Abstract
Rationale
Mitochondrial-derived hydrogen peroxide (H2O2) regulates flow-induced dilation (FID) in microvessels from patients with coronary artery disease (CAD). The relationship between ceramide, an independent risk factor for CAD and a known inducer of mitochondrial reactive oxygen species (ROS), and FID is unknown.
Objective
We examined the hypothesis that exogenous ceramide induces a switch in the mediator of FID from nitric oxide (NO) to H2O2.
Methods and Results
Internal diameter changes of resistance arterioles from human adipose and atrial tissue were measured by videomicroscopy. Mitochondrial H2O2 production was assayed in arterioles using MitoPeroxy Yellow 1 (Mito PY1). PEG-catalase, rotenone, and Mito-TEMPO, impaired FID in healthy adipose arterioles pre-treated with ceramide whereas Nω-Nitro-L-arginine methyl ester (L-NAME) had no effect. Mitochondrial H2O2 production was induced in response to flow in healthy adipose vessels pre-treated with ceramide and this was abolished in the presence of PEG-catalase. Immunohistochemistry demonstrated ceramide accumulation in arterioles from both healthy and CAD patients. L-NAME reduced vasodilation to flow in adipose as well as atrial vessels from patients with CAD incubated with GW4869, a neutral sphingomyelinase (NSmase) inhibitor, whereas PEG-catalase had no effect.
Conclusion
Our data indicate that ceramide has an integral role in the transition of the mediator of FID from NO to mitochondrial-derived H2O2 and that inhibition of ceramide production can revert the mechanism of dilation back to NO. Ceramide may be an important target for preventing and treating vascular dysfunction associated with atherosclerosis.
Keywords: Ceramide, shear stress, mitochondria, reactive oxygen species, nitric oxide, endothelial dysfunction, sphingomyelinase
INTRODUCTION
Vasodilation to shear stress (flow-induced dilation; FID), is an endothelium-dependent process important for maintaining vascular homeostasis. Impaired FID is a powerful predictor of future cardiovascular events.1-3 Increased shear during flow stimulates endothelial release of vasoactive substances including nitric oxide (NO), prostacyclin, and endothelial derived hyperpolarizing factors (EDHFs).4-6 We have previously shown that risk factors for, or the presence of coronary artery disease (CAD), evokes a transition in the endothelial mediator of FID from NO to mitochondrial-derived hydrogen peroxide (H2O2).7-9 Although both factors elicit smooth muscle relaxation, the non-vasomotor effects of each are generally opposite with NO stimulating anti-inflammatory, anti-thrombotic, and anti-proliferative pathways, compared to the pro-inflammatory, pro-thrombotic, and pro-atherogenic nature of H2O2. 10 The mechanism by which this transition occurs with the onset of disease represents a major gap in our understanding of microvascular control.
Accumulating evidence reveals that a class of bioactive metabolites, known as sphingolipids, play an essential role in cardiovascular pathophysiology. Their function is diverse and ranges from modulating cell proliferation and angiogenesis to atherosclerosis and cell death. Ceramide, a prototypical sphingolipid product of sphingomyelinase is produced in endothelial cells, found in human plasma, and is a risk factor for atherosclerosis.11-13 Furthermore ceramide is known to stimulate mitochondrial ROS production.12 Therefore we tested whether ceramide might play a role in the switch from NO to H2O2 in the mechanism of FID in the human microcirculation. We addressed this question by examining (1) whether exogenous ceramide can convert the mediator of FID from NO to H2O2 in healthy non-CAD arterioles, (2) whether ceramide induces mitochondrial production of H2O2, and (3) whether inhibition of ceramide production restores NO as the mediator of dilation in arterioles from patients with chronic CAD.
METHODS
Tissue acquisition
Fresh human adipose tissue (visceral, subcutaneous, peritoneal) and right atrial appendages from patients undergoing surgical procedures were obtained as discarded surgical specimens. Tissues were placed in ice-cold HEPES buffer or cardioplegia solution for adipose and atrial tissue, respectively. De-identified patient demographic data was collected using the Generic Clinical Research Database (GCRD) at the Medical College of Wisconsin. All protocols were approved by the local Institutional Review Board at the Medical College of Wisconsin and the Zablocki VA Medical Center.
Measurement of FID by videomicroscopy
Briefly, isolated arterioles were cannulated on glass micropipettes and secured in an organ chamber with circulating Kreb’s buffer. Following equilibration, endothelin-1 was added to assess viability and to preconstrict the vessels by 30-50% of their passive diameter. Internal diameters were measured at steady-state before and during intraluminal flow at pressure gradients of 5 to 100cmH2O. The following inhibitors were added to the bath 30 minutes prior to initiation of flow; nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine (L-NAME; 10−4 mol/L), H2O2 scavenger polyethylene glycol-catalase (PEG-Catalase; 500U/mL), mitochondrial antioxidant Mito-TEMPO (10−5 mol/L), or electron transport chain (ETC) complex I inhibitor rotenone (10−6 mol/L ). C-2 Ceramide (10−5 mol/L ) or the neutral sphingomyelinase inhibitor GW4869 (4×10−6 mol/L) was incubated with arterioles from healthy patients and patients with CAD, respectively, for 16-20hrs prior to the experiment. Papaverine, (10−4 mol/L ) an endothelium-independent vasodilator, was added at the end of each experiment to determine the vessel’s maximal diameter, then the direction of maximal flow was reversed in the presence of maximal dilation, to confirm matched impedance between pipettes.
Fluorescence detection of mitochondrial H2O2
Mito Peroxy Yellow 1 (Mito PY1) was used to evaluate mitochondrial-derived H2O2 in vessels during flow. Following cannulation in a warmed chamber (37°C) containing HEPES buffer (pH 7.4), arterioles were perfused intraluminally with Mito PY1 (10−5 mol/L for 1 hour). Vessels pre-treated with vehicle or ceramide (10−5 mol/L, 16-20hrs) were then exposed to either no flow (remained pressurized at 80cmH2O, 0 gradient) or flow at a pressure gradient of 100cmH2O in the presence or absence of PEG-catalase (500U/mL). Changes in mitochondrial H2O2 fluorescence probed by MitoPY1 were examined under fluorescence microscopy equipped with a krypton/argon laser fluorescent microscope (model TE 200 Nikon Eclipse) using an excitation wavelength of 488nm and measured emission between 530-590nm. Baseline measurements of fluorescence were obtained in the absence of flow and every min during 5 min of intraluminal flow. Images were then analyzed for fluorescence intensity in arbitrary units using Metamorph (Universal Imaging Corp) subtracting background fluorescence. Relative average fluorescence intensity was normalized for surface area and presented as percent change from baseline (prior to initiation of flow). All vessels used for comparison on a given day were obtained from the same patient and studied using the same imaging parameters.
Immunohistochemistry
Immunohistochemistry was performed to visualize ceramide expression in human arterioles from discarded adipose tissue as previously described.9 Briefly, isolated arterioles (~200µm in diameter) were fixed in zinc formalin buffer for 24-72hrs and processed for paraffin embedding. Samples were sectioned on a HMS355 microtome at 4µm. Immunolabeling was performed using a mouse anti-human monoclonal antibody against ceramide. Immunostaining was performed using a Leica Bond MAX Immunostainer. Slides were deparaffinized and subject to heat-induced epitope retrieval (HIER) for 10 minutes at pH 6.0. The primary antibody was optimal at 1:200 using the Bond Refine-HRP detection system. Slides were scanned with a NanoZoomer HT slide scanner (Hamamatsu, Japan). Staining was quantified within the vessel wall using Metamorph (Universal Imaging Corp) and reported as average area percent or the total stained area divided by the total wall area (µm2).
Materials
C-2 Ceramide, (Cayman Chemical, Ann Arbor, Michigan), and GW4869 (Sigma-Aldrich, Saint Louis, MO) were prepared in DMSO. Mitochondria Peroxy Yellow 1 (MitoPY1) was obtained from Tocris Bioscience (Bristol, UK). Mito-TEMPO was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY). All other chemicals were purchased from Sigma-Aldrich and prepared in distilled water with the exception of endothelin-1 which was prepared in 1% bovine serum albumin (BSA). Vehicle control studies indicated that the final concentration of DMSO had no effect on basal tone or function of the arterioles. The ceramide primary antibody was purchased from Enzo Life Sciences (Farmingdale, NY).
Statistical analyses
Data are expressed as means +/− SEM. Flow-induced dilation is expressed as a percentage of maximal relaxation from endothelin-1 constriction, with 100% representing full relaxation to the maximal diameter obtained by the addition of papaverine. To compare flow–response relationships, a 2-way ANOVA was used with flow gradient and treatment as parameters. When a significant difference was observed between curves (p<0.05), responses at individual concentrations were compared using a Holm-Sidak multiple comparison test. Fischer’s exact test was used to compare baseline characteristics for nonCAD and CAD patients. All analyses were performed using SigmaStat, version 3.5. Statistical significance was defined as p < 0.05.
RESULTS
Discarded human tissue was collected from a total of 56 patients. Results were tabulated using adipose arterioles from 42 patients without CAD and 14 patients with CAD. In those with CAD, 10 vessels were from adipose and 4 from atrial appendages. The diameters of arterioles before and after (passive diameter) administration of papaverine are as follows (mean±SD); 171±56 and 174±62 respectively for adipose vessels from healthy patients, 176±62 and 185±72 respectively for adipose vessels from patients with CAD, and 81±33 and 91±30 respectively for coronary arterioles from CAD patients. Patient demographic information is summarized in Table 1.
Table 1.
Patient demographics.
| Characteristics | Healthy (n=42) | CAD (n=14) |
|---|---|---|
| Sex, M/F | 13/29 | 10/4 |
| Age, years (average+SD) | 52±14 | 67±8 |
| Underlying diseases/risk factors | ||
| Coronary Artery Disease* | 0 | 14 |
| Hypertension* | 1 | 12 |
| Hypercholesterolemia* | 4 | 10 |
| Diabetes Mellitus* | 0 | 6 |
| Congestive Heart Failure | 0 | 2 |
| None of the Above | 37 | 0 |
CAD, coronary artery disease.
p<0.05 for healthy vs. CAD patients. n indicates number of patients.
Ceramide induces a transition in the mediator of FID
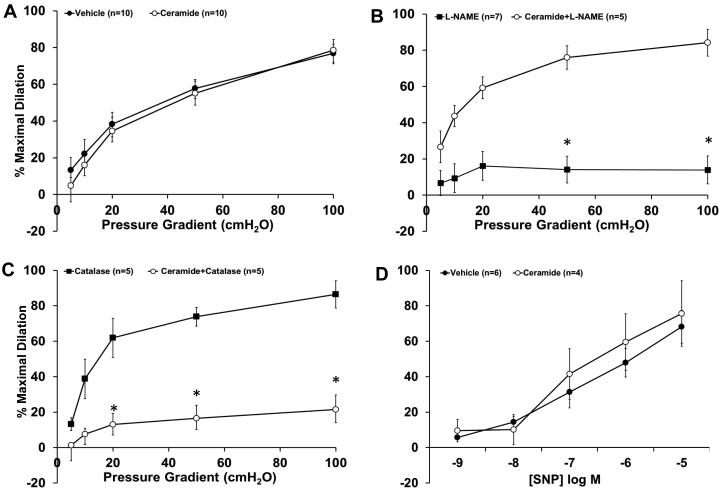
As shown in Figure 1, after incubating human adipose arterioles overnight (16-20hrs) with C-2 ceramide, FID was maintained compared to vehicle-treated control (%maximal dilation [MD] 78.6±5.8, n=10; ceramide vs. 76.8±4.9, n=10; vehicle). Inhibition of nitric oxide synthase with L-NAME inhibited FID in the vehicle group (%MD 13.8±7.7, n=7) but had no effect on FID in the ceramide-treated group (%MD 84.1±7.4, n=5). PEG-catalase abolished dilation in the ceramide-incubated vessels (%MD 21.4±8.2, n=5) but had no effect on vehicle-treated vessels (%MD 86.4±7.7, n=5). When arterioles were treated acutely (30 min) with ceramide, FID remained NO-dependent (%MD 16.3±3.7, n=5, data not shown). Overnight treatment with ceramide did not affect dilation to sodium nitroprusside (SNP), indicating endothelial-specificity to the effects of ceramide.
Figure 1. Effect of exogenous ceramide on FID.
A, The magnitude of dilation is not affected with overnight incubation of ceramide alone compared to vehicle-treated control (n= 10 and 10, respectively). B, FID in healthy adipose arterioles is reduced in the presence of L-NAME however is maintained if first pre-incubated with ceramide (n= 7 and 5, respectively). C, PEG-catalase has minimal effect on FID in healthy arterioles, however impairs FID in ceramide-treated arterioles (n= 5 and 5, respectively). D, SNP-induced dilation is not reduced by ceramide compared to vehicle-treated control (n=4 and 6, respectively). *p<0.05 vs. vehicle at specific pressure gradients. n indicates number of patients.
Ceramide increases mitochondrial H2O2 in response to flow
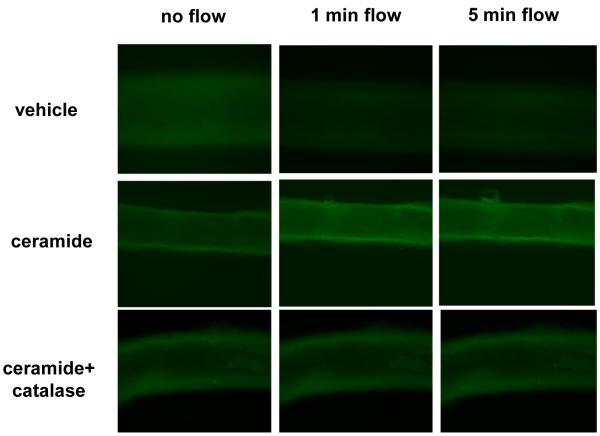
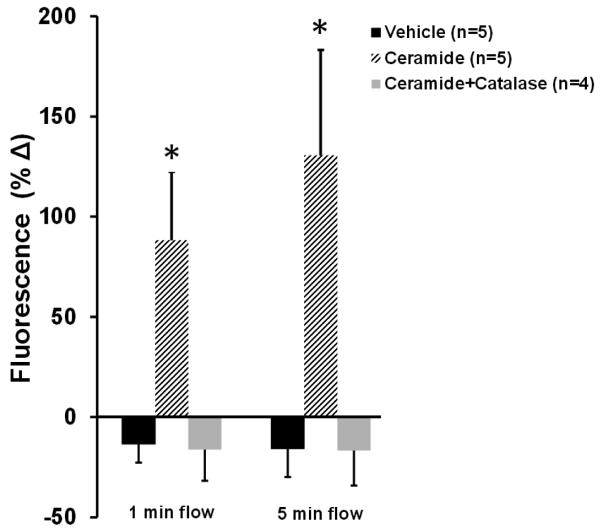
To directly assess whether ceramide induces mitochondrial-derived H2O2 in response to increases in shear, semiquantitative analysis of MitoPY1, a fluorescent probe used for imaging H2O2 specifically in the mitochondria14, was performed. As shown in Figure 2, an increase in mitochondrial-derived H2O2 was observed at both 1 min (% change from baseline [%Δ], 88±34, n=5; ceramide vs. −14±9.2, n=5; vehicle) and 5 min of maximal flow (100cmH2O) (%Δ130±52.6, n=5; ceramide vs. −16±14, n=5; vehicle) in healthy human adipose arterioles pre-incubated with ceramide compared to vehicle. To confirm that the increase in MitoPY1 fluorescence was attributable to H2O2 as opposed to other peroxide species, additional studies were performed in the presence of PEG-catalase. PEG-catalase completely blocked the increase in MitoPY1 fluorescence in ceramide-treated arterioles exposed to max flow at 1 min (%Δ−16.3±15.3, n=4) and 5 min (%Δ−16.7%±17.4) compared to ceramide alone (%Δ88±34, n=5).
Figure 2. Effect of ceramide on production of mitochondrial H2O2 in response to flow.
A, Representative images of cannulated vessels incubated in vehicle or ceramide overnight +/-PEG-catalase, following intraluminal incubation with MitoPY1 at baseline, 1 min, and 5 min of max intraluminal flow. B, Quantification of mitochondrial H2O2 expressed as percent change in MitoPY1 fluorescence from baseline at 1 min and 5 min of max flow. * p<0.05 vs. vehicle, † p<0.05 vs. ceramide+catalase.
Mitochondria is the source of FID-induced H2O2 release in ceramide-treated vessels
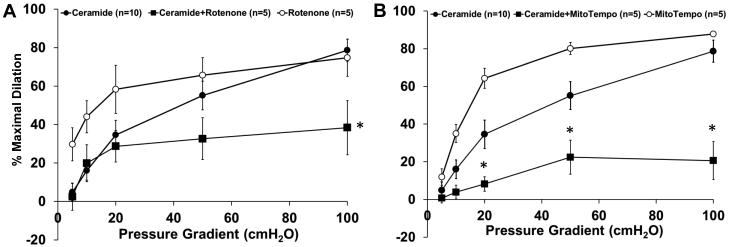
To determine whether the source of H2O2 is mitochondrial, dilation was measured in the presence of the electron transport chain (ETC) Complex I inhibitor rotenone (1µM). Figure 3A shows that rotenone alone does not affect FID (%MD 74.7±9.6, n=5) whereas vasodilation is significantly reduced in ceramide-treated arterioles in the presence of rotenone (%MD 38.3±14.1, n=5) compared to ceramide alone (%MD 78.6±5.8, n=10). Treatment with the mitochondrial-targeted antioxidant, Mito-TEMPO, resulted in a similar reduction in FID (Figure 3B). Mito-TEMPO decreased FID in arterioles incubated overnight with ceramide (%MD 20.6±10.1, n=5; Mito-TEMPO vs. 78.6±5.8, n=10; ceramide alone). Mito-TEMPO alone did not affect FID (%MD 87.8±1.5, n=5).
Figure 3. Role of mitochondria in FID in ceramide exposed vessels.
A, Vasodilation in response to flow is impaired in arterioles pre-treated with ceramide and rotenone compared to ceramide alone. Rotenone alone had no effect on FID (n=5, 10, and 5, respectively). B, Mito-TEMPO significantly decreased the response to flow in ceramide-treated vessels compared to ceramide alone. Mito-TEMPO alone had no effect (n=5, 10, and 5, respectively). † p<0.05 vs. ceramide curve. *p<0.05 vs. ceramide at specific pressure gradients.
Ceramide presence in human arterioles
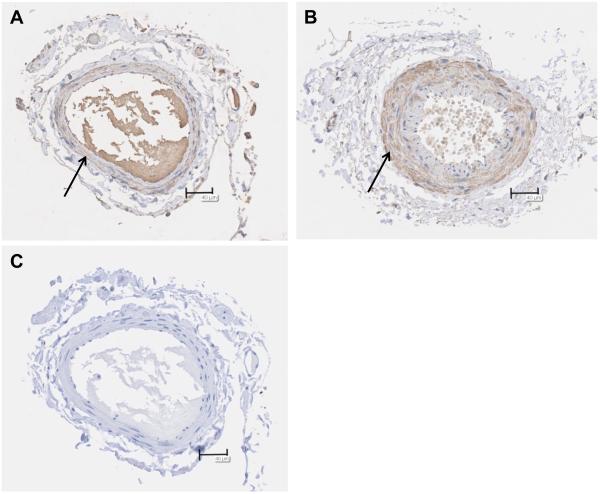
Immunohistochemistry was utilized to evaluate the presence of ceramide in arterioles from healthy patients (nonCAD) and those with CAD. Figure 4 is representative of 3 experiments. Interestingly, ceramide appears to be predominantly located in the smooth muscle layer. The average area percent of staining did not differ between the two groups (27.6%±8.7, nonCAD and 25.3%±6.2, CAD; mean±SD), nor was there a difference in staining density between the two groups (data not shown). However the total stained area was greater in the CAD arterioles (5503µm2±1802) versus nonCAD (1994µm2±619). Likewise, the total vascular cross-sectional area was larger in CAD versus nonCAD vessels (2.2×104µm2±0.4×104 vs. 0.7×104 µm2±0.3×104, respectively). The secondary antibody was specific (Fig 4, panel C).
Figure 4. Ceramide accumulation in arterioles from healthy versus CAD patients.
Representative images from 3 patients (3 healthy, 3 CAD). The total area of staining is decreased in arterioles from healthy patients (A) versus patients with CAD (B), however area stained/total area did not differ between groups. Specificity of the antibody was examined by removal of the primary antibody (C). Bar=40 μm.
Inhibition of NSmase reverts mechanism of FID from H2O2 to NO in arterioles from patients with CAD
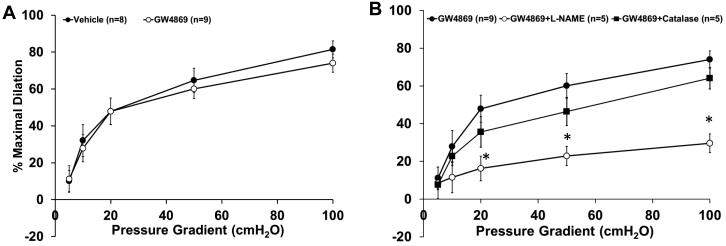
Adipose arterioles from patients with CAD were incubated overnight with the specific non-competitive NSmase inhibitor GW4869 (4×10−6 mol/L, 16-20hrs). In these vessels, dilation to flow was attenuated by l-NAME (Figure 5, %MD 29.5±4.9, n=5) but not by PEG-catalase (%MD 64.1±5.6, n=5). Incubation with GW4869 did not alter the magnitude of FID (%MD 73.9±4.9, n=9; vs. 81.5±4.6, vehicle; n=8). Thus inhibiting ceramide production reverts the mechanism of dilation from H2O2 to NO in vessels from subjects with CAD.
Figure 5. Inhibition of NSmase reverts the mediator of FID back to NO in vessels from adipose tissue from patients with CAD.
A, Incubation with the specific NSmase inhibitor GW4869 (4μM, 16-20hrs) did not affect the overall magnitude of dilation to flow compared to vehicle-treated control (n=9 and 8, respectively). B, The response to flow was inhibited in CAD vessels first incubated with GW4869 in the presence of L-NAME (100μM) compared to GW4869 alone, whereas PEG-catalase (500U) had no effect (n=5, 9, and 5, respectively). *p<0.05 vs. GW4869 at specific pressure gradients.
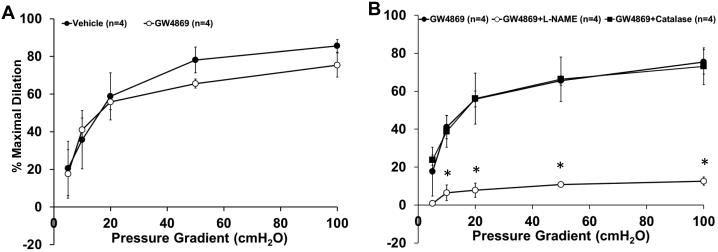
To determine if a similar switch in mechanism occurs in the coronary circulation, arterioles isolated from right atrial appendages from patients with CAD were treated in a similar fashion. As shown in Figure 6A, incubation with GW4869 alone had no effect on dilation (%MD 75.4±6.4, n=4; GW4869 vs. 85.6±3.4, n=4; vehicle). Inhibition of NOS with l-NAME reduced FID in CAD atrial vessels treated with GW4869, whereas PEG-catalase had no effect. (Figure 6B; %12.6±2.2, n=4; vs. 73.1±9.7, n=4; respectively)
Figure 6. Inhibition of NSmase reverts the mediator of FID back to NO in atrial vessels from patients with CAD.
Incubation with the specific NSmase inhibitor GW4869 (4μM, 16-20hrs) had no effect on FID compared to vehicle (n=4). B, The response to flow was inhibited in CAD vessels first incubated with GW4869 in the presence of L-NAME (100μM), whereas PEG-catalase (500U) had no effect (n=4). *p<0.05 vs. GW4869 at specific pressure gradients.
DISCUSSION
This study is the first to demonstrate that ceramide plays a pivotal role in switching the primary mediator of FID in human arterioles with the onset of CAD. The major novel findings are 3-fold. First, overnight exposure to exogenous ceramide can evoke a transition from NO to H2O2 as the mediator of FID. Second, ceramide-induced increases in H2O2 require an intact mitochondrial electron transport chain. Third, inhibition of the ceramide-producing enzyme NSmase can restore NO as the mediator of FID in patients with chronic CAD. These findings show that ceramide is a critical mechanistic component of the transition that takes place from the NO-mediated pathway of microvascular dilation observed in healthy individuals to an H2O2-driven signaling pathway of FID seen in patients with atherosclerotic disease.
Vasodilation to shear stress is not only important to maintain tissue perfusion, but is highly predictive of future cardiovascular events.15 Endothelial dependent dilation is mediated by hyperpolarization or by release of vasoactive factors which include NO, prostacyclin (PGI2), and other endothelium-derived hyperpolarizing factors (EDHFs, e.g. epoxyeicosatrienoic acid or H2O2). 16,17,3 Although each factor is capable of eliciting dilation, each has a different effect on vascular biology. For instance, in vessels from subjects with CAD, endothelial release of H2O2 creates an environment that promotes inflammation, thrombosis, and atherosclerosis.
Ceramide and mitochondrial H2O2-dependent dilation
Prior evidence suggests that the endothelial mitochondria are the source of H2O2 responsible for FID in patients with CAD.8 Elevated levels of plasma sphingomyelin, the precursor to ceramide, are an independent risk factor for CAD18 and a known inducer of mitochondrial dysfunction.12 This is the first study to our knowledge demonstrating a link between ceramide and mitochondrial-derived H2O2 in microvessels from patients with CAD.
Ceramide consists of a family of bioactive sphingolipids formed via multiple pathways. The most rapid generation of ceramide occurs through sphingomyelin hydrolysis by acid or neutral spingomyelinases.19 Ceramide can also be formed by reacylation of sphinogsine, known as the salvage pathway, as well as de novo by the condensation of palmitate and serine.20 Ceramidase enzymes found within the cytosol are responsible for the catabolism of ceramide back to sphingosine.21 This complexity of ceramide formation and removal implies that cellular levels of ceramide are tightly regulated as is true with many key signaling molecules. Overall the de novo synthesis of ceramide contributes little to the overall amount of ceramide within the cell, with most of its generation coming from the sphingomyelinases found in the cell membrane (NSmase), or within lysosomes (ASmase).22 The current study examined specifically the role of NSmase as this is the only ceramide-producing enzyme found within the sphingomyelin-rich caveolae, allowing close contact to luminal flow and intimate connection with a primary signaling location in the cell membrane. Cznary and colleagues demonstrated that NSmase activity dramatically increases within the first two minutes of increased flow in the membrane fraction of the rat pulmonary artery, whereas there is no change in ASmase activity.23
Previous studies have shown that ceramide can increase ROS levels through activation of NADPH oxidase, xanthine oxidase, and the mitochondrial electron transport chain (ETC), specifically at the Qi site of complex III.12 Zhang and colleagues previously showed that administration of exogenous ceramide increases NADPH oxidase activity, resulting in decreased vasodilation to bradykinin in small bovine arterioles.24 However, prior evidence in human tissue suggests that the primary source of H2O2 that mediates FID in microvessels from patients with CAD is the mitochondrial electron transport chain.8 The current study supports the mitochondria as a predominant source of ceramide-induced ROS formation, but it is recognized that cross talk exists between intracellular sites of ROS production, in the form of ROS-induced ROS-release (RIRR). 25 It is possible that ceramide exerts its effect on multiple cellular sites of ROS formation. However the mitochondria appear to play an obligatory role in the ROS generation during shear based on the effectiveness of the ETC complex I inhibitor rotenone and the mitochondrial-specific antioxidant Mito-TEMPO, in abolishing FID in arterioles from healthy subjects that were pre-treated with ceramide.
NO-dependent FID was not affected by acute exposure (30min) to ceramide whereas vessels exposed to ceramide chronically (16-20hrs) transitioned from an NO to a H2O2-dependent mechanism of dilation. This highlights the complexity involved in this transition. For instance it is known that there exists a constant flux between formation of the cell-damaging ceramides and the more benign sphingosines such as sphingosine-1-phosphate, known as the sphingolipid rheostat.22 It is feasible that expression of ceramidase, the enzyme that converts ceramide to sphingosine, may decrease during chronic exposure. Likewise, longer exposure may be necessary to elicit changes in NO bioavailability. A study by Zhang and colleagues showed that ceramide accumulation, in vivo and in vitro, increases the association of protein phosphatase 2A (PP2A) with eNOS, subsequently decreasing phosphorylation at Ser1177 and Ser617, thus decreasing the amount of available NO.26 Although ceramide is known to increase mitochondrial ROS directly, NO is capable of inhibiting mitochondrial-derived ROS27, therefore ceramide-induced decreases in NO likely contribute to this alteration in dilation as well. Future studies are needed to understand how ceramide metabolism and regulation of NO contribute to this transition in mechanism as this could provide novel avenues for drug discovery.
Interestingly, our study suggests that ceramide concentrates predominantly in the smooth muscle layer of arterioles from patients with CAD. This observation is in agreement with a study by Auge et al which demonstrated that activation of neutral sphingomyelinase and subsequent ceramide accumulation is associated with smooth muscle cell proliferation.28 While ceramide may be responsible for hyperplasia associated with disease, the medial layer might also serve a reservoir for lipid actions in the endothelium. This finding is also supported by the fact that vascular endothelial cells tend to not accumulate cholesterol or lipid as compared to other various cell types such as smooth muscle cells or macrophages. Studies have shown that following exposure to elevated levels of lipid, endothelial cells upregulate specific transporters such as ATP-binding cassette G1.29 While the average percent area of ceramide staining did not differ between the two groups, and the average vascular wall area was larger in the CAD arterioles, the total stained area was greater in the CAD vessels suggesting that the overall amount of ceramide is elevated in arterioles from CAD patients.
The use of an inert form of ceramide would be beneficial in determining the specificity of ceramide in modifying vascular function. Often the precursor to ceramide, dihydroceramide, which lacks the trans-4,5 double bond, is used as a negative control, however the data has been conflicting since dihydroceramide can reproduce some of the effects elicited by ceramide, an effect that might be dependent of the type of cell.12 Treatment with dihydroceramide produced inconclusive results (data not shown).
Potential study limitations
An important limitation to studies in isolated arterioles is the inability to quantify vascular cell target molecules. Small sample volumes prevented us from quantifying ceramide levels in the tissue. The diacylgylcerol (DAG) kinase assay has been used to measure ceramide levels between 25pmol to 2nmol, however the specificity of this assay has been questioned.30 The amount of starting sample required for other methods such as thin-layer chromatography, high performance liquid chromatography, and mass spectrometry surpasses the total amount that can be isolated from a patient sample making accurate measurement of ceramide from human microvessels a challenge.
By necessity our “healthy” samples of tissue were collected from subjects with a variety of diseases and who may be taking one or more medications. There may also be patients who have subclinical atherosclerotic plaque and are misclassified as “normal.” To minimize this risk, only subjects with no more than one risk factor for CAD and no evidence of CAD (see Table 1) were classified as not having CAD. We argue that the benefit of directly examining responses in human vessels outweighs the methodological limitations described. However we further try to minimize these restrictions by washing vessels with 60mL of buffer prior to experimentation to eliminate most pharmacological agents and by searching for confounding effects of related diagnoses, age, and gender.
Fluorescent probes such as dichlorodihydrofluorescein (DCF), dihydroethidium (DHE), and mitoSOX, have been used extensively to measure production of H2O2, superoxide (O2−·), and mitochondrial-derived O2−·, respectively31. While each has the ability to detect changes in intracellular ROS, they have limitations and shortcomings as well32. The present study utilized a newer fluorescent probe, MitoPY1, to detect levels of mitochondrial-derived H2O2. This boronate-based probe contains a triphenylphosphonium group similar to mitoSOX that targets the fluorophore to the mitochondria, allowing it to react with H2O2. It is possible that MitoPY1 may react with other forms of ROS, however in the present study PEG-catalase, which is specific for H2O2, abolished the increase in fluorescence indicating that the majority of ROS being detected was H2O2.
Conclusions and clinical implications
The present study confirms that exogenous ceramide can cause a shift in vasoactive mediators from NO to mitochondrial-derived H2O2 and that inhibition of neutral sphingomyelinase, a key enzyme in ceramide production, can revert the mechanism of dilation back to NO. While the majority of clinically significant lesions involve the coronary conduit arteries, microvascular dysfunction is a key risk factor for cardiovascular events, even in the absence of large artery disease.33 Accumulating evidence suggests a correlation between elevated ceramide levels and type 2 diabetes34 as well as smoking,35 both potent instigators of endothelial microvascular dysfunction. The ability to decrease overall ceramide levels through inhibition of NSmase or via activation of ceramidase, can have a profound impact on multiple clinical scenarios that are attributable to oxidative stress including vascular inflammation and thrombosis. Therefore thorough understanding of ceramide signaling and regulatory mechanisms within the vasculature may allow for the development of new therapeutics and a means to improve microcirculation in disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Impairment of flow-induced dilation (FID), the ability of vessels to dilate in response to increases in blood flow, is associated with cardiovascular events.
The primary mediator of FID in healthy adults is nitric oxide (NO), whereas in patients with coronary artery disease (CAD), the mediator is mitochondrial-derived hydrogen peroxide (H2O2).
Ceramide, a biologically active lipid, known to induce mitochondrial H2O2, is elevated in the plasma of patients with CAD.
What New Information Does This Article Contribute?
Exogenous ceramide administered to arterioles from patients without CAD causes a transition in mechanism of FID from NO to H2O2, the same mediator of FID observed in vessels from patients with CAD.
Inhibition of ceramide formation in diseased arterioles from patients with CAD reverts the mediator of FID to NO, as observed in patients without CAD.
Ceramide acts as a pathological and reversible switch for endothelial production of either NO or H2O2 in response to endothelial shear stress in the microcirculation.
Flow-induced dilation, the ability of the vasculature to dilate to increased shear stress is critically dependent upon an intact endothelium and is inversely related to future cardiovascular events. CAD and its risk factors induce a chronic change in the mediator of FID from NO to H2O2. This study for the first time identifies ceramide as a central signaling molecule which is both necessary and sufficient for this switch. The long term effect of endothelial release of NO, which is athero-protective and anti-inflammatory, versus H2O2 which is pro-atherogenic and begets inflammation suggests a benefit to therapies designed to prevent or reverse H2O2 as the released vasoactive substance during flow. Ceramide and sphingomyelinase emerge as promising candidate pathways for intervention.
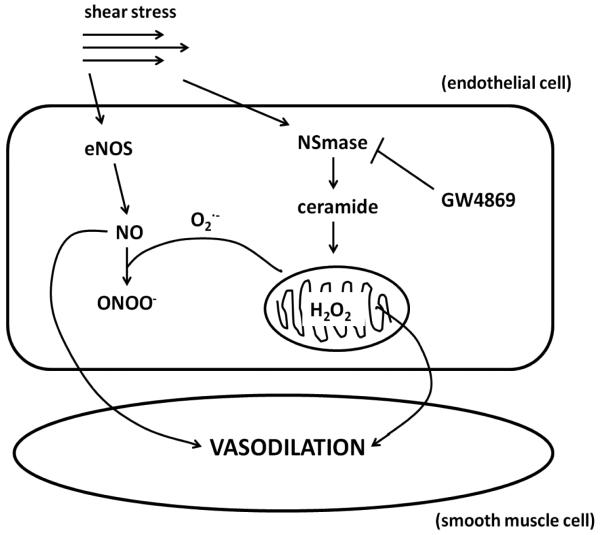
Figure 7. A schematic diagram illustrating the involved pathways.
Under normal conditions in healthy adults, exposure of the endothelial layer to shear stress activates endothelial nitric oxide synthase (eNOS) causing elevation of nitric oxide (NO) which primarily serves as the mediator of smooth muscle dilation. In the disease state, increased levels of ceramide formed via neutral sphingomyelinase (NSmase) trigger mitochondrial reactive oxygen species (ROS) formation which both decrease the bioavailability of NO and ultimately change the mediator of vasodilation to hydrogen peroxide (H2O2). Inhibition of NSmase with the specific non-competitive inhibitor GW4869 can revert the mechanism of dilation back to NO.
ACKNOWLEDGEMENTS
The authors wish to thank the surgeons and nurses at Froedtert Memorial Lutheran Hospital, the Division of Cardiothoracic Surgery at the Medical College of Wisconsin, Cardiothoracic Surgery Division at the Zablocki VA Medical Center in Milwaukee, the Aurora Medical Group Cardiovascular and Thoracic Surgery, the Cardiothoracic Surgery Group of Milwaukee for providing tissue, the Wheaton Franciscan Healthcare Group, and Glenn Slocum for his expertise in microscopy.
SOURCES OF FUNDING
This work was supported by a National Institutes of Health RO1 HL113612-02 (to D.D. Gutterman) and a T-32 Physician Scientist Training Grant GM089586 (to J.R. Kersten).
Nonstandard Abbreviations and Acronyms
- EDHF
endothelial-derived hyperpolarizing factor
- ETC
electron transport chain
- FID
flow-induced dilation
- H2O2
hydrogen peroxide
- L-NAME
Nω-nitro-l-arginine
- mitoPY1
mito peroxy yellow 1
- NSmase
neutral sphingomyelinase
- NO
nitric oxide
- nonCAD
non-coronary artery disease
- ROS
reactive oxygen species
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 2.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. The international journal of cardiovascular imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 3.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr., Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: Important role of ca(2+)-activated k(+) channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 4.Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circulation research. 1990;67:529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- 5.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. The American journal of physiology. 1991;261:H1706–1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 6.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via bk(ca) channels: Implications for soluble epoxide hydrolase inhibition. American journal of physiology. Heart and circulatory physiology. 2006;290:H491–499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2o2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circulation research. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of h2o2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circulation research. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 9.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circulation research. 2003;92:e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 10.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovascular research. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, Kojo S. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. The Journal of biological chemistry. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 13.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. American journal of respiratory cell and molecular biology. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. Journal of the American Chemical Society. 2008;130:9638–9639. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The cardiovascular health study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 16.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 17.Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. The American journal of physiology. 1994;267:H326–332. doi: 10.1152/ajpheart.1994.267.1.H326. [DOI] [PubMed] [Google Scholar]
- 18.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 19.Kogot-Levin A, Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2013 doi: 10.1016/j.biochi.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Tidhar R, Futerman AH. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochimica et biophysica acta. 2013;1833:2511–2518. doi: 10.1016/j.bbamcr.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 22.Arana L, Gangoiti P, Ouro A, Trueba M, Gomez-Munoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids in health and disease. 2010;9:15. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czarny M, Liu J, Oh P, Schnitzer JE. Transient mechanoactivation of neutral sphingomyelinase in caveolae to generate ceramide. The Journal of biological chemistry. 2003;278:4424–4430. doi: 10.1074/jbc.M210375200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of nadph oxidase and endothelial dysfunction in small coronary arteries. American journal of physiology. Heart and circulatory physiology. 2003;284:H605–612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 25.Zinkevich NS, Gutterman DD. Ros-induced ros release in vascular biology: Redox-redox signaling. American journal of physiology. Heart and circulatory physiology. 2011;301:H647–653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by pp2a-mediated dephosphorylation of the enos-akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free radical biology & medicine. 2002;33:1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- 28.Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, Krell HW, Salvayre R, Negre-Salvayre A. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. 2004;110:571–578. doi: 10.1161/01.CIR.0000136995.83451.1D. [DOI] [PubMed] [Google Scholar]
- 29.Hassan HH, Denis M, Krimbou L, Marcil M, Genest J. Cellular cholesterol homeostasis in vascular endothelial cells. The Canadian journal of cardiology. 2006;22(Suppl B):35B–40B. doi: 10.1016/s0828-282x(06)70985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts JD, Gu M, Polverino AJ, Patterson SD, Aebersold R. Fas-induced apoptosis of t cells occurs independently of ceramide generation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7292–7296. doi: 10.1073/pnas.94.14.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxidants & redox signaling. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free radical biology & medicine. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the nhlbi wise study. American heart journal. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 34.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in ldl are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral sphingomyelinase 2: A novel target in cigarette smoke-induced apoptosis and lung injury. American journal of respiratory cell and molecular biology. 2011;44:350–360. doi: 10.1165/rcmb.2009-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.