Abstract
Background and Aims Glacier foreland plants are highly threatened by global warming. Regeneration from seeds on deglaciated terrain will be crucial for successful migration and survival of these species, and hence a better understanding of the impacts of climate change on seedling recruitment is urgently needed to predict future plant persistence in these environments. This study presents the first field evidence of the impact of climate change on recruitment success of glacier foreland plants.
Methods Seeds of eight foreland species were sown on a foreland site at 2500 m a.s.l., and at a site 400 m lower in altitude to simulate a 2·7 °C increase in mean annual temperature. Soil from the site of origin was used to reproduce the natural germination substrate. Recruitment success, temperature and water potential were monitored for 2 years. The response of seed germination to warming was further investigated in the laboratory.
Key Results At the glacier foreland site, seedling emergence was low (0 to approx. 40 %) and occurred in summer in all species after seeds had experienced autumn and winter seasons. However, at the warmer site there was a shift from summer to autumn emergence in two species and a significant increase of summer emergence (13–35 % higher) in all species except two. Survival and establishment was possible for 60–75 % of autumn-emerged seedlings and was generally greater under warmer conditions. Early snowmelt in spring caused the main ecological factors enhancing the recruitment success.
Conclusions The results suggest that warming will influence the recruitment of glacier foreland species primarily via the extension of the snow-free period in spring, which increases seedling establishment and results in a greater resistance to summer drought and winter extremes. The changes in recruitment success observed here imply that range shifts or changes in abundance are possible in a future warmer climate, but overall success may be dependent on interactions with shifts in other components of the plant community.
Keywords: Adaptation, alpine plants, climate change, glacier foreland plants, global warming, seed germination, seedling recruitment, seedling survival
INTRODUCTION
Among the world’s ecosystems, mountains and their biota are considered highly threatened by climate change because they are predicted to experience above-average warming (Diaz and Bradley, 1997; Beniston, 2003) and because they are characterized by species adapted to low temperatures (Körner, 2003). In many parts of the European Alps, for example, temperatures have risen by up to 2 °C between 1901 and 2000 (Beniston et al., 1997) which is well above the observed global increase in temperatures of 0·7 °C (Jones and Moberg, 2003). Warming is expected to continue for global mountain systems as a whole between 3·2 and 2·1 °C for 2055 (Nogués-Bravo et al., 2007).
The ability of species to respond to these changes will depend largely on their ability to colonize new territory, or to modify their physiology and seasonal behaviour to adapt to the changed conditions (Thuiller et al., 2005). In this regard, seeds are thought to be the main vehicle for plant migration in alpine ecosystems (Parolo and Rossi, 2008; Vittoz et al., 2009), where the movement of species to higher elevations is clearly one of the responses to climate warming (Pauli et al., 2012). Additionally, the substantial variation in both genetic and phenotypic plasticity for seed dormancy and germination may help to adapt species to future climates (Walck et al., 2011). In this latter case, persistence within tolerance ranges will play a crucial role for species unable to migrate fast enough to track the rapidly changing climate of the future. Understanding the impacts of climate change on regeneration from seedlings is therefore urgently needed to predict future plant population dynamics and migration (Leishman et al., 1992; Ibáñez et al., 2007; Jeltsch et al., 2008; Morin and Thuiller, 2009; De Frenne et al., 2010). In alpine ecosystems several studies have found high rates of seedling establishment (Niederfriniger and Erschbamer, 2000; Forbis, 2003) and diversity of genotypes (Jonsson et al., 1996; Gabrielsen and Brochmann, 1998), arguing for the importance of recruitments in the maintenance of plant populations. Seedling recruitment of alpine plants is under strong environmental control, being affected largely by low temperatures, short growing season and soil drought during summer (Billings and Mooney, 1968; Marcante et al., 2014), indicating that climate change will inevitably affect recruitment success.
Previous studies on the effects of climatic change on seedling emergence and survival of arctic and alpine species have yielded ambiguous results (see below). Such inconsistencies can be related to differences between habitats (Graae et al., 2009), including moisture availability (Bell and Bliss, 1980; Welling and Laine, 2002), geology and climate (Körner, 2003) or interspecific differences (Lambrecht et al., 2007). Interestingly, the contradictory responses to warming in arctic/alpine plants are strongly reduced when sorting the studies for the approach used (i.e. grouping the studies with similar methodology). For example, emergence and survival were reduced when warming was applied during a specific season (e.g. in summer, Graae et al., 2009; Shevtsova et al., 2009; Hoyle et al., 2013), while repeated field observations during a natural warming trend (e.g. in Diemer, 2002; Cooper et al., 2004), or under simulated all-season warming in the laboratory (Milbau et al., 2009; Mondoni et al., 2012) showed higher recruitment. Assuming that the latter two include both direct and indirect effects of warming, it is possible that temperature warming per se may obscure the true responses to climate change, at least in early spring, when most seedlings emerge.
Supporting this view, Körner (1992) suggested that climate warming might operate on alpine plants primarily via indirect effects, e.g. via responses attributable to the extension of the snow-free period. With shortening of winter (Dong et al., 2010) seeds may remain partially dormant (Walck et al., 1997), or if they germinate, the precocious emergence may increase the chances of young seedlings being exposed to freezing episodes. Conversely, a longer (and warmer) growing season should also improve seedling recruitment if water is available (Crawford, 2008a, b). However, such possibilities remain to be investigated.
Moreover, the effect of increased temperatures on seedling emergence and survival of alpine glacier foreland plants is unknown. However, alpine species at higher elevations are expected to be at the greatest level of threat due to climate change (Engler et al., 2011), as shown by the decreases of subnival species cover and concomitant increases in the cover of thermophilous alpine species detected in the European mountains (Pauli et al., 2007; Gottfried et al., 2012; Stanisci et al., 2015). Facing global warming the species that live close to the glacier might disappear because of the changed climatic conditions and/or the increased competition with species migrating from lower altitude. However, the retreat of the glacier might provide new terrain for expansion for these species (Crawford, 2008a, b) and the possibility that they can physiologically thrive in a warmer climate cannot be ruled out [i.e. fundamental vs. realized niche (e.g. Guisan and Thuiller, 2005)]. Hence, plant regeneration from seeds will play a key role for the successful persistence and/or migration of glacier foreland species in a future climate.
Here we present the first field evidence of the impact of climate change on seedling emergence and establishment of plants from the glacier foreland. To investigate how and whether seedling emergence and establishment could withstand a warming scenario of a 2·7 °C increase in mean annual temperature, seeds of eight glacier foreland species were sown at the current growing site and 400 m of altitude downward in the study area located in the central Italian Alps. At both sites, seeds were sown on soil collected from the site of origin to reproduce the natural germination substrate of the glacier foreland (nutrient-poor deglaciated soil) (Chapin et al., 1994). Seedling emergence and survival, soil surface temperature and water potential were monitored for two years (2012 and 2013). Further laboratory experiments were set up to investigate the effects of different autumn and spring simulated temperatures (derived from measurements taken at the study sites) on seed germination. Specific research questions were: does autumn warming elicit seedling emergence of alpine plants in the wild? Are autumn-emerged seedlings able to survive during winter? Does early spring emergence, stimulated by an anticipated snowmelt, enhance seedling establishment? Do seedlings survive an increase of summer drought and temperature?
MATERIAL AND METHODS
Study species
Seeds were collected at the time of natural dispersal (Hay and Smith, 2003) on 5 September 2011 and on 7 September 2012 from the following eight species selected on their occurrence and abundance in the 150-year ice-free moraine of the glacier Dosdè (46 °24′N, 10 °12′E; approx. 2500 m a.s.l.), in the Alps of Lombardy (Sondrio, northern Italy): Poa laxa Haenke subsp. laxa (Poaceae), Geum reptans L. (Rosaceae), Luzula alpino-pilosa (Chaix) Breistr. (Juncaceae), Veronica alpina L. (Plantaginaceae), Cerastium pedunculatum Gaud. ex Ser. (Caryophyllaceae), Oxyria digyna Hill (Polygonaceae), Saxifraga bryoides L. (Saxifragaceae) and Gnaphalium supinum L. (Asteraceae). These species are some of the most common taxa that colonize the glacier foreland in the Alps (Pirola and Credaro, 1994). Seeds collected in September 2011 were sown in the wild (and hence subjected to observations of seedling emergence and establishment), while those collected in September 2012 were exposed to different temperature and light treatments in the laboratory (see below and Table 1). For simplicity, each species is referred to hereafter by its genus name.
Table 1.
Temperatures (°C) during the different weeks of the incubation treatments; different text styling indicates the simulated seasons of autumn (normal text), winter (italic) and spring/summer (bold)
| Week of theexperiments | Equivalent timeof the year | 2500 m | 2100 m |
|---|---|---|---|
| 1–4 | September | 12/5 | 16/9 |
| 5–32 | October–April | 0 | 0 |
| 33–36 | May | 0 | 14/7 |
| 37–40 | June | 0 | 16/10 |
| 41–44 | July | 15/9 | 18/11 |
| 45–48 | August | 15/10 | 19/12 |
Emergence phenology in the wild
We simulated a climate warming scenario using the natural increase in temperature with decreasing altitude at the study area. From the time of collection, seeds were exposed to environmental conditions occurring at the species growing site (at 2500 m a.s.l.) and 400 m lower in altitude. This difference of altitude was chosen to correspond to an approx. 2·4 °C difference of mean annual temperature (considering a decrease of approx. 0·6 °C/100 m in the Alps; Körner, 2003), reflecting a less pessimistic scenario of temperature increase in the next century for mountain ecosystems (2·9–5·3 °C; Nogués-Bravo et al., 2007). Both sites were chosen in SW-facing open areas, so as to be representative of the ecological niche of the target species.
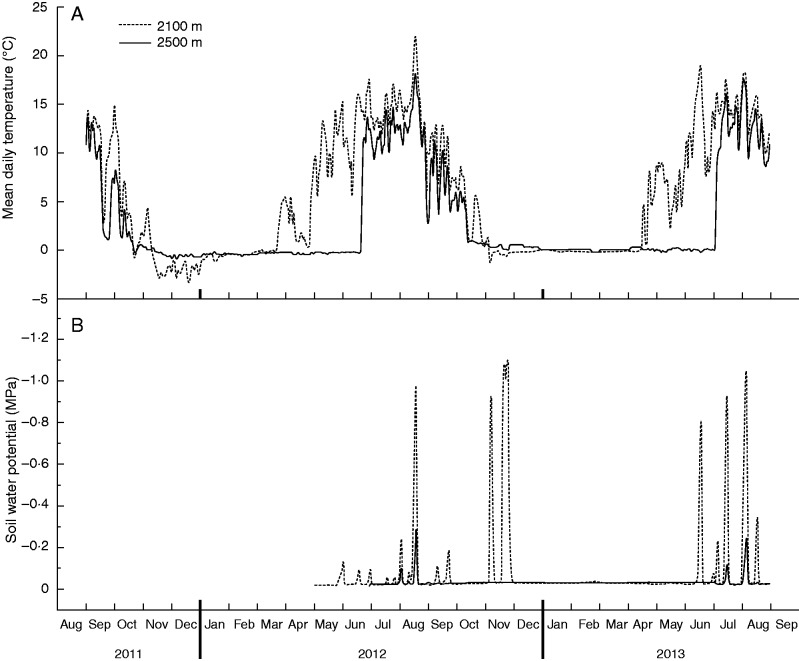
In each site, experiments involved sowing four replicates of 100 seeds each on 10 × 10-cm square blocks of natural soil collected at the site of species origin. A further four blocks were left empty to serve as controls, so that a total of 72 blocks (36 per site: 8 species × 4 replictes plus 4 controls) were established. To reproduce and standardize the natural soil profile of the species growing site, the soil used for the experiments was sieved using three different meshes (of 3, 6 and 10 mm). In each study site, seeds were sown over the 3-mm-size sieved soil and then covered with a thin layer of gravel obtained using the larger mashes (6 and 10 mm). This latter layer was found to normally cover the bare ground surface of the recent deglaciated area in the study site (our pers. observ.) and were thought to play a role in limiting water evaporation during summer. Finally, the surface of each block was covered with a 5-mm plastic square grid that was used as a reference to mark the position and follow the fate of each seedling into the blocks. Seedling emergence (number of emerged seedlings/number of seed sown), survival (number of survived seedlings/number of emerged seedlings) and establishment (number of survived seedlings/number of seed sown) were monitored at about 10-d intervals during the snow-free period for two years (from September 2011 to September 2013). Tiny Tag data loggers (Gemini, Chichester, UK), recording temperature at hourly intervals, were buried 2 cm deep in the soil for the entire length of the experiment at both sites. Further data loggers recording soil water potential (SWP) at hourly intervals (MicroLog SP3, Environmental Measuring Systems, Brno, Czech Republic) were subsequently added at each site from June 2012. The first snow-free day after winter and the length of the growing season (daily mean temperature ≥2 °C) were derived from the temperature data. Mean annual soil surface temperature at 2100 m was about 2·7 °C higher than at 2500 m, both in 2012 and in 2013 (calculated from 1 January to 31 December, each year), in accordance with our expectation of temperature increase (see above). In these years the growing season (considered as the snow-free period) was about 2 months longer at 2100 m (approx. May–October) than at 2500 m (July–October), mostly because an earlier snow melt in spring (end of April, Fig. 1A).
Fig. 1.
Mean daily temperature (A) and water potential (B) about 2 cm deep in the soil at the species growing site (2500 m a.s.l. continuous line) and 400 m lower (2100 m a.s.l., dashed line), between September 2011 (May 2012 in the case of water potential) and August 2013.
Germination experiments in the laboratory
The laboratory treatments investigated the effects of autumn and spring temperatures on seed germination under controlled conditions of water availability and light. From the time of collection (September 2012), seeds were subjected to temperatures simulating mean monthly day (0800–2000 h) and night time (2000–0800 h) changes at 2100 and 2500 m (Table 1). Temperatures were based on measurements taken at hourly intervals by data loggers buried at the two sites (2500 and 2100 m a.s.l) during the first year of the study (1 September 2011 to 1 September 2012). As we expected a certain level of germination in autumn, we used a full population of ungerminated seeds to assess the effects of different spring/summer scenarios; hence, the cycles listed in Table 1 were investigated also in the absence of autumn conditions.
In each case, three replicates of 30 seeds each were placed on agar held in 90-mm-diameter Petri dishes. All treatments were carried out in temperature- and light-controlled incubators using a 12-h daily photoperiod (photosynthetically active radiation 40– 50 µmol m–2 s–1) and illumination was provided during the warm phase, except during the cold stratification period (0 °C) which was conducted in complete darkness. Germination was defined as radicle protrusion and elongation of more than 2 mm and was monitored at weekly intervals. To test the effect of light on germination, subsamples of seeds were exposed to complete darkness under temperature conditions previously found to elicit significant germination at 12-h daily photoperiod (20/10 °C after 16 weeks of cold/wet stratification at 0 °C). The numbers of seeds and replications for each test, method of sowing and observations were as described above.
Data analysis
The responses of total seedling emergence (number of emerged seedlings/number of seed sown), seedling survival (number of survived seedlings/number of emerged seedlings) and seedling establishment (number of survived seedlings/number of seeds sown) to the different climate experience in the two study sites (hereafter referred to as climate change) were analysed by means of a generalized linear mixed model with binomial error and logit-link function. Fixed effects were species, sites, season (autumn and spring) and their interaction; replicates were considered as random effects.
To study the effects of climate change on speed of seedling emergence the mean emergence time (MET) was calculated using the formula:
where ni is the number of seeds that emerged within consecutive intervals of time, ti is the time between the beginning of the test and the end of a particular interval of measurement, and N is the total number of seeds that emerged.
MET in spring was calculated using the first snow-free day after winter as initial time, derived from the temperature data (daily mean temperature ≥2°C). The MET was normally distributed and its responses to the different climate experience in the two study sites was analysed by means of a linear mixed model, in which fixed and random effects were as described above. Species that failed to germinate were excluded from the analysis, although still present on the figures for comparative purposes.
All analyses were performed using MASS and nlme packages in R software (version 3.1.1; R Core Team, 2014).
Additionally, to show how seedling survival was affected during each month after emergence, the mean monthly mortality (number of dead seedlings in a given month/number of seedlings available up to that month) was calculated for each species and growing site (results based on fewer than five seedlings were not considered).
RESULTS
Emergence phenology
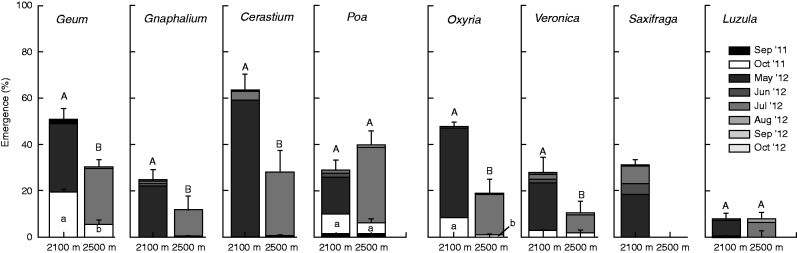
Seedling emergence (number of seedlings emerged/number of seed sown) was significantly different across species, the growing site (2100 and 2500 m), and season (autumn and spring) (Table 2). Because of the strong species × site, species × season and site × season interactions, species-, site- and season-specific analyses were performed. In general, total seedling emergence was higher at 2100 m than at 2500 m in all species, except in Poa and Luzula (Fig. 2). At the species growing site (2500 m), during the time of seed dispersal (autumn, September–October), seedling emergence was similar in Geum and Poa (approx. 5 %) and very low (≤2 %) or null in the other species. At the warmer sowing site (2100 m) autumn emergence remained low (or null) in all species, but increased significantly to approx. 20 % in Geum (t21 = –3·942; P = 0·001) and to 10 % in Oxyria (t21 = –2·909; P = 0·008). Monitoring of seedling emergence across the winter was not possible because of snow cover and was re-started as soon as snow melted the following spring. After winter, further seedling emergence occurred only in spring/summer 2012 (i.e. no further germination was observed in spring/summer 2013), mostly during the first month after snow melt (i.e. in May at 2100 m and in July at 2500 m; Fig. 2), after which there was only minor emergence, except in Saxifraga that continued to germinate in June and July (at 2100 m). At 2100 m, final emergence (i.e. autumn- and spring-emerged seedlings) was highest in Cerastium, Geum and Oxyria (approx. 50–60 %), followed by Gnaphaliam, Poa, Veronica, Saxifraga (approx. 25–28 %) and Luzula (approx. 8 %). At the species growing site (2500 m) final emergence was highest in Poa, Geum and Cerastium (approx. 30–40 %), followed by Oxyria (approx. 20 %), Gnaphaliam, Veronica and Luzula (approx. 8–10 %), while no seedling emergence occurred in Saxifraga. Unlike Gnaphaliam, Cerastium, Saxifraga and Luzula, which emerged only in spring/summer, total seedling emergence of Geum, Poa, Veronica and Oxyria was determined by using a different proportion of seeds germinating in autumn 2011 and/or spring/summer 2012. In these latter species, fitting the model using spring/summer-emerged seedlings (proportioned to the available un-germinated seeds at the end of winter) did not change the result except in Geum, which showed the same proportion of spring/summer emergence between growing sites (t39 = –1·295; P = 0·20).
Table 2.
Results of a generalized linear mixed model on the effects of site of sowing, season (autumn and spring) and species identity on seedling emergence
| Factors | χ2 | d.f. | P |
|---|---|---|---|
| Species | 85·594 | 6 | <0·0001 |
| Site | 30·23 | 1 | <0·0001 |
| Season | 80·612 | 1 | <0·0001 |
| Species × site | 31·536 | 6 | <0·0001 |
| Species × season | 21·14 | 6 | 0·002 |
| Site × season | 5·308 | 1 | 0·021 |
Fig. 2.
Cumulative monthly seedling emergence percentage (means ± s.e.) of each species in the wild at 2100 and 2500 m. Emergence from November to April has been omitted because of snow cover. Different letters indicate significant differences of emergence at P < 0·05 at the end of October 2011 (lower-case) and October 2012 (upper-case).
Mean time to emerge
MET was calculated only when seedling emergence was ≥5 %. Under autumn conditions, MET was similar across either species (Geum, Poa and Oxyria) or site (only in Geum), varying between about 15 and 20 d (F2,6 = 2·676; P = 0·15) (Supplementary Data Fig. S1).
To investigate the effects of the different spring/summer conditions at the two study sites (at 2100 and 2500 m) on MET, the beginning of the test (see ‘Data analysis’) was considered as the timing of snow melt at each site (27 April 2012 at 2100 m and 21 June 2012 at 2500 m). Spring/summer MET was significantly different across both species (L4 = 37·56; P < 0·0001), site of sowing (L9 = 4·814; P = 0·028) and their interactions (L10 = 13·512; P = 0·035). Intraspecific difference of MET between growing sites was significant only in Luzula, which germinated faster at 2100 m (Fig. S1). In general, MET was fastest in Oxyria and Geum (approx. 15–17 d), followed by Veronica, Cerastium, Gnaphalium and Luzula at 2100 m, Poa (approx. 20–26 d), Luzula (at 2500 m) and Saxifraga (only at 2100 m) (approx. 31–36 d).
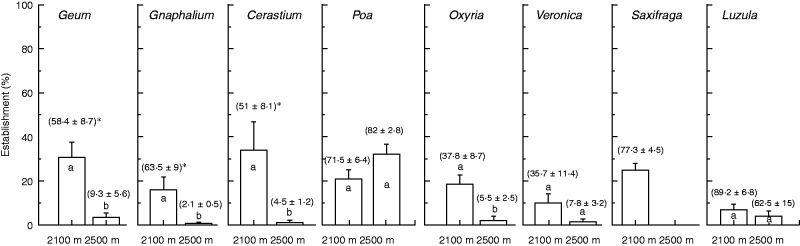
Seedling establishment and survival
Seedling establishment (number of survived seedlings/number of seed sown) was significantly different across both species and sites of sowing, with strong species × site interaction (Table 3; Fig. 3). In Geum, Cerastium and Oxyria, seedling establishment was significantly higher at 2100 m than at 2500 m, while it was similar in the other species between the two growing sites. At 2100 m, Geum, Cerastium, Oxyria, Poa and Saxifraga showed similarly the highest establishment (approx. 20–30 %), followed by Gnaphalium, Veronica and Luzula (approx. 10–15 %). At the species growing site seedling establishment was very low (0–5 %), except in Poa (approx. 20 %).
Table 3.
Results of a generalized linear mixed model on the effects of site of sowing and species identity on seedling establishment and survival
| Factors | χ2 | d.f. | P |
|---|---|---|---|
| Establishment | |||
| Species | 43·003 | 6 | <0·000 |
| Site | 8·861 | 1 | 0·003 |
| Species × site | 34·407 | 6 | <0·000 |
| Survival | |||
| Species | 40·89 | 6 | <0·0001 |
| Site | 18·431 | 1 | <0·0001 |
| Species × site | 16·316 | 6 | 0·012 |
Fig. 3.
Seedling establishment (bars, means ± s.e.) and survival (numbers in parentheses, means ± s.e.) 2 years after sowing (September 2011 to September 2013) of each species in the wild at 2100 and 2500 m. Different lower-case letters and an asterisk indicate significant differences of establishment and survival, respectively, between growing sites at P < 0·05.
In general, the survival (number of survived seedlings/number of emerged seedlings) differed across both species and site of sowing, with significant species × site interaction (Table 3). In Geum, Gnaphalium and Cerastium, seedling survival was significantly higher at 2100 m than at 2500 m; in the other species, although there were differences these were not significant: Oxyria and Veronica survived better at 2100 m (Fig. 3). At 2100 m, Poa, Saxifraga and Luzula showed the highest survival (73–84 %), followed by Geum, Gnaphalium, Cerastium (53–63 %), Veronica and Oxyria (38 %). At the species growing site (2500 m) seedling survival was in general low (3– 14 %), except in Luzula and Poa (53 and 81 %, respectively).
At 2100 m, about 42 and 25 % of autumn-emerged seedlings (in 2011) did not survive across the winter in Geum and Oxyria, respectively (i.e. sum of dead seedlings in autumn and winter). In the other species (and/or growing site) autumn germination was null or very low (e.g. < 5 %), so seedling survival is not presented. In general, seedling mortality was highest the first summer after emergence (e.g. August 2012) mostly at 2500 m (Supplementary Data Fig. S2).
Germination phenology in the laboratory
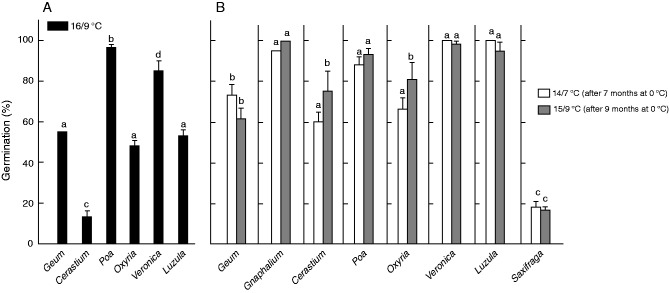
Under simulated autumn mean monthly day and night time changes in temperature at 2100 m (16/9 °C, September) seed germination differed across species (χ25 = 175·7; P < 0·001; Fig. 4A). Poa showed consistently the highest germination (e.g. ≥90 %), followed by Veronica (approx. 85 %), Luzula, Oxyria and Geum (approx. 50 %). Conversely, germination was low in Cerastium (<10 %) and did not occur in Gnaphalium or Saxifraga. The effects of simulated autumn temperature on seed germination at the species growing site were not investigated, as no (or very low) emergence occurred at this location in the wild (see Fig. 2).
Fig. 4.
Percentage germination (means ± s.e.) of each species at the end of simulated autumn (A) and spring (B) temperature treatments in the laboratory. See Table 1 for the incubation treatments. Different lower-case letters indicate significant differences of germination at P < 0·05 level.
When winter-treated seeds were transferred to simulated spring/summer temperatures at 2100 and 2500 m, seed germination occurred within the first temperature experienced, whereupon there was no further germination. Significant differences in the germination percentage were observed between species (χ24 = 156·84; P < 0·001), incubation temperatures (χ21 = 4·74; P < 0,029) and species × temperature interactions (χ24 = 13·34; P < 0·009). However, species-specific analysis revealed that differences between incubation temperatures were significant only in Cerastium and Oxyria (Fig. 4B). Seeds of Gnaphalium, Poa, Veronica and Luzula showed consistently the highest germination (e.g. 80–90 %), followed by Geum, Oxyria, Cerastium (approx. 60–70 %) and Saxifraga (approx. 20 %). Finally, seed germination was strongly reduced under dark condition in all species (on average approx. 55 %; χ21 = 185·2; P < 0·001), except in Geum (data not shown).
DISCUSSION
Our results show for the first time that an increase of about 2·7 °C in mean annual temperature (at soil surface level) may lead to a significant increase of seedling emergence and establishment of several species of the glacier foreland. Indeed, a warmer and longer growing season (at 2100 m) significantly enhanced recruitment success (Fig. 3) except in the graminoids (Poa and Luzula), which showed similar responses regardless of the climatic scenarios experienced. Confirming previous observations (Körner, 2003; Schwienbacher et al., 2011) spring germination prevailed in our species, mostly occurring within the first month after snow melted. However, under a warmer climate (at 2100 m) there was a significant increase in autumn emergence in Geum and Oxyria, supporting the view of Mondoni et al. (2012) that warming may lead to a shift from spring to autumn germination in some alpine plants. About 60 and 75 % of autumn-emerged seedlings could survive across the winter (in Geum and Oxyria, respectively), showing an interesting adaptation of alpine plants to cope with winter temperatures even at these early stages of development. This is an important and novel observation, indicating that alpine plants are able to regenerate despite the effects of climate warming. The fundamental regeneration niche of our species is therefore wider than that realized, suggesting that their absence at lower/warmer altitude is probably due to competition.
Under controlled laboratory conditions the effects of autumn warming were exacerbated, increasing the level and the number of autumn germinating species (Fig. 4), compared with what was observed in the field. Similarly, spring germination was higher in the laboratory than in the field under both simulated climatic scenarios. Supporting the view of Müller et al. (2011) that germination of alpine/arctic plants is high in the laboratory but low in the field, the reduced field emergence observed here may be due to deviations of temperature, water availability and light conditions from the optimal for germination. In this regard, seeds of our species showed a strong light requirement for germination, which could not be controlled in the field, where seeds were buried. Furthermore, the possibility that the proportion of emerged seedlings in the field may not have reflected the real germination due to an early mortality cannot be ruled out. On the other hand, the similar germination responses under simulated spring/summer temperatures in the laboratory observed here suggest that conditions in May at 2100 m and in July at 2500 m are relatively similar. Hence, while caution should be taken when evaluating species recruitment potential based on laboratory studies alone (i.e. laboratory experiments could overestimate it), our results highlight the importance of combining laboratory and field investigations to better explain species germination ecology.
Intraspecific differences in spring emergence between sowing sites in the field seems not to be related to temperature differences, nor to water availability, as at both altitudes spring temperatures and soil water potential were similar during the period of highest recruitment (i.e. 4 weeks after snow melted at both altitudes, Fig. 1A, B; Table 1). Furthermore, the higher spring emergence shown at 2100 m suggests that seeds received sufficient chilling to satisfy pre-germination stratification requirements (see Walck et al., 1997), despite the fact that at this altitude winter was 2 months shorter due to earlier snow melt (Fig. 1). A possible explanation of the higher emergence at 2100 m is that seeds started to germinate during the early spring warming recorded between 25 March and 10 April 2012 (but not at 2500 m, Fig. 1A) and therefore they could rely on longer suitable conditions for germination at this lower altitude. Indeed, such warming periods indicate a temporary reduction of snow thickness and/or presence, which may have a large influence on phenological phases (Price and Waser, 1998; Inouye et al., 2002; Inouye, 2008), including seedling emergence (Milbau et al., 2009; Griffith and Loik, 2010). After about the first month of snow-free period (i.e. end of May at 2100 m, end of July at 2500 m), germination was almost halted at both altitudes, probably due to the onset of summer drought episodes (see Fig. 1B).
In alpine environments, winter warming is expected to result in a reduction in snow cover duration and snowfall (Valt and Cianfarra, 2010; IPCC, 2013), which may have significant influence on the existence of alpine species (Gottfried et al., 2002). Interestingly, here we have shown that a reduction of snow cover duration (of about 2 months) increases recruitment success, but not the soil surface temperature early in the spring, when most of the emergence occurred (Fig. 1B and Table 1). Early snowmelt might therefore play a key role for the successful recruitment of alpine plants, favouring both seedling emergence and seedling survival. In this regard, the results presented here can be generalized to establishment in the absence of summer frost and on bare ground, as we excluded possible indirect effects from the resident vegetation. Neighbouring plants could have both negative and positive effects on seedling establishment, increasing the competition on the one hand, or providing a shelter for healthy growth on the other, respectively (e.g. Ryser, 1993; Cichini et al., 2011). However, bare ground will presumably be the regeneration niche for glacier foreland plants, being the pioneer colonizer following deglaciation (Matthews and Whittaker, 1987), but in this environment (i.e. nival zone) summer frost of –7 to –9 °C may occur as a consequence of cold waves (Larcher et al., 2010), causing seedling injuries.
Our findings agree with the assumption that glacier foreland species establish at low rates due to high seedling mortality (Welling et al., 2005; Erschbamer et al., 2008; Marcante et al., 2009), but indicate that a warmer climate might alleviate recruitment limitations, favouring species ability to colonize patches of unvegetated ground. The highest seedling mortality found here occurred at the glacier foreland, mostly in August 2012 (Supplementary information 2) and concurrently with peaks of temperatures and very low water potentials (Fig. 1A, B). Summer drought and heat are indeed known as the main cause of seedling mortality in alpine ecosystems (Forbis, 2003; Körner, 2003), both directly through physical damage and indirectly through water and nutrient shortage when top soil dries out (Graae et al., 2009). Interestingly, at 2100 m seedling establishment and survival were higher than at 2500 m, although summer drought and heat were stronger at this lower altitude, showing mean daily temperatures and soil water potential of approx. 25 °C (but peaks of 40 °C, data not shown) and –1 MPa, respectively (e.g. August 2012; Fig. 1A, B). A possible explanation is that early spring-emerged seedlings at 2100 m have more developed root systems than those at 2500 m, enabling better survival of drought conditions during the critical summer heat/drought periods. Such environmental extremes are not surprising in alpine ecosystems, especially on bare soil where temperatures often reach 50–55 °C (Kronfuss, 1972) and although it is known that adult alpine plants are adapted to cope with such extremes (Körner, 2003), recent observations show similar capability even at stages of young seedlings (up to 40–50 °C; see Marcante et al., 2014). Similarly, survival across winter 2012 was less problematic at 2100 m than at 2500 m (Supplementary information 2), despite subtle frost (–4°C) and drought (soil water potential –1·2 MPa) episodes at the former (November 2012, Fig. 1); these extremes did not occur at the species growing site, where winter was about 2 months longer. Seedlings of alpine pioneer species have been shown to be particularly frost-resistant in the wild (up to –5 °C; Marcante et al., 2012) and, interestingly, particularly those from populations experiencing early spring snowmelt (Briceño et al., 2014). Accordingly, our observations at 2100 m show that early spring-emerged seedlings (in May) survived well to the following winter, but those at 2500 m highlight that late spring emergence (in July) combined with long exposure to winter and low stages of development may strongly reduce such capability.
The results presented here clearly demonstrate that regeneration of pioneer alpine species might be favoured by a moderate temperature warming except for the graminoids (Poa and Luzula), which showed similar recruitment regardless of the climate scenario experienced (Fig. 3). Graminoids in general are predicted to become more productive in a warmer climate (Dormann and Woodin, 2002), and hence their expansion should be expected in nival environments. However, while higher seedling emergence at 2100 m may lead to an increase in recruitment, it will reduce the soil seed bank. A persistent soil seed bank in several alpine species is thought to be an ecological adaptation to the low chance of establishment in these environments (Schwienbacher et al., 2010), which delays germination until favourable conditions are present. Higher germination may therefore not necessarily be beneficial for alpine plants, as summer drought is common in alpine habitats (see above) (and presumably will increase under climate change), reducing the likelihood of seedling survival. However, in this study seed germination occurred only the first spring/summer after seed sowing (i.e. May–August, 2012), while no emergence was observed the following growing period (i.e. May–August, 2013), suggesting that the species studied here may not form a persistent soil seed bank, as shown for Geum reptans and Oxyria digyna by Schwienbacher et al. (2010).
The differences of seedling establishment across species observed here may result in range shift and/or changes of local dominance in the glacier foreland plant communities. In this regard, some species (e.g. Geum, Cerastium and Saxifraga) will significantly increase their establishment, although their real expansion will subsequently depend also on the capacity to compete with each other. Furthermore, climate warming may promote the range expansion of glacier foreland species in deglaciated areas, but at the same time may contribute to the range expansion of low-altitude species in nival communities, causing further threats (Pauli et al., 2007). Indeed, glacier foreland plants are known to be very weak competitors (Körner, 2003). Further studies should therefore focus on the role of competition (with more thermophilous plants) as a limiting factor for the actual and future distribution of glacier foreland species. Moreover, long-term monitoring of seedling recruitment at the glacier foreland is also urgently needed to better understand the ecological factors involved in the migration, contraction or expansion of alpine plants in the future.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: mean time to emerge in autumn and spring/summer for each species at the two sites. Fig. S2: mean monthly and winter mortality for each species at the two sites.
ACKNOWLEDGEMENTS
This project received funding from the European Union’s Seventh Framework Programme (FP7), through the programme ‘People’ (Marie Curie Action – COFUND) and the Provincia Autonoma di Trento. Further support was provided by the Ev-K2-CNR SHARE, Bergamo and Next Data (Italian CNR). We thank Dr Roberto Sacchi for the statistical analysis and Professor Scott Wilson for helpful criticism and valuable suggestions to the manuscript.
LITERATURE CITED
- Bell KL, Bliss LC. 1980. Plant reproduction in a High Arctic Environment. Arctic and Alpine Research 12: 1–10. [Google Scholar]
- Beniston M. 2003. Climatic change in mountain regions: a review of possible impacts. Climatic Change 59: 5–31. [Google Scholar]
- Beniston M, Diaz HF, Bradley RS. 1997. Climatic change at high elevation sites: an overview. In: Diaz HF, Beniston M, Bradley RS, eds. Climatic change at high elevation sites. Dordrecht: Springer, 233–251. [Google Scholar]
- Billings WD, Mooney HA. 1968. The ecology of arctic and alpine plants. Biological Reviews 43: 481–529. [Google Scholar]
- Briceño VF, Harris-Pascal D, Nicotra AB, Williams E, Ball MC. 2014. Variation in snow cover drives differences in frost resistance in seedlings of the alpine herb Aciphylla glacialis. Environmental and Experimental Botany 106: 174–181. [Google Scholar]
- Chapin FS, III, Walker LR, Fastie CL, Sharman LC. 1994. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecological Monographs 64: 149–175. [Google Scholar]
- Cichini K, Schwienbacher E, Marcante S, Seeber GUH, Erschbamer B. 2011. Colonization of experimentally created gaps along an alpine successional gradient. Plant Ecology 212: 1613–1627. [Google Scholar]
- Cooper EJ, Alsos IG, Hagen D, Smith FM, Coulson SJ, Hodkinson ID. 2004. Plant recruitment in the High Arctic: seed bank and seedling emergence on Svalbard. Journal of Vegetation Science 15: 115–124. [Google Scholar]
- Crawford RMM. 2008a. Plants at the margin ecological limits and climate change. Cambridge: Cambridge University Press. [Google Scholar]
- Crawford RMM. 2008b. Cold climate plants in a warmer world. Plant Ecology & Diversity 1: 285–297. [Google Scholar]
- Diaz HF, Bradley RS. 1997. Temperature variations during the last century at high elevation sites. In: Diaz HF, Beniston M, Bradley RS, eds. Climatic change at high elevation sites. Dordrecht: Springer, 21–47. [Google Scholar]
- Diemer M. 2002. Population stasis in a high-elevation herbaceous plant under moderate climate warming. Basic and Applied Ecology 3: 77–83. [Google Scholar]
- Dong W, Jiang Y, Yang S. 2010. Response of the starting dates and the lengths of seasons in Mainland China to global warming. Climatic Change 99: 81–91. [Google Scholar]
- Dormann CF, Woodin SJ. 2002. Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Functional Ecology 16: 4–17. [Google Scholar]
- Engler R, Randin CF, Thuiller W, et al. 2011. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17: 2330–2341. [Google Scholar]
- Erschbamer B, Niederfriniger Schlag R, Winkler E. 2008. Colonization processes on a central Alpine glacier foreland. Journal of Vegetation Science 19: 855–862. [Google Scholar]
- Forbis TA. 2003. Seedling demography in an alpine ecosystem. American Journal of Botany 90: 1197–206. [DOI] [PubMed] [Google Scholar]
- De Frenne P, Graae BJ, Kolb A, et al. 2010. Significant effects of temperature on the reproductive output of the forest herb Anemone nemorosa L. Forest Ecology and Management 259: 809–817. [Google Scholar]
- Gabrielsen TM, Brochmann C. 1998. Sex after all: high levels of diversity detected in the arctic clonal plant Saxifraga cernua using RAPD markers. Molecular Ecology 7: 1701–1708. [Google Scholar]
- Gottfried M, Pauli H, Reiter K, Grabherr G. 2002. Potential effects of climate change on alpine and nival plants in the Alps. In: Körner C, Spehn E, eds. Mountain biodiversity – a global assessment. London: Parthenon Publishing, 2133–223. [Google Scholar]
- Gottfried M, Pauli H, Futschik A, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2: 111–115. [Google Scholar]
- Graae BJ, Ejrnæs R, Marchand FL, et al. 2009. The effect of an early-season short-term heat pulse on plant recruitment in the Arctic. Polar Biology 32: 1117–1126. [Google Scholar]
- Griffith AB, Loik ME. 2010. Effects of climate and snow depth on Bromus tectorum population dynamics at high elevation. Oecologia 164: 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecology Letters 8: 993–1009. [DOI] [PubMed] [Google Scholar]
- Hay FR, Smith RD. 2003. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linnington SH, Pritchard HW P, eds. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew, 97–133. [Google Scholar]
- Hoyle GL, Venn SE, Steadman KJ, et al. 2013. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Global Change Biology 19: 1549–1561. [DOI] [PubMed] [Google Scholar]
- Ibáñez I, Clark JS, LaDeau S, Lambers JHR. 2007. Exploiting temporal variability to understand tree recruitment response to climate change. Ecological Monographs 77: 163–177. [Google Scholar]
- Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89: 353–362. [DOI] [PubMed] [Google Scholar]
- Inouye D, Morales M, Dodge G. 2002. Variation in timing and abundance of flowering by Delphinium barbeyi Huth (Ranunculaceae): the roles of snowpack, frost, and La Niña, in the context of climate change. Oecologia 130: 543–550. [DOI] [PubMed] [Google Scholar]
- IPCC. 2013. Climate change 2013: the physical science basis. IPCC, http://www.climatechange2013.org/report/ [Google Scholar]
- Jeltsch F, Moloney KA, Schurr FM, Köchy M, Schwager M. 2008. The state of plant population modelling in light of environmental change. Perspectives in Plant Ecology, Evolution and Systematics 9: 171–189. [Google Scholar]
- Jones PD, Moberg A. 2003. Hemispheric and large-scale surface air temperature variations: an extensive revision and an update to 2001. Journal of Climate 16: 206–223. [Google Scholar]
- Jonsson BO, Jonsdottir IS, Cronberg N. 1996. Clonal diversity and allozyme variation in populations of the arctic sedge Carex bigelowii (Cyperaceae). Journal of Ecology 84: 449–459. [Google Scholar]
- Körner C. 1992. Response of alpine vegetation to global climate change. In: International Conference on Landscape Ecological Impact of Climate Change. Reiskirchen: Catena Verlag, 85–96. [Google Scholar]
- Körner C. 2003. Alpine plant life. Functional plant ecology of high mountain ecosystems (C Körner, ed.). Heidelberg: Springer. [Google Scholar]
- Kronfuss H. 1972. Kleinklimatische Vergleichsmessungen an zwei subalpinen Standorten. Mitteilungen der Forstlichen Bundes-Versuchsanstalt Wien 96: 159–176. [Google Scholar]
- Lambrecht SC, Loik ME, Inouye DW, Harte J. 2007. Reproductive and physiological responses to simulated climate warming for four subalpine species. The New Phytologist 173: 121–134. [DOI] [PubMed] [Google Scholar]
- Larcher W, Kainmüller C, Wagner J. 2010. Survival types of high mountain plants under extreme temperatures. Flora - Morphology, Distribution, Functional Ecology of Plants 205: 3–18. [Google Scholar]
- Leishman M, Hughes L, French K, Armstrong D, Westoby M. 1992. Seed and seedling biology in relation to modelling vegetation dynamics under global climate change. Australian Journal of Botany 40: 599–613. [Google Scholar]
- Marcante S, Winkler E, Erschbamer B. 2009. Population dynamics along a primary succession gradient: do alpine species fit into demographic succession theory? Annals of Botany 103: 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcante S, Sierra-Almeida A, Spindelböck JP, Erschbamer B, Neuner G. 2012. Frost as a limiting factor for recruitment and establishment of early development stages in an alpine glacier foreland? Journal of Vegetation Science 23: 858–868. [Google Scholar]
- Marcante S, Erschbamer B, Buchner O, Neuner G. 2014. Heat tolerance of early developmental stages of glacier foreland species in the growth chamber and in the field. Plant Ecology 215: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JA, Whittaker RJ. 1987. Vegetation succession on the Storbreen Glacier Foreland, Jotunheimen, Norway: a review. Arctic and Alpine Research 19: 385–395. [Google Scholar]
- Milbau A, Graae BJ, Shevtsova A, Nijs I. 2009. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany 104: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert RJ. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X, Thuiller W. 2009. Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90: 1301–1313. [DOI] [PubMed] [Google Scholar]
- Müller E, Cooper EJ, Alsos IG. 2011. Germinability of arctic plants is high in perceived optimal conditions but low in the field. Botany 89: 337–348. [Google Scholar]
- Niederfriniger SR, Erschbamer B. 2000. Germination and establishment of seedlings on a glacier foreland in the Central Alps, Austria. Arctic, Antarctic, and Alpine Research 32: 270–277. [Google Scholar]
- Nogués-Bravo D, Araújo MB, Errea MP, Martínez-Rica JP. 2007. Exposure of global mountain systems to climate warming during the 21st century. Global Environmental Change 17: 420–428. [Google Scholar]
- Parolo G, Rossi G. 2008. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology 9: 100–107. [Google Scholar]
- Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13: 147–156. [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, et al. 2012. Recent plant diversity changes on Europe’s mountain summits. Science 336: 353–355. [DOI] [PubMed] [Google Scholar]
- Pirola A, Credaro V. 1994. Osservazioni sul dinamismo della vegetazione di morena recente nel Gruppo del Bernina. Fitosociologia 27: 139–149. [Google Scholar]
- Price MV, Waser NM. 1998. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79: 1261–1271. [Google Scholar]
- Ryser P. 1993. Influences of neighbouring plants on seedling establishment in limestone grassland. Journal of Vegetation Science 4: 195–202. [Google Scholar]
- Schwienbacher E, Marcante S, Erschbamer B. 2010. Alpine species seed longevity in the soil in relation to seed size and shape – A 5-year burial experiment in the Central Alps. Flora 205: 19–25. [Google Scholar]
- Schwienbacher E, Navarro-Cano JA, Neuner G, Erschbamer B. 2011. Correspondence of seed traits with niche position in glacier foreland succession. Plant Ecology 213: 371–382. [Google Scholar]
- Shevtsova A, Graae BJ, Jochum T, et al. 2009. Critical periods for impact of climate warming on early seedling establishment in subarctic tundra. Global Change Biology 15: 2662–2680. [Google Scholar]
- Stanisci A, Frate L, Morra Di Cella U, et al. 2015. Short-term signals of climatechange in Italian summit vegetation: observations at two GLORIA sites. Plant Biosystems doi: 10.1080/11263504.2014.968232 [Google Scholar]
- Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. 2005. Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences of the United States of America 102: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valt M, Cianfarra P. 2010. Recent snow cover variability in the Italian Alps. Cold Regions Science and Technology 64: 146–157. [Google Scholar]
- Vittoz P, Dussex N, Wassef J, Guisan A. 2009. Diaspore traits discriminate good from weak colonisers on high-elevation summits. Basic and Applied Ecology 10: 508–515. [Google Scholar]
- Walck JL, Baskin JM, Baskin CC. 1997. A comparative study of the seed germination biology of a narrow endemic and two geographically-widespread species of Solidago (Asteraceae). 1. Germination phenology and effect of cold stratification on germination. Seed Science Research 7: 47–58. [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17: 2145–2161. [Google Scholar]
- Welling P, Laine K. 2002. Regeneration by seeds in alpine meadow and heath vegetation in sub-arctic Finland. Journal of Vegetation Science 13: 217–226. [Google Scholar]
- Welling P, Tolvanen A, Laine K. 2005. Plant traits: their role in the regeneration of alpine plant communities in sub-arctic Finland. Journal of Vegetation Science 16: 183–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.