Abstract
Traumatic brain injury (TBI) is a multifaceted injury and a leading cause of death in children, young adults, and increasingly in Veterans. However, there are no neuroprotective agents clinically available to counteract damage or promote repair after brain trauma. This study investigated the neuroprotective effects of normobaric oxygen (NBO) after a controlled cortical impact in rats. The central hypothesis was that NBO treatment would reduce lesion volume and functional deficits compared with air-treated animals after TBI by increasing brain oxygenation thereby minimizing ischemic injury. In a randomized double-blinded design, animals received either NBO (n=8) or normal air (n=8) after TBI. Magnetic resonance imaging (MRI) was performed 0 to 3 hours, and 1, 2, 7, and 14 days after an impact to the primary forelimb somatosensory cortex. Behavioral assessments were performed before injury induction and before MRI scans on days 2, 7, and 14. Nissl staining was performed on day 14 to corroborate the lesion volume detected from MRI. Contrary to our hypothesis, we found that NBO treatment increased lesion volume in a rat model of moderate TBI and had no positive effect on behavioral measures. Our results do not promote the acute use of NBO in patients with moderate TBI.
Keywords: MRI, normobaric oxygen, mitochondria, oxidative stress, TBI, vasogenic edema
Introduction
Traumatic brain injury (TBI) as a result of a physical blow to the head is a leading cause of death world-wide.1 Each year in the United States alone, at least 1.7 million people have TBI and an estimated 3.2 to 5.3 million people live with the long-term physical, cognitive, and psychological health disabilities of TBI, with annual direct and indirect costs estimated at over $60 billion.2 Traumatic brain injury can range from a mild to severe injury and is classically characterized using the Glascow coma scale.3 Despite the tremendous efforts invested in understanding the pathophysiology of TBI and testing new treatments, the ability to minimize neurologic damage remains extremely limited. There are currently no neuroprotective drugs to treat TBI.
Traumatic brain injury is a progressive injury categorized into two phases: primary and secondary injury. The initial physical impact results in damage to blood vessels, axonal shearing, and contusions to the brain. Little can be done to prevent the effects of the primary injury after TBI and therefore the majority of studies focus on the secondary injury phase. In the hours to days after the primary injury there is a cascade of secondary injuries that are an indirect result of the initial impact and include mitochondrial dysfunction, neuronal degeneration, inflammation, blood brain barrier dysfunction, tissue hypoxia, ischemia, and edema formation.4, 5 These secondary injuries can ultimately lead to progressive cell death and impaired functional outcomes and is dependent on the location of injury.
Cerebral hypoxia, a condition of inadequate oxygen supply in the brain, has been shown to have a critical role in the cascade of events leading to cell death after brain trauma.6 After a head injury, many patients present with intracranial hypertension that decreases cerebral perfusion pressure, resulting in impaired oxygen delivery to the tissue.7, 8 The result of decreased O2 delivery, due to decreased cerebral blood flow (CBF) and by reduced O2 diffusion to cells, leads to inefficient maintenance of oxidative cerebral metabolism and edema formation.9, 10, 11 Anaerobic metabolism takes over,12 but it is relatively inefficient and results in the depletion of cellular energy, mitochondrial dysfunction, and prolonged hypometabolism.13, 14, 15 However, it is believed that the degree to which cerebral oxidative metabolism is restored correlates with clinical outcome.15 Therefore, means to increase tissue oxygenation could help to normalize aerobic metabolism thereby promoting increased survival of neural tissue.
It is thus reasonable to hypothesize that oxygen therapy could improve oxidative metabolism. Several studies have shown that normobaric oxygen (NBO) therapy increases brain tissue PO2.16, 17 Further studies show that NBO decreases microdialysate lactate levels, which likely indicates improved tissue oxygenation.16, 17, 18 However, it is possible that NBO is not beneficial at all phases of TBI progression. The clinical potential of NBO treatment after TBI has yet to be demonstrated. Normobaric oxygen is also cost effective and is a potentially attractive therapy because of its ease of administration by emergency responders onsite. It can potentially be used to ‘buy' time and expand the treatment time window in TBI patients. Additionally, NBO could be used in combination with other treatments.
The goal of this study was to investigate the neuroprotective effect of NBO therapy after moderate TBI in rats. We used a controlled cortical impactor to induce a moderate open-skull TBI in the primary forelimb somatosensory cortex. Magnetic resonance imaging (MRI) was used to longitudinally measure lesion volume, T2 relaxation time constant, CBF, apparent diffusion coefficient and fractional anisotropy. Behavioral outcomes were assessed using the forelimb asymmetry placement and forelimb foot-fault tests. We hypothesized that NBO treatment decreases lesion volume and improves functional outcomes after TBI.
Materials and methods
Animal Preparations
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and were performed according to the IACUC, NIH, and ARRIVE guidelines. Male Sprague Dawley rats (3 to 5 months of age, 250 to 350 g, n=14) were anesthetized initially with 5% isoflurane mixed with room air and maintained using 1.2% isoflurane throughout all surgical and imaging procedures. The animal was secured in a stereotaxic frame and a Ø5-mm craniotomy was created over the left primary forelimb somatosensory cortex (S1FL: +0.25 mm anterior and 3.5 mm lateral to bregma), exposing the dura matter. The intact dura matter was impacted using a pneumatic controlled cortical impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA, USA) fitted with a Ø3-mm tip (5.0 m/s, 250 μs dwell time, 1 mm depth) to mimic a moderate focal TBI. The cranial opening was gently sealed with bone wax after the impact, and the scalp sutured closed and antibiotic ointment applied. The animal was subsequently moved into the MRI scanner for imaging while remaining under 1.2% isoflurane. Buprenex (0.05 mg/kg) was given subcutaneously every 12 hours for 3 days for pain.
In a randomized double-blinded design, air (n=7) or oxygen (n=7) was administered via face mask (100% FiO2) from 0.5 to 3.5 hours after TBI. Magnetic resonance imaging was performed on the day of the TBI procedure (1 to 3 hours after TBI), and 1, 2, 7, and 14 days after TBI. The physiology of each animal was monitored continuously using the MouseOx system (Starr Life Sciences Corp., Oakmont, PA, USA) during both surgery and all MRI procedures.
Behavioral assessments were made 1 to 3 days before TBI and again 1, 2, 7, and 14 days after TBI before MRI acquisition in the same animals. Behavioral tests were not performed on the day of TBI induction due to incomplete recovery from anesthetic. Nissl staining was performed after MRI on day 14 after TBI to verify the lesion volume seen on MRI. The 14-day end point was selected based on a subset of studies in which no apparent differences in lesion volume between 14 and 28 days after TBI were observed.
Magnetic Resonance Imaging
Magnetic resonance imaging (Bruker Biospec 7-Tesla/30 cm) was performed to longitudinally monitor lesion volume. The animal was anesthetized with 1.2% isoflurane and secured in an MRI-compatible stereotaxic holder with ear and tooth bars. A transceiver surface coil of 2 cm in diameter was placed on top of the rat's head.
Cerebral blood flow map (6 minutes)
Perfusion-weighted images were attained using the continuous arterial spin labeling19 technique with a single-shot, gradient-echo, echo-planar imaging sequence with partial Flourier (5/8) acquisition. Continuous arterial spin labeling used a 2.7-second square radio frequency pulse to the labeling coil. The other parameters were seven 1.0-mm coronal images, field of view=2.56 × 2.56 cm, matrix 96 × 96 reconstructed to 128 × 128, field of view=2.56 × 2.56 cm, repetition time=3 seconds (90° flip angle), echo time=10.2 ms, and 60 repetitions.
Diffusion tensor-weighted magnetic resonance imaging (3.5 minutes)
Diffusion tensor images were obtained with a single low b value (10 s/mm2) in 30 directions with a 1,200 s/mm2 bmax value. Echo-planar imaging scans with partial Fourier (5/8) were also acquired using the following settings: seven 1.0-mm coronal images, field of view=2.56 × 2.56 cm, matrix 96 × 96 reconstructed to 128 × 128, single shot, repetition time=3 seconds, echo time=32 ms, Δ=14 ms, δ=5 ms, and 2 transients for signal averaging.
T2 map (9.5 minutes)
T2-weighted images were acquired using a fast spin-echo sequence with repetition time=3 seconds (90° flip angle), effective echo time=18, 54, 90, and 126 ms, 4 echo train lengths, where the center of the echo train was taken as the effective echo time for the T2 calculation. The other parameters were seven 1.0-mm coronal images, field of view=2.56 × 2.56 cm, matrix 96 × 96 reconstructed to 128 × 128, and 8 transients for signal averaging. The contralateral hemisphere T2 was used as the internal control (note that although there are changes in the molecular or cellular levels in the contralateral hemisphere, these changes did not alter T2 values).19
Image Analysis
Cerebral blood flow, T2, ADC, and fractional anisotropy maps were calculated as previously described.20 Images were coregistered across time points using QuickVol and MRIAnalysisPak software.20 The lesion volumes were determined using Stimulate software based on established procedures used in our previous stroke and TBI studies.21, 22, 23 Briefly, lesion volume was determined using a threshold region of interest (ROI) approach where an ROI was drawn in the contralateral S1 cortex to determine the mean value of intensity. This value plus 2 standard deviations was used as a threshold value. An ROI was then drawn in the S1 region on the ipsilesional side around the highlighted region. The number of pixels within the ipsilesional region was then counted and constituted the lesion volume. This process was repeated for all time points and all subjects. The ROIs used to determine T2 lesion volumes were also used to tabulate the CBF, T2, ADC, and fractional anisotropy values across all time points.23
Functional Assessment
Sensorimotor function was assessed using the asymmetry forelimb placement (cylinder) test and foot-fault test 1 to 3 days before TBI and again 1, 2, 7, and 14 days after TBI as described previously.22 The forelimb asymmetry placement test was performed with videotaping to assess the use of forelimbs. The rat was placed in a transparent cylinder (20 cm diameter, 30 cm height) for 5 minutes or until 30 placements were made. A mirror was positioned under the cylinder to enable the video recorder to see directly into the cylinder. The behavior was scored by counting the number of left or right individual forelimb placements, and the number of simultaneous right and left forelimb placements onto the wall of the cylinder during rearing. The forelimb asymmetry index was calculated as (the number of forelimb placements for each individual limb)+½(number of both placements) divided by the total number of placements.
The foot-fault test was performed with videotaping to assess limb misplacement during locomotion. The rat was placed on an elevated grid floor (e.g., size 18 in × 11 in with grid openings of ~1.56 in2 and 1 in2) for 5 minutes or until 50 steps were taken with one (right hind) limb. The rat was allowed to move freely on the grid and the total number of steps and the number of times each limb fell below the grid opening were counted. The percentage of foot faults for each limb was calculated as the number of right or left forelimb or hind-limb foot faults divided by the total number of steps taken.
Nissl Staining
Nissl staining was used to assess morphologic changes and final lesion volume.23, 24 Anesthetized rats were perfused with ice-cold heparinized phosphate-buffered saline, followed by ice-cold 4% buffered paraformaldehyde on day 14 after TBI. Brains were removed and fixed for 2 to 5 hours at 4°C and subsequently cryopreserved in 30% sucrose for 48 hours. Coronal sections (25-μm thick) were cut on a cryostat and affixed to gelatin-coated slides and dried overnight at 37°C. Slides were hydrated through a series of graded alcohols to distilled water followed by 0.1% cresyl violet acetate for 7 minutes. Brain sections were then dehydrated, cleared in xylene, and coverslipped with mounting medium.
Images were acquired on an Olympus BX60F microscope (Olympus Corporation, Tokyo, Japan). A 10 × objective was used to acquire images for mosaic full brain images assimilated using Microsoft ICE software (Microsoft Corporation, Redmond, WA, USA). Four slices from each brain were selected for analysis by determining corresponding slices to the images acquired from T2 MRI using stereotaxic coordinates. The slices were 1 mm apart to ensure adequate sampling throughout the lesioned area. Results were expressed as the average lesion volume for each animal.
Statistical Analysis
Unpaired t-tests were used to compare T2 and Nissl defined lesion volumes between vehicle- and NBO-treated groups. Unpaired t-tests were also used to compare the percent differences of CBF, T2, ADC, and FA abnormality of the ipsi- and contra-lesional sides between the between vehicle- and NBO-treated groups. Mann–Whitney U tests were used to compare differences in asymmetrical limb use and the percentage of foot faults between vehicle- and NBO-treated animals. Values are presented as mean±s.e.m. Statistical significance was set at P<0.05.
Results
Normobaric Oxygen Worsened Lesion Volume
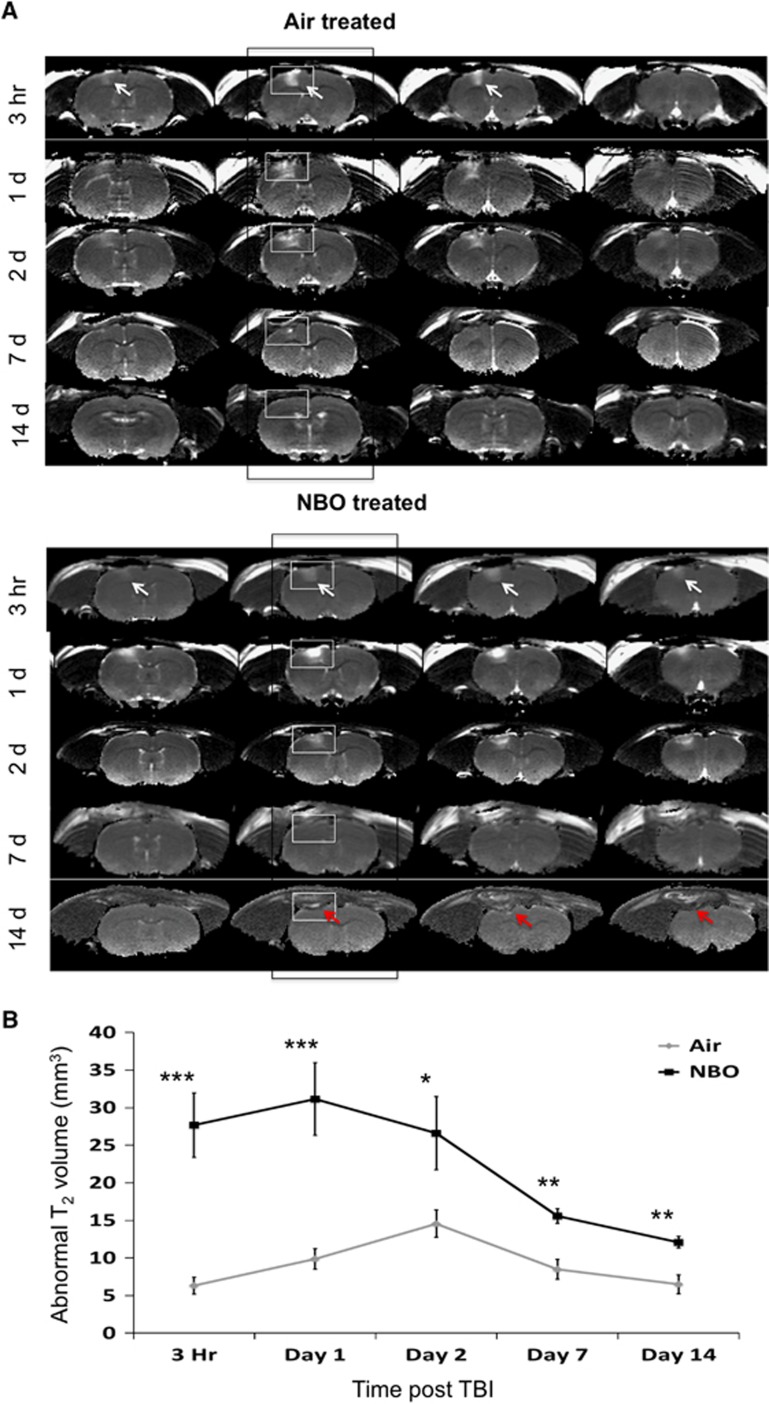
Multislice T2 maps at 3 hours, and 1, 2, 7, and 14 days after TBI for both air- and NBO-treated animals are shown in Figure 1A. The hyperintense area in the S1FL cortex (arrows) indicates the lesion. Magnetic resonance imaging lesions became apparent 3 hours after TBI in the air-treated group. The air-treated animals had a lesion present spanning three slices, while the NBO animals had lesions covering up to four slices as indicated by the white arrows. Additionally, NBO-treated animals had an obvious loss of tissue by day 14 as noted by the red arrows (Figure 1A). In the air-treated group, the abnormal T2 volume peaked on day 2, and decreased by day 14 after TBI (Figure 1B). In the NBO-treated group, by contrast, abnormal T2 volumes remained significantly higher than the air-treated group at all time points studied, also peaking on day 2 after TBI. These results indicate that NBO treatment increased TBI lesion volume and vasogenic edema.
Figure 1.
T2 magnetic resonance imaging (MRI). (A) Representative T2 maps are shown for air- and normobaric oxygen therapy (NBO)-treated animals at 3 hours, and 1, 2, 7, and 14 days after traumatic brain injury (TBI). The arrows indicated hyperintense lesions in the ipsilesional cortex. (B) Line graphs show the progression of the abnormal T2 volumes in NBO- and air-treated animals (mean±s.e.m., n=7 each group, *P<0.05; **P<0.01; ***P<0.001).
Normobaric Oxygen Effects on Magnetic Resonance Imaging Measured Parameters
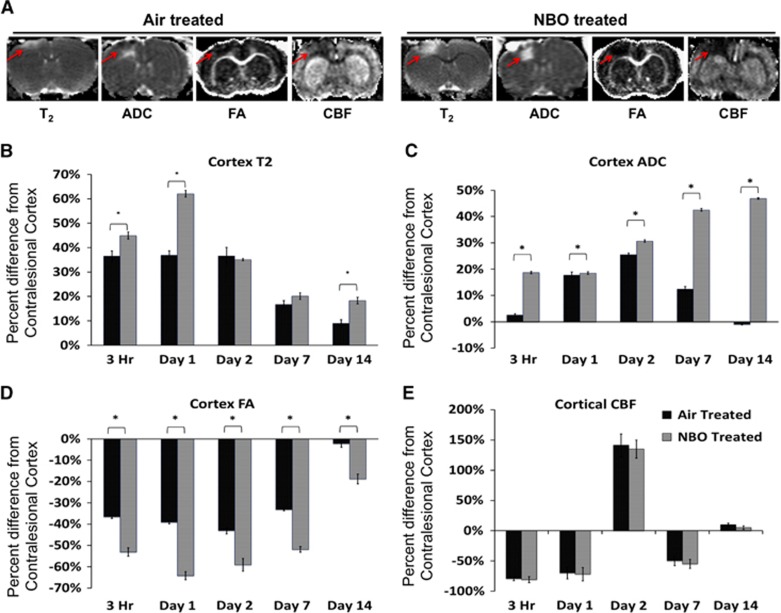
T2, ADC, FA, and CBF maps of air- and NBO-treated TBI animals on day 2 after TBI revealed variations in the detection of lesion volumes (Figure 2A, red arrows).
Figure 2.
(A) Representative cerebral blood flow (CBF), T2, apparent diffusion coefficient, and fractional anisotropy maps are shown for air- and normobaric oxygen (NBO) therapy-treated animals 2 days after traumatic brain injury (TBI). The lesioned area is indicated by the red arrows. (B) Bar graph showing the percent difference in ipsilesional cortex CBF values from contralesional cortex values for air- and NBO-treated animals at 3 hours, 1, 2, 7, and 14 days after TBI. (C) Bar graph showing the percent difference in ipsilesional cortex T2 values from contralesional cortex values for air- and NBO-treated animals at 3 hours, 1, 2, 7, and 14 days after TBI. (D) Bar graph showing the percent difference in ipsilesional cortex ADC from contralesional cortex values for air- and NBO-treated animals at 3 hours, 1, 2, 7, and 14 days after TBI. (E) Bar graph showing the percent difference in ipsilesional cortex FA from contralesional cortex values for air- and NBO-treated animals at 3 hours, 1, 2, 7, and 14 days after TBI (mean±s.e.m., n=7 each group, *P<0.05).
The evolution of T2, ADC, FA, and CBF of the S1FL region in air-treated and NBO-treated animals was analyzed using ROI analysis. The data are expressed as a percent difference from the contralesional cortex.
In air-treated TBI rats the ipsilesional T2 was elevated compared with the contralesional T2 at 3 hours, and on days 1 and 2 after TBI, but returned toward contralesional values on days 7 and 14 (Figure 2B). The NBO treatment exaggerated ipsilesional T2 within 3 hours of injury and was further increased on day 1. On day 2, the ipsilesional T2 returned toward values of air-treated animals but remained significantly increased compared with air-treated animals on day 14.
In air-treated TBI rats, the ipsilesional ADC increased acutely, peaked on day 2, and then returned toward contralesional values by day 14. In contrast, the ipsilesional ADC was extremely elevated in NBO-treated animals at 3 hours, and continued to steadily increased through day 14 after TBI with significant increases compared with air-treated animals at all time points evaluated (Figure 2C). The increased ADC values suggest more prevalent and persistent edema formation after NBO treatment and correlates with a greater lesion volume.
The ipsilesional FA was significantly lower at 3 hours and continued to decrease through day 14 in both air- and NBO-treated animals. Normobaric oxygen-treated animals were significantly decreased compared with air-treated animals at all time points (Figure 2D). Decreased FA values suggest a loss in white-matter integrity.
Cerebral blood flow measurements indicated a substantial decrease in both air- and NBO-treated animals acutely (within 3 hours of injury) and persisted through 24 hours after injury. By day 2, however, CBF values significantly increased in both treatment groups to a similar level indicating increased blood flow to the impacted area. The CBF values decreased on day 7 after injury and returned to normal values by day 14 (Figure 2E). The data suggest that TBI induces a biphasic decrease in CBF with a significant increase peaking on day 2.
The combined analysis of T2, ADC, FA, and CBF indicates exacerbated lesion severity in NBO-treated animals compared with air-treated animals that may be dominated by edema formation in NBO-treated animals.
Gross Dissection
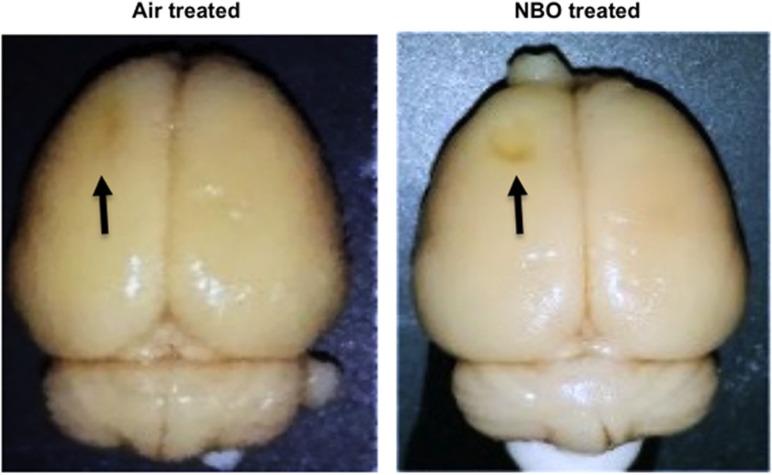
The brains of both air- and NBO-treated animals were removed for immunohistochemical detection of alterations in neuronal morphology and apoptosis on day 14 after TBI. Gross dissection revealed an obvious lesion in both animals. However, the NBO-treated animals had markedly more brain tissue loss in the area of the initial impact (S1FL cortex) (Figure 3).
Figure 3.
Gross dissection of air- and normobaric oxygen (NBO) therapy-treated animals 14 days after injury reveals a more severe tissue loss in NBO-treated animals. The lesion is indicated by the black arrows.
Immunohistochemistry
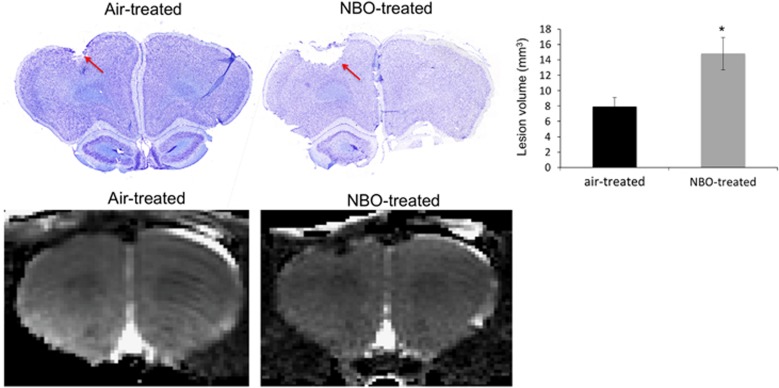
Nissl staining for both the air- and NBO-treated animals at 14 days showed an obvious lesion defined as loss of tissue below the impact site (Figure 4). The NBO-treated animals had a significantly larger area of tissue loss compared with the air-treated animals in all sections analyzed. The average lesion volume assessed 14 days after injury was 7.9±1.2 mm3 in air-treated animals and 14.8±2.1 mm3 in NBO-treated animals. The Nissl determined lesion volumes were similar to those determined by T2 abnormality.
Figure 4.
Representative images of Nissl-stained brain sections from an air-treated traumatic brain injury (TBI) and normobaric oxygen therapy (NBO)-treated TBI animal on day 14 after injury with the corresponding magnetic resonance imaging (MRI) scan shown below. The lesion is indicated by the red arrows in both Nissl sections. The histogram shows the average lesion volume between air- and NBO-treated animals (mean±s.e.m., n=7 each group, *P<0.05).
Normobaric Oxygen did not Improve Behavioral Outcomes
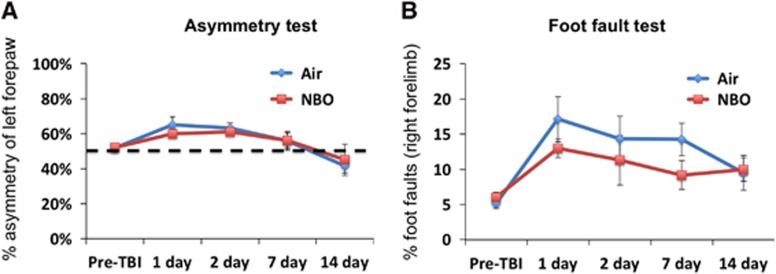
Sensorimotor function was assessed using the asymmetry forelimb placement (cylinder) and foot-fault tests. In the air-treated group, asymmetry scores worsened on days 1 and 2 after TBI, indicating increased utilization of the left (unaffected) forelimb in the air-treated animals. The asymmetry scores returned toward pre-TBI values on days 7 and 14. In the NBO-treated group, the forelimb asymmetry scores were not significantly different from those of the air-treated group (Figure 5A). In fact, the two treatment groups tracked each other almost identically at all time points assessed.
Figure 5.
Line graphs of the (A) cylinder and (B) foot-fault tests for the air- and normobaric oxygen therapy (NBO)-treated animals at pre-TBI, 1, 2, 7, and 14 days after TBI (mean±s.e.m., n=7 each group). TBI, traumatic brain injury.
In both the air- and NBO-treated groups, foot-fault scores worsened in the right forelimb dramatically on day 1, persisted on days 2 and 7 after TBI, and improved slightly on day 14. The NBO-treated group did not reached the severity observed in the air-treated group, with slightly lower faults however they were not significantly different from that of the air-treated animals. Together, these data indicated that NBO did not reduce sensorimotor deficits after TBI. These data also suggest that there were likely functional compensations in both groups by day 14 after TBI (Figure 5B).
Discussion
This is the first study to test whether NBO treatment reduces lesion volume and improves functional recovery using a cortical impact model of moderate TBI in rats assessed by MRI, behavioral outcomes, and ex vivo lesion volume measures in the same animal. Contrary to our hypothesis, we found that (1) NBO exacerbates lesion volume and vasogenic edema compared with air-treated animals and (2) NBO treatment did not improve behavioral outcome measures compared with air-treated animals.
Magnetic Resonance Imaging Determined Neuropathologic Changes Associated with Traumatic Brain Injury and Normobaric Oxygen Therapy
Traumatic brain injury induces CBF disturbances that could lead to hypoxic and ischemic injury. While normobaric and hyperbaric oxygen therapy have been studied in TBI, the results have been equivocal, likely due to the variability in injury severity seen in a patient population.25 In our study, CBF was impaired initially at the site of the impact and the borders of perfusion deficit extended beyond that of the T2 determined injury area in both air- and NBO-treated animals. The hypoperfusion seen in the injured cortex within 1 to 24 hours after TBI is consistent with other reports using MRI26 and 14C-IPIA autoradiography measures of CBF.27, 28 Interestingly, we found no statistically significant differences between the air- and NBO-treated animals in terms of CBF, with both being impaired equally at all time points.
ADC values were calculated from the same ROI used to determine lesion volume. ADC after TBI increased acutely (up to 2 days) but declined at later time points, while NBO-treated animals continued to rise suggesting increased persistent vasogenic edema formation. Interestingly, in some animals the core of the impact with decreased ADC values was surrounded by an area of increased ADC, which was exacerbated by NBO treatment when compared with air-treated animals. Previous studies have shown that the contusion area is ischemic and that decreased ADC is associated with cytotoxic edema.29 The rim of increased ADC suggests vasogenic edema formation and may be due to a transient breakdown in the BBB.30, 31 We also assessed FA changes in association with TBI that describe microstructural alterations in the cortical region, and found decreased FA values in both air- and NBO-treated animals. However, the decrease was exaggerated even further by NBO treatment suggesting an increased loss of tissue integrity within the lesioned area. In TBI, FA reduction has been shown in multiple brain regions depending on the site of injury. It is thought that axonal injury may be widespread in TBI with studies showing global decreases in gray-matter FA values; however, the identification of the fibers affected is still unclear.23
Exacerbation of Lesion Volume and Behavioral Deficits after Normobaric Oxygen
Gross examination on day 14 after injury revealed significant differences in lesion volume with the NBO-treated animals showing a much larger lesion compared with air-treated animals. End point histologic evaluation using Nissl confirmed the apparent difference in lesion volumes between the two treatment groups. Furthermore, the temporal profile using T2 correlated with gross and histologic findings. Surprisingly, we found no statistical difference in behavioral outcomes between NBO- and air-treated animals across all time points. However, both groups of animals recovered behaviorally by day 14, which suggests functional compensation or spontaneous recovery. It is possible that the behavioral tests utilized in this study may not be sensitive enough to detect changes long term and may be more appropriate to detect early treatment effects. Therefore, further studies using more sensitive behavioral tests should be performed in the future to validate these findings.
Immunohistochemical Validation of Magnetic Resonance Imaging Findings
In the current study, we also performed immunohistologic staining. In particular, we performed Nissl staining to corroborate MRI determined lesion volumes between treatment groups. We observed that NBO treatment increased tissue loss when compared with the air-treated group. There is much variability in the outcomes reported by other studies exploring the use of NBO after TBI. Zhou et al.32 observed no statistical significance in neuronal loss between NBO- and air-treated groups. Palzur et al.33 observed a slight reduction in the number of dead cells with normobaric hyperoxia compared with air treatment after dynamic cortical deformation. Another group, however, found immediate 3-hour exposure to NBO after fluid percussive injury resulted in a reduction in cell death compared with untreated animals.34 The variations seen between our study and others are likely due to variations in the TBI model utilized, the severity of injury induced, the concentration of oxygen utilized, and the timing and duration of NBO treatment. Many of the studies showing positive effects of NBO treatment after TBI have been done using more severe TBI models or were observed in clinical populations.35 The current study used a moderate model of TBI and therefore the severity of the lesion may not have been great enough to cause disruptions in oxygenation or cerebral metabolism. Therefore, increased oxygenation may lead to a counterproductive increase in the production of reactive oxygen species (ROS) thereby potentially exaggerating injury severity.
Normobaric Oxygen in Human Traumatic Brain Injury
Normobaric oxygen is a potentially attractive treatment option due to its availability and ease of administration. The pioneering study that led to a number of human clinical trails exploring NBO treatment after TBI was performed by Menzel et al.,16 where they showed that NBO increases brain tissue PO2 and decreased microdialysate extracellular lactate levels (indicating improved tissue oxygenation). However, NBO treatment after TBI in human subjects since this study have been met with conflicting results, which could be due to variations in treatment concentration, timing, and duration. The largest and most cited study is from Tolias et al.,17 where they administered 100% FIO2 within 6 hours of TBI for 24 hours and measured brain oxidative metabolism by microdialysis and intracranial pressure. The NBO treatment compared with historic controls showed decreased intracranial pressure, increased glucose, decreased glutamate and lactate levels, and decreased ratio of lactate/glucose and lactate/pyruvate.17 Other clinical studies however, have noted deleterious effects of NBO treatment including increased production of ROS, markedly reduced antioxidant defenses, and increased cell death.25, 36 Our studies indicate that NBO is not effective and in fact causes an increase in lesion volume.
Mitochondrial Dysfunction and Normobaric Oxygen
Mitochondria are crucial to the function of the central nervous system as their function is tightly coupled to neuronal activity and energy production. Damage to mitochondria leads to metabolic dysfunction and is well reported in both human and experimental models of TBI.14, 37 Negative outcomes after NBO shown here could be the result of NBO increasing ROS production. The timing of NBO treatment in the studies presented here is within the time frame of known mitochondrial dysfunction, which may further exacerbate the injury effects seen with NBO. Incomplete reduction of oxygen increases ROS, which are known to impair the structural integrity of the inner mitochondrial membrane result in a reduction of the efficiency of the electron transport chain.38 Furthermore, increased ROS oxidizes both proteins and DNA thus promoting apoptosis.39 In addition to the potential central nervous system toxicity from increased ROS generation, increasing oxygenation could also lead to cerebral vasoconstriction, resulting in reduced perfusion in the brain.40 The result of decreased perfusion and reduced O2 diffusion to cells would further lead to inefficient maintenance of oxidative cerebral metabolism and edema formation.9, 10, 11 While anaerobic metabolism takes over12 its' inefficiency could further deplete cellular energy, increase mitochondrial dysfunction, and prolong hypometabolism.13, 14, 15 The combination of impaired mitochondria increased ROS production and decreased cerebral perfusion are likely mechanisms contributing to the detrimental effect of NBO found in our study and should be further examined.
Conclusions
Contrary to our hypothesis, we found that NBO treatment increases lesion volume in a rat model of moderate TBI and had no additional positive effects on behavioral measures. We also found that NBO treatment increases T2, ADC, FA, and CBF abnormality after TBI. These results do not promote the acute use of NBO in patients with moderate TBI. Further studies adjusting the concentration of O2, altering the timing, or extending the duration of NBO exposure along with varying the range of injury severity will be necessary to improve the understanding of the potential benefits or limitations of NBO therapy after TBI.
Acknowledgments
The authors thank Timothy Schallert and Theresa Jones of UT Austin for their assistance in the setup of the behavioral assays utilized in this study.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by NIH/NINDS R01 NS45879 (TQD), a TL1 grant (JAL) and KL2 TR001118 (LTW) via the Clinical Translational Science Awards (CTSA, parent grant 8UL1TR000149 and TL1TR001119). Immunohistochemical images were generated in the Core Optical Imaging Facility, which is supported by UTHSCSA, NIH/NCI P30CA54174, and NIH/NIA P01AG19316. This work was supported by the CTSA KL2 (TR001118).
References
- 1Nortje J, Menon DKN. Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol 2004; 17: 711–718. [DOI] [PubMed] [Google Scholar]
- 2Center for Disease Control and Prevention Injury Prevention and Control: Traumatic brain Injury. http://www.cdc.gov/TraumaticBrainInjury.
- 3Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil 1982; 63: 118–123. [PubMed] [Google Scholar]
- 4Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995; 65: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 5Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl 1998; 71: 104–106. [DOI] [PubMed] [Google Scholar]
- 6Bardt TF, Unterberg AW, Hartl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl 1998; 71: 153–156. [DOI] [PubMed] [Google Scholar]
- 7Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA et al. Diffusion limited oxygen delivery following head injury. Crit Care Med 2004; 32: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 8Stein SC, Graham DI, Chen XH, Smith DH. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery 2004; 54: 687–691, discussion 691. [DOI] [PubMed] [Google Scholar]
- 9Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 1991; 75: 685–693. [DOI] [PubMed] [Google Scholar]
- 10Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg 1992; 77: 360–368. [DOI] [PubMed] [Google Scholar]
- 11Vigue B, Ract C, Benayed M, Zlotine N, Leblanc PE, Samii K et al. Early SjvO2 monitoring in patients with severe brain trauma. Intensive Care Med 1999; 25: 445–451. [DOI] [PubMed] [Google Scholar]
- 12Bergsneider M, Hovda DA, McArthur DL, Etchepare M, Huang SC, Sehati N et al. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J Head Trauma Rehabil 2001; 16: 135–148. [DOI] [PubMed] [Google Scholar]
- 13Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 2004; 4: 705–713. [DOI] [PubMed] [Google Scholar]
- 14Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma 2001; 18: 977–991. [DOI] [PubMed] [Google Scholar]
- 15Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab 2003; 23: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 16Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg 1999; 91: 1–10. [DOI] [PubMed] [Google Scholar]
- 17Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR. Normobaric hyperoxia—induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg 2004; 101: 435–444. [DOI] [PubMed] [Google Scholar]
- 18Magnoni S, Ghisoni L, Locatelli M, Caimi M, Colombo A, Valeriani V et al. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J Neurosurg 2003; 98: 952–958. [DOI] [PubMed] [Google Scholar]
- 19Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab 2003; 23: 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab 2005; 25: 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol 2004; 55: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Talley Watts L, Long JA, Chemello J, Van Koughnet S, Fernandez A, Huang S et al. Methylene blue is neuroprotective against mild traumatic brain injury. J Neurotrauma 2014; 31: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Long JA, Watts LT, Chemello J, Huang S, Shen Q, Duong TQ. Multiparametric and longitudinal MRI characterization of mild Traumatic Brain Injury in rats. J Neurotrauma 2014, doi:10.1089/neu.2014.3563; e-pub ahead of print. [DOI] [PMC free article] [PubMed]
- 24Talley Watts L, Sprague S, Zheng W, Garling RJ, Jimenez D, Digicaylioglu M et al. Purinergic 2Y1 receptor stimulation decreases cerebral edema and reactive gliosis in a traumatic brain injury model. J Neurotrauma 2013; 30: 55–66. [DOI] [PubMed] [Google Scholar]
- 25Kumaria A, Tolias CM. Normobaric hyperoxia therapy for traumatic brain injury and stroke: a review. Br J Neurosurg 2009; 23: 576–584. [DOI] [PubMed] [Google Scholar]
- 26Long JA, Watts LT, Chemello J, Shen Q, Duong TQ. MRI reveals widespread disruptions in CBF and vascular reactivity following focal TBI. J Neurotrauma 2014; 31: A27–A27. [Google Scholar]
- 27Yamakami I, McIntosh TK. Alterations in regional cerebral blood flow following brain injury in the rat. J Cereb Blood Flow Metab 1991; 11: 655–660. [DOI] [PubMed] [Google Scholar]
- 28Bryan RM, Jr., Cherian L, Robertson C. Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth Analg 1995; 80: 687–695. [DOI] [PubMed] [Google Scholar]
- 29Kuroiwa T, Nagaoka T, Miyasaka N, Akimoto H, Zhao F, Yamada I et al. Time course of trace of diffusion tensor [Trace(D)] and histology in brain edema. Acta Neurochir Suppl 2000; 76: 191–194. [DOI] [PubMed] [Google Scholar]
- 30Donkin JJ, Vink R. Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr Opin Neurol 2010; 23: 293–299. [DOI] [PubMed] [Google Scholar]
- 31Katayama Y, Kawamata T. Edema fluid accumulation within necrotic brain tissue as a cause of the mass effect of cerebral contusion in head trauma patients. Acta Neurochir Suppl 2003; 86: 323–327. [DOI] [PubMed] [Google Scholar]
- 32Zhou Z, Daugherty WP, Sun D, Levasseur JE, Altememi N, Hamm RJ et al. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J Neurosurg 2007; 106: 687–694. [DOI] [PubMed] [Google Scholar]
- 33Palzur E, Vlodavsky E, Mulla H, Arieli R, Feinsod M, Soustiel JF. Hyperbaric oxygen therapy for reduction of secondary brain damage in head injury: an animal model of brain contusion. J Neurotrauma 2004; 21: 41–48. [DOI] [PubMed] [Google Scholar]
- 34Muthuraju S, Pati S, Rafiqul M, Abdullah JM, Jaafar H. Effect of normabaric hyperoxia treatment on neuronal damage following fluid percussion injury in the striatum of mice: a morphological approach. J Biosci 2013; 38: 93–103. [DOI] [PubMed] [Google Scholar]
- 35Beynon C, Kiening KL, Orakcioglu B, Unterberg AW, Sakowitz OW. Brain tissue oxygen monitoring and hyperoxic treatment in patients with traumatic brain injury. J Neurotrauma 2012; 29: 2109–2123. [DOI] [PubMed] [Google Scholar]
- 36Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res 2002; 51: 571–578. [DOI] [PubMed] [Google Scholar]
- 37Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111). Neurol Res 1997; 19: 334–339. [DOI] [PubMed] [Google Scholar]
- 38Mecocci P, Beal MF, Cecchetti R, Polidori MC, Cherubini A, Chionne F et al. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol Chem Neuropathol 1997; 31: 53–64. [DOI] [PubMed] [Google Scholar]
- 39Wang X. The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15: 2922–2933. [PubMed] [Google Scholar]
- 40Bulte DP, Chiarelli PA, Wise RG, Jezzard P. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab 2007; 27: 69–75. [DOI] [PubMed] [Google Scholar]