Abstract
Background and Aims Many individual studies have shown that the timing of leaf senescence in boreal and temperate deciduous forests in the northern hemisphere is influenced by rising temperatures, but there is limited consensus on the magnitude, direction and spatial extent of this relationship.
Methods A meta-analysis was conducted of published studies from the peer-reviewed literature that reported autumn senescence dates for deciduous trees in the northern hemisphere, encompassing 64 publications with observations ranging from 1931 to 2010.
Key Results Among the meteorological measurements examined, October temperatures were the strongest predictors of date of senescence, followed by cooling degree-days, latitude, photoperiod and, lastly, total monthly precipitation, although the strength of the relationships differed between high- and low-latitude sites. Autumn leaf senescence has been significantly more delayed at low (25° to 49°N) than high (50° to 70°N) latitudes across the northern hemisphere, with senescence across high-latitude sites more sensitive to the effects of photoperiod and low-latitude sites more sensitive to the effects of temperature. Delays in leaf senescence over time were stronger in North America compared with Europe and Asia.
Conclusions The results indicate that leaf senescence has been delayed over time and in response to temperature, although low-latitude sites show significantly stronger delays in senescence over time than high-latitude sites. While temperature alone may be a reasonable predictor of the date of leaf senescence when examining a broad suite of sites, it is important to consider that temperature-induced changes in senescence at high-latitude sites are likely to be constrained by the influence of photoperiod. Ecosystem-level differences in the mechanisms that control the timing of leaf senescence may affect both plant community interactions and ecosystem carbon storage as global temperatures increase over the next century.
Keywords: Autumn phenology, climate change, growing season, leaf senescence, temperature, deciduous tree, woody plants
INTRODUCTION
Air temperatures around the globe are increasing (Jones et al., 2012), which has measureable effects on a variety of plant processes. Specifically, the effects of climate change on the phenology of vegetation have received increased attention over the past several decades as any factor that alters the timing of early growing season leaf-out and senescence has the potential to affect a variety of ecosystem properties. For example, the timing of leaf-out and senescence of deciduous plants has been shown to affect plant competition (Fridley, 2012), plant growth (Myneni et al., 1997) and ecosystem carbon uptake (Barichivich et al., 2012). Changes in phenology also affect surface albedo through differences in reflectance between closed and bare canopies (Richardson et al., 2013). While leaf-out has been shown to advance over the past century as a result of increasing air temperatures (Linderholm, 2006; Polgar and Primack, 2011), the relationship between temperature and leaf senescence remains less well understood (García-Plazaola et al., 2003; Richardson et al., 2013). Leaf senescence completes the growing season for deciduous trees and therefore factors that delay leaf-off can lengthen the period of plant photosynthesis and increase rates of gross primary productivity (Richardson et al., 2010). Thus, an extension of the growing season can contribute to reduced atmospheric CO2 concentrations due to enhanced carbon sequestration in terrestrial plants (Penuelas et al., 2009; Richardson et al., 2013). However, the increase in carbon uptake may be partially offset by increased rates of ecosystem respiration (Piao et al., 2008).
Efforts to examine the effects of increasing air temperatures on leaf senescence have employed a wide array of methodologies and have measured senescence across a range of scales, from ground-based observations of individual trees to regional-scale remote sensing. Ge et al. (2014) conducted a meta-analysis of ground-based phenological data sets spanning >20 years throughout China and found that leaf senescence in trees and woody shrubs was delayed by 1·98 d per decade from 1960 to 2011. Menzel et al. (2006) used long-term, ground-based observations to determine that autumn senescence in Europe was delayed by 1·3 d per decade and 1·0 d °C–1 between 1971 and 2000; however, these trends were not unidirectional as only 15 % of individual sites demonstrated significantly delayed senescence, 12 % showed senescence dates that advanced and 83 % did not change significantly over time. Through the use of 8 × 8 km2 grid remote-sensing data, Piao et al. (2006) demonstrated that senescence was delayed by 0·37 d year–1 over the 27 year period between 1982 and 2009 in deciduous forests in the northern hemisphere. Jeong et al. (2011) found evidence of advanced senescence in temperate North America using satellite data from 1982 to 2011. Several additional studies also show weak or insignificant relationships between the timing of autumn senescence and air temperatures (Menzel, 2003; Menzel et al., 2006; Pudas et al., 2008).
The lack of consistent relationships between air temperature and timing of leaf senescence suggests that autumn senescence may be influenced by a variety of factors that obscure its relationship with temperature. The date of first frost in the autumn months in temperate and boreal ecosystems is highly variable and does not always follow a linear cooling trend (Way and Montgomery, 2014). It is therefore likely that the timing of leaf senescence responds to a suite of environmental factors, although the genetic basis of senescence activity and physiology remains unknown (Tuskan et al., 2006; Way and Montgomery, 2014). For example, photoperiod (Hanninen and Tanino 2011; Jeong et al., 2011; Way, 2011) and local meteorological factors such as wind and humidity (Staelens et al., 2003; Travers and Eldridge, 2013) have all been shown to affect dates of senescence. The factors that most strongly influence the onset of senescence may also differ across ecosystems, with high-latitude plants considered to be more responsive to photoperiod and low-latitude plants more responsive to temperature (Stinziano and Way, 2014; Way and Montgomery, 2014). In addition to site-level meteorological factors, recent studies also demonstrate a relationship between the timing of spring leaf-out and the timing of autumn leaf senescence (Fu et al., 2014; Keenan and Richardson, 2015).
Changes in vegetation dynamics have major implications for the global carbon cycle, yet the current generation of terrestrial biosphere models often fail to simulate changes in autumn phenology accurately (Richardson et al., 2012). Many terrestrial biosphere models use temperature as a primary predictor of leaf senescence despite its inconsistency as a strong driver in observational studies (Menzel, 2002; Menzel et al., 2006; Richardson et al., 2013). For example, the Integrated Biosphere Simulator (IBIS) model induces leaf drop when mean daily air temperatures fall below 5 °C (Foley et al., 2010), while other models use combinations of both mean air temperature and threshold temperatures to predict leaf shedding (Estrella and Menzel, 2006). Growing degree-days or cooling degree-days are employed in some models, and provide an integrated measure of temperature history over a growing season (White and Nemani, 2003; Jolly et al., 2005; Richardson et al., 2012). Other models predict leaf drop through an integration of photoperiod and mean air temperature thresholds (BIOME-BGC; White et al., 1997), or photoperiod and cooling degree-day summation (Delpierre et al., 2009). The lack of consensus in phenology modelling schemes reflects the fact that the mechanisms that drive autumn phenology remain poorly understood (Vitasse et al., 2011).
Leaf senescence data are widely collected, although integrating these observations into an interpretable framework poses a challenge due to the variability in measurement methods and criteria used to determine the date of senescence. There is no consistent definition of leaf senescence, nor a standard methodology used for monitoring the process across studies (Gallinat et al. 2015). Some data networks standardize methodologies over specific regions or habitat types in an effort to synthesize senescence trends over time and space (e.g. Ibanez et al., 2010; Panchen et al., 2015). Despite these large-scale efforts, there are numerous individual studies that have examined controls on the timing of leaf senescence at local and regional scales that vary in their methodology. In many cases the definition of leaf senescence depends on the plant species examined, as some species’ life history traits inherently lend themselves to particular definitions. For instance, the genus Quercus typically holds senescing leaves longer than other genera, which may make the date of 50 % leaf colour change the best description of leaf senescence, while members of the genus Paulownia drop green leaves, making leaf drop a preferable metric to characterize the end of the growing season. Some studies integrate physiological measurements such as photosynthetic rate and chlorophyll content to delineate the date of senescence (Nagai et al., 2011). While the metrics chosen by individual research groups often optimize senescence estimates for a particular genus or location, the variation in methodology across ecosystems makes large-scale generalization challenging.
Here we synthesize publications from the peer-reviewed literature to evaluate larger scale trends in dates of leaf senescence across leaf senescence studies in the northern hemisphere. We present a meta-analysis to describe patterns of leaf senescence in boreal and temperate deciduous trees and examine relationships between dates of leaf senescence and meteorological measurements to assess physical drivers of leaf senescence. The primary goals of this synthesis were to: (1) characterize the methodologies employed in observations of autumn senescence in boreal and temperate deciduous trees in the northern hemisphere; (2) determine the influence of temperature, precipitation, latitude and photoperiod on the timing of leaf senescence in high- and low-latitude ecosystems; (3) identify how senescence trends differ among taxa and regions; and (4) provide recommendations for future research by identifying biologically relevant methods for monitoring leaf senescence and characterizing key areas of uncertainty in predicting senescence phenology.
MATERIALS AND METHODS
Data collection
We assembled a database of peer-reviewed publications using the Web of Science search engine. We used all combinations of the following search terms as ‘topics’: ‘autumn’ or ‘fall’; ‘phenology’ or ‘senescence’; ‘tree’ or ‘plant’ or ‘forest’; and ‘leaf’. We limited the search to English-language, peer-reviewed journal articles published before 25 February 2013. The search resulted in 760 publications, but we limited our subsequent analysis to all publications that report either (1) the date of tree leaf senescence in a given year (for one year or a series of years) or (2) a rate of change in tree leaf senescence dates over time or temperature for a reported measurement period. We limited the data set to publications that report estimates of autumn leaf senescence for ecosystems with one annual senescence period. To optimize data resolution, we included only those publications in which researchers monitored phenology more frequently than 14 day intervals throughout the senescence period. We included measurements from both young and mature trees and, in the cases of experimental studies, used data from reference treatments only. Within publications, we considered unique sites and plant species to represent independent data. We extracted data from tables and text when available and used DataThief (version 1.6; Tummers, 2006) to extract values presented in the figures.
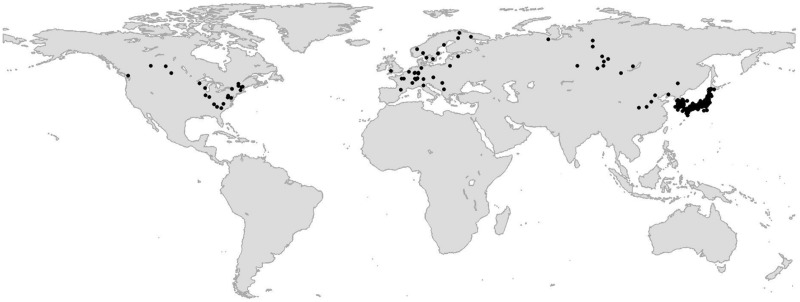
Studies were distributed throughout North and South America, Europe and Asia, with data concentrated in the eastern USA, Europe and Japan (Fig. 1). The distribution of studies was heavily skewed toward the northern hemisphere, with only two studies located in the southern hemisphere. Due to lack of sufficient replication in the southern hemisphere, we focus our analysis on senescence trends in the northern hemisphere alone. We identified 64 publications and 1121 independent measurements that met our criteria.
Fig. 1.
Distribution of sites used in the meta-analysis. Studies that do not report a specific site location or co-ordinates are not presented on the map.
We compiled data into two categories based on the response type: (1) date of senescence (DOS) and (2) change in senescence. The DOS data set includes 56 publications (Appendix 1), all of which report a date of leaf senescence for a single year or a single mean date of leaf senescence over multiple years. The change in senescence data set includes 21 publications (Appendix 2), all of which report a rate of change in senescence over time or temperature (d year–1 or d °C–1, respectively). The change in senescence data set includes five publications that did not self-report a rate of change for which we calculated the change in senescence over time from the dates of senescence for more than five continuous years (Appendix 2). Twelve of the 64 publications reported data that fell into both DOS and change in senescence categories.
Analyses
Date of senescence (DOS).
In order to make meaningful comparisons across studies reporting the date of leaf senescence and to assess autumn leaf senescence trends accurately on a large scale, we characterized the metrics used to measure the date of leaf senescence and identified the metrics most widely presented in the literature (Table 1). In some cases, publications reported DOS using multiple metrics (e.g. both 50 and 100 % leaf fall), and we report these to demonstrate the full spectrum of metrics used to estimate date of leaf senescence in the literature (Table 1). For cases in which the authors report a mean date of leaf senescence over a range of years, we used the median year of the measurement period to approximate leaf fall year. We assessed the relationship of colour change-based metrics and abscission-based metrics (leaf fall), the two main classes of senescence measurements, with site latitude.
Table 1.
Metrics used to estimate date of senescence across publications reporting the date of fall leaf senescence in the northern hemisphere (DOS studies only, n = 56 publications and 861 independent measurements total)
| Leaf senescence metric | No. of publications | Fraction of publications | No. of observations | Fraction of observations |
|---|---|---|---|---|
| 50 % leaf fall | 20 | 0·36 | 291 | 0·34 |
| 100 % leaf fall | 12 | 0·21 | 162 | 0·19 |
| NDVI | 6 | 0·11 | 83 | 0·1 |
| 80 % leaf fall | 4 | 0·07 | 62 | 0·07 |
| 50 % green | 3 | 0·05 | 3 | 0 |
| 50 % colour change | 2 | 0·04 | 7 | 0·01 |
| 0 % green | 2 | 0·04 | 2 | 0 |
| 50 % yellow | 2 | 0·04 | 3 | 0 |
| PAR transmission plateau | 2 | 0·04 | 66 | 0·08 |
| Peak colour change | 2 | 0·04 | 94 | 0·11 |
| Start of leaf fall | 2 | 0·04 | 68 | 0·08 |
| 10 % colour change in 10 % of trees | 1 | 0·02 | 8 | 0·01 |
| 10 % colour change in 90 % of trees | 1 | 0·02 | 8 | 0·01 |
| 10 % leaf fall | 1 | 0·02 | 48 | 0·06 |
| 50 % Amax | 1 | 0·02 | 6 | 0·01 |
| 50 % colour change or leaf fall | 1 | 0·02 | 103 | 0·12 |
| 75 % leaf fall | 1 | 0·02 | 9 | 0·01 |
| 90 % leaf fall | 1 | 0·02 | 9 | 0·01 |
| Eddy covariance | 1 | 0·02 | 2 | 0 |
| Landsat 50 % cover | 1 | 0·02 | 1 | 0 |
| More yellow than green leaves | 1 | 0·02 | 4 | 0 |
| Photo ‘greenness’ index | 1 | 0·02 | 1 | 0 |
| Photosynthetic rate = 0 | 1 | 0·02 | 16 | 0·02 |
| Peak red | 1 | 0·02 | 1 | 0 |
The number and total percentage of published studies and independent observations that used each metric are reported.
Note that many studies employed multiple metrics, therefore the number of entries in the table is greater than the total number of publications included in Appendix 1.
See Appendix 1 for list of publications included here.
LAI, leaf area index; PAR, photosnthetically acive radiation; NDVI, normalized difference vegetation index.
Meteorological data collection.
We examined relationships of air temperature, cooling degree-days (CDDs), precipitation, photoperiod and site latitude to the date of leaf senescence. In particular, we sought to determine the temporal window (e.g. 1 month or 3 months prior to leaf fall) over which meteorological data (i.e. air temperature and precipitation) were averaged that explained the greatest variation in leaf fall dates. Based on the latitude and longitude of the individual sites, we obtained daily meteorological data for each study location from either the National Climate Data Center’s (NCDC) Global Summary of the Day (GSOD) database (National Climate Data Center, 2013) or the Global Historical Climatology Network (GHCN) Daily database (Menne et al., 2012). We excluded observations that did not report location co-ordinates or a specific site location, and those which did not have station-based meteorological data reported within 2 ° latitude/longitude for the reported period. For each study site, we used the closest meteorological station (median distance 14 km) reporting daily minimum temperature, maximum temperature and precipitation with >70 % temporal coverage (22 days per month) for the years referenced in the corresponding study. Mean temperature was calculated by taking the mean of the daily minimum and maximum temperatures (World Meteorological Organization, 2011). At each site, we calculated the mean temperature (°C), latitude-based photoperiod (h) and total precipitation (mm) for three periods: 1–31 August, 1–30 September and 1–31 October. CDDs were calculated using mean daily temperature from 1 August to 31 October. CDDs represent the thermal sum below a base temperature of 20°C based on the method of Richardson et al. (2006).
We examined the relationships between meteorological variables and date of 50 % leaf fall, the most common metric employed across DOS studies (Table 1). We binned senescence observations by site latitude (low latitude, 25–49°N; high latitude, 50–70°N; roughly corresponding to the distribution of temperate and boreal forests, respectively) and regressed mean DOS at each site against mean August, September and October temperature (°C), cumulative CDDs (°C), photoperiod on the date of leaf senescence (h), site latitude and total monthly precipitation (mm) for August, September and October. In order to assess the relative effect of temperature and photoperiod on the date of senescence across high and low latitude sites, we then used partial correlation analysis to assess the relationship between the DOS and temperature (mean August, September, October and cumulative CDDs) while removing the effect of photoperiod.
Change in senescence.
While all studies completed their observation period between 1993 and 2010, the start of senescence observations varied widely across studies from 1931 to 2005 (Appendix 2). In order to evaluate the influence of when observations were initiated and the study duration on study-specific estimates of change in senescence over time or temperature, we binned studies by decades based on the start of the measurement period. We further binned studies by continent (North America, Europe and Asia), major plant order (all orders with more than five independent measurements) and site latitude (low latitude, 25–49°N; high latitude, 50–70°N) to evaluate the relationships between spatial and taxonomic factors and the date of senescence. We computed the mean change in the date of senescence over time (years) and temperature (°C) across all studies and used one-way analyses of variance (ANOVAs; P < 0·05) and Tukey’s tests of post-hoc intergroup comparison to assess differences in the change in senescence across continents, plant orders, site latitude range and time periods. All statistical analyses were conducted in R (version 3.1.1; R Development Core Team, 2013).
RESULTS
Date of senescence
Among DOS studies, we identified 24 distinct methods of estimating date of autumn leaf senescence across 56 publications (Table 1; Appendix 1). These publications utilized a variety of metrics, including leaf fall, leaf colour change and annual changes in plant physiology as measures of leaf senescence. Date of senescence was significantly earlier at higher latitudes, regardless of whether colour change or percentage leaf fall was used as a measure of date of senescence (P < 0·01 for both; Fig. 2). However, latitude explained more variation in the date of leaf senescence in measurements utilizing colour change (R2 = 0·55; e.g. 50 % colour change, 50 % green, peak colour change, more yellow than green leaves; Fig. 2A) compared to percentage leaf fall (R2 = 0·26; e.g. 10, 50, 80, 90 and 100 % leaf fall; Fig. 2B).
Fig. 2.
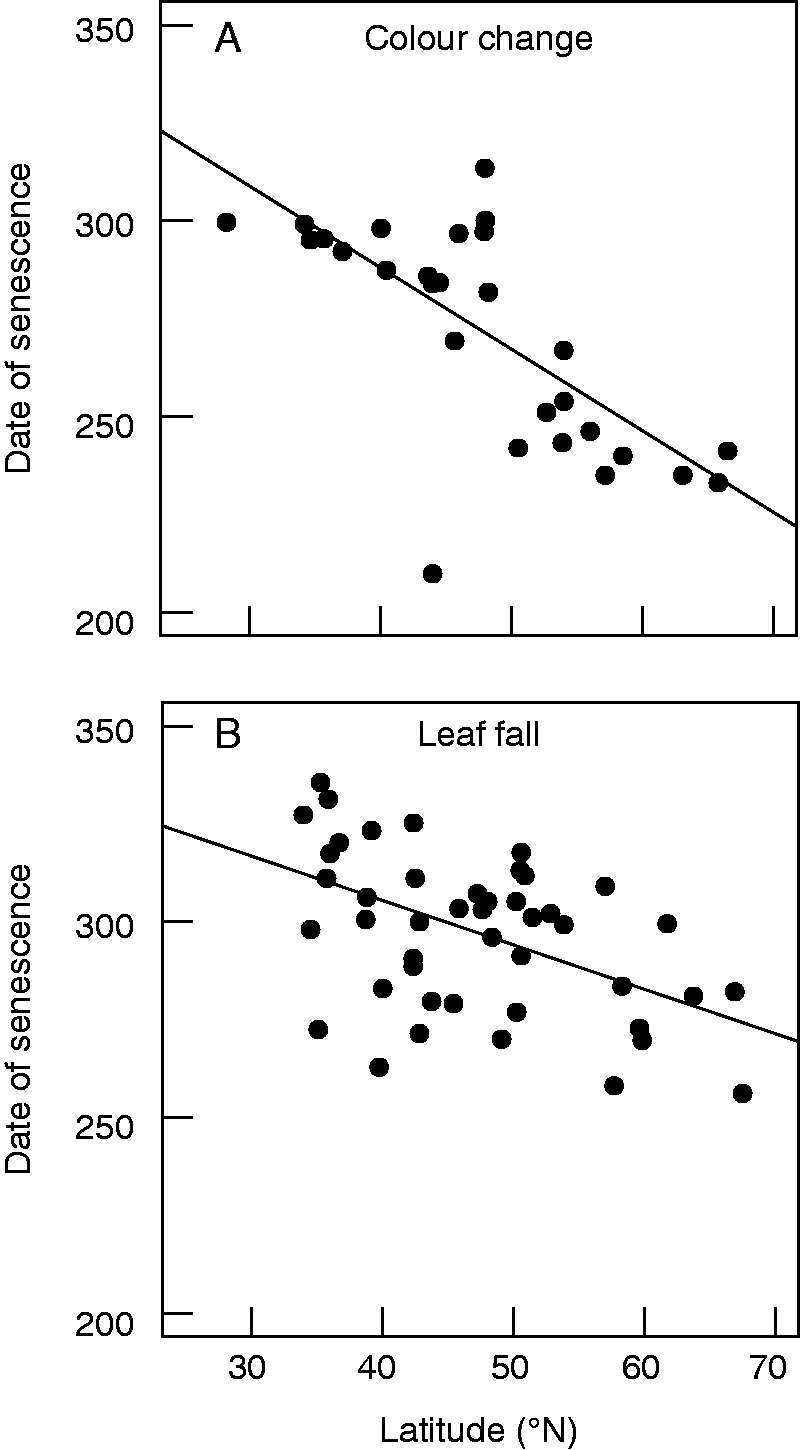
Relationship between latitude and the date of leaf senescence by studies that estimate date of senescence using (A) leaf colour change and (B) percentage leaf fall. Points represent all studies that estimate the date of leaf senescence using either method. Measurements using leaf colour change explain more variation in the relationship between latitude and the date of leaf senescence than measurements using percentage leaf fall (colour change, R2 = 0·55, P < 0·001; leaf fall, R2 = 0·26, P < 0·001).
We limited our analysis of the relationship between meteorological variables and the date of leaf senescence to studies reporting the date of 50 % leaf fall in order to compare a common metric of leaf senescence among published studies (20 publications). The date of 50 % leaf fall was the most common metric utilized and accounted for 36 % of publications and 34 % of individual observations of DOS. Thirteen of the other 24 methods used to characterize the date of leaf senescence were unique to a single study; it was therefore not feasible to examine effects of meteorological measurements across these studies together.
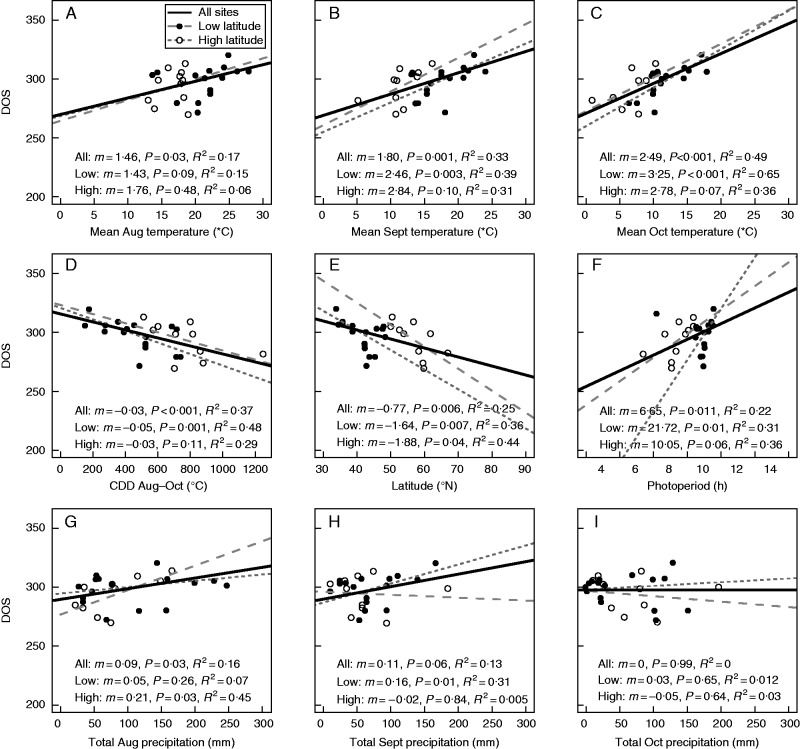
Simple linear regression models highlighted relationships between site-level meteorological data and the date of 50 % leaf fall across all sites examined in the northern hemisphere (Fig. 3). Compared with other meteorological variables, mean monthly temperature explained the most variation in the date of 50 % leaf fall, and the relationship strengthened as the temperature window examined shifted later in the year, with a delay of 2·49 ± 0·48 (mean ± s.e.) d °C–1 in mean October temperature (R2 = 0·49, P < 0·001; Fig. 3C). Sites with greater CCDs senesced earlier (R2 = 0·37, P < 0·001; Fig. 3D). Latitude and photoperiod on the date of 50 % leaf fall explained 25 and 22 % of the variation in the date of 50 % leaf fall, respectively (Fig. 3E, F). Greater precipitation in August through October weakly delayed or did not change leaf fall timing significantly, with precipitation alone explaining ≤16 % of the variability in the date of 50 % leaf fall (Fig. 3G–I).
Fig. 3.
Relationship between monthly averaged site temperature (°C), cooling degree-days (CDDs, °C), photoperiod (h) on date of senescence (DOS), site latitude, total monthly precipitation (mm) and the date of 50 % leaf fall. Points represent the average date of 50 % leaf fall by site. Low-latitude and high-latitude sites are as indicated in the key in (A).
The relationships between date of 50 % leaf fall and all measures of temperature (August, September, October mean temperature and cumulative CDDs) were weaker in high-latitude compared with low-latitude sites (Table 2; Fig. 3A–D). The relationship between date of 50 % leaf fall and each measure of temperature weakened (i.e. P-values increased) once the effect of photoperiod through partial correlation analysis was removed for high-latitude sites (CDD: R = –0·54, P = 0·068; Rpartial = –0·064, P = 0·87; mean October temperature: R = 0·59, P = 0·068; Rpartial = 0·25, P = 0·50; Table 2). In contrast, the relationship between each measure of temperature and date of 50 % leaf fall in low-latitude sites remained statistically significant when the effect of photoperiod was removed (CDD: R = –0·69, P = 0·001; Rpartial = –0·49, P = 0·02; mean October temperature: R = 0·81, P < 0·001; Rpartial = 0·73, P < 0·001; Table 2).
Table 2.
Partial correlation coefficients associated with simple linear regression models
| Parameter | Effect removed | Low latitude |
High latitude |
||
|---|---|---|---|---|---|
| R | P-value | R | P-value | ||
| Cooling degree-days (°C) | None | –0·69 | 0·001 | –0·54 | 0·068 |
| Photoperiod | –0·49 | 0·02 | –0·064 | 0·87 | |
| August temperature (°C) | None | 0·39 | 0·098 | 0·25 | 0·48 |
| Photoperiod | –0·31 | 0·19 | –0·072 | 0·85 | |
| September temperature (°C) | None | 0·63 | 0·4 | 0·55 | 0·097 |
| Photoperiod | 0·35 | 0·14 | 0·012 | 0·96 | |
| October temperature (°C) | None | 0·81 | <0·001 | 0·59 | 0·068 |
| Photoperiod | 0·73 | <0·001 | 0·25 | 0·5 | |
Change in senescence
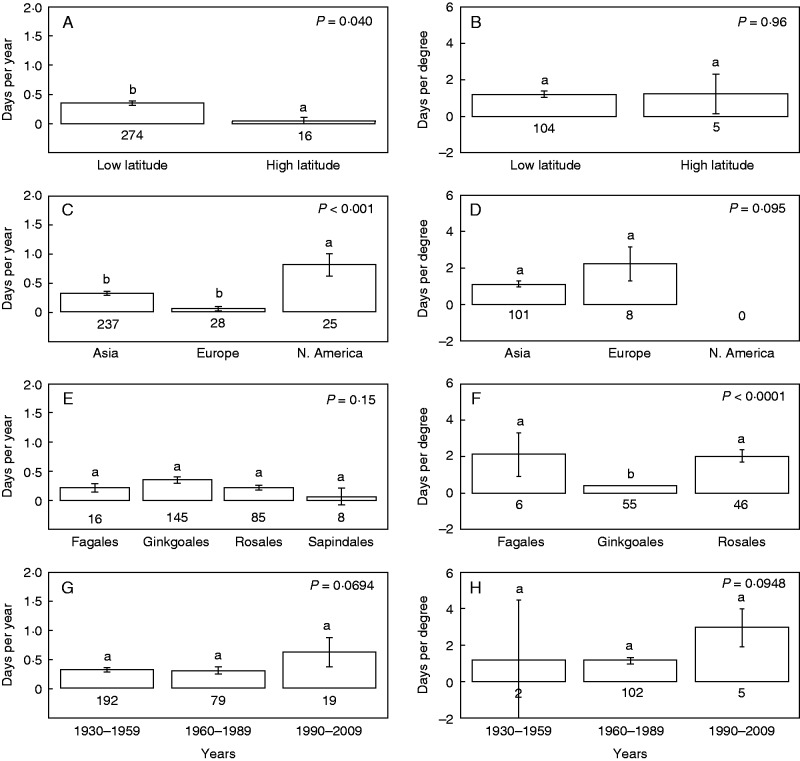
Six publications reported changes in autumn leaf senescence with temperature (109 independent observations) and 18 publications (291 independent observations) reported changes in autumn leaf senescence with time (Appendix 2). Examining all studies together, we found that leaf senescence was delayed by 0·33 ± 0·03 d year–1 and 1·20 ± 0·17 d °C–1 (mean ± 1 s.e.). Date of senescence did not vary over time at high-latitude sites, but was significantly more delayed at low-latitude sites over time (P = 0·040; Fig. 4A). The relationship between date of senescence and temperature did not differ significantly between high and low latitudes (P = 0·96; Fig 4B). In all sites (low- and high-latitude), date of senescence was delayed with increased temperatures (Fig. 4B). Senescence was delayed with time across all three northern hemisphere continents examined, but the delay was significantly larger with time in North America than in Europe and Asia (P <0·001; Fig. 4C). Both Europe and Asia showed delayed leaf senescence with increased temperatures (Fig. 4D), but there was not a statistically significant difference in response to temperature between continents (P = 0·095, Fig. 4B). Both Rosales and Fagales were significantly more delayed per °C than Ginkogales (P < 0·0001; Fig. 4F). The rate of senescence delay (d year–1 and d degree–1) was higher in studies initiated in recent decades (1990s–2000s) than those initiated in 1930–1980, although the difference was not statistically significant (Fig. 4G, P = 0·069; Fig. 4H, P = 0·095, respectively).
Fig. 4.
Change in date of senescence by latitude range, continent, plant order and decade. Shown are orders with more than five independent observations. Time periods in (G) and (H) show the decade in which senescence observations began, with all studies ending between 1993 and 2010. Values are means ± s.e.; different letters indicate statistically significant differences between groups. Values below the bars show the number of independent observations.
DISCUSSION
Drivers of autumn leaf senescence
Among the meteorological measurements examined, October temperatures were the strongest predictors of date of 50 % leaf fall, followed by CDDs, latitude, photoperiod and, lastly, total monthly precipitation, although the strength of the relationships differed between high- and low-latitude sites (Figs 3 and 4; Table 2). Temperature continued to explain significant variation in the date of 50 % leaf fall in low-latitude sites after the effect of photoperiod was removed, but did not explain significant variation in high-latitude leaf fall timing following removal of photoperiod from regression models (Table 2). These results suggest that while temperature alone may be a reasonable predictor of the date of 50 % leaf fall in general (mean October temperature: R2 = 0·49 across all sites), the factors that most strongly control leaf fall may differ by site location. The lack of change in date of 50 % leaf fall over time at high latitudes despite large changes in temperature (Fig. 4A; Jones et al., 2012) is possibly due to the strong constraint photoperiod imposes on the timing of leaf fall in these latitudes (Stinziano and Way, 2014; Way and Montgomery, 2014). Our results suggest that while temperature and photoperiod are two important variables that could be incorporated in models to predict the date of leaf senescence, it is unlikely that model parameters can be applied uniformly at the global scale. Temperature alone may provide a reasonable predictor for the date of 50 % leaf fall at low latitudes, but the inclusion of the effect of photoperiod in models may be important to assess the timing of leaf-off at high latitudes.
Compared with low-latitude ecosystems, high-latitude ecosystems may continue to experience the largest increases in temperature over the next century (Jones et al., 2012), while photoperiod will remain the same over time. These asynchronous changes in meteorological factors may affect plant communities and their role in carbon sequestration (Stinziano and Way, 2014). For example, as the ranges of more southerly plant species migrate north in response to rising temperatures (Beck et al., 2011), these temperature-responsive species may lengthen their photosynthetic period, while native, photoperiod-dependent northerly species may show less flexibility in the timing of leaf senescence, affecting competitive dynamics within plant communities (Way and Montgomery, 2014). The strong influence of photoperiod on leaf senescence at high latitudes could also influence ecosystem-scale carbon uptake. Photoperiod-induced leaf senescence will end an individual growing season’s period of ecosystem carbon uptake, but carbon losses through soil respiration may remain high with warmer temperatures (Piao et al., 2008). While warming-induced changes in leaf senescence delay the end of the growing season and result in enhanced net carbon uptake in temperate forests (Keenan et al., 2014), these patterns may not hold true in higher latitude boreal forests if trees do not extend their period of photosynthesis at the end of the growing season. In other words, the stronger constraint of photoperiod on date of leaf senescence in high-latitude boreal deciduous forests may limit the response of forest carbon uptake to increases in temperature.
There was a statistically significant delay in leaf senescence with time (days per year) across all three continents in the northern hemisphere, but delays were stronger in North America than in Europe and Asia. This pattern is similar to the results of Piao et al. (2007), who used large-scale remote-sensing techniques and quantified senescence delays of 0·28 d year–1 in North America but only 0·11 d year–1 in Eurasia over the last two decades. Some of the differences observed across continents may be due to differences in tree species composition and the number of species assessed. Differences in geographical distribution of studies within each continent may also explain some of the variation observed. For example, while European studies were distributed throughout the continent, studies tended to be concentrated in coastal regions in Asia and North America (Fig. 1). In addition, our analysis found that high-latitude regions have smaller changes in senescence over time than low-latitude regions (Fig. 4). As the majority of high-latitude studies included in this analysis were conducted in Europe, it is not surprising that we found larger delays in North America and Asia than in Europe. Unlike satellite-derived measurements of senescence that show an intensifying delay in senescence in recent decades (Jeong et al., 2011), we did not find a statistically significant change in the rate of delay over time (Fig. 4G).
Recommendations for future research
Looking forward, as the study of leaf senescence phenology garners additional research interest, one important take-away from this meta-analysis is the need to identify and use common, biologically relevant definitions of the date of leaf senescence. Among the publications we examined in our meta-analysis, date of senescence was estimated by a variety of metrics, including leaf fall, colour change and chlorophyll content, and was measured on a variety of scales, including leaf, branch, individual tree and total canopy. Fifty per cent leaf fall comprised over one-third of senescence measurements, and we show that it is a useful metric to compare date of senescence observations across multiple studies. Leaf fall is easily characterized in the field and contributes to measurements of forest productivity, making it a practical ecological measure. While 50 % leaf fall is the metric most commonly used across studies of fall leaf senescence, our results demonstrate that senescence dates estimated using leaf colour change are more strongly correlated with latitude than percentage leaf fall (Fig. 2). Because the senescence process is a combination of biodegradation and nutrient resorption, deciduous plants often show a large decline in photosynthetic activity long before they drop their leaves (Wilson et al., 2001). Additionally, species-specific relationships between the end of the photosynthetic period and date of leaf fall may vary, adding bias to patterns not accounted for in this analysis. Therefore, metrics involving leaf colour change may represent more biologically meaningful measures of the end of the growing season for deciduous plants compared with percentage leaf fall. We suggest that researchers consider the biological relevance of senescence metrics when designing future studies to allow for robust cross-study comparisons and enable understanding of large-scale trends.
Deciduous trees in the northern hemisphere are well represented by leaf senescence studies, especially in eastern North America, Europe and parts of Asia (Fig. 1). Additional studies in other regions in North America and the southern hemisphere are needed. This enlarged geographic range of studies would allow researchers to disentangle differences in the response of autumn leaf senescence to changes in temperature at finer resolution across space and deciduous forest biomes (temperate vs. boreal). Due to small sample sizes, we found it was not feasible to estimate senescence trends at the species level. We therefore focused on the level of plant order and found that plants in the order Rosales and Fagales respond to increases in air temperature with later dates of senescence compared with Ginkgoales.
Regional, national and global networks of on-the-ground and remotely sensed data provide important information about changes in autumn phenology, but there is an opportunity to augment these data through smaller scale, independent studies as well. In particular, we recommend that global change experiments that are currently underway to examine the effects of elevated CO2, warmer temperatures, drought, elevated tropospheric ozone or other global change factors, as well as multifactor investigations, include measurements of leaf senescence. Despite the noted underprediction of changes in spring leaf-out by experimental manipulations compared with observational studies (Wolkovich et al., 2012), ongoing or new experiments could provide additional critical information by disentangling the various environmental and physiological drivers of autumn phenology.
Conclusions
To our knowledge, this is the first study to synthesize existing senescence data derived from individual studies of deciduous trees throughout the northern hemisphere. Our findings demonstrate that across published studies, leaf senescence is delayed over time and in response to temperature, although low-latitude sites show significantly stronger delays in senescence over time than high-latitude sites. We find that while temperature alone may be a reasonable predictor of the date of leaf senescence when examining a broad suite of sites, it is important to consider that temperature-induced changes in senescence at high-latitude sites are likely to be constrained by the influence of photoperiod. Ecosystem-level differences in the mechanisms that control the timing of leaf senescence may affect both plant community interactions and ecosystem carbon storage as global temperatures increase over the next century.
ACKNOWLEDGEMENTS
We thank Richard Primack, Michael Hanley and two anonymous reviewers for helpful comments on earlier drafts of this manuscript. We thank Amanda Vieillard for help with the early stages of this work, and the Boston University Biogeoscience Program for providing a venue to bring our group together. This work was supported by awards from the United States Department of Energy Office of Science Graduate Fellowship Program administered by the Oak Ridge Institute for Science and Education (DE-AC05-06OR23100 to A.L.G.) and the United States National Science Foundation Graduate Fellowship Program (DGE-1247312 to A.J.R. and A.S.G.) and CAREER program (DEB 1149929 to P.H.T.).
Appendix 1 Publications included in date of senescence analysis
This table includes all publications that met search criteria for date of senescence studies and were included in the senescence metric tabulation in Table 1. Studies highlighted in bold used the most common senescence metric (50 % leaf fall) and were included in the regression and partial correlation analyses. (*) indicates a paper that reported a mean date of leaf senescence over a range of years, and (**) indicates that the range of years varied by site within the study.
| No. | Paper | Observations | Continent | Site | Latitude | Longitude | Biome | Order | Year range | Senescence metric |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Asshoff et al. (2006) | 9 | Europe | Swiss Canopy Crane Reserve | 47·46667 | 7·5 | Temperate | Fagales | 2002–2004 | 75 % LF |
| 2 | Augspurger et al. (2005) | 5 | North America | Trelease Woods, understorey | 40·15 | –88·16667 | Temperate | Sapindales | 1996–1998* | 0 % LAI |
| Trelease Woods, edge | ||||||||||
| Trelease Woods, canopy | Mixed | |||||||||
| 3 | Barr et al. (2004) | 16 | North America | Old Aspen Site | 53·7 | –106·2 | Boreal | Fagales | 1997–2003 | PAR transmission plateau, Px = 0 |
| Malpighiales | 1994, 1996–2003 | |||||||||
| 4 | Blanke and Kunz (2011) | 3 | Europe | Klein-Altendorf Research Centre | 50·62 | 6·988 | Temperate | Mixed | 1988–2010* | 50 % LF |
| 1958–2007* | ||||||||||
| 1958–1987* | ||||||||||
| 5 | Brown et al. (1997) | 4 | North American | Orland E. White Arboretum | 39·0638 | –78·0646 | Temperate | Fagales | 1994 | 50 % LF |
| 6 | Chen et al. (2005) | 6 | Asia | Beijing | 40·01667 | 116·3333 | Temperate | Mixed | 1982–1993* | NDVI |
| Gaixian | 40·4333 | 122·3333 | 1982–1993* | |||||||
| Harbin | 45·7 | 126·6667 | 1982–1993* | |||||||
| Luoyang | 34·6667 | 112·41667 | 1982–1993* | |||||||
| Mudanjiang | 44·4333 | 129·6667 | 1982–1993* | |||||||
| Xi'an | 34·21667 | 108·96667 | 1982–1993* | |||||||
| Xingtai | 37·0667 | 114·5 | 1982–1993* | |||||||
| 7 | Chmielewski and Rötzer (2001) | 11 | Europe | Dinaric Mountain Region | 42·9 | 20·3 | Temperate | Mixed | 1969–1984* | 50 % LF |
| Great Hungarian Lowlands | 46 | 19·6 | ||||||||
| North Alpine Foreland | 47·8 | 10·9 | ||||||||
| Southern Central European Highlands | 48·2 | 8·2 | ||||||||
| Bohemian Highlands | 48·6 | 15·5 | ||||||||
| Northern Central European Highlands | 50·4 | 8·7 | ||||||||
| British Isles | 51·6 | –3·8 | Temperate | |||||||
| North Sea/Central European Lowlands | 52·9 | 10·1 | Temperate | |||||||
| Baltic Sea Region | 57·2 | 15·2 | Temperate | |||||||
| North Atlantic Mountain Region | 61·9 | 8·1 | Boreal | |||||||
| North Scandinavia | 67·1 | 26·7 | Boreal | |||||||
| 8 | Chmielewski et al. (2005) | 3 | Europe | Saxony | NA | NA | Temperate | Sapindales | 1961–2000* | 50 % CC |
| Fagales | 1961–2000* | |||||||||
| 9 | Connor et al. (1994) | 6 | North America | Orland E. White Arboretum | 39·0638 | −78·0646 | Temperate | Fagales | 1989–1991 | 50 % LF |
| 10 | Delbart et al. (2005) | 10 | Asia | Turuhansk | 65·8 | 88 | Boreal | Fagales | 1999 | NDVI |
| Taseevo | 57·18333 | 94·833333 | Boreal | |||||||
| Vehme | 63·08333 | 88 | Boreal | |||||||
| Podtsesovo | 58·51666 | 92·216666 | Boreal | |||||||
| Aksarka | 66·5 | 67·816666 | Boreal | |||||||
| Ilir | 50·5 | 100·7 | Boreal | |||||||
| Kuragino | 53·9 | 92·7 | Boreal | |||||||
| Krasnoyarsk | 56 | 93 | Boreal | |||||||
| Tachtip | 52·733 | 90 | Boreal | |||||||
| Volchno | 54 | 81 | Boreal | |||||||
| 11 | Dillen et al. (2012) | 2 | North America | Harvard Forest | 42·533 | –72·183 | Temperate | Fagales | 2010 | 50 % LF |
| Fagales | ||||||||||
| 12 | Doi (2012) | 53 | Asia | Japan meteorological sites (53 sites) | NA | NA | Temperate | Rosales | 1953–2005 | 80 % LF |
| 13 | Doi and Takashi (2008) | 2 | Asia | Japan meteorological sites | 35·4 | 135·8 | Temperate | Ginkgoales | 1953–2005* | 80 % LF |
| Sapindales | ||||||||||
| 14 | Dragoni et al. (2011) | 10 | North America | Morgon Monroe State Forest | 39·317 | –86·417 | Temperate | Mixed | 1999–2008 | 0 % LAI |
| 15 | Dragoni and Rahman (2012) | 48 | North America | 28·25°N | 28·25 | NA | Temperate | Mixed | 1989–1999 | NDVI |
| 35·75°N | 35·75 | NA | ||||||||
| 48·25°N | 48·25 | NA | ||||||||
| 16 | Elmore et al. (2012) | 1 | North America | Cheasapeake Bay | 39·87 | –77·75 | Temperate | Mixed | 2004–2008* | Landsat 50 % cover |
| 17 | Fridley (2012) | 6 | North America | Syracuse, NY | 43·05 | –76·15 | Temperate | Mixed | 2008–2010 | 50 % LF, 90 % LF |
| 18 | Gibson et al. (2001) | 1 | North America | Clemson, SC | 34·68 | –82·84 | Temperate | Rosales | 1998 | 50 % LF |
| 19 | Gill et al. (1998) | 4 | North America | Hubbard Brook Experimental Forest | 43·94 | –71·72 | Temperate | Fagales | 1989 | 50 % LF |
| Lamiales | ||||||||||
| Sapindales | ||||||||||
| 20 | Glinwood and Pettersson (2000) | 1 | Uppsala | 59·816667 | 17·633333 | Temperate | Rosales | 1998 | 50 % LF | |
| 21 | Grassi and Magnani (2005) | 6 | Europe | Nonantola Experimental Site | 44·683 | 11·033 | Temperate | Lamiales | 2001–2003 | 50 % Amax |
| Fagales | ||||||||||
| 22 | Heide (2003) | 7 | Europe | Agricultural University of Norway | 60 | 10·783 | Boreal | Fagales | 1996–2002* | 50 % LF |
| 23 | Isaacson et al. (2012) | 8 | North America | Flambeau River State Forest | 45·733 | –90·75 | Temperate | Lamiales | 2009 | NDVI |
| Kettle Moraine State Forest | 43·598 | –88·280 | Sapindales | |||||||
| 24 | Juknys et al. (2011) | 92 | Europe | Vytautus Magnus University | 54·083 | 23·083 | Temperate | Malvales | 1956–60, 1968–1997, 2000–2010 | 50 % LF, 50 % CC |
| 25 | Kafaki et al. (2009) | 4 | Asia | Guilan Province | NA | NA | Temperate | Fagales | 2003–2006 | 100 % LF |
| 26 | Keskitalo (2005) | 1 | Europe | Umea University | 63·820539 | 20·303591 | Boreal | Malpighiales | 2003 | 100 % LF |
| 27 | Kodani et al. (2002) | Japan | Tohoku Research Center | 36·76667 | 141·13333 | Temperate | Fagales | 1999 | 0 % LAI | |
| 28 | Kozlov and Berlina (2002) | 60 | Europe | Lapland Biosphere Reserve | 67·65 | 32·61667 | Boreal | Mixed | 1931–1941, 1946–1952, 1957–1998 | Start of LF |
| 29 | Kramer (1995) | 9 | Europe | International Phenological Gardens | NA | NA | Temperate | Malpighiales | 1955–1987 | 50 % LF |
| Malvales | ||||||||||
| Pinales | ||||||||||
| Fagales | ||||||||||
| 30 | Laurila et al. (2001) | 2 | Europe | Petsikko | 69·47 | 27·23 | Boreal | Fagales | 1997–1998 | Eddy covariance |
| 31 | Lebourgeois et al. (2010) | 8 | Europe | 102 sites | NA | NA | Temperate | Pinales | 1997–2006 | 10 % CC in 10 % of trees, 10 % CC in 90 % of trees |
| Fagales | ||||||||||
| 32 | Lee et al. (2003) | 88 | North America | Harvard Forest | 42·53 | –72·18 | Temperate | Fagales | 1991–1999 | 50 % LF |
| Cornales | ||||||||||
| Lamiales | ||||||||||
| Fagales | ||||||||||
| Sapindales | ||||||||||
| Rosales | ||||||||||
| 33 | Nagai et al. (2011) | 9 | Asia | Takayama | 36·1466 | 137·423 | Temperate | Fagales | 2005–2007 | 0 % LAI |
| Sapindales | ||||||||||
| 34 | Nakamura et al. (2010) | 1 | Asia | Tomakomai Experimental Forest | 42·667 | 141·6 | Temperate | Fagales | 2008 | 80 % LF |
| 35 | Nicolai (2010) | 8 | Europe | Marburg, Hesse | 50·816667 | 8·6 | Temperate | Fabales | 2002–2009 | Start of LF |
| 36 | Niinemets and Tamm (2005) | 6 | Europe | Karkna | 58·46667 | 26·633333 | Temperate | Sapindales | 1997 | 50 % LF, 80 % LF |
| Fagales | ||||||||||
| Malpighiales | ||||||||||
| 37 | Norby et al. (2003) | 4 | North America | Oak Ridge | 35·9 | –84·333333 | Temperate | Sapindales | 1995–1996 | 50 % LF |
| 38 | Parker and Tibbs (2004) | 11 | North America | Anapolis, MD | 38·883333 | –76·55 | Temperate | Fagales | 1994 | 50 % LF |
| Cornales | ||||||||||
| Fagales | ||||||||||
| Saxifragales | ||||||||||
| Magnoliales | ||||||||||
| 39 | Pataki and Oren (2003) | 6 | North America | Duke Forest | 36·017 | –79·982 | Temperate | Fagales | 1997 | 50 % LF, 100 % LF |
| Saxifragales | ||||||||||
| Magnoliales | ||||||||||
| 40 | Pellis et al. (2004) | 50 | Europe | Antwerp | 51·083 | 4·366 | Temperate | Malpighiales | 1996–1998, 2001 | 100 % LF, plateau PAR ratio |
| 41 | Richardson et al. (2009) | 1 | North America | Bartlett Experimental Forest | 44·064 | –71·288 | Temperate | Mixed | 2007 | Photo ‘greenness’ index, peak red colour |
| 42 | Rolshausen and Schaefer (2007) | 2 | Europe | Freiberg | 48 | 8 | Temperate | Rosales | 2004 | Peak CC |
| 43 | Schaberg et al. (2003) | 1 | North America | USDA Forest Service Northern Research Station | 44·5 | –73·2 | Temperate | Sapindales | 1999 | 50 % green, 50 % yellow, 0 % green |
| 44 | Schreiber et al. (2013) | 4 | North America | Alberta-Pacific Forest Industries | – | –113 | Temperate | Malpighiales | 2010 | More yellow than green leaves |
| 45 | Sonnentag et al. (2012) | 1 | North America | Harvard Forest | 42·5 | –72·2 | Temperate | Mixed | 2010 | 100 % LF, 50 % green, 0 % green |
| 46 | Soolanayakana-hally et al. (2013) | 2 | North America | Vancouver | 49·26 | –123·25 | Temperate | Malpighiales | 2009 | 100 % LF, 50 % yellow |
| Indian Head, Saskatchewan | ||||||||||
| 47 | Soudani et al. (2012) | 9 | Europe | Fontainebleau | 48 | 2 | Temperate | Fagales | 2005–2009** | NDVI |
| Hesse | 48 | 7 | ||||||||
| Fougeres | 48 | 1 | ||||||||
| 48 | Tartachnyk and Blanke (2001) | 3 | Europe | Bonn | 50·733 | 7·0833 | Temperate | Rosales | 1997–1999 | 90 % LF |
| 49 | Tateno et al. (2005) | 48 | Asia | Ashui Forest Research Station | 35·3 | 135·72 | Temperate | Cornales | 2001 | 10 % LF |
| Ericales | ||||||||||
| Sapindales | ||||||||||
| Aquifoliales | ||||||||||
| Apiales | ||||||||||
| mixed | ||||||||||
| Fagales | ||||||||||
| Rosales | ||||||||||
| Lamiales | ||||||||||
| Magnoliales | ||||||||||
| Laurales | ||||||||||
| 50 | Uddling et al. (2005) | 2 | Europe | Ostad Field Station | 57·9 | 12·4 | Temperate | Fagales | 1997–1998 | 100 % LF |
| 51 | Vitasse et al. (2009) | 103 | Europe | Pyrenees | 46 | 6 | Temperate | Fagales | 2005–2007 | 50 % CC or LF |
| Lamiales | ||||||||||
| Sapindales | ||||||||||
| 52 | Waddell et al. (2001) | 3 | North America | Sesquicentennial State Park (high elevation) | 34·091 | –80·907 | Temperate | Fagales | 1994 | 50 % LF |
| Sesquicentennial State Park (low elevation) | 34·091 | –80·907 | ||||||||
| Clemson University | 34·132 | –80·869 | ||||||||
| 53 | Wang et al. (1992) | 23 | North America | Mont St. Hilaire Nursery | 45·533 | –73·133 | Temperate | Fagales | 1988–1989* | 50 % LF |
| Lamiales | ||||||||||
| Malvales | ||||||||||
| Rosales | ||||||||||
| Malpighiales | ||||||||||
| Sapindales | ||||||||||
| 54 | White et al. (1997) | 2 | North America | Hubbard Brook | 43·933 | –71·67 | Temperate | Mixed | 1991–1992 | NDVI |
| 55 | Zhang et al. (2011) | 4 | North America | Harvard Forest | 42·533 | –72·183 | Temperate | Mixed | 2001–2004 | 50 % LF, 50 % colour change |
| 56 | Zhao et al. (2006) | 71 | Asia | Henan Meteorological Bureau sites | NA | NA | Temperate | Malpighiales | 1986–2003 | 100 % LF |
| Fabales | ||||||||||
| Sapindales | ||||||||||
CC, colour change, LAI, leaf area index; LF, leaf fall; PAR, photosynthetically acive radiation; NA, not available; NDVI, normalized difference vegetation index.
Appendix 2 Publications included in change in senescence analysis
(*) indicates that the slope was calculated based on >5 years of DOS data reported in the study, and (**) indicates that the range of years for which data are reported is less than the 5 year threshold period, but the study reported senescence change in d °C–1 across an elevation gradient. All studies were included in the analysis regardless of senescence metric.
| No. | Paper | Observations | Continent | Biome | Order | Year range | Senescence metric | Regression type |
|---|---|---|---|---|---|---|---|---|
| 1 | Barr et al. (2004)* | 1 | North America | Boreal | Fagales | 1994–2003 | PAR transmission plateau | Time |
| 2 | Chen and Xu (2012) | 46 | Asia | Temperate | Rosales | 1986–2005 | “Almost all leaves fallen” | Time and temperature |
| 3 | Chen et al. (2005) | 5 | Asia | Temperate | Mixed | 1982–1993 | NDVI | Time |
| 4 | Chmielewski and Rötzer. (2005) | 11 | Europe | Temperate/boreal | Mixed | 1969–1998 | 50 % LF | Time |
| 5 | Chmielewski et al. (2005) | 3 | Europe | Temperate | Sapindales | 1961–2000 | 50 % CC | Time |
| Fagales | ||||||||
| 6 | Dragoni et al. (2011) | 3 | North America | Temperate | Mixed | 1998–2008 | 0 % LAI | Time |
| 7 | Dragoni and Rahman (2012) | 9 | North America | Temperate | Mixed | 1989–2008 | NDVI | Time |
| 8 | Ibanez et al. (2010) | 183 | Asia | Temperate | Ginkgoales | 1953–2005 | 80 % LF | Time |
| Rosales | ||||||||
| 9 | Juknys et al. (2012a) | 3 | Europe | Temperate | Sapindales | 1956–2010 | 50 % LF | Time |
| Fagales | ||||||||
| Malvales | ||||||||
| 10 | Juknys et al. (2012b) | 2 | Europe | Temperate | Fagales | 1956–2010 | 50 % LF | Temperature |
| Malvales | ||||||||
| 11 | Kozlov and Berlina (2002)* | 1 | Europe | Boreal | mixed | 1931–1941, 1946–1952, 1957–1998 | ‘Begininning of leaf fall’ | Time |
| 12 | Kunz and Blanke (2011) | 1 | Europe | Temperate | Rosales | 1958–2008 | 50 % LF | Time |
| 13 | Lee et al. (2003)* | 11 | North America | Temperate | Rosales | 1991–1998 | 50 % LF | Time |
| Sapindales | ||||||||
| Lamiales | ||||||||
| Fagales | ||||||||
| 14 | Matsumoto (2010) | 55 | Asia | Temperate | Ginkgoales | 1961–2000 | 80 % LF | Temperature |
| 15 | Menzel et al. (2001) | 4 | Europe | Temperate | Sapindales | 1951–1996 | Start of CC | Time |
| Fagales | ||||||||
| 16 | Menzel et al. (2006) | 1 | Europe | Temperate | mixed | 1971–2000 | 50 % CC | Time and temperature |
| 17 | Nicolai (2010)* | 1 | Europe | Temperate | Fabales | 2002–2009 | Start of LF | Time |
| 18 | Pudas et al. (2008) | 3 | Europe | Boreal | Fagales | 1997–2006 | 50 % colour change | Time and temperature |
| 19 | Richardson et al. (2006) | 1 | North America | Temperate | Sapindales | 1957–2004 | Photo greenness index | Time |
| 20 | Vitasse et al. (2009) | 2 | Europe | Temperate | Fagales | 2005–2007** | 50 % CC or LF | Temperature |
| 21 | Zhao et al. (2006)* | 4 | Europe | Temperate | Sapindales | 1986–2003 | 100% LF | Time |
| Malpighiales | ||||||||
| Fabales |
LAI, leaf area index; LF, leaf fall; PAR, photosnthetically acive radiation; NDVI, normalized difference vegetation index.
LITERATURE CITED
- Asshoff R, Zotz G, Korner C. 2006. Growth and phenology of mature temperate forest trees in elevated CO2. Global Change Biology 12: 848–861. [Google Scholar]
- Augspurger CK, Cheeseman JM, Salk CF. 2005. Light gains and physiological capacity of understorey woody plants during phenological avoidance of canopy shade. Functional Ecology 19: 537–546. [Google Scholar]
- Barichivich J, Briffa KR, Osborn TJ, Melvin TM, Caesar J. 2012. Thermal growing season and timing of biospheric carbon uptake across the Northern Hemisphere. Global Biogeochemical Cycles 26: GB4015. [Google Scholar]
- Barr AG, Black TA, Hogg EH, Kljun N, Morgenstern K, Nesic Z. 2004. Inter-annual variability in the leaf area index of a boreal aspen–hazelnut forest in relation to net ecosystem production. Agricultural and Forest Meteorology 126: 237–255. [Google Scholar]
- Beck PS, Juday GP, Alix C, et al. 2011. Changes in forest productivity across Alaska consistent with biome shift. Ecology Letters 14: 373–379 [DOI] [PubMed] [Google Scholar]
- Blanke MM, Kunz A. 2011. Effects of climate change on pome fruit phenology and precipitation. Acta Horticulturae 922: 381–386. [Google Scholar]
- Brown JL, Vargo S, Connor EF, Nuckols MS. 1997. Causes of vertical stratification in the density of Cameraria hamadryadella. Ecological Entomology 22: 16–25. [Google Scholar]
- Chen X, Xu L. 2012. Phenological responses of Ulmus pumila (Siberian Elm) to climate change in the temperate zone of China. International Journal of Biometeorology 56: 695–706. [DOI] [PubMed] [Google Scholar]
- Chen X, Hu B, Yu R. 2005. Spatial and temporal variation of phenological growing season and climate change impacts in temperate eastern China. Global Change Biology 11: 1118–1130. [Google Scholar]
- Chmielewski F, Rötzer T. 2001. Response of tree phenology to climate change across Europe. Agricultural and Forest Meteorology 108: 101–112. [Google Scholar]
- Chmielewski F, Muller A, Kuchler W. 2005. Possible impacts of climate change on natural vegetation in Saxony (Germany). International Journal of Biometeorology 50: 96–104. [DOI] [PubMed] [Google Scholar]
- Connor EF, Adams-Manson RH, Carr TG, Beck MW. 1994. The effects of host plant phenology on the demography and population dynamics of the leaf-mining moth. Ecological Entomology 19: 111–120. [Google Scholar]
- Delbart N, Kergoat L, Le Toan T, Lhermitte J, Picard G. 2005. Determination of phenological dates in boreal regions using normalized difference water index. Remote Sensing of Environment 97: 26–38. [Google Scholar]
- Delpierre N, Dufrene E, Soudani K, et al. 2009. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agricultural and Forest Meteorology 149: 938–948. [Google Scholar]
- Dillen SY, de Beeck MO, Hufkens K, Buonanduci M, Phillips NG. 2012. Seasonal patterns of foliar reflectance in relation to photosynthetic capacity and color index in two co-occurring tree species, Quercus rubra and Betula papyrifera. Agricultural and Forest Meteorology 160: 60–68. [Google Scholar]
- Doi H. 2012. Response of the Morus bombycis growing season to temperature and its latitudinal pattern in Japan. International Journal of Biometeorology 56: 895–902. [DOI] [PubMed] [Google Scholar]
- Doi H, Takashi M. 2008. Latitudinal patterns in the phenological responses of leaf colouring and leaf fall to climate change in Japan. Global Ecology and Biogeography 17: 556–561. [Google Scholar]
- Dragoni D, Rahman AF. 2012. Trends in fall phenology across the deciduous forests of the Eastern USA. Agricultural and Forest Meteorology 157: 96–105. [Google Scholar]
- Dragoni D, Schmid HP, Wayson CA, Potter H, Grimmond C, Randolph JC. 2011. Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south-central Indiana, USA. Global Change Biology 17: 886–897. [Google Scholar]
- Elmore AJ, Guinn SM, Minsley BJ, Richardson AD. 2012. Landscape controls on the timing of spring, autumn, and growing season length in mid-Atlantic forests. Global Change Biology 18: 656–674. [Google Scholar]
- Estrella N, Menzel A. 2006. Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Climate Research 32: 253–267. [Google Scholar]
- Foley JA, Prentice IC, Ramankutty N, et al. 2010. An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Global Biogeochemical Cycles 10: 603–628. [Google Scholar]
- Fridley JD. 2012. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485: 359–362. [DOI] [PubMed] [Google Scholar]
- Fu YSH, Campioli M, Vitasse Y, et al. 2014. Variation in leaf flushing date influences autumn senescence and next year’s flushing date in two temperate tree species. Proceedings of the National Academy of Sciences, USA 111: 7344–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat AS, Primack RB, Wagner DL. 2015. Autumn, the neglected season in climate change research. Trends in Ecology and Evolution 30: 169–176. [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hernández A, Olano JM, Becerril JM. 2003. The operation of the lutein epoxide cycle correlates with energy dissipation. Functional Plant Biology 30: 319–324. [DOI] [PubMed] [Google Scholar]
- Ge Q, Wang H, Rutishauser T, Dai J. 2014. Phenological response to climate change in China: a meta-analysis. Global Change Biology 21: 1–10. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Reighard PG, Scott SW, Oullette DR. 2001. Using graft transmissible agents in Y-trained peach systems. Acta Horticulturae 557: 139–144. [Google Scholar]
- Gill DS, Amthor JS, Bormann FH. 1998. Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiology 18: 281–289. [DOI] [PubMed] [Google Scholar]
- Glinwood R, Pettersson J. 2000. Movement by mating females of a host alternating aphid: a response to leaf fall. Oikos 90: 43–49. [Google Scholar]
- Grassi G, Magnani F. 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell and Environment 28: 834–849. [Google Scholar]
- Hänninen H, Tanino K. 2011. Tree seasonality in a warming climate. Trends in Plant Science 16: 412–416. [DOI] [PubMed] [Google Scholar]
- Heide OM. 2003. High autumn temperature delays spring bud burst in boreal trees, counterbalancing the effect of climatic warming. Tree Physiology 23: 931–936. [DOI] [PubMed] [Google Scholar]
- Ibanez I, Primack RB, Miller-Rushing AJ, et al. 2010. Forecasting phenology under global warming. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3247–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson BN, Serbin SP, Townsend PA. 2012. Detection of relative differences in phenology of forest species using Landsat and MODIS. Landscape Ecology 27: 529–543. [Google Scholar]
- Jeong S-J, Ho C-H, Gim H-J, Brown ME. 2011. Phenology shifts at start vs. end of growing season in temperate vegetation over Northern Hemisphere for the period of 1982–2008. Global Change Biology 17: 2385–2399. [Google Scholar]
- Jolly WM, Nemani R, Running SW. 2005. A generalized, bioclimatic index to predict foliar phenology in response to climate. Global Change Biology 11: 619–632. [Google Scholar]
- Jones PD, Lister DH, Osborn TJ, Harpham C, Salmon M, Morice CP. 2012. Hemispheric and large-scale land-surface air temperature variations: an extensive revision and an update to 2010. Journal of Geophysical Research 117: D05127. [Google Scholar]
- Juknys R, Sujetoviene G, Zeimavicius K, Gustainyte J. 2011. Effects of climate warming on timing of lime (Tilia cordata L.) phenology. Environmental Engineering 1: 139–143. [Google Scholar]
- Juknys R, Sujetoviene G, Zeimavicius K, Sveikauskaite I. 2012a. Comparison of climate warming induced changes in silver birch (Betula pendula Roth) and lime (Tilia cordata MIll.) phenology. Baltic Forestry 18: 25–32. [Google Scholar]
- Juknys R, Zeimavicius K, Sujetoviene G, Gustainyte J. 2012b. Response of tree seasonal development to climate warming. Polish Journal of Environmental Studies 21: 107–113. [Google Scholar]
- Kafaki SB, Mataji A, Hashemi SA. 2009. Hornbeam trees phenological characteristics of mountain forest by using ground observation and satellite data in Iran. Environmental Science and Sustainability: 121–127. [Google Scholar]
- Keenan TF, Gray J, Friedl MA, et al. 2014. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nature Climate Change 4: 598–604. [Google Scholar]
- Keenan TF, Richardson AD. 2015. The timing of autumn senescence is affected by the time of spring phenology: implications for predictive models. Global Change Biology (in press). [DOI] [PubMed] [Google Scholar]
- Keskitalo J. 2005. A cellular timetable of autumn senescence. Plant Physiology 139: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani E, Awaya Y, Tanaka K, Matsumura N. 2002. Seasonal patterns of canopy structure, biochemistry and spectral reflectance in a broad-leaved deciduous Fagus crenata canopy. Forest Ecology and Management 167: 233–249. [Google Scholar]
- Kozlov MV, Berlina NG. 2002. Decline in length of the summer season on the Kola Peninsula, Russia. Climatic Change 54: 387–398. [Google Scholar]
- Kramer K. 1995. Phenotypic plasticity of the phenology of seven European tree species in relation to climatic warming. Plant, Cell and Environment 18: 93–104. [Google Scholar]
- Kunz A, Blanke MM. 2011. Effects of global climate change on apple ‘Golden Delicous’ phenology – based on 50 years of meteorological and phenological data in Klein-Altendorf. Acta Horticulturae 903: 1121–1126. [Google Scholar]
- Laurila T, Soegaard H, Lloyd CR, Aurela M, Tuovinen JP, Nordstroem C. 2001. Seasonal variations of net CO2 exchange in European Arctic ecosystems. Theoretical and Applied Climatology 70: 183–201. [Google Scholar]
- Lebourgeois F, Pierrat J-C, Perez V, Piedallu C, Cecchini S, Ulrich E. 2010. Simulating phenological shifts in French temperate forests under two climatic change scenarios and four driving global circulation models. International Journal of Biometeorology 54: 563–581. [DOI] [PubMed] [Google Scholar]
- Lee DW, O’Keefe J, Holbrook NM, Feild TS. 2003. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Applications 18: 677–694. [Google Scholar]
- Linderholm HW. 2006. Growing season changes in the last century. Agricultural and Forest Meteorology 137: 1–14. [Google Scholar]
- Matsumoto K. 2010. Causal factors for spatial variation in long-term phenological trends in Ginkgo biloba L. in Japan. International Journal of Climatology 2010: 1280–1288. [Google Scholar]
- Menne MJ, Durre I, Vose RS, Gleason BE, Houston TG. 2012. An overview of the global historical climatology network-daily database. Journal of Atmospheric and Oceanic Technology 29: 897–910. [Google Scholar]
- Menzel A. 2002. Phenology: its importance to the global change community. Climatic Change 54: 379–385. [Google Scholar]
- Menzel A. 2003. Plant phenological anomalies in Germany and their relation to air temperature and NAO. Climatic Change 57: 243–263. [Google Scholar]
- Menzel A, Estrella N, Fabian P. 2001. Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Global Change Biology 7: 657–666. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. 1997. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386: 698–702. [Google Scholar]
- Nagai S, Maeda T, Gamo M, Muraoka H, Suzuki R, Nasahara KN. 2011. Using digital camera images to detect canopy condition of deciduous broad-leaved trees. Plant Ecology and Diversity 4: 79–89. [Google Scholar]
- Nakamura M, Muller O, Tayanagi S, Nakaji T, Hiura T. 2010. Experimental branch warming alters tall tree leaf phenology and acorn production. Agricultural and Forest Meteorology 150: 1026–1029. [Google Scholar]
- National Climatic Data Center. 2013. Global surface summary of day, 2013. (ftp://ftp.ncdc.noaa.gov/pub/data/gsod). Asheville, NC. [Google Scholar]
- Nicolai V. 2010. Plasticity in voltinism of the successful European Neozoon Phyllonorycter robiniella (Lepidoptera: Gracillariidae). Entomologia Generalis 32: 301–309. [Google Scholar]
- Niinemets U, Tamm U. 2005. Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiology 25: 1001–1014. [DOI] [PubMed] [Google Scholar]
- Norby RJ, Hartz-Rubin JS, Verbrugge MJ. 2003. Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Global Change Biology 9: 1792–1801. [Google Scholar]
- Panchen Z, Primack RB, Gallinat A, et al. 2015. Substantial variation in leaf senescence times among 1360 temperate woody plant species: implications for phenology and ecosystem processes. Annals of Botany (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GG, Tibbs DJ. 2004. Structural phenology of the leaf community in the canopy of a Liriodendron tulipifera L. forest in Maryland, USA. Forest Ecology and Management 50: 387–397. [Google Scholar]
- Pataki DE, Oren R. 2003. Species differences in stomatal control of water loss at the canopy scale in a mature bottomland deciduous forest. Advances in Water Resources 26: 1267–1278. [Google Scholar]
- Pellis A, Laureysens I, Ceulemans R. 2004. Genetic variation of the bud and leaf phenology of seventeen poplar clones in a short rotation coppice culture. Plant Biology 6: 38–46. [DOI] [PubMed] [Google Scholar]
- Penuelas J, Rutishauser T, Filella Y. 2009. Phenology feedbacks on climate change. Science 324: 887–888. [DOI] [PubMed] [Google Scholar]
- Piao S, Fang J, Zhou L, Ciais P, Zhu B. 2006. Variations in satellite-derived phenology in China’s temperate vegetation. Global Change Biology 12: 672–685. [Google Scholar]
- Piao S, Friedlingstein P, Ciais P, Viovy N, Demarty J. 2007. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Global Biogeochemical Cycles 21: GB3018. [Google Scholar]
- Piao S, Ciais P, Friedlingstein P, et al. 2008. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451: 49–52. [DOI] [PubMed] [Google Scholar]
- Polgar CA, Primack RB. 2011. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist 191: 926–941. [DOI] [PubMed] [Google Scholar]
- Pudas E, Leppälä M, Tolvanen A, Poikolainen J, Venäläinen A, Kubin E. 2008. Trends in phenology of Betula pubescens across the boreal zone in Finland. International Journal of Biometeorology 52: 251–259. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org. [Google Scholar]
- Richardson AD, Bailey AS, Denny EG, Martin CW, O’Keefe J. 2006. Phenology of a northern hardwood forest canopy. Global Change Biology 12: 1174–1188. [Google Scholar]
- Richardson AD, Braswell BH, Hollinger DY, Jenkins JP, Ollinger SV. 2009. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecological Applications 19: 1417–1428. [DOI] [PubMed] [Google Scholar]
- Richardson AD, Black AT, Ciais P, et al. 2010. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 3227–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Anderson RS, Altaf Arain M, et al. 2012. Terrestrial biosphere models need better representation of vegetation phenology: results from the North American Carbon Program Site Synthesis. Global Change Biology 18: 566–584. [Google Scholar]
- Richardson AD, Keenan TF, Migliavacca M, Ryu Y, Sonnentag O, Toomey M. 2013. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agricultural and Forest Meteorology 169: 156–173. [Google Scholar]
- Rolshausen G, Schaefer HM. 2007. Do aphids paint the tree red (or yellow) – can herbivore resistance or photoprotection explain colourful leaves in autumn? Plant Ecology 191: 77–84. [Google Scholar]
- Schaberg PG, van den Berg AK, Murakami PF, Shane JB, Donnelly JR. 2003. Factors influencing red expression in autumn foliage of sugar maple. Tree Physiology 23: 325–333. [DOI] [PubMed] [Google Scholar]
- Schreiber SG, Hamann A, Hacke UG, Thomas BR. 2013. Sixteen years of winter stress: an assessment of cold hardiness, growth performance and survival of hybrid poplar clones at a boreal planting site. Plant, Cell and Environment 36: 419–428. [DOI] [PubMed] [Google Scholar]
- Sonnentag O, Hufkens K, Teshera-Sterne C, et al. 2012. Digital repeat photography for phenological research in forest ecosystems. Agricultural and Forest Meteorology 152: 159–177. [Google Scholar]
- Soolanayakanahally RY, Guy RD, Silim SN, Song M. 2013. Timing of photoperiodic competency causes phenological mismatch in balsam poplar (Populus balsamifera L.). Plant, Cell and Environment 36: 116–127. [DOI] [PubMed] [Google Scholar]
- Soudani K, Hmimina G, Delpierre N, et al. 2012. Ground-based network of NDVI measurements for tracking temporal dynamics of canopy structure and vegetation phenology in different biomes. Remote Sensing of Environment 123: 234–245. [Google Scholar]
- Staelens J, Nachtergale L, Luyssaert S, Lust N. 2003. A model of wind-influenced leaf litterfall in a mixed hardwood forest. Canadian Journal of Forest Research 33: 201–209. [Google Scholar]
- Stinziano JR, Way DA. 2014. Combined effects of rising [CO2] and temperature on boreal forests: growth, physiology, and limitations. Botany 92: 425–436. [Google Scholar]
- Tartachnyk I, Blanke MM. 2001. Environmental effects on apple tree physiology. Acta Horticulturae 557: 465–472. [Google Scholar]
- Tateno R, Aikawa T, Takeda H. 2005. Leaf-fall phenology along a topography-mediated environmental gradient in a cool-temperate deciduous broad-leaved forest in Japan. Journal of Forest Research 10: 269–274. [Google Scholar]
- Travers SK, Eldridge DJ. 2013. Increased rainfall frequency triggers an increase in litter fall rates of reproductive structures in an arid eucalypt woodland. Austral Ecology 38: 820–830. [Google Scholar]
- Tummers D. 2005. DataThief III. http://datathief.org/ [Google Scholar]
- Tuskan GA, DiFazio S, Jansson S, et al. 2006. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Uddling J, Karlsson PE, Glorvigen A, Sellden G. 2005. Ozone impairs autumnal resorption of nitrogen from birch (Betula pendula) leaves, causing an increase in whole-tree nitrogen loss through litter fall. Tree Physiology 26: 113–120. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Potre AJ, Kremer A, Michalet R, Delzon S. 2009. Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia 161: 187–198. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, François C, Delpierre N, et al. 2011. Assessing the effects of climate change on the phenology of European temperate trees. Agricultural and Forest Meteorology 151: 969–980. [Google Scholar]
- Waddell KJ, Fox CW, White KD, Mousseau TA. 2001. Leaf abscission phenology of a scrub oak: consequences for growth and survivorship of a leaf mining beetle. Oecologia 127: 251–258. [DOI] [PubMed] [Google Scholar]
- Wang J, Ives NE, Lechowicz MJ. 1992. The relation of foliar phenology to xylem embolism in trees. Functional Ecology 6: 469–475. [Google Scholar]
- Way DA. 2011. Tree phenology responses to warming: spring forward, fall back? Tree Physiology 31: 469–471. [DOI] [PubMed] [Google Scholar]
- Way DA, Montgomery RA. 2014. Photoperiod constraints on tree phenology, performance and migration in a warming world.Plant, Cell and Environment (in press). [DOI] [PubMed] [Google Scholar]
- White MA, Nemani RR. 2003. Canopy duration has little influence on annual carbon storage in the deciduous broad leaf forest. Global Change Biology 9: 967–972. [Google Scholar]
- White MA, Thornton PE, Running SW. 1997. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Global Biogeochemical Cycles 11: 217–234. [Google Scholar]
- Wilson KB, Baldocchi D, Hanson PJ. 2001. Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant, Cell and Environment 24: 571–583. [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485: 494–497. [DOI] [PubMed] [Google Scholar]
- World Meteorological Organization. 2011. Guide to climatological practices. Geneva, Switzerland: WMO-No. 100.2011 edition. [Google Scholar]
- Zhang X, Goldberg MD. 2011. Monitoring fall foliage coloration dynamics using time-series satellite data. Remote Sensing of Environment 115: 382–391. [Google Scholar]
- Zhao G, Zheng Y, Liu J, Zhang X, Chen H, Wang J. 2006. The relation investigatino on climate change and woody plant phenophase in Zhengzhou City of China In: Gao G, Ustin SL, eds. Remote sensing and modeling of ecosystems for sustainability III 6298: 629822. [Google Scholar]