Summary
Alterations in estrogen-mediated cellular signaling play an essential role in the pathogenesis of endometriosis. In addition to higher estrogen receptor (ER)β levels, enhanced ERβ activity was detected in endometriotic tissues, and the inhibition of enhanced ERβ activity by an ERβ-selective antagonist suppressed mouse ectopic lesion growth. Notably, gain of ERβ function stimulated the progression of endometriosis. As a mechanism to evade endogenous immune surveillance for cell survival, ERβ interacts with cellular apoptotic machinery in the cytoplasm to inhibit TNFα-induced apoptosis. ERβ also interacts with components of the cytoplasmic inflammasome to increase interleukin-1β and thus enhance its cellular adhesion and proliferation properties. Furthermore, this gain of ERβ function enhances epithelial-mesenchymal transition signaling, thereby increasing the invasion activity of endometriotic tissues for establishment of ectopic lesions. Collectively, we reveal how endometrial tissue generated by retrograde menstruation can escape immune surveillance and develop into sustained ectopic lesions via gain of ERβ function.
Introduction
Endometriosis is a medical condition in which endometrial cells are deposited and grow outside the uterine cavity (Bulun, 2009; Giudice, 2010). Severe symptoms of endometriosis are typically observed in 6 – 10 % of reproductive-aged women (Simoens et al., 2007). Among patients with endometriosis, approximately 50% have major pelvic pain, and 40–50% have fertility problems (Eskenazi and Warner, 1997; Ozkan et al., 2008). In these patients, endometriosis-associated symptoms negatively impact their health and quality of life (Moradi et al., 2014).
To improve the efficiency of endometriosis therapies, it is important to dissect the unique molecular properties of endometriotic tissues compared with normal endometrium. Previous studies identified several endocrine properties associated with endometriotic tissues. Altered estrogenic signaling pathways have been reported in endometriosis pathogenesis (Bulun, 2009). Endometriotic lesions have been reported to contain higher 17β-estradiol levels than normal endometrium due to the elevated expression of 17β-hydroxysteroid dehydrogenase-1 and aromatase genes compared with normal endometrium (Acien et al., 2007; Delvoux et al., 2009). These higher levels of local 17β-estradiol could play a role in the proliferation of endometriotic tissues (Zhang et al., 2010). This increased 17β-estradiol binds and activates ERs in endometriotic tissues to stimulate estrogen-dependent their growth. There are two different forms of the ER, usually referred to as α and β, each encoded by a different gene, ESR1 and ESR2, respectively. Prior studies with ERα (−/−), ERβ (−/−) mouse models and selective estrogen receptor modulators revealed essential roles of both ERα and ERβ in mouse ectopic lesion development (Burney, 2013; Zhao et al., 2015). However, each ER isoform has an unique expression pattern between endometriotic tissues and normal endometrium. In case of ERα, it is controversial whether ERα has an endometriotic tissue specific pattern (Han and O’Malley, 2014). In contrast to ERα, however, the mRNA level of ERβ is significantly higher in endometriotic tissues than in normal uterine endometrium (Bulun et al., 2012). Aberrant ERβ levels in endometriotic tissues have been associated with a distinct epigenomic profile in the ERβ genomic locus: a hypomethylated promoter of the ERβ gene was detected in endometriotic tissues compared with normal endometrium and correlates with increased ERβ mRNA levels (Xue et al., 2007).
What is the role of ER isoforms in the pathogenesis of endometriosis? Unfortunately, the detail molecular mechanism regarding specific contribution of each ER isoform in the endometriosis progression is not clearly elucidated, yet. Only partial information is available. For examples, ERα(−/−) mouse with endometriosis revealed that ESR1 gene is required for attachment, inflammation and proliferation of ectopic lesions (Burns et al., 2012). ERβ directly induces Ras-like estrogen-regulated growth inhibitor gene expression in an estrogen-dependent manner to enhance the proliferative activity of endometriotic tissues (Monsivais et al., 2014). In addition, ERβ directly binds to the ERα promoter region to repress ERα gene expression, which can lead to a state of progesterone resistance in the endometriotic tissues by suppressing ERα-mediated progesterone receptor (PR) expression in endometriotic tissues (Bulun et al., 2012). However, we believe the complete repertoire of ERβ functions to be more complicated because greatly elevated levels of ERβ exist in both nuclear and cytoplasmic locations in endometriotic tissues (Cheng et al., 2011). We believed a more detailed investigation should be carried out to fully understand the mechanisms of ERβ action in endometriosis progression.
In addition to its genomic functions, we propose a new cytoplasmic ERβ protein network that promotes endometriosis pathogenesis in a non-genomic manner. Together with our previously observed SRC-1 coactivator isoform, these two drivers of endometriotic disease cooperate to render endometriosis a therapeutically complex disease.
Results
Mouse endometriotic tissues have elevated ERβ levels similar to those in human endometriotic cells
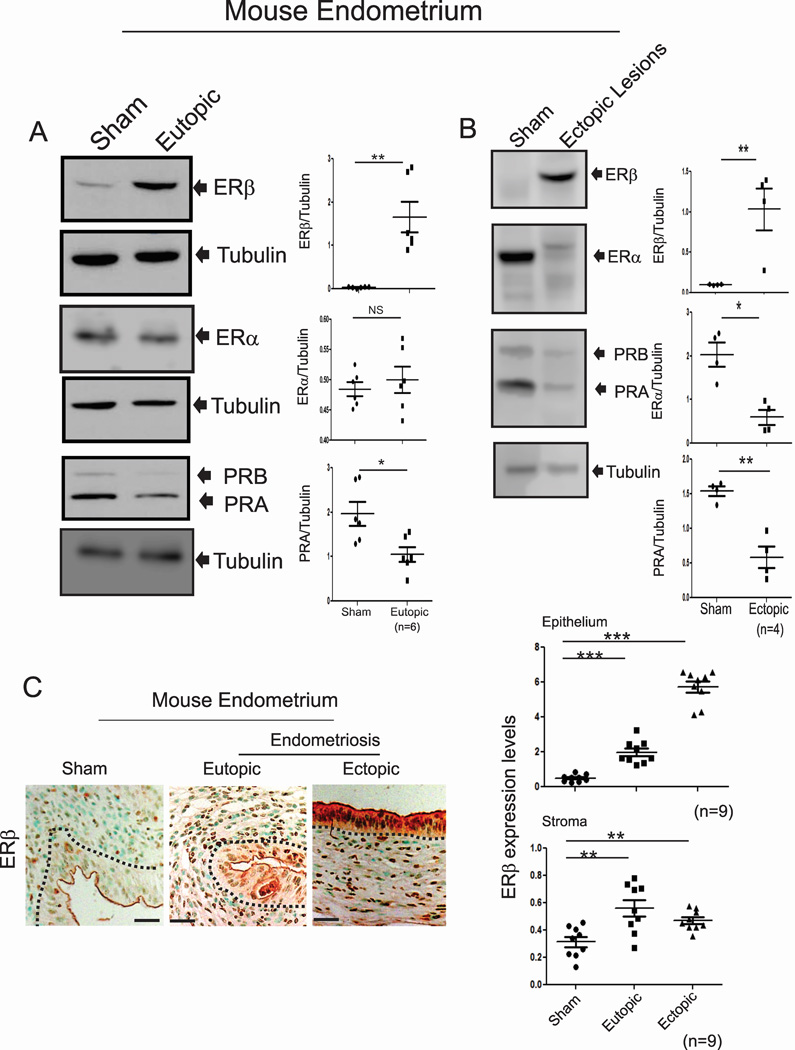
Human endometriotic cells isolated from endometriosis patients have higher levels of ERβ, but not ERα, than do normal human endometrial cells (Han et al., 2012). Consistent with human endometriotic cells, both eutopic and ectopic endometria from mice with endometriosis also had markedly higher ERβ levels compared with the uteri of sham-treated mice (Figures 1A and 1B). In contrast to ERβ, however, the levels of ERα did not differ in eutopic endometria but were reduced in ectopic lesions compared with sham-treated uteri (Figures 1A and 1B). Levels of PR were reduced in both ectopic lesions and eutopic endometria of mice with endometriosis compared with the uteri of sham-treated mice (Figures 1A and 1B). Immunohistochemistry (IHC) using an ERβ antibody (validation of its specificity in Figure 4B) revealed elevated ERβ levels in the epithelial and stromal compartments of both ectopic lesions and eutopic endometria compared with those compartments in normal endometrium (Figure 1C). Therefore, the ERβ levels are elevated in endometriotic tissues of mice with endometriosis similar to the levels observed in human endometriotic cells.
Figure 1. Mouse Endometriotic Tissues Have Elevated Levels of ERβ.
(A–B) The expression levels of ERβ, ERα, PR and tubulin in the uteri of sham-treated C57BL/6J mice and the eutopic endometria (A) and ectopic lesions (B) of C57BL/6J mice with endometriosis.
(C) IHC and quantitative analyses of ERβ levels in the uteri of sham-treated C57BL/6J mice and ectopic and eutopic endometria of C57BL/6J mice with endometriosis.
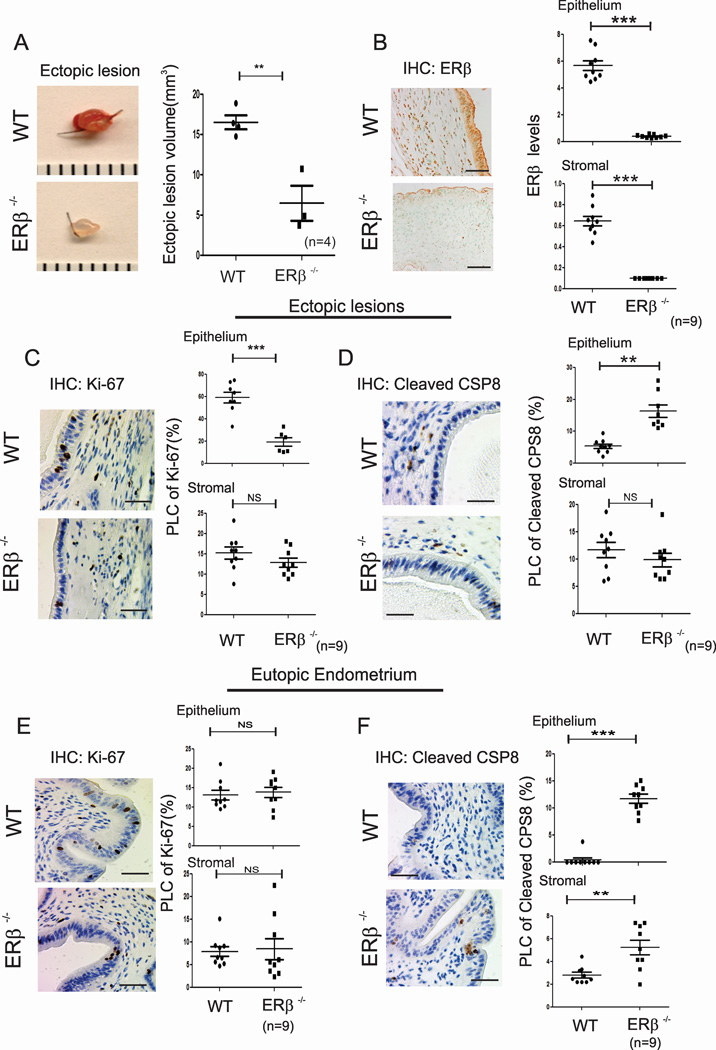
Figure 4. The Loss of ERβ Function Prevents Ectopic Lesion Growth.
(A) Ectopic lesions isolated from C57BL/6J (WT) and ERβ−/− mice with endometriosis.
(B) IHC analyses and quantification of the ERβ levels in ectopic lesions isolated from WT and ERβ−/− mice with endometriosis.
(C–F) IHC and quantitative analyses of Ki-67 (C and E) and cleaved CSP8 (D and F) in the epithelial and stromal compartments of ectopic lesions (C and D) and eutopic endometrium (E and F) of WT and ERβ−/− mice with endometriosis.
Endometriotic tissues have enhanced ERβ activity compared with normal endometrium
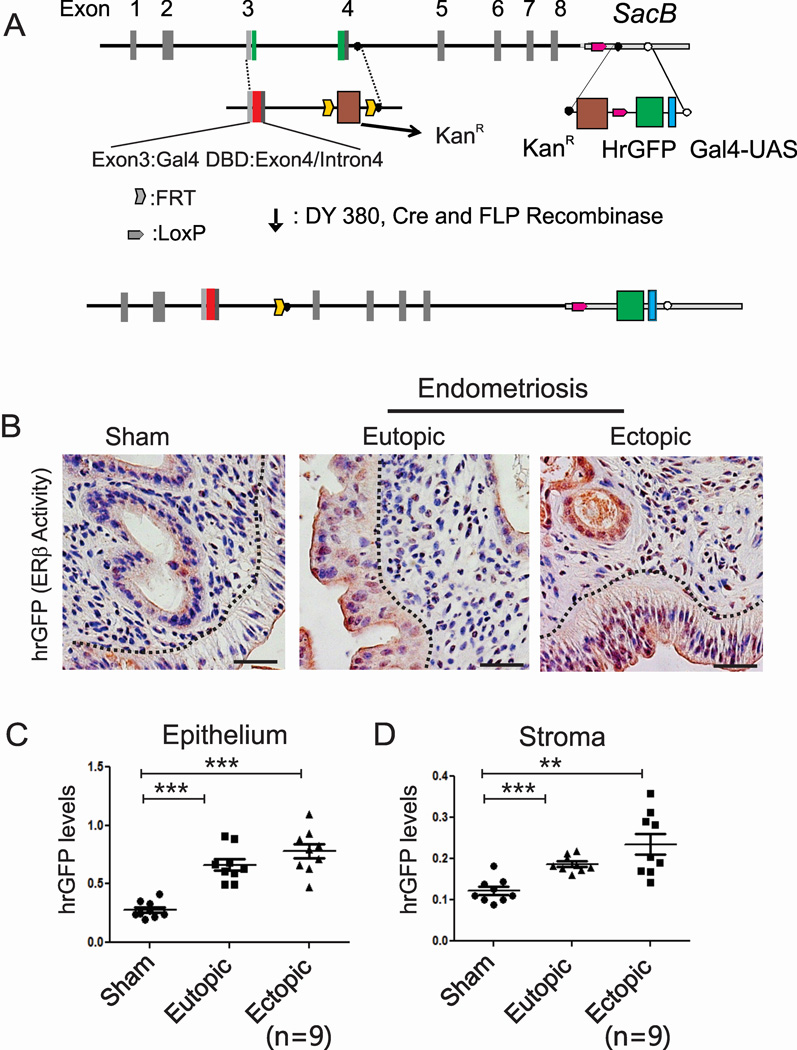
To determine ERβ activity in endometriotic tissues in vivo, we generated an ERβ activity indicator (ERBAI) mouse containing a modified ERβ bacterial artificial chromosome clone that has a Gal4 DNA-binding domain (DBD) instead of its own DBD and a hrGFP reporter controlled by the Gal4-upstream activating sequence (UAS) according to our prior protocol (Han et al., 2009) (Figures 2A and S1). Therefore, Gal4-ERβ binds to Gal4-UAS, transcribing hrGFP gene expression in response to hormone. Detailed information regarding the generation and validation of the ERBAI mouse model is described in the Supplemental Information (Figure S1).
Figure 2. Enhanced ERβ Activity is Detected in the Endometriotic Tissues of Mice with Endometriosis Compared to Normal Endometrium.
(A) Generation of a modified ERβ bacterial artificial clone that has a Gal4 DNA-binding domain and the Gal4-UAS-hrGFP reporter. DY380, Bacterial recombination strain. KanR, kanamycin-resistant gene. DBD, DNA-binding domain. Gal4-UAS, Gal4-upstream activating sequence. FLP, flippase. hrGFP, humanized renilla GFP.
(B) IHC analyses of hrGFP levels in the uteri of sham-treated ERBAI mice and ectopic and eutopic endometria of ERBAI mice with endometriosis.
(C and D) The quantification of hrGFP levels in the epithelial (C) and stromal compartment (D) of each type of endometrium in panel B. See also Figures S1.
To investigate potential alterations in ERβ activity in endometriotic tissues during endometriosis progression, endometriosis was surgically induced using an ERBAI mouse model through autotransplantation; to monitor ERβ activity, the hrGFP levels were determined in ectopic and eutopic endometria of ERBAI mice with endometriosis and in the uteri of sham-treated ERBAI mice. Elevated hrGFP levels were detected in epithelial and stromal cells of ectopic and eutopic endometria compared with those found in the normal uteri of sham-treated ERBAI mice (Figures 2B, 2C and 2D). Therefore, enhanced ERβ activities were detected in the stromal and epithelial compartments of endometriotic tissues compared with normal endometrium.
Enhanced ERβ activity is required for ectopic lesion growth in mice with endometriosis
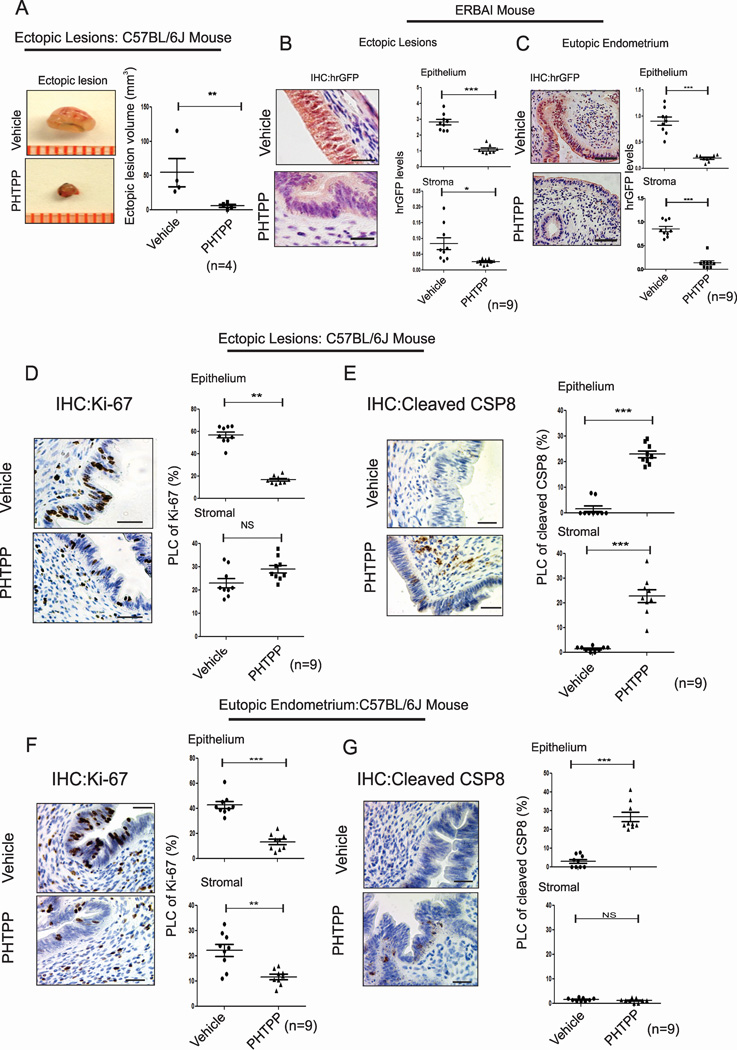
Although enhanced ERβ activity was detected in endometriotic tissues, it was not clear whether the enhanced ERβ activity was required for ectopic lesion growth. Therefore, PHTPP, an ERβ-selective antagonist (Compton et al., 2004), was employed to address it. Ectopic lesions were surgically developed in ovariectomized C57BL/6J mice containing an Estradiol (E2) pellet. On the 21st day after endometriosis induction, PHTPP or vehicle was administered to endometriosis-induced mice (Figure S2A). Compared with vehicle treatment, PHTPP treatment significantly suppressed ectopic lesion growth in mice with endometriosis (Figure 3A). For the stimulation of ectopic lesion growth, endometriotic tissues recruit immune cells (CD163-positive monocyte/macrophage cells) to enhance immune cell-mediated cytokine signaling (Figure S2B, arrowhead). However, PHTPP-treated ectopic lesions did not recruit immune cells compared with vehicle-treated ectopic lesions (Figure S2B). In addition, PHTPP treatment clearly diminished ERβ activity in the epithelial and stromal compartments of ectopic lesions and the eutopic endometrium of mice with endometriosis compared with vehicle (Figures 3B and 3C). Collectively, PHTPP inhibits ERβ activity, which leads to endometriotic lesion growth in mice with endometriosis.
Figure 3. ERβ-Specific Antagonist Regresses Ectopic Lesion Growth.
(A) Ectopic lesions isolated from C57BL/6J mice with endometriosis subcutaneously treated with vehicle or PHTPP.
(B and C) IHC and quantitative analyses of hrGFP levels in ectopic lesions (B) and eutopic endometria (C) of ERBAI mice with endometriosis subcutaneously treated with vehicle or PHTPP.
(D and E) IHC and quantitative analyses of the expression patterns of Ki-67 (D) and cleaved CSP8 (E) in ectopic lesions of C57BL/6J mice with endometriosis subcutaneously treated with vehicle or PHTPP.
(F and G) IHC and quantitative analyses of the levels of Ki-67 (F) and cleaved CSP8 (G) in the eutopic endometria of C57BL/6J mice with endometriosis subcutaneously treated with vehicle or PHTPP. PLC, percentage of labeled cells. CSP8, caspase 8. See also Figures S2.
Anti-apoptosis signaling and the acceleration of proliferation are typical molecular properties associated with the survival of endometriotic tissues (Pellegrini et al., 2012; Salmassi et al., 2011). Because endometriotic tissues consist of epithelial and stromal compartments, signaling communication between these compartments plays an essential role in endometriotic lesion progression (Kim et al., 2013). Therefore, functional defects involving hyperproliferation and anti-apoptosis signaling in either compartment in endometriotic tissues should impair cellular processes in the other compartment, ultimately leading to the suppression of ectopic lesion growth. PHTPP treatment reduced proliferative activity as determined by Ki-67 in the epithelial, but not stromal, compartments of ectopic lesions in C57BL/6J mice with endometriosis compared with vehicle treatment (Figure 3D). In the case of apoptosis signaling as determined by cleaved caspase 8 levels, PHTPP treatment significantly enhanced apoptotic signaling in both the epithelial and stromal compartments of ectopic lesions of C57BL/6J mice with endometriosis compared with vehicle treatment (Figure 3E). In addition to ectopic lesions, PHTPP suppressed proliferation and anti-apoptosis signaling in the epithelium and inhibited proliferation in stromal cells in the eutopic endometria of mice with endometriosis (Figure 3F and 3G).
In addition to ERβ, PHTPP can inhibit ERα activity in vivo, although its effects on ERα are minimal (Compton et al., 2004). To address this issue, the levels of mouse uterine ERα direct target genes (such as PR, CDKN1A and ERRFI1) were examined in ovariectomized mice upon E2 and/or PHTPP treatment. PHTPP partly reduced the expression of direct ERα target genes stimulated by E2 (Figure S2C, S2D and S2E). Interestingly, a female mouse fertility assay revealed that PHTPP did not reduce reproductive activity in female mice, whereas an ERα-selective antagonist, MPP dihydrochloride, significantly reduced the fertility of female mice compared with vehicle treatment (Figure S2F). Therefore, PHTPP does not disrupt the fertility of female mice, though it partly suppresses uterine ERα activity. In contrast to the effects of PHTPP, ERB-041 (ERβ-specific agonist) treatment enhanced the mouse ectopic lesion growth compared with vehicle (Figure S2G).
To address the effects of ERB-041 and PHTPP in human endometriotic lesion growth, we employed two types of human endometrial cells: EMosis-CC/TERT cells, which are immortalized human endometriotic epithelial cells isolated from ovarian endometriomas (Bono et al., 2012), and immortalized human endometrial stromal cells (iHESCs) (Krikun et al., 2004). For simplification, EMosis-CC/TERT cells are called immortalized human endometriotic epithelial cells (iHEECs) hereafter. For noninvasive bioluminescence imaging analyses of ectopic lesions in SCID mice, luciferase reporters expressing iHEECs (iHEECs/Luc) and iHESCs (iHESCs/Luc) were generated using a lentiviral expression system. To induce endometriosis, a mixture of epithelial and stromal cells (iHEECs/Luc plus iHESCs/Luc) was injected into ovariectomized SCID mice with an E2 pellet. On the 21st day after endometriosis induction, endometriosis-induced SCID mice were treated with ERB-041 or PHTPP for another 21 days (Figure S2A). Ectopic lesion image analyses revealed that ERB-041 treatment stimulated human ectopic lesion growth, whereas PHTPP treatment decreased ectopic lesion growth in SCID mice (Figure S2H). Moreover, ERBAI mice with endometriosis also revealed that ERB-041 enhanced ERβ activity in ectopic lesions compared with vehicle treatment (Figure S2I). Collectively, enhanced ERβ activity is required for the pathogenesis of endometriosis (Table 1).
Table 1.
Proliferation and apoptosis in ectopic lesions and eutopic endometrium of PHTPP-treated, ERβ−/− and ERβ:OE mice with endometriosis.
| Cellular Process | Type of endometrium |
Compartment | PHTPP | ERB−/− | ERB:OE |
|---|---|---|---|---|---|
|
Proliferative Activity |
Ectopic lesions | Epithelium | − | − | + |
| Stromal | 0 | 0 | + | ||
| Eutopic Endometrium |
Epithelium | − | 0 | + | |
| Stromal | − | 0 | + | ||
|
Apoptosis Signaling |
Ectopic lesions | Epithelium | + | + | − |
| Stromal | + | 0 | − | ||
| Eutopic Endometrium |
Epithelium | + | + | 0 | |
| Stromal | 0 | + | 0 | ||
| Ectopic lesion Volume | − | − | + | ||
(+: Increased, 0: No change, -:Decreased compared to vehicle treatment, wild type, or control mice)
Loss of ERβ function suppresses ectopic lesion growth in mice with endometriosis
To directly investigate the loss of ERβ function in the pathogenesis of endometriosis, endometriosis was surgically induced via the auto-transplantation of uterine tissue using ERβ−/−(Krege et al., 1998) and wild-type (WT) mice. The sizes of the ERβ−/− ectopic lesions were reduced significantly compared with WT ectopic lesions (Figure 4A). IHC using an ERβ antibody (Saji et al., 2000) validated the fact that ERβ−/− ectopic lesions did not exhibit ERβ expression compared with WT ectopic lesions (Figure 4B).
To investigate how loss of the ERβ function impacts ectopic lesion progression, cell proliferation and apoptotic signals in each type of ectopic lesion were examined. The reduced levels of epithelial, but not stromal, proliferation were detected in ERβ −/− ectopic lesions compared with WT ectopic lesions (Figure 4C and Table 1). In contrast to proliferation, however, loss of ERβ functions significantly elevated epithelial, but not stromal, apoptosis in ERβ−/− ectopic lesions (Figure 4D and Table 1).
Regarding eutopic endometrium, loss of ERβ function did not impair the proliferation of ERβ−/− eutopic endometria compared with WT eutopic endometria during endometriosis progression (Figure 4E and Table 1). However, apoptosis signaling was elevated in both compartments of the eutopic endometrium in the absence of the ERβ gene compared with WT eutopic endometrium (Figure 4F and Table 1).
Gain of ERβ function stimulates ectopic lesion growth in mice with endometriosis
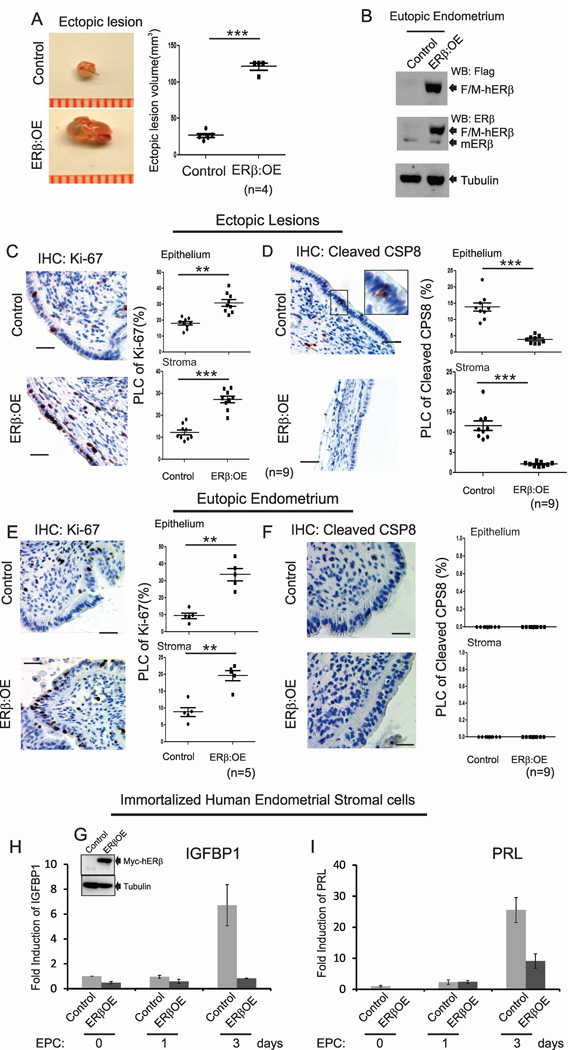
To mimic ERβ elevation in human and mouse endometriotic tissues, an endometrium-specific ERβ-overexpressing mouse model was generated and validated (Figure S3). For simplification, ROSALSL:ERβ/+ monogenic mice, which do not express exogen ous ERβ, and endometrium-specific ERβ-overexpressing (ROSALSL:ERβ/+:PRCRE/+) bigenic mice are hereafter referred to as control and ERβ:OE mice, respectively (Figure S3A).
To determine whether endometrium-specific ERβ overexpression impacts ectopic lesion growth, endometriosis was surgically induced by auto-transplantation using ovariectomized control and ERβ:OE mice containing E2 pellets. ERβ:OE ectopic lesions had much larger volumes than did control ectopic lesions in mice with endometriosis (Figure 5A). Exogenous Flag/Myc-tagged ERβ expression and elevated levels of total ERβ were determined in ERβ:OE ectopic lesions compared with control ectopic lesions (Figure 5B and S3). Thus, elevated ERβ levels in ectopic lesions enhanced ectopic lesion growth. The overexpression of nuclear receptors can induce ligand-independent effects (Weigel and Zhang, 1998). However, neither control nor ERβ:OE endometrial tissue fragments successfully developed into ectopic lesions in ovariectomized mice without the administration of an E2 pellet (Figure S3F). Therefore, gain-of-ERβ-function-mediated stimulation of ectopic lesion growth is an estrogen-dependent process.
Figure 5. The Gain of ERβ Function Stimulates Ectopic Lesion Growth.
(A) Ectopic lesions isolated from control and ERβ:OE mice with endometriosis.
(B) Exogenous Flag/Myc-tagged human ERβ (F/M-hERβ) protein levels in the eutopic endometria of control and ERβ:OE mice with endometriosis. mERβ, endogenous mouse ERβ.
(C–F) IHC and quantitative analyses Ki-67 (C and E) and cleaved CSP8 (D and F) in the epithelial and stromal compartments of ectopic lesions (C and D) and eutopic endometrium (E and F) of control and ERβ:OE mice with endometriosis. Higher magnification views of the boxed regions.
(G) Exogenous Myc-tagged human ERβ (Myc-hERβ) protein levels in iHESCs/ERβ as determined with a Myc antibody.
(H and I) The quantification of relative changes in the mRNA levels of decidualization marker genes, IGFBP1 (H) and PRL (I), in iHESCs (Control) and iHESCs/ERβ (ERβOE) upon estrogen/medroxyprogesterone/db-cAMP (ECP) treatment at the indicated day. See also Figures S3.
The proliferative activity was significantly elevated in both the epithelial and stromal compartments of ERβ:OE ectopic lesions compared with control ectopic lesions (Figure 5C and Table 1). However, apoptosis was significantly reduced in both the epithelial and stromal compartments of ERβ:OE ectopic lesions compared with control lesions (Figure 5D and Table 1). In addition to ectopic lesions, both the epithelial and stromal compartments of the ERβ:OE eutopic endometrium demonstrated enhanced proliferative activity compared with control eutopic endometrium (Figure 5E and Table 1). However, no alteration in apoptosis signaling was detected in ERβ:OE eutopic endometrium compared with control eutopic endometrium (Figure 5F and Table 1). Thus, the eutopic endometria of endometriosis patients appear primarily to be in a hyperproliferative state due to elevated ERβ levels. Notably, breeding trials designed to assess mating success revealed that ERβ:OE mice were infertile compared with control mice (Figure S3G). Moreover, ERβ-overexpressing immortalized human endometrial stromal cells (iHESCs) lose their decidualization response because the induction of decidual cell marker genes, such as insulin-like growth factor-binding protein 1 and prolactin, were significantly reduced upon estradiol-medroxyprogesterone-cAMP treatment compared with parental iHESCs (Figure 5G, 5H and 5I). Therefore, endometriosis-associated ERβ overexpression in eutopic endometrium might impair the decidualization process in women with endometriosis, leading to infertility.
ERβ interacts with the cytoplasmic apoptosis and inflammasome machinery in ectopic lesions to enhance ectopic lesion survival
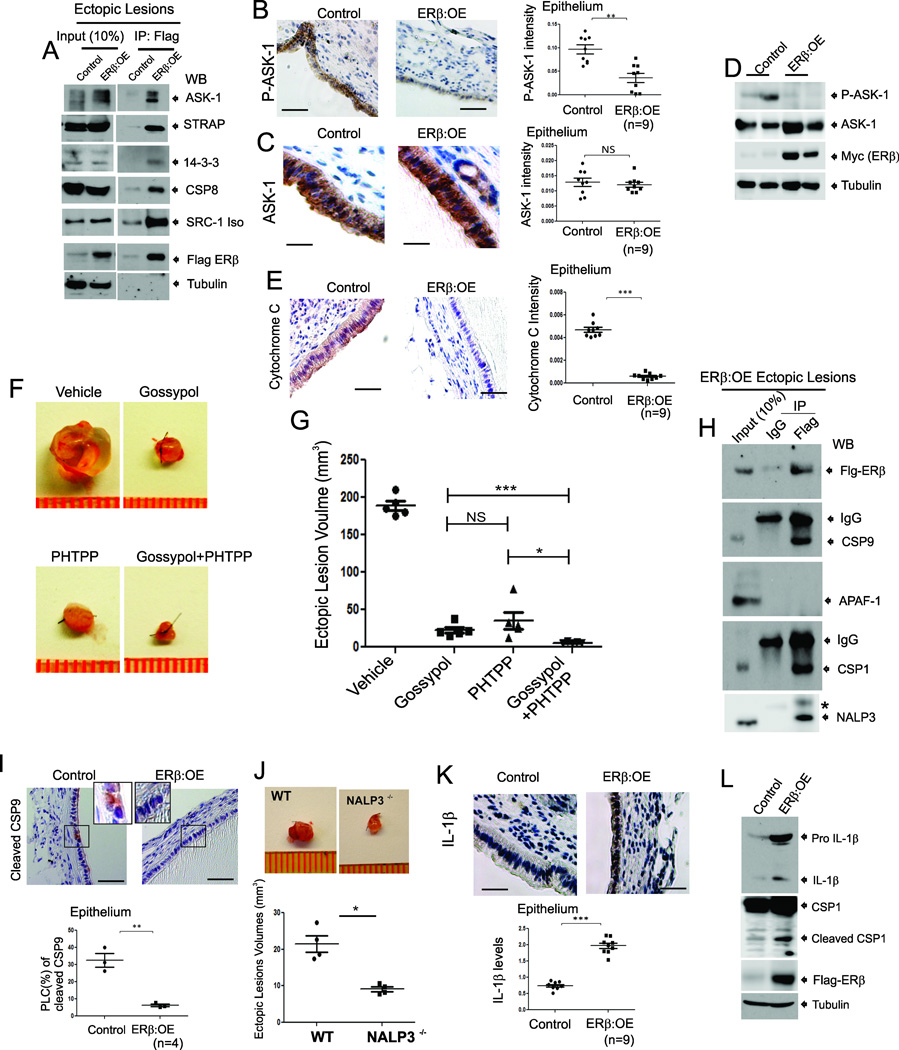
To further dissect the molecular mechanisms of ERβ in endometriosis progression, Flag-tagged ERβ-containing protein complexes were immunoprecipitated (IPed) from the eutopic endometria of ERβ:OE mice with endometriosis using a Flag antibody. In IP/Mass analyses, a primary consideration is to separate out proteins that non-specifically interact with beads from the list of proteins that are associated with the target protein. For this purpose, we employed control mice which had the same genetic background as ERβ:OE mice and had extremely low levels of Flag-tagged exogenous ERβ compared with ERβ:OE mice (Figure 6A). Therefore, proteins co-IPed with the Flag antibody from endometriotic tissues of control mice are considered as non-specific bead-binding proteins. To specifically identify ERβ-interacting proteins, proteins IPed from the eutopic endometria of control mice were removed from the proteins that IPed from the eutopic endometria of ERβ:OE mice. Gene Ontology analyses with endometriotic tissue-specific ERβ-interacting proteins revealed that large numbers of proteins involved in inflammation and apoptosis signaling were specifically co-IPed with ERβ from ERβ:OE eutopic endometrium (Figure S4A and S4B). To validate these interactions in ectopic lesions, the ERβ complex was isolated from control and ERβ:OE ectopic lesions using a Flag antibody, and ERβ-interacting proteins were analyzed further by Western blot analyses (Figure 6A). Western blot analyses revealed that only a very weak Flag-ERβ signal was detected in control ectopic lesions that developed in ROSALSL:ERβ/+ monogenic mice; this small amount is likely attributable to the leaky expression of exogenous ERβ in the ROSALSL:ERβ/+ monogenic mouse (Figure 6A).
Figure 6. ERβ Interacts with TNFα-induced Apoptosis Complexes and the Inflammasome in Endometriotic Tissues of Mice with Endometriosis.
(A) Flag-ERβ complexes immunoprecipitated (IPed) with a Flag antibody from ectopic lesions of Control and ERβ:OE mice with endometriosis followed by western blotting (WB) with antibodies against ASK-1, STRAP, 14-3-3, CSP8, SRC-1, Flag and tubulin.
(B–C) IHC and quantitative analyses of phospho-Thr845-ASK-1 (P-ASK-1) (B) and total ASK-1 (C) in Control and ERβ:OE ectopic lesions.
(D) Western blot analyses of phospho- Thr845-ASK-1 (P-ASK-1), total ASK-1, ERβ and tubulin in Control and ERβ:OE ectopic lesions.
(E) IHC and quantitative analyses of cytochrome C levels in Control and ERβ:OE ectopic lesions.
(F and G) Regression of ectopic lesion growth in endometriosis-induced C57BL/6J mice subcutaneously treated with Gossypol, PHTPP or their combination compared to vehicle (F). Quantification of ectopic lesion volume in panel F is shown in the graph (G).
(H) The IPed Flag-ERβ complex from ERβ:OE ectopic lesions with a Flag antibody or IgG followed by western blotting with antibodies against Flag, CSP 9, APAF1, CSP1 and NRLP3. *, Non-Specific Protein.
(I) IHC and quantitative analyses of cleaved CSP9 levels in Control and ERβ:OE ectopic lesions. Higher magnification views of the boxed regions.
(J) Ectopic lesions isolated from C57BL/6J (WT) and NALP3−/− mice with endometriosis.
(K) IHC and quantitative analyses of IL-1β levels in Control and ERβ:OE ectopic lesions.
(L) Western blot analyses of levels of IL-1β, CSP1, Flag-tagged ERβ and tubulin (as a protein loading control) in ectopic lesions of control and ERβ:OE mice with surgically induced endometriosis. See also Figure S4 and S5.
Apoptosis signal-regulating kinase 1 (ASK1) was found to interact prominently with ERβ in ectopic lesions (Figure 6A). ASK-1 is a component of TNFα-induced apoptosis complex I, and its activation is required for TNFα-induced apoptosis in multiple cell types (Tobiume et al., 2001). In addition to ASK-1, serine/threonine kinase receptor-associated protein (STRAP) and 14-3-3 were also specifically co-IPed with ERβ from the ERβ:OE ectopic lesions, but not from control ectopic lesions (Figure 6A). To prevent TNFα-induced apoptosis, STRAP and 14-3-3 proteins interact with ASK-1 to disrupt associations between TNF receptor-associated factor 2 (TRAF2) and ASK-1 upon TNFα stimulation (Hatai et al., 2000). These data imply that ERβ may induce ASK-1/STRAP/14-3-3 complex formation to prevent the activation of TNFα/ASK-1-mediated apoptosis in endometriotic tissues. To validate this hypothesis, the levels of ASK-1 phosphorylation at Thr845 were determined for each type of ectopic lesion because ASK-1 phosphorylation at Thr845 is associated with ASK-1 activation to enhance TNFα-induced apoptosis (Tobiume et al., 2002). The phospho-Thr845 ASK-1 levels were significantly reduced in ERβ:OE ectopic lesions compared with control ectopic lesions without alternation of total ASK-1 levels (Figures 6B, 6C and 6D). In contrast, the levels of total ASK-1 and phospho-ASK-1 were significantly elevated in ERβ−/− ectopic lesions compared with WT ectopic lesions (Figure S5A, S5B and S5C). Collectively, ERβ induced ASK-1/STRAP/14-3-3 complex formation to prevent ASK-1 activation in ectopic lesions, thereby promoting ectopic lesion survival. TNFα-induced ASK1 activation also increases mitochondrial cytochrome C levels to activate caspase 9 (Hatai et al., 2000). The cytochrome C levels in ERβ:OE ectopic lesions were significantly lower than those in control ectopic lesions (Figure 6E). Therefore, the gain of ERβ function prevented the TNFα/ASK-1/cytochrome C signaling pathway in ectopic lesions to promote lesion survival.
After the initiation of TNFα-induced apoptosis by apoptosis complex I, the Tumor Necrosis Factor Receptor (TNFR)1 -associated death domain (TRADD) protein, a component of complex I, is shuttled from TNFR to the cytoplasm and then interacts with the Fas-Associated via Death Domain (FADD) protein and caspase 8 to generate apoptosis complex II to amplify TNFα-induced apoptosis (Micheau and Tschopp, 2003). The endometriotic 70-kDa SRC-1 isoform also interacts with caspase 8 to inhibit caspase 8 activation in ectopic lesions to promote their survival (Han et al., 2012). Interestingly, we also found that ERβ interacted with caspase 8 and this SRC-1 isoform in ectopic lesions (Figures 6A and Figure S4C). Moreover, ERβ:OE ectopic lesions contained significantly reduced levels of cleaved caspase 8 compared with control ectopic lesions (Figure 5D). Therefore, we suggest that ERβ also interacts with caspase 8 along with the SRC-1 isoform; this combined interaction strongly inhibits caspase 8 activation in ectopic lesions to effectively prevent activation of TNFα-induced apoptosis complex II in ectopic lesions. However, this SRC-1 isoform/ERβ/caspase8 complex did not interact with components of TNFα-induced apoptosis complex I and the apoptosome in ectopic lesions (Figure S4D).
To validate synergism between the SRC-1 isoform and ERβ for the progression of endometriosis, a combination of Gossypol that reduces the transcriptional activity and stability of SRC-1 (Wang et al., 2011) and PHTPP was employed to suppress ectopic lesion growth in mice with endometriosis. This combination of Gossypol and PHTPP treatment significantly reduced ectopic lesion growth compared with individual treatments (Figures 6F and 6G). Therefore, cooperative interactions between the ERβ and SRC-1 isoforms effectively appear to drive the pathogenesis of endometriosis.
The cytochrome C effectively induces the formation of the apoptosome, which consists of caspase 9 and apoptotic peptidase-activating factor1 (APAF1), to activate caspase 9 (Bratton and Salvesen, 2010). In ERβ:OE ectopic lesions, the interaction of caspase 9 and APAF1 was not detected (Figures 6H and S5D). The cleaved caspase 9 levels were significantly reduced in ERβ:OE ectopic lesions compared with those in control ectopic lesions (Figures 6I). In ERβ−/− ectopic lesions, however, the interaction of caspase 9 and APAF-1 was detected (Figure S5D). These data suggest that ERβ prevented TNFα-induced apoptosome formation in endometriotic cells by disrupting the interaction of caspase 9 and APAF1 through a competitive ERβ interaction with caspase 9. Collectively, ERβ synergistically inhibited the activation of apoptosis complex I, complex II and apoptosome formation in ectopic lesions to effectively prevent TNFα-induced apoptosis in endometriotic tissues for ectopic lesions survival.
Caspase 1 and the NLR family pyrin domain-containing 3 (NALP3) were also co-IPed with ERβ from ERβ:OE ectopic lesions (Figure 6H and S4B). Both caspase 1 and NALP3 are components of the inflammasome, which is involved in the maturation of IL-1β formation from pro-IL-1β (Willingham et al., 2009). Interestingly, the NALP3-mediated inflammasome has an essential role in endometriosis progression because NALP3−/− ectopic lesion volume was significantly reduced compared with WT ectopic lesions of mice with endometriosis (Figure 6J). IL-1β is a key cytokine involved in both the adhesion and proliferation of endometrial cells (Cao et al., 2005; Sillem et al., 1999). ERβ:OE ectopic lesions had higher IL-1β levels than control ectopic lesions because cleaved caspase 1 levels were highly elevated in ERβ:OE ectopic lesions compared with controls (Figure 6K and 6L). However, cleaved caspase 1 and IL-1β levels were reduced in ERβ−/− ectopic lesions compared with WT lesions of mice with endometriosis (Figure S5A). Therefore, the combinational interactions of ERβ with caspase 1 and NRLP3, the activation of caspase 1, and the elevation of IL-1β levels in ectopic lesions supported our conclusion that ERβ also enhances inflammasome activity in ectopic lesions for their survival.
This Flag-ERβ interacting protein network may not accurately recapitulate the endogenous ERβ-interacting protein network in endometriotic tissues because this network was generated by overexpressed exogenous ERβ. To further address this issue, ERβ complexes were isolated from ectopic lesions of C57BL/6J mice with endometriosis because these ectopic lesions had elevated endogenous ERβ levels (Figure 1B). The SRC-1 isoform, caspase 8, caspase 9, caspase 1 and ASK-1 were also co-IPed with endogenous ERβ from ectopic lesions similar to exogenous Flag-ERβ, though the IP efficiency of ERβ antibody (SC-8794, Santa Cruz) is low (Figure S4E). Therefore, we concluded that the overexpressed ERβ complex is similar to the endogenous ERβ complex in endometriotic tissues.
Gain of ERβ function prevents TNFα-induced apoptosis and enhances proliferation, invasion and adhesion activities of immortalized human endometriotic epithelial cells
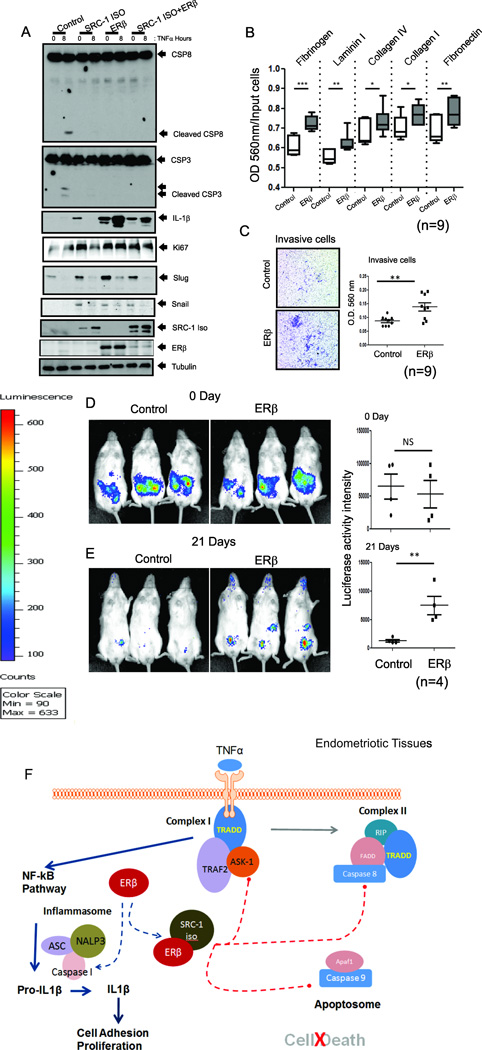
To investigate the functions of ERβ and the ERβ/SRC-1 isoform complex, iHEECs stably expressing ERβ (iHEECs/ERβ) and the SRC-1 isoform (iHEECs/SRC-1Iso) were generated separately and together (iHEECs/ERβ/SRC-1Iso) (Figure 7A, in bottom). TNFα treatment increased the levels of cleaved caspase 8 and cleaved caspase 3 in control iHEECs compared with vehicle (Figure 7A). However, TNFα treatment did not enhance the levels of the above apoptosis markers in iHEECs/ERβ, iHEECs/SRC-1Iso or in the combined iHEECs/SRC- 1Iso/ERβ (Figure 7A). Therefore, ERβ and ERβ/SRC-1 isoform complex effectively prevented TNFα-induced apoptosis. The gain of ERβ, but not the SRC-1 isoform, function elevated the IL-1β levels in iHEECs in the presence or absence of TNFα treatment (Figure 7A). These data were also generated via the artificial overexpression of ERβ. To support that gain of ERβ function is not artificial, primary human endometriotic stromal cells isolated from human endometriosis patients were employed because these cells also had elevated levels of endogenous ERβ compared with normal human endometrial stromal cells (Figure S4F). Primary human endometriotic stromal cells also have elevated levels of IL-1B and anti-apoptosis signaling upon TNFα treatment compared with normal endometrial stromal cells (Figure S4G). Therefore, ERβ plays a critical role in anti-apoptosis signaling and inflammasome activation in ectopic lesions. In addition, ERβ enhanced the cell adhesion and proliferative activities of iHEECs compared with control cells in the presence of TNFα (Figure 7A and 7B). Therefore, it is likely that the increased IL-1β observed with elevated ERβ induces adhesion and proliferation activities of endometrial tissue fragments in the peritoneal area of endometriosis patients to initiate ectopic lesion development. In addition to IL-1β, the levels of several cytokines (such as MIP-2, IL-16, MIP-1a, MCP-5, TREM-1 and BLC) were significantly elevated in ERB:OE ectopic lesions compared with control ectopic lesions (Figure S6A and S6C). Previous studies also revealed that levels of these cytokines are elevated in the peritoneal fluid of women with endometriosis (Ahn et al., 2015). In contrast, some cytokine levels (MIG, M-CSF, TNFα, KC and IP-10) were reduced in ERB:OE ectopic lesions compared to control ectopic lesions (Figure S6A and S6B). Collectively, the gain of ERβ function broadly alters the cytokine milieu in ectopic lesions in concert with promotion of endometriotic lesion growth. Consistent with SRC-1 isoform, ERβ also increased the expression of EMT markers, such as Slug and Snail, and enhanced invasion activity in iHEECs (Figures 7A and 7C). Therefore, the increased EMT and invasion activity of ectopic lesions again occurs through the effective cooperation of the ERβ and SRC-1 isoforms. However, vascular endothelial growth factor (VEGF) levels were not changed in ERβ:OE ectopic lesions and iHEECs/ERβ compared with their control (Figure S6D and S6E). Therefore, the angiogenesis of ectopic lesions is not regulated by ERβ.
Figure 7. Gain of ERβ Function Prevents TNFα-Induced Apoptosis Signaling but Stimulates Proliferation, Adhesion and Invasion Activities of Human Endometriotic Cells.
(A) Levels of cleaved CSP8, cleaved CSP3, IL-1β, Ki67, Slug, Snail, and SRC-1 isoform (determined by a Flag antibody), ERβ (determined using a Myc antibody) and tubulin in iHEECs (Control), iHEECs/SRC-1Iso (SRC-1ISO), iHEECs/ERβ (ERβ), or iHEECs/SRC-1Iso/ERβ (SRC-1ISO+ERβ) upon 50 ng/ml TNFα plus 10 µg/ml cycloheximide treatment for 0 and 8 hours.
(B) Cell adhesion activities of paternal iHEECs (Control) and iHEECs/ERβ (ERβ) against various extracellular matrices in the presence of 50 ng/ml TNFα.
(C) Invasion activities of iHEECs (Control) and iHEECs/ERβ (ERβ) for 2 days using a Transwell plate assay. The amounts of invasive cells in each group were determined using a crystal violet staining protocol and are shown in the graph.
(D–E) Bioluminescence and quantitative analyses of iHEECs/Luc (Control) and iHEECs/ERβ/Luc (ERβ) in SCID mice at 0(D) and 21(E) days after the induction of endometriosis.
(F) Working model for the non-genomic action of ERβ in endometriosis progression.
See also Figures S6 and S7.
To further support the gain of ERβ function in human ectopic lesion development, iHEECs/Luc, iHEECs/ERβ/Luc and iHESCs/Luc were employed for noninvasive bioluminescence imaging analysis of ectopic lesion growth in SCID mice. To induce endometriosis, a mixture of iHESCs/Luc plus iHEECs/ERβ/Luc was injected into ovariectomized SCID mice with an E2 pellet. For controls, a mixture of iHESCs/Luc and iHEECs/Luc was injected. Bioluminescence image analysis on injection day 0 revealed that similar amounts of human endometrial cells for each group were injected into recipient SCID mice (Figure 7D). Comparative bioluminescent analysis on the 21st day after injection revealed that human ectopic lesions with ERβ overexpression exhibited stronger bioluminescent activity compared with control ectopic lesions (Figure 7E). Therefore, ERβ enhanced the in vivo survival rate of human endometriotic cells and promoted their development into human ectopic lesions in SCID mice.
In addition to ERβ, ERα plays an essential role in the pathogenesis of endometriosis in the mouse model (Burns et al., 2012). To determine the functional difference between ERα and ERβ in endometriosis progression, iHEECs expressing Myc-tagged human ERα genes (iHEECs/ERα) were generated (Figure S7A). In contrast to iHEECs/ERβ, however, gain of ERα function did not prevent TNFα-induced apoptosis signaling and not induce IL-1β expression, proliferative activity, expression of EMT markers (Slug and Snail) and VEGF in iHEECs/ERα compared with parental iHEECs upon TNFα treatment (Figure S7A). Unlike ERβ, therefore, ERα is not involved directly in the evasion of immune surveillance or in the invasion and IL-1β mediated proliferation of ectopic lesions. For the combination of ERα and ERβ, ERα did not interfere with ERβ-mediated anti-apoptotic activity in IHEECs upon TNFα treatment (Figure S7B). In fact, ERα inhibited ERβ-mediated IL-1β production (Figure S7B). Therefore, ERα might be involved in the negative regulation of ERβ -mediated inflammasome activation.
Taken together, the gain of ERβ and SRC-1 isoform function in endometrial fragments generated by retrograde menstruation prevents TNFα-induced apoptosis complex activity to evade immune surveillance. After evasion, ERβ interacts with the inflammasome complex to induce IL-1β in endometrial fragments that have evaded immune surveillance to facilitate attachment to and growth at target sites (Figure 7F). In addition, ERβ also induces EMT and invasion activity in corporation with the SRC-1 isoform to establish endometriotic lesions (Figure 7F).
Discussion
ERβ has non-genomic action for anti-apoptosis and inflammasome activation
The physiological effects of estrogen are mediated by estradiol binding to one of ER isoform, ERα and ERβ. Estrogen-liganded ER isoform then binds to specific DNA sequence called estrogen response elements. Interestingly, phenotype analyses of ERα(−/−), ERβ(−/−), ERα(−/−):ERβ(−/−) bigenic mouse models have revealed that ER isoforms have overlapping but also unique roles in estrogen-dependent action in vivo (Walker and Korach, 2004). For their unique function, ERα and ERβ have different transcriptional activities in certain ligand, cell-type, and promoter contexts. In case of endometriotic tissues, both ER isoforms are expressed in endometriotic tissues and required for endometriotic lesions growth. The gain of ERβ function study revealed that ERβ prevents apoptosis singling and enhances adhesion, invasion, proliferation, inflammasome activity and inflammation signaling in ectopic lesions for their growth. The study with ERα(−/−) mice with endometriosis revealed that ERα drives proliferation, adhesion and angiogenesis and also modulates inflammation signaling in ectopic lesions (Burns et al., 2012). To establish endometriotic lesions, collectively, both ERα and ERβ might synergistically contribute the regulation of proliferation, adhesion and inflammation signaling in ectopic lesions. However, ERα mainly drives angiogenesis and ERβ has a predominant role in anti-apoptosis and activation of inflammasome and invasion in ectopic lesions for their survival.
Based on a retrograde menstruation model for endometriosis (Hughesdon, 1958), endometrial tissue and erythrocytes are shed through the fallopian tubes into the peritoneal cavity during menses. In healthy women, refluxed endometrial fragments that appear during retrograde menstruation are cleared by inflammatory mediated cell-death signaling, such as caspase-1-mediated pyroptosis (Miao et al., 2011). However, endometriosis patients have an immunity that prevents them from clearing the refluxed endometrial fragments and then potentiates the development and severity of endometriosis. For survival, endometrial tissue fragments must evade the immune surveillance system, particularly peritoneal macrophages (Nasu et al., 2009). During the early steps of evasion from the immune surveillance system, ERβ generates a cytoplasmic protein network to rapidly prevent TNFβ-mediated apoptosis by inactivating TNFα-induced apoptosis complex I and II and the apoptosome. We believe that a key synergism exists between ERβ and the SRC-1 isoform during this evasion of the immune surveillance system because the SRC-1 isoform also prevents TNFα-induced apoptosis in ectopic lesions. Our combined observations lead us to propose that ERβ and the SRC-1 isoform act cooperatively together to affect a potent anti-apoptotic state in endometriotic tissues.
The formation of the inflammasome and the activity of caspase-1 determine the balance between pathogen resolution and disease pathology. How is the inflammasome involved in the pathogenesis of endometriosis? The NALP3 gene has an essential role in endometriosis progression because NALP3−/− mice have a defect in ectopic lesion growth under endometriosis. ERβ involves in upregulation of NALP3 inflammasome in hepatocellular carcinoma cells upon estrogen stimulation even though the interaction of ERβ to NALP3 is not clearly demonstrated (Wei et al., 2015). Here, we revealed that ERβ interacts with inflammasome components and enhances inflammasome activity through the activation of caspase 1 activity. Activation of the inflammasome results in highly elevated IL-1β levels in endometriotic tissues compared with normal endometrium, and enhanced IL-1β signaling can influence the adhesion activity of endometriotic tissues and proliferative activities of human endometrial cells.
The gain of ERβ function may lead to female infertility
One of the primary symptoms associated with endometriosis is dysfunction of the normal endometrium, leading to endometriosis-associated infertility (Holoch and Lessey, 2010). In addition to ectopic lesions, we found that eutopic endometrium demonstrated elevated levels of ERβ compared with normal endometrium. We believe that ERβ overexpression could increase endometriosis-associated infertility by preventing the decidualization response in the stromal compartment of eutopic endometrium. Thus, targeting ERβ could have dual potential benefits in patients with endometriosis: regression of ectopic lesion growth and enhancement of fecundity of women with endometriosis.
A combination therapy using antagonists of ERβ and the SRC-1 isoform represents a proof-of-principle for the next generation of endometriosis therapy
Inhibitors of estrogen signaling and estrogen synthesis as well as inflammatory inhibitors (COX-2 inhibitors) have been employed, given the dependence on estrogen and the inflammatory response of ectopic lesions. However, these treatments can be associated with undesirable side effects. In addition to substantiating an infertile state in young women, long-term estrogen deficiency therapies can have harmful side effects on other estrogen target tissues, such as the brain and bone (Shah et al., 1987; Vanderschueren et al., 1997). Therefore, a greater choice of alternate therapies that more specifically target endometriotic causal modes is needed.
Our observations proposed that the targeting ERβ activity should increase the specificity and efficiency of endometriosis treatment and could be an alternative combinational approach for endometriosis treatment in lieu of current estrogen-deficiency therapy. As a proof of principle, the application of an ERβ-selective antagonist, such as PHTPP, significantly suppressed ectopic lesion growth by inhibiting ERβ activity in ectopic lesions of mice with endometriosis without side effects on fertility. The minimal inhibitory effects of PHTPP against uterine ERα could be also another advantage to minimize side effects. We note that a previous study stated that ERB-041, an ERβ-specific agonist, caused regression of ectopic lesion growth in an endometriosis animal model system (Harris et al., 2005). The reason for this discrepancy could potentially be related to differential ERβ expression in ectopic lesions. The expression of ERβ was not detected in human ectopic lesions that developed in athymic nude mice in Harris’ study. PHTPP treatment demonstrated certain differential effects in endometriotic tissues compared with ERβ knockout tissues. For example, proliferation of eutopic endometrium and apoptosis in the stromal compartment of ectopic and eutopic endometrium were differentially regulated between them. This differential regulation might be attributable to the differences between pharmacological inhibition and genetic knockout.
Collectively, we propose that the SRC-1 isoform/ERβ complex could be a next-generation endometriosis therapeutic target with reduced side effects compared to current endometriosis treatment because 1) ERβ and the SRC-1 isoform show endometriotic tissue-specific expression but have little expression in normal endometrium; 2) both play an essential role in the early stages of endometriosis pathogenesis; and 3) targeting both of these drivers allows the marked suppression of ectopic lesion growth in animals compared with either individual agent alone.
Experimental Procedures
Mouse information
Five-week-old normal (C57BL/6J), ERβ−/− (B6;129P2-Esr2tm1Unc/J), NALP3−/− (B6.129S6-Nlrp3tm1Bhk /J) and SCID (NOD.CB17-Prkdcscid/J) mice were purchased from Jackson Laboratory. ROSALSL:ERβ/+ and ERBAI mice were generated. The ROSALSL:ERβ/+ :PRCre/+ mice were generated by crossing ROSALSL:ERβ/+ with PRCre/+ mice (Soyal et al., 2005). All animal care was controlled by the ethical regulations approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Immortalized human endometrial cells
Immortalized human endometrial stromal cells (iHESCs) and EMosis-CC/TERT1 (immortalized human endometriotic epithelial cells) were employed and confirmed by Short Tandem Repeat profiling; these cells were not contaminated with mycoplasma.
Surgically induced endometriosis
Endometriosis in mice was surgically induced under aseptic conditions under anesthesia. Details on surgically induced endometriosis are found in the Supplemental Experimental Procedures.
Generation of ERBAI and ERβ:OE mice
Details on these mice are found in in the Supplemental Experimental Procedures.
In vivo analysis of human ectopic lesion growth in SCID mice
The bioluminescence image of human ectopic lesions developed with IHESCs/Luc plus IHEECs/Luc (or IHEECs/ERβ/Luc) in SCID mice were determined. Details on this are found in the Supplemental Experimental Procedures.
For basic procedures, see the Supplemental Experimental Procedures.
Statistical Analyses
The data are expressed as the mean ± s.d. Significance was assessed using an independent two-tailed Student’s t test; A P value of less than 0.1 was considered statistically significant. NS, non-specific. * P<0.1, ** P<0.01, *** P<0.005 by Student’s t test.
Supplementary Material
Acknowledgements
This work was supported by grants from the US National Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD0077495 and 5K12HD050128 to S.M.H. U54HD007495 to F.D., and R01 HD082786, R01 HD07857, R01 HD08188 and Clayton Fdn. to B.W.O., U54HD007495 pilot grant to S.J.H., R01 HD-042311 to J.P.L), a grant from NIDDK (U19 DK62434 to M.-J.T, S.Y.T and F.J.D.) and a grant from CPRIT (CPRIT RPI10471 to M.-J.T. and S.Y.T). We also thank CPRIT (RP120092) Facilities Support Award and NCI P30 CA123125 Cancer Center Support Grant for the Proteomics Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
S.J.H. led the project and designed and performed most of the experiments. S.Y.J., MJ.P. and J.Q. provided technical expertise. S.M.H., and S.K. provided the human endometrial cells. S.-P.W, S.Y.T., M.-J.T, J.P.L; and F.J.D generated the mouse models and supervised data evaluation. B.W.O. supervised the entire project and data evaluation, and SJ.H. and B.W.O wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- Acien P, Velasco I, Gutierrez M, Martinez-Beltran M. Aromatase expression in endometriotic tissues and its relationship to clinical and analytical findings. Fertil Steril. 2007;88:32–38. doi: 10.1016/j.fertnstert.2006.11.188. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed research international. 2015;2015:795976. doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono Y, Kyo S, Takakura M, Maida Y, Mizumoto Y, Nakamura M, Nomura K, Kiyono T, Inoue M. Creation of immortalised epithelial cells from ovarian endometrioma. British journal of cancer. 2012;106:1205–1213. doi: 10.1038/bjc.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. Journal of cell science. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. The New England journal of medicine. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-beta in endometriosis. Seminars in reproductive medicine. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO. The genetics and biochemistry of endometriosis. Current opinion in obstetrics & gynecology. 2013;25:280–286. doi: 10.1097/GCO.0b013e3283630d56. [DOI] [PubMed] [Google Scholar]
- Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153:3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WG, Morin M, Metz C, Maheux R, Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol Reprod. 2005;73:565–570. doi: 10.1095/biolreprod.104.038331. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Licence D, Cook E, Luo F, Arends MJ, Smith SK, Print CG, Charnock-Jones DS. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. The Journal of pathology. 2011;224:261–269. doi: 10.1002/path.2852. [DOI] [PubMed] [Google Scholar]
- Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor beta antagonist activity. Journal of medicinal chemistry. 2004;47:5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- Delvoux B, Groothuis P, D’Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17beta-estradiol in endometriosis lesions is the result of impaired metabolism. The Journal of clinical endocrinology and metabolism. 2009;94:876–883. doi: 10.1210/jc.2008-2218. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O’Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nature medicine. 2012;18:1102–1111. doi: 10.1038/nm.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, O’Malley BW. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Human reproduction update. 2014;20:467–484. doi: 10.1093/humupd/dmu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, O’Malley BW, DeMayo FJ. Genesis. Vol. 47. New York, NY: 2009. An estrogen receptor alpha activity indicator model in mice; pp. 815–824. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Human reproduction. 2005;20:936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Holoch KJ, Lessey BA. Endometriosis and infertility. Clinical obstetrics and gynecology. 2010;53:429–438. doi: 10.1097/GRF.0b013e3181db7d71. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Endometriosis and retrograde menstruation; case report and review. J Obstet Gynaecol Br Emp. 1958;65:944–953. doi: 10.1111/j.1471-0528.1958.tb08588.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine reviews. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A Novel Immortalized Human Endometrial Stromal Cell Line with Normal Progestational Response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Monsivais D, Dyson MT, Yin P, Coon JS, Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, et al. ERbeta- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Molecular endocrinology. 2014;28:1304–1315. doi: 10.1210/me.2013-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women’s lives: a qualitative study. BMC Womens Health. 2014;14:123. doi: 10.1186/1472-6874-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu K, Yuge A, Tsuno A, Nishida M, Narahara H. Involvement of resistance to apoptosis in the pathogenesis of endometriosis. Histology and histopathology. 2009;24:1181–1192. doi: 10.14670/HH-24.1181. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Annals of the New York Academy of Sciences. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, Fiche M, Canny GO. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertility and sterility. 2012;98:1200–1208. doi: 10.1016/j.fertnstert.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci U S A. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmassi A, Acar-Perk B, Schmutzler AG, Koch K, Pungel F, Jonat W, Mettler L. Apoptosis resistance in endometriosis. BioImpacts : BI. 2011;1:129–134. doi: 10.5681/bi.2011.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Roberts T, McQueen IN, Graham JG, Walker K. Danazol and benign intracranial hypertension. British medical journal (Clinical research ed) 1987;294:1323. doi: 10.1136/bmj.294.6583.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillem M, Prifti S, Monga B, Arslic T, Runnebaum B. Integrin-mediated adhesion of uterine endometrial cells from endometriosis patients to extracellular matrix proteins is enhanced by tumor necrosis factor alpha (TNF alpha) and interleukin-1 (IL-1) Eur J Obstet Gynecol Reprod Biol. 1999;87:123–127. doi: 10.1016/s0301-2115(99)00114-1. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Genesis. Vol. 41. New York, NY: 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor; pp. 58–66. 2000. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO reports. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, van Herck E, Nijs J, Ederveen AG, De Coster R, Bouillon R. Aromatase inhibition impairs skeletal modeling and decreases bone mineral density in growing male rats. Endocrinology. 1997;138:2301–2307. doi: 10.1210/endo.138.6.5216. [DOI] [PubMed] [Google Scholar]
- Walker VR, Korach KS. Estrogen Receptor Knockout Mice as a Model for Endocrine Research. ILAR Journal. 2004;45:455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O’Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011;25:2041–2053. doi: 10.1210/me.2011-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, et al. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Laboratory investigation; a journal of technical methods and pathology. 2015;95:804–816. doi: 10.1038/labinvest.2015.63. [DOI] [PubMed] [Google Scholar]
- Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med (Berl) 1998;76:469–479. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biology of reproduction. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB-dependent pathway. Molecular and cellular endocrinology. 2010;317:31–43. doi: 10.1016/j.mce.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Science translational medicine. 2015;7 doi: 10.1126/scitranslmed.3010626. 271ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.