Abstract
Background and Aims Plants growing under elevated atmospheric CO2 concentrations often have reduced stomatal conductance and subsequently increased leaf temperature. This study therefore tested the hypothesis that under long-term elevated CO2 the temperature optima of photosynthetic processes will shift towards higher temperatures and the thermostability of the photosynthetic apparatus will increase.
Methods The hypothesis was tested for saplings of broadleaved Fagus sylvatica and coniferous Picea abies exposed for 4–5 years to either ambient (AC; 385 µmol mol−1) or elevated (EC; 700 µmol mol−1) CO2 concentrations. Temperature response curves of photosynthetic processes were determined by gas-exchange and chlorophyll fluorescence techniques.
Key Results Initial assumptions of reduced light-saturated stomatal conductance and increased leaf temperatures for EC plants were confirmed. Temperature response curves revealed stimulation of light-saturated rates of CO2 assimilation (Amax) and a decline in photorespiration (RL) as a result of EC within a wide temperature range. However, these effects were negligible or reduced at low and high temperatures. Higher temperature optima (Topt) of Amax, Rubisco carboxylation rates (VCmax) and RL were found for EC saplings compared with AC saplings. However, the shifts in Topt of Amax were instantaneous, and disappeared when measured at identical CO2 concentrations. Higher values of Topt at elevated CO2 were attributed particularly to reduced photorespiration and prevailing limitation of photosynthesis by ribulose-1,5-bisphosphate (RuBP) regeneration. Temperature response curves of fluorescence parameters suggested a negligible effect of EC on enhancement of thermostability of photosystem II photochemistry.
Conclusions Elevated CO2 instantaneously increases temperature optima of Amax due to reduced photorespiration and limitation of photosynthesis by RuBP regeneration. However, this increase disappears when plants are exposed to identical CO2 concentrations. In addition, increased heat-stress tolerance of primary photochemistry in plants grown at elevated CO2 is unlikely. The hypothesis that long-term cultivation at elevated CO2 leads to acclimation of photosynthesis to higher temperatures is therefore rejected. Nevertheless, incorporating acclimation mechanisms into models simulating carbon flux between the atmosphere and vegetation is necessary.
Keywords: Climate change, CO2 assimilation, elevated CO2 acclimation, European beech, Fagus sylvatica, Norway spruce, photorespiration, photosystem II photochemistry, Picea abies, Rubisco carboxylation, thermotolerance
INTRODUCTION
Global climate models predict a gradual increase in atmospheric CO2 concentration and global temperature by as much as 700 µmol CO2 mol−1 and 2·2 °C, respectively, by 2100 (RCP6.0 scenario; IPCC, 2013). In addition, more intense, more frequent and longer lasting heat waves are predicted in the 21st century by a global coupled climate model (Meehl and Tebaldi, 2004). It is therefore important to assess the effect on plants of elevated atmospheric CO2 concentrations (EC), elevated temperatures and their interactions; in particular, whether EC will cause changes in the temperature sensitivity of plants’ metabolism, growth and development and whether EC will increase plants’ heat stress tolerance remain open questions.
It is well documented that EC enhances photosynthetic CO2 uptake under sufficient light intensities in C3 plants, while photorespiration rate and stomatal conductance are usually reduced (Long, 1991; Jarvis, 1998; Ainsworth and Rogers, 2007; Urban et al., 2014). Long-term, field-based studies on European forest tree species have indicated a significant decrease in stomatal conductance (by 21 % on average) for trees grown under EC. The decrease was stronger in young trees than in old trees, in deciduous trees than in coniferous trees, and in water-stressed trees than in nutrient-stressed trees (Medlyn et al., 2001). In addition, stomatal density (i.e. the number of stomata per unit of leaf area) may decrease with increasing CO2 concentration (Woodward and Bazzaz, 1988; Tricker et al., 2005), which is related to an overexpression of the HIC (high carbon dioxide) gene encoding an enzyme involved in a negative regulation of stomatal development (Gray et al., 2000). These restrictions lead to a reduced dissipation of latent heat via transpiration followed by an increase in leaf temperature that is often observed in plants grown under EC (Siebke et al., 2002; Barker et al., 2005; Leuzinger and Körner, 2007).
Temperature affects photosynthetic processes influencing the composition of thylakoid membranes and the reaction kinetics of related biochemical processes. Plants grown at low temperatures have lower temperature optima for CO2 assimilation (Berry and Björkman, 1980; Sage and Kubien, 2007), as well as a lower electron transport rate (June et al., 2004) and other photosynthesis-related processes (Hikosaka et al., 2006; Sage and Kubien, 2007) than do plants grown at higher temperatures. Plants that are native to or grown in warm environments usually have increased heat stress tolerance, i.e. injuries to plant tissues and metabolism occur at higher temperatures than they do for plants from cool environments (Ghouil et al., 2003; Hikosaka et al., 2006; Wahid et al., 2007; Crous et al., 2013). High temperatures can, among other things, impair quantum yield of photosystem II (Taub et al., 2000; June et al., 2004; Wang et al., 2012) and Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase) enzymatic activity (Crafts-Brandner and Salvucci, 2000; Ainsworth and Rogers, 2007; Urban et al., 2012). Whether an EC expected in the future has a potential, through a reduced transpiration and increased leaf temperature, to induce temperature acclimation of photosynthesis and increase a tolerance of plants to heat waves remains, however, unclear.
It has been shown that the rate and temperature optima (Topt) of light-saturated photosynthesis are considerably higher at saturating CO2 concentration than ambient CO2 concentration irrespective of the temperature at which a plant was grown (cf. Berry and Björkman, 1980). Temperature optima instantaneously increase with CO2 concentration due to a shift of photosynthesis limitation by Rubisco carboxylation activity to a limitation by RuBP (ribulose-1,5-bisphosphate) regeneration capacity, Rubisco kinetics and reduction of photorespiration (reviewed by Hikosaka et al., 2006). Therefore, the relative increase in CO2 assimilation rate under elevated CO2 concentration is greater at high temperatures than it is at low temperatures (Ainsworth and Ort, 2010). Under heat stress, the reported effects of EC on plant photosynthesis and growth are, however, very variable and differ among functional groups of plants (reviewed by Wang et al., 2012).
In this study, we tested two hypotheses: (1) plants grown under EC conditions experience higher leaf temperatures than AC plants; and (2) higher leaf temperatures subsequently lead to temperature acclimation of carbon assimilation. Such temperature acclimation is defined here as an increase in temperature optima that does not disappear after exposing EC plants to ambient CO2 concentrations. The hypotheses were tested in two distinctive temperate-zone tree species (broadleaved Fagus sylvatica and coniferous Picea abies) under the growth CO2 concentrations (385 versus 700 µmol CO2 mol−1) as well as those concentrations in reverse. The study’s specific objectives were to investigate (1) temperature response curves of CO2 assimilation rate, (2) temperature response curves of photorespiration rate and (3) thermal stability of photosystem II photochemistry in plants exposed to ambient and elevated atmospheric CO2 concentrations.
MATERIAL AND METHODS
Plants and experimental design
The experiment was carried out at the Bílý Kříž experimental research site in the Beskydy Mountains, Czech Republic (49°30′N, 18°32′E, 908 m a.s.l.). The area has a cool (annual mean air temperature 6·8 °C) and humid (annual mean relative humidity 84 %) climate with high annual precipitation (average for 1998–2011 was 1293 mm). In the present study, we compared photosynthetic activity in two widespread temperate-zone tree species: broadleaved European beech (Fagus sylvatica) and coniferous Norway spruce (Picea abies). Three-year-old saplings were planted and thereafter grown at ambient (385 µmol CO2 mol−1; hereafter AC) and elevated (700 µmol CO2 mol−1; hereafter EC) atmospheric CO2 concentrations for 4–5 years using glass dome facilities (Urban et al., 2001). Carbon dioxide enrichment under the glass dome was continuous from April to November each year. The saplings’ mean height (± s.d.) was 2·6 ± 0·5 m (F. sylvatica) and 1·5 ± 0·3 m (P. abies) at the start of measurements in 2010. Saplings were grown in their native soil. The geological bedrock was formed of Mesozoic Godula sandstone (flysch type) and is overlain by ferric podzols. Environmental air conditions inside the domes were maintained by an air-conditioning device together with an adjustable window system enabling also throughput of incident rainfall. In case of differences between soil moisture outside and inside the domes, irrigation was provided by an automatic system. A detailed description of the glass dome microclimate can be found in Urban et al. (2014).
Monthly climatic conditions for AC and EC saplings are given in Table 1. Growth conditions 7 d prior to individual measuring campaigns are given in Supplementary Data Table S1. To assess leaf surface temperature (Tleaf) and its spatial distribution, images from a Ti55FT thermal camera (Fluke, Mississauga, Ontario, Canada) were collected during a sunny day at 1-h intervals (Fig. 1). At least 100 points (individual beech leaves or spruce shoots) were manually selected from each thermal image for temperature analysis. Only evenly sunlit leaves and shoots were selected.
Table 1.
Daily mean (s.d.) and minimum–maximum air temperatures (Tair; °C) and relative humidities (RH; %) for individual months of the 2010 and 2011 growing seasons inside glass domes with ambient (AC) and elevated (EC) CO2 concentrations. Sums of precipitation (Psum; mm) originate from a nearby meteorological station. Measurements were made automatically at 10-min frequency
| May | June | July | August | September | October | |
|---|---|---|---|---|---|---|
| 2010 | ||||||
| Tair AC | 10·0 (3·37) | 15·1 (4·26) | 18·5 (4·24) | 16·3 (3·56) | 10·4 (2·97) | 5·1 (3·4) |
| 0·9–22·4 | 6·6–32·9 | 7·9–35·1 | 6·5–29·4 | 1·6–21·9 | –2·2 to 22·9 | |
| Tair EC | 10·3 (3·48) | 15·7 (4·43) | 18·9 (4·33) | 16·5 (3·60) | 10·7 (3·05) | 5·4 (3·46) |
| 1·1–23·5 | 6·8–33·4 | 8·4–35·5 | 6·6–30·8 | 1·8–23·3 | –1·9 to 21·5 | |
| RH AC | 92 (7·6) | 80 (13·0) | 76 (15·5) | 84 (9·1) | 89 (8·6) | 86 (9·4) |
| 44·1–99·8 | 32·8–99·9 | 39·6–99·8 | 39·6–99·8 | 38·7–99·8 | 28·4–99·7 | |
| RH EC | 92 (7·6) | 80 (13·0) | 77 (15·51) | 84·1 (9·2) | 89 (8·6) | 86 (9·4) |
| 44·4–99·9 | 33·1–99·9 | 28·8–99·9 | 39·9–99·9 | 39·0–99. | 28·6–99·9 | |
| Psum | 394·2 | 115·8 | 155·6 | 221·4 | 203·2 | 29·8 |
| 2011 | ||||||
| Tair AC | 12·7 (5·04) | 15·5 (2·56) | 15·2 (4·0) | 17·4 (3·39) | 14·4 (2·79) | 7·0 (4·11) |
| –1·9 to 29·6 | 7·8–27·3 | 6·0–29·0 | 6·4–31·1 | 5·3–28·2 | –2·4 to 23·8 | |
| Tair EC | 12·8 (5·14) | 15·6 (2·66) | 15·4 (4·12) | 17·9 (3·54) | 14·8 (2·89) | 7·2 (4·48) |
| –2·2 to 29·2 | 7·6–28·4 | 5·9–29·9 | 6·3–32·7 | 5·5–29·7 | –2·6 to 28·4 | |
| RH AC | 69 (13·6) | 82 (8·3) | 86 (10·6) | 80 (8·4) | 79 (9·5) | 85 (10·23) |
| 24·2–99·7 | 42·8–99·7 | 39·6–99·8 | 38·1–99·8 | 34·7–99·3 | 32·4–99·7 | |
| RH EC | 68 (14·3) | 81 (9·4) | 85 (18·8) | 79 (9·6) | 77 (11·3) | 84 (12·4) |
| 24·4–99·9 | 37·6–99·9 | 37·7–99·9 | 21·2–99·9 | 29·7–99·9 | 27·0–99·9 | |
| Psum | 122.6 | 145·8 | 256·6 | 84·4 | 41·0 | 57·4 |
Fig. 1.
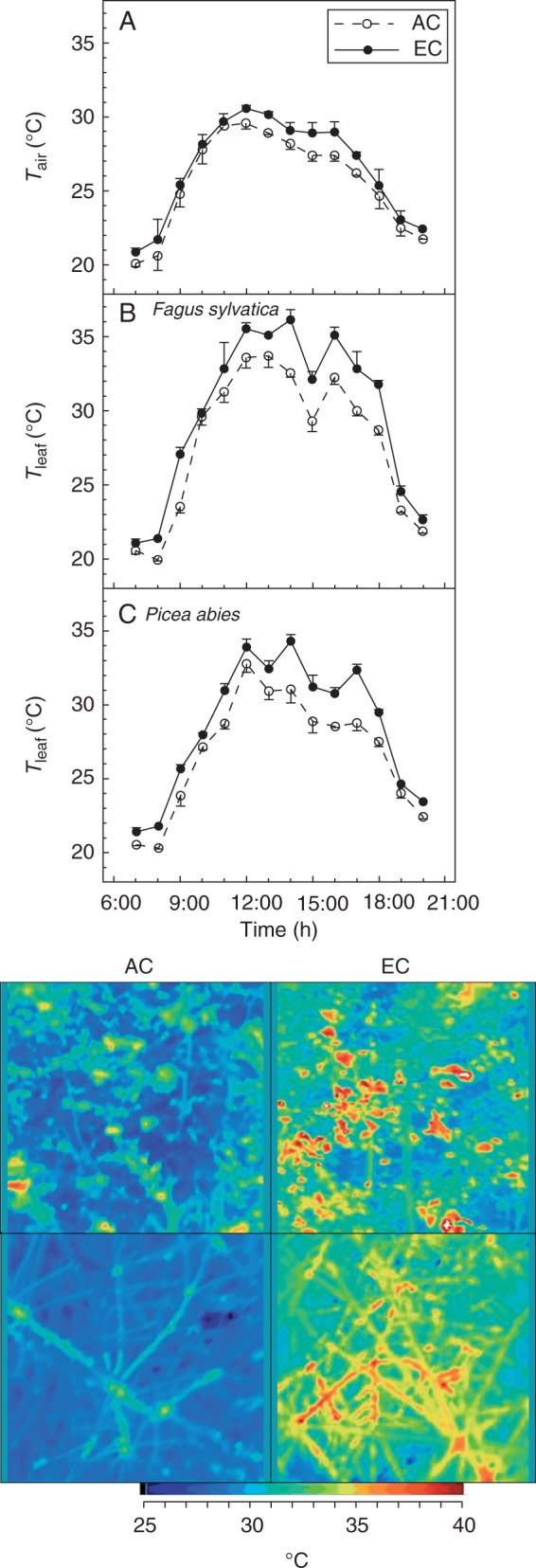
Diurnal courses of (A) air temperature (Tair) and leaf temperature (Tleaf) of (B) Fagus sylvatica and (C) Picea abies in glass domes with ambient (AC) and elevated (EC) atmospheric CO2 concentrations. Points indicate hourly averages (with error bars for standard deviation) from 15-s readings of Tair and Tleaf estimates from thermal camera images. The scans were taken during two consecutive days (23–24 August 2011). Example thermal images (bird’s-eye view) acquired at the time of greatest differences between leaf temperature of trees from the AC and EC conditions (14:00 local mean time) are shown.
Physiological measurements
Thirty-six spruce and 63 beech saplings per treatment were planted in the layout of an equilateral triangle (side length 1·20 m). Saplings within each dome (AC and EC domes) were split into three plots (each with an area of 33 m2) per dome in a south–north orientation. Each dome plot consisted of 12 spruce and 21 beech saplings and was considered as a separate replication.
In situ physiological measurements were carried out during mid-season (July and August) in two consecutive years (2010, 2011) on sun-exposed and fully developed beech leaves and current-year spruce shoots. Two saplings per plot were evaluated and the average from these two measurements was used for statistical analyses. Investigated saplings were selected among those of average height and stem diameter with similar leaf chlorophyll content estimated in vivo using an SPAD-502 Chlorophyll Meter (Konica Minolta, Osaka, Japan).
Gas-exchange measurements
Temperature response curves of basic photosynthetic characteristics (CO2 assimilation rate A, stomatal conductance GS and intercellular CO2 concentration Ci) were measured on intact leaves using the Li-6400 gas-exchange system (Li-Cor, Lincoln, NB, USA) within a Tleaf range from 10 to 45 °C. Target Tleaf was controlled using an integrated Peltier thermoelectric module. Vapour pressure deficit (VPD) values varied naturally along with temperature from 0·6 kPa at 10 °C to 4·2 kPa at 45 °C. These changes in VPD were, however, identical for both CO2 concentration treatments and both species studied (Supplementary Data Fig. S1).
Light-saturated rates of CO2 assimilation (Amax) and gross CO2 assimilation rate (Agross) were measured after 10 min exposure to a saturating irradiance (1400 µmol photons m−2 s−1) and growth CO2 concentration (i.e. 385 µmol CO2 mol−1 for AC plants and 700 µmol CO2 mol−1 for EC plants). Agross was measured using air supply with 2 % O2. In 2011, measurement of the temperature response curve of Amax at growth CO2 concentrations was coupled with an estimation of Amax at the opposite CO2 concentration (i.e. EC plants were exposed to 385 µmol CO2 mol−1 and AC plants to 700 µmol CO2 mol−1). The aim was to assess the instantaneous effect of CO2 concentration on temperature responses of CO2 assimilation rate and to assess the sensitivity of Rubisco-limited and RuBP-limited rates of CO2 assimilation to temperature.
The photorespiration rate at saturating irradiance (RL) was quantified as the difference between the CO2 assimilation rate measured under 2 % oxygen (Agross) and that measured under normal 21 % oxygen (Amax). Due to an inhibition of oxidative phosphorylation in photosynthesizing cells by competition for available ADP, mitochondrial respiration (the Krebs cycle activity) in light was assumed to be negligible compared with Amax and RL (Sharkey, 1988).
To estimate the rate of in vivo Rubisco carboxylation (VCmax), the initial linear phase of the A/Ci response curves was measured at low Ci (50–250 µmol CO2 mol−1) and saturating irradiance (1400 µmol photons m−2 s−1). VCmax values were subsequently calculated according to the equations of Farquhar et al. (1980). The temperature dependence of the Michaelis–Menten constants of Rubisco for carboxylation (Kc) and oxygenation (Ko), which are key parameters of Farquhar’s photosynthetic model, were calculated as Parameter = exp(c – ΔHa/RTleaf), where R is the molar gas constant (8·314 J mol−1 K−1), c represents a scaling constant (38·05 for Kc and 20·30 for Ko) and ΔHa is activation energy (79·43 for Kc and 36·38 kJ mol−1 for Ko) as suggested by Bernacchi et al. (2001).
Chlorophyll fluorescence measurement
Temperature responses of chlorophyll a fluorescence (Chl-F) parameters were estimated on the dark-adapted detached needles or leaf discs using a pulse amplitude-modulated fluorometer (PAM 101/103; Heinz Walz, Effeltrich, Germany). The system for linear heating of needle or leaf samples consisted of a temperature-controlled chamber (LD2/2 leaf-disc oxygen electrode chamber; Hansatech Instruments, King’s Lynn, UK) equipped with an optical lid for the PAM fibre-optic guide and connected to an ME-4 programmable temperature-controlled water bath (Julabo, Seelbach, Germany) adjusted to produce approximately 1 °C min−1 heating of the needle or leaf surface within the temperature range 20–48 °C. Needle or leaf disc surface temperature was continuously monitored using a thermocouple during the heating regime. The Chl-F measurements were carried out in darkness to estimate potential efficiency of photosystem (PS) II photochemistry [FV/FM = (FM – F0)/FM] or under a moderate actinic light intensity (250 µmol photons m−2 s−1) to assess the allocation of light absorbed by PS II to photochemical reactions: actual yield of PS II photochemistry [P = (FM′ – FT)/FM′] and thermal energy dissipation [D = 1 – (FM′ – F0′)/FM′] according to Demmig-Adams et al. (1996). Samples were inserted into the measuring chamber on water-soaked foam (to avoid desiccation during measurement) and pre-acclimated at 20 °C for 10 min prior to the start of heating, either in darkness (only at a weak measuring light; <0·1 µmol photons m−2 s−1) or at the given actinic illumination (to reach steady-state Chl-F signal). Saturation pulses (duration 0·8 s; intensity approx. 5000 µmol photons m−2 s−1) were applied at 20 °C and then approx. every 2 min (immediately following a 2 °C increase in leaf surface temperature) to determine a maximum Chl-F in dark-adapted (FM) and/or light-adapted (FM′) states. Before application of the saturation pulse, readings of the minimum Chl-F in the dark-adapted state (F0) and/or actual Chl-F in the light-adapted state (FT) were taken. In light-adapted samples, the actinic light was switched off 10–15 s after a saturation pulse for 5 s and the minimum Chl-F in the light-adapted state (F0′) was estimated as the lowest Chl-F intensity during that period.
Modelling of temperature response curves
The instantaneous rates (k; µmol CO2 m−2 s−1) of Amax and Agross were modelled as a general parabolic function of actual leaf temperature (T; °C) according to Säll and Pettersson (1994):
| (1) |
where Topt is optimal leaf temperature at which k achieves its highest value, kopt is the assimilation rate at Topt and parameter b defines the spread of the parabola.
The asymmetric temperature responses of VCmax and RL were fitted by a modified Arrhenius function including a component to take account of the deactivation processes that occur above the optimum temperature (Dreyer et al., 2001):
| (2) |
where kr is the parameter value at reference temperature Tr (25 °C, 298·15 K), ΔHa is the activation energy (J mol−1), ΔHd is the deactivation energy of the given parameter (J mol−1), ΔS is entropy (J K−1 mol−1), R is the molar gas constant (8·314 J mol−1 K−1) and T (K) is leaf temperature.
The optimal temperature Topt (°C) of either VCmax or RL can be computed from eqn (2) as:
| (3) |
Statistical data analysis
The aforementioned models were fitted (using Microsoft Office Excel 2010 with the Solver add-in and R statistical programming language) to the data points of individual samples (n = 6) to allow for statistical testing between treatments. Estimated parameters were tested for normal distribution (Shapiro–Wilk test) within treatments and for equality of variances between the pair of treatments (F-test). Testing for statistical differences between means was performed using R software (R Development Core Team, 2014) with the Wilcoxon test for non-normal data and a two-sample t-test or Welch’s two-sample t-test for normal data with equal or unequal variances, respectively.
RESULTS
Mean values of stomatal conductance under saturating irradiance (GSmax) were obtained from temperature response measurements during campaigns in 2010 and 2011 as the average GSmax for the entire measured temperature range. We found a decrease in GSmax for EC-treated plants compared with AC plants of 13–30 %, although these differences were only significant (P < 0·05) for beech in 2010 (Table 2).
Table2.
Mean values (±s.d.) of stomatal conductance at saturating irradiation (Gsmax) in mol H2O m−2 s−1 estimated across the entire temperature range (10–45 °C) for Fagus sylvatica and Picea abies grown under ambient (AC) and elevated (EC) CO2 concentrations
| AC 2010 | EC 2010 | AC 2011 | EC 2011 | |
|---|---|---|---|---|
| F. sylvatica | 0·24 ± 0·11 | 0·18 ± 0·09* | 0·12 ± 0·04 | 0·11 ± 0·06 |
| P. abies | 0·13 ± 0·05 | 0·11 ± 0·04 | 0·11 ± 0·07 | 0·08 ± 0·05 |
Measurements were performed during campaigns in 2010 (July) and 2011 (August) at growth CO2 concentration.
*Statistically significant differences between AC and EC treatments at P < 0·05, n = 35.
Analysis of thermal camera images (Fig. 1) revealed differences in Tleaf between AC and EC plants. On average, EC plants recorded 2 °C higher Tleaf compared with AC plants, with the greatest difference (3·5 °C) coming in the afternoon of a sunny day (Fig. 1B, C). These differences can be attributed only in part to an increase in air temperature (Tair) under EC, because Tair observed in the EC dome was higher by 0·9 °C on average as compared with that for the AC condition (Fig. 1A).
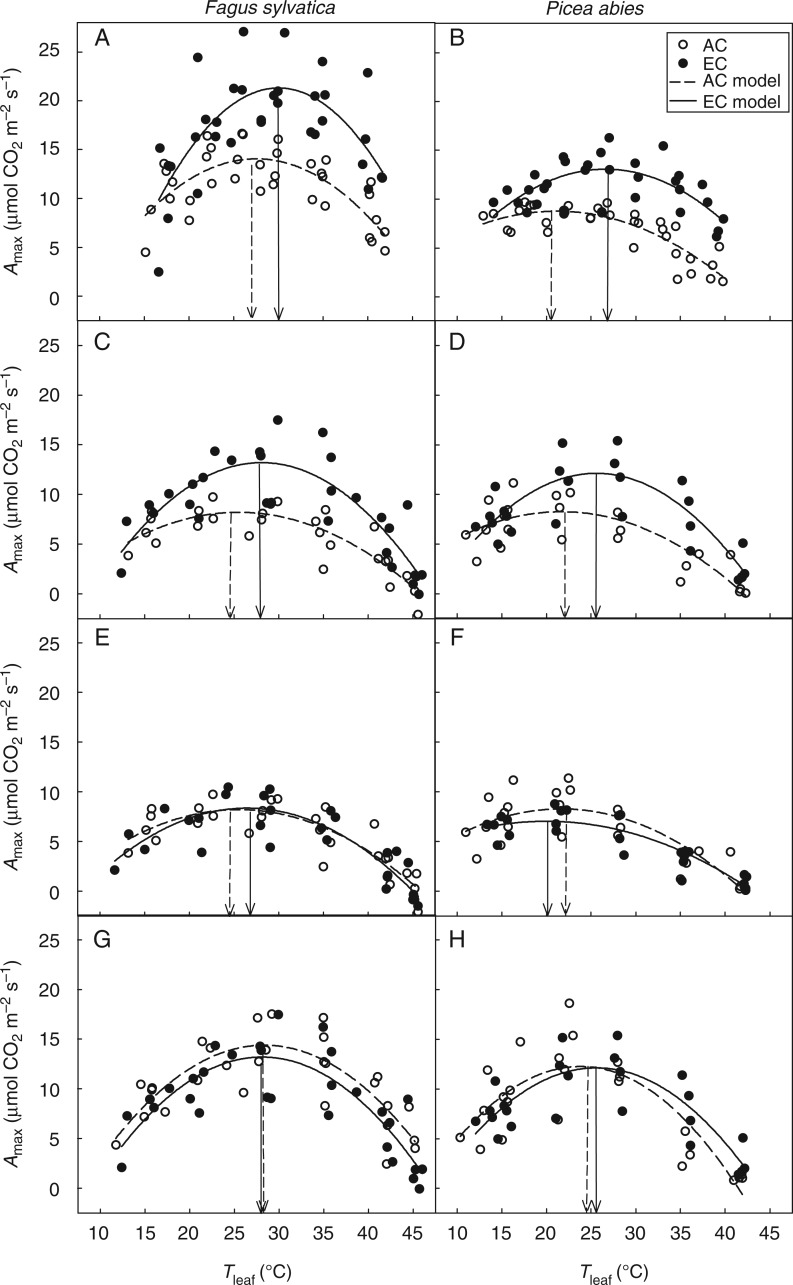
The EC condition led to the stimulation of Amax in both tree species (Fig. 2A–D). For example, significant (P < 0·05) stimulations of Amax at optimal temperature (Aopt) were found for beech (53–74 %) and spruce (22–47 %) saplings cultivated under EC conditions (Table 3). This stimulatory effect was, however, negligible or reduced at low and high temperatures (approx. below 15 °C and above 40 °C, respectively). During the two consecutive seasons, we observed significantly higher temperature optima for Amax [Topt(Amax)] in EC beech (by 2·9–3·5 °C) and spruce (by 3·3–6·0 °C) in comparison with their AC counterparts (Fig. 2A–D; Table 3). However, an estimation of both Aopt and Topt(Amax) for saplings from both CO2 concentration treatments revealed no significant differences (P > 0·05) between AC and EC saplings when the saplings were measured at the same CO2 concentration (Fig. 2E–H; Table 3).
Fig. 2.
Temperature response of net CO2 assimilation rate at saturating irradiance (Amax) in Fagus sylvatica and Picea abies grown at ambient (AC) and elevated (EC) atmospheric CO2 concentrations. Measurements were performed during campaigns in 2010 (A, B) and 2011 (C, H) at growth (A–D), ambient (E, F) and elevated (G, H) CO2 concentrations. Parabolic fits for all samples of individual treatments and respective estimated temperature optima are displayed for AC and EC. The individual measured values are shown; R2 ranged from 0·43 to 0·79 (P < 0·01). The vertical line indicates the optimum temperature of the fit.
Table 3.
Parameters (mean ± s.d.) of the temperature response curves of light-saturated CO2 assimilation rate (Amax) and gross CO2 assimilation rate under 2 % oxygen (Agross) calculated for individual leaves of Fagus sylvatica and Picea abies grown at ambient (AC) and elevated (EC) CO2 concentrations
| Treatment |
Amax |
Agross |
|||||
|---|---|---|---|---|---|---|---|
| Aopt | Topt | b | Aopt | Topt | b | ||
| 2010 | |||||||
| F. sylvatica | AC 385 | 13·8 ± 2·1 | 27·0 ± 1·0 | 0·03 ± 0·01 | 20·2 ± 1·9 | 27·8 ± 0·3 | 0·06 ± 0·01 |
| EC 700 | 21·1 ± 1·7** | 29·9 ± 0·9* | 0·06 ± 0·02* | 26·2 ± 2·9* | 31·4 ± 0·8** | 0·06 ± 0·01 | |
| P. abies | AC 385 | 8·9 ± 1·0 | 20·8 ± 1·5 | 0·02 ± 0·01 | 11·7 ± 0·8 | 24·0 ± 0·7 | 0·04 ± 0·01 |
| EC 700 | 13·2 ± 1·4* | 26·8 ± 0·3** | 0·03 ± 0·01 | 15·1 ± 2·0* | 28·5 ± 0·7** | 0·04 ± 0·02 | |
| 2011 | |||||||
| F. sylvatica | AC 385 | 7·6 ± 1·9 | 24·6 ± 1·1 | 0·02 ± 0·01 | 10·7 ± 1·3 | 26·6 ± 0·4 | 0·03 ± 0·01 |
| EC 700 | 13·2 ± 1·3* | 27·9 ± 0·8* | 0·03 ± 0·01 | 15·1 ± 1·9* | 30·5 ± 1·8* | 0·04 ± 0·01 | |
| AC 385 | 7·6 ± 1·9 | 24·6 ± 1·1 | 0·02 ± 0·01 | 10·7 ± 1·3 | 26·6 ± 0·4 | 0·03 ± 0·01 | |
| EC 385 | 7·5 ± 2·0 | 26·8 ± 0·9 | 0·02 ± 0·01 | 10·1 ± 1·1 | 27·4 ± 1·2 | 0·03 ± 0·01 | |
| AC 700 | 14·1 ± 1·3 | 28·1 ± 0·3 | 0·03 ± 0·01 | 17·1 ± 2·5 | 29·0 ± 0·9 | 0·05 ± 0·01 | |
| EC 700 | 13·2 ± 1·3 | 27·9 ± 0·8 | 0·03 ± 0·01 | 15·1 ± 1·9 | 30·5 ± 1·8 | 0·04 ± 0·01 | |
| P. abies | AC 385 | 8·5 ± 1·4 | 22·2 ± 1·0 | 0·03 ± 0·01 | 8·8 ± 1·9 | 22·5 ± 0·7 | 0·03 ± 0·01 |
| EC 700 | 12·5 ± 2·0* | 25·6 ± 0·5** | 0·04 ± 0·01 | 14·4 ± 0·4** | 25·6 ± 0·6** | 0·04 ± 0·01 | |
| AC 385 | 8·5 ± 1·4 | 22·2 ± 1·0 | 0·03 ± 0·01 | 8·8 ± 1·9 | 22·5 ± 0·7 | 0·03 ± 0·01 | |
| EC 385 | 7·1 ± 1·0 | 20·0 ± 1·3 | 0·02 ± 0·01* | 8·9 ± 1·3 | 23·4 ± 1·8 | 0·02 ± 0·01 | |
| AC 700 | 13·0 ± 2·2 | 24·6 ± 0·5 | 0·04 ± 0·01 | 13·5 ± 1·7 | 24·3 ± 0·6 | 0·05 ± 0·01 | |
| EC 700 | 12·5 ± 2·0 | 25·6 ± 0·5 | 0·04 ± 0·01 | 14·4 ± 0·4 | 25·6 ± 0·6 | 0·04 ± 0·01 | |
A general parabolic function (eqn 1) was fitted to Amax and Agross data: Aopt, assimilation rate at optimal temperature (µmol CO2 m−2 s−1); Topt, optimal leaf temperature (°C). Measurements were performed under 385 µmol CO2 mol−1 (385; corresponding to AC) and/or 700 µmol CO2 mol−1 (700; corresponding to EC) CO2 concentrations. Statistically significant differences between AC and EC treatments are indicated as follows: *0·01 < P < 0·05, **P < 0·01; n = 3.
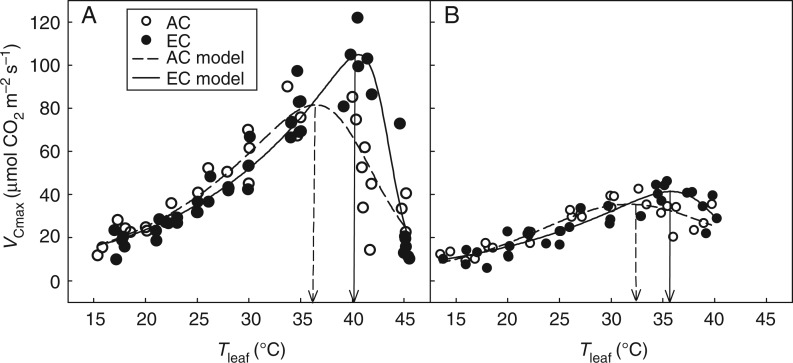
Temperature response curves of VCmax (Fig. 3) showed a significant (P < 0·05) shift of temperature optima for Rubisco carboxylation rate by 3·4–3·7 °C for EC saplings of both species compared with their AC counterparts. The values of VCmax at reference leaf temperature 25 °C were lower for EC plants compared with AC plants by 12–13 % for both species (Table 4). These differences were not, however, statistically significant.
Fig. 3.
Temperature dependences of light-saturated Rubisco carboxylation rate (VCmax) in Fagus sylvatica (A) and Picea abies (B) grown at ambient (AC) and elevated (EC) atmospheric CO2 concentration. The modified Arrhenius function (eqn 2) was fitted to the data. The individual measured values are shown; R2 ranged from 0·80 to 0·89 (P < 0·01). The vertical lines indicate modelled temperature optima of VCmax for AC and EC trees.
Table 4.
Selected parameters (mean ± s.d.) of temperature response curves of light-saturated rate of Rubisco carboxylation (VCmax) and light-saturated rate of photorespiration (RL) calculated for individual leaves of Fagus sylvatica and Picea abies grown at ambient (AC) and elevated (EC) CO2 concentration
| Parameter | Units |
F. sylvatica |
P. abies |
||
|---|---|---|---|---|---|
| AC | EC | AC | EC | ||
| VCmax,25 | µmol m−2 s−1 | 40·1 ± 3·9 | 34·4 ± 2·8 | 26·5 ± 1·4 | 22·6 ± 2·6 |
| VCmax at Topt | µmol m−2 s−1 | 82·6 ± 5·7 | 105·7 ± 10·6* | 37·2 ± 2·8 | 42·2 ± 3·1 |
| Topt(VCmax) | °C | 36·3 ± 0·9 | 40·0 ± 0·9** | 32·4 ± 0·3 | 35·8 ± 0·3** |
| RL,25 | µmol m−2 s−1 | 6·3 | 3·2 | 3·3 | 0·1 |
| RL at Topt | µmol m−2 s−1 | 6·9 | 6·4 | 3·3 | 3·6 |
| Topt(RL) | °C | 29·8 | 35·3 | 25·3 | 30·9 |
VCmax,25 and RL,25 are VCmax and RL rates at reference leaf temperature 25 °C; Topt(VCmax) and Topt(RL) are temperature optima of VCmax and RL. Measurements were performed at growth CO2 concentration and 2 % oxygen (RL) or at intercellular CO2 concentrations ranging between 50 and 250 µmol CO2 mol−1 (VCmax). Statistically significant differences between AC and EC treatments are indicated as follows: *0·01 < P < 0·05, **p < 0·01; n = 3. See Supplementary Table S2 for the complete list of parameters of the modified Arrhenius function (eqn 2) fitted to the VCmax and RL data.
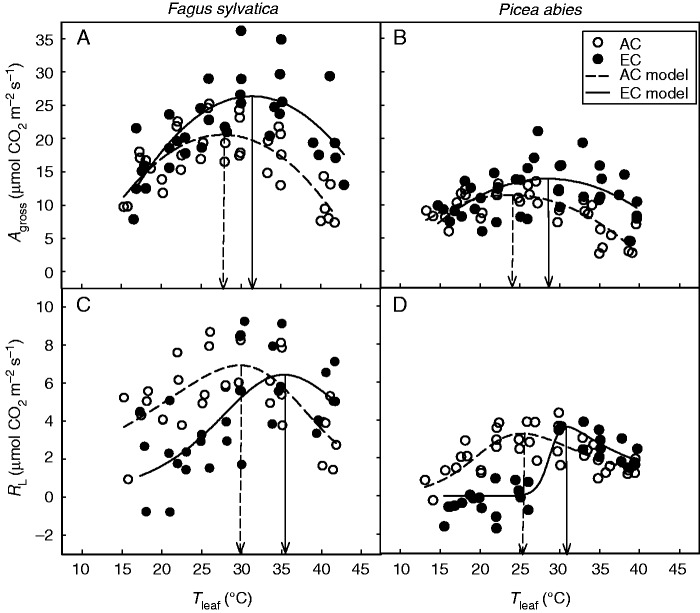
The values of Agross showed a similar temperature curve pattern as those for Amax (Fig. 4A, B). In both species, EC stimulated Agross only at high temperatures, while differences in Agross between AC and EC saplings were negligible at temperatures below 25 °C. Accordingly, significant (P < 0·05) stimulations of Agross at optimal temperature (Aopt) were found for beech (30–41 %) and spruce (29–64 %) saplings cultivated under EC conditions (Table 3). A significant increase in Topt(Agross) due to the EC treatment was confirmed for beech (by 3·5–3·6 °C; P < 0·05) as well as spruce (3·1–4·5 °C; P < 0·01) in the two consecutive years studied (Table 3). Suppression of photorespiration at low temperatures led to a slight increase in Topt(Agross) compared with Topt(Amax), particularly for spruce (Tables 3 and 4).
Fig. 4.
Temperature response curves of light-saturated rate of gross CO2 assimilation (Agross; A, B) and photorespiration (RL; C, D) in Fagus sylvatica (A, C) and Picea abies (B, D) grown under ambient (AC) and elevated (EC) atmospheric CO2 concentration. Gas-exchange measurements were made at growth CO2 concentration and 2 % O2 in the atmosphere. The parabolic function (eqn 1) and modified Arrhenius function (eqn 2) were fitted to the Agross (R2 = 0·38–0·65; P < 0·01) and RL (R2 = 0·42–0·84; P < 0·01) data, respectively. Individual measured values are shown. The vertical lines indicate modelled temperature optima of VCmax for AC and EC trees.
Values of RL were estimated as the difference between Agross and Amax rates. EC had a similar effect on the pattern of temperature response curves of photorespiration in both species (Fig. 4C, D). While the maximum rates of RL were the same for both CO2 treatments, EC saplings achieved maximum RL at temperatures 5·5–5·6 °C higher than those for AC saplings (Table 4). Therefore, significantly lower RL values in EC plants as compared with AC saplings were observed only in a relatively narrow temperature range of 15–30 °C. At low and high temperatures (approx. below 15 °C and above 30 °C, respectively), the RL values of AC and EC saplings tended to converge. In contrast to RL, dark mitochondrial respiration (RD) and its temperature response curve of RD were not influenced by CO2 treatment (see Supplementary Data Fig. S2).
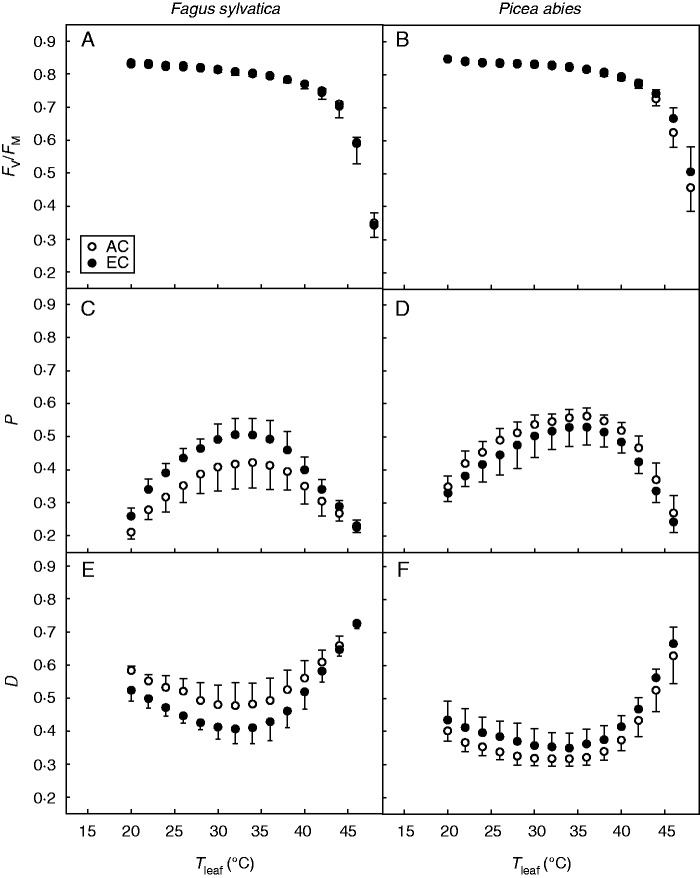
The EC treatment led to slightly higher FV/FM values at 46 and 48 °C in spruce as compared with the AC treatment (Fig. 5B), but that was not the case in beech (Fig. 5A). Irrespective of treatment, spruce had higher FV/FM than did beech at such high temperatures. Similarly, slightly higher temperature optima for actual yield of PS II photochemistry, P (Fig. 5C, D), and thermal energy dissipation, D (Fig. 5E, F), were observed in spruce than in beech. Long-term acclimation to EC, however, had no effect on the temperature response curve of P and D in either tree species across the entire temperature range studied.
Fig. 5.
Temperature response curves of potential efficiency of photosystem (PS) II photochemistry in dark-adapted leaves (FV/FM; A, B), actual yield of PS II photochemistry (P; C, D) and thermal energy dissipation (D; E, F) in Fagus sylvatica (A, C, E) and Picea abies (B, D, F) grown at ambient (AC) and elevated atmospheric CO2 concentration (EC). Mean values with error bars for standard deviation are shown.
DISCUSSION
In accordance with previous studies (Siebke et al., 2002; Barker et al., 2005; Leuzinger and Körner, 2007), we have confirmed the hypothesis that growth under EC increases foliage temperature in both broadleaved and coniferous trees species as compared with AC conditions by decreasing stomatal conductance (Fig. 1). We therefore further hypothesized parallel acclimation of photosynthesis to EC and temperature and that growth of plants under EC would lead to a shift of temperature optima for CO2 assimilation rate to higher temperatures and to enhanced heat stress tolerance. Transitory (more intense and frequent heat waves) or continually (increase in global air temperature) high temperatures may cause an array of injuries at different hierarchical levels of plants (Sage and Kubien, 2007; Wahid et al., 2007; Way and Sage, 2008), which may subsequently affect plant growth, development, economic yield and/or geographical distribution. It remains an open question whether long-term growth under EC induces temperature acclimation of photosynthesis and increases the heat stress tolerance of plants.
In addition, it has been shown that simulated carbon flux between the atmosphere and vegetation can dramatically differ between versions of models that do and do not include acclimation (Smith and Dukes, 2013). In contrast to instantaneous responses, mechanisms of acclimation to increasing atmospheric CO2 concentration and temperature are, however, rarely presented and incorporated into the models.
To test this hypothesis, we investigated sensitivities of photosynthesis-related processes within the temperature range 10–45 °C. Although it was impossible to maintain low VPD values at high temperatures, this change was identical for both the AC and the EC conditions (Supplementary Data Fig. S1). High VPD values may lead to increased stomatal limitation of photosynthesis. However, it has been shown in many coniferous and broadleaved tree species that the sensitivity of stomata to VPD is not affected by growth under EC conditions (Jarvis, 1998). The presented differences between the AC and EC treatments are therefore not affected by changing VPD. In addition, the relatively slow response of stomata to different environmental parameters, particularly in coniferous P. abies (Košvancová et al., 2009), leads to the presumption that the effect of VPD on the photosynthetic temperature response curve is relatively small.
Temperature responses of photosynthetic processes
Similarly to previous studies (e.g. Berry and Björkman, 1980; Long, 1991; Hikosaka et al., 2006), considerably higher rates and temperature optima of Amax and Agross were found under EC than under AC (Figs 2A–D and 4A, B). The effect of EC on assimilation rate was, however, reduced at high (>40 °C) and particularly low temperatures (<15 °C). As CO2 concentration increases, photosynthesis is increasingly limited by the capacity for RuBP regeneration, i.e. by photosynthetic electron transport chain capacity (Farquhar et al., 1980). Hikosaka et al. (2006) noted that the optimal temperature of the RuBP-limited assimilation rate is higher than that of the Rubisco-limited assimilation rate in many species, thus leading to an increase in Topt(Amax) at high CO2 concentrations (Fig. 2). In addition, temperature-stimulated electron flow through PS II (estimated here by P; Fig. 5C, D) and reduced photorespiration under EC (Fig. 4C, D) consequently lead to an increase in the optimal temperature of the Rubisco-limited CO2 assimilation rate (Franco and Lüttge, 2002; Sage and Kubien, 2007; Yamori et al., 2008). In our experiment, the shift in Topt observed for AC versus EC saplings under growth CO2 concentrations, however, was considerably reduced or completely disappeared when the saplings were exposed to the same CO2 concentration, i.e. 385 or 700 µmol CO2 mol−1 (Fig. 2E–H; Table 3). Based on these results, we conclude that EC has a significant, instantaneous and reversible effect on Topt, but long-term cultivation under EC does not lead to typical photosynthetic acclimation to elevated temperatures as reported in reviews by Berry and Björkman (1980) and Sage and Kubien (2007).
Nevertheless, the observed shift of the VCmax optimum to higher temperatures in both species when EC treated as compared with when AC treated (Fig. 3; estimated at low Ci of 50–250 µmol CO2 mol−1) indicates certain temperature acclimation of Rubisco’s kinetic properties. Possible mechanisms contributing to the reduction in Rubisco activity at high temperatures include an increase of mesophyll resistance to CO2 diffusion followed by Rubisco decarbamylation (Bernacchi et al., 2001), reduction of Rubisco activation state due to Rubisco activase constraint (Crafts-Brandner and Salvucci, 2000) and/or increased synthesis of Rubisco inhibitor, xylulose-1,5-bisphosphate (Newman and Gutteridge, 1994), and an insufficiency of Pi in chloroplast stroma followed by a limitation of ATP production (June et al., 2004). In our previous studies using the same tree species (Košvancová et al., 2009; Urban et al., 2012), we have shown that high intercellular CO2 concentration in EC plants protects Rubisco against decarbamylation and maintains a higher proportion of Rubisco in its active form in comparison with AC plants. In contrast, the often reported inaccessibility of Pi in EC plants may reduce the ATP/ADP ratio and subsequently lead to reduced Rubisco activase activity (Crafts-Brandner and Salvucci, 2000). Such contrasting CO2-dependent mechanisms of Rubisco regulation may consequently lead to the reported species-specific and seasonal variability in VCmax temperature dependence (Ziska, 2000; Urban et al., 2012; Crous et al., 2013).
Rubisco oxygenase activity, measured here as photorespiration rate (RL; Fig. 4C, D), was significantly reduced by EC, particularly at temperatures between 20 and 25 °C. Further increase in Tleaf, however, resulted in a sharp increase in RL values to the level of those observed for AC plants. Such increase in RL is probably due to the reduced solubility of CO2 compared with O2 (Ehleringer and Björkman, 1977) and/or reduced activity of carbonic anhydrase (Badger and Price, 1994) at high temperatures. The EC treatment consequently resulted in a significant shift of photorespiration temperature optima in both species studied (Fig. 4C, D; Table 4). Our results thus show, in contrast to earlier predictions (Long, 1991; Bowes, 1996), that the effects of EC-reduced photorespiration on the stimulation of CO2 uptake are relatively small or even negative at temperatures above 30 °C.
Thermal stability of PS II photochemistry
The steeper decline in FV/FM in Tleaf above 40 °C demonstrates the lower thermostability of PS II in beech leaves under both CO2 concentration treatments as compared with spruce needles (Fig. 5A, B). We observed a smaller decline in FV/FM for EC spruce needles as compared with AC needles at temperatures above 40 °C, but no such decline for beech leaves. These data suggest that EC’s effect on the enhancement of PS II thermal stability is negligible. Growth under EC has been shown, however, to protect Quercus suber (Faria et al., 1996) as well as Pinus taeda and Quercus rubra (Ameye et al., 2012) from short-term heat shock at the levels of both PS II photochemical efficiency and CO2 assimilation rate. Also, Taub et al. (2000) found enhanced PS II thermotolerance, measured as FV/FM decline, in both woody and herbaceous species and in both monocotyledonous and dicotyledonous species cultivated under EC conditions. Although the exact mechanisms responsible for increased PS II thermotolerance in plants grown at elevated CO2 remain unclear, enhanced thermotolerance probably relates to an increased production of heat shock proteins, chemical composition of the thylakoid membrane and chloroplast stroma, as well as isoprene production and its integration into the thylakoid membrane (reviewed by Taub et al., 2000).
A number of authors (Ghouil et al., 2003; Daas et al., 2008; Way and Sage, 2008; Yamori et al., 2008) have observed significant increases in Topt of P and thermotolerance of PS II when growth temperature increased by more than 8 °C. The sensitivity to temperature of Chl-F parameters that we present in this study (Fig. 5C–F) demonstrates that the EC-related increase in Tleaf was below the threshold level for inducing acclimatory changes resulting in significantly enhanced PS II thermostability and shift of Topt to higher temperatures.
To conclude, we have confirmed higher temperature optima (Topt) of Amax for EC plants than for AC plants when measured at their growth CO2 concentrations. This is caused mainly by reduced photorespiration and limitation of photosynthesis by RuBP regeneration under EC. However, the instantaneous differences in Topt between AC and EC disappeared when the plants were exposed to identical CO2 concentrations. Enhanced thermostability of PS II in EC saplings was not confirmed. We therefore rejected the hypothesis that EC conditions led to temperature acclimation of photosynthesis in both species studied.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: relationships between vapour pressure deficit and air temperature inside the assimilation chamber of the gas-exchange system during measurement of the temperature response curve of photosynthesis. Figure S2: temperature response curves of dark mitochondrial respiration (RD) in Fagus sylvatica and Picea abies grown under ambient and elevated atmospheric CO2 concentrations. Table S1: comparison of daily air temperature and relative humidity statistics between glass domes with ambient and elevated CO2 concentrations for the 7 d preceding individual measurement periods. Table S2: parameters of temperature response curves of light-saturated rate of Rubisco carboxylation and light-saturated rate of photorespiration calculated for individual leaves of Fagus sylvatica and Picea abies grown at ambient and elevated CO2 concentrations.
ACKNOWLEDGEMENTS
This work is part of research supported by the Grant Agency of the Czech Republic (grants GAP501/10/0340, 13-28093S) and MEYS CR (LO1415). Participation of P.H., K.K., M.Š., C.C. and O.U. was supported by the EfCOP – IPo project ENVIMET (CZ.1.07/2.3.00/20.0246). L.Š. was supported by the University of Ostrava (SGS20/PřF/2014) and Helmholtz Research School MICMoR. The experimental system for CO2 fumigation comprises a part of the National Infrastructure CzeCos/ICOS (LM2010007). Our particular thanks go to Professor S. Linder for critical reading and valuable comments.
LITERATURE CITED
- Ainsworth EA, Ort DR. 2010. How do we improve crop production in a warming world? Plant Physiology 154: 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell and Environment 30: 258–270. [DOI] [PubMed] [Google Scholar]
- Ameye M, Wertin TM, Bauweraerts I, McGuire MA, Teskey RO, Steppe K. 2012. The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytologist 196: 448–461. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD. 1994. The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 45: 369–392. [Google Scholar]
- Barker DH, Loveys BR, Egerton JJG, Gorton H, Williams WE, Ball MC. 2005. CO2 enrichment predisposes foliage of a eucalypt to freezing injury and reduces spring growth. Plant, Cell and Environment 28: 1506–1515. [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Jr, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24: 253–259. [Google Scholar]
- Berry J, Björkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491–543. [Google Scholar]
- Bowes G. 1996. Photosynthetic responses to changing atmospheric carbon dioxide concentration. In: Baker NR, ed. Photosynthesis and the environment. Advances in photosynthesis and respiration. Dordrecht: Kluwer Academic Publishers, 387–407. [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. 2000. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences of the United States of America 97: 13430–13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous KY, Quentin AG, Lin YS, et al. 2013. Photosynthesis of temperate Eucalyptus globulus trees outside their native range has limited adjustment to elevated CO2 and climate warming. Global Change Biology 19: 3790–3807. [DOI] [PubMed] [Google Scholar]
- Daas C, Montpied P, Hanchi B, Dreyer E. 2008. Responses of photosynthesis to high temperatures in oak saplings assessed by chlorophyll-a fluorescence: inter-specific diversity and temperature-induced plasticity. Annals of Forest Science 65: 305–401. [Google Scholar]
- Demmig-Adams B, Adams WW, III, et al. 1996. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiologia Plantarum 98: 253–264. [Google Scholar]
- Dreyer E, Le Roux X, Montpied P, Daudet FA, Masson F. 2001. Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiology 21: 223–232. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O. 1977. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2, and O2 concentration. Plant Physiology 59: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria T, Wilkins D, Besford RT, Vaz M, Pereira JS, Chaves MM. 1996. Growth at elevated CO2 leads to down-regulation of photosynthesis and altered response to high temperature in Quercus suber L. seedlings. Journal of Experimental Botany 47: 1755–1761. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Franco A, Lüttge U. 2002. Midday depression in savanna trees: coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 131: 356–365. [DOI] [PubMed] [Google Scholar]
- Ghouil H, Montpied P, Epron D, Ksontini M, Hanchi B, Dreyer E. 2003. Thermal optima of photosynthetic functions and thermostability of photochemistry in cork oak seedlings. Tree Physiology 23: 1031–1039. [DOI] [PubMed] [Google Scholar]
- Gray JE, Holroyd GH, van der Lee FM, et al. 2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. 2006. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany 57: 291–302. [DOI] [PubMed] [Google Scholar]
- Jarvis PG. 1998. European Forests and Global Change. The Likely Impacts of Rising CO2 and Temperature . Cambridge: Cambridge University Press. [Google Scholar]
- IPCC. 2013. Summary for policymakers. In: Stocker TF, Qin D, Plattner G-K, et al., eds. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge, UK and New York, NY, USA: Cambridge University Press, 3–29. [Google Scholar]
- June T, Evans JR, Farquhar GD. 2004. A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Functional Plant Biology 31: 275–283. [DOI] [PubMed] [Google Scholar]
- Košvancová M, Urban O, Šprtová M, et al. 2009. Photosynthetic induction in broadleaved Fagus sylvatica and coniferous Picea abies cultivated under ambient and elevated CO2 concentrations. Plant Science 177: 123–130. [Google Scholar]
- Leuzinger S, Körner C. 2007. Tree species diversity affects canopy leaf temperatures in a mature temperate forest. Agricultural and Forest Meteorology 146: 29–37. [Google Scholar]
- Long SP. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell and Environment 14: 729–739. [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, et al. 2001. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytologist 149: 247–264. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997. [DOI] [PubMed] [Google Scholar]
- Newman J, Gutteridge S. 1994. Structure of an effector-induced inactivated state of ribulose 1,5-bisphosphate carboxylase/oxygenase: the binary complex between enzyme and xylulose 1,5-bisphosphate. Structure 2: 495–502. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; www.r-project.org. [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- Säll T, Pettersson P. 1994. A model of photosynthetic acclimation as a special case of reaction norms. Journal of Theoretical Biology 166: 1–8. [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration in leaves. Physiologia Plantarum 73: 147–152. [Google Scholar]
- Siebke K, Ghannoum O, Conroy JP, von Caemmerer S. 2002. Elevated CO2 increases the leaf temperature of two glasshouse-grown C4 grasses. Functional Plant Biology 29: 1377–1385. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2013. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology 19: 45–63. [DOI] [PubMed] [Google Scholar]
- Taub DR, Seemann JR, Coleman JS. 2000. Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant, Cell and Environment 23: 649–656. [Google Scholar]
- Tricker PJ, Trewin H, Kull O, et al. 2005. Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2. Oecologia 143: 652–660. [DOI] [PubMed] [Google Scholar]
- Urban O, Janouš D, Pokorný R, et al. 2001. Glass domes with adjustable windows: a novel technique for exposing juvenile forest stands to elevated CO2 concentration. Photosynthetica 39: 395–401. [Google Scholar]
- Urban O, Hrstka M, Zitová M, et al. 2012. Effect of season, needle age and elevated CO2 concentration on photosynthesis and Rubisco acclimation in Picea abies. Plant Physiology and Biochemistry 58: 135–141. [DOI] [PubMed] [Google Scholar]
- Urban O, Klem K, Holišová P, et al. 2014. Impact of elevated CO2 concentration on dynamics of leaf photosynthesis in Fagus sylvatica is modulated by sky conditions. Environmental Pollution 185: 271–280. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. 2007. Heat tolerance in plants: an overview. Environmental and Experimental Botany 61: 199–223. [Google Scholar]
- Wang D, Heckathorn SA, Wang X, Philpott SM. 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169: 1–13. [DOI] [PubMed] [Google Scholar]
- Way DA, Sage RF. 2008. Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) B.S.P.]. Global Change Biology 14: 624–636. [Google Scholar]
- Woodward FI, Bazzaz FA. 1988. The responses of stomatal density to CO2 partial pressure. Journal of Experimental Botany 39: 1771–1781. [Google Scholar]
- Yamori W, Noguchi K, Kashino Y, Terashima I. 2008. The role of electron transport in determining the temperature dependence of the photosynthetic rate in spinach leaves grown at contrasting temperatures. Plant Cell Physiology 49: 583–591. [DOI] [PubMed] [Google Scholar]
- Ziska LH. 2000. The impact of elevated CO2 on yield loss from a C3 and C4 weed in field-grown soybean. Global Change Biology 6: 899–905. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.