment
In September 2007, 40 open-top chambers (OTCs) measuring 1 × 1 × 1 m were randomly deployed (at a minimum spacing of 3 m) in a fenced, flat area of ≈1·0 ha. The sides of 20 OTCs were covered with thin (<0·1 mm) steel screen with a mesh size of 0·2 × 0·2 mm. The other 20 OTCs were covered with clear, smooth polycarbonate sheeting, which provided a warming effect the steel screening did not (Wu et al., 2re covered with thin (<0·1 mm) steel screen with a mesh size of 0·2 × 0·2 mm. The other 20 OTCs were covered with clear, smooth polycarbonate sheeting, which provided a warming effect the steel screening did not (Wu et al., 2011). The average transparency of the steel screen was 84 % (n = 5) under full light conditions, slightly less than that of the polycarbonate sheet (86 %, n = 5) in full light in mid-July (t = 0·974, P = 0·358). Each OTC was sunk 10–15 cm into the soil and firmly stabilized to withstand extremely windy conditions, which occur often at the site. Grazing was high in adjoining areas but large herbivores were excluded from the study site by fencing.
Measurements using a DS1921G model thermometer (Maxim Integrated Products, Sunnyvale, CA, USA) from 2007 to 2013 showed that the mean annual temperature 30 cm above ground was 0·7 (±0·05) °C higher in the warmed OTCs than in the ambient OTCs during the growing season (May to September) and was 2·7 (±0·17) °C higher in the non-growing season (October to April). Night-time (19:00 to 7:00, Beijing time) temperatures (30 cm above ground) were 2·8 (±0·38)° C higher in the warmed OTCs than in the ambient OTCs during the period from 1 June to 31 August, when S. nigrescens flowered. The daytime (7:00 to 19:00) temperatures were also 1·6 (± 0·17) °C higher in the ambient OTCs during the same time period (Supplementary Data Table S1). In addition, measurements made on three sunny days (between 10:00 and 14:00) showed that the air temperature difference 30 cm above the soil surface was 1·97 ± 0·11 °C, while capitulum tissue was 1·02 ± 0·40 °C warmer in warmed compared with ambient OTCs (n = 20 for each treatment). There was no variation in soil moisture recorded at a depth of 5 cm once every month during the non-freezing periods (April to October in 2012; Supplementary Data Fig. S1) or relative humidity between the warmed and ambient OTCs (81·6 ± 8·45 and 84·7 ± 1·57 %, respectively).
Nectar measurements
Floret nectar volumes and concentrations were measured for plants in the warmed and ambient OTCs from late July to early August in both 2012 and 2013, when most mature plants were observed flowering. We chose five to ten vigorous, medium-sized plants in each OTC and tagged and enclosed one capitulum (in which all florets were closed) per plant with bridal veiling to exclude insect visitors (Real and Rathcke, 1991). Previous work has shown that the nectar volume peaks when anthers become white in appearance (Mu et al., 2014) and we used this visual cue as an indicator of when to measure floret nectar volumes and concentrations (from 10:00 to 15:00 on sunny days). Nectar volume was measured for a minimum of five florets per capitulum using 1- or 5 -µl micropipettes (Hirschmann Laborgeräte, Germany) and nectar (sucrose) concentration was concurrently measured with a hand refractometer (Eclipse; Bellingham and Stanley, UK) following Johnson et al. (2006). About 4500 florets were used to monitor the nectar volume and concentration.
Non-structural carbohydrate depletion
To determine whether night-time warming significantly affected leaf non-structural carbohydrate levels, we measured non-structural carbohydrate for representative leaves sampled just after sunset (when non-structural carbohydrate values were at their maximum) and leaves sampled just before sunrise (when non-structural carbohydrate values were at their minimum). The leaves were removed from plants growing in ten warmed OTCs and ten ambient OTCs on 5 and 6 August 2013. The samples were kept in a portable icebox with dry ice and taken to the laboratory, where non-structural carbohydrate levels were measured following the procedure of Hansen and Møller (1975) and Yoshida et al. (1976). We calculated the non-structural carbohydrate depletion by subtracting the non-structural carbohydrate content measured at sunset from the non-structural carbohydrate content measured at sunrise from plants growing in the same OTCs.
Plant morphology
In addition to measuring nectar volume and quantity and non-structural carbohydrate content, we tracked plant density and flowering phenology by recording the total number of plants and the number of flowering plants in each OTC during the flowering season of both 2012 and 2013. The total number of plants per OTC was taken as plant density. The flowering onset and offset times were both defined as the dates when 10 of all plants were flowering (Sun and Frelich, 2011). The percentage of flowering plants was calculated with respect to the total number of flowering plants divided by the total number of plants in each OTC. We investigated 20 ambient OTCs and 20 warmed OTCs in both years using these protocols.
We also measured plant height and aboveground biomass for a minimum of three plants per OTC. Plant height was measured from the soil surface to the apex of the terminal shoot to the nearest 0·5 cm (Nagashima et al., 1995). We then harvested aboveground parts, which were dried to constant mass and weighed in the laboratory. The biomass of leaves, stems and capitula (fruits) was measured per plant, as was the number of capitula. Ripened capitula were removed and the number of aborted ovules and sound seeds was recorded. The seeds were weighed (to 0·1 mg) for each capitulum. Total seed number per plant was calculated as the product of seed number per capitulum and the number of capitula per plant. Seed set was calculated as the percentage of ovules that matured into sound seeds. We used 420 plants to measure these morphological and reproductive features.
Statistical analyses
Data for each of the measured traits were tested for normality before analysis. The floret nectar volume was log10-transformed and the proportion of flowering plants per OTC was arcsine-transformed to achieve normality. The differences in nectar volume and concentration, floret number per capitulum, the number of capitula per plant, plant density, plant height, and aboveground biomass between treatments were determined using three-way mixed ANOVA with OTC identity as the random factor and warming and study year as the fixed factors. The treatment differences in plant density, proportion of flowering plants, nectar productivity and flowering phenology were determined using two-way ANOVA with study year and warming as the fixed factors. Once a significant warming effect was detected, post hoc Tukey’s tests were used to determine the difference in each study year. The difference in non-structural carbohydrate depletion between warmed and ambient OTCs were assessed with t-tests. In addition, correlation analyses were conducted to determine the relationships among the above parameters across treatments for both study years.
Plant nectar productivity was gauged on the basis of nectar volume per floret (FNV), floret number per capitulum (FN), capitulum number per plant (CN), plant density (total number of plants [TNP] per OTC) and the proportion of flowering plants (PFP): plant nectar productivity = FNV × FN × CN × TNP ×PFP. The difference in plant nectar productivity between warmed and ambient OTCs was determined using two-way ANOVA with warming and study year as the fixed factors. In order to determine the extent to which the five different variables contributed to the variation in plant nectar productivity, variance component analysis (varcomp in R; www.r-project.org) was used to partition plant nectar productivity variance among the variables. Bootstrapping was used to calculate the 95 % confidence intervals of variance components for each factor. We constructed a data set by randomly sampling the data points from the original data set with replacement, and then the variance component for each factor was calculated by the method mentioned above. This procedure was repeated 1000 times and each result was stored. The 95 % confidence interval was calculated as the values corresponding to 0·025 and 0·975 percentiles. All statistical analyses were performed in R (www.R-project.org).
RESULTS
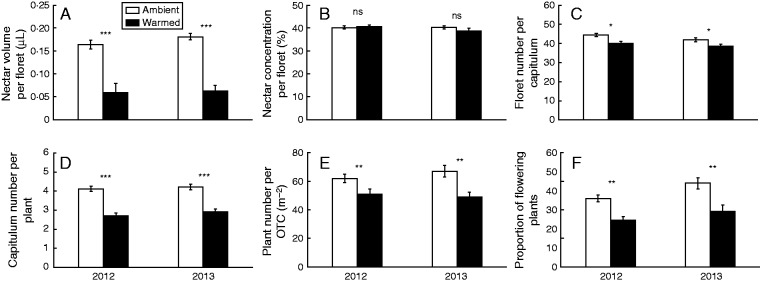
Artificial warming significantly reduced floret nectar volume, floret number per capitulum, capitulum number per plant, proportion of flowering plants and plant density in both study years (Table 1; Fig. 1).
TABLE 1.
Summary of ANOVA for the effects of warming (ambient versus warmed) and year (2012 and 2013) on nectar production and concentration, vegetative and reproductive traits, flowering phenology of the Tibetan alpine Asteraceae (S. nigrescens). The open top chamber (OTC) was assigned as a random factor in the analyses, except for flowering phenology, plant density, proportion of flowering plants and nectar productivity
| d.f. | F-value | P-value | |
|---|---|---|---|
| Floret nectar volume | |||
| Warming | 1 | 74·080 | <0·001 |
| Year | 1 | 0·698 | 0·406 |
| Warming × year | 1 | 0·253 | 0·617 |
| Residuals | 75 | ||
| Nectar concentration | |||
| Warming | 1 | 0·560 | 0·457 |
| Year | 1 | 0·181 | 0·672 |
| Warming × year | 1 | 0·623 | 0·432 |
| Residuals | 75 | ||
| Floret number per capitulum | |||
| Warming | 1 | 17·419 | <0·001 |
| Year | 1 | 3·851 | 0·053 |
| Warming × year | 1 | 0·404 | 0·527 |
| Residuals | 75 | ||
| Capitulum number per plant | |||
| Warming | 1 | 97·588 | <0·001 |
| Year | 1 | 0·678 | 0·413 |
| Warming × year | 1 | 0·237 | 0·628 |
| Residuals | 75 | ||
| Nectar productivity | |||
| Warming | 1 | 200·941 | <0·001 |
| Year | 1 | 6·206 | <0·05 |
| Warming × year | 1 | 3·043 | 0·085 |
| Residuals | 76 | ||
| Plant height | |||
| Warming | 1 | 0·080 | 0·775 |
| Year | 1 | 0·027 | 0·870 |
| Warming × year | 1 | 0·095 | 0·759 |
| Residuals | 75 | ||
| Aboveground vegetative biomass | |||
| Warming | 1 | 0·156 | 0·694 |
| Year | 1 | 0·754 | 0·388 |
| Warming × year | 1 | 0·015 | 0·904 |
| Residuals | 75 | ||
| Seed number per plant | |||
| Warming | 1 | 48·30 | <0·001 |
| Year | 1 | 0·010 | 0·922 |
| Warming × year | 1 | 0·910 | 0·343 |
| Residuals | 75 | ||
| Seed set | |||
| Warming | 1 | 2·299 | 0·134 |
| Year | 1 | 0·739 | 0·393 |
| Warming × year | 1 | 0·102 | 0·751 |
| Residuals | 75 | ||
| Seed mass per plant | |||
| Warming | 1 | 134·377 | <0·001 |
| Year | 1 | 5·045 | <0·05 |
| Warming × year | 1 | 2·359 | 0·129 |
| Residuals | 75 | ||
| Flowering onset time | |||
| Warming | 1 | 1·176 | 0·282 |
| Year | 1 | 2·305 | 0·133 |
| Warming × year | 1 | 0·228 | 0·635 |
| Residuals | 76 | ||
| Flowering offset time | |||
| Warming | 1 | 3·358 | 0·071 |
| Year | 1 | 0·089 | 0·766 |
| Warming × year | 1 | 0·089 | 0·766 |
| Residuals | 76 | ||
| Plant density | |||
| Warming | 1 | 16·84 | <0·001 |
| Year | 1 | 0·144 | 0·705 |
| Warming × year | 1 | 0·827 | 0·366 |
| Residuals | 76 | ||
| Proportion of flowering plants | |||
| Warming | 1 | 25·413 | <0·001 |
| Year | 1 | 5·856 | <0·05 |
| Warming × year | 1 | 0·406 | 0·526 |
| Residuals | 76 | ||
FIG. 1.
Floret nectar volume and concentration (A, B), floret number per capitulum (C), capitulum number per plant (D), plant number per open-top chamber (OTC) (E) and proportion of flowering plants (F) for warmed and ambient OTCs in 2012 and 2013. ***P < 0.001; **P < 0.01; *P < 0.05 (one-way ANOVA).
The proportion of flowering plants was significantly smaller in 2012 than 2013 (Supplementary Data Table S2), but no significant difference was found in floret nectar volume, capitulum number per plant or plant density (Tables 1 and 2).
TABLE 2.
Means (± s.e.) of vegetative and reproductive traits, flowering phenology of the Tibetan Asteraceae Saussurea nigrescens between ambient and warmed open top-chambers (OTCs) in 2012 and 2013. All means are for the individual plant level. Plant height, aboveground vegetative biomass and capitulum number per plant were all based on single measurements per plant
| Ambient OTCs | Warmed OTCs | |
|---|---|---|
| 2012 | ||
| Plant height (cm) | 29·03 ± 0·56a | 30·14 ± 0·68a |
| Aboveground vegetative biomass (g) | 1·49 ± 0·05a | 1·38 ± 0·06a |
| Seed number per plant | 78·10 ± 4·63a | 49·73 ± 5·10b |
| Seed set (%) | 61·80 ± 1·62a | 66·60 ± 2·64a |
| Seed mass per plant (mg) | 185·00 ± 9·33a | 77·00 ± 8·40b |
| Flowering onset time | 212·10 ± 0·69a | 211·20 ± 0·55a |
| Flowering offset time | 228·50 ± 0·56a | 227·20 ± 0·76a |
| Nectar productivity (µl) | 668·32 ± 63·64a | 59·74 ± 13·54b |
| 2013 | ||
| Plant height (cm) | 28·86 ± 0·63a | 30·74 ± 0·70a |
| Above-ground vegetative biomass (g) | 1·52 ± 0·06a | 1·46 ± 0·10a |
| Seed number per plant | 83·07 ± 5·08a | 45·67 ± 3·94b |
| Seed set (%) | 65·00 ± 2·42a | 68·00 ± 3·33a |
| Seed mass per plant (mg) | 225·80 ± 14·79a | 84·40 ± 9·20b |
| Flowering onset time | 211·00 ± 0·46a | 210·60 ± 0·58a |
| Flowering offset time | 228·10 ± 0·49a | 227·20 ± 0·48a |
| Nectar productivity (µl) | 875·67 ± 72·91a | 96·29 ± 25·64b |
Different letters after means denote significant differences in values (P < 0·05; two-way ANOVA followed by post hoc Tukey test.
All data are normally distributed (Shapiro–Wilk test, P > 0·1 for each OTC); n = 20 for both ambient and warmed treatments.
Although the difference in the nectar concentration was indistinguishable between warmed and ambient OTCs in both study years (Table 1; Fig. 1), nectar productivity was significantly lower (by >90 %) in the warmed OTCs compared with the ambient OTCs in both years (Tables 1 and 2). Moreover, nectar productivity was significantly smaller in 2012 than 2013 (Table 2; Supplementary Data Table S2).
Warming had no impact on flowering phenology; the flowering onset time and flowering offset time were indistinguishable between warm and ambient OTCs in both study years (Tables 1 and 2). Likewise, warming had no significant effect on seed set, plant height or aboveground biomass in both years (Tables 1 and 2). Nevertheless, warming significantly reduced total seed number per plant and seed mass per plant in both years (Tables 1 and 2).
Depletion of leaf non-structural carbohydrates was significantly higher in the warmed than ambient OTCs (Fig. 2). This was likely linked with the reduction of floret nectar volume, as indicated by the significantly negative relationship between depletion and nectar volume (Fig. 3). Floret nectar volume was positively correlated with capitulum number per plant, which was positively associated with aboveground plant biomass (Supplementary Data Table S3). Moreover, plant nectar production was positively correlated with floret nectar volume, proportion of flowering plants and capitulum number per plant (Supplementary Data Table S3).
FIG. 2.
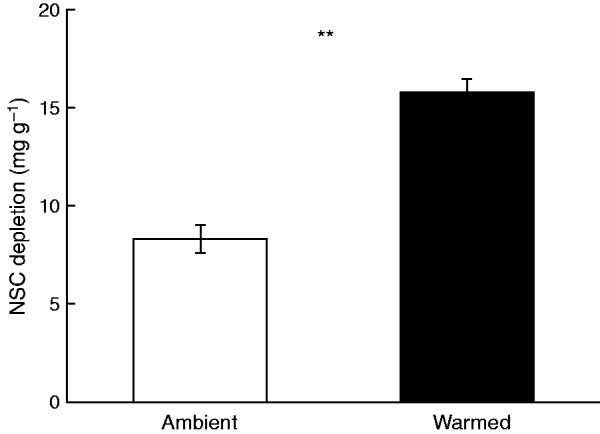
Non-structural carbohydrate (NSC) depletion between ambient and warmed open-top chambers in 2013. **P < 0.01 (one-way ANOVA).
FIG. 3.
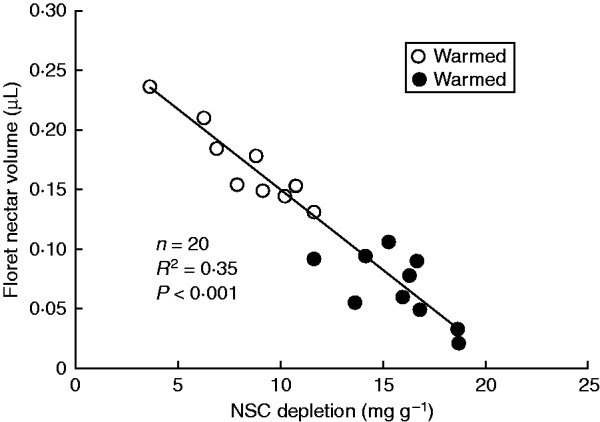
Regression of floret nectar volume on non-structural carbohydrate (NSC) depletion across open-top chambers.
Variance component analysis showed that the five factors (floret nectar volume, floret number per capitulum, capitulum number per plant, total number of plants and proportion of flowering plants) could explain >90 % of the variation in plant nectar productivity (Table 3). Floret nectar volume accounted for most of the variation (60 %), followed by the proportion of flowering plants (11 %) and capitulum number per plant (10 %).
TABLE 3.
Variance components of plant nectar productivity (PNP) for five factors
| Factor | Variance of the response (%) (95 % CIa) |
|---|---|
| Floret nectar volume | 60 (36, 83) |
| Floret number per capitulum | 0·91 (2·21e–03, 7·58) |
| Capitulum number per plant | 10 (0·54, 26) |
| Total number of plants per OTC | 7·4 (1·6, 15) |
| PFP proportion of flowering plants | 11 (3·3, 19) |
| Residuals | 9·8 (5·6, 13) |
aCalculated by bootstrapping.
DISCUSSION
We show that warming (most markedly elevated winter and summer night-time temperatures) had negative impacts on nectar yield and thus on the likely consequences for plant–pollinator interactions during the peak summer flowering period of S. nigrescens. An important ancillary observation is that the reduction in nectar was associated with the depletion of non-structural carbohydrates, as indicated by the negative relationship between the depletion of non-structural carbohydrates and floret nectar volume across OTCs. Night-time warming can increase nocturnal respiration and thus deplete carbohydrates (Ryan, 1991; Griffin et al., 2002; Wan et al., 2009) that would otherwise be allocated to the production of nectar. Our data also show that asymmetric warming has other negative effects, such as reductions in flower number per capitulum, capitula number per plant, the proportion of flowering plants and plant density. These phenomena, which are likely linked to devernalization and competition among viable plants, have negative effects on the resources plants make available to pollinators and thus possibly on the density and foraging behaviour of pollinators (Hanley et al., 2008, 2014; Gilman et al., 2012).
Large differences in daytime and night-time temperatures are known to favour nectar production in many other species (McDade and Weeks, 2004; Wolff, 2006). For example, Jakobsen and Kristjánsson (1994) found that low night-time temperatures (∼10 °C) can increase nectar production but only with increased daytime temperatures. Conversely, when temperatures exceed a threshold daily maximum, nectar production can decrease, often drastically (Nicolson, 1995; Petanidou and Smets, 1996). The underlying mechanism for this phenomenon is that high daytime temperatures can enhance photosynthesis and carbon gain, whereas low night-time temperatures tend to reduce respiration and the depletion of stored carbohydrates. As a result, non-structural carbohydrate content and flower or fruit quality (as gauged by sugar content; Percival, 1961) are often improved by increasing the night–day temperature difference. In our case, asymmetric warming greatly increased the depletion of non-structural carbohydrate content and decreased nectar volume per floret despite the lack of significant difference in illumination, relative humidity or soil moisture between warmed and ambient open top chambers.
Asymmetric warming also affects vernalization and thus population demographics. Cold winters are often necessary for flower formation in perennial herbaceous species growing in temperate regions, because many of these species flower only after they experience an extended period of cold temperatures (Bernier et al., 1981; Michaels and Amasino, 2000), likely as an adaptation to a growing season and the benefits conferred by rapid flowering and seed production (Aarssen and Jordan, 2001). For these species, even a slight increase in winter temperatures can have significant negative consequences (Chouard, 1960; Scaven and Rafferty, 2013). For example, Yu et al. (2010) report that the ‘spring greening’ phenology has been delayed in the latest two decades due to winter temperature increases, while Liu et al. (2012) also report reductions in the proportion of flowering shoots and seed output for a variety of herbaceous species subjected to artificial warming. This observation is consistent with the decline in the proportion of flowering plants and the number of capitula per plant observed for our study species and are likely a result of weakened vernalization due to the winter warming (Scaven and Rafferty, 2013). This explanation is also consistent with many previous reports in that higher than normal temperatures result in what has been called devernalization (Bernier et al., 1981; Saavedra et al., 2003; Bokhorst et al., 2008), which depresses flower formation (Chouard, 1960; Hideyuki and Takashi, 2003; Liu et al., 2012).
In this context, other potential factors appear not to have contributed to the phenomenon reported here. For example, studies frequently indicate that some species flower only after they gain a threshold height or biomass and only when the timing of flower bud formation is appropriate. Nevertheless, we did not find a significant shift in plant phenology or plant growth between warmed and ambient chambers, possibly because the warming effect on plant phenology might not have emerged during the study years. Furthermore, early physiological experiments show that water stress can induce flowering, presumably as a result of an evolutionary advantage of producing seeds before ageing (Southwick and Davenport, 1986; Ekanayake et al., 1988). Our data show that this potential stress had no effect (as indicated by comparisons of plant height and biomass). In addition, the effect of the physical setting on pollinators should be similar between warmed and ambient chambers, as indicated by the similar levels of seed set between treatments. This can be attributed to the facts that the OTCs (for both warmed and ambient treatments) served as similar physical barriers preventing migration of most insect species (due to small mesh size of the ambient OTCs) and that the major pollinator species for S. nigrescens, the domesticated honeybee (Apis mellifera), was extremely abundant in the study site (Mu et al., 2014).
Artificial asymmetric warming also resulted in a significant decline in plant density, except for the reduction in floret nectar volume and flowering potential. Decreased seed production per plant might have contributed to the reduction in plant density in Saussurea, because seedling density was often positively associated with seed density in the study region (Xi et al., 2015). The reduction in seed production is likely due to the negative effect of warming on capitulum/floret number per plant, but it cannot be accounted for by the indistinguishable difference in bee visitation rates and seed set.
In summary, our results show that asymmetric warming can reduce nectar provision in S. nigrescens via reduced nectar production of individual florets, fewer florets per capitulum and capitula per plant, and a lower proportion of flowering plants within a reduced overall plant density. We speculate that climate change will likely have increasingly negative effects on plant-pollinator interactions (Roulston and Goodell, 2011; Scaven and Rafferty, 2013), but highlight the pressing need for future research to address how climate-induced changes in plant physiology and resource allocation affect pollinator reward provision more generally.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: monthly means of soil moisture for the duration of the experiment. Table S1: differences in the temperature 30 cm above ground in warmed and ambient open-top chambers from 2007 to 2013. Table S2: results of Tukey post hoc tests showing the effects of warming and year on nectar production and concentration, vegetative and reproductive traits and flowering phenology. Table S3: matrix of the correlation coefficients among the variables studied.
ACKNOWLEDGEMENTS
We thank Jiyan Zhao, Yan Li, Jie Xiong, Hongli Chen, Yongpin Li, Rui Cao, Yangheshan Yang, Xincheng Li and Kai He for field and laboratory assistance. This study was funded by the 973 Program (2013CB956302) and the National Science Foundation of China (31270513, 31100397 and 31325004).
LITERATURE CITED
- Aarssen LW, Jordan CY. 2001. Between-species patterns of covariation in plant size, seed size and fecundity in monocarpic herbs. EcoScience 8: 471–477. [Google Scholar]
- Alston DG, Tepedino VJ, Bradley BA, Toler TR, Griswold TL, Messinger SM. 2007. Effects of the insecticide phosmet on solitary bee foraging and nesting in orchards of Capitol Reef National Park, Utah. Environmental Entomology 36: 811–816. [DOI] [PubMed] [Google Scholar]
- Bernier G, Kinet J-M, Sachs RM. 1981. The physiology of flowering, Vol. II. Boca Raton, FL: CRC Press. [Google Scholar]
- Bokhorst SJ, Bjerke W, Bowles FW, Callaghan TV, Phoenix GK. 2008. Impacts of extreme winter warming in the sub-Arctic: growing season responses of dwarf shrub heathland. Global Change Biology 14: 2603–2612. [Google Scholar]
- Bonsal BR, Zhang X, Vincent LA, Hogg WD. 2001. Characteristics of daily and extreme temperatures over Canada. Journal of Climate 14:1959–1976. [Google Scholar]
- Brandenburg A, Kuhlemeier C, Bshary R. 2012. Hawkmoth pollinators decrease seed set of a low-nectar Petunia axillaris line through reduced probing time. Current Biology 22: 1–5. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Alarcón R. 2011. The future of plant–pollinator diversity: understanding interaction networks across time, space, and global change. American Journal of Botany 98: 1–11. [DOI] [PubMed] [Google Scholar]
- Chouard P. 1960. Vernalization and its relations to dormancy. Annual Review of Plant Physiology 11: 191–238. [Google Scholar]
- Cook BI, Wolkovich EM, Parmesan C. 2012. Divergent responses to spring and winter warming drive community level flowering trends. Proceedings of the National Academy of Sciences of the USA 109: 9000–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster DL, Conlan S, Holmes EC, et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318: 283–287. [DOI] [PubMed] [Google Scholar]
- Davis AR, Pylatuik JD, Paradis JC, Low NH. 1998. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta 205: 305–318. [DOI] [PubMed] [Google Scholar]
- Ekanayake IJ, de Dattta SK, Steponkus PL. 1988. Spikelet sterility and flowering response of rice to water stress in anthesis. Annals of Botany 63: 257–264. [Google Scholar]
- Fortuna MA, Bascompte J. 2006. Habitat loss and the structure of plant-animal mutualistic networks. Ecology Letters 9: 278–283. [DOI] [PubMed] [Google Scholar]
- Gilman RT, Fabina NS, Abbott KC, Rafferty NE. 2012. Evolution of plant–pollinator mutualisms in responses to climate change. Evolutionary Applications 5: 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KL, Turnbull M, Murthy R, Lin G, Adams J, et al. 2002. Leaf respiration is differentially affected by leaf vs. stand-level night-time warming. Global Change Biology 8: 479–485. [Google Scholar]
- Hanley ME, Franco M, Pichon S, Darvill B, Goulson D. 2008. Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Functional Ecology 22: 592–598. [Google Scholar]
- Hanley ME, Awbi AJ, Franco M. 2014. Going native? Flower use by bumblebees in English urban gardens. Annals of Botany 113: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Møller IB. 1975. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Analytical Biochemistry 68: 87–94. [DOI] [PubMed] [Google Scholar]
- Hennessy KJ, Clayton-Greene 1995. Greenhouse warming and vernalization of high-chill fruit in southern Australia. Climate Change 30: 327–348. [Google Scholar]
- Hideyuki S, Takashi S. 2003. Effects of high temperature interruption during vernalization on the inflorescence formation in turnip plants. Journal of the Japanese Society for Horticultural Science 72: 329–334. [Google Scholar]
- Hoover SER, Ladley JJ, Shchepetkina AA, et al. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant–pollinator mutualism. Ecology Letters 15: 227–234. [DOI] [PubMed] [Google Scholar]
- Jakobsen HB, Kristjánsson K. 1994. Influence of temperature and floret age on nectar secretion in Trifolium repens L. Annals of Botany 74: 327–334. [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. 2006. Dark, bitter-tasting nectar functions as filter of flower visitors in a bird-pollinated plant. Ecology 87: 2706–2716. [DOI] [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong TJ. 1990. Effects of plant size, plant density and sex differential nectar reward on pollinator visitation in the protandrous Echium vulgare (Boraginaceae). Oikos 57: 399–405. [Google Scholar]
- Kudo G, Harder LD. 2005. Floral and inflorescence effects on variation in pollen removal and seed production among six legume species. Functional Ecology 19: 245–254. [Google Scholar]
- Lake JC, Hughes L. 1999. Nectar production and floral characteristics of Tropaeolum majus L. grown in ambient and elevated carbon dioxide. Annals of Botany 84: 535–541. [Google Scholar]
- Larsson M, Franzen M. 2007. Critical resource levels of pollen for the declining bee Andrena hattorfiana (Hymenoptera, Andrenidae). Biology Conservation 134: 405–414. [Google Scholar]
- Li GY, Liu YZ, Frelich LE, Sun SC. 2011. Experimental warming induces degradation of Tibetan alpine meadow through tropic interactions. Journal of Applied Ecology 48: 659–667. [Google Scholar]
- Liu YZ, Mu JP, Li GY, Sun SC. 2012. Global warming may reduce plant reproductive effort for temperate multi-flowered species. New Phytologist 195: 427–436. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Ayre DJ, Whelan RJ. 2002. A rapid and accurate visual assessment of nectar production can reveal patterns of temporal variation in Banksia ericifolia (Proteaceae). Australian Journal of Botany 50: 595–600. [Google Scholar]
- Macior LW, Tang Y, Zhang CJ. 2001. Reproductive biology of Pedicularis (Scrophulariaceae) in the Sichuan Himalaya. Plant Species Biology 16: 83–89. [Google Scholar]
- McDade LA, Weeks JA. 2004. Nectar in hummingbird-pollinated Neotropical plants I: patterns of production and variability in 12 species. Biotropica 36: 196–215. [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. 2007. Global warming and the disruption of plant–pollinator interactions. Ecology Letters 10:710–717. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. 2000. Memories of winter: vernalization and the competence to flower. Plant, Cell and Environment 23: 1145–1153. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KJ, Bell JM. 2004. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology 18: 116–124. [Google Scholar]
- Mu JP, Peng YH, Xi XQ, et al. 2014. Domesticated honeybees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology 95: 3161–3172. [Google Scholar]
- Nagashima H, Terashima I, Katoh S. 1995. Effects of plant density on frequency distributions of plant height in Chenopodium album stands: analysis based on continuous monitoring of height-growth of individual plants. Annals of Botany 75: 173–180. [Google Scholar]
- Nicolson SW. 1995. Direct demonstration of nectar reabsorption in the flowers in Grevillea robusta (Proteaceae). Functional Ecology 9: 584–588. [Google Scholar]
- Percival MS. 1961. Types of nectar in angiosperms. New Phytologist 3: 235–281. [Google Scholar]
- Petanidou T, Smets E. 1996. Does temperature stress induce nectar secretion in Mediterranean plants? New Phytologist 133: 513–518. [Google Scholar]
- Petanidou T, Goethals V, Smets E. 1999. The effect of nutrient and water availability on nectar secretion and nectar structure of the dominant Labiatae species of phrygana. Systematics and Geography of Plants 68: 233–244. [Google Scholar]
- Potts SG, Vulliamy B, Roberts S, et al. 2004. Nectar resource diversity organises flower-visitor community structure. Entomologia Experimentalis et Applicata 113: 103–107. [Google Scholar]
- Rafferty NE, Ives AR. 2012. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology 93: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real LA, Rathcke BJ. 1991. Individual variation in nectar production and its effects on fitness in Kalmia latifolia. Ecology 72: 149–155. [Google Scholar]
- Roulston TH, Goodell K. 2011. The role of resources and risks in regulating wild bee population. Annual Review of Entomology 56: 293–312. [DOI] [PubMed] [Google Scholar]
- Ryan MG. 1991. Effects of climate change on plant respiration. Ecological Applications 1: 157–167. [DOI] [PubMed] [Google Scholar]
- Saavedra F, Inouye DW, Price MV, Harte J. 2003. Changes in flowering and abundance of Delphinium nuttallianum (Ranunculaceae) in response to a subalpine climate warming experiment. Global Change Biology 9: 885–894. [Google Scholar]
- Saure MC. 1985. Dormancy release in deciduous fruit trees. Horticultural Reviews 7: 239–300. [Google Scholar]
- Scaven V, Rafferty NE. 2013. Physiological effects of climate warming on flowering plants and insect pollinator and potential consequences for their interaction. Current Zoology 59: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbar A, Bonsal B. 2003. An assessment of changes in winter cold and warm spells over Canada. Natural Hazards 29: 173–188. [Google Scholar]
- Singh AK. 2013. Abundance of honeybees and nectar sugar concentration in mustard flower. Environment and Ecology 31: 1111–1115. [Google Scholar]
- Solomon S, Qin D, Manning M, et al. 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Southwick SM, Davenport TL. 1986. Characterization of water stress and low temperature effects on flower induction in Citrus. Plant Physiology 81: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Plattner G-K, et al. 2013. Climate change 2013: the physical science basis. Contribution of Working Group I to the IPCC Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Stout J, Morales CL. 2009. Ecological impacts of invasive alien species on bees. Apidologie 40: 388–409. [Google Scholar]
- Sun SC, Frelich LE. 2011. Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate, and stem tissue mass density in herbaceous grassland species. Journal of Ecology 99: 991–1000. [Google Scholar]
- USDA. 2013. U.S. honey production down one percent. American Bee Journal. http://www.dadant.com/news/u-s-honey-production-down-1-percent. [Google Scholar]
- Wallisdevries MF, van Swaay CAM, Plate CL. 2012. Changes in nectar supply: a possible cause of widespread butterfly decline. Current Zoology 58: 384–391. [Google Scholar]
- Wan SQ, Xia JY, Liu WX, Niu SL. 2009. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 90: 2700–2710. [DOI] [PubMed] [Google Scholar]
- Warner RM, Erwin JE. 2006. Prolonged high-temperature exposure differentially reduces growth and flowering of 12 Viola × wittrockiana Gams. cvs. Horticulturae 108: 295–302. [Google Scholar]
- Wolff D. 2006. Nectar sugar composition and volumes of 47 species of Gentianales from a southern Ecuadorian montane forest. Annals of Botany 97: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XW, Duffy JE, Reich PB, Sun SC. 2011. A brown-world cascade in the dung decomposer food web of an alpine meadow: effects of predator interactions and warming. Ecology Monographs 81: 313–328. [Google Scholar]
- Xi XQ, Mu JP, Peng YH, Eisenhauer N, Sun SC. 2015. Capitulum density-dependent effects generate peak seed yield at an intermediate density of a Tibetan lotus. Journal of Plant Ecology doi: 10.1093/jpe/rtv025. [Google Scholar]
- Xia JY, Chen JQ, Piao SL, Ciais P, Luo YQ, Wan SQ. 2014. Terrestrial carbon cycle affected by non-uniform climate warming. Nature Geoscience 7: 173–180. [Google Scholar]
- Yoshida S, Forno D, Cock J, Gomez KA. 1976. Laboratory manual for physiological studies of rice. Manila, Philippines: International Rice Research Institute. [Google Scholar]
- You QL, Fraedrich K, Ren GY, Pepin N, Kang SH. 2013. Variability of temperature in the Tibetan Plateau based on homogenized surface stations and reanalysis data. International Journal of Climatology 33: 1337-1347. [Google Scholar]
- Yu H, Luedeling E, Xu J. 2010. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proceedings of the National Academy of Sciences, USA 107: 22151–22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.