Abstract
Adaptive immunity critically depends on the functional compartmentalization of secondary lymphoid organs. Mesenchymal stromal cells create and maintain specialized niches that support survival, activation and expansion of T and B cells, and integrated analysis of lymphocytes and their niche has been instrumental in understanding adaptive immunity. Lymphoid organs are also home to type 3 innate lymphoid cells (ILC3), innate effector cells essential for barrier immunity. However, a specialized stromal niche for ILC3 has not been identified. A novel lineage-tracing approach now identifies a subset of murine fetal lymphoid tissue organizer cells that gives rise exclusively to adult marginal reticular cells (MRC). Moreover, both cell types are conserved from mouse to human and co-localize with ILC3 in secondary lymphoid tissues throughout life. In sum, we provide evidence that fetal stromal organizers give rise to adult MRC and form a dedicated stromal niche for innate ILC3 in adaptive lymphoid organs.
Introduction
Mesenchymal stromal cells are essential for the development of secondary lymphoid organs (reviewed in (1)). In adult mice, mesenchymal stromal cells maintain the specialized microenvironments in lymphoid tissues where T cells encounter their cognate antigen presented by dendritic cells and B cells generate high affinity antibodies during germinal center reactions (2). To date, several specialized subsets of stromal cells have been identified. T zone fibroblastic reticular cells (TRCs) provide structural support for the migration of T cells and deliver survival and recruitment signals to the migrating T cells in the form of IL-7 and CCL19/CCL21 (3). Follicular dendritic cells (FDCs) are located in B cell follicles and are essential for generation of high affinity antibodies by presenting immune-complexes to B cells and for supporting B cell migration and homeostasis through expression of BAFF and non-classical CD40 ligands (4-8). Functional deficiency of either TRC or FDC severely impact adaptive immunity, highlighting the essential role of stromal cell-derived microenvironments in the immune system (9-13).
Rorγt+ type 3 innate lymphoid cells (ILC3) are a part of a family of innate-like immune cells with important roles in barrier immunity to enteric pathogens, cancer surveillance and intestinal homeostasis (reviewed in (14)). Rorγt+ ILC3 reside in mucosal lymphoid and non-lymphoid tissues, where they are characterized by expression of the natural cytotoxicity receptor (NCR) NKp46 in mice and NKp44 in human (15-18). In addition, ILC3 are also found in peripheral lymphoid organs as NCR− cells (15, 16). Even though ILC3 are found in most lymphoid tissues, it has yet to be established whether ILC3 reside in specialized micro domains, analogous to T and B cells. If so, this would provide novel insights into ILC3 function and regulation.
During murine embryogenesis, a specialized population of stromal cells termed lymphoid tissue organizer (LTo) cells interacts with a subset of ILC3 also known as Lymphoid Tissue inducer (LTi) cells, at prospective lymph node anlagen (19-22). This interaction induces activation of LTo cells through lymphotoxin-β receptor (LTβR) and TNF receptor (TNFR) signaling (22). Activated LTo cells express ICAM-1, VCAM-1 and MAdCAM-1, and secrete the three homeostatic chemokines CXCL13, CCL21 and CCL19 that are essential for stromal cell-driven organization of the developing lymph node. While LTi cell – LTo cell interactions are crucial for mouse lymph node and Peyer’s patch development, splenic white pulp development is LTi cell independent (23-25).
In adult lymph nodes, a stromal cell population exists that phenotypically resembles fetal stromal LTo cells (26, 27). These marginal reticular cells (MRC) express VCAM-1, ICAM-1 and MAdCAM-1 (26) and cell lines generated from MRC produce CXCL13 upon LTβR stimulation (28). In vivo, MRC can differentiate into FDC after immune activation (29). Due to their phenotypic resemblance to fetal LTo cells, it was postulated that MRC represent direct descendants of the fetal stromal organizer cells (30). However, stromal cell lineage relationships between LTo and MRC remain experimentally unaddressed.
Here we set out to identify and characterize a specialized stromal cell niche for ILC3 in human and mouse secondary lymphoid organs. We show that LTo and MRC are conserved from mouse to man, and that they co-localize with ILC3. Using a novel lineage tracing approach we provide evidence that a subset of fetal LTo cells give rise exclusively to MRC, suggesting that the LTo – MRC lineage provides a life-long stromal framework for human and mouse ILC3.
Materials and Methods
Mice
Il7Cre, (31) and Rosa26 eYFP mice were bred and maintained in a specific pathogen-free facility at the University of York under Home Office license. All animal research was approved by the University of York Ethical Review Process and conducted in accord with ARRIVE guidelines. Superovulated female Rosa26eYFP mice were mated with IL-7 Cre males and day of plug was defined as day 0.5.
Human tissue
Use of all human tissues was approved by the Medical Ethical Commission of the Erasmus University Medical Center Rotterdam and was contingent on informed consent. Fetal lymph nodes and spleens were obtained from elective abortions and gestational age was determined by ultrasonic measurement of the skull or femur and ranged from 8 to 22 weeks. Adult hepatic lymph nodes were obtained during multi organ donation procedures.
Immunohistochemistry
Murine tissues were PFA fixed and passed through sucrose gradient before embedding in OCT (Tissue-Tek Sakura). 8-10 μm thick cryosections were stained with antibodies against GFP (Invitrogen), gp38, CD21/35, VCAM-1 (eBioscience) Desmin (AbCam), α-Smooth Muscle Actin (α -SMA) (Sigma) and the relevant secondary antibodies (Molecular Probes). Sections were mounted in Prolong®Gold Antifade Reagent (Invitrogen). For total LN overviews 5×7 individual images were merged by using tile scan under x40 magnification by Zeiss M710 confocal microscope. Cryosections (6 mm) of human tissues were fixed in acetone for 5 min and air-dried for an additional 10 min. After rehydration, sections were blocked with 1% (wt/vol) blocking reagent (TSAkits; Molecular Probes) and 10% (vol/vol) normal human and donkey serum, followed by treatment with a streptavidin-biotin blocking kit (Vector Laboratories). Sections were incubated with primary antibodies for 30 min. at RT, followed by 30 min. incubation with Alexa Fluor–labeled antibodies (Molecular Probes) and 5% normal human serum. Biotinylated primary antibodies were incubated overnight at 4°C and visualized using Tyramide Signal Amplification Kits (Molecular Probes) according to the manufacturer’s instructions. Sections were embedded in Vectashield (Vector Labs). Antibodies used were: rabbit-anti-human-CD20 (Epitomics), rabbit-anti-human-CD3ε (Epitomics), mouse-anti-human-podoplanin Covance), rabbit-anti-human-Lyve-1 (Abcam), mouse-anti-human-MAdCAM-1 (HBT), mouse-anti-human-VCAM-1-bio (eBioscience), mouse-anti-human-RANKL-bio (eBioscience), rat anti-human Rorγt (eBioscience) and goat-anti-human-CXCL13-bio (R&D systems).
Flow Cytometry and cell sorting
Single cell suspensions of murine tissues were prepared from lymph nodes by dicing them into small pieces and then disaggregating with Liberase CI / Dnase1 (Roche Diagnostics GmbH), blocked with FcBlock (ebioscience) and then stained with gp38 (Biolegend), CD31, anti-CD45 biotin (ebioscience), and Streptavidin Pacific Blue (Invitrogen). Stained cells were sorted using a Moflo high speed cell sorter (Beckman Coulter). For flow cytometric analysis of human fetal LNs the following antibodies were used: Alexa Fluor-488-conjugated mouse-anti-podoplanin Biolegend), PE-conjugated mouse-anti-human RANKL (eBioscience), PE-Cy5.5–conjugated mouse-anti-CD45 (eBioscience), PE-Cy7-conjugated mouse-anti-human CD34 (BD Biosciences) and PE-TexasRed-conjugated mouse-anti-human CD31 (ImmunoTools). A FACSAria (Becton Dickinson) was used for cell sorting. Data were analyzed with FlowJo software (TreeStar).
Quantitative RT-PCR Analysis
RNA was extracted from murine tissues using the RNeasy Mini kit (Qiagen). RNA was converted to cDNA using Superscript III First Strand cDNA synthesis kit (Invitrogen). qPCR was then performed for each sorted cell subset in triplicate on ABI 7300 Real Time PCR system using primers to various genes specific to stromal cell subsets (mice or human) (Table 1). Gene expression was quantified relative to the endogenous housekeeping gene HPRT using 7000 System software from ABI. RNA from human tissues was extracted using the RNA-XS kit (Machery Nagel) followed by reverse-transcription with random hexamer primers. A Neviti Thermal Cycler (Applied Biosystems) and DyNAmo Flash SYBR Green qPCR kit (Finnzymes) were used for quantitative PCR, with the addition of MgCl2 to a final concentration of 4 mM. All reactions were done in duplicate and are normalized to the expression of GAPDH (glyceraldehyde phosphate dehydrogenase). Relative expression was calculated by the cycling threshold (CT) method as 2−Δt.
Table 1. Primer sequences.
| Gene | Forward primer | Reverse Primer |
|---|---|---|
| gapdh | GTC GGA GTC AAC GGA TT | AAG CTT CCC GTT CTC AG |
| tnfsf11 | GTG CAA AAG GAA TTA CAA CA | CGG TGG CAT TAA TAG TGA G |
| cxcl13 | CCT CCA GAC AGA ATG AAG TT | AGG GTC CAC ACA CAC AAT |
| ccl21 | GTA CAG CCA AAG GAA GAT TC | GGG GAT GGT GTC TTG TC |
| ccl19 | AGC CTG CTG GTT CTC TG | TGC AGC CAT CCT TGA T |
| il7 | CCT CCC CTG ATC CTT GTT CT | CGA GCA GCA CGG AAT AAA AA |
| GAPDH | GTC GGA GTC AAC GGA TT | AAG CTT CCC GTT CTC AG |
| TNFSF11 | GTG CAA AAG GAA TTA CAA CA | CGG TGG CAT TAA TAG TGA G |
| CXCL13 | CCT CCA GAC AGA ATG AAG TT | AGG GTC CAC ACA CAC AAT |
| CCL21 | GTA CAG CCA AAG GAA GAT TC | GGG GAT GGT GTC TTG TC |
| CCL19 | AGC CTG CTG GTT CTC TG | TGC AGC CAT CCT TGA T |
| IL7 | CCT CCC CTG ATC CTT GTT CT | CGA GCA GCA CGG AAT AAA AA |
Statistical analysis
Significance values were calculated using an unpaired two-tailed Student’s T test. P values of less than 0.05 were considered statistically significant.
Results
Identification of human fetal stromal organizer cells
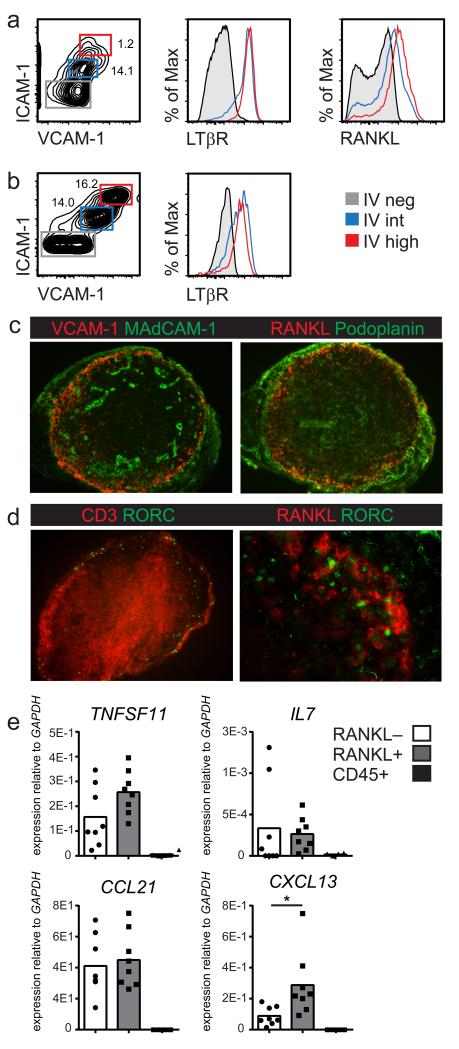
The stromal cells driving lymphoid organ development have been identified in mice, but it is unknown whether similar cells have been conserved in humans. To identify putative fetal stromal LTo cells in humans, we analyzed the stromal composition of developing human lymph nodes during first trimester pregnancy. At 9 weeks gestation, which is prior to the appearance of T cells in the circulation (32), mesenteric lymph nodes develop within the mesentery (33). At this age individual lymph nodes are undetectable using dissection microscopes (33), but upon isolation of total first trimester mesenteries we detected CD45− CD31− mesenchymal stromal cells that expressed ICAM-1 and VCAM-1 at intermediate or high levels. In addition, these stromal cells expressed LTβR and RANKL, very reminiscent of murine LTo cells (Figure 1A) (19, 22). To verify that these human stromal cells are indeed associated with lymphoid tissues we subsequently isolated individual lymph nodes from second trimester mesenteries (Figure 1B). Similar populations of ICAM-1 and VCAM-1 expressing cells were present in the fetal lymph nodes, and these cells also co-expressed LTβR. Having established the presence of a phenotypic stromal organizer population we asked whether these cells had the ability to function as LTo cells. First, as LTo interact with LTi cells, the spatial distribution of stromal cells and Rorγt+CD3− LTi cells in human fetal developing lymph nodes was determined (Figure 1C). Both Rorγt+CD3− LTi cells and stromal cells expressing VCAM-1 and MadCAM-1 as well as RANKL and Podoplanin were preferentially located in the outer cortex of the lymph node. The RANKL+ LTo-like cells were closely associated with the Rorγt+ LTi cells (Figure 1D). Second, the functional hallmark of murine LTo cells is their production of the homeostatic chemokines CXCL13, CCL21 and CCL19. We used the characteristic expression of RANKL to purify these cells by flow cytometry and compared chemokine transcripts by qPCR to sorted CD45−CD31− RANKL-Podoplanin+ stromal cells, which contain both TRC and FDC (Figure 1E). Transcript analysis revealed that, similar to the equivalent murine subset (3), TRC/FDC expressed transcripts for CCL19 and CCL21, most likely in TRC, and transcripts for CXCL13, most likely from FDC. In line with mouse cells, human RANKL+ stromal cells contained transcripts for all 3 homeostatic chemokines: CCL19, CCL21 and CXCL13, a hallmark of stromal organizer cells, as well as for IL7. Collectively these data identify VCAM-1+ICAM-1+RANKL+ stromal cells as human equivalents to murine LTo cells.
Figure 1. Stromal LTo cells in human fetal lymph nodes.
Fetal human lymph nodes and mesenteries were analyzed for presence of cells resembling murine LTo cells. (a) Expression of VCAM-1, ICAM-1, LTβR and RANKL on CD45-CD31− stromal cells from first trimester mesenteries. (n=3; age range: 8-10 weeks gestation) (b) Expression of VCAM-1, ICAM-1 and LTβR on CD45−CD31− stromal cells dissected from second trimester lymph nodes. (n=5; age range: 8-10 weeks gestation) (c) Localization of VCAM-1+ and RANKL+ stromal cells directly underneath the Podoplanin expressing subcapsular sinus (magnification 100x) (d) Co-localization of Rorγt+ cells and RANKL+ cells in fetal lymph nodes (magnification 100x/250x) (n=5) (e) Transcript analysis by qPCR of CD45−CD31− RANKL+ and RANKL− stromal cells purified from second trimester lymph nodes (n=8) compared to total CD45+ cells (n=4). (c-e: second trimester; age range 15-22 weeks gestation)
Stromal organizers in the fetal human spleen
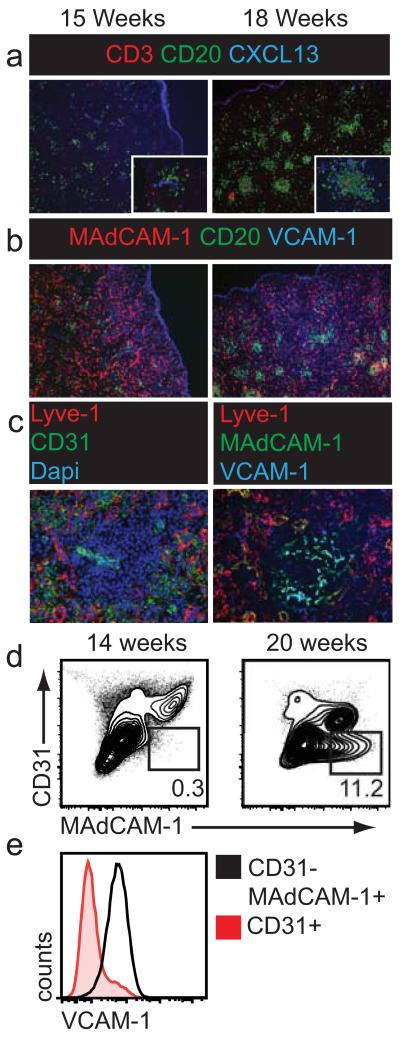
Having identified stromal LTo cells in human fetal lymph nodes we then assessed whether a putative stromal organizer population could also be identified in developing human spleens. In fetal spleens of 15 weeks gestation, primordial white pulp was apparent by perivascular clusters of B cells surrounding arterioles displaying strong CXCL13 labeling. At this gestational age only few scattered T cells were present (Figure 2A; left panel). At 18 weeks gestation, B cell clusters had increased in number and size and were now interspersed with T cells (Figure 2A; right panel). At both gestational ages no segregation of T and B cells was observed, in accordance with previous reports describing the appearance of delineated splenic B cell follicles from 24 weeks gestation onwards (34-37).
Figure 2. Stromal organizer cells in fetal human spleen.
Fetal human spleens were analyzed for the presence of putative stromal organizer cells (a) Appearance of perivascular lymphocyte clusters in fetal human spleens of 15 to 18 weeks gestation. (b) Appearance of MAdCAM-1+ cells in developing white pulp at week 18 (c) MAdCAM-1+Lyve-1−CD31− stromal cells in spleens of 18 weeks gestation. (d) Flow cytometric analysis of fetal spleens showing the appearance of CD45−CD31− MAdCAM-1+ stromal cells (n=3).
We hypothesized that putative stromal organizer cells should be contained within the perivascular lymphocyte clusters and might share phenotypic characteristics with lymph node organizers. In contrast to fetal lymph nodes we did not detect any RANKL-expressing cells in fetal spleens. On the other hand, MAdCAM-1 was abundantly expressed at all ages analyzed, albeit not by stromal cells but by endothelial cells contained within the fetal red pulp (Figure 2B). However, from 18 weeks gestation onwards a subset of non-hematopoietic cells, located exclusively within the primordial white pulp, initiated expression of MAdCAM-1 (Figure 2B; right panel). These MAdCAM-1+ cells were neither vascular nor lymphatic endothelium as shown by the lack of CD31 and Lyve-1 expression respectively (Figure 2C; left panel). Moreover, in line with lymph node organizers, the MAdCAM-1+ stromal cells co-expressed VCAM-1 (Figure 2C; right panel). To confirm the temporal regulation of MAdCAM-1 expression on splenic stromal cells flow cytometric analysis of fetal spleens was performed (Figure 2D). In support of the histological data, a distinct population of MAdCAM-1+ stromal cells was present at 20 weeks gestation yet virtually absent at 14 weeks gestation (Figure 2D). In addition to MAdCAM-1, these stromal cells were also uniformly positive for VCAM-1 (Figure 2E).
Fetal splenic organizers transcribe LTo-associated genes
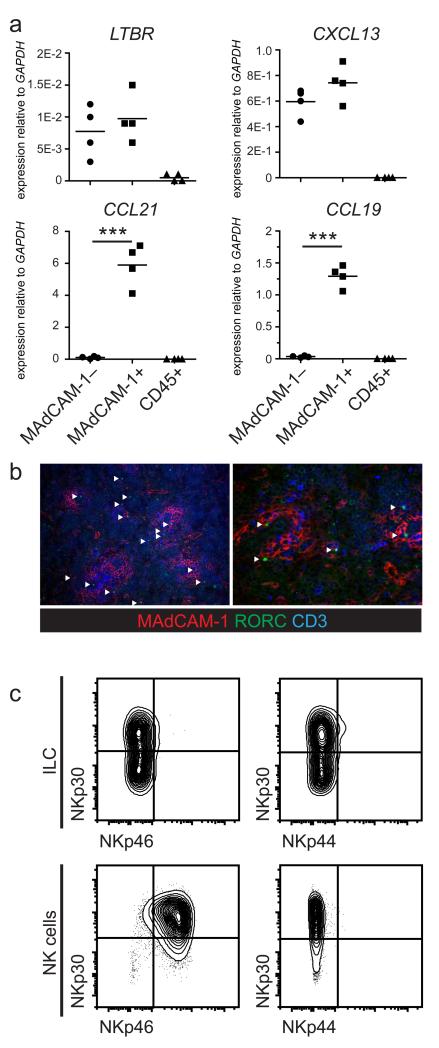
As the MAdCAM-1+ VCAM-1+ stromal cells that localize specifically to the developing white pulp showed phenotypic resemblance to lymph node LTo cells we sought to gain mechanistic insight into a possible organizing function of these cells. Therefore CD45−CD31−MAdCAM-1− and CD45−CD31−MAdCAM-1+ cells were sorted to purity and analyzed by qPCR for genes involved in lymphoid tissue organization (Figure 3A). Even though the MadCAM-1 negative populations contains the majority of all splenic reticular cells, including erythroblasts, both populations contained transcripts for LTBR and CXCL13. The T zone-specific transcripts CCL21 and CCL19 were almost exclusively expressed in MAdCAM-1+ cells, suggesting involvement of this stromal cell population in organization of developing T cell areas in the spleen. These data indicate that MAdCAM-1+ stromal cells in fetal spleen share phenotypic and functional characteristics with lymph node LTo cells and that these cells could be involved in formation and organization of splenic T cell areas.
Figure 3. LTo and ILC in human fetal spleen.
(a) Comparison of transcript levels for LTBR, CXCL13, CCL21 and CCL19 in CD45−CD31−MAdCAM-1− and MAdCAM-1+ stromal cells as well as total CD45+ cells from fetal human spleens. (n>3; age 14-22 weeks) (b) Localization of Rorγt+CD3− ILC3 in fetal spleens (arrowheads indicate Rorγt+ cells, (magnification 100x/250x) (n=3; age 14-22 weeks) (c) Phenotype of fetal splenic Lineage−CD117+CD127+Rorγt+ ILC3 compared to fetal splenic CD56+CD3− NK cells (n=3; age 14-22 weeks).
We have previously reported the presence of a small but distinct population of Rorγt+ ILC3 in the fetal spleen (33), but their phenotype and in-situ location are undetermined. Since in lymph nodes we observed a striking co-localization between stromal LTo cells and ILC, we analyzed fetal spleen sections for Rorγt+CD3− cells in spatial relation to the MAdCAM-1+ stromal cells (Figure 3B) In situ, Rorγt+ ILC3 appeared evenly distributed throughout the red and white pulp of the developing spleen. Within the white pulp, ILC3 often co-localized with MAdCAM-1+ stromal cells, although this co-localization was far less exclusive compared to the developing lymph nodes. Detailed analysis of the ILC3 phenotype revealed that fetal splenic IL7Rα+CD117+ ILC3 were phenotypically very similar to ILC3 from fetal and adult lymph nodes (15): expression of NKp44 and NKp46 was absent, yet approximately half of the cells expressed NKp30 (Figure 3C; fetal splenic NK cells as comparison). Collectively these data indicate that ILC3 are present in human developing spleens, resemble lymph node resident ILC3, but are not exclusively localized to regions containing stromal organizers.
MRC are a distinct stromal subset in adult human lymph nodes
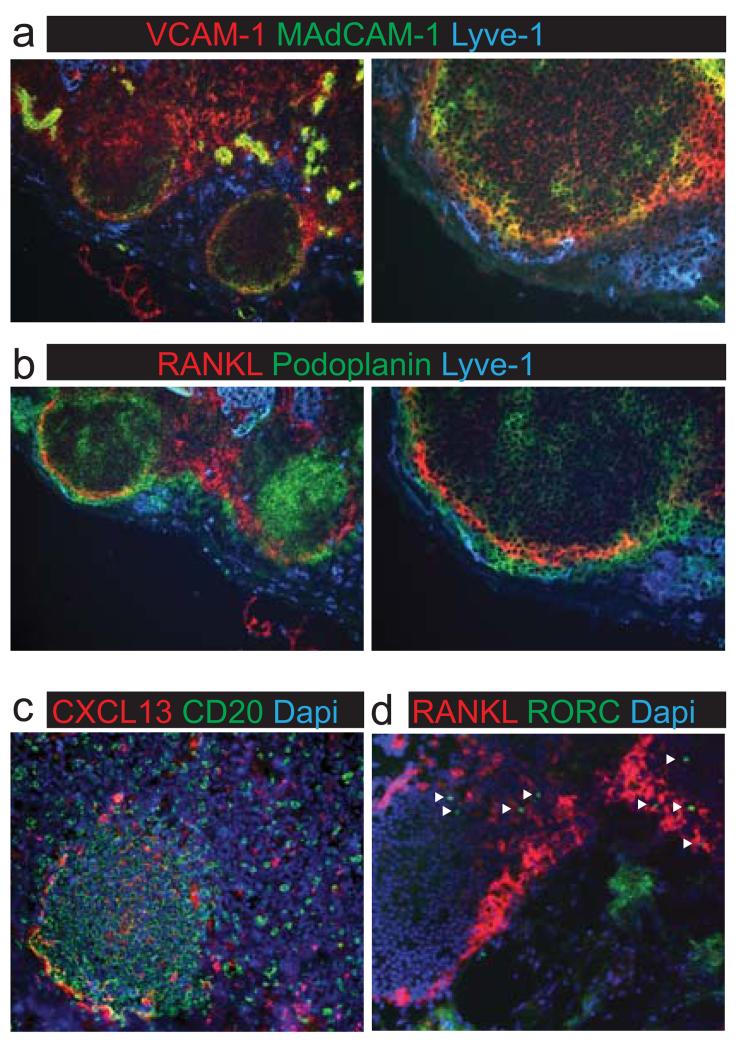
The identification of human fetal LTo cells prompted us to determine whether MRC, the phenotypic equivalents to fetal LTo in adult mouse lymph nodes, were also conserved in humans. In resting adult hepatic lymph nodes harvested during multi-organ donation procedures, B cell follicles were evident by Podoplanin staining on FDCs. In the inter-follicular area, HEVs were marked by MAdCAM-1 and VCAM-1 (Figure 4A). MRC-like cells expressing high levels of VCAM-1 and MAdCAM-1 as well as RANKL and Podoplanin were found directly underlying the Lyve-1+ subcapsular sinus, on the apical side of B cell follicles and prominently in inter-follicular areas (Figure 4A-B). As mouse MRC can secrete CXCL13, immunohistochemistry was performed to detect CXCL13 protein on human MRC and these experiments showed that adult lymph node-derived RANKL+ stromal cells expressed CXCL13 protein (Figure 4C). Finally, we assessed whether the fetal co-localization of RANKL+ stromal cells and Rorγt+ ILC3 was maintained into adulthood. Figure 4D shows that Rorγt+ ILC3 reside mainly in the inter-follicular areas of adult human lymph nodes (38), in close proximity to RANKL+ MRC. These data establish that the phenotype and localization of human MRC is phenotypically similar to human fetal LTo cells and corresponds to that of murine MRC. Moreover, these findings establish the continuous presence of ILC – stromal cell co-localization in specialized niches of both fetal and adult human lymph nodes.
Figure 4. Stromal MRC in adult human lymph nodes.
Human non-inflamed adult lymph nodes were analyzed for presence of MRC. (a) Stromal cells expressing VCAM-1 and MAdCAM-1 in adult human lymph nodes. (b) Stromal cells expressing RANKL in adult human lymph nodes (magnification 100x/250x). (c) CXCL13 expression by stromal cells at the follicle boundary (magnification 100x/250x). (d) Roryt+ cells in the inter-follicular areas of adult human lymph nodes co-localize with RANKL+ stromal cells (arrowheads indicate Rorγt+ cells, magnification 100x) (n>4).
IL7 marks a subset of murine fetal LTo cells
The conserved presence in human lymph nodes of fetal LTo cells and adult MRC, and the co-localization of both these stromal subsets with Rorγt+ ILC3 raised the question whether there might be a precursor – progeny relationship between LTo and MRC. If so, this would imply the presence of a life-long stromal niche for ILC3 in lymph nodes.
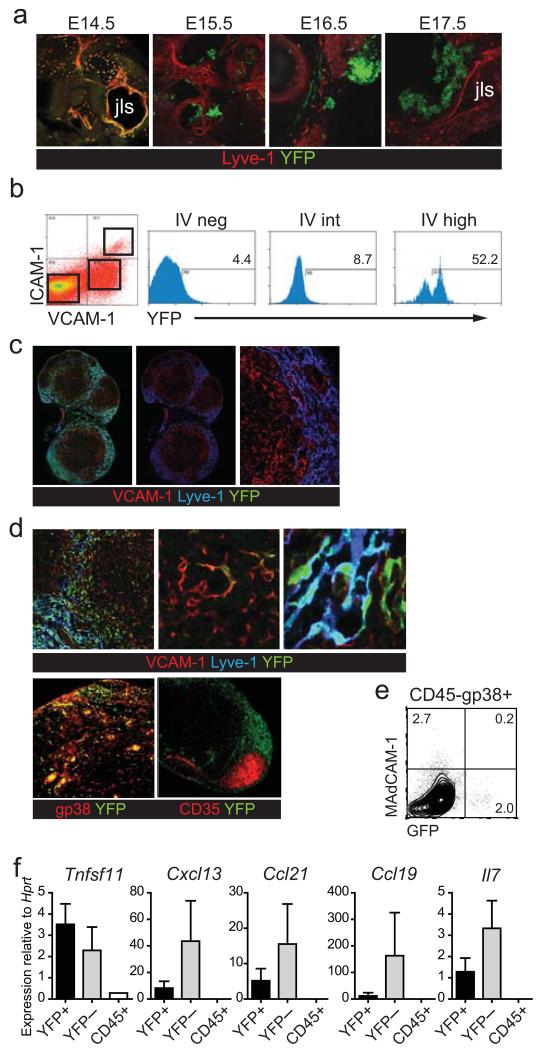
To gain insight into the precursor-progeny relationship between fetal organizers and MRC we set out to identify reporter mice marking stromal organizer cells in mouse fetal lymph nodes. IL-7 is expressed by LTo cells in fetal Peyer’s patches and lymph node anlagen and functions by enhancing LTα1β2 expression on LTi cells (39). To determine if IL-7 could be used to genetically mark fetal LTo cells we analyzed the fetal lymph node anlagen of Il7-Cre BAC transgenic Rosa26eYFP F1 mice where Cre has replaced the first exon of the Il7 gene and eYFP is expressed under the control of the inducible ubiquitous Rosa26 promoter (31). Expression of Il7 indelibly marks cells that have expressed this cytokine at sufficient levels to allow Cre-mediated recombination of the Rosa locus (40). Il7-cre+ Rosa26eYFP+ embryos were sectioned by vibratome into 200-400 μm sections and the cervical region was imaged. As seen in figure 4A, YFP+ cells were found scattered around the Lyve-1+ jugular lymph sac (jls) as early as E14.5. These YFP+ cells gradually condensed in mesenchymal clusters at E15.5 and E16.5 (Figure 5A) (41). At E17.5, an organized mass of YFP+ cells can be seen along the boundary of the jugular lymph sac (Figure 5A), which matures into a lymph node during progressing gestation. These data show that in Il7-cre+ Rosa26eYFP+ mice stromal cells in the developing lymph nodes are marked by YFP. To determine the nature of the stromal cells expressing YFP, MLN were isolated from Il7-cre+ Rosa26eYFP+ mice at E16.5 and analyzed by flow cytometry for YFP expression in CD45−CD31− stromal cells (Figure 5B). YFP was virtually undetectable in stromal cells lacking expression of ICAM-1 and VCAM-1 or in LTo cells expressing these adhesion molecules at intermediate levels. In contrast, approximately half of the ICAM-1/VCAM-1(I/V) high stromal LTo cells were marked by YFP, indicating that during fetal lymph node development a subset of stromal LTo cells transcribes sufficiently high levels of Il7, and therefore Cre, to recombine the Rosa locus, allowing for lineage tracing of this population.
Figure 5. Lineage tracing of fetal LTo cells.
(a) Condensation of YFP+ mesenchyme in the jugular lymph sac (jls) region of embryonic IL7-cre+ Rosa26eYFP+ mice at the indicated embryonic age in days (n=3). (b) Flow cytometric analysis of CD45−CD31− stromal cells from E16.5 MLN from IL7-cre+ Rosa26eYFP+ mice (n=2). (c) No co-expression of VCAM-1 and Lyve-1 in lymph nodes from adult IL7-cre+ Rosa26eYFP+ mice. (d) Lymph nodes from adult IL7-cre+ Rosa26eYFP+ mice show co-expression of YFP and Lyve-1 and of YFP and VCAM-1 or gp38. CD35+ FDC do not express YFP (n=5). (e) representative flow cytometry plot of CD45−CD31−gp38+ lymph nodes from adult IL7-cre+ Rosa26eYFP+ mice labelled with MAdCAM-1 (f) Transcript analysis of sorted CD45−CD31−gp38+YFP+ and YFP− stromal cells and total CD45+ cells from adult lymph nodes of IL7-cre+ Rosa26eYFP+ mice (n=2-4).
Fetal YFP+ LTo cells give rise to MRC
IL7-driven expression of Cre in I/Vhigh fetal stromal organizer cells in the Il7-Cre+ Rosa26eYFP+ mice allowed us to perform lineage tracing of IL7 expressing stromal organizers to provide evidence for a possible precursor-progeny relationship between this subset of fetal stromal organizers and MRC in adult lymph nodes. Detailed characterization of lymph nodes isolated from adult Il7-cre+ Rosa26eYFP+ mice by histology revealed that YFP marked two distinct non-hematopoietic cell types (Figure 5C). First, Lyve-1+ lymphatic endothelial cells were brightly YFP positive (Figure 5C and D), in line with previous work describing a role for IL-7 production by lymphatic endothelial cells during lymph node restructuring (42). Second, a subset of VCAM-1+ stromal cells beneath the sub-capsular lymphatic sinus expressed YFP (Figure 5C and D). VCAM-1 is not expressed by the Lyve-1+ endothelium allowing for the discrimination of stromal and endothelial cells based on these two markers (Figure 5C). This characteristic localization suggested that the cells marked in the IL-7 reporter are indeed MRC and this was confirmed by the co-expression Podoplanin (gp38) (Figure 5D). CD35-expressing FDC were YFP negative (Figure 5D). Strikingly, YFP+ cells did not express MadCAM-1 (Figure 5E), underscoring the fact that YFP marks a subset of MRC in adult LNs. which is in line with a recent study showing that in steady state lymph nodes FDC do not derive from MRC (29). As TRC are known to transcribe Il7, the absence of YFP in these cells was unexpected and is likely due to absence of regulatory elements in the BAC. While this renders the Il7-YFP mice less suitable for lineage tracing TRC, they clearly show that the fetal YFP+ stromal organizers did not give rise to adult TRC. To identify the YFP+ cells on a functional level we purified YFP-expressing CD45−CD31− stromal cells and CD45−CD31− stromal TRC/FDC from adult lymph nodes of IL7-cre+ Rosa26eYFP mice for transcript analysis (Figure 5F). The YFP+ cells contained transcripts for Tnfsf11 (encoding Rankl), Cxcl13, Ccl21 and Ccl19, positively identifying them as MRCs. (Figure 5D). Total LN CD45+ cells are shown as negative control. Collectively these data indicate that part of the I/Vhigh stromal LTo cells found early in lymph node development can mature into a subset of MRCs in the adult lymph nodes.
Discussion
In this study we provide evidence that adult MRC derive from a subset of fetal ICAM-1highVCAM-1high LTo cells, that both fetal LTo and adult MRC are conserved from mouse to human and that the LTo-MRC lineage co-localizes with lymph node-resident Rorγt+ ILC3 throughout life.
Lymph node development and function is critically dependent on functionally distinct stromal cell networks (1, 43). In contrast to the well-defined functions of TRC and FDC, the function of MRC is largely unknown. MRC were identified based on their resemblance to stromal organizer cells that interact with LTi cells in fetal lymph node anlagen, and it was proposed that these cells might have organizing functions in adult lymph nodes (26, 30). We have previously described the presence of fetal LTi cells in human developing lymph nodes and the presence of ILC3 in adult human lymph nodes and spleen (15, 33). Here we present data showing first that fetal human lymph nodes also contain stromal LTo cells that share phenotypic and functional characteristics with their mouse counterparts and second that adult human lymph nodes contain distinct stromal MRC. In both fetal and adult lymph nodes Rorγt+ ILC3 were found in close association with LTo / MRC. In developing human spleens we also identified a stromal cell population with possible organizing potential. MAdCAM-1+ stromal cells appear in the perivascular lymphocyte clusters of the human spleen during second trimester and show specific transcription of the T zone chemokines CCL19 and CCL21. In line with mouse data, these splenic organizers do not exclusively co-localize with Rorγt+ ILC, suggesting that the ILC3-independent organization of the murine spleen might be conserved in humans. Finally we sought to gain insight into a possible precursor – progeny relationship between fetal LTo and adult MRC. If so, this would establish a mesenchymal cell lineage providing a life-long stromal niche for ILC in lymph nodes. Using Il7 lineage trace mice (31) we were able to perform lineage tracing of a population of fetal stromal organizer cells. Our analysis revealed that IL7-YFP is selectively induced in a subset of fetal LTo cells that co-express high levels of ICAM-1 and VCAM-1. As both these adhesion molecules are downstream of the LTβR it is tempting to speculate that commitment to the MRC lineage is restricted to stromal cells that were activated by LTα1β2-expressing ILC3. In adult lymph nodes of IL7 lineage trace mice, a subset of MRC are YFP positive while both FDC and TRC are not marked by the IL7 lineage tracer. This suggests that at least part of the MRC found in adult LNs are direct descendants of a population of fetal stromal organizers. On the other hand, steady state TRC and FDC are derived from IL7-negative mesenchymal precursors. Such precursor cells be either the YFP− ICAM-1highVCAM-1high cells that we observed in the developing lymph nodes, or additional distinct YFP- stromal populations. This again is in line with recent data describing the development of MRC into FDC after immune stimulation, but not at steady state (29).
The evidence for a precursor-progeny relationship between stromal organizers and MRC provided here suggests that these cells could also be functionally related. In line with the function of fetal organizers one can envision a role for MRC in either maintaining lymphoid tissue organization or in restoring lymphoid tissue architecture after insult. Additional strategies aimed at the targeted ablation of MRC are needed to address these issues.
The findings presented in this study raise the possibility of the existence of a specialized innate niche in otherwise primarily adaptive secondary lymphoid tissues. The physiological function of ILC3 in lymphoid tissues is still unclear, and an integrated view of the cross communication between specialized stromal cells and ILC3 is likely needed to unravel such role. With the identification of a stromal lineage that co-localizes with ILC3 in lymphoid tissues an essential first step towards this integrated analysis has now been made.
Acknowledgments
We are grateful to the staff of the CASA clinics in Leiden and Rotterdam for collecting fetal tissues, to Dr. Jaap Kwekkeboom (Dept. of Gastroenterology, ErasmusMC) for coordinating the acquisition of adult lymph nodes, to Ellen Richie for the IL-7cre mice, to Dimitris Kioussis for mice and reagents and to Paul Kaye for critical feedback.
Abbreviations
- MRCs
Marginal Reticular Cells
- FRCs
T cell zone Fibroblastic Reticular Cells
- LTi
Lymphoid Tissue inducers
- LTo
Lymphoid Tissue Organizers
- FDC
Follicular Dendritic Cells
- ILC
Innate Lymphoid Cells
Footnotes
Financial support
This work was supported by an ErasmusMC grant to TC and by ZenithII grant #93512004 awarded by the Netherlands genomics Initiative to TC, Medical Research Council grant G0601156 to MCC, by Human Frontier Science Program grant #RGP0006/2009-C to TC and MCC and by the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013 under REA grant agreement no. 289720.
References
- 1.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 2.Crivellato E, Vacca A, Ribatti D. Setting the stage: an anatomist’s view of the immune system. Trends in Immunology. 2004;25:210–217. doi: 10.1016/j.it.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 4.Bajénoff M, Egen JG, Koo LY, Laugier Jean P., Brau F, Glaichenhaus N, Germain RN. Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspal FM, McConnell FM, Kim MY, Gray D, Kosco-Vilbois MH, Raykundalia CR, Botto M, Lane PJ. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. European journal of immunology. 2006;36:1665–1673. doi: 10.1002/eji.200535339. [DOI] [PubMed] [Google Scholar]
- 6.Hase H, Kanno Y, Kojima M, Hasegawa K, Sakurai D, Kojima H, Tsuchiya N, Tokunaga K, Masawa N, Azuma M, Okumura K, Kobata T. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood. 2004;103:2257–2265. doi: 10.1182/blood-2003-08-2694. [DOI] [PubMed] [Google Scholar]
- 7.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, Choi YS. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–788. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 9.Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nature immunology. 2014;15:973–981. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, Cyster JG. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. The Journal of experimental medicine. 2011;208:2497–2510. doi: 10.1084/jem.20111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick J, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 12.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nature immunology. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 15.Hoorweg K, Peters CP, Cornelissen F, Aparicio-Domingo P, Papazian N, Kazemier G, Mjosberg JM, Spits H, Cupedo T. Functional Differences between Human NKp44(−) and NKp44(+) RORC(+) Innate Lymphoid Cells. Front Immunol. 2012;3:72. doi: 10.3389/fimmu.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escaliere B, Renauld JC, Dussurget O, Cossart P, Lecuit M, Vivier E, Tomasello E. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat- lymphoid cells. Embo J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E. Influence of the transcription factor ROR[gamma]t on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 18.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. ROR[gamma]t and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 20.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3-LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 21.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 22.White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, Jenkinson E, Anderson G, Caamano JH. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. 2007;110:1950–1959. doi: 10.1182/blood-2007-01-070003. [DOI] [PubMed] [Google Scholar]
- 23.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. PNAS. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 25.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S-I, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 26.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-Like Reticular Stromal Cell Layer Common to Adult Secondary Lymphoid Organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 27.Park SM, Angel CE, McIntosh JD, Brooks AES, Middleditch M, Chen C-JJ, Ruggiero K, Cebon J, Rod Dunbar P. Sphingosine-1-phosphate lyase is expressed by CD68+ cells on the parenchymal side of marginal reticular cells in human lymph nodes. Eur. J. Immunol. 2014;44:2425–2436. doi: 10.1002/eji.201344158. [DOI] [PubMed] [Google Scholar]
- 28.Suto H, Katakai T, Sugai M, Kinashi T, Shimizu A. CXCL13 production by an established lymph node stromal cell line via lymphotoxin-beta receptor engagement involves the cooperation of multiple signaling pathways. Int Immunol. 2009;21:467–476. doi: 10.1093/intimm/dxp014. [DOI] [PubMed] [Google Scholar]
- 29.Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, Klauschen F, Bajenoff M. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. 2014;211:1109–1122. doi: 10.1084/jem.20132409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol. 2012;3:200. doi: 10.3389/fimmu.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repass JF, Laurent MN, Carter C, Reizis B, Bedford MT, Cardenas K, Narang P, Coles M, Richie ER. IL7-hCD25 and IL7-Cre BAC transgenic mouse lines: new tools for analysis of IL-7 expressing cells. Genesis. 2009;47:281–287. doi: 10.1002/dvg.20497. [DOI] [PubMed] [Google Scholar]
- 32.Pahal GS, Jauniaux E, Kinnon C, Thrasher AJ, Rodeck CH. Normal development of human fetal hematopoiesis between eight and seventeen weeks’ gestation. Am J Obstet Gynecol. 2000;183:1029–1034. doi: 10.1067/mob.2000.106976. [DOI] [PubMed] [Google Scholar]
- 33.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 34.Steiniger B, Ulfig N, Risse M, Barth PJ. Fetal and early post-natal development of the human spleen: from primordial arterial B cell lobules to a non-segmented organ. Histochem Cell Biol. 2007;128:205–215. doi: 10.1007/s00418-007-0296-4. [DOI] [PubMed] [Google Scholar]
- 35.Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987;60:603–609. [PMC free article] [PubMed] [Google Scholar]
- 36.Vellguth S, von Gaudecker B, Muller-Hermelink HK. The development of the human spleen. Ultrastructural studies in fetuses from the 14th to 24th week of gestation. Cell Tissue Res. 1985;242:579–592. doi: 10.1007/BF00225424. [DOI] [PubMed] [Google Scholar]
- 37.Satoh T, Sakurai E, Tada H, Masuda T. Ontogeny of reticular framework of white pulp and marginal zone in human spleen: immunohistochemical studies of fetal spleens from the 17th to 40th week of gestation. Cell Tissue Res. 2009;336:287–297. doi: 10.1007/s00441-009-0757-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117(+) CD3(−) CD56(−) OX40L(high) cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur J Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol. 2014;167:441–452. doi: 10.1111/bjh.13113. [DOI] [PubMed] [Google Scholar]
- 41.Herbers AH, de Haan AF, van der Velden WJ, Donnelly JP, Blijlevens NM. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2014;16:279–285. doi: 10.1111/tid.12195. [DOI] [PubMed] [Google Scholar]
- 42.Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, Cupedo T, Coles M, Ludewig B. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012 doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]