Abstract
Purpose of review
To provide an overview of recent advances and future possibilities for therapeutic tolerance.
Recent findings
Allograft survival despite complete immunosuppressant withdrawal has been demonstrated in selected renal transplant recipients with haematopoietic chimerism. Early clinical trials of mesenchymal stromal cell therapy have shown promising results in several autoimmune diseases. Regulatory T-cells show potential benefit in graft versus host disease, although challenges to ex vivo expansion remain. Targeted modulation of T-cell function in vivo with monoclonal antibodies have shown beneficial effects in phase II/III trials of multiple sclerosis (alemtuzumab) and type I diabetes mellitus (teplizumab, otelixizumab). Emerging data from animal models suggests an important role for the commensal microbiome in the maintenance and disruption of immune tolerance with parallels in human studies.
Summary
After years of slow progress, recent research has reduced the translational gap between animal models and clinical therapeutic tolerance. Early detection of autoimmunity, potentially at preclinical stages, offers a window of opportunity for tolerogenic therapy. Reliable biomarkers of tolerance are urgently needed to provide objective measurements of the effectiveness of tolerogenic therapies, and to allow for intelligent immunosuppressant withdrawal in patients whose autoimmune disease is stable.
Keywords: therapeutic tolerance, immune tolerance, autoimmune, regulatory T-cell, therapy
Introduction
Immunosuppression has been the mainstay of treatment for autoimmune diseases for the past half century. Traditional therapeutics, such as corticosteroids or cytotoxic agents, generally provide blanket suppression of the immune response. More recently, monoclonal antibodies have provided the opportunity to target specific aspects of the immune cascade at a molecular level. However, these immunosuppressant approaches fail to distinguish between responses to self and non-self antigens and hence carry a burden of increased infection, and in certain cases, malignancy.
The resetting of immune tolerance to self antigens, or the exploitation of tolerance towards alloantigens in the setting of transplantation, without the need for immunosuppression has been termed “therapeutic tolerance” [Figure 1]. This concept has been demonstrated in numerous animal models, although translation to humans has historically been lacking. In this review, we will focus on recent advances towards clinical therapeutic tolerance [Figure 2].
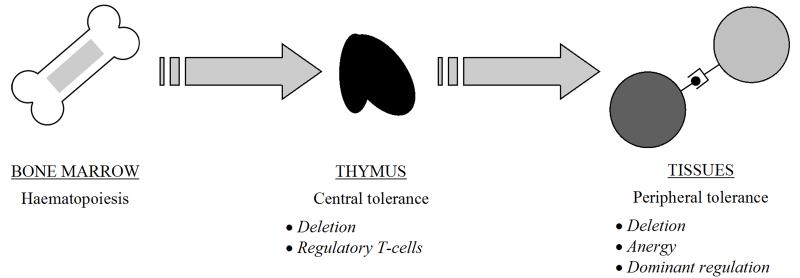
Figure 1.
Overview of central and peripheral tolerance mechanisms. Thymocytes are produced by the bone marrow from early foetal life and travel to the thymus for maturation. In the thymus, promiscuous expression of self-antigens from a range of tissue types (mediated by the AIRE gene) and affinity-based mechanisms result in deletion of autoreactive T cells and the generation of regulatory T-cells. In the periphery, further deletion and anergy can occur if T cells encounter antigen in the absence of sufficient co-stimulatory “danger” signals. Furthermore, unwanted autoreactivity can be directly suppressed by regulatory T-cells (dominant regulation).
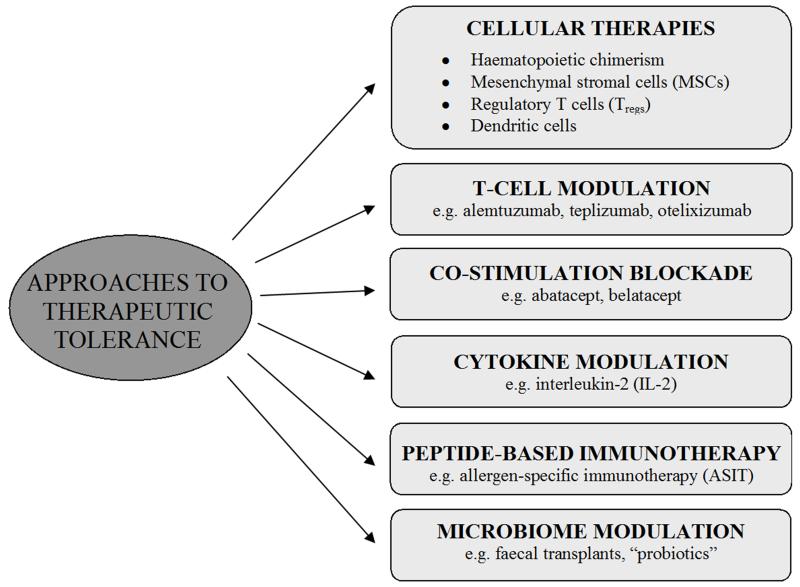
Figure 2.
Approaches to therapeutic tolerance induction.
Cellular therapies
Four main cellular therapies have shown promise in human tolerogenesis - haematopoietic chimerism, mesenchymal stromal cells, regulatory T cells, and dendritic cells. The latter is the focus of a detailed review in this issue, and hence will not be discussed further here.
Haematopoietic chimerism
The pioneering work of Medawar and Owen over 60 years ago established that therapeutic tolerance in the transplant setting could be conferred by prior recipient conditioning during foetal life with donor haematopoietic cells [1, 2]. The key requirement for success was the achievement of haematopoietic chimerism, with a “dual” immune system derived from both donor and recipient haematopoietic elements. Later work identified that this could also be achieved in adults by prior myeloablation; however, initial protocols achieved this through extreme whole-body irradiation with severe and often fatal side-effects. Combined with the risks of graft versus host disease (GvHD), these limitations have severely limited the adoption of bone marrow transplantation (BMT) for tolerance induction in humans [3].
An exception to this is in the context of haematological malignancy, where the toxic effects of BMT are an acceptable price to pay for cure and where graft versus leukaemic effect is beneficial. Several small clinical studies in patients with multiple myeloma and end-stage renal failure confirmed the possibility of drug-free renal allograft survival in combination with BMT [4]. These promising results have provided impetus for the development of less toxic myelodepletive conditioning in solid organ transplantation outside of the setting of haematological malignancy. Indeed, several such recent proof-of-concept human studies have demonstrated complete withdrawal of immunosuppressive drugs in significant proportion of recipients [Table 1], and long term follow-up data are eagerly awaited.
Table 1.
Recent studies of haematopoietic chimerism in human solid organ transplantation.
| Study | Organ | Number of patients | Protocol | Outcomes |
|---|---|---|---|---|
| Scandling et al. (2012) [5] | HLA-matched living donor kidney transplant | 16 | Conditioning: ATG, CS, TLI (10 × 80-120cGy) HSCT (CD34 enriched) day +11 MI: MMF, CYS | 12 patients achieved durable peripheral blood donor chimerism, of which 11 have been fully withdrawn from immunosuppression (median 21 months, range 1 - 40) No GvHD, no graft loss 1 patient suddenly died at 42 months, 1 patient developed breast cancer (possibly unrelated) |
| Leventhal et al. (2013) [6] | HLA-mismatched living donor kidney transplant | 15 | Conditioning: FLU, CYC, TBI (200cGy) HSCT (facilitating cell enriched) day +1 MI: TAC, MMF | 9 patients achieved durable peripheral blood donor chimerism, of which 6 have been fully withdrawn from immunosuppression (median 13.5 months, range 6-22) No GvHD 1 patient developed bone marrow failure with graft loss and viral sepsis |
| Schneeberger et al. 2013 [7] | HLA-matched cadaveric upper limb transplant | 4 | Conditioning: ATZ, CS BMT (unmodified) day +14 MI: TAC | Graft survival in all 4 patients on TAC monotherapy (vs. standard TAC/MMF/CS regimen) No GvHD No patients achieved durable donor chimerism |
ATG = anti-thymocyte globulin, ATZ = alemtuzumab, BMT = bone marrow transplant, CS = corticosteroid, CYC = cyclophosphamide, CYS = cyclosporin, FLU = fludarabine, GvHD = graft versus host disease, HSCT = haematopoietic stem cell transplant, MI = maintenance immunosuppression, MMF = mycophenolate mofetil, TAC = tacrolimus, TBI = total body irradiation, TLI = total lymphoid irradiation.
Mesenchymal stromal cells
Mesenchymal stromal cells (MSCs) are multipotent stem cells which can differentiate into a range of mesodermal lineages including haematopoietic cells, adipocytes, chondrocytes and osteocytes [8]. Initially studied for their regenerative properties, MSCs have been the recent focus of intense interest due to their immunomodulatory properties both in vitro and in vivo in several animal models of human autoimmune disease. The exact mechanisms by which MSCs regulate the immune response are unclear, though studies suggest soluble factors (such as transforming growth factor β (TGFβ), prostaglandin E2, indoleamine 2,3-dioxygenase (IDO) and human leukocyte antigen (HLA) G5) as well as direct cell-contact are important [8]. Traditionally MSCs are harvested from bone marrow samples [9], although they can now also be obtained by less invasive approaches including from adipose tissue [10], oral mucosa [11] and umbilical cord [12].
The safety of intravenous MSC infusion in autoimmune disease has been confirmed in several early-phase clinical trials, with promising suggestions of beneficial therapeutic effects in these small studies [13] Table 2]. However, observations that MSCs can differentiate to sarcoma cells in vitro [21] and can potentiate the immune evasion of breast cancer cells [22] raise significant safety concerns. Furthermore, the in vivo longevity of MSCs may be limited. In a recent human autopsy study of patients who had received intravenous MSC infusions before death, 9 of 13 patients had detectable donor MSC DNA where the infusion was given within the past 50 days, compared to only 2 of 8 patients who had received the infusion more than 50 days previously [23]. Nevertheless, it is possible that a transient induction of tolerance could be maintained far beyond the life-span of the MSC through mechanisms such as infectious tolerance, as will be discussed later.
Table 2.
Studies of mesenchymal stromal cell (MSC) therapy for autoimmune disease in humans.
| Study | Disease | Number of patients | MSC source | Outcomes |
|---|---|---|---|---|
| Ra et al. (2011) [14] | Various (multiple sclerosis, polymyositis, rheumatoid arthritis, atopic eczema, autoimmune hearing loss) | 10 | Autologous adipose-derived, intravenous delivery | Safety, some evidence of improved clinical outcomes |
| Duijvestein et al. (2010) [15] | Crohn’s disease | 10 | Autologous bone marrow-derived, intravenous delivery | Safety, three patients achieved significant clinical response |
| Garcia-Olmo et al. (2009) [16] | Crohn’s disease | 49 | Autologous adipose derived, topical delivery with fibrin glue | Safety, improved peri-anal fistula healing in fibrin-MSC versus fibrin-only control group (71% vs. 16% - study included non-immune causes of fistulation) |
| Ciccocioppo et al. (2011) [17] | Crohn’s disease | 12 | Autologous bone marrow-derived, intra-fistula injection | Safety, complete fistula closure in 7 patients and reduced clinical disease severity |
| Connick et al. (2012) [18] | Multiple sclerosis | 10 | Autologous bone marrow-derived, intravenous delivery | Safety, improvement in visual acuity, visual evoked response latency and optic nerve area |
| Karussis et al. (2010) [19] | Multiple sclerosis | 34 | Autologous bone marrow-derived, intravenous (n=14) and intrathecal (n=34) delivery | Safety, improved neurological disability scores (study also included patients with amyotrophic lateral sclerosis) |
| Sun et al. (2009) [20] | Systemic lupus erythematosus | 4 | Allogenic living relative bone marrow-derived, intravenous delivery | Safety, significant improvements in SLEDAI scores and proteinuria levels |
Adapted from Bernado & Fibbe [13]. SLEDAI = systemic lupus erythematosus disease activity index.
Regulatory T cells
T cell depletion and functional anergy underpinned early theories of tolerance induction. A revolutionary paradigm shift came with the recognition of “dominant” tolerance mechanisms, whereby regulatory T cell (Treg) subsets actively and specifically suppress or regulate potentially harmful immune responses. In addition to offering a potentially powerful in vivo effector mechanism for tolerogenic therapies [3], an alternative approach is the ex vivo isolation of Tregs, either from the patient or an allogenic donor, and subsequent use as a therapeutic agent per se [24]. Autologous ex vivo expanded Treg therapies circumvent the potential negating “host versus graft” effect, although the bespoke manufacturing process is significantly more time-consuming and costly compared to their “off-the-shelf” allogenic Treg counterparts. Indeed, intravenous infusions enriched with either donor-specific or allogenic Tregs have shown potentially beneficial effects in the prevention of GvHD in human BMT [25, 26], although this is yet to be translated to other human diseases.
Much of the difficulty in this translation lies with a lack of reliable Treg surface markers, with an over-reliance on functional assays and the potential risk of unintentional inclusion of pro-inflammatory T cells within therapeutic isolates [24]. However, recent epigenetic studies have suggested that expansion of naïve CD45RA+ Tregs may provide a more stable regulatory phenotype compared with unselected Treg populations [27]. Furthermore, in vivo animal studies and in vitro studies with human cells have confirmed the ability of Tregs to potentiate a regulatory phenotype in other effector T cells in a process termed “infectious tolerance’ [28], which may amplify the therapeutic effect. However, the in vivo stability of a regulatory phenotype in human Treg therapy remains to be established, and observations that Tregs can potentiate the metastatic spread of breast cancer in mouse models [29] raises potential safety concerns. Both issues will require careful consideration and will likely be critical to the success of Tregs as a future cellular tolerogenic therapy.
T-cell modulation
In addition to the use of exogenous Tregs as a cellular therapy, it is also possible to manipulate the existing T cell population in vivo towards a tolerogenic state. One of the earliest and least subtle approaches is the mass depletion of lymphocytes through the use of animal-derived anti-thymocyte globulins and monoclonal antibodies such as alemtuzumab (anti-CD52). These agents are now routinely used as induction agents in solid organ transplantation, reducing the incidence of acute allograft rejection [30] and enabling graft survival with reduced maintenance immunosuppression in a phenomenon termed “prope tolerance” [31]. In recent clinical trials, alemtuzumab has been shown to reduce relapse rate in multiple sclerosis [32], though at the expense of a paradoxical increase in other forms of autoimmunity during immune reconstitution, most notably autoimmune hyperthyroidism [33].
In a similar approach, monoclonal antibodies directed against the T-cell receptor (anti-CD3: teplizumab and otelixizumab) have shown promise in the treatment of type I diabetes mellitus. When used in early disease, anti-CD3 therapy can improve endogenous insulin secretion and reduce exogenous insulin requirements as demonstrated in phase I/II clinical trials [34]. Pre-clinical data suggest an induction of Tregs following anti-CD3 treatment [35], though the precise mode of action remains elusive. Randomised controlled phase III trials have however failed to reach primary endpoints, possibly reflecting under-dosing and heterogeneity in clinical response [34], and further research continues.
Co-stimulation blockade
The priming of a naïve T-cell response requires the presence of both an antigen-specific signal via the T-cell receptor, and a co-stimulatory signal provided by the antigen-presenting cell (APC). Both of these signals are mediated by receptor-ligand interactions at the so-called “immunological synapse” [36]. By manipulating the co-stimulatory signals, it is possible to abrogate the priming of an effector T-cell response or even shift priming towards a Treg phenotype [37].
Two of the best-characterised co-stimulatory interactions take place between CD80 and CD86, which are expressed on the surface of APCs, and CD28 on the surface of T cells [37]. Abatacept and belatacept are CTLA-4:Ig Fc fusion proteins which compete with CD28 for CD80/86 and hence block this co-stimulatory signal. Abatacept is licensed for use in rheumatoid arthritis, and has also been trialled in several other autoimmune diseases including inflammatory bowel disease [38], multiple sclerosis [39] and systemic lupus erythematosus [40] though with less promising results.
Given the pivotal role of co-stimulatory signals in the initial activation of T cells, therapy aimed at co-stimulatory blockade may be most effective when delivered early in the establishment of autoimmune disease. Indeed, in a recent randomised double-blinded trial of 112 patients with a recent (<100 days) diagnosis of type I diabetes, abatacept delayed the reduction in endogenous insulin secretion by around 9 months, although subsequent disease progression in both groups was largely similar [41]. Furthermore, in patients with early undifferentiated inflammatory arthritis, 6 months of abatacept therapy reduced the rate of progression to rheumatoid arthritis at 2 years by 20%, though falling just short of statistical significance in this small study [42]. The enhanced detection of autoimmunity at very early or even preclinical stages may therefore provide a “window of opportunity” for co-stimulatory blockade and other approaches in future tolerogenic strategies [43].
Interleukin-2
Interleukin-2 (IL-2) is a key player in T-cell activation and proliferation, and has been the focus of recent interest as a possible target for therapeutic tolerance. Early phase clinical trials of daclizumab, a monoclonal antibody directed against the α-subunit of the IL-2 receptor (CD25), have shown promise in the treatment of multiple sclerosis [44]. There is a surprising heterogeneity of action observed at a cellular level - daclizumab not only blocks IL-2 binding to CD25 on the surface of T-cells, but has also been shown to block the trans-presentation of IL-2 by binding to CD25 on the surface of dendritic cells [45] and also appears to upregulate natural killer (NK) cell mediated T-cell destruction [46]. Furthermore, IL-2 is important for the proliferation of Tregs [47], with deficiency of CD25 resulting in autoimmunity in humans [48]. In a recent phase 1/2a clinical study of hepatitis C virus-induced cryoglobulinaemic vasculitis, low-dose IL-2 led to an in vivo expansion of CD4+CD25hiFOXP3+ Tregs with associated clinical improvements [49]. With such diverse and at times opposing functions, it is unlikely that unselective IL-2 blockade will provide a reliable tolerogenic strategy in humans, though may prove to be a useful tool in the conditioning of cellular therapies ex vivo.
Allergen-specific immunotherapy (ASIT)
Allergic reactions are characterised by a Th2 skewed response to a specific allergen, mediated largely by IgE bound to IgE receptors on the surface of innate immune cells including mast cells and basophils. Upon contact with the cognate allergen, IgE cross-linking triggers the release of chemokines and vasoactive compounds, leading to an immuno-inflammatory cascade resulting in rapid bronchoconstriction and widespread vascular permeability with potentially fatal consequences [50]. Although mechanistically different from autoimmunity, recent advances in allergy immunotherapy may hold important lessons for therapeutic tolerance.
The traditional management of allergic disorders has focussed on allergen avoidance, and the use of drugs to counter the allergic response such as anti-histamines, corticosteroids and adrenaline. An alternative approach is desensitisation therapy with graded allergen challenge. Numerous studies have identified the central importance of IL-10 production by T cells (termed Tr1 cells) in this process, with consequent down-regulation of pro-inflammatory cytokine production and shifting of B cell antibody production away from IgE and towards IgG4 [51]. Furthermore, so-called “regulatory B-cells” may also play a role in IL-10 production and allergen presentation, though this relatively novel concept is somewhat controversial [52].
Precisely which factors promote the differentiation and stimulation of Tr1 cells in this context remain elusive, though low followed by gradually escalating allergen doses and sustained allergen exposure appears to be important, particularly if delivered by the oral route. In two randomised placebo-controlled and double-blinded trials of oral ASIT therapy for children with peanut allergy [53, 54], patients in the treatment arms showed significantly reduced skin-prick responses and loss of atopy to oral allergen challenge after 1 year of ASIT compared to placebo. One study also demonstrated higher levels of FoxP3+CD4+CD25+ Tregs in the ASIT group [54]. Similarly, in an observational study of hen’s egg oral ASIT [55], allergen tolerance was associated with an increase in a hypo-proliferative CD4+CD38+CD45RO- T cell subset.
Although not equivalent to tolerance induction, ASIT provides proof-of-principle for the potential to therapeutically modulate antigen-specific responses in vivo. In terms of tolerance, the induction of an immunoregulatory response to one autoantigen can catalyse a similar response to other autoantigens in a process termed “epitope spreading”. Therefore, although the nature of the antigens that drive human autoimmunity remain elusive, antigen-specific therapy may yet hold promise.
The microbiome in autoimmunity and tolerance
Genetic predisposition to autoimmunity is important but by no means absolute - for example, monozygotic twin studies show a concordance of only 12-15% for rheumatoid arthritis [56]. Recent interest in identifying the environmental triggers and perpetuators of autoimmunity has focussed on interactions with the human microbial flora, or “microbiome” [57]. It is estimated that there are at least 10 times as many microbes within the gut than cells within the human body [57], and recent advances in high-throughput techniques have revealed approximately 65% variation in gut microbial genes between individuals [58].
Strong evidence from animal models supports a role for the microbiome in the breakdown of immune tolerance. In the K/BxN murine arthritis model, arthritis activity is greatly attenuated in mice reared in germ-free environments, combined with a reduction in autoantibody titres and pro-inflammatory Th17 T-cell populations [59]. However, subsequent introduction of a single species of gut microorganism (segmented filamentous bacteria) was sufficient to recreate autoimmunity, and this effect could be inhibited by antibiotics effective against the microbe [59]. In human observational studies, colonisation by specific bacteria such as Porphyromonas gingivalis has been shown to correlate with the presence of anti-citrullinated protein antibodies in rheumatoid arthritis [60], and antibodies against citrullinated P. gingivalis peptides have been shown to cross-react with citrullinated self-proteins [61].
Contrary to the above, there is also evidence to support a pro-tolerogenic role for the microbiome in preventing autoimmunity. Colonisation by a mixture of Clostridium species has been demonstrated to increase colonic Treg populations in mice, which were subsequently resistant to chemical-induced colitis [62]. Furthermore, murine colonic Tregs have been demonstrated to show antigen-specificity for microbial antigens in vitro [63], and the recent findings of specific Treg-promoting receptors on the luminal surface of gut lamina propria in mice raises the intriguing possibility of microbial ligands for host immunoregulatory mechanisms [64].
Given its emerging importance in the regulation of immunity, microbiome modulation offers an enticing target for therapeutic tolerance. The simplest form of microbiome-based therapies are antibiotics, which have been shown to afford benefit, albeit limited, in rheumatoid arthritis [65] and inflammatory bowel disease [66]. A more intelligent and perhaps more powerful approach would be a focussed rebalance of a pathogenic microbiome towards a tolerogenic state, such as by the use of faecal matter from healthy individuals in a process termed “faecal transplantation” [67, 68]. Faecal transplants have proven efficacy in the treatment of Clostridium difficile colitis [67], and there are increasing numbers of case reports of beneficial effects in small cohorts of patients with autoimmune disease such as inflammatory bowel disease and multiple sclerosis [67, 68]. In a recent pre-clinical study, Clostridium isolates from healthy human stool were shown to increase intestinal Treg populations in germ-free mice, and also provided a protective effect against experimentally-induced colitis [69]. Randomised controlled clinical trials in this rapidly evolving field are awaited with keen interest.
Looking to the future - biomarkers of tolerance
A major obstacle to the advancement of tolerogenesis in human studies is the lack of reliable biomarkers that distinguish immune tolerance from a state of immunosuppression. Such biomarkers would allow for objective and direct measurements of the effectiveness of tolerogenic therapies, and also would allow for intelligent withdrawal of immunosuppression in patients whose autoimmune disease is stable [70].
Intriguing observations in the field of transplantation have shown that immune tolerance is perhaps more achievable than previously believed. A minority of transplant patients will not reject their allograft despite, often unintentional, cessation of their immunosuppressive medication, yet can still mount potent immune responses to other non-self antigens in a phenomenon termed “operational tolerance”[71]. Encouraged by these findings, prospective immunosuppression weaning studies have demonstrated the ability to achieve operational tolerance in as many as 40% of stable liver transplant recipients [72, 73], albeit with strict exclusion criteria. Studies of international cohorts of operationally-tolerant renal and liver transplant patients have identified distinct tolerance signatures of gene expression in peripheral blood microarray analyses (reviewed by Chandrasekharan et al. [70]), which are able to identify operationally tolerant individuals with high specificity. Interestingly, comparisons of operational tolerance in renal and liver transplant patients have shown non-overlapping gene expression signatures, with a predominance of NK cell gene enrichment in liver transplantation compared to B-cell signatures in renal transplantation [74]. Although the analysis of small microarray samples is statistically challenging [71], this does raise the distinct possibility of allograft-specific tolerance mechanisms. Some of these may indeed act in the local allograft microenvironment and be undetectable at the systemic level, as suggested by murine transplantation experiments [75]. Nevertheless, the results from these observational studies are encouraging, and the validation of putative biomarkers in prospective immunosuppressant withdrawal studies is awaited with much interest.
Conclusion
Immune tolerance has long been attainable in animal models, although translation to humans has been frustratingly elusive. However, recent evidence from human transplantation provides clear proof-of-principle that therapeutic tolerance is an attainable target, and a diverse range of tolerogenic strategies have shown promise in early clinical trials. It is increasingly apparent that the mechanisms of immune tolerance are multifaceted, with the relative dominance of each mechanism dependent on the disease-specific state of immune dysregulation. Key to the advancement of tolerogenesis is the use of tolerance biomarkers, allowing for disease-specific and patient-tailored therapy. With such continuing advances it is surely possible to move immune tolerance away from the current horizon of therapeutic possibility and towards future clinical reality.
Supplementary Material
Key Points.
Recent research has helped to narrow the translational gap between experimental animal models and clinical therapeutic tolerance
The survival of transplanted allografts despite complete withdrawal of immunosuppression is possible with haematopoietic chimerism, and is observed sporadically in a small number of transplant recipients in a phenomenon termed “operational tolerance”
Cellular therapies including regulatory T cell and mesenchymal stromal cell transfer show promise in the treatment of several autoimmune diseases in recent early-phase clinical trials
The enhanced detection of autoimmunity at very early or even preclinical stages can provide a window of opportunity for therapeutic tolerance
Reliable biomarkers of tolerance are urgently needed to provide objective measurements of the effectiveness of tolerogenic therapies, and to allow for intelligent immunosuppressant withdrawal in patients whose autoimmune disease is stable.
Acknowledgements
Funding Disclosure: K.F.B. is supported by a Wellcome Trust and Newcastle University Clinical Research Fellowship in Translational Medicine and Therapeutics. Work in the Musculoskeletal Research Group is supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
No conflicts of interest
References
- [1].Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- [2].Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- [3].*; Issa F, Wood KJ. Translating tolerogenic therapies to the clinic - where do we stand? Front Immunol. 2012;3:254. doi: 10.3389/fimmu.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A topical review summarising current advances in the field of therapeutic tolerance, with a specific focus on cellular therapies.
- [4].Spitzer TR, Sykes M, Tolkoff-Rubin N, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–676. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].**; Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12:1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].**; Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95:169–176. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** The above two clinical trials provide important proof-of-concept evidence for the effectiveness of haematopoietic chimerism in solid organ transplantation. Conditioning followed by haematopoietic stem cell transplantation allowed for complete withdrawal of immunosuppression in a large proportion of renal allograft recipients.
- [7].Schneeberger S, Gorantla VS, Brandacher G, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].*; Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Ying and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91:12–18. doi: 10.1038/icb.2012.60. [DOI] [PubMed] [Google Scholar]; * This succinct review summarises current evidence on the immunomodulatory functions of mesenchymal stromal cells and their interaction with regulatory T cells, with a specific focus on clinical applications.
- [9].Hanley PJ, Mei Z, da Graca Cabreira-Hansen M, et al. Manufacturing mesenchymal stromal cells for phase I clinical trials. Cytotherapy. 2013;15:416–422. doi: 10.1016/j.jcyt.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davies LC, Lönnies H, Locke M, et al. Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev. 2012;21:1478–1487. doi: 10.1089/scd.2011.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karlsson H, Erkers T, Nava S, et al. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167:543–555. doi: 10.1111/j.1365-2249.2011.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N.Y. Acad Sci. 2012;1266:107–117. doi: 10.1111/j.1749-6632.2012.06667.x. [DOI] [PubMed] [Google Scholar]
- [14].Ra JC, Kang SK, Shin IS, et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med. 2011;9:181. doi: 10.1186/1479-5876-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- [16].Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- [17].Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulating Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- [18].Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rubio R, Gutierrez-Aranda I, Sáez-Castillo AI, et al. The differentiation stage of p53-Rb-deficient bone marrow mesenchymal stem cells imposes the phenotype of in vivo sarcoma development. Oncogene. 2012 Dec 10; doi: 10.1038/onc.2012.507. doi: 10.1038/onc.2012.507. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [22].Patel SA, Meyer JR, Greco SJ, et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- [23].*; von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]; * In this autopsy study of patients who had received mesenchymal stromal cell therapy before death, no evidence of malignancy or ectopic tissue formation was demonstrated. There was however a negative correlation between detection of donor MSC DNA and time from infusion to autopsy.
- [24].Sagoo P, Lombardi G, Lechler RI. Relevance of regulatory T cell promotion of donor-specific tolerance in solid organ transplantation. Front Immunol. 2012;3:184. doi: 10.3389/fimmu.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- [27].Schmidl C, Hansmann L, Andreesen R, et al. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naïve Treg. Eur J Immunol. 2011;41:1491–1498. doi: 10.1002/eji.201041067. [DOI] [PubMed] [Google Scholar]
- [28].*; Gravano DM, Vignali DA. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci. 2012;69:1997–2008. doi: 10.1007/s00018-011-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A well-written review that summarises the concept of “infectious tolerance” and its importance in tolerance induction.
- [29].Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].*; Zhang X, Huang H, Han S, et al. Alemtuzumab induction in renal transplantation: a meta-analysis and systemic review. Transpl Immunol. 2012;27:63–68. doi: 10.1016/j.trim.2012.08.006. [DOI] [PubMed] [Google Scholar]; * This meta-analysis of six RCTs demonstrates superiority of alemtuzumab over alternative antibody induction agents in reducing the incidence of acute rejection in renal transplantation. This benefit however may not extend to patients at high risk of rejection, and does not clearly translate to increased long-term graft or patient survival.
- [31].Calne R, Watson CJ. Some observations on prope tolerance. Curr Opin Organ Transplant. 2011;16:353–358. doi: 10.1097/MOT.0b013e328348b44c. [DOI] [PubMed] [Google Scholar]
- [32].*; Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]; * An important phase III RCT which demonstrates superiority of alemtuzumab above interferon therapy for multiple sclerosis, with relapse rates of 22% in the alemtuzumab group versus 40% in the interferon group.
- [33].Aranha AA, Amer S, Reda ES, et al. Autoimmune Thyroid Disease in the Use of Alemtuzumab for Multiple Sclerosis: A Review. Endocr Pract. 2013 Jun 11;:1–25. doi: 10.4158/EP13020.RA. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [34].Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol. 2013 May;:11. doi: 10.1016/j.clim.2013.05.001. pii: S1521-6616(13)00120-4. doi: 10.1016/j.clim.2013.05.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [35].Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–2022. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hivroz C, Chemin K, Tourret M, Bohineust A. Crosstalk between T lymphocytes and dendritic cells. Crit Rev Immunol. 2012;32:139–155. doi: 10.1615/critrevimmunol.v32.i2.30. [DOI] [PubMed] [Google Scholar]
- [37].Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sandborn WJ, Colombel JF, Sands BE, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62–69. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- [39].Viglietta V, Bourcier K, Buckle GJ, et al. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71:917–924. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- [40].Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- [41].Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Emery P, Durez P, Dougados M, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial) Ann Rheum Dis. 2010;69:510–516. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Isaacs JD. The changing face of rheumatoid arthritis: sustained remission for all? Nat Rev Immunol. 2010;10:605–611. doi: 10.1038/nri2804. [DOI] [PubMed] [Google Scholar]
- [44].Wiendl H, Gross CC. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol. 2013;9:394–404. doi: 10.1038/nrneurol.2013.95. [DOI] [PubMed] [Google Scholar]
- [45].Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Goudy K, Aydin D, Barzaghi F, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146:248–261. doi: 10.1016/j.clim.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- [50].Greenberger PA, Ditto AM. Chapter 24: Anaphylaxis. Allergy Asthma Proc. 2012;33(Suppl 1):S80–83. doi: 10.2500/aap.2012.33.3557. [DOI] [PubMed] [Google Scholar]
- [51].Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012;2:2. doi: 10.1186/2045-7022-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Smits HH. B cells in allergic diseases: bad or better? Autoimmunity. 2012;45:415–426. doi: 10.3109/08916934.2012.665525. [DOI] [PubMed] [Google Scholar]
- [53].Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–646. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fuentes-Aparicio V, Alonso-Lebrero E, Zapatero L, et al. Oral immunotherapy in hen’s egg-allergic children increases a hypo-proliferative subset of CD4+ T cells that could constitute a marker of tolerance achievement. Pediatr Allergy Immunol. 2012;23:648–653. doi: 10.1111/j.1399-3038.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- [56].Reveille JD. Genetic studies in the rheumatic diseases: present status and implications for the future. J Rheumatol Suppl. 2005;72:10–13. [PubMed] [Google Scholar]
- [57].Proal AD, Albert PJ, Marshall TG. The human microbiome and autoimmunity. Curr Opin Rheumatol. 2013;25:234–240. doi: 10.1097/BOR.0b013e32835cedbf. [DOI] [PubMed] [Google Scholar]
- [58].Atarashi K, Honda K. Microbiota in autoimmunity and tolerance. Curr Opin Immunol. 2011;23:761–768. doi: 10.1016/j.coi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [59].Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T Helper 17 Cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Arvikar SL, Collier DS, Fisher MC, et al. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;15:R109. doi: 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yeoh N, Burton JP, Suppiah P, et al. The role of the microbiome in rheumatic diseases. Curr Rheumatol Rep. 2013;15:314. doi: 10.1007/s11926-012-0314-y. [DOI] [PubMed] [Google Scholar]
- [62].Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].**; Kim SV, Xiang WV, Kwak C, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340:1456–1459. doi: 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this important preclinical study, the G-protein coupled receptor GPR15 controlled the migration of FoxP3+ Tregs to the large intestine lamina propria in mice. The expression of GPR15 was modulated by gut microbiota, providing evidence of a molecular link between the microbiome and the maintenance of tolerance.
- [65].Smith CJ, Sayles H, Mikuls TR, Michaud K. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res Ther. 2011;13:R168. doi: 10.1186/ar3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- [67].**; Smits LP, Bouter KE, de Vos WM, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- [68].*; Vrieze A, de Groot PF, Kootte RS, et al. Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol. 2013;27:127–137. doi: 10.1016/j.bpg.2013.03.003. [DOI] [PubMed] [Google Scholar]
- * These two recent reviews provide an easily accessible overview of the clinical evidence and practicalities for faecal transplantation.
- [69].**; Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]; ** In this exciting study, mice administered Clostridium isolates from healthy human stool showed increased intestinal Treg populations, and were resistant to experimental colitis. This raises the possibility of utilising probiotic organisms isolated from human stool in future clinical tolerance strategies.
- [70].Chandrasekharan D, Issa F, Wood KJ. Achieving operational tolerance in transplantation: how can lessons from the clinic inform research directions? Transpl Int. 2013;26:576–589. doi: 10.1111/tri.12081. [DOI] [PubMed] [Google Scholar]
- [71].Londoño MC, Danger R, Giral M, et al. A need for biomarkers of operational tolerance in liver and kidney transplantation. Am J Transplant. 2012;12:1370–1377. doi: 10.1111/j.1600-6143.2012.04035.x. [DOI] [PubMed] [Google Scholar]
- [72].**; Benítez C, Londoño MC, Miquel R, et al. Prospective multi-center clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013 Mar 26; doi: 10.1002/hep.26426. doi: 10.1002/hep.26426. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [73].**; Bohne F, Martínez-Llordella M, Lozano JJ, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122:368–382. doi: 10.1172/JCI59411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** In these two prospective clinical trials, stable liver transplant recipients were weaned off maintenance immunosuppression with approximately 40% achieving operational tolerance. Through analysis of biomarkers prior to immunosuppression withdrawal it may be possible in the future to predict which patients can successfully achieve operational tolerance.
- [74].Lozano JJ, Pallier A, Martinez-Llordella M, et al. Comparison of transcriptional and blood cell-phenotypic markers between operationally tolerant liver and kidney recipients. Am J Transplant. 2011;11:1916–1926. doi: 10.1111/j.1600-6143.2011.03638.x. [DOI] [PubMed] [Google Scholar]
- [75].Cobbold SP, Adams E, Waldmann H. Biomarkers of transplantation tolerance: more hopeful than helpful? Front Immunol. 2011;2:9. doi: 10.3389/fimmu.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.