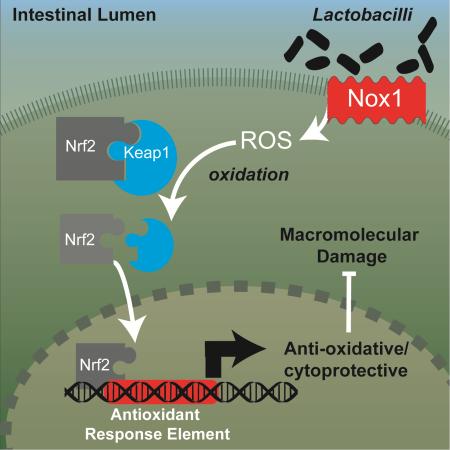
Summary
An optimal gut microbiota influences many beneficial processes in the metazoan host. However, the molecular mechanisms that mediate and function in symbiont-induced host responses have not yet been fully characterized. Here, we report that cellular ROS enzymatically generated in response to contact with lactobacilli in both mice and Drosophila has salutary effects against exogenous insults to the intestinal epithelium via the activation of Nrf2 responsive cytoprotective genes. These data show that the xenobiotic inducible Nrf2 pathway participates as a signaling conduit between the prokaryotic symbiont and the eukaryotic host. Indeed, our data imply that the capacity of lactobacilli to induce redox signaling in epithelial cells is a highly conserved hormetic adaptation to impel cellular conditioning to exogenous biotic stimuli. These data also highlight the role the microbiota plays in eukaryotic cytoprotective pathways, and may have significant implications in the characterization of a eubiotic microbiota.
Keywords: Nrf2, lactobacillus, Reactive oxygen species, microbiota, cytoprotection, hormesis
Graphical Abstract
Introduction
The prokaryotic microbiota of virtually all metazoans participate in many symbiotic functions, including regulation of cellular growth and survival (Neish and Jones, 2014). However, the host intrinsic mechanisms that mediate non-immune symbiont-induced effects are largely unknown. A well-studied and evolutionarily highly conserved system for transducing exogenous stimuli into eukaryotic transcriptional responses is the Nrf2 pathway. Nrf2 activation upregulates a regulon of genes including those involved in xenobiotic and reactive oxygen species (ROS) detoxification, as well as pro-restitutive function. This pathway has attracted considerable attention because small molecule inducers of Nrf2 have cytoprotective effects against oxidant and electrophilic environmental stressors (Kobayashi et al., 2009). Our research group has recently reported that lactobacilli, which are constituents of the microbiota in many metazoans and well-known probiotic agents, are highly adapted to induce the generation of physiological levels of ROS in cells of the intestinal epithelium and consequent induction of cell proliferation (Jones et al., 2013).
Gut bacteria stimulate reactive oxygen species (ROS) production in epithelial cells by an enzymatic mechanism analogous to the pathogen-induced respiratory burst in phagocytes (Alam et al., 2013; Jones et al., 2013). Cellular ROS are generated by the catalytic action of NADPH oxidases. The first and exemplary member of this family of enzymes, Nox2, was characterized in phagocytes, and is well known to function in neutrophil microbicidal ROS generation in response to pathogens. Thereafter, isozymes of Nox2 were identified in nonphagocytic tissues, including Nox1, which is highly expressed in colonic enterocytes of both flies and mice (Lambeth and Neish, 2014). Interestingly, enzymatically generated ROS in the epithelia is stimulated not only by potential pathogens, but also by symbiotic bacteria, especially members of the lactobacilli taxon, and promotes cell proliferation and migration (Wentworth et al., 2011; Wentworth et al., 2010), accelerates restitution post injury (Swanson et al., 2011), and modifies epithelial NF-κB signaling (Kumar et al., 2007).
The Nrf2 system is a well-characterized ubiquitin dependent signaling pathway that responds to oxidative stress and electrophilic xenobiotics, and is recognized as a major regulator of cytoprotective responses against such environmental cellular stresses (Jones et al., 2012). Components of the Nrf2 signaling are functionally conserved even in lower metazoans such as C. elegans, and are fully developed in Drosophila and mammals. Disruption of Nrf2 signaling results in increased sensitivity to oxidative-stress in flies (Sykiotis and Bohmann, 2008), and increased sensitivity to radiological insult in mice (McDonald et al., 2010). The Nrf2 pathway has been implicated in proliferative control of gut stem cells (Hochmuth et al., 2011). At low levels of ROS, Nrf2 is bound to its cytoplasmic inhibitor Keap1 which suppresses the activity of Nrf2 by targeting it for constitutive polyubiquitination by a Cullin3-based E3 ligase complex and consequent proteasomal degradation. Keap1 contains redox-sensitive cysteine residues that under oxidative stresses conditions react and alter the functional conformation of Keap1, thereby abolishing the inactivation of Nrf2. Thereafter, Nrf2 translocates to the nucleus, binds to Antioxidant Response Element (ARE) sequences and initiates the transcription of a battery of antioxidant enzymes and detoxifying proteins, known as the “Phase 2 detoxification response”. We hypothesized that symbiont-induced ROS may activate epithelial Nrf2 pathway signaling, and thereby mechanistically mediate the beneficial influences of a eubiotic microbiota.
Results
Lactobacillus plantarum induces cytoprotection against oxidative insult and potentiates upregulation of CncC pathway responsive genes in Drosophila
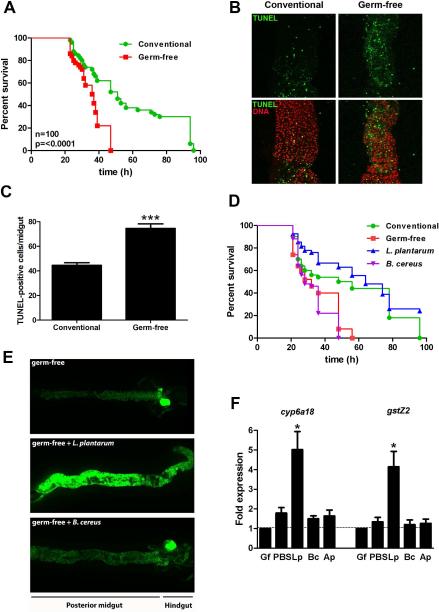
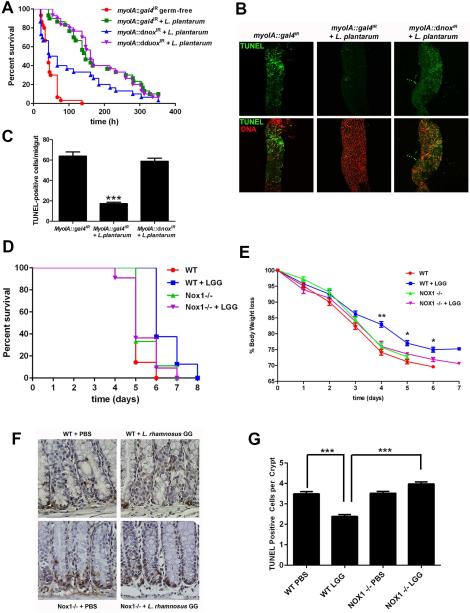
We first implicated a role for the indigenous microbiota in cytoprotection against oxidative injury in the intestine by showing that adult germ-free Drosophila, compared to conventionally raised controls, are significantly more sensitive to Paraquat-induced toxicity (Fig. 1A), and exhibit elevated numbers of apoptotic cells in the highly proliferative Posterior 3 (P3) (Marianes and Spradling, 2013) section of the midgut (Fig. 1B and 1C). To identify if, or which, specific bacterial genera contribute to cytoprotection, we studied germ-free Drosophila gnotobiotically colonized with monocultures of bacterial species that we previously isolated from the luminal content of adult Drosophila (Jones et al., 2013), and examined the extent to which individual members of the microbiota influenced cytoprotection. Midguts were dissected and comparable levels of bacterial ingestion confirmed by plate count. Of those tested, only flies gnotobiotically colonized with pure Lactobacillus plantarum cultures significantly protected Drosophila from oxidative injury (Fig. 1D) and (Fig. S1). To demonstrate the beneficial hormetic effect of pre-exposure of germ-free adult Drosophila to lower levels of ROS, we show that lower doses of paraquat, with 5mM for 24 hours being optimal, can protect against ensuing severe insults triggered by 25mM paraquat feeding (Fig S2). Because we also previous showed that only L plantarum possessed the capacity to induce ROS generation in Drosophila enterocytes following colonization (Jones et al., 2013), these data suggested the possibility of redox signaling in mediation of commensal mediated cytoprotection. To examine this notion we used a GFP reporter fly bearing an antioxidant response element (ARE) dependent promoter (gstD1-GFP) that responds to Nrf2 (known as CncC in Drosophila) (Sykiotis and Bohmann, 2008). Third instar larva were utilized as their feeding behavior allows rapid colonization and synchronous induction of signaling events. We observed that colonization of either germ-free larvae or adult Drosophila harboring a CncC-responsive reporter was activated following L. plantarum, but not B. cereus ingestion (Fig. 1E and Fig S2). To assess gene expression associated with L. plantarum–induced cytoprotection, we employed microarray analysis on dissected larval midgut tissue following L. plantarum ingestion by germ-free Drosophila. Gene ontology analysis identified highly represented transcripts in L. plantarum fed larva (compared to germ-free) (Table S1), many of which were found to be located downstream of a promoter region harboring a putative antioxidant response element (ARE) homologous to the 10 bp core element that lies at the center of the 20 bp sequence ARE consensus sequence (Nioi et al., 2003). Many of these genes have a known cytoprotective function, including glutathione S-transferase zeta 1 GstZ1, GstZ2 and GstD10, as well as a number of Cytochrome P450, E-class, group I enzymes, including Cyp4p1, Cyp4ac1 and Cyp4ac3. Consistently, upregulation of Cytochrome P450 family of genes was detected in midgut sections P2, P3 and P4 in other recent investigations (Marianes and Spradling, 2013). Real time quantitative PCR (RT-qPCR) analysis corroborated the detection of the increased representation of transcripts for the aforementioned proteins in midgut tissues of larva fed L. plantarum, but not in the midgut regions of larvae colonized with other, non-ROS inducing bacteria (Fig. 1F). These data indicate that the transcriptional response is specific to L. plantarum ingestion, and based on transcriptional profile analysis, that the mechanism of L. plantarum-induced cytoprotection may by as a result of the activation of genes under the regulatory control of an ARE promoter sequence.
Fig. 1. Lactobacillus plantarum induces cytoprotection against oxidative insult and potentiates upregulation of CncC pathway responsive genes in Drosophila.
(A) Relative survival of 5-day-old germ-free and conventionally raised adult Drosophila in response to Paraquat challenge. Log-Rank test P=<0.001, n=100. (B) TUNEL analysis of posterior midgut tissues dissected from Drosophila described in (A) at 24 hours following Paraquat challenge. Note the high concentration of TUNEL positive cells in the Posterior 3 (P3) section of the midgut. (C) Numeration of TUNEL-positive cells per midgut examined in 1B. *** = P<0.001, n=10. (D) Relative survival of germ-free adult Drosophila gnotobiotically monocolonized with L. plantarum or B. cereus in response to Paraquat challenge. Note increased survival of flies monocolonized with L. plantarum (Log-Rank test for g-f vs. g-f + L. plantarum, P=<0.0001, n=100). Note also significantly increased survival of germ-free fed L. plantarum compared to B. cereus fed flies. (Log-Rank for g-f + L. plantarum vs. g-f + B. cereus, P=<0.0001, n=100). (E) Detection of ARE dependent GFP expression in the midgut of germ-free PgstD1-gfp third instar larvae following ingestion of L. plantarum. (F) Real time quantitative PCR (RT-qPCR) analysis for the detection of cyp6a18, and gstZ2 transcript enrichment in the midgut of germ-free third instar larvae at 4 hours following ingestion of either L. plantarum (Lp), B. cereus (Bc), A. piechaudii (Ap). *= P<0.05, n=30.
CncC pathway signaling mediates Lactobacillus plantarum induced cytoprotection against oxidative insult in Drosophila
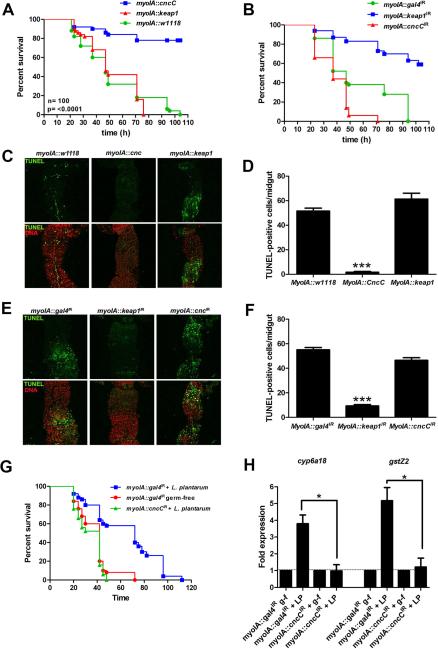
To mechanistically substantiate that CncC signaling is mediating L. plantarum-induced cytoprotection, we employed the bipartite –GAL4/UAS- system to modulate CncC pathway activity by constitutively driving the expression of Keap1 or CncC, or by using RNAi constructs against CncC as previously described (Sykiotis and Bohmann, 2008), under the enterocyte specific myoIA-GAL4 driver fly. Depletion of transcript levels in the midgut of these genotypes were confirmed by qPCR (Fig S3). Constitutive enterocyte specific expression of CncC (Fig. 2A), or depletion of Keap1 levels (Fig. 2B), both of which result in Nrf2 pathway activation, significantly enhanced survival of adult Drosophila in response to Paraquat-induced toxicity, correlating with the extent of apoptotic cells observed in segments P3 and P4 of the midgut (Marianes and Spradling, 2013) (Fig. 2C and 2D). Conversely, constitutive activation of Keap1 (Fig. 2A), or depletion of CncC transcripts (UAS-cncC-RNAi) (Fig. 2B) (both of which inhibit pathway activation) in the midgut rendered flies significantly more susceptible to Paraquat-induced toxicity, again with correlative apoptotic damage to the midgut (Fig. 2E and 2F). The cytoprotective function of CncC in enterocytes was also detected in germ-free adult Drosophila (Fig. S3). Importantly, depletion of CncC, while having no effect on bacterial numbers (see materials and methods), essentially abolished L. plantarum-induced cytoprotection against Paraquat to levels observed for germ-free adult Drosophila (Fig. 2G). Indeed, enterocyte-specific depletion of CncC-levels significantly reduced L. plantarum-induced activation of cyp6a18 and gstZ2 in the adult Drosophila midgut (Fig. 2H) together indicating that L. plantarum-induced protection against oxidative insult was dependent on CncC pathway signaling.
Fig. 2. CncC pathway signaling mediates Lactobacillus plantarum induced cytoprotection against oxidative insult in Drosophila.
(A) Relative survival in response to Paraquat challenge of conventionally raised adult Drosophila either constitutively expressing cncC (UAS-CncC) or constitutive expressing keap1 under the enterocyte specific myoIA-GAL4 driver. (Log-Rank test for myoIA-GAL4 w1118 vs. myoIA-GAL4 UAS-cncC, P=<0.0001, n=100). (B) Survival in response to Paraquat challenge of 5-day-old conventionally raised adult Drosophila where the levels of cncC (UAS-cncCIR) or Keap1 (UAS-keap1IR) are diminished. (Log-Rank test for myoIA-GAL4 w1118 vs. myoIA-GAL4 UAS-cncCIR, P=<0.0001, n=100). (C) TUNEL analysis of posterior midgut dissected from adult Drosophila listed in (A), and exposed to Paraquat challenge for 48 hours. (D) Numeration of TUNEL-positive cells per midgut examined in 2C. *** = P<0.001, n=10. (E) TUNEL analysis of posterior midgut tissues dissected from adult Drosophila described in (C), following 48 hours exposure to Paraquat challenge. (F) Numeration of TUNEL-positive cells per midgut examined in 2E. *** = P<0.001, n=10. (G) Survival in response to Paraquat challenge of germ-free adult Drosophila monocolonized with L. plantarum where the levels of CncC (UAS-cncCIR) are diminished under the enterocyte specific myoIA-GAL4 driver, (Log-Rank for myoIA-GAL4;UAS-gal4IR + L. plantarum vs. myoIA-GAL4;UAS-cncCIR + L. plantarum, P=<0.0001, n=100). (H) Real time quantitative PCR (RT-qPCR) analysis for the detection of cyp6a18 and gstZ2, transcript enrichment in the midgut of adult Drosophila treated as described in (G). *= P<0.05, n=30.
Colonization of the murine intestine with Lactobacillus rhamnosus GG induces Nrf2-dependent cytoprotection
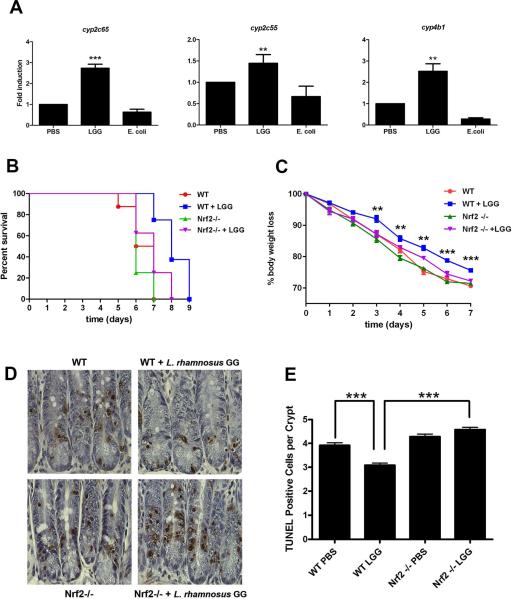
Nrf2 signaling is highly conserved across metazoans. To determine whether lactobacilli-induced and Nrf2 pathway mediated cytoprotection occurs in mammals, we recapitulated our investigations in the murine model. Transcriptional profiling and gene ontology analysis identified highly represented transcripts in the colonic tissues of germ-free mice orally gavaged with L. rhamnosus GG, a mammalian adapted lactobacillus, when compared to transcripts in the colonic tissues of germ-free mice orally gavaged with equivalent inoculum of E. coli, which in our previous studies could not induce detectable generation of ROS in intestinal epithelial cells (Jones et al., 2013). Interestingly, L. rhamnosus GG induced transcripts cluster distinct from transcripts induced by E. coli or vehicle (Fig. S4). Indeed, comparison of L. rhamnosus GG-fed to E. coli-fed tissues identified a set of enriched transcripts from the Cytochrome P450 family of proteins which have conserved upstream ARE promoter sequences, consistent with our observations in the analogous Drosophila experiments (Table S2). Specifically, increased transcript representation of Cyp2c65, Cyp2c55 and Cyp4b1 was detected, and confirmed in these samples by qPCR (Fig. 3A). Importantly, qPCR analysis also confirmed that transcripts for these three Cytochrome P450 genes were not enriched in colonic tissues of E. coli fed mice, further supporting our observation that of the bacteria tested, the upregulation of these genes are specifically induced in response to lactobacilli (Fig. 3A). Previous reports have described the cytoprotective effects of L. rhamnosus GG in mice against radiological insult (Ciorba et al., 2012). We corroborate these data by confirming that mice fed L. rhamnosus GG have significantly enhanced survival, are protected against weight loss, and have fewer apoptotic cells at within colonic crypts following irradiation (Fig. 3B to 3E). Strikingly, each of the measured cytoprotective influences of L. rhamnosus GG against irradiation were completely abolished in Nrf2−/− mice (Fig. 3B to 3E). Note also that we did not detect any changes in the response to irradiation insult between untreated wild type and Nrf2−/− mice. Together, these data show that Nrf2 is required for the optimal cytoprotective influences of L. rhamnosus GG in the intestine in conventionally raised mice.
Fig. 3. Colonization of the murine intestine with Lactobacillus rhamnosus GG induces Nrf2-dependent cytoprotection.
(A) Quantitative (q)PCR analysis to measure the abundance of mRNA transcripts expressed from cyp2c65, cyp2c55 and cyp4b1 in colonic tissues of wild type B6 germ-free mice fed either 2×109 cfu total L. rhamnosus GG, or E.coli by oral gavage for 4 hours. (B) Survival of 7-week-old Nrf2−/− or and wild type littermates fed L. rhamnosus GG (LGG) following 12 Gy irradiation insult. Note significantly increased survival of irradiated WT mice fed LGG in response to irradiation (Log-Rank test for WT vs. WT+LGG, P=0.0013, n=8). Also note significantly deceased survival rate of Nrf2−/− mice fed LGG compared to WT mice fed LGG (Log-Rank test for WT+LGG vs. Nrf2−/− +LGG, P=0.0142, n=8), and no significant increase in survival of Nrf2−/− mice fed LGG compared to unfed WT (Log-Rank test for WT vs. Nrf2−/− +LGG, P=0.2150, n=8), (C) Percent body weight loss of mice described in (B). Statistical analysis represents comparison of WT+LGG vs. Nrf2−/− +LGG on each respective day. *=P<0.05, **=P<0.01, n=8. (D) Detection of TUNEL-positive cells within colonic tissues harvested from mice described in (A) and (B). (E) Quantification of TUNEL-positive cells in (c) ***=P<0.001, n=5.
Nox1 is required for optimal lactobacilli-induced cytoprotection
We previously showed that Nox1 in mice and the orthologous dNox in Drosophila are required for lactobacilli-induced ROS generation in intestinal enterocytes (Jones et al., 2013). To show that Nox1-generated ROS function in lactobacilli-induced cytoprotection, we employed the same bipartite –GAL4/UAS- system as above to expressed RNAi constructs against dNox under the enterocyte specific myoIA-GAL4 driver fly. Depletion of transcript levels in the midgut of these genotypes were confirmed in (Jones et al., 2013). Constitutive depletion of dnox transcripts (UAS-dnox-RNAi), but not dduox, while again having no effect on bacterial numbers (see materials and methods), significantly reduced L. plantarum-induced cytoprotection against Paraquat (Fig. 4A) with correlative apoptotic damage to the midgut (Fig. 4B and 4C). These data corroborate our previous observations in the fly that symbiotic lactobacilli-induced ROS generation is mediated via the enzymatic activity of dNox, and not by dDuox, which by contrast is reported to function in the Drosophila anti-microbial response to pathogens (Ha et al., 2005). To show that Nox1 is also required for L. rhamnosus GG –induced cytoprotection against irradiation in mice, we used the intestinal epithelial cell-specific Nox1-deficient knockout (B6.Nox1ΔIEC) (Leoni et al., 2013). Consistent with our previous data, L. rhamnosus GG – induced enhanced survival and reduced loss of body weight was significantly abrogated in the B6.Nox1ΔIEC compared to wild type littermates (Fig 4D to 4G). In summary, these data are compelling evidence showing that enterocyte-expressed ROS generation catalyzed by Nox1 is required for lactobacilli-potentiated cytoprotection.
Fig. 4. Nox1 is required for optimal lactobacilli-induced cytoprotection.
(A) Survival in response to Paraquat challenge of 5-day-old conventionally raised adult Drosophila where the levels of dnox (UAS-dnoxIR) are diminished, under the enterocyte specific myoIA-GAL4 driver. (Log-Rank test for myoIA-GAL4; UAS-gal4IR + L. plantarum vs. myoIA-GAL4;UAS-dnoxIR + L. plantarum, P=0.0185, n=100). (B) TUNEL analysis of posterior midgut tissues dissected fromadult Drosophila listed in (A), and exposed to Paraquat challenge for 48 hours. (C) Numeration of TUNEL-positive cells per midgut examined in 4B. *** = P<0.001, n=10. (D) Survival of 7-week-old intestinal epithelial cell-specific Nox1-deficient (B6.Nox1ΔIEC) and wild type littermates fed L. rhamnosus GG (LGG) following 12 Gy irradiation insult. Note significantly increased survival of irradiated WT mice fed LGG in response to irradiation (Log-Rank test for WT vs. WT+LGG, P=0.0012, n=8). Also note significantly deceased survival rate of B6.Nox1ΔIEC mice fed LGG compared to WT mice fed LGG (Log-Rank test for WT+LGG vs. B6.Nox1ΔIEC + LGG, P=0.0118, n=8). Finally, note no significantly increased survival rates of irradiated WT unfed mice and B6.Nox1ΔIEC mice fed LGG compared (Log-Rank test for WT vs. B6.Nox1ΔIEC + LGG, P=0.4465, n=8). (E) Percent body weight loss of mice described in (D). Statistical analysis represents comparison of WT+LGG vs. B6.Nox1ΔIEC+LGG on each day, *=P<0.05, **=P<0.01, n=8. (F) Detection of TUNEL-positive cells within colonic tissues harvested from mice described in (C) and (D). (G) Quantification of TUNEL-positive cells in (E) ***=P<0.001, n=5.
Discussion
In this study, we identified the Nrf2 pathway as a distinct system for the non-immune perception and response to specific members of the microbiota, namely lactobacilli. In addition, we show that this ROS-sensitive signal transduction system mediates cytoprotection, consistent with our previous observations of lactobacilli-induced enzymatic generation of ROS in the gut in mammals and Drosophila (Jones et al., 2013). Members of the diverse Lactobacillus taxon have long been exploited by humans in the production of fermented dairy products and are commonly employed as candidate probiotic agents. They are characteristic early colonists of the neonatal mammalian gut and are fructose fermenters present in decaying fruit that form the major energy source of Drosophila. Lactobacilli-specific symbiotic functions include cytoprotective in the mouse (Ciorba et al., 2012), and effects in Drosophila ranging from mate selection (Sharon et al., 2010), to metabolic regulation (Storelli et al., 2011). These effects likely involve the mucus binding adhesive properties of these bacteria (Ardita et al., 2014). Thus, members of the genus lactobacilli may have evolved symbiotic relationships where microbial induced generation of ROS functions as a transducer of bacterial signals into host gene regulatory events that potentiate multiple effects in disparate biological systems. We describe a molecular mechanism by which lactobacilli can elicit their beneficial influences on host gut tissues, wherein ROS generated by Nox following bacterial contact activates downstream cytoprotective signaling. These observations were particularly striking in germ-free Drosophila mono-associated with L. plantarum. Importantly, this influence of lactobacilli was also observed in conventionally raised mice indicating that animals do not necessarily have to be germ-free to benefit from lactobacilli-induced cytoprotection.
One intriguing observation is that flies depleted of dnox transcripts and monoassociated with L. plantarum have a similar sensitivity as germ-free flies over the first half of the experimental time assayed, and thereafter exhibit a degree of resistance to oxidative stress over the second half of the assay (Fig. 4A). This infers that lactobacilli-Nox-Nrf2 signaling may not be the only evolved mechanism that lactobacilli exploited to elicit cytoprotective influences on the host. Indeed, other pathways reported to be activated in response to symbiotic bacteria include the TOR pathway in Drosophila (Storelli et al., 2011). Consistent with our study Storelli et al. 2011 concluded that mono-ingestion of L. plantarum is sufficient to recapitulate the effects of the normal microbiota. In addition, lactobacilli were reported to protect the murine intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner (Ciorba et al., 2012). Going forward, it will be interesting to determine the extent to which both TOR signaling, and TLR-MAPK signaling synergistically interacts with ROS generated by Nox in cytoprotection. Lactobacilli's impact on host physiology has been found to be strain-specific (Storelli et al., 2011). In our experiments, we utilized a single L. plantarum strain isolated from the intestine of Drosophila from our fly stock. Whether the influence of L. plantarum in Drosophila, (or the influence of L. rhamnosus GG in mice) is strain and species specific, or a more general phenomenon of lactobacilli is a subject of intense focus within research group.
Taken together, these observations are consistent with the concept of “hormesis”, wherein low, near threshold levels of a stressor is protective against more intense or prolonged stimuli, which is explicitly demonstrated in figure 1E. This concept has been implicated in ROS induced, Nrf2 mediated protection against irradiation injury (in the bone marrow) (Kim et al., 2014) and cytotoxic mucosal injury (in the airway) (Paul et al., 2014). The microbiota, despite having a beneficial relationship with the host, nevertheless is an extrinsic influence, and ROS stimulated by microbes is a potent signaling stressor. Thus, hormesis, as a response to xenobiotic and environmental stimuli, evidently extends to the acquisition of and adaption to exogenous microbiota, illustrating a mechanism of co-evolution and symbiosis between host cells and microbes.
Experimental Procedures
Drosophila Paraquat resistance assays
Whole animal cytoprotection in Drosophila was measured in response to Methyl viologen dichloride (Paraquat™) -induced oxidative stress. Groups of 10 per vial, of 5-day-old adult Drosophila of assayed genotypes were starved for 3 hours and then fed a solution of 5% sucrose containing a semi-lethal dose of Paraquat (25 mM). Survivors were scored for up to 5 days, or until 100% lethality. Percent surviving flies were recorded and compared by log-rank Martel–Cox test. From each genotype and gender, triplicate assays of initial surviving 100 flies were scored. Gut tissue cytoprotection in response (Paraquat) -induced oxidative stress was analyzed following dissection of the fly midgut, fixing the tissue in 4% paraformaldehyde, followed by TUNEL assay analysis using In Situ Cell Death Detection Kit (Roche).
Supplementary Material
Acknowledgments
RMJ is supported in part by NIH Grant R01DK098391, and ASN is supported, in part, by National Institutes of Health Grant R01DK071604 and RO1AI064462. We thank April Reedy for assistance with the designing of the graphical abstract.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
RMJ and ASN conceived and designed the experiments. RMJ, LL and ESK performed experiments on the Drosophila animal model. RMJ, CD and CSA performed experiments using the Nrf2 null murine model, and TMD performed experiments using the Nox1 null murine model. RMJ, CD, CDS, ESK and AAW analyzed the data. RMJ and ASN wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
See Supplemental Information on other Experimental Procedures
References
- Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, Swanson PA, Lambeth JD, Jones RM, Nusrat A, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal immunology. 2013 doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardita CS, Mercante JW, Kwon YM, Luo L, Crawford ME, Powell DN, Jones RM, Neish AS. Epithelial Adhesion Mediated by Pilin SpaC Is Required for Lactobacillus rhamnosus GG-Induced Cellular Responses. Appl Environ Microbiol. 2014;80:5068–5077. doi: 10.1128/AEM.01039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Walker MR, Marinshaw JM, Stappenbeck TS, Stenson WF. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–838. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell stem cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. The EMBO journal. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Mercante JW, Neish AS. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Current medicinal chemistry. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T, et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124:730–741. doi: 10.1172/JCI70812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. The EMBO journal. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianes A, Spradling AC. Physiological and stem cell compartmentalization within the Drosophila midgut. Elife (Cambridge) 2013;2:e00886. doi: 10.7554/eLife.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky L, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neish AS, Jones RM. Redox signaling mediates symbiosis between the gut microbiota and the intestine. Gut Microbes. 2014;5:250–253. doi: 10.4161/gmic.27917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al. Dynamic Changes in Intracellular ROS Levels Regulate Airway Basal Stem Cell Homeostasis through Nrf2-Dependent Notch Signaling. Cell Stem Cell. 2014;15:199–214. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell metabolism. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Swanson PA, 2nd, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci U S A. 2011;108:8803–8808. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth CC, Alam A, Jones RM, Nusrat A, Neish AS. Enteric commensal bacteria induce ERK pathway signaling via formyl peptide receptor (FPR)-dependent redox modulation of Dual specific phosphatase 3 (DUSP3). The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.268938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth CC, Jones RM, Kwon YM, Nusrat A, Neish AS. Commensalepithelial signaling mediated via formyl peptide receptors. The American journal of pathology. 2010;177:2782–2790. doi: 10.2353/ajpath.2010.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.