Abstract
Background and Aims Although extreme climatic events such as drought are known to modify forest dynamics by triggering tree dieback, the impact of extreme cold events, especially at the low-latitude margin (‘rear edge’) of species distributional ranges, has received little attention. The aim of this study was to examine the impact of one such extreme cold event on a population of Scots pine (Pinus sylvestris) along the species’ European southern rear-edge range limit and to determine how such events can be incorporated into species distribution models (SDMs).
Methods A combination of dendrochronology and field observation was used to quantify how an extreme cold event in 2001 in eastern Spain affected growth, needle loss and mortality of Scots pine. Long-term European climatic data sets were used to contextualize the severity of the 2001 event, and an SDM for Scots pine in Europe was used to predict climatic range limits.
Key Results The 2001 winter reached record minimum temperatures (equivalent to the maximum European-wide diurnal ranges) and, for trees already stressed by a preceding dry summer and autumn, this caused dieback and large-scale mortality. Needle loss and mortality were particularly evident in south-facing sites, where post-event recovery was greatly reduced. The SDM predicted European Scots pine distribution mainly on the basis of responses to maximum and minimum monthly temperatures, but in comparison with this the observed effects of the 2001 cold event at the southerly edge of the range limit were unforeseen.
Conclusions The results suggest that in order to better forecast how anthropogenic climate change might affect future forest distributions, distribution modelling techniques such as SDMs must incorporate climatic extremes. For Scots pine, this study shows that the effects of cold extremes should be included across the entire distribution margin, including the southern ‘rear edge’, in order to avoid biased predictions based solely on warmer climatic scenarios.
Keywords: Dendrochronology, climate change, drought, extreme climate, freeze event, Pinus sylvestris, range-shifts, rear edge, Scots pine, species distribution model
INTRODUCTION
Increasing concentrations of atmospheric greenhouse gases will lead to global climate warming, but the frequency, severity and even the nature of extreme climatic events are also expected to change (IPCC, 2013). Extreme climatic events are unusual and unpredictable drivers of forest community dynamics (Gutschick and BassiriRad, 2003), yet, in order to account fully for the effects of climate extremes on forests and other plant communities, their extremity must be documented from both the ‘driver’ and the ‘response’ perspective (Smith, 2011). To do this, a statistical framework using climatic records should be used to quantify how unusual (extreme) an event is, before the responses of impacted forests are characterized to determine how intense the impact is. The rarity and unpredictability of climate extremes preclude recording these responses when they happen. Therefore, retrospective approaches are used to facilitate reconstruction of post-event impacts on tree growth, mortality and vigour (Strain, 1966; Dobbertin, 2005).
Climate warming and more extreme temperatures could increase the risk of cold-induced damage on forests if mild conditions induce phenological alterations (late hardening, early dehardening or premature bud break), making tree tissues more vulnerable to low temperatures by reducing frost hardiness (Hänninen, 1991; Leinonen, 1996). The main abiotic drivers of plant responses to winter conditions are the variability of air and soil temperatures and the extent and timing of snow cover (Marchand, 1996; Carlson et al. 2015) coupled with phenological timing, the physiological sensitivity of emerging tissues to rapid drops in air temperatures (Sakai and Larcher, 1987; Mayr et al., 2003; Augspurger, 2009; Zwicke et al., 2015). Forest dieback, characterized by massive defoliation (needle loss), growth decline and rising mortality rates, often occurs in response to extreme climatic events such as droughts or heat waves severely limiting water availability (Camarero et al., 2015). However, cold-induced forest dieback (Mayr et al., 2003) is also a widespread phenomenon occurring after very cold and dry winter conditions (i.e. with reduced snow depth), although most examples are geographically biased towards sub-alpine or boreal forests (Tranquillini, 1979; Kullman, 1989, 1991; Pomerleau, 1991). Nevertheless, cold-induced dieback is not exclusive to such cold biomes since it has been described in dry and continental areas including Mediterranean forests (Soulé and Knapp, 2007; Jalili et al., 2010; Matusick et al., 2014).
Here we describe the response of Mediterranean Scots pine forests to a cold-induced dieback which occurred in winter 2001–2002 and affected 14 000 ha of forests situated in the Spanish Sistema Ibérico range (Voltas et al., 2013). This represents the approximate southernmost limit (‘rear edge’; cf. Hampe and Petit, 2005) of the species’ distribution in Europe (Fig. 1). Dieback was most prevalent in south-facing sites located at mid elevations with shallow and rocky soils, and defoliation was also more important in the south-facing side of crowns (Camarero and Sancho-Benages, 2006). Affected trees showed xylem embolism which was probably caused by cavitation due to freeze–thaw cycles (Peguero-Pina et al., 2011). This indicates that winter conditions could be critical for tree growth and survival (e.g. Pederson et al., 2004), even in the rear edge. Moreover, in the Iberian Peninsula, recent warming has been linked to strong decreases in the frequency of cold extremes (Brunet et al., 2007), which makes rare and extreme cold events potentially more harmful to trees experiencing warmer winters. Understanding how cold-induced dieback impairs rear-edge stands is critical for conserving these genetically unique populations (Hampe and Petit, 2005).
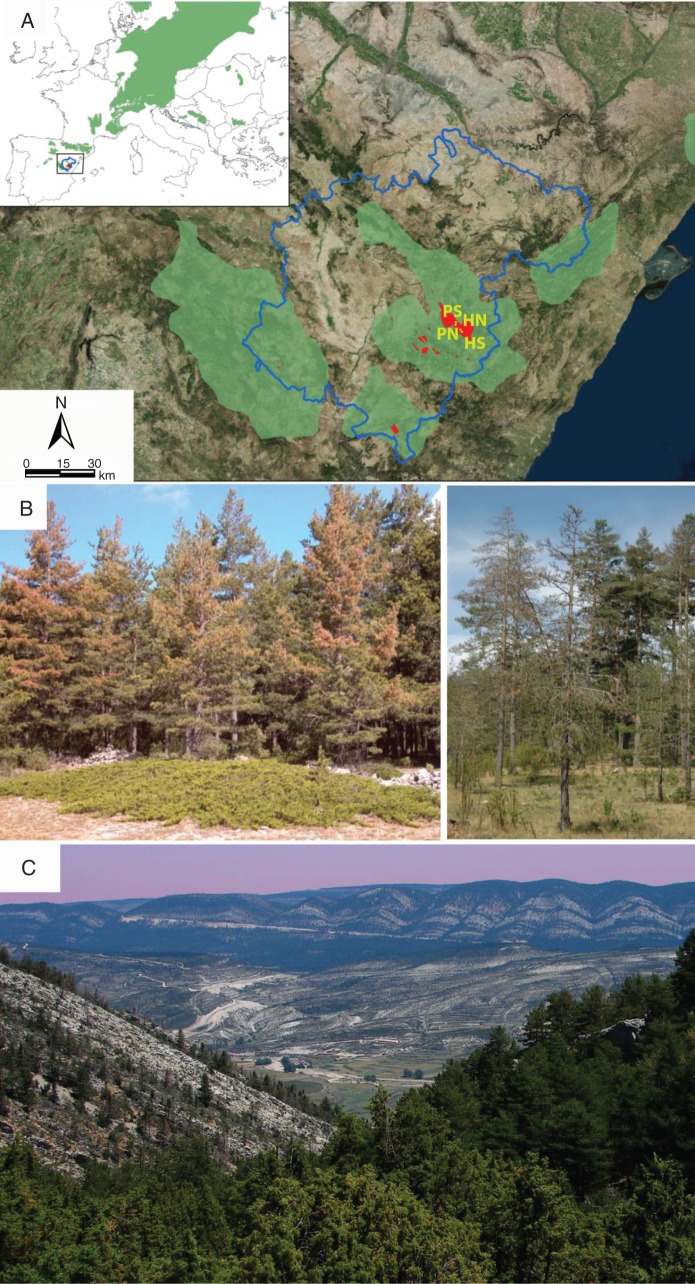
Fig. 1.
Natural range of Scots pine [A; Pinus sylvestris (denoted by green areas); the inset shows the European range] and location of study sites (denoted by red) in Teruel Province (blue line), eastern Spain to examine the impacts of an extreme cold event in 2001 on tree dieback and mortality predominantly affecting trees (B) located in southern-oriented sites (C). Note that shrubby junipers (Juniperus sabina) were not affected by the dieback (B).
We test the hypothesis that cold-induced dieback following extreme low temperatures dictates the ‘rear-edge’ distribution of Scots pine forests in continental dry conditions. Our specific objectives were to: (1) quantify the extremeness of the 2001 climatic event; (2) investigate stand and tree features predisposing them to cold-induced dieback; and (3) characterize tree responses (growth, defoliation and mortality). In addition, since most previous research has focused solely on the negative effects of extreme hot and dry conditions (e.g. drought) on rear-edge Scots pine populations (e.g. Richter et al., 1991; Matías and Jump, 2012; Sánchez-Salguero et al., 2012, 2015), we used a climate-based species distribution model (SDM) to compare the effects of the extreme winter 2001 climatic event with predictions of distribution based solely on long-term climate variability.
MATERIALS AND METHODS
Study area and species
The study was conducted in Scots pine (Pinus sylvestris) forests situated in the Sierra de Gúdar, Sistema Ibérico range, Aragón, eastern Spain (Table 1). The Scots pine populations in the region are found at elevations varying from 1600 to 2000 m a.s.l., and constitute the southernmost continuous forests for the current distribution of one of the most widespread conifers worldwide (Richardson, 1998). Soils in the area are shallow (particularly in areas with low forest cover) and are derived from underlying limestone bedrock. These forests experienced strong land-use pressures (logging, grazing), but this tendency reversed with the extensive abandonment of cultivated land following the migration of the rural population to cities in the 1950s. In fact, the region is currently one of the least densely populated in Europe (http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_r_d3dens&lang=en). There has been significant warming throughout the 20th century in the region, which intensified from the 1980s onwards, when severe droughts affecting the area occurred in the 1990s and 2000s (Voltas et al., 2013).
Table 1.
Geographical, topographical, structural and edaphic features of Pinus sylvestris from four study sites located in eastern Spain showing cold-induced dieback (means ± s.e.)
| Site code | Latitude (N) | Longitude (W) | Elevation (m a.s.l.) | Slope (°) | Basal area (m2 ha–1) | Diameter at 1·3 m (cm) | Height (m) | Soil depth (cm) |
|---|---|---|---|---|---|---|---|---|
| Barranco de la Hoya-North | 40°26′28′′ | 00°30′50′′ | 1620 | 10 | 16·54 | 10·4 ± 0·8a | 8·5 ± 0·5 | 26·0 ± 1·1b |
| Barranco de la Hoya-South | 40°26′31′′ | 00°30′51′′ | 1650 | 22 | 7·40 | 14·7 ± 1·4b | 7·8 ± 0·5 | 20·6 ± 2·1a |
| Peñacerradilla-North | 40°28′02′′ | 00°31′58′′ | 1700 | 15 | 18·64 | 12·2 ± 0·8a | 6·7 ± 0·3 | 24·9 ± 2·7 |
| Peñacerradilla-South | 40°28′14′′ | 00°31′54′′ | 1650 | 27 | 12·48 | 16·2 ± 2·7b | 6·7 ± 0·6 | 22·0 ± 3·1 |
Different letters indicate significant differences between plots of the same site (Mann–Whitney U-tests).
The forest cover changes according to aspect, since northern slopes are dominated by relatively dense Scots pine forests, whereas southern slopes are characterized by scattered trees (Fig. 1). The ground flora also changes according to aspect, with shrubs and junipers dominating the southern-oriented sites (Genista scorpius, Juniperus communis, Berberis vulgaris and Juniperus sabina), while trees or shrubs inhabit northern-oriented slopes (Taxus bacatta and Amelanchier ovalis). Scattered Pinus nigra subsp. salzmannii trees appear in southern-oriented slopes.
The 2001 cold snap was characterized by a rapid drop in minimum temperatures lasting from 16 to 29 December (Supplementary Data Fig. S1). The first visual symptoms of dieback appeared in early spring 2002, when many Scots pine trees started to display needle yellowing, followed by a massive loss of dead buds, shoots and needles (Fig. 1). The dieback affected approx. 30 % of Scots pine forests in the area (Camarero and Sancho-Benages, 2006). In winter 2014, the average standing tree mortality of the most affected stands was about 10 % (pers. obs.). Pinus sylvestris was the only species to show post-event dieback; neither junipers nor P. nigra trees, for instance, showed any signs of defoliation after the 2001 cold event (Fig. 1).
Field sampling
Two study areas (Barranco de la Hoya, henceforth area ‘H’; and Peñacerradilla, henceforth area ‘P’) located in the region most intensively affected by post-2001 forest dieback were selected (Table 1). In each study area, two study sites located in slopes with northern (N) or southern (S) exposure were chosen and abbreviated accordingly (area H, sites HN and HS; area P, sites PN and PS) (Table 1). Field sampling was carried out in spring to summer 2005 (i.e. 4 years after cold-induced forest dieback started); and thus we recognize that not all tree mortality was necessarily due to the 2001 extreme cold event. In each site, a 30 × 30 m plot was established in a representative location of the forest. All trees located within each plot were tagged, measured [spatial co-ordinates, diameter at breast height (dbh) at 1·3 m, and tree height] and the degree of defoliation based on a semi-quantitative scale of five levels (0, 0–20 %; 1, 21–40 %; 2, 41–60 %; 3, 61–80 %; 4, 81–100 %; cf. Müller and Stierlin, 1990) estimated using binoculars. Since estimates of defoliation vary among places, the defoliation data compared every tree with a nearby, fully foliated reference tree. A competition index was calculated for each tree by modifying the influence index proposed by Woods (2000):
| (1) |
where Dn is the dbh of an individual neighbouring tree n, and distn,f is the distance between neighbouring and focal (f) trees. The summation is calculated over all neighbours within 7 m of the focal tree. To avoid edge effects, we replicated the tree positions around the plot limits. Finally, we estimated the soil depth beneath each sampled tree by inserting a 1·5 m long metal rod until we detected rocky substrate. The rod was introduced into the ground at four points located at 0·5 m from the main stem following the cardinal directions. This method provides better estimates of actual soil depth than more sophisticated tools such as ground-penetrating radar because the rocky substrate in the study area is heterogeneous and cracks abound.
Dendrochronology
Dendrochronology was used to characterize secondary growth trends of trees retrospectively (Fritts, 2001). Growth was measured by extracting two radial cores per tree at 1·3 m using a Pressler increment borer. We measured bark thickness and the length of the sapwood in the extracted cores in the field. Sapwood length measured in the two cores of each tree was averaged and transformed into sapwood area. Wood samples were air-dried and sanded until tracheids were visible and then visually cross-dated. Once dated, we measured the tree ring widths to the nearest 0·01 mm using a binocular scope and a LINTAB measuring device (Rinntech, Heidelberg, Germany). The accuracy of visual cross-dating was checked with the program COFECHA, which calculates moving correlations between each individual series and the mean site series (Holmes, 1983).
Tree-ring width series were converted into basal area increment (BAI) which accounts for the geometrical constraint of adding a volume of wood to a stem of increasing radius and it is theoretically related to changes in canopy area (Biondi and Qaedan, 2008). Tthe BAI was obtained by using the formula:
| (2) |
where rt and rt – 1 are the radii corresponding to years t and t – 1, respectively. In cores without pith, we estimated the length of the missing part of the radius by fitting a geometric pith locator to the innermost rings.
To quantify how trees responded to the 2001 event in terms of growth, we calculated a BAI recovery which is defined as the ratio between the BAI averages of the 3 year long periods following (2002–2004) and preceding (1999–2001) the event (Camarero and Sancho-Benages, 2006). To estimate the amount and timing of mortality, we considered that trees were dead when both radial cores showed no ring formation since 2001–2003 up to the sampling year (2005). Then, the annual percentage of dead trees in the four study sites was calculated.
Climatic data sets
First, a long-term (1877–2004) series of daily temperatures from stations located in Teruel (40°20′38′′N, 01°06′33′′W, 915 m a.s.l.), the longest climatic record in the study area, were used to determine how extreme were the low temperatures recorded in winter 2001. This data set allowed calculation of a 100 year return level (plus 95 % confidence intervals), which corresponds to an event that has a 1/100 chance of occurrence in any given year assuming stationary conditions (Gilleland and Katz, 2011). Secondly, to obtain a regional climatic series characterizing the study area and relate it to growth series, local data from 16 meteorological stations were combined into a regional mean for the period 1950–2004 (Supplementary Data Table S1). To estimate the missing data for each station and to combine them, we used the MET program from the Dendrochronology Program Library (Holmes, 1994). For each station, monthly variables (temperature and total precipitation) were transformed into normalized standard deviations to give each station the same weight in calculating the average monthly values for each year. These data allowed us to quantify the monthly climatic anomalies observed in 2001. Thirdly, to put the 2001 event in a geographical European context, we used station and gridded air temperature data from the European Climate Assessment data set, which contains long and quality-controlled climatic records from meteorological stations covering the whole continent (Klein Tank et al., 2002). This data set has been used to develop the E-OBS gridded climate data set at 0·25 ° resolution, and both are accessible through the internet (http://www.ecad.eu/). We also used this data set to map the December 2001 diurnal temperature range (DTR) which captured the exceptional thermal contrast recorded in the study area (difference between the daily maximum and minimum temperatures for that month).
Climate-based model of Scots pine distribution in Europe
An SDM of Scots pine across Europe was fitted based on presence-only data using MaxEnt (Elith et al., 2011). This method evaluates the environmental tolerance of the species by establishing a probability distribution as a function of a set of constraints (drivers of species occurrence) derived from data occurrence (Phillips and Dudík, 2008), generating an estimate of probability of presence for the species ranging between 0 and 1 (Phillips et al., 2006). We used as constraints different bioclimatic variables (see http://www.worldclim.org/bioclim) obtained for the European continent from the WorldClim data set (Hijmans et al., 2005) gridded at 2·5 arc-min resolution. In addition, the model was fitted using information about the current presence of Scots pine available at the Euforgen webpage (data available at http://www.euforgen.org/distribution-maps/). The model was calibrated with the test data set (n = 3600 presences) and its adjustment was assessed employing the area under the receiver operating characteristic curve (AUC), with higher AUC values corresponding to better models (Phillips et al., 2006). We reserved a randomly selected 20 % of pixels to validate the models, which were fitted using the Maxent software, ver. 3.3.3 (http://www.cs.princeton.edu/∼schapire/maxent/).
Statistical analyses
To estimate the 100 year return interval of the minimum temperatures registered in December 2001, we fitted the December minimum temperatures to generalized extreme value distribution through maximum likelihood methods using the package extRemes (Gilleland and Katz, 2011). Climatic anomalies during the year 2001 were compared with the 1961–1990 averages using one-way t-tests.
The comparison of tree variables between sites was performed using the Mann–Whitney U-test. We used χ2 tests to compare the distribution of dead trees between sites. The associations between climatic variables and BAI residuals were quantified using Pearson correlation coefficients. Correlations between climatic variables and BAI residuals were calculated from previous (year t – 1) to current (years t of tree-ring formation) August based on previous analyses (Voltas et al., 2013).
To evaluate the influence of the extreme climate event on tree growth, we calculated the ratio between the BAI before (1999–2001) and after (2002–2004) the extreme event. Index values <1 (hereafter growth recovery) indicate a decrease in growth after the extreme event, whereas values >1 indicate enhanced growth.
The influence of site conditions on tree growth recovery was evaluated by applying generalized least square (GLS) models (Venables and Ripley, 2002). We considered four variables with a potential influence on growth recovery: soil depth, competition index, defoliation degree and sapwood area. Separate analyses were carried out for each plot. In order to select the best sub-set of predictors, we applied a multimodel inference approach based on information theory (Burnham and Anderson, 2002). All the potential models that can be generated with the set of predictor variables were ranked according to their Akaike information criterion (AIC) value. After that, the model with the lowest AIC was selected. We also calculated the difference in AIC between each model and the best one (ΔAIC), and the relative probability that model i was the best one for the observed data (Wi). The fit of the model was evaluated by graphical examination of the residual and fitted values (Zuur et al., 2009).
Generalized additive mixed models (GAMMs; Wood, 2006) were used to characterize the BAI of Scots pine trees in the four studied plots. GAMMs are a semi-parametric case of generalized linear models in which the response variable depends on smooth functions of the explanatory variable (Hastie and Tibshirani, 1990). Thus, they represent a flexible method to characterize non-linear trends in BAI data (e.g. Camarero et al., 2015).
To study the BAI trajectories of trees for the common period 1950−2004 in each plot and the influence of site conditions, a two-step approach was used: (1) we proposed a model to reveal the main BAI trends in each site and then (2) evaluated the influence of site factors. For the first approach, we proposed a GAMM of the form:
| (3) |
in which the BAI of a tree i is modelled as smooth functions (s) of three predictors (dbh, tree age, year of tree-ring formation) and tree identity (ZiBi) considered as a random effect since multiple measurements were performed for each individual tree during its life. Since BAI of year t depends on the BAI of the previous year (t – 1), we also included in the model an error term (εi) with a first-order temporal autocorrelation structure (AR1, p = 1, q = 0). The smooth terms were represented using default settings of the function gamm in the R statistical software (Wood, 2006). For the second approach, we added to the model presented above four predictor variables: defoliation degree, competition index, soil depth and sapwood area. Among these four predictor variables, in order to select the best sub-set of predictors, a multimodel inference approach based on information theory (Burnham and Anderson, 2002) was applied.
To study the influence of climate on tree growth, the residual variation of BAI was correlated with monthly climate data. First, the residual values of the first GAMM fitted in each site was obtained. With these values, we calculated an averaged residual BAI series in each plot for the period 1950–2004. The residual BAI series were correlated with monthly climatic variables (mean minimum and maximum temperatures, total precipitation) from the previous to current August. This temporal window was chosen based on previous analyses (Richter et al., 1991). All statistical analyses were performed using the R statistical software (www.r-project.org). The nlme package was used to calculate the GLS (Pinheiro et al., 2015). The mgcv package was used to calculate the GAMMs (Wood, 2006), and the MuMIn package was used to perform the multimodel selection (Bartoń, 2009).
RESULTS
The extremeness of the 2001 event
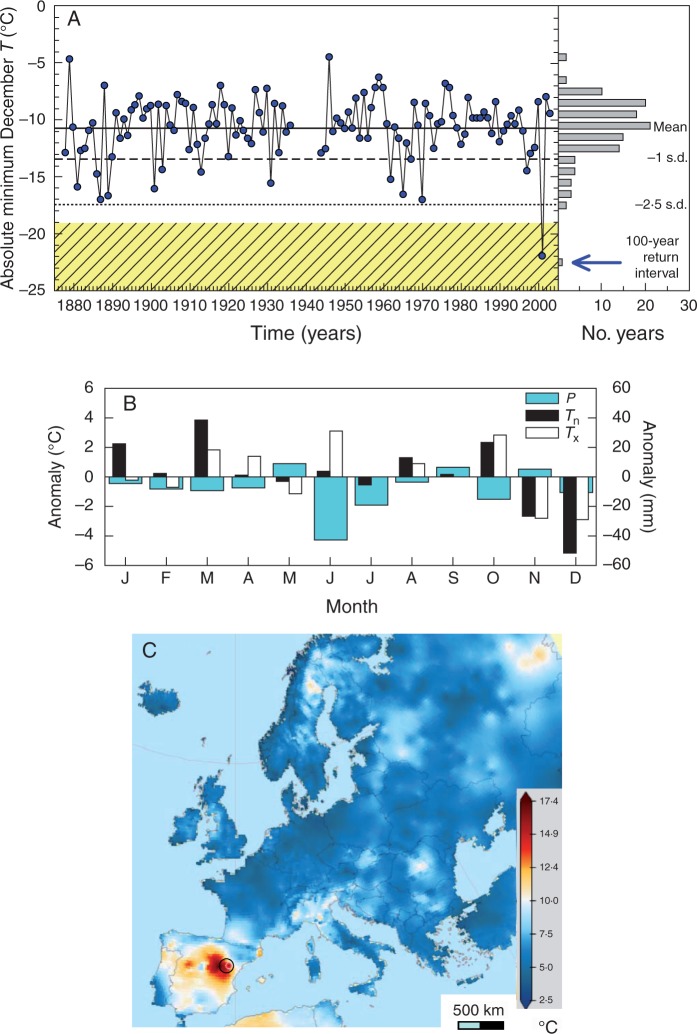
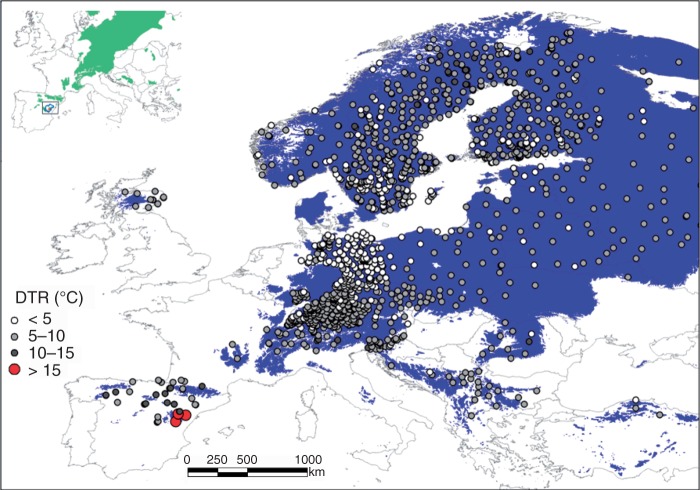
The absolute minimum December temperatures recorded in Teruel station (–22 °C) were the lowest recorded for that month since the late 19th century, and were below the threshold of the 100 year return interval (Fig. 2A). Note that this measurement in the city underestimated the actual thermal conditions in the forests since values of −25 °C were recorded in villages located in the study area (pers. obs.). The year 2001 was characterized by significantly extreme negative anomalies in the case of mean minimum December temperatures (anomaly = –5·1 °C, t = 20·5, P < 0·001) as compared with the 1961–1990 average (Fig. 2B). Both November and December mean maximum temperatures were also very low (anomalies between –2·8 and −2·9 °C), but June (anomaly = +3·1 °C, t = 12·7, P < 0·001) and October (anomaly = +2·8 °C, t = 9·9, P < 0·001) maximum temperatures were anomalously warm. Regarding precipitation, June was also exceptionally dry (anomaly = –42·3 mm, t = 9·3, P < 0·001). Across Europe, the study area registered the highest December 2001 diurnal temperature ranges, with values between 15·0 and 17·5 °C (Fig. 2C). This elevated thermal contrast was produced by high-pressure conditions leading to warm October conditions and followed by a cold front advancing from the Arctic up to north-east Iberia and other transitional areas (e.g. northern Italy). This caused a rapid drop of temperatures in late December 2001 and produced elevated diurnal temperature ranges (Supplementary Data Fig. S1).
Fig. 2.
Temporal (A, B) and spatial (C) contexts defined for the extreme December 2001 cold event which caused dieback in Scots pine (Pinus sylvestris) forests in eastern Spain. The uppermost plot shows the absolute minimum temperatures recorded in Teruel station (note the gap in the 1930s to 1940s) with its distribution and mean, standard deviation (s.d.) and estimated 100 year return interval (hatched area). The middle plot shows the 2001 monthly anomalies with respect to the 1961–1990 average for precipitation (P) and mean minimum (Tn) and maximum (Tx) temperatures. In the lowermost plot, the gridded December 2001 diurnal temperature range is shown across Europe (the circle indicates the study area). The map was built using the E-OBS gridded climate data set (Haylock et al., 2008).
Stand structure, defoliation and mortality
Tree diameter was the only variable that was significantly higher in southerly than in northerly exposed sites in each study area, whereas soil depth was significantly lower in the southern- than in the northern-facing sites from the H study area (Table 1). Severe defoliation (e.g. trees with defoliation >40 % of the crown) was more widespread in trees from southern- (PS, 60 %; HS, 37 %) than from northern-oriented slopes (PN, 4 %; HN, 3 %; Table 1). Mortality peaked in 2001 in site PS, affecting 20 % of trees, while in 2002 12 % of trees died in site HS (Fig. 3). Considering years with dead trees in both study sites, the distribution frequencies of deaths differed between southern- and northern-facing sites in area P (χ2 = 17·7, P = 0·001) but not in area H (χ2 = 0·6, P = 0·30).
Fig. 3.
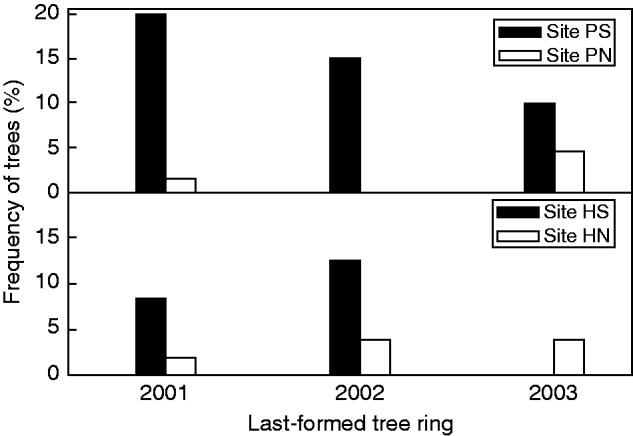
Scots pine (Pinus sylvestris) mortality following an extreme cold event in eastern Spain in 2001. The proportion of dead trees was calculated by dating the last-formed tree ring of all trees sampled in the four study sites.
Growth trends and responses to climate and impact of the 2001 cold event
Trees from south-facing sites possessed thicker stems since they usually grew more than trees from north-facing aspects (Table 2). Growth started decreasing in southerly aspect sites since the 1980s, particularly in the case of area P (Fig. 4). In site PS, growth showed a high year to year variability and started a long-term decline since the 1980s when climate became warmer and drier (results not shown). From the 1990s onwards, growth was similar between south- and north-facing sites. We also detected a growth drop in 1998 which corresponds to a similar cold event occurring in winter 1997–1998, but which was not as extreme as the 2001 event (Supplementary Data Fig. S1).
Table 2.
Tree-ring data for Pinus sylvestris from four study sites located in eastern Spain showing cold-induced dieback
| Site | No. of trees | No. of cores | Age at 1·3 m (years) | Most replicated period | Mean tree-ring width (mm) | BAI recovery |
|---|---|---|---|---|---|---|
| Barranco de la Hoya-North | 64 | 122 | 60 ± 7 | 1947–2005 | 1·56 ± 0·01a | 1·16 ± 0·04b |
| Barranco de la Hoya-South | 24 | 46 | 49 ± 5 | 1940–2005 | 1·76 ± 0·02b | 0·58 ± 0·02a |
| Peñacerradilla-North | 65 | 134 | 70 ± 5 | 1945–2005 | 1·18 ± 0·01a | 0·79 ± 0·03b |
| Peñacerradilla-South | 26 | 53 | 76 ± 6 | 1942–2005 | 1·87 ± 0·03b | 0·64 ± 0·02a |
The basal area increment (BAI) recovery was calculated as the ratio between the BAI means for the periods following (2002–2004) and preceding (1999–2001) an extreme 2001 cold event.
Different letters indicate significant differences between plots of the same site (Mann–Whitney U-tests).
Fig. 4.
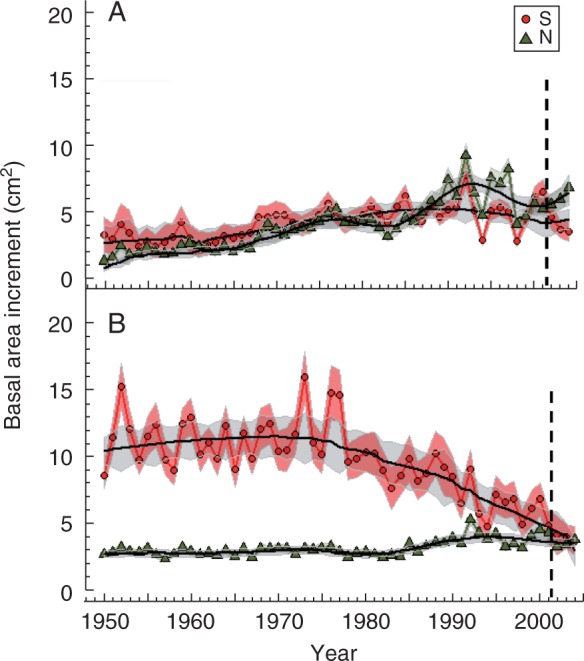
Observed and modelled basal area increment (BAI) patterns for Scots pine (Pinus sylvestris) for two study areas located in eastern Spain [Barranco de la Hoya (H; A) and Peñacerradilla (P; B)] varying in slope aspect (southerly ‘S’ denoted by circles and northerly ‘N’ by triangles). Observed and modelled BAI values are indicated as lines with or without symbols, respectively. The vertical dashed line highlights the 2001 extreme cold event.
The BAI recovery after the 2001 cold event was higher in the north- than in south-facing sites, where growth decline was marked (Table 2). The ability of trees to recover BAI values similar to those observed before the 2001 event decreased as defoliation increased in both southern-exposed sites (Table 3). In contrast BAI trends were associated positively with the amount of sapwood area produced (Table 4).
Table 3.
Summary of models selected to explain basal area increment (BAI) recovery of Pinus sylvestris from four study sites in eastern Spain after a 2001 climatic extreme cold event
| Site | Defoliation | Competition | Sapwood area | Soil depth | ΔAIC | Wi |
|---|---|---|---|---|---|---|
| Barranco de la Hoya-North | – | – | – | – | 1·38 | 0·35 |
| Barranco de la Hoya-South | −7·45* | – | – | – | 0·23 | 0·33 |
| Peñacerradilla-North | – | – | – | – | 1·73 | 0·30 |
| Peñacerradilla-South | −8·08* | – | – | 0·82 | 2·12 | 0·41 |
Generalized least square (GLS) models were used to explain BAI recovery in each site using as explanatory variables: defoliation level, competition index, sapwood area and soil depth.
The asterisks (*) indicate that the variable has a significant (P < 0·05) influence on BAI recovery.
Table 4.
Summary of models selected to explain basal area increment (BAI) patterns of Pinus sylvestris from four study sites in eastern Spain affected by an extreme cold event
| Site | Defoliation | Competition | Sapwood area | Soil depth | ΔAIC | Wi |
|---|---|---|---|---|---|---|
| Barranco de la Hoya-North | – | −0·87 | 1·22 | – | 0·61 | 0·27 |
| Barranco de la Hoya-South | – | – | 2·04* | 0·75 | 2·10 | 0·76 |
| Peñacerradilla-North | – | – | 5·65* | – | 1·74 | 0·53 |
| Peñacerradilla-South | – | – | 2·84* | 0·71 | 0·71 | 0·39 |
A regression model was proposed to explain BAI patterns in each study site using as explanatory variables: defoliation level, competition index, sapwood area and soil depth.
The table shows the t statistic associated with each variable in the selected model.
The asterisks (*) indicate that the variable has a significant (P < 0·05) influence on BAI recovery.
Growth was enhanced by cool and wet conditions in summer and by high precipitation in the previous autumn and winter, particularly in the PS and HN sites (Table 5). Growth was also favoured by warm spring conditions, but decreased if minimum January temperatures increased; this association was stronger in south- than in north-facing sites.
Table 5.
Correlations calculated between monthly climatic variables (mean minimum and maximum temperatures, precipitation) for eastern Spain with basal area increment residuals representing the year to year growth variability of Scots pine (Pinus sylvestris)
| Variable | Year | Month | HS | HN | PS | PN |
|---|---|---|---|---|---|---|
| Mean minimum temperature | Previous | a | 0·144 | 0·222 | –0·049 | 0·006 |
| s | 0·042 | –0·083 | –0·150 | –0·003 | ||
| o | –0·168 | –0·064 | –0·058 | –0·225 | ||
| n | –0·093 | 0·072 | 0·059 | –0·112 | ||
| d | –0·090 | –0·108 | 0·205 | 0·009 | ||
| Current | J | –0·272 | –0·190 | –0·284 | –0·233 | |
| F | –0·183 | 0·025 | 0·007 | –0·125 | ||
| M | –0·053 | –0·099 | –0·177 | –0·175 | ||
| A | 0·157 | 0·321 | 0·148 | 0·139 | ||
| M | 0·081 | 0·196 | 0·420 | 0·361 | ||
| J | –0·184 | –0·019 | –0·043 | –0·073 | ||
| J | –0·247 | –0·132 | –0·211 | –0·034 | ||
| A | –0·156 | –0·171 | –0·106 | 0·003 | ||
| Mean maximum temperature | Previous | a | 0·074 | –0·067 | –0·204 | 0·188 |
| s | 0·058 | –0·207 | –0·235 | –0·021 | ||
| o | 0·017 | 0·079 | –0·008 | –0·242 | ||
| n | –0·072 | 0·075 | –0·054 | 0·073 | ||
| d | –0·224 | –0·050 | 0·034 | 0·016 | ||
| Current | J | –0·247 | –0·148 | –0·233 | –0·237 | |
| F | 0·143 | 0·219 | –0·030 | 0·124 | ||
| M | –0·226 | –0·035 | 0·051 | –0·18 | ||
| A | 0·273 | 0·235 | 0·280 | 0·204 | ||
| M | –0·005 | 0·397 | 0·222 | 0·314 | ||
| J | –0·114 | –0·326 | –0·203 | –0·057 | ||
| J | –0·280 | –0·483 | –0·415 | –0·249 | ||
| A | –0·149 | –0·218 | –0·109 | 0·121 | ||
| Precipitation | Previous | a | –0·020 | 0·125 | 0·183 | –0·166 |
| s | –0·057 | 0·145 | 0·318 | 0·151 | ||
| o | 0·048 | –0·163 | –0·216 | 0·07 | ||
| n | 0·101 | 0·274 | 0·137 | –0·025 | ||
| d | 0·093 | 0·255 | 0·224 | –0·021 | ||
| Current | J | –0·002 | 0·312 | 0·283 | –0·134 | |
| F | –0·181 | –0·097 | –0·026 | 0·007 | ||
| M | –0·052 | –0·188 | –0·044 | –0·013 | ||
| A | 0·095 | –0·004 | 0·271 | –0·091 | ||
| M | 0·021 | 0·044 | 0·075 | –0·040 | ||
| J | 0·050 | 0·241 | 0·394 | 0·225 | ||
| J | 0·201 | 0·342 | 0·412 | 0·123 | ||
| A | 0·088 | 0·154 | 0·152 | –0·096 |
Months abbreviated by lower case and upper case letters correspond to the previous and current year, respectively.
Values shown in bold are significant (P < 0·05).
Model of Scots pine presence in Europe
The SDM facilitated prediction of the climatic range limits P. sylvestris in Europe to contextualize the severity of the 2001 event from a biogeographical point of view. The best fitted model (AUC = 0·778) selected the following explanatory variables for Scots pine presence: annual mean temperature; minimum temperature of the coldest month; mean temperature of the coldest quarter; maximum temperature of the warmest month; and the ratio between actual and potential evapotranspiration of the second quarter of the year (Fig. 5). The maximum temperature of the warmest month accounted for 55 % of the predictive power, and the annual mean temperature and the minimum temperature of the coldest month accounted for 41 %. When compared with the SDM predictions for European Scots pine forests mainly based on maximum and minimum monthly temperatures, the effects of the 2001 cold event at the southerly range limit were unforeseen.
Fig. 5.
Predicted distribution area of Scots pine (Pinus sylvestris) in Europe (blue area), and mean diurnal temperature range (DTR) recorded during December 2001 in meteorological stations located over this area showing maximum DTR values in eastern Spain. The DTR is the difference between the daily maximum and minimum temperatures. The inset shows the Scots pine distribution area and the area affected by winter-drought-induced dieback in winter 2001 situated in eastern Spain, near the low-latitude margin (rear edge) of the species distribution area.
DISCUSSION
The extreme climate conditions of winter 2001–2002 triggered a cold-induced dieback in the rear edge of the Scots pine distribution area, with defoliation (needle loss), mortality and growth being the major responses. However, these responses varied depending on site and tree conditions since the most affected sites were stands located in south-facing areas, and the most intense dieback parameters (defoliation, mortality rate and growth reduction) were apparent in trees formerly showing high growth rates in these sites (Figs 3 and 4). The climate-based model (SDM) selected maximum and minimum temperatures as the best predictors of Scots pine distribution across Europe but failed to account for the effects of climatic extremes such as the 2001 cold event, particularly at the southerly range limit of the species (Fig. 5).
Climatic drivers of dieback related to the 2001 extreme cold event
The low December temperatures recorded in the study site were unusual and extreme (they surpassed the 100 year return interval), as were the resulting high diurnal temperature ranges in the European spatial context. However, this sudden drop in winter temperatures was preceded by very warm autumn conditions and also a dry summer, which complicates determination of the physiological drivers of the dieback (Voltas et al., 2013).
It has been postulated that repeated freeze–thaw events due to the extreme low temperatures and the high and rapid temperature changes in December caused xylem embolism and reduced the cavitation resistance of tracheids (Peguero-Pina et al., 2011). A similar explanation has been posed in the alpine treeline, where the number of days with freeze–thaw cycles was always higher in sun- than in shade-exposed twigs of Norway spruce, contributing to a higher loss of hydraulic conductivity in sun-exposed branches (Mayr et al., 2003).
These physiological explanations are consistent with the observation of more intense damage in south-facing sites and branches, and also with the existence of shallow soils (Table 1). This discrepancy emphasizes the complexity of measuring the actual soil amount available to trees since the dominant substrates in the study area are fractured limestones with deep soil pockets, whereas upper soil layers are usually very shallow (10–25 cm). In fact, long-term BAI trends were not significantly explained by soil depth, which indicates that better and continuous measures of soil water available to growth are needed, for instance using soil water isotopes.
Additional winter factors associated with the anticyclonic conditions probably contributed to the dieback since high radiation levels and leaf to air temperature differences enhance water loss by transpiration if soil temperatures surpass the +8 °C threshold (Mellander et al., 2004). In contrast, soil water availability was very low in 2001/2002 when winter snow cover was also shallow (pers. obs.). A shallow or scarce snow pack is an important driver of cold-induced dieback because the lack of snow insulation makes soil minimum temperatures drop rapidly (e.g. Kullman, 1989; Carlson et al. 2015). As a consequence, soil water uptake is constrained when upper soil layers are cool or frozen during winter or early spring due to a decrease in root permeability (Mellander et al., 2006). In fact, snow removal experiments performed in the study area from winter 2007 to 2011 reduced the minimum soil temperatures from −5 to −7·5 °C (Supplementary Data Fig. S2). Additionally, the warm October conditions could also lead to a late hardening of buds and needles (Leinonen, 1996), but the fact that damage concentrated in southerly exposures makes this explanation less plausible than that based on drought stress due to localized xylem embolism.
Forest dieback responses to climatic extremes depend on site conditions
Recent competition intensity did not explain BAI recovery or BAI trends, suggesting that growth was not constrained by the available space in the study sites. Defoliation was related to BAI recovery in the southern-facing sites (Table 3), which were the stands most affected by dieback, confirming that needle loss caused growth decline and the ability to take up carbon as wood. In the long term, BAI mainly depended on sapwood production which reflects the conductive area of stem, and indirectly the transpiring area of the crown (Galván et al., 2012). Growth in southern-exposed sites was less than in northern-exposed sites, reversing previous trends. Less vigorous trees that had previously exhibited high growth rates were also highly susceptible to mortality and reduced growth following the cold-induced dieback, particularly in the most affected PS site (Figs 3 and 4). In a nearby site which experienced the same dieback process, less vigorous trees grew more and possessed superior hydraulic conductivity (inferred from isotope and anatomical analyses of wood), than normal vigour trees up to 25 years prior to the dieback (Voltas et al., 2013). This suggests that the cold-induced dieback starting in 2001 invoked such physiological differences by triggering dieback in trees with higher growth rates, as the production of sapwood made them more vulnerable to hydraulic failure. Trees more susceptible to the winter-drought dieback were mostly from south-facing slopes where the diurnal temperature ranges were the highest.
Forest growth in sites subject to continental Mediterranean conditions is mainly constrained by reduced soil moisture availability triggered by warm temperatures and elevated spring and summer evapotranspiration (Camarero et al., 2010). Therefore, high diurnal temperature ranges during the growing season are linked to reduced growth of Scots pine (Büntgen et al., 2013). Dry summer 2001 conditions probably contributed to the dieback triggered in winter 2001–2002. Low summer water availability is the major constraint on Scots pine growth in the study sites (Table 5), so we cannot discard the possibility that trees affected by the cold-induced dieback were previously stressed by drought. Previous episodes similar to the one detected in winter 1997–1998 were also preceded by dry summer conditions, which are usual in the study area. This suggests that summer drought followed by winter cold together contribute to dieback at the Scots pine rear edge. Furthermore, warm nights and dry conditions in January were associated with narrow tree rings, particularly in the most affected southern-exposed sites (Table 5), confirming that a wide thermal range in winter is associated with decreased growth, perhaps by enhancing carbohydrate consumption during winter in evergreen conifers (Gimeno et al., 2012).
Implications for forest response to climatic extremes
Climatic extreme events are fundamental drivers of forest dynamics; here we show that rear-edge Scots pine forests subjected to atypical continental and summer-dry Mediterranean conditions experienced cold-induced dieback. The December 2001 cold event was extreme in eastern Spain considering long temporal (100 year return interval) and wide spatial (European distribution of Scots pine) scales. However, the impacts of such climatic extremes on forests depend on site conditions, since here some south-facing sites were the most affected by cold-induced dieback in terms of defoliation, mortality and growth. This is related to the highest diurnal temperature ranges experienced by trees growing in these sites, which promote xylem embolism through recurrent freeze–thaw events. Additional drivers contributed to the winter-drought-induced dieback such as previous summer dry conditions, shallow soils and reduced snow pack.
We used an SDM of Scots pine to illustrate how such climate-based models do not fully account for the effects of climate extremes on forests responses. Specifically, this ‘naïve’ model selected maximum and minimum temperatures as the best predictors of Scots pine distribution and predicted reasonably well the European distribution of P. sylvestris (Fig. 5). However, the model also allowed the importance of climate variability on present and future species ranges and the need to incorporate stochastic climate extremes such as the 2001 cold event into SDMs to be highlighted (cf. Zimmermann et al., 2009; Rasztovits et al., 2014). We conclude that climatic extremes are pivotal for tree species distributions, and argue that because anthropogenic climate change could alter the inherent variability of these weather phenomena, forests will be impacted in unexpected places such as lower latitude (‘rear edge’) distribution limits (Seneviratne et al., 2012). Mediterranean conifer forests are shaped by the strong seasonality in water availability and usually present drought-induced dieback in response to long dry spells and high temperatures (Camarero et al., 2015), but, as we show here, these forests can also react to cold-induced dieback by showing elevated defoliation levels, growth decline and high tree mortality rates.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: daily temperatures recorded in the Teruel station during October and December 2001 compared with the period 1950–2004, and mean diurnal temperature range for December 2001 expressed as anomalies or deviations from the 1961–1990 average. Figure S2: effects of snow removal on soil minimum temperatures at the study site as related to air temperatures recorded from November to March from 2007 to 2011. Table S1: characteristics of local meteorological stations used to build the regional mean series.
ACKNOWLEDGEMENTS
A. Gazol is supported by a Postdoctoral grant from MINECO (Contrato Formacion Postdoctoral MINECO - FPDI 2013-16600, FEDER funds). We thank all colleagues from ‘Laboratorio de Sanidad Forestal’ and CITA who helped and contributed to field data. We acknowledge the E-OBS data set from the EU-FP6 project ENSEMBLES (http://ensembles-eu.metoffice.com) and the data providers in the ECA&D project (http://www.ecad.eu). We thank the reviewers, the Handling Editor (Dr Mick Hanley) and the Chief Editor (Professor Heslop-Harrison) for their comments on previous versions of the manuscript.
LITERATURE CITED
- Augspurger CK. 2009. Spring 2007 warmth and frost: phenology, damage and refoliation in a temperate deciduous forest. Functional Ecology 23: 1031–1039. [Google Scholar]
- Bartoń K. 2009. MuMIn: multi-model inference. http://CRAN.R-project.org/, R package, version 1.0.0. [Google Scholar]
- Biondi F, Qaedan F. 2008. A theory-driven approach to tree-ring standardization: defining the biological trend from expected basal area increment. Tree-Ring Research 64: 81–96. [Google Scholar]
- Brunet M, Jones PD, Sigró J, et al. 2007. Temporal and spatial temperature variability and change over Spain during 1850–2005. Journal of Geophysical Research 112: D12117. [Google Scholar]
- Büntgen U, Martínez-Peña F, Aldea J, et al. 2013. Declining pine growth in Central Spain coincides with increasing diurnal temperature range since the 1970s. Global and Planetary Change 107: 177–185. [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. New York: Springer. [Google Scholar]
- Camarero JJ, Sancho-Benages S. 2006. Dendroecología, decaimiento del bosque y relaciones no lineares entre clima y crecimiento radial. In: Cuadrat JM, Saz MA, Vicente Serrano SM, Lanjeri S, de Luis M, González-Hidalgo JC, eds. Clima, Sociedad y Medio Ambiente. Zaragoza: Librería General, 1–9. [Google Scholar]
- Camarero JJ, Olano JM, Parras A. 2010. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytologist 185: 471–480. [DOI] [PubMed] [Google Scholar]
- Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. 2015. To die or not to die: early-warning signals of dieback in response to a severe drought. Journal of Ecology 103: 44–57. [Google Scholar]
- Carlson BZ, Choler P, Renaud J, Dedieu J-P, Thuiller W. 2015. Modelling snow cover duration improves predictions of functional and taxonomic diversity for alpine plant communities. Annals of Botany 116: 1023– 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbertin M. 2005. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. European Journal of Forest Research 124: 319–333. [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. 2011. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions 17: 43–57 [Google Scholar]
- Fritts HC. 2001. Tree rings and climate, 2nd edn Caldwell: Blackburn Press. [Google Scholar]
- Galván JD, Camarero JJ, Sangüesa-Barreda G, Alla AQ, Gutiérrez E. 2012. Sapwood area drives growth in mountain conifer forests. Journal of Ecology 100: 1233–1244. [Google Scholar]
- Gilleland E, Katz RW. 2011. New software to analyze how extremes change over time. Eos 92: 13–14. [Google Scholar]
- Gimeno TE, Camarero JJ, Granda E, Pías B, Valladares F. 2012. Enhanced growth of Juniperus thurifera under a warmer climate is explained by a positive carbon gain under cold and drought. Tree Physiology 32: 326–336. [DOI] [PubMed] [Google Scholar]
- Gutschick VP, BassiriRad H. 2003. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytologist 160: 21–42. [DOI] [PubMed] [Google Scholar]
- Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8: 461–467. [DOI] [PubMed] [Google Scholar]
- Hänninen H. 1991. Does climatic warming increase the risk of frost damage to northern trees? Plant, Cell and Environment 14: 449–454. [Google Scholar]
- Hastie T, Tibshirani R. 1990. Generalized additive models. New York: Chapman & Hall. [DOI] [PubMed] [Google Scholar]
- Haylock MR, Hofstra N, Klein Tank AMG, Klok EJ, Jones PD, New M. 2008. A European daily high-resolution gridded dataset of surface temperature and precipitation. Journal of Geophysical Research (Atmospheres) 113: D20119. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Holmes RL. 1983. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin 43: 68–78. [Google Scholar]
- Holmes RL. 1994. Dendrochronology program library users manual. University of Arizona: Tucson, AZ. [Google Scholar]
- IPCC. 2013. Summary for policymakers. In: Stocker TF, Qin D, Plattner G-K, et al., eds. Climate Change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge: Cambridge University Press, 3–29. [Google Scholar]
- Jalili A, Jamzad Z, Thompson K, et al. 2010. Climate change, unpredictable cold waves and possible brakes on plant migration. Global Ecology and Biogeography 19: 642–648. [Google Scholar]
- Klein Tank AMG, Wijngaard JB, Konnen GP, et al. 2002. Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. International Journal of Climatology 22: 1441–1453. [Google Scholar]
- Kullman L. 1989. Cold-induced dieback of montane spruce forests in the Swedish Scandes – a modern analogue of paleoenvironmental processes. New Phytologist 113: 377−389. [DOI] [PubMed] [Google Scholar]
- Kullman L. 1991. Cataclysmic response to recent cooling of a natural boreal pine (Pinus sylvestris L.) forest in Northern Sweden. New Phytologist 117: 351–360. [Google Scholar]
- Leinonen I. 1996. A simulation model for the annual frost hardiness and freeze damage of Scots pine. Annals of Botany 78: 687–693. [Google Scholar]
- Marchand PJ. 1996. Life in the cold, 3rd edn Hanover, NH: University Press of New England. [Google Scholar]
- Matías L, Jump AS. 2012. Interactions between growth, demography and biotic interactions in determining species range limits in a warming world: the case of Pinus sylvestris. Forest Ecology and Management 282: 10–22. [Google Scholar]
- Matusick G, Ruthrof KX, Brouwers NC, Hardy GSJ. 2014. Topography influences the distribution of autumn frost damage on trees in a Mediterranean-type Eucalyptus forest. Trees-Structure and Function 28: 1449–1462. [Google Scholar]
- Mayr S, Schwienbacher F, Bauer H. 2003. Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in Stone pine? Plant Physiology 131: 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellander PE, Bishop K, Lundmark T. 2004. The influence of soil temperature on transpiration: a plot scale manipulation in a young Scots pine stand. Forest Ecology and Management 195: 15–28. [Google Scholar]
- Mellander PE, Stahli M, Gustafsson D, Bishop K. 2006. Modelling the effect of low soil temperatures on transpiration by Scots pine. Hydrological Processes 20: 1929–1944. [Google Scholar]
- Müller HER, Stierlin HR. 1990. Sanasilva tree crown photos with percentages of foliage loss. Birmensdorf: WSL. [Google Scholar]
- Pearce RS. 2001. Plant freezing and damage. Annals of Botany 87: 417–424. [Google Scholar]
- Pederson N, Cook ER, Jacoby G, Peteet DM, Griffin KL. 2004. The influence of winter temperatures on the annual radial growth of six northern range margin tree species. Dendrochronologia 22: 7–29. [Google Scholar]
- Peguero-Pina JJ, Alquézar-Alquézar JM, Mayr S, Cochard H, Gil-Pelegrín E. 2011. Embolism induced by winter drought may be critical for the survival of Pinus sylvestris L. near its southern distribution limit. Annals of Forest Science 68: 565–574. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modelling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- Phillips SJ, Dudík M. 2008. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161–175. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2015. nlme: Linear and nonlinear mixed effects models. http://CRAN.R-project.org/, R package version 3.1-120, package=nlme. [Google Scholar]
- Pomerleau R. 1991. Experiments on the causal mechanisms of dieback on deciduous forests in Quebec. Sainte-Foy: Forestry Canada. [Google Scholar]
- Rasztovits E, Berki I, Mátyás C, Czimber K, Pötzelsberger E, Moricz N. 2014. The incorporation of extreme drought events improves models for beech persistence at its distribution limit. Annals of Forest Science 71: 201–210. [Google Scholar]
- Richardson DM. 1998. Ecology and biogeography of Pinus. Cambridge: Cambridge University Press. [Google Scholar]
- Richter K, Eckstein D, Holmes RL. 1991. The dendrochronological signal of pine trees (Pinus spp.) in Spain. Tree Ring Bulletin 51: 1–13. [Google Scholar]
- Sakai A, Larcher W. 1987. Frost survival of plants: responses and adaptation to freezing stress. New York: Springer-Verlag. [Google Scholar]
- Sánchez-Salguero R, Navarro-Cerillo RM, Camarero JJ, Fernández-Cancio A. 2012. Selective drought-induced decline of pine species in southeastern Spain. Climatic Change 113: 767–785. [Google Scholar]
- Sánchez-Salguero R, Camarero JJ, Hevia A, et al. 2015. What drives growth of Scots pine in continental Mediterranean climates: drought, low temperatures or both? Agricultural and Forest Meteorology 206: 151–162. [Google Scholar]
- Seneviratne SI, Nicholls N, Easterling D, et al. 2012. Changes in climate extremes and their impacts on the natural physical environment. In: Field CB, Barros V, Stocker TF, et al., eds. Managing the risks of extreme events and disasters to advance climate change adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC SREX Report). Cambridge: Cambridge University Press, 109–230. [Google Scholar]
- Smith MD. 2011. An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. Journal of Ecology 99: 656–663. [Google Scholar]
- Soulé PT, Knapp PA. 2007. Topoedaphic and morphological complexity of foliar damage and mortality within western juniper (Juniperus occidentalis var. occidentalis) woodlands following an extreme meteorological event. Journal of Biogeography 34: 1927–1937. [Google Scholar]
- Strain BR. 1966. The effect of a late spring frost on the radial growth of variant quaking aspen biotypes. Forest Science 12: 334–337. [Google Scholar]
- Tranquillini W. 1979. Physiological ecology of the alpine timberline. Tree existence at high altitudes with special reference to the European Alps. Berlin: Springer. [Google Scholar]
- Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York: Springer. [Google Scholar]
- Voltas J, Camarero JJ, Carulla D, Aguilera M, Ortiz A, Ferrio JP. 2013. A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant, Cell and Environment 36: 1435–1448. [DOI] [PubMed] [Google Scholar]
- Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- Woods KD. 2000. Dynamics in late-successional hemlock–hardwood forests over three decades. Ecology 81: 110–126. [Google Scholar]
- Zimmermann NE, Yoccoz NG, Edwards TC, et al. 2009. Climatic extremes improve predictions of spatial patterns of tree species. Proceedings of the National Academy of Sciences, USA 106: 19723–19728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith G. 2009. Mixed effects models and extensions in ecology with R. New York: Springer. [Google Scholar]
- Zwicke M, Picon-Cochard C, Morvan-Bertrand A, Prud’homme M-P, Volaire F. 2015. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Annals of Botany 116: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.