Abstract
Extracellular vesicles (EVs), a term that includes both exosomes of endocytic origin and vesicles derived from plasma membranes, are continuously secreted by cells to the extracellular environment, and represent a novel vehicle for cell-cell communication. Exosomes contain specific repertoires of proteins and RNAs, indicating the existence of mechanisms that control the sorting of molecules into them. Although the molecular mechanisms that regulate the loading of proteins into exosomes have been studied for years, the sorting of RNA has been elusive until recently. Here we review the molecular mechanisms that control the sorting of molecules into exosomes, with special attention to the sorting of RNA. We also discuss how the cellular context affects the composition of exosomes, and thus the outcome of the communication between the exosome-producer and recipient cells, with particular focus on the communication between tumor cells and with cells of the tumor microenvironment.
Keywords: extracellular vesicles, exosomes, sorting, ESCRT, tetraspanins, hnRNPs, microRNAs, stress, tumor
INTRODUCTION
Cells continuously secrete extracellular vesicles (EVs) to the extracellular environment. These EVs can originate either through the fusion of specific endosomal compartments called multivesicular bodies (MVB) with the plasma membrane (exosomes) or by direct shedding from the plasma membrane. Cells can secrete both types of EVs simultaneously, and once released they cannot be separated by physical means. For this reason, EV samples usually contain a mixture of exosomes and shedding vesicles, and it is difficult to find specific markers that allow them to be discriminated. EVs can adhere to the surface of recipient cells and induce specific signals [1, 2], and can also be internalized by recipient cells and release their content into them [3-7]. Therefore, EVs act as mediators of cell-cell communication in many different contexts and pathologies [8].
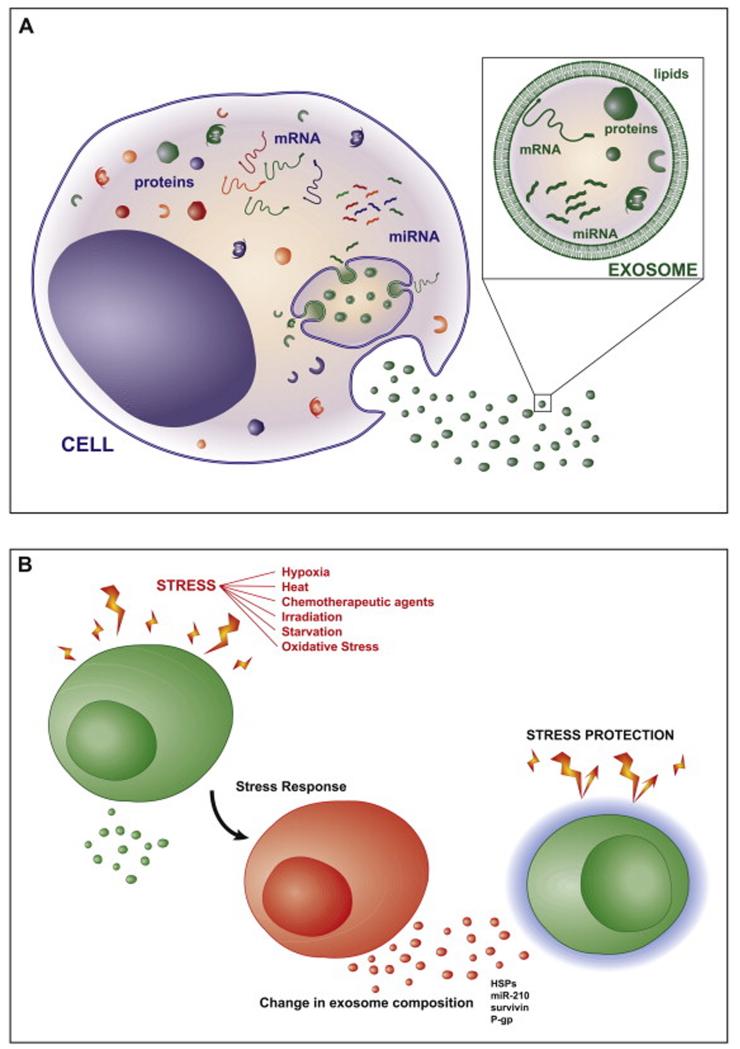
The molecular composition of exosomes is not a mere reflection of the cell. On the contrary, exosomes are enriched in specific proteins, lipids and RNAs, whereas others are absent, indicating the existence of specialized mechanisms that control the sorting of molecules into exosomes (Fig. 1A). Exosome membranes are enriched in cholesterol, sphingolipids, glycerophospholipids and ceramide [9], and bear both intraluminal and transmembrane proteins, with the same orientation as the plasma membrane. Proteins enriched in exosomes include tetraspanins (CD63, CD81) and associated proteins such as integrins, immunoglobulins and growth factor receptors; cytoskeletal proteins (tubulin, actin); ESCRT-related proteins (Alix, tsg101); heat-shock proteins (hsp70, hsp90), and proteins involved in vesicle trafficking such as Rab GTPases proteins, annexins and flotillin (reviewed in [10]. Exosomes are also enriched in small RNA species, including vaultRNA, tRNA and miRNAs [11]. The profile of miRNAs in exosomes is specific, since particular repertoires of miRNAs are selectively sorted into exosomes, while other miRNAs are usually excluded. Additionally, exosomes can contain components that reflect the nature and even the state of the producer cell.
Figure 1. Exosomes contain specific repertoires of proteins, RNAs and lipids.
(A) The molecular composition of exosomes is not a reflection of the cell. Exosomes are enriched in specific proteins, lipids and RNAs, whereas others are absent, indicating the existence of specialized mechanisms that control the sorting of molecules into exosomes. (B) The stress-induced changes in exosomal RNA and protein composition can influence the response of distant cells to stress by providing protective signals (surveillance, drug resistance, etc.).
The specific sorting of proteins and RNA molecules into exosomes is controlled through a variety of pathways, most of which are not fully understood. What is clear is that the exosome composition will determine the outcome of the communication. Exosome-mediated communication is very important for tumor cells, which secrete exosomes constitutively. These exosomes play a key role in the modulation of the immune response against the tumor [12-16], the induction of angiogenesis [17, 18], and cell invasion and metastasis [19]. Tumor cells are continuously subjected to a range of stressors such as hypoxia, starvation or chemotherapeutic agents (Fig. 1B), and cancer progression depends on the ability of cells to sense and adapt to these situations. Here we review the mechanisms that control the protein and RNA composition of exosomes, the effect of stress on exosome composition, and how these stress-induced changes affect exosome-mediated intercellular communication.
SORTING OF CARGO INTO EXOSOMES
Protein cargo
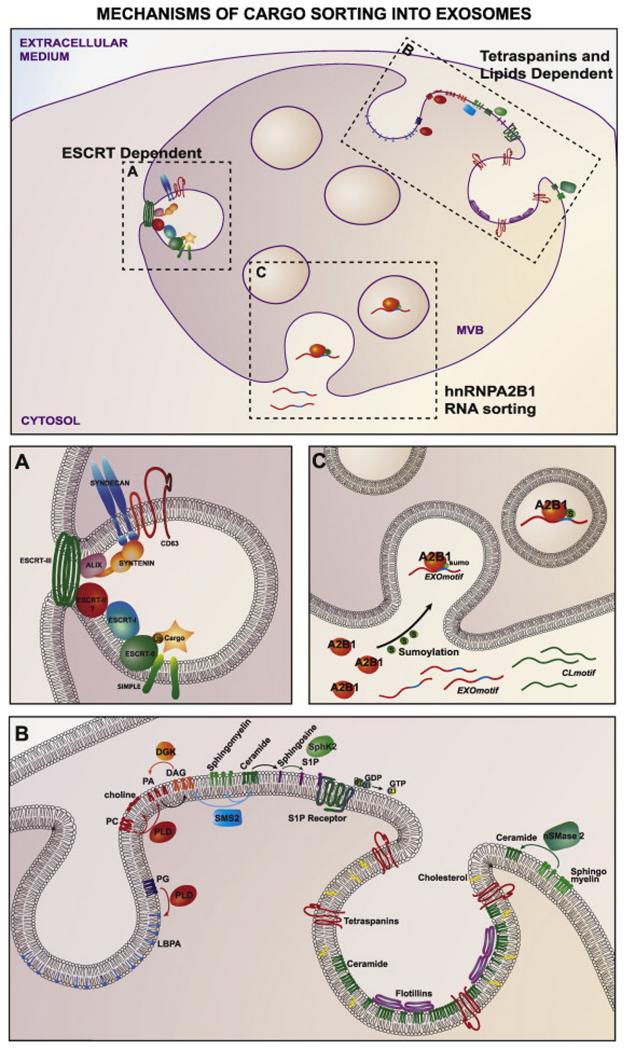
Different mechanisms have been involved in the specific sorting of proteins into exosomes, including ESCRT, tetraspanins and lipid-dependent mechanisms (Fig. 2).
Figure 2. Mechanisms that control the sorting of cargo into exosomes.
(A) Endosomal Sorting Complexes Required for Transport (ESCRT) 0, I and III, and Alix accessory protein control the sorting of ubiquitinated proteins into the intraluminal vesicles of multivesicular bodies (MVBs). Syntenin and the Small integral membrane protein of the lysosome/late endosome (SIMPLE) are also involved in exosome secretion through their interaction with specific components of the ESCRT complex. (B) Tetraspanins (CD81, CD9, CD63) play a key role in the composition of ESCRT-independent exosomes. Different lipids and lipid-related enzimes control the secretion of these exosomes. nSMase2: neutral sphingomyelinase 2; S1P: sphingosine-1-phosphate; SphK2: sphingosine kinase 2; DAG: diacylglycerol; SMS2: sphingomyelin synthase 2; PA: phosphatidic acid; PC: phosphatidyl choline; PLD: phospholipase D; LBPA: lysophosphatidic acid. (C) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific short motifs (EXOmotif).
ESCRT-dependent mechanisms
Endosomal Sorting Complexes Required for Transport (ESCRT) have been shown to control the sorting of ubiquitinated proteins into the intraluminal vesicles (ILV) that are later degraded upon the fusion of MVBs with lysosomes [20]. However, the role of this complex in the sorting of cargo into ILVs that are secreted as exosomes is more controversial. ESCRT complex is composed of several subcomplexes that work coordinately to produce ILV budding and sort ubiquitinated proteins into them (Fig. 2A). ESCRT-0 recognizes ubiquitinated proteins and is recruited to endosomal membranes through the interaction of the Hrs FYVE domain with PtdIns3P (phosphatidyl inositol 3-phosphate). ESCRT-0 recruits ESCRT-I machinery through the interaction of Hrs PSAP domains with the ESCRT-I subunit tsg101 (tumor susceptibility gene 101). ESCRT-I then recruits ESCRT-II proteins, which recruit and activate the ESCRT-III complex. The ESCRT-III component protein Snf7 forms oligomeric assemblies that promote vesicle budding. Snf7 also recruits the adaptor protein Alix (ALG-2-interacting protein X), which stabilizes ESCRT-III assembly. Once assembled, ESCRT-III requires energy to dissociate from the membrane, which is provided by the ATPase Vps4 (vacuolar protein 4) (Reviewed in [20]).
A recent study shows the effect of silencing twenty-three components of the ESCRT machinery by RNAi in MHC-II-expressing HeLa cells [21]. The silencing of Hrs, STAM1 and tsg101 (ESCRT-0/I) decreased the production of exosomes bearing CD63, CD81 and MHC-II. Hrs was also shown to be required for the secretion of exosomes in dendritic cells (DCs) [22]. Conversely, Vps4B (ESCRT-III) or Alix silencing seemed to increase exosome release [21]. However, the effect of Alix silencing was restricted to the secretion of exosomal MHC-II, which is also upregulated at the mRNA level in cells, so this increase cannot be attributed exclusively to an increase in exosome production.
ESCRT-dependent exosome biogenesis was recently shown to involve syntenin and syndecans [23] (Fig. 2A). Syndecans are transmembrane proteins that represent the main source of heparan sulfate (HS) in cell membranes. Syndecans interact with syntenin, which, in turn, interacts with CD63 and Alix through LYPX(n)L motifs. Silencing of syntenin or syndecan decreases the number of released exosomes and reduces exosomal accumulation of Alix, hsp70 and CD63, but has no effect on flotillin-positive EVs. Alix silencing also decreases the release of exosomes, whereas CD63 silencing does not show any effect. Syntenin-induced exosome release is dependent on its interaction with Alix. Curiously, a Vps4 dominant negative mutant did not affect the secretion of exosomes, while its silencing did, suggesting that Vps4 may have a direct role in membrane abscission, independently of its ATPase domain and its role in recycling ESCRT-III components. Exosome secretion is also affected by the silencing of the ESCRT-III component charged multivesicular body protein 2A (CHMP2A) [23]. Syntenin secretion in exosomes is driven by cargo-induced oligomerization of syndecan, which induces heparan sulfate clustering. The overexpression of heparanase, which cleaves heparan sulphate, also enhances exosomal secretion of flotillin, clathrin and CD63 [24].
The Small integral membrane protein of the lysosome/late endosome (SIMPLE) has recently been shown to play a role in exosome secretion in several cell types [25] (Fig. 2A). SIMPLE is present in the ILVs of MVB and in exosomes, and its overexpression increases exosome release and exosomal accumulation of Alix and CD63, but had no effect in flotillin secretion. Patients of Charcot-Marie-Tooth 1C (CMT1C) disease with mutations in SIMPLE and genetic mouse models of this disease show decreased exosome production and altered MVB formation. Secretion of SIMPLE in exosomes is decreased by mutations in the di-Leu or YKRL motifs (signature motifs of endocytic functions) or the PSAP motif (mediator of SIMPLE interaction with tsg101) [25].
Ubiquitination is involved in the ESCRT-complex-mediated sorting of proteins into ILVs for their later degradation [20]; however, its involvement in the sorting of cargo into exosomes is not so clear. Nedd4 binds and ubiquitinates proteins containing PPXY motifs and appears to be a necessary step for recruitment of viral proteins (Gag, LMP2A) to MVBs. Overexpression of Nedd4 Family-interacting Protein 1 (Ndfip1) results in higher (ubiquitinated and total) protein levels in exosomes [26]. However, mutations in the PPxY motif of SIMPLE, which mediates its binding to E3 ubiquitin ligases, enhances the secretion of SIMPLE in exosomes [25], possibly by impairing lysosomal targeting of the vesicles. Similarly, the loading of MHC-II into exosomes in DCs after T cell activation is, in contrast to lysosomal targeting, independent of MHC ubiquitination and correlates with its incorporation into CD9-enriched domains [27].
The role of lipids
ESCRT-independent exosome secretion has also been described. For example, proteolipid (PLP)-positive exosome secretion is not affected by Hrs, Alix or Tsg101 silencing, or by Vps4 dominant negative mutant overexpression [9], while the sorting of EGFR into MVB is affected. Accordingly, MVBs are still present when Hrs, tsg101, Vsp22 and Vps24 are simultaneously silenced in mammals, whereas EGF-induced MVB formation is impaired [28]. The PLP-positive exosomes co-localize in endosomal compartments with flotillin and GPI, but not with Hrs. They are enriched in cholesterol and ceramide, and their secretion is dependent on ceramide production by neutral sphingomyelinase 2 (nSMase2) [9]. Sphingolipid-metabolizing enzymes, including nSMase2 and sphingomyelin synthase 2 (SMS2), were also demonstrated to control the secretion of Aβ-peptide-bearing exosomes in neurons [29]. Additionally, the transfer of CD63-positive exosomes from T cells to antigen presenting cells during the immune synapse is ceramide-dependent [30]. Ceramide causes spontaneous curvature of the endosomal membrane and coalescence of microdomains, providing a mechanism that would reasonably explain ILV budding, but not how cargo is selectively sorted into the vesicles (Fig. 2B).
Another sphingomyelin metabolite, sphyngosine-1-phosphate (S1P), was recently shown to play a key role in MVB biogenesis [31]. This study shows that Gi-coupled S1P receptors co-localize with MVB markers, and that these receptors are in a continuously activated state on endosomal membranes. Furthermore, silencing of sphingosine kinase 2 (Sphk2) or S1P1 receptors impairs the formation of CD63, CD81, or flotillin-positive ILVs and exosomes. The authors also suggest that the effect of ceramide on the maturation of MVBs and the production of exosomes might be an indirect consequence of its further metabolization to S1P. Diacylglycerol (DAG), another lipid second messenger, also seems to have a role in the formation of exosomes, since the inhibition of DGK (which metabolizes DAG into phosphatidic acid) induces the release of CD63 and FasL-positive exosomes from T cells [32] (Fig. 2B).
Phospholipase D (PLD), which hydrolyzes phosphatidylcholine to generate choline and phosphatidic acid, is present in endosomal compartments and exosomes, and its activity regulates exosome secretion [33]. PLD2 is also involved in lysophosphatidic acid (LBPA) biosynthesis through the production of its precursor phosphatidylglycerol (PG) by transphosphatidylation [34]. LBPA is highly enriched in MVBs and ILVs [35], and promotes inward budding of vesicles and ILV formation [36]. Interestingly, LBPA interacts with both Alix [36] and hsp70 [37]. The accumulation of endosomal cholesterol also enhances the secretion of flotillin-positive exosomes [38]. Finally, ABCA3, a transporter of phosphatidylcholines, is also involved in exosome production [39].
Tetraspanins
Tetraspanins are integral membrane proteins highly enriched in exosomes [40]. Through their interaction with other transmembrane proteins, cytosolic proteins and lipids, tetraspanins organize membranes into tetraspanin-enriched domains (TEMs) [41]. Tetraspanin CD81 plays a key role in exosome composition, not only through the physical organization of membranes in microdomains, but also through the interactome of its cytoplasmic domain [42]. Similarly, the loading of metalloproteinase CD10 in exosomes is dependent on its interaction with the CD9 cytoplasmic domain [43]. Exosome biogenesis and protein loading also involve tetraspanin CD63; for example, this tetraspanin controls the loading of EBV protein LMP1 into exosomes [44], and also regulates the loading of PMEL into ILVs during melanogenesis [45], a process independent of the ESCRT-complex and ubiquitination [46].
Vesicle heterogeneity
The existence of ESCRT-dependent and independent mechanisms for the loading of proteins into exosomes is not necessarily contradictory, but rather points to the presence of heterogeneous populations of MVB and exosomes. This heterogeneity becomes more evident when exosome production is enhanced [47] or inhibited [48], since the different stimuli or the silencing of specific components of the exosome biogenesis machinery, do not affect all exosomal markers in the same way. It has also been shown that breast cancer cells secrete several types of exosomes, which differ on their miRNA composition, size and CD44 content [49]. Finally, distinct populations of exosomes released from the apical or basolateral sides of epithelial cells have been identified [50, 51]. In a colon carcinoma cell line, these populations could not be distinguished by electron microscopy and contained stereo-typical exosome markers (Tsg101, Alix, and hsp70), but were differently enriched in specific markers (CD63, mucin 13, sucrase isomaltase and prominin 1 at the apical side; early endosome antigen 1, ADP-ribosylation factor, and clathrin at basolateral side) [51]. The asymmetry of secretion was also observed for αβ crystallin in the retinal pigment epithelium, and this was disrupted by severe oxidative stress [50]. The existence of different populations of exosomes released from apical and basolateral cell surfaces not only demonstrates the heterogeneity of vesicles, but also strongly suggests the existence of very specialized mechanisms to control the selective sorting of cargo into these vesicles.
Protein cargo in shedding vesicles
The ESCRT machinery has also been implicated in the sorting of protein cargo into vesicles derived from the plasma membrane. For example, Vps4 is involved in the secretion of shedding vesicles enriched in arrestin-domain-containing proteins (ARRDC) [52]. These vesicles are CD63-negative and their secretion seems to be driven by interaction of the PSAP motif of ARRDC with the UEV motif of tsg101. Ubiquitination of ARRDC also seems to be important for the secretion of ARRDC-bearing shedding vesicles [52].
Sorting of proteins into shedding vesicles also involves plasma membrane protein oligomerization [53]. Oligomerization of plasma membrane proteins induced by a cross-linking agent promotes their sorting into shedding vesicles in T cells. Also, appendage of an acylation tag to the highly oligomeric protein Tya appears to be sufficient for its secretion in shedding vesicles [53]. This is apparently due to the addition of a myristoylation site that anchors the protein to the plasma membrane [54]. However, it seems that targeting of Tya to the endosomal membrane by fusion to a PI3P binding domain does not produce the same effect [54].
Lipid cargo
The composition of exosomes also suggests the existence of specific sorting mechanisms for lipids. Compared with the plasma membrane of parental cells, exosomes are enriched in cholesterol, sphingomyelin, saturated molecular species of phosphatidyl choline and phosphatyletanolamine. In contrast, the lipid composition of shedding vesicles is quite similar to the plasma membrane. The type of lipids vectorized by exosomes and the role of lipids in exosome fate and bioactivity have recently been reviewed in [55].
RNA cargo
Exosomes contain functional RNAs that can be incorporated into recipient cells [56]. The transfer of RNA-loaded vesicles plays a key role in cell-cell communication in many different contexts and pathologies [3-6, 30, 57].
The RNA content of exosomes is enriched in small RNA species including miRNAs, which have been demonstrated to be functional in the recipient cells [3, 4, 30, 58, 59]. Some miRNAs are enriched in exosomes while others are barely present, pointing to the existence of a machinery that regulates the active sorting of specific sets of miRNAs into exosomes [11, 30, 57, 60, 61]. In T cells, a subset of miRNAs that share specific sequence features was found to be always enriched in exosomes, independently of the activation state of the cell [61]. In silico analysis of overrepresented motifs and directed mutagenesis experiments allowed the identification of specific EXOmotifs that control the loading of these miRNAs into exosomes. These EXOmotifs mediate the binding to the heterogeneous ribonucleoprotein A2B1 (hnRNPA2B1), which controls the loading of these miRNAs into exosomes (Fig. 2C). Interestingly, hnRNPA2B1 is mostly sumoylated in exosomes, and this modification is essential for the loading of miRNAs into exosomes [61]. HnRNPA2B1 is a ubiquitous protein that has been previously shown to control the intracellular trafficking of specific mRNAs to distal sites in neurons [62]. This RNA transport function of hnRNPA2B1 is mediated through its binding to an RNA transport signal (RTS or A2RE) present in the 3′UTR [63] and that, interestingly, contains the EXOmotifs identified in exosomal miRNAs. HnRNPA2B1 also regulates the cytoplasmic transport of HIV genomic RNA to packaging sites [64] through its binding to A2RE sequences present in gag and vpr ORFs [65]. Another sequence present in HIV genomic RNA (nucleotides 557-663, corresponding to gag ORF) has been shown to be necessary for the loading of unspliced HIV RNA into exosomes [66]. Anexin-2 is another protein that might play a role in RNA sorting into exosomes, since it is able to bind specific RNAs [67-69] and it is highly abundant in exosomes [70].
In addition to miRNAs, exosomes carry mRNAs [56], which also show a selective enrichment [18]. Exosomes content in mRNAs seems to be enriched in 3'UTR fragments [71], that might be important for the sorting of specific mRNAs into these vesicles [72]. For example, fusion of the 3'UTR end of the exosomal-enriched GalR3 mRNA with GFP mRNA promotes its incorporation into exosomes. This enrichment is impaired when the short motif CTGCC or the miR-1289 target sequence in GalR3 is mutated. Additionally, overexpression of miR-1289 increases the packaging of GalR3 mRNA into extracellular vesicles, suggesting that miRNAs might contribute to the selective sorting of RNA cargo into exosomes. The CTGCC motif and the miR-1289 target sequence are both shared by other mRNAs enriched in glioblastoma exosomes [72]. Other nucleotide patterns have been found to be enriched in exosomal mRNAs, and apparently correlate negatively with RNA stability [73].
Next generation sequencing (NGS) analysis of exosomal RNA revealed that the most abundant RNA species are not mRNAs or miRNAs, but small ribosomal RNA (rRNA) and the structural RNAs vRNA, Y-RNA and SRP-RNA [11]. NGS also detects a high abundance of tRNAs, which are preferentially fragmented. Interestingly, SRP-RNA binding to the SRP protein core is mediated by the GGAG tetraloop [74], the exact same sequence as the recently identified EXOmotif.
RNAs are thus not randomly loaded into exosomes. Specific proteins, such as hnRNPA2B1, act in coordination with cis-acting elements in the RNA sequence to control the sorting of RNAs into these vesicles. However, there are still many unanswered questions regarding the sorting of RNAs into extracellular vesicles and the regulation of this process. For example, the presence and role of RISC proteins in exosomes is still unclear, and it is unknown whether miRNAs and mRNAs are sorted together into exosomes. Further research is also needed to determine the involvement of other RNA-binding proteins and RNA motifs.
EXOSOME RELEASE
Upon movement of MVBs to the plasma membrane and subsequent fusion, the internal vesicles are released into the extracellular space as exosomes. Exosome release involves contributions from several Rab proteins, a subfamily of small GTPases with more than 60 known members, involved in the regulation of intracellular vesicle transport [75] through the interaction with specific effector molecules [76]. Rab proteins reversibly associate with membranes via geranylgeranyl modifications and localize at different membrane-bound compartments, where they regulate sequential steps in membrane traffic—such as vesicle budding, transport, tethering, and fusion—through cycling between an active GTP-bound form and an inactive guanosine diphosphate (GDP)-bound form [77]. Rab4 and Rab5 decorate early endosomes, Rab11 is used to define the juxtanuclear recycling endosomes, and Rab7 and Rab9 are late endosomal markers. Thus, Rab5 and their interacting proteins determine the function of early endosomes, whereas Rab7 and its corresponding factors have a similar role in MVBs and lysosomes [78]. Endosome maturation involves a switch in content from Rab5 to Rab7 [79-81], which can be blocked by overexpression of a constitutively active mutant form of Rab5 (Q79L), resulting in the formation of hybrid endosomal compartments with markers of both early endosomes and MVBs.
The mechanisms regulated by Rab GTPases are cell-specific, depending on the differential expression and function of particular effectors. Rab4, Rab5, Rab11, Rab35, Rab27a and Rab27b have been implicated in different steps of exosome release on different cell types [82-85]. Regarding the immune system, Rab27 family and Munc13-4 (their effector in leukocytes) have an important role in the exocytosis of lytic granules of CTLs, NKs cells and neutrophils, and genetic defects in Rab27a or Munc13-4 genes lead to immunodeficiencies in humans [86]. In flies, interfering Rab11, but not Rab35, prevents exosome release, whereas in C. elegans the combined knockdown of Rab35 and −11 leads to a dramatically enhanced intracellular accumulation of endosomal cargo, suggesting that Rab35 and −11 play a dual role in endocytic recycling [87]. It is also important to note that inhibition of some of these Rab proteins, like Rab27a, could also affect the secretion of non-exosome-associated proteins [88]. A screening of Rab GTPase-activating proteins (GAP) identified the TBC1D10 family members as regulators of exosome secretion. Rab35 and its GAPs TBC1D10A–C regulate exosome release of PLP in oligodendroglial cells [82].
The subcellular location of MVBs is dependent on their interaction with the actin and microtubule cytoskeleton, which is also regulated by Rab proteins and their effectors. Rab effectors include motor proteins that facilitate the movement of the Rab-tethered vesicle along both the actin and/or microtubule cytoskeleton [89]. A good example of this is Rab11, which can separately recruit myosin Vb via its effector FIP2 (Rab11 family-interacting protein 2) and cytoplasmic dynein via FIP3 [90]. The involvement of the actin cytoskeleton in exosome release has been studied in cancer and immune cells. Recent work has shown that invadopodia—actin-rich subcellular structures formed by invasive cancer cells that protrude into the extracellular matrix—are key docking sites for MVBs and control exosome secretion. By inhibiting N-WASp, a critical protein for actin polymerization at invadopodia sites, and the signaling scaffolder Tks5, the authors demonstrated that polarized delivery of MVBs to invadopodia is an important step in exosomal secretion [91]. In a similar way, during the formation of a immune synapse, which is also dependent on active actin polymeration, MVBs are polarized and docked towards the T cell side of the synapse, releasing exosomes and their RNA content to the antigen presenting cell [30].
Regarding the role of the microtubule network, maturation of late endosomes is governed by their movement along microtubules towards the cell center in a dynein-dependent fashion, whereas localization of MVBs to the plasma membrane requires kinesin-dependent movement towards microtubule plus ends [92]. The movement of MVBs along microtubules is in part controlled by the cholesterol content of the cells [55]. Once MVBs are docked to the plasma membrane, Rabs are also implicated in the fusion of the limiting membrane of MVBs with the plasma membrane. This is accomplished by direct or indirect regulation of SNARE proteins (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) [93], through the pairing of a SNARE on a transport vesicle (v-SNARE) with its cognate SNARE-binding partner (t-SNARE) on the appropriate target membrane [94, 95]. In K562 erythroleukemic cells, the vSNARE VAMP7 and the ATPase NSF, a protein involved in SNARE disassembly, participate in the fusion of MVB with the plasma membrane [96]. In C elegans, the Vacuolar H+-ATPase (V-ATPase) mediates apical secretion of exosomes containing Hedgehog-related proteins, independently of its contribution to the V-ATPase proton pump activity [97].
EXOSOME UPTAKE
The elucidation of the mechanisms of exosome targeting and uptake by recipient cells remains an important challenge. The binding of exosomes to the surface of recipient cells is mediated by the classical adhesion molecules involved in cell-cell interactions, such as integrins and ICAMs. However, other molecular pairs more specific to the exosome membrane, such as TIM-binding phosphatidylserines, carbohydrate/lectin receptors and heparan sulfate proteoglycans (HSPGs), could be involved as well. ICAM-1-LFA-1 interactions are involved in exosome uptake by immune cells [98, 99], and tetraspanins also contribute to exosome binding to target cells. For example, the integrin CD49d and Tspan8 contribute to exosome adhesion to endothelial cells [100]. MHC-TCR interactions can also facilitate the binding of exosomes derived from T cells to dendritic cells and vice versa [101]. Additionally, cells can use Tim4 and Tim1 as phosphatidylserine receptors for the engulfment of exosomes [102]. Exosomes are enriched in specific mannose- and sialic acid-containing glycoproteins; for example, α2,3-linked sialic acid is enriched on B cell-derived exosomes, and allows their capture by CD169+ macrophages in both spleen and lymph node. Exosome access to the lymphoid system is thus dysregulated in CD169 knock-out mice, resulting in aberrant trafficking of exosomes into the splenic red pulp or lymph node cortex [103]. In contrast, sialic acid removal causes a small but non-significant increase in uptake. The uptake of exosomes by macrophages is also mediated by a C-type lectin expressed in macrophages and by galectin-5 exposed on exosomes [104]. Virus and lipoproteins are internalized via HSPGs. In a similar way, HSPGs function as a receptor for cancer-derived exosomes [105]. Experiments with exogenous HS, pan-PG deficient cells, and pharmacological inhibitors of HSPG have shown that cell-surface HS is required for efficient exosome uptake [105].
To deliver their content, exosomes attached to a recipient cell can either fuse with the cell membrane, directly releasing their cargo into the cytoplasm, or get internalized by endocytic pathways. Fusion of exosomes with another membrane is more likely to occur at the acidic pH of the endosome rather than at the neutral pH of the plasma membrane [55]. Depending in the phagocytic and endocytic capacity of the recipient cells, exosomes can be internalized by clathrin- dynamin- caveolae-dependent endocytosis, pinocytosis, or phagocytosis. Neurons and microglia internalize oligodendroglial exosomes effectively, whereas uptake by oligodendrocytes and astrocytes occurs only sporadically. Microglia take up exosomes non-specifically by macropinocytosis [106], while internalization of oligodendroglial exosomes by neurons is mediated by selective clathrin- and dynamin-dependent endocytosis [107]. Exosomes released from EBV-infected B cells are internalized via caveola-dependent endocytosis [108]. Dynamin has been also involved in mediating the endocytosis of exosomes [104, 109]. For example, exosomes containing anthrax toxin are taken up by a dynamin-dependent mechanism that transports them to the endocytic pathway. The anthrax toxin is then released into the recipient-cell cytoplasm by ILV back fusion in an Alix- and Tsg101-dependent manner [110]. Fusogenic lipids, such as LBPA, also seem to be required for fusion between exosomes and the recipient late endosome membrane [55].
THE EFFECT OF STRESS ON EXOSOME COMPOSITION
The composition, biogenesis and secretion of exosomes are firmly regulated processes that are influenced by environmental challenges, such as activation or stress conditions. The secretion of exosomes is moreover an efficient adaptive mechanism by which cells modulate intracellular stress situations and modify the surrounding environment. Stress situations are frequent in the tumor microenvironment, and need to be overcome by cancer cells to enable tumor progression. Exosome-mediated signaling in tumors is thus affected by a variety of stress conditions [111], and promotes tumor progression through communication between the tumor and surrounding stromal tissue, activation of proliferative and angiogenic pathways, promotion of immune suppression, and initiation of pre-metastatic niches [12-19].
Tumor cells are exposed to hypoxia due to the limited oxygen availability in the tumor microenvironment. Tumor hypoxia induces adaptive mechanisms that promote endothelial cell function, angiogenesis, proliferation and survival [112]. Importantly, hypoxia and anoxia enhance the release of microvesicles containing CD63, CD9 and the hypoxia-related miR-210 [113]; and hypoxia additionally modulates the microenvironment and facilitates angiogenesis and metastasis by inducing the secretion of exosomes containing proteins associated with cell migration, extracellular matrix (ECM) degradation, growth hormone signaling, clathrin-mediated endocytosis and VGEF signaling [114, 115]. Exosomes derived from cells grown under hypoxic conditions accelerate tumor growth and angiogenesis more potently than normoxia-derived exosomes in malignant brain tumor gliobastoma [116]. Also, hypoxic cancer cells secrete exosomes bearing tissue factor (TF)/VIIa, a major initiator of coagulation-dependent PAR signaling. These exosomes are involved in the hypercoagulation found in highly malignant tumors and mediate paracrine activation of protease-activated receptor type 2 (PAR-2) on endothelial cells [117].
Acidic conditions are characteristic of tumor microenvironments, and modulate the release, function and trafficking of tumor shedding vesicles and exosomes. Acidic microenvironments enhance exosome release and uptake efficiency by modulating exosome lipid composition, enriching them in sphingomyelin and ganglioside GM3, which increase their rigidity and fusion efficiency with recipient cells [118]. Acidosis also triggers the rupture of shedding vesicles and therefore increases the availability of VEGF [119] and promotes the activation of the proenzyme cathepsin B to facilitate tumor invasion [120].
Tumor-derived exosomes promote immunosuppression through the induction of T cell apoptosis, inhibition of DC differentiation and NK cell cytotoxicity, and the induction of immunosuppressive myeloid suppressor cells (MDSC) and regulatory T cells [10]. Certain situations, such as thermal and oxidative stress, can enhance the release of immunosuppressive exosomes from leukemia and lymphoma T and B cells [1] and promote changes in the proteomic and genetic content of exosomes that influence the response to stress in receptor cells [121, 122]. Interestingly, heat stress may revert tumor exosome functions, leading to antitumor immunity by inducing the release of exosomes bearing Heat Shock Proteins (HSPs), which provide danger signals for the immune system. Heat might directly affect lipid metabolism and alter the lipid composition of the vesicles, increasing the presence of chemokines associated with cholesterol-rich lipid rafts [123]. Moreover, heat can also induce extracellular ATP release, which in turn mobilizes calcium influx and induces exosome release [124]. Calcium signaling triggers exosome release in many cell types, including lymphocytes and other cell types such as neurons or oligodendrocytes [125]. For instance, calcium entry through NMDA and AMPA receptors triggers oligodendroglial exosome secretion, which improves viability of the recipient neuronal cells upon exposure to conditions of cell stress [107].
Other exogenous stress stimuli might influence exosome composition and secretion. For example, hepatocellular carcinoma cells respond to anticancer drugs by releasing HSP-bearing exosomes that efficiently stimulate the NK cell response and granzyme B production by upregulating the expression of the inhibitory receptor CD94 and downregulating the expression of CD69, NKGD2 and NKp44 [126]. Interestingly, exposure of ovarian carcinoma cells to cisplatin increases the secretion of cisplatin-bearing exosomes [127], suggesting that they might be involved in the expulsion of the drug from the cell. In fact, exosomes derived from drug resistant cells have been shown to contain multidrug resistance (MDR)-related proteins such as P-glycoprotein 1 (P-gp), ABCG2, ABCB1 and ABCC2 [128, 129], which facilitates the sequestration of drugs in these vesicles, thereby reducing their available concentration in cells and preventing binding to their targets. Sublethal doses of proton irradiation have the potential to increase the release of exosome-packaged survivin, an anti-apoptotic protein involved in cellular proliferation, survival and tumor cell invasion [130]. Irradiation therapy can induce a senescent phenotype associated with increased production and release of exosome-like vesicles into the microenvironment that can potentially influence tumor progression. [131, 132]. The increased exosome secretion requires increased expression of the transmembrane protein tumor suppressor-activated pathway 6 (TSAP6), which is increased by activation of the transcription factor p53, among many other physiological changes [132]. p53-dependent secreted proteins include soluble growth factors and metalloproteinases that can alter the host microenvironment or change the ECM to block angiogenesis or cell migration.
The stress-induced changes in exosomal RNA and protein composition can influence the response of distant cells by providing protective signals (Fig. 1B). For example, during neuronal development, oligodendrocytes secrete exosomes that are taken up by neurons and induce neuronal viability under conditions of oxidative stress or starvation [107]. Additionally, mesenchymal stem cells (MSCs) and cardiac progenitor cells release exosomes that promote tissue vascularization and angiogenesis, and have a cardioprotective effect during ischemia/reperfusion (I/R) injury through the modulation of cell bioenergetics, reduction of oxidative stress and the activation of prosurvival signals [133]. MSC-derived exosome treatment reduces inflammation after I/R by decreasing neutrophil and macrophage infiltration [134]. Interestingly, the presence of 20S proteasome within exosomes has been shown to mediate cardioprotection by reducing the amount of misfolded proteins during acute myocardial infarction [135]. Exosomes also mediate the crosstalk between cells at the vascular endothelium, preventing cell cycle arrest and providing signals for angiogenesis and the activation and differentiation of endothelial cells. Interestingly, atheroprotective shear stress induces the exosome-mediated transfer of the miR-143/145 cluster from endothelial cells to smooth muscle cells, where they target gene expression and reduce atherosclerotic lesion formation in ApoE (−/−) mice [4]. Finally, exosomes have been suggested to play a role in the transfer of MDR in cancer. For example, functional P-gp and MRP-1 can be transferred from drug resistant to drug sensitive cells through exosomes [136-139], resulting in an increase in their substrates efflux and MDR acquisition. The transfer of MDR-related mRNAs and miRNAs from drug-resitant to drug-sensitive cells may also play a role in MDR spreading in cancer cells [140].
CONCLUDING REMARKS
Protein and RNA molecules are not randomly loaded into exosomes. On the contrary, specialized mechanisms act to ensure a very specific composition which will define the outcome of communication between the exosome-producer and the recipient cell. Exosomal protein composition is controlled by ESCRT-dependent and -independent mechanisms, and key roles in the biogenesis of exosomes and the sorting of specific proteins into them are also played by tetraspanins and lipids. All these mechanisms seem to act differently depending on the cell type, and also point to the existence of different subsets of exosomes produced within the same cell. Whether these different types of exosomes come from the same MVB, or reflect the existence of different MVB subsets in cells is still unknown. The mechanisms that control the sorting of RNA molecules has also remained elusive. Recently, short sequence motifs present in exosomal miRNAs have been shown to control their loading into exosomes through binding to hnRNPA2B1. Sumoylation (which, unlike ubiquitination, does not target proteins to degradation) seems to be important for hnRNPA2B1-mediated transport of miRNAs into exosomes. However, it is unknown whether this modification affects the localization of the protein or its RNA binding ability or both. It must be taken into account that, although hnRNPA2B1 is a ubiquitous protein, not all miRNAs enriched in exosomes contain EXOmotifs, so there must be additional mechanisms for miRNA sorting. Additionally, miRNAs themselves have been proposed to act as trans-acting elements controlling the loading of mRNAs into exosomes.
Exosome composition is widely affected by cellular state, and can reflect changes in the cell microenvironment. Stress situations such as hypoxia, starvation or oxidative stress can alter exosome content, and thus the message they carry. Interestingly, several reports indicate that exosomes produced under specific stress situations can confer protection on recipient cells. Further study of these processes will provide new insights into how tumor cells adapt and overcome the multiple stresses they are subject to, and may offer new targets for anti-tumor therapies. Exosomes are moreover potential tools for the treatment of diseases such as myocardial infarction, in which cell survival in stress situations is vital.
ACKNOWLEDGEMENTS
The authors thank Dr. S. Bartlett for assistance with English editing. This work was supported by SAF2011-25834 from the Spanish Ministry of Science and Innovation, INDISNET-S2011/BMD-2332 from the Comunidad de Madrid, Red Cardiovascular RD 12-0042-0056 from Instituto Salud Carlos III (ISCIII), ERC-2011-AdG 294340-GENTRIS and COST-Action BM1202. C.V.B. was supported by FPU program (Spanish Ministry of Education). M.M. was supported by the Instituto de Salud Carlos III.
REFERENCES
- [1].Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011;6(2):e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J, Anel A. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167(12):6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- [3].Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123(5):2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- [5].Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121(19):3997–4006. S3991–3915. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- [7].Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, Zhou X, Yuan Z. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14(8):793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- [8].Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- [10].Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251(1):125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nolte-'t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195(10):1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- [15].Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- [18].Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, et al. Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- [21].Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- [22].Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, Ueno Y, Shimosegawa T, Sugamura K. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399(3):384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- [23].Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- [24].Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu H, Guariglia S, Yu RY, Li W, Brancho D, Peinado H, Lyden D, Salzer J, Bennett C, Chow CW. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol Biol Cell. 2013;24(11):1619–1637. S1611–1613. doi: 10.1091/mbc.E12-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Putz U, Howitt J, Lackovic J, Foot N, Kumar S, Silke J, Tan SS. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J Biol Chem. 2008;283(47):32621–32627. doi: 10.1074/jbc.M804120200. [DOI] [PubMed] [Google Scholar]
- [27].Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- [28].Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- [29].Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287(14):10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- [32].Alonso R, Rodriguez MC, Pindado J, Merino E, Merida I, Izquierdo M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem. 2005;280(31):28439–28450. doi: 10.1074/jbc.M501112200. [DOI] [PubMed] [Google Scholar]
- [33].Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles JP, Bonnerot C, Perret B, Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572(1-3):11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- [34].Bollag WB, Xie D, Zheng X, Zhong X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol. 2007;127(12):2823–2831. doi: 10.1038/sj.jid.5700921. [DOI] [PubMed] [Google Scholar]
- [35].Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392(6672):193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- [36].Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303(5657):531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- [37].Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, Zylicz A, Knudsen J, Sandhoff K, Arenz C, Kinnunen PK, Nylandsted J, Jaattela M. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature. 2010;463(7280):549–553. doi: 10.1038/nature08710. [DOI] [PubMed] [Google Scholar]
- [38].Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, Simons M, Schneider A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem. 2010;285(34):26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L, Wulf GG. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- [41].Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [42].Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vazquez J, Yanez-Mo M. The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J Biol Chem. 2013;288(17):11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mazurov D, Barbashova L, Filatov A. Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. Febs J. 2013;280(5):1200–1213. doi: 10.1111/febs.12110. [DOI] [PubMed] [Google Scholar]
- [44].Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, Kieff E, Geerts D, van der Kant R, Neefjes J, Middeldorp JM, Pegtel DM. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-kappaB activation. Embo J. 2011;30(11):2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van der Vlist EJ, Arkesteijn GJ, van de Lest CH, Stoorvogel W, Nolte-'t Hoen EN, Wauben MH. CD4(+) T cell activation promotes the differential release of distinct populations of nanosized vesicles. J Extracell Vesicles. 2012;1:18364. doi: 10.3402/jev.v1i0.18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML, Duelli DM. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40(18):9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5(10):e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12(3):587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- [56].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [57].Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [58].Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180(5):3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- [59].Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5(10):e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Munro TP, Magee RJ, Kidd GJ, Carson JH, Barbarese E, Smith LM, Smith R. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J Biol Chem. 1999;274(48):34389–34395. doi: 10.1074/jbc.274.48.34389. [DOI] [PubMed] [Google Scholar]
- [63].Hoek KS, Kidd GJ, Carson JH, Smith R. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry. 1998;37(19):7021–7029. doi: 10.1021/bi9800247. [DOI] [PubMed] [Google Scholar]
- [64].Levesque K, Halvorsen M, Abrahamyan L, Chatel-Chaix L, Poupon V, Gordon H, DesGroseillers L, Gatignol A, Mouland AJ. Trafficking of HIV-1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic. 2006;7(9):1177–1193. doi: 10.1111/j.1600-0854.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- [65].Beriault V, Clement JF, Levesque K, Lebel C, Yong X, Chabot B, Cohen EA, Cochrane AW, Rigby WF, Mouland AJ. A late role for the association of hnRNP A2 with the HIV-1 hnRNP A2 response elements in genomic RNA, Gag, and Vpr localization. J Biol Chem. 2004;279(42):44141–44153. doi: 10.1074/jbc.M404691200. [DOI] [PubMed] [Google Scholar]
- [66].Columba Cabezas S, Federico M. Sequences within RNA coding for HIV-1 Gag p17 are efficiently targeted to exosomes. Cell Microbiol. 2013;15(3):412–429. doi: 10.1111/cmi.12046. [DOI] [PubMed] [Google Scholar]
- [67].Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM. Annexin A2 is a novel RNA-binding protein. J Biol Chem. 2004;279(10):8723–8731. doi: 10.1074/jbc.M311951200. [DOI] [PubMed] [Google Scholar]
- [68].Hollas H, Aukrust I, Grimmer S, Strand E, Flatmark T, Vedeler A. Annexin A2 recognises a specific region in the 3'-UTR of its cognate messenger RNA. Biochim Biophys Acta. 2006;1763(11):1325–1334. doi: 10.1016/j.bbamcr.2006.08.043. [DOI] [PubMed] [Google Scholar]
- [69].Mickleburgh I, Burtle B, Hollas H, Campbell G, Chrzanowska-Lightowlers Z, Vedeler A, Hesketh J. Annexin A2 binds to the localization signal in the 3' untranslated region of c-myc mRNA. Febs J. 2005;272(2):413–421. doi: 10.1111/j.1742-4658.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- [70].Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- [71].Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3'-untranslated regions. Biol Direct. 2013;8:12. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, Fan JB, Breakefield XO, Saydam O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011;12(Suppl 3):S18. doi: 10.1186/1471-2164-12-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wild K, Sinning I, Cusack S. Crystal structure of an early protein-RNA assembly complex of the signal recognition particle. Science. 2001;294(5542):598–601. doi: 10.1126/science.1063839. [DOI] [PubMed] [Google Scholar]
- [75].Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- [76].Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22(4):461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149(4):901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Huotari J, Helenius A. Endosome maturation. Embo J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141(3):497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- [80].Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- [81].Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3(7):e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115(Pt 12):2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- [85].Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. 11–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- [86].Catz SD. Regulation of vesicular trafficking and leukocyte function by Rab27 GTPases and their effectors. J Leukoc Biol. 2013;94(4):613–622. doi: 10.1189/jlb.1112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sato M, Sato K, Liou W, Pant S, Harada A, Grant BD. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. Embo J. 2008;27(8):1183–1196. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- [89].Kelly EE, Horgan CP, Goud B, McCaffrey MW. The Rab family of proteins: 25 years on. Biochem Soc Trans. 2012;40(6):1337–1347. doi: 10.1042/BST20120203. [DOI] [PubMed] [Google Scholar]
- [90].Ducharme NA, Ham AJ, Lapierre LA, Goldenring JR. Rab11-FIP2 influences multiple components of the endosomal system in polarized MDCK cells. Cell Logist. 2011;1(2):57–68. doi: 10.4161/cl.1.2.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Martin-Cofreces NB, Baixauli F, Sanchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 2014;24(1):61–72. doi: 10.1016/j.tcb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- [94].Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4(3):220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- [95].Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- [96].Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793(12):1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [97].Liegeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol. 2006;173(6):949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A. 2003;100(11):6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr., Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- [100].Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- [101].Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191(7):1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- [103].Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2013 doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- [105].Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- [107].Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87(18):10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- [110].Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5(4):986–996. doi: 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1:18396. doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Noman MZ, Messai Y, Carre T, Akalay I, Meron M, Janji B, Hasmim M, Chouaib S. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31(5):357–377. doi: 10.1615/critrevimmunol.v31.i5.10. [DOI] [PubMed] [Google Scholar]
- [113].King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7):e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A. 2011;108(32):13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Taraboletti G, D'Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D, Giavazzi R, Pavan A, Dolo V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8(2):96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Giusti I, D'Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, Pavan A, Dolo V. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia. 2008;10(5):481–488. doi: 10.1593/neo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319(13):2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5(12):e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186(4):2219–2228. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- [124].Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- [125].Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- [126].Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4(10):1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- [128].Goler-Baron V, Assaraf YG. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS One. 2011;6(1):e16007. doi: 10.1371/journal.pone.0016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Gong J, Luk F, Jaiswal R, George AM, Grau GE, Bebawy M. Microparticle drug sequestration provides a parallel pathway in the acquisition of cancer drug resistance. Eur J Pharmacol. 2013;721(1-3):116–125. doi: 10.1016/j.ejphar.2013.09.044. [DOI] [PubMed] [Google Scholar]
- [130].Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- [133].Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431(3):566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [135].Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23(9):1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- [137].Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O'Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7(12):e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pasquier J, Galas L, Boulange-Lecomte C, Rioult D, Bultelle F, Magal P, Webb G, Le Foll F. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J Biol Chem. 2012;287(10):7374–7387. doi: 10.1074/jbc.M111.312157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lu JF, Luk F, Gong J, Jaiswal R, Grau GE, Bebawy M. Microparticles mediate MRP1 intercellular transfer and the re-templating of intrinsic resistance pathways. Pharmacol Res. 2013;76:77–83. doi: 10.1016/j.phrs.2013.07.009. [DOI] [PubMed] [Google Scholar]
- [140].Jaiswal R, Gong J, Sambasivam S, Combes V, Mathys JM, Davey R, Grau GE, Bebawy M. Microparticle-associated nucleic acids mediate trait dominance in cancer. Faseb J. 2012;26(1):420–429. doi: 10.1096/fj.11-186817. [DOI] [PubMed] [Google Scholar]