Abstract
The euryarchaeon Thermococcus prieurii inhabits deep-sea hydrothermal vents, one of the most extreme environments on Earth, which is reduced and enriched with heavy metals. Transmission electron microscopy and cryo-electron microscopy imaging of T. prieurii revealed the production of a plethora of diverse membrane vesicles (MVs) (from 50 nm to 400 nm), as is the case for other Thermococcales. T. prieurii also produces particularly long nanopods/nanotubes, some of them containing more than 35 vesicles encased in a S-layer coat. Notably, cryo-electron microscopy of T. prieurii cells revealed the presence of numerous intracellular dark vesicles that bud from the host cells via interaction with the cytoplasmic membrane. These dark vesicles are exclusively found in conjunction with T. prieurii cells and never observed in the purified membrane vesicles preparations. Energy-Dispersive-X-Ray analyses revealed that these dark vesicles are filled with sulfur. Furthermore, the presence of these sulfur vesicles (SVs) is exclusively observed when elemental sulfur was added into the growth medium. In this report, we suggest that these atypical vesicles sequester the excess sulfur not used for growth, thus preventing the accumulation of toxic levels of sulfur in the host's cytoplasm. These SVs transport elemental sulfur out of the cell where they are rapidly degraded. Intriguingly, closely related archaeal species, Thermococcus nautili and Thermococcus kodakaraensis, show some differences about the production of sulfur vesicles. Whereas T. kodakaraensis produces less sulfur vesicles than T. prieurii, T. nautili does not produce such sulfur vesicles, suggesting that Thermococcales species exhibit significant differences in their sulfur metabolic pathways.
Keywords: Archaea, Thermococcales, Membrane vesicles, Detoxification, Sulfur
Highlights
-
•
We report the presence of sulfur vesicles from Thermococcales.
-
•
Sulfur vesicles are formed from elemental sulfur added to the growth medium.
-
•
Sulfur vesicles are rapidly degraded outside the cell.
-
•
The production of sulfur vesicles is not a general phenomenon in Thermococcales.
-
•
Sulfur vesicles play a key role in sulfur detoxification.
1. Introduction
The production and release of membrane vesicles (MVs) is a widespread mechanism in the three domains of life [1]. Active investigations of MVs have increased dramatically over the last years, as emphasized by the creation in 2011 of the International Society for Extracellular Vesicles (ISEV). Study of extracellular membrane vesicles represents an exciting field of research in cellular interactions, horizontal gene transfer and biological evolution. MVs contain various bioactive molecules such as proteins, lipids and nucleic acids. They can mediate cell–cell communication and are involved in very diverse biological processes [2], [3]. In particular, they participate in transfer and delivery of their components: DNA and RNA, proteins such as virulence factors, toxins and other molecules such as quorum sensing factors [4], [5], [6].
The trafficking of extracellular MVs and their biological roles are well-studied processes in eukaryotes and an increasing number of new studies are being carried out in diverse bacteria [7]. In archaea, the production of MVs has been mainly studied in species of the genus Sulfolobus and Thermococcus [8], [9], [10], [11], [12], [13], [14]. Archaeal MVs are typically 50 nm – 250 nm spherical bodies that originate by protrusion from the cell envelope. Sulfolobus and Thermococcus MVs indeed mainly contain membrane proteins, lipids and S-layer proteins. Interestingly, Sulfolobus MVs can transport antimicrobial proteins named sulfolobicins, which inhibit the growth of other Sulfolobus species [8], [12], [13]. MVs produced by Thermococcus are associated with genomic/plasmidic DNA and can be confused with viral particles in epifluorescence microscopy analyses [10], [15]. MVs from Thermococcus can transfer DNA between cells at high temperatures, at least between cells of the same species (Thermococcus kodakaraensis) [14], [16]. Interestingly, some MVs produced by Thermococcus nautili harbor a plasmid, pTN3, corresponding to the genome of a defective virus. These unique biological entities have been named viral membrane vesicles: vMVs [17]. It has been speculated that vMVs can serve as vehicles for the transport of viral genome in the absence of viral infection [18]. In addition to MVs, Thermococcus species produce large numbers of tubular structures named nanopods or nanotubes formed by long strings of MVs surrounded by S-layer [10], [14].
In eukaryotes and bacteria, several studies have shown that membrane vesicles can play a role in detoxification [19], [20]. This phenomenon was first observed in eukaryotic marine organisms such as mollusks and crustaceans which accumulate cadmium. Storage and excretion of cadmium are performed by MVs as a detoxifying mechanism [21]. Later it was found that another eukaryotic microorganism, Dictyostelium discoideum, can get rid of Hoechst 33342 or drugs like hypericin, used in some cancer diagnosis, by secretion of MVs embedding these molecules [22], [23], [24].
Notably, numerous bacteria produce vesicles containing sulfur (referred to as sulfur globules in some publications) [25], [26]. Sulfur is an important element for microbial life present in deep-sea environments and is metabolized by a wide variety of microorganisms [27], which transiently store sulfur in intracellular vesicles. The production of sulfur vesicles (SVs) has been observed in many free-living bacteria of the Proteobacteria division [28] and magnetotactic bacteria [29], [30] but also in bacterial endosymbionts of animals such as the vestimentiferan Riftia pachyptila or the ciliate Zoothamnium niveum living in sulfidic deep-sea environments [31], [32]. More recently, it has been shown that bacterial endosymbionts of the marine tubeworms Sclerolinum contortum of the Siboglinidae family produce globules, which could also play a key role in sulfide detoxification [33]. The hydrogen sulfide naturally present in deep-sea hydrocarbon seeps is an energy source for the symbionts but it is also highly toxic for the host. The endosymbionts thus produce many globules containing sulfur crystals non-toxic to the host. The sulfur crystals inside globules originate from the excess of hydrogen sulfide in tubeworm cells [33].
Here, we report the discovery of vesicles containing sulfur (sulfur vesicles) produced by Thermococcus species. This finding was made during the course of transmission and cryo-electron microscopy analyses of MVs produced by the hyperthermophilic archaeon Thermococcus prieurii. T. prieurii was isolated from hydrothermal chimney sample collected from the East Pacific Rise, at 2700 m depth [34]. We have previously shown that T. prieurii produces a virus named TPV1 [35] and harbors also two other extrachromosomal elements: the small rolling-circle (RC) plasmids pTP1 and pTP2 [36]. Here, we show that, in addition to TPV1 virions, T. prieurii produces abundant MVs, especially long nanotubes filled with small MVs and sulfur vesicles. These sulfur vesicles are only observed when elemental sulfur was added to the growth medium of the host and were never observed in purified MVs preparation, suggesting that they are degraded as soon as they are released into the growth medium. We suggest that these dark vesicles accumulate excess of sulfur and transport it outside the host cell as a detoxifying mechanism. Interestingly, the strain T. kodakaraensis also produces sulfur vesicles but less than T. prieurii and we did not observe sulfur vesicles in a parallel study of MVs produced by Thermococcus nautili. This indicates that production of sulfur vesicles is not a general phenomenon in Thermococcus species, but could be related to some specific sulfur metabolic pathway characteristic of few Thermococcales.
2. Material and methods
2.1. Strain and growth conditions
Thermococcus prieurii, Thermococcus kodakaraensis and Thermococcus nautili were cultivated at 85 °C with shaking, in Ravot medium supplemented with elemental sulfur (10 g/L) as previously described [34], [37]. Cultures were also grown in Ravot medium with l-cystine (10 g/L) to replace elemental sulfur.
2.2. Isolation and purification of membrane vesicles from culture medium
Purified membranes vesicles from T. nautili and T. prieurii were prepared as previously described [18]; briefly, a 10-ml culture (stationary phase) of each strain was centrifuged twice at 8000 × g for 20 min, then at 16,000 × g for 20 min, to remove debris and cells. Supernatants were collected and centrifuged at 120,000 × g for 2 h at 8 °C in a Beckman 45 Ti rotor. The pellets containing vesicles were suspended in 100 μl of buffer (10 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2). Preparations of isolated vesicles were examined by transmission electron microscopy and cryo-electron microscopy.
2.3. Transmission electron microscopy (TEM)
To prepare samples for TEM, 4 ml cultures (stationary phase) were centrifuged at 5000 × g for 10 min. Supernatants were ultracentrifuged at 120 000 × g for 2 h. The pellets were then resuspended with 100 μl of buffer containing 10 mM Tris-HCl, 100 mM NaCl and 5 mM CaCl2). 20 μl droplets of samples were adsorbed onto a carbon-coated copper grid for 1 min. After removing the excess liquid, the samples were negatively stained with 2% uranyl acetate for 1 min, as previously described by Soler and colleagues [10]. Specimens were examined using a JEOL electron microscope (JEM 100 CX II, operating at 120 kV).
2.4. Cryo-electron microscopy (Cryo-EM)
To prepare samples for Cryo-EM, 10 ml cultures (stationary phase) were centrifuged at 5000 × g for 20 min. The pellets were resuspended with 50 μl of buffer (10 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2). 10 μl droplets of preparations were adsorbed onto a grid covered with a perforated fine layer of carbon (QUANTIFOIL®, R2/4). After removing the excess liquid with Whatman® paper, the grids were quickly immersed in liquid ethane and transferred under liquid nitrogen into the microscope using a side entry nitrogen-cooled (Gatan, 626-DH cryotransfer system) cryoholder. The observations were performed with a Jeol JEM-2100 transmission cryo-electron microscope with an acceleration voltage of 200 kV, a nominal magnification of 10,000 K. Images were recorded under low dose conditions with ultra-scan 1000 camera (Gatan, 2 × 2).
2.5. Energy dispersive X-Ray spectroscopy (EDS) analysis
EDS (Jeol) was carried out to detect compounds that were either adsorbed to the cell surface or entrapped in the membrane vesicles.
3. Results
3.1. Morphology and heterogeneity of T. prieurii extracellular membrane vesicles
We have previously shown that T. prieurii produces lemon shaped virions containing the genome of the UV-inducible virus TPV1 [35]. TPV1 virions were isolated by ultracentrifugation either in linear Iodixanol gradients or in CsCl buoyant density gradients. In order to investigate whether T. prieurii also produces membrane vesicles (MVs), we used the protocol set up to isolate T. nautili vesicles that only involves ultracentrifugation. Preparations were then examined by transmission electron microscopy as previously described [10]. In addition, we examined these preparations by cryo-electron microscopy, a technique that we have not used previously to study vesicles from Thermococcus species.
TEM examination of purified MVs revealed that T. prieurii produces abundant pleomorphic and heterogenous MVs with sizes ranging from 50 to 400 nm (Fig. 1A and B) as well as tubular structures containing long chain of small internal vesicles (50 nm) (Fig. 1A and B). The tubular structures (nanopods/nanotubes) were especially long compared to those produced by other Thermococcus species. As many as 35 vesicles could be counted in these structures. Notably, we only observed a few TPV1 viral particles in our preparations (Fig. 1C). Cryo-electron microscopy examination (Cryo-EM) of purified vesicles confirmed the heterogeneity and size of MVs (Fig. 1D–F) allowing a more detailed view of their internal structure. Most T. prieurii MVs were not covered by S-layer and appeared “naked” (Fig. 1D–F). On Fig. 1E, one can observe a vesicle with a larger envelope (about 30 nm). Furthermore, Fig. 1E shows that free MVs can probably also fuse to form super-vesicles (more than 400 nm) (Fig. 1F).
Fig. 1.
Micrographs of purified vesicles isolated from T. prieurii. A, B, C: Electron micrographs of purified vesicles and nanotubes negatively stained with 2% uranyl acetate showing the diversity of vesicles; arrows indicate the presence of nanotubes (A, B); only one TPV1 viral particle among vesicles indicated by an arrow (C), bar: 200 nm. D, E, F: Cryo-electron micrographs of purified vesicles showing the size diversity (D), the merged vesicles (E) and a super-vesicle (F), bar: 100 nm.
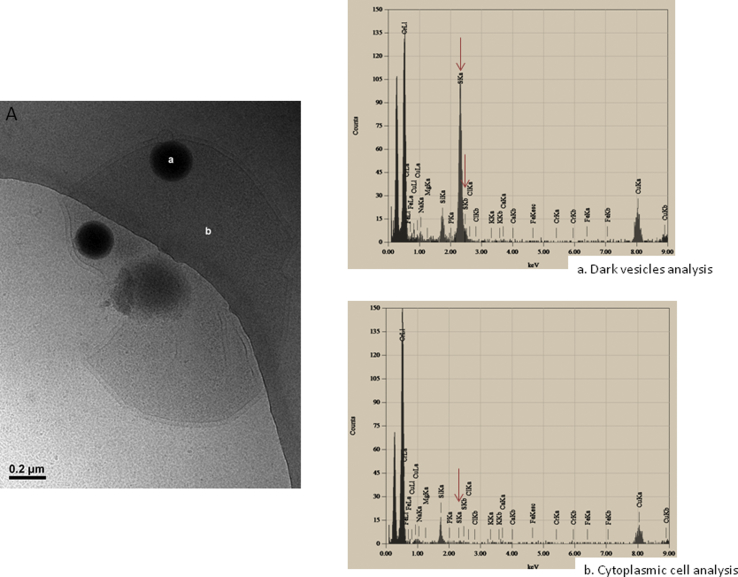
To gain further insights on the mechanism of MV production, we analyzed whole cells of T. prieurii using Cryo-EM. Surprisingly, we then observed numerous dark vesicles in our cell preparations (Fig. 2A–C). These darks vesicles, which have sizes ranging from 50 to 150 nm, were always observed in association with the host cells (between 4 and 16 dark vesicles for 16 cells examined). They were very rarely detected in a free form (Fig. 2A) in the cultures or in preparations of purified vesicles (Fig. 1). As shown in Fig. 2, these dark vesicles are released by budding through the cell envelopes (Fig. 2A1–C1). Thus, whereas dark vesicles appeared naked inside the cells, they were surrounded by the cell S-layer during the budding process and shortly after their release (compare Fig. 2B2 and C2). In order to determine the atomic content of these unusual intracellular dark vesicles, we performed EDX elemental spectroscopic analyses of isolated dark vesicles. As shown in Fig. 3Aa, this analysis revealed that dark vesicles contain basically pure sulfur. In contrast, the characteristic peaks of sulfur were not detectable in vesicle-free control regions of the cell cytoplasm (Fig. 3Ab). Carbon, copper and silica peaks were present in both samples due to the coating of the grid.
Fig. 2.
Cryo-electron micrographs of T. prieurii cells associated with numerous dark vesicles (A, B, C). A1, B1 and C1: Close up of extrusion of dark vesicles through the cell envelope. B2: Close up of dark vesicles surrounded by S-layer indicated by the white arrow. C3: Close up of intracellular dark vesicles not surrounded by S-layer; bar: 200 nm.
Fig. 3.
A: Cryo-electron micrographs of T. prieurii cell with dark vesicles, bar: 200 nm a, b: Representative EDX analysis. a: EDX spectrum of dark vesicles with peaks at 2.33 keV corresponding to sulfur Kα lines indicated by red arrow. b: EDX spectrum of cell cytoplasm.
In our experiments, T. prieurii was cultivated in Ravot medium supplemented with elemental sulfur. To support our hypothesis that the sulfur present in these dark vesicles does indeed originate from the elemental sulfur added to the growth medium, we replaced elemental sulfur by l-cystine in the growth medium and repeated the same analyses. Fig. 4A–C show that the number of sulfur vesicles was greatly reduced (only one or two dark vesicles per cell) in the first subculture of Ravot medium containing l-cystine. Intriguingly, these SVs were much larger (from 150 nm to 250 nm) than those previously observed in the medium supplemented with sulfur. EDX analyses confirmed the presence of sulfur in these larger dark vesicles (data not shown). In the second subculture of Ravot medium with l-cystine, T. prieurii continued to produce vesicles. However all vesicles observed by Cryo-EM were clear vesicles (Fig. 4D–F). Indeed, EDX analysis revealed that these clear vesicles do not contain sulfur in detectable amounts (data not shown).
Fig. 4.
Cryo-electron micrographs of T. prieurii cells, bar: 200 nm. A, B, C: First subculture of T. prieurii into Ravot medium supplemented with l-cystine. Arrows indicate the presence of only one large dark sulfur vesicle. D, E, F: Second subculture of T. prieurii into Ravot medium supplemented with l-cystine. Arrows indicate the presence of only clear vesicles devoid of sulfur.
Whole cells of T. kodakaraensis, a model organism for which numerous genetic tools are available, were also examined by Cryo-EM. We observe that T. kodakaraensis produces also sulfur vesicles; this is confirmed by EDX analyses (Fig. 5). Interestingly, the number of SVs produced by T. kodakaraensis (up to 2 SVs per cell) is less than the number of SVs produced by T. prieurii (up to 16 SVs per cell) in the same culture conditions. In the first subculture of Ravot medium containing l-cystine, T. kodakaraensis did not produce sulfur vesicles (Fig. S1).
Fig. 5.
Cryo-electron micrographs of T. kodakaraensis cells with dark vesicles (A, B), bar: 200 nm a, b: Representative EDX analysis. a: EDX spectrum of dark vesicles with peaks at 2.33 keV corresponding to sulfur Kα lines indicated by red arrow. b: EDX spectrum of cell cytoplasm.
To determine if our model Thermococcus species, Thermococcus nautili [37], [38] also produces sulfur vesicles, we examined T. nautili cells directly by Cryo-EM. As expected, Fig. 6A shows the presence of numerous and heterogeneous membrane vesicles from T. nautili. Cryo-EM imaging of purified vesicles confirmed that these vesicles were derived from the host's cell membrane. The MVs are clearly surrounded by the cell S-layer, with diameters ranging between 50 and 200 nm (Fig. 6B). Notably, no dark vesicles were observed for the T. nautili samples using Cryo-EM (Fig. 6C, C1 and D). Furthermore, the clear vesicles observed in T. nautili samples do not contain sulfur in detectable amounts (data not shown). In contrast to sulfur vesicles, individual clear vesicles are observed in the culture medium and their structure is similar to those of purified vesicles of T. nautili.
Fig. 6.
Micrographs of purified vesicles isolated from T. nautili. A: Electron micrographs of purified vesicles and nanotubes negatively stained with 2% uranyl acetate showing the diversity of vesicles; bar: 200 nm. B: Cryo-electron micrographs of purified vesicles showing vesicles surrounded by the host S-Layer; bar: 100 nm. C: Cryo-electron micrographs of T. nautili cells associated with its vesicles. C1 and D: Close up of extrusion of vesicles through the cell envelope; bar: 200 nm.
4. Discussion
Based on TEM and Cryo-EM observations, we revealed that T. prieurii, in addition to producing numerous membrane vesicles and especially long strings of MVs surrounded by S-layer, also produces sulfur vesicles (SVs). The latter are almost always observed in association with the cells. It appears that their presence is directly dependent on the elemental sulfur added into the growth medium since they disappeared when cells were cultivated in a medium without elemental sulfur. We suspect that SVs accumulate excess elemental sulfur within the cells and transport it outside the cell, thus avoiding the accumulation of toxic concentrations of sulfur in the cytoplasm. These SVs were never observed in purified MVs preparations and very rarely outside cells in Cryo-EM preparations, suggesting that they are rapidly degraded outside the cell. Further studies such as XANES spectroscopy will be required to reveal the chemical speciation of sulfur inside the SVs [39] and in the cytoplasm of T. prieurii and T. kodakaraensis since Raman spectroscopy used in this study did not give informations about the nature of intracellular sulfur due to fluorescence issues (Fig. S2).
Thermococcales gain energy by fermentation using peptides as the carbon source and most of them require elemental sulfur (S°) as an electron acceptor [40], [41]. The S° is reduced to hydrogen sulfide [42]. It has been shown that magnetotactic bacteria and few alpha-proteobacteria produce sulfur globules when cells are grown on sulfide [29], [30], [43]. However, it is unlikely that SVs from T. prieurii are produced in response to excess hydrogen sulfide. Indeed, presence of either l-cystine or elemental sulfur in the growth medium results in the generation of hydrogen sulfide [34], [37], [44], [45]. This is not true for the SVs, which disappear when the elemental sulfur is replaced by l-cystine. We thus suspect that T. prieurii SVs are formed in response to excess elemental sulfur.
The main mechanism of the fermentation-based S° reduction in Thermococcales involves two enzymes: NAD(P)H elemental sulfur oxidoreductase (NSR also called CoADR for Coenzyme A Disulfide Reductase) and MBX (membrane-bound oxidoreductase) [46], [47], [48], [49], [50], [51]. As elemental sulfur is poorly soluble in water [52] and not known at higher temperature, the sulfur particles probably have direct physical contact with the cells as already described for Allochromantium vinosum [53], [54]. However, very little is known about the precise mechanisms of sulfur uptake into the cell [46]. Thermococcales contain also two distinct S°- reducing enzymes, which are cytoplasmic: a sulfhydrogenase and an iron-sulfur flavor protein named sulfide dehydrogenase [55]. Substrates by these enzymes are polysulfides. Moreover soluble polysulfides are generated from S° combined with sulfide [56]. This suggest that the intracellular sulfur could be formed by an excess of polysulfides in the cytoplasm.
In A. vinosum, a direct contact between the sulfur and the cell leads to the formation of sulfur globules [53]. In their studies, Schut and colleagues demonstrated that the enzyme NSR can be saturated in vitro due to an excess of elemental sulfur (at a concentration of 6.4 g/L) [46]. It is possible that NSR is saturated in the conditions of our study, leading to the rapid accumulation of sulfur in the cytoplasm. The formation of SVs could prevent possible damaging effects of this accumulation by sequestering excess of sulfur not immediately used for the growth.
Notably, the production of SVs does not seem to be a common phenomenon in Thermococcales, since we never observed SVs in the case of T. nautili. This suggests some differences in the sulfur metabolic pathways between T. Prieurii and T. kodakaraensis on the one hand and T. nautili on the other hand. We speculate that the NSR of T. nautili is not saturated by the sulfur concentration used in this study and/or that other enzymes (such as the two sulfide dehydrogenases: SudH I and SudH II) could be used for S° reduction [48]. The genome of T. prieurii is currently being sequenced and this should help in the future the identification of differences in the sulfur metabolic pathways of these Thermococcales.
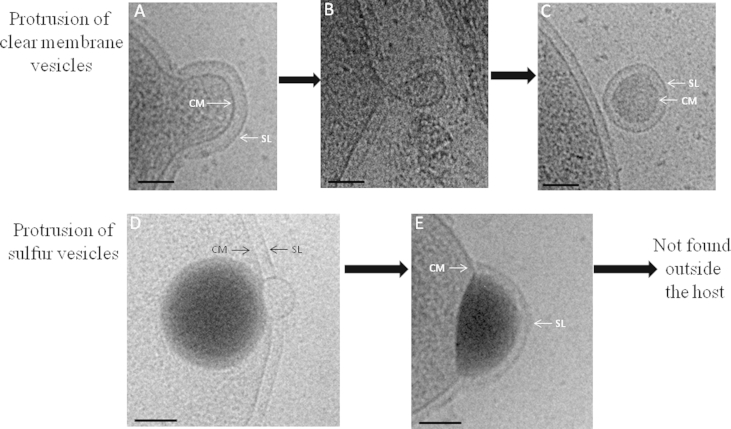
None SVs were observed outside the cell nor in purified MVs preparations indicating that, in contrast to classical MVs, SVs are not stable and are rapidly degraded in the extracellular medium. This cannot be explained by the absence of S-layer around SVs since MVs without S-layer are frequently observed in the extracellular medium. Many sulfur-oxidizing bacteria from the Proteobacteria division form and accumulate intracellular sulfur globules [25], [26], which are covered by a protein membrane [28]. It is still unclear whether the SVs of T. prieurii are surrounded by any membrane avoiding a direct contact between sulfur and cellular components. Fig. 7A–E show striking differences in the processes of MVs and SVs production. When a MV is released, the cell S-layer bulges out and forms a curved structure (Fig. 7A). This structure is pinched off to form the clear MVs (Fig. 7B and C), such as described for the formation of OMVs in Bacteria [57]. In contrast, the intracellular SVs, possibly covered by a thin envelope, move to the surface of the cell where they interact with the cell envelope. During their release, a bleb is formed (Fig. 7D) and the SVs are covered by the S-layer, but close examination suggests that SVs are not covered by the cytoplasmic membrane (Fig. 7E). Direct interaction between sulfur and S-layer proteins could be unstable leading to disruption of the SV. Because of their rapid degradation outside the cells, we cannot isolate sulfur vesicles to determine their exact chemical composition and sulfur speciation.
Fig. 7.
Comparison of the extrusion of membrane vesicles (MVs) and sulfur vesicles (SVs) from Thermococcales. A, B, C: Protrusion of clear membrane vesicles; D, E: Protrusion of sulfur vesicles. Bar: 100 nm. CM: Cytoplasmic membrane, SL: S-layer.
5. Conclusion
A number of bacteria form extracellular and/or intracellular SVs (also called sulfur globules) [25] from different sources of sulfur compounds such as elemental sulfur, sulfide and thiosulfate. These SVs behave differently depending of the species that have been investigated, suggesting a great diversity of mechanisms for SV production. In some bacteria, SVs are transient and completely degraded after oxidation of sulfur to sulfate [53]. In others, SVs are produced to prevent a toxic accumulation of sulfur, and then released in the extracellular medium, thus playing a key role in sulfur detoxification [33]. This seems to be the case for T. prieurii and T. kodakaraensis SVs, extending the role of vesicles in the physiology of hyperthermophilic archaea.
Acknowledgments
AG and collaboration between PF and CG were funded by the Agence Nationale de la Recherche, project Thermovésicules (ANR 12-BSV3-003-01). PF and SG were supported by the European Research council, project EVOMOBIL (FP/2007–2013) – ERC Grant Agreement no. 340440 to PF. This work benefited from the facilities and expertise of the Imagif Cell Biology Unit of the Gif campus (www.imagif.cnrs.fr) which is supported by the “Conseil Général de l'Essonne”. We thank Sylvain Bernard for his assistance in Raman spectroscopy.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biochi.2015.07.026.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Fig. S2.
References
- 1.Deatherage B.L., Cookson B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulp A., Kuehn M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.György B., Szabó T.G., Pásztói M., Pál Z., Misják P., Aradi B., László V., Pállinger E., Pap E., Kittel A., Nagy G., Falus A., Buzás E.I. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mause S.F., Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald I.A., Kuehn M.J. Offense and defense: microbial membrane vesicles play both ways. Res. Microbiol. 2012;163:607–618. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni H.M., Jagannadham M.V. Biogenesis and multifaceted roles of outer membrane vesicles from gram-negative bacteria. Microbiology. 2014;160:2109–2121. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 7.Haurat M.F., Elhenawy W., Feldman M.F. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol. Chem. 2015;396:95–109. doi: 10.1515/hsz-2014-0183. [DOI] [PubMed] [Google Scholar]
- 8.Prangishvili D., Holz I., Stieger E., Nickell S., Kristjansson J.K., Zillig W. Sulfolobicins, specific proteinaceous toxins produced by strains of the extremely thermophilic archaeal genus Sulfolobus. J. Bacteriol. 2000;182:2985–2988. doi: 10.1128/jb.182.10.2985-2988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachel R., Wyschkony I., Riehl S., Huber H. The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea. 2002;1:9–18. doi: 10.1155/2002/307480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soler N., Marguet E., Verbavatz J.M., Forterre P. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. Microbiol. 2008;159:390–399. doi: 10.1016/j.resmic.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Soler N., Gaudin M., Marguet E., Forterre P. Plasmids, viruses and virus-like membrane vesicles from Thermococcales. Biochem. Soc. Trans. 2011;39:36–44. doi: 10.1042/BST0390036. [DOI] [PubMed] [Google Scholar]
- 12.Ellen A.F., Albers S.V., Huibers W., Pitcher A., Hobel C.F., Schwarz H., Folea M., Schouten S., Boekema E.J., Poolman B., Driessen A.J. Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles. 2009;13:67–79. doi: 10.1007/s00792-008-0199-x. [DOI] [PubMed] [Google Scholar]
- 13.Ellen A.F., Rohulya O.V., Fusetti F., Wagner M., Albers S.V., Driessen A.J. The sulfolobicin genes of Sulfolobus acidocaldarius encode novel antimicrobial proteins. J. Bacteriol. 2011;193:4380–4387. doi: 10.1128/JB.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marguet E., Gaudin M., Gauliard E., Fourquaux I., du Plouy S. le Blond, Matsui I., Forterre P. Membrane vesicles, nanopods and/or nanotubes produced by hyperthermophilic archaea of the genus Thermococcus. Biochem. Soc. Trans. 2013;41:436–442. doi: 10.1042/BST20120293. [DOI] [PubMed] [Google Scholar]
- 15.Forterre P., Soler N., Krupovic M., Marguet E., Ackermann H.W. Fake virus particles generated by fluorescence microscopy. Trends Microbiol. 2013;21:1–5. doi: 10.1016/j.tim.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Gaudin M., Gauliard E., Schouten S., Houel-Renault L., Lenormand P., Marguet E., Forterre P. Hyperthermophilic archaea produce membrane vesicles that can transfer DNA. Environ. Microbiol. Rep. 2013;5:109–116. doi: 10.1111/j.1758-2229.2012.00348.x. [DOI] [PubMed] [Google Scholar]
- 17.N. Soler, M. Krupovic, E. Marguet, P. Forterre, Membrane vesicles in natural environments: a major challenge in viral ecology, ISME J., http://dx.doi.org/10.1038/ismej.2014.184. [DOI] [PMC free article] [PubMed]
- 18.Gaudin M., Krupovic M., Marguet E., Gauliard E., Cvirkaite-Krupovic V., Le Cam E., Oberto J., Forterre P. Extracellular membrane vesicles harbouring viral genomes. Environ. Microbiol. 2014;16:1167–1175. doi: 10.1111/1462-2920.12235. [DOI] [PubMed] [Google Scholar]
- 19.Sterling K.M., Mandal P.K., Roggenbeck B.A., Ahearn S.E., Gerencser G.A., Ahearn G.A. Heavy metal detoxification in crustacean epithelial lysosomes: role of anions in the compartmentalization process. J. Exp. Biol. 2007;210:3484–3493. doi: 10.1242/jeb.008300. [DOI] [PubMed] [Google Scholar]
- 20.Shifrin D.A., Jr., McConnell R.E., Nambiar R., Higginbotham J.N., Coffey R.J., Tyska M.J. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr. Biol. 2012;22:627–631. doi: 10.1016/j.cub.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray S. Bioaccumulation of cadmium in marine organisms. Exp. Suppl. 1986;50:65–75. doi: 10.1007/978-3-0348-7238-6_9. [DOI] [PubMed] [Google Scholar]
- 22.Tatischeff I., Lavialle F., Pigaglio-Deshayes S., Péchoux-Longin C., Chinsky L., Alfsen A. Dictyostelium extracellular vesicles containing hoechst 33342 transfer the dye into the nuclei of living cells: a fluorescence study. J. Fluoresc. 2008;18:319–328. doi: 10.1007/s10895-007-0271-4. [DOI] [PubMed] [Google Scholar]
- 23.Lavialle F., Deshayes S., Gonnet F., Larquet E., Kruglik S.G., Boisset N., Daniel R., Alfsen A., Tatischeff I. Nanovesicles released by dictyostelium cells: a potential carrier for drug delivery. Int. J. Pharm. 2009;380:206–215. doi: 10.1016/j.ijpharm.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Tatischeff I. Assets of the non-pathogenic microorganism dictyostelium discoideum as a model for the study of eukaryotic extracellular vesicles. F1000Research. 2013;2:73. doi: 10.12688/f1000research.2-73.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl C., Prange A. Bacterial sulfur globules: occurrence, structure and metabolism. In: Shively J.M., editor. Inclusions in procayotes. Springer; Heidelberg, Germany: 2006. pp. 21–51. [Google Scholar]
- 26.Maki J.S. Bacterial intracellular sulfur globules: structure and function. J. Mol. Microbiol. Biotechnol. 2013;23:270–280. doi: 10.1159/000351335. [DOI] [PubMed] [Google Scholar]
- 27.Cao H., Wang Y., Lee O.O., Zeng X., Shao Z., Qian P.Y. Microbial sulfur cycle in two hydrothermal chimneys on the Southwest Indian Ridge. MBio. 2014;5 doi: 10.1128/mBio.00980-13. e00980–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prange A., Engelhardt H., Trüper H.G., Dahl C. The role of the sulfur globule proteins of Allochromatium vinosum: mutagenesis of the sulfur globule protein genes and expression studies by real-time RT-PCR. Arch. Microbiol. 2004;182:165–174. doi: 10.1007/s00203-004-0683-3. [DOI] [PubMed] [Google Scholar]
- 29.Bazylinski D.A., Dean A.J., Williams T.J., Long L.K., Middleton S.L., Dubbels B.L. Chemolithoautotrophy in the marine, magnetotactic bacterial strains MV-1 and MV-2. Arch. Microbiol. 2004;182:373–387. doi: 10.1007/s00203-004-0716-y. [DOI] [PubMed] [Google Scholar]
- 30.Bazylinski D.A., Williams T.J., Lefèvre C.T., Trubitsyn D., Fang J., Beveridge T.J., Moskowitz B.M., Ward B., Schübbe S., Dubbels B.L., Simpson B. Magnetovibrio blakemorei gen. nov., sp. nov., a magnetotactic bacterium (Alphaproteobacteria: Rhodospirillaceae) isolated from a salt marsh. Int. J. Syst. Evol. Microbiol. 2013;63:1824–1833. doi: 10.1099/ijs.0.044453-0. [DOI] [PubMed] [Google Scholar]
- 31.Pflugfelder B., Fisher C.R., Bright M. The color of the trophosome: elemental sulfur distribution in the endosymbionts of Rifia pachyptila Jones, 1981 (Vestimentifera, Siboglinidae) Mar. Biol. 2005;146:895–901. [Google Scholar]
- 32.Maurin L.C., Himmel D., Mansot J.L., Gros O. Raman microspectrometry as a powerful tool for a quick screening of thiotrophy: an application on mangrove swamp meiofauna of Guadeloupe (F.W.I.) Mar. Environ. Res. 2010;69:382–389. doi: 10.1016/j.marenvres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Eichinger I., Schmitz-Esser S., Schmid M., Fisher C.R., Bright M. Symbiont-driven sulfur crystal formation in a thiotrophic symbiosis from deep-sea hydrocarbon seeps. Environ. Microbiol. Rep. 2014;6:364–372. doi: 10.1111/1758-2229.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorlas A., Alain K., Bienvenu N., Geslin C. Thermococcus prieurii sp. nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2013;63:2920–2926. doi: 10.1099/ijs.0.026419-0. [DOI] [PubMed] [Google Scholar]
- 35.Gorlas A., Koonin E.V., Bienvenu N., Prieur D., Geslin C. TPV1, the first virus isolated from the hyperthermophilic genus Thermococcus. Environ. Microbiol. 2012;14:503–516. doi: 10.1111/j.1462-2920.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorlas A., Krupovic M., Forterre P., Geslin C. Living side by side with a virus: characterization of two novel plasmids from Thermococcus prieurii, a host for the spindle-shaped virus TPV1. Appl. Environ. Microbiol. 2013;79:3822–3828. doi: 10.1128/AEM.00525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorlas A., Croce O., Oberto J., Gauliard E., Forterre P., Marguet E. Thermococcus nautili sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal deep-sea vent. Int. J. Syst. Evol. Microbiol. 2014;64:1802–1810. doi: 10.1099/ijs.0.060376-0. [DOI] [PubMed] [Google Scholar]
- 38.Oberto J., Gaudin M., Cossu M., Gorlas A., Slesarev A., Marguet E., Forterre P. Genome sequence of a hyperthermophilic archaeon, Thermococcus nautili 30-1, that produces viral vesicles. Genome Announc. 2014;27:2. doi: 10.1128/genomeA.00243-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franz B., Lichtenberg H., Hormes J., Dahl C., Prange A. The speciation of soluble sulphur compounds in bacterial culture fluids by X-ray absorption near edge structure spectroscopy. Environ. Technol. 2009;30:1281–1289. doi: 10.1080/09593330903055635. [DOI] [PubMed] [Google Scholar]
- 40.Adams M.W., Holden J.F., Menon A.L., Schut G.J., Grunden A.M., Hou C., Hutchins A.M., Jenney F.E., Jr., Kim C., Ma K., Pan G., Roy R., Sapra R., Story S.V., Verhagen M.F. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 2001;183:716–724. doi: 10.1128/JB.183.2.716-724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kletzin A. Metabolism of inorganic sulfur compounds in archaea. In: Garrett R.A., Klenk H.P., editors. Archaea: Evolution, Physiology, and Molecular Biology. Blackwell; Oxford, UK: 2007. pp. 261–274. [Google Scholar]
- 42.Zillig W. The order Thermococcales. In: Balows A., Trüper H.G., Dworkin M., Harder W., Schleifer K.H., editors. The Prokaryotes. second ed. Springer; New York: 1992. pp. 702–706. [Google Scholar]
- 43.Bazylinski D.A., Williams T.J. Ecophysiology of magnetotactic bacteria. In: Schüler D., editor. Magnetoreception and Magnetosomes in Bacteria: Microbiology Monographs. Springer; Berlin: 2006. pp. 37–75. [Google Scholar]
- 44.Ma K., Loessner H., Heider J., Johnson M.K., Adams M.W. Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, non-heme iron alcohol dehydrogenase. J. Bacteriol. 1995;177:4748–4756. doi: 10.1128/jb.177.16.4748-4756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godfroy A., Meunier J.R., Guezennec J., Lesongeur F., Raguénès G., Rimbault A., Barbier G. Thermococcus fumicolans sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent in the north Fiji Basin. Int. J. Syst. Bacteriol. 1996;46:1113–1119. doi: 10.1099/00207713-46-4-1113. [DOI] [PubMed] [Google Scholar]
- 46.Schut G.J., Bridger S.L., Adams M.W. Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J. Bacteriol. 2007;189:4431–4441. doi: 10.1128/JB.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobori H., Ogino M., Orita I., Nakamura S., Imanaka T., Fukui T. Characterization of NADH oxidase/NADPH polysulfide oxidoreductase and its unexpected participation in oxygen sensitivity in an anaerobic hyperthermophilic archaeon. J. Bacteriol. 2010;192:5192–5202. doi: 10.1128/JB.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bridger S.L., Clarkson S.M., Stirrett K., DeBarry M.B., Lipscomb G.L., Schut G.J., Westpheling J., Scott R.A., Adams M.W. Deletion strains reveal metabolic roles for key elemental sulfur-responsive proteins in Pyrococcus furiosus. J. Bacteriol. 2011;193:6498–6504. doi: 10.1128/JB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Beer L.L., Whitman W.B. Sulfur metabolism in archaea reveals novel processes. Environ. Microbiol. 2012;14:2632–2644. doi: 10.1111/j.1462-2920.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 50.Herwald S., Liu A.Y., Zhu B.E., Sea K.W., Lopez K.M., Sazinsky M.H., Crane E.J. Structure and substrate specificity of the pyrococcal coenzyme A disulfide reductases/polysulfide reductases (CoADR/Psr): implications for S(0)-based respiration and a sulfur-dependent antioxidant system in Pyrococcus. Biochemistry. 2013;52:2764–2773. doi: 10.1021/bi3014399. [DOI] [PubMed] [Google Scholar]
- 51.Harnvoravongchai P., Kobori H., Orita I., Nakamura S., Imanaka T., Fukui T. Characterization and gene deletion analysis of four homologues of group 3 pyridine nucleotide disulfide oxidoreductases from Thermococcus kodakarensis. Extremophiles. 2014;18:603–616. doi: 10.1007/s00792-014-0643-z. [DOI] [PubMed] [Google Scholar]
- 52.Steudel R. On the nature of the ‘elemental sulfur’ (S°) produced by sulfur-oxidizing bacteria – a model for S° globules. In: Schlegel H.G., Bowien B., editors. Autotrophic Bacteria. Science Tech; Madison: 1989. pp. 289–303. [Google Scholar]
- 53.Franz B., Lichtenberg H., Hormes J., Modrow H., Dahl C., Prange A. Utilization of solid “elemental” sulfur by the phototrophic purple sulfur bacterium Allochromatium vinosum: a sulfur K-edge X-ray absorption spectroscopy study. Microbiology. 2007;153:1268–1274. doi: 10.1099/mic.0.2006/003954-0. [DOI] [PubMed] [Google Scholar]
- 54.Franz B., Gehrke T., Lichtenberg H., Hormes J., Dahl C., Prange A. Unexpected extracellular and intracellular sulfur species during growth of Allochromatium vinosum with reduced sulfur compounds. Microbiology. 2009;155:2766–2774. doi: 10.1099/mic.0.027904-0. [DOI] [PubMed] [Google Scholar]
- 55.Ma K., Schicho R.N., Kelly R.M., Adams M.W.W. Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc. Natl. Acad. Sci. 1993;90:5341–5344. doi: 10.1073/pnas.90.11.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schauder R., Kröger A. Bacterial sulphur respiration. Arch. Microbiol. 1993;159:491–497. [Google Scholar]
- 57.Kadurugamuwa J.L., Beveridge T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.