Abstract
Using cortical neuronal cultures and glutamic acid excitotoxicity and oxygen-glucose deprivation (OGD) stroke models, we demonstrated that poly-arginine and arginine-rich cell-penetrating peptides (CPPs), are highly neuroprotective, with efficacy increasing with increasing arginine content, have the capacity to reduce glutamic acid-induced neuronal calcium influx and require heparan sulfate preotoglycan-mediated endocytosis to induce a neuroprotective effect. Furthermore, neuroprotection could be induced with immediate peptide treatment or treatment up to 2 to 4 hours before glutamic acid excitotoxicity or OGD, and with poly-arginine-9 (R9) when administered intravenously after stroke onset in a rat model. In contrast, the JNKI-1 peptide when fused to the (non-arginine) kFGF CPP, which does not rely on endocytosis for uptake, was not neuroprotective in the glutamic acid model; the kFGF peptide was also ineffective. Similarly, positively charged poly-lysine-10 (K10) and R9 fused to the negatively charged poly-glutamic acid-9 (E9) peptide (R9/E9) displayed minimal neuroprotection after excitotoxicity. These results indicate that peptide positive charge and arginine residues are critical for neuroprotection, and have led us to hypothesize that peptide-induced endocytic internalization of ion channels is a potential mechanism of action. The findings also question the mode of action of different neuroprotective peptides fused to arginine-rich CPPs.
Keywords: arginine-rich peptides, cell-penetrating peptides, endocytosis, neuroprotection, poly-arginine, stroke
Introduction
Currently there are no clinically effective agents that provide neuroprotection in stroke. Recently, we reported that the arginine-rich cationic cell-penetrating peptides (CPPs), poly-arginine-9 (R9: RRRRRRRRR) and penetratin (RQIKIWFQNRRMKWKK) were neuroprotective in in vitro neuronal cell stroke models.1 This finding followed several earlier reports from our laboratory and other laboratories demonstrating that the arginine-rich CPP TAT45–57 (TAT: GRKKRRQRRR) displays neuroprotective actions in both in vitro and in vivo stroke models.2, 3, 4, 5 However, our more recent data revealed that R9 and penetratin were 17- and 4.6-fold, respectively, more potent than TAT.1
The recent TAT, R9 and penetratin neuroprotective findings are highly significant, as prior studies have shown that different neuroprotective peptides fused to CPPs, including TAT, are efficacious in a range of acute neurodegenerative disorders, including stroke, traumatic brain injury, and perinatal hypoxia–ischemia.6, 7, 8 What our recent study1 demonstrates is that carrier peptides (e.g., R9, penetratin) also display a high level of neuroprotection. This increases the possibility that the mechanism of action of a neuroprotective peptide fused to a CPP is largely, if not exclusively, the result of an enhanced neuroprotective effect of the carrier peptide. To illustrate this, in one of our earlier studies, we showed that the addition of three amino acids to TAT (PKIGRKKRRQRRRG; AM8D-TAT) increased peptide potency considerably (IC50 decreased from >15 μmol/L to 1.1 μmol/L) in a glutamic acid excitotoxicity model.2 Furthermore, the mechanism by which arginine-rich CPPs exert their neuroprotective action may be linked to endocytosis, a predominant carrier-peptide cellular uptake route, rather than by an interaction with a specific cytoplasmic target. In contrast, a neuroprotective peptide fused to a carrier peptide entering a cell by endocytosis, must first escape the endosome, which is known to be a highly inefficient process,9, 10 before it can interact with its cytoplasmic target, thereby rendering it unlikely that the peptide can act through interaction with its intended target.
Therefore, given our previous TAT, R9 and penetratin findings, and recognition of the potential of cargo peptides to enhance the effects of CPPs, this current study focuses on further characterizing the neuroprotective properties of poly-arginine and arginine-rich peptides, and neuroprotective peptides fused to CPPs with the goal of gaining a better understanding of the mechanism of neuroprotection.
Materials and Methods
Neuronal Cultures
Establishment of rat primary cortical cultures in Neurobasal/B27 supplement (Life Technologies, Melbourne, VIC, Australia) using cortical tissue obtained directly from E18-day embryos was as previously described;1 however, some cultures were established from cortical tissue stored in Hibernate-E (Life Technologies)/2% B27 supplement for 2 to 7 days at 5 °C. Neurons were seeded into 96-well-sized glass wells (7 mm diameter, Grace, Melbourne, VIC, Australia), 96-well plastic plates (Nunc, Thermo Fisher Scientific, Melbourne, VIC, Australia), or 96-well plastic strip-plates (Costar, Sigma-Aldrich, Castle Hill, NSW, Australia) and maintained in a CO2 incubator (5% CO2, 95% air balance, 98% humidity) at 37 °C until use on day in vitro 10 to 14. Under these conditions, cultures routinely consist of >97% neurons and 1% to 3% astrocytes.
Peptides
Peptides used in the study are summarized in Table 1. All peptides were synthesized by China Peptides (Shanghai, China), except for K10, TAT-NR2B9c, JNKI-1-TATD, and PYC36-TAT (Mimotopes, Clayton, VIC, Australia), XIP (Peptide 2.0, Chantilly, VA, USA), NCXBP3 (Pepmic, Suzhou, China), and TAT, BEN0254, BEN0540, BEN1079 (Pepscan, Lelystad, The Netherlands). The peptides were high-performance liquid chromatography purified to attain 88% to 98% purity. The R9D and JNKI-1-TATD peptides were synthesized in the protease-resistant D-isoform, synthesized from D-amino acids. All the peptides were prepared as 100 × stocks (500 μmol/L) in water (Baxter, Old Toongabbie, NSW, Australia) and assessed in a concentration range anywhere from 0.1 to 20 μmol/L, dependent upon the peptide and injury model used.
Table 1. List of peptides used in study.
| Peptide name | Peptide sequence | AA residues: arginine residues | Net peptide charge at pH 7 |
|---|---|---|---|
| R1 | H-R-OH | 1: 1 | 1 |
| R3 | H-RRR-OH | 3: 3 | 3 |
| R6 | H-RRRRRR-OH | 6: 6 | 6 |
| R7 | H-RRRRRRR-OH | 7: 7 | 7 |
| R8 | H-RRRRRRRR-OH | 8: 8 | 8 |
| R9 | H-RRRRRRRRR-OH | 9: 9 | 9 |
| R9D | H-rrrrrrrrr-NH2 | 9: 9 | 10 |
| R10 | H-RRRRRRRRRR-OH | 10: 10 | 10 |
| R11 | H-RRRRRRRRRRR-OH | 11: 11 | 11 |
| R12 | H-RRRRRRRRRRRR-OH | 12: 12 | 12 |
| R13 | H-RRRRRRRRRRRRR-OH | 13: 13 | 13 |
| R14 | H-RRRRRRRRRRRRRR-OH | 14: 14 | 14 |
| R15 | H-RRRRRRRRRRRRRRR-OH | 15: 15 | 15 |
| R18 | H-RRRRRRRRRRRRRRRRRR-OH | 18: 18 | 18 |
| R9/X7/R9 | H-RRRRRRRRRPGRVVGGRRRRRRRRR-OH | 25: 19 | 19 |
| E9/R9 | H-EEEEEEEEE-RRRRRRRRR-OH | 18: 9 | 0 |
| K10 | H-KKKKKKKKKK-OH | 10: 0 | 10 |
| PTD-4a | H-YARAAARQARA-OH | 11: 3 | 3 |
| TAT | Ac-GRKKRRQRRRG-NH2 | 11: 6 | 8 |
| TAT-NR2B9cb | H-GRKKRRQRRR-KLSSIESDV-OH | 19: 6 | 7 |
| JNKI-1-TATDc | H-tdqsrpvqpflnlttprkprpp-rrrqrrkkrG-NH2 | 32: 9 | 12 |
| TAT-JNKI-1c | H- GRKKRRQRRR-PPRPKRPTTLNLFPQVPRSQDT-OH | 32: 9 | 11 |
| kFGF-JNKI-1 | H-AAVALLPAVLLALLAP-PPRPKRPTTLNLFPQVPRSQDT-OH | 38: 3 | 3 |
| kFGFd | H-AAVALLPAVLLALLAP-OH | 16: 0 | 0 |
| PYC36-TATe | H-GRKKRRQRRR-GGLQGRRRQGYQSIKP-NH2 | 26: 9 | 13 |
| NCXBP3f | H-RRERRRRSCAGCSRARGSCRSCRR-NH2 | 24: 11 | 10.8 |
| XIPg | H-RRLLFYKYVYKRYRAGKQRG-OH | 20: 5 | 8 |
| BEN0254h | Ac-WGCCGRSSRRRRTR-NH2 | 14: 6 | 5.9 |
| BEN0540h | Ac-PFLKRVPACLRLRR-NH2 | 14: 4 | 5 |
| BEN1079h | Ac-RCGRASRCRVRWMRRRRI-NH2 | 18: 8 | 8.9 |
At the N terminus, H indicates free amine, and Ac indicates acetyl. At the C terminus, OH indicates free acid and NH2 indicates amide. Lower case single-letter code indicates D-isoform of the amino acid (AA).
Peptide described in Ho et al.12
NR2B9c-TAT peptide described by Aarts et al.6
Peptide described in Borsello et al.37
Peptide described in Lin et al.14
Peptide isolated by JLC from phylomer library (Phylogica Pty Ltd).
XIP described by He et al.38
Peptides isolated by Phylogica Pty Ltd.
Glutamic Acid/N-Methyl-d-Aspartate Excitotoxicity and Peptide Treatments
Peptides were added to culture wells 15 minutes before glutamic acid (l-glutamic acid; Sigma-Aldrich) or NMDA (Tocris, R&D Systems, Minneapolis, MN, USA) exposure by removing media and adding 50 μL of Neurobasal/2% B27 containing peptide. To induce excitotoxicity, 50 μL of Neurobasal/2% B27 containing glutamic acid (200 μmol/L; final concentration 100 μmol/L) or N-methyl-d-aspartate (NMDA; 100 μmol/L; final concentration 50 μmol/L) was added to the culture wells and incubated at 37 °C in the CO2 incubator for 5 minutes (note: peptide concentration reduced by half during this step). After the 5-minute exposure, media were replaced with 100 μL of Neurobasal/2% B27 and cultures incubated for a further 20 to 24 hours at 37 °C in the CO2 incubator. Untreated controls with or without glutamic acid treatment underwent the same incubation steps and media additions.
For pre-glutamic acid exposure experiments, neurons were exposed to peptide for a 10-minute period, immediately before or 1, 2, 3, 4, or 5 hours before glutamic acid exposure. This was performed by removing media and adding 50 μL of Neurobasal/2% B27 containing peptide. After the 10 minutes at 37 °C in the CO2 incubator, media were removed and replaced with 100 μL of Neurobasal/2% B27 (for immediate glutamic acid exposure media contained glutamic acid; 100 μmol/L). At the relevant peptide pre-treatment time, media were removed and replaced with 100 μL of Neurobasal/2% B27 containing glutamic acid (100 μmol/L). After 5-minute glutamic acid exposure, neuronal culture wells were treated as described above. For all the experiments, untreated controls with or without glutamic acid treatment underwent the same incubation steps and media additions.
Heparin Experiments
Heparin (for injection) was obtained from Pfizer (1000 IU/mL). Two different heparin experiments were performed: (1) Peptides were incubated with heparin (20 IU/mL) in Neurobasal/B27 for 5 minutes at room temperature before addition to culture wells (50 μL) for 15 minutes at 37 °C in the CO2 incubator. After the incubation period, media in wells were removed and replaced with 100 μL of Neurobasal/2% B27 containing glutamic acid (100 μmol/L), and subsequently treated as described above; (2) Media in wells were replaced with Neurobasal/2% B27 containing heparin (50 μL; 40 IU/mL) and incubated for 5 minutes at 37 °C in the CO2 incubator. After the incubation period, peptides or glutamate receptor blockers (MK801/CNQX) in Neurobasal/2% B27 (50 μL) were added to the culture wells and cultures incubated for a further 10 minutes at 37 °C in the CO2 incubator. After the incubation period, media in wells were removed and replaced with 100 μL of Neurobasal/2% B27 containing glutamic acid (100 μmol/L), and subsequently treated as described above. For all the experiments, non-heparin-treated peptide controls with glutamic acid treatment underwent the same incubation steps and media additions.
Oxygen-Glucose Deprivation and Peptide Incubation
For oxygen-glucose deprivation (OGD), culture media were removed from wells (glass 96-well plate format) and washed with 300 μl of glucose free balanced salt solution (BSS; mmol/L: 116 NaCl, 5.4 KCl, 1.8 CaCl2, 0.8 MgSO4, 1 NaH2PO4; pH 6.9) before the addition of 60 μl BSS and incubation in an anaerobic incubator (Don Whitely Scientific, North Gosford, NSW, Australia; atmosphere of 5% CO2, 10% H2, and 85% argon, 98% humidity) at 37 °C for 50 minutes. On removal from the anaerobic incubator, 60 μl of Neurobasal/2% B27 was added to the wells containing peptides and cultures incubated for 15 minutes or 24 hours at 37 °C in the CO2 incubator. After the 15-minute peptide exposure, media were removed and replaced with 100 μL of Neurobasal/2% B27 and cultures incubated for 24 hours at 37 °C in the CO2 incubator. For pre-OGD exposure experiments, the procedure was the same as described in the glutamic acid model, except that the 10-minute peptide pre-treatment was performed using 100 μL Neurobasal/2% B27. Control cultures underwent the same BSS wash procedures and media additions as OGD-treated cultures.
Intracellular Calcium Kinetics
Intracellular calcium influx was monitored in neuronal culture wells (glass wells) using Fura-2 AM in real-time using a fluorescent plate reader. The aim of these experiments was to determine the relative change in intracellular calcium before and after glutamic acid exposure. Cells were loaded with the fluorescent calcium ion indicator Fura-2-AM (5 μmol/L) in 50 μl NB/B27, 0.1% pluronic F-127, for 20 minutes at 37 °C in the CO2 incubator. Fura-2-AM solution was removed from wells, replaced with 50 μL NB/B27 containing peptide (5 μmol/L) or glutamate receptor blockers (5 μmol/L MK801 and 5 μmol/L CNQX) and incubated for 10 minutes at 37 °C in the CO2 incubator. Control cultures received 50 μL of NB/B27 only. After the 10-minute incubation period, media in wells were replaced with 50 μL of BSS and wells were transferred to a spectrophotometer (BMG Labtec, CLARIOstar, Mornington, VIC, Australia) while maintaining temperature at 37 °C. Fifty microliters of NB/B27 containing glutamic acid (200 μmol/L; 100 μmol/L final concentration) was added to wells, and every 5 seconds, starting 30 seconds before and for 2 minutes after glutamic acid addition, spectrophotometer measurements (excitation: 355 nm/emission 495 nm) were recorded. Experiments were performed in triplicate.
Intracellular Calcium Imaging
Intracellular calcium levels were also assessed in neuronal culture wells (strip wells) using Fura-2 AM and epi-fluorescent microscope. Neuronal cultures were loaded with Fura-2 AM and treated with peptides or glutamate receptor blockers as described above. After the 10-minute incubation period, 50 μL of NB/B27 containing glutamic acid (200 μmol/L; final concentration 100 μmol/L) was added to the wells before incubation for 5 minutes at 37 °C in the CO2 incubator. After the incubation period, media in wells were replaced with 100 μL BSS, and wells incubated for a further 5 minutes at 37 °C in the CO2 incubator, before examination and image acquisition using an Olympus IX70 inverted microscope (Notting Hill, VIC, Australia), fitted with a digital camera (DP70; Olympus), under software control (DP controller; Olympus).
Neuronal Viability Assessment and Statistical Analysis
At different times after treatments (e.g., 0.5 to 4 hours and 18 to 24 hours), cultures were examined by light microscopy for qualitative assessment of neuronal cell viability. Neuronal viability was quantitatively measured by MTS (3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt) assay (Promega, Sydney, NSW, Australia). The MTS absorbance data were converted to reflect proportional cell viability relative to both the untreated (no insult) and treated (glutamic acid/OGD) controls, with the untreated control taken as 100% viability. After glutamic acid and OGD exposure, cell death in these controls wells typically ranges from 2% to 10% and 5% to 20%, respectively, based on light microscopy; all data are presented as mean±s.d. Viability data were analyzed by analysis of variance, followed by post hoc Fisher's protected least significant difference test, with P<0.05 values considered statistically significant. At least four wells were used in all the assays and usually repeated a minimum of two times independently.
Rat Permanent Middle Cerebral Artery Stroke Model
This study was approved by the Animal Ethics Committee of the University of Western Australia and follows guidelines outlined by the Australian code for the care and use of animals for scientific purposes. Male Sprague–Dawley rats weighing 270 to 320 g were kept under controlled housing conditions with 12 hours light–dark cycle with free access to food and water. Experimental animals were fasted overnight and subjected to filament permanent middle cerebral artery occlusion (MCAO) and physiologic measurements as previously described.11 The procedure was considered successful with a >25% decrease from baseline of cerebral blood flow (CBF) after insertion of filament, as measured by laser Doppler flowmetry. Thirty minutes post-MCAO rats were intravenously treated with R9D (1 μmol/kg in 600 μL over 5 minutes) or vehicle (normal saline for injection; 600 μL over 5 minutes). Treatments were randomized and all procedures were performed masked to treatment. Twenty-fours hours post-MCAO infarct area assessment was performed11 and presented as mean±s.d. The t-test was used to compare mean infarct volume in vehicle and peptide treatment groups. A total of 29 animals were used in the trial. Two animals were euthanased because of subarachnoid hemorrhage, one animal was excluded because of insufficient decrease in CBF, one animal was excluded because of pyrexia, and one died during surgical recovery for an unknown reason.
Results
Effects of Poly-Arginine Peptides on Cultured Neurons Exposed to Glutamic Acid Excitotoxicity
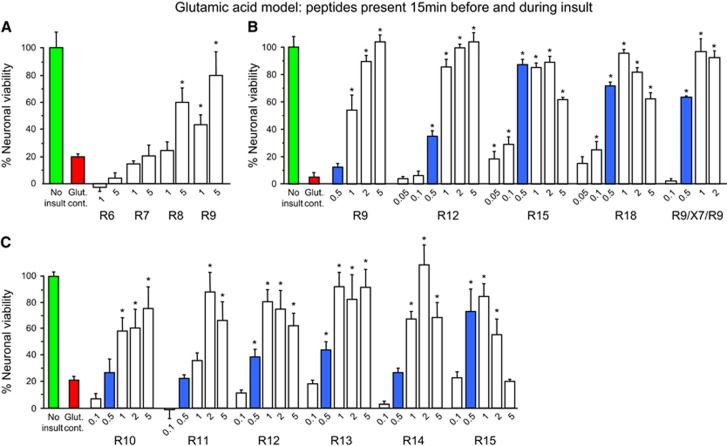
We first examined the influence of poly-arginine length (R1, R3, R6–R15, R18) in circumstances where peptides were present in neuronal cultures 15 minutes before, and during a 5-minute glutamic acid insult. We observed that R1, R3, R6, and R7 peptides had no neuroprotective effects, while based on efficacy at lower concentrations (0.1 to 1 μmol/L) for R8 and longer peptides, neuroprotective potency increases, with increasing length, except that the longer peptides (R15 and R18) cause cell death at higher concentrations (Figures 1A–C; data not shown for R1, R3). On the basis of the level of neuroprotection obtained at a concentration of 0.5 μmol/L, R15 and R18 were identified as the two most potent peptides.
Figure 1.
Glutamic acid excitotoxicity model; poly-arginine peptide dose response experiments. (A–C) Peptides present in neuronal cultures for 15 minutes before and during 5-minute glutamic acid exposure. Neuronal viability measured 20 to 24 hours after glutamic acid exposure. Concentration of peptide in μmol/L. MTS data were expressed as percentage neuronal viability with no insult control taken as 100% viability (mean±s.d.; n=4; *P<0.05). MTS, 3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt.
Effects of Other Arginine-Rich Peptides on Cultured Neurons Exposed to Glutamic Acid Excitotoxicity
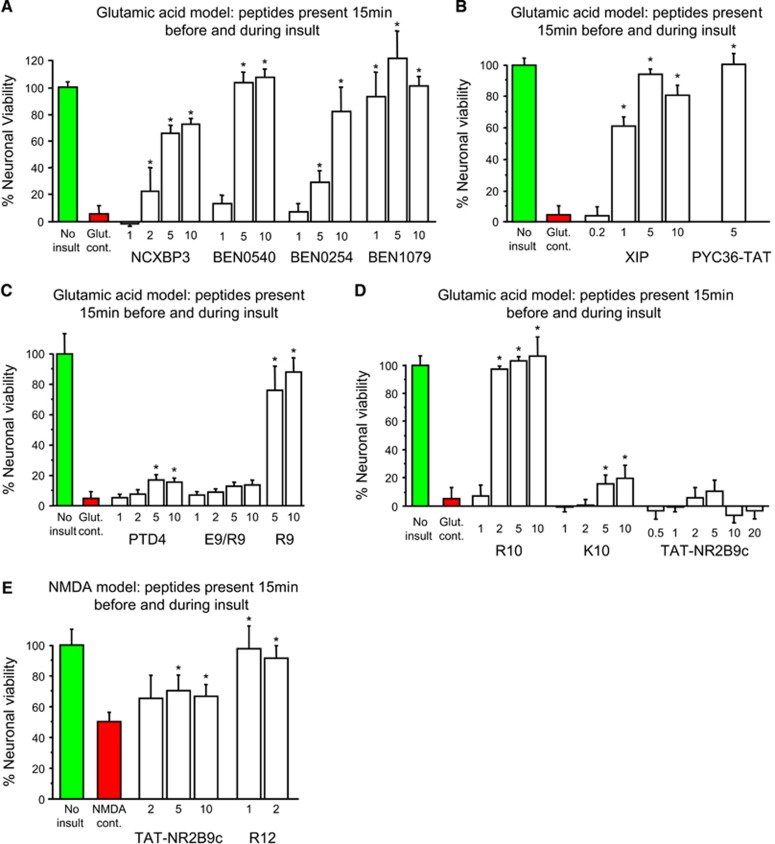
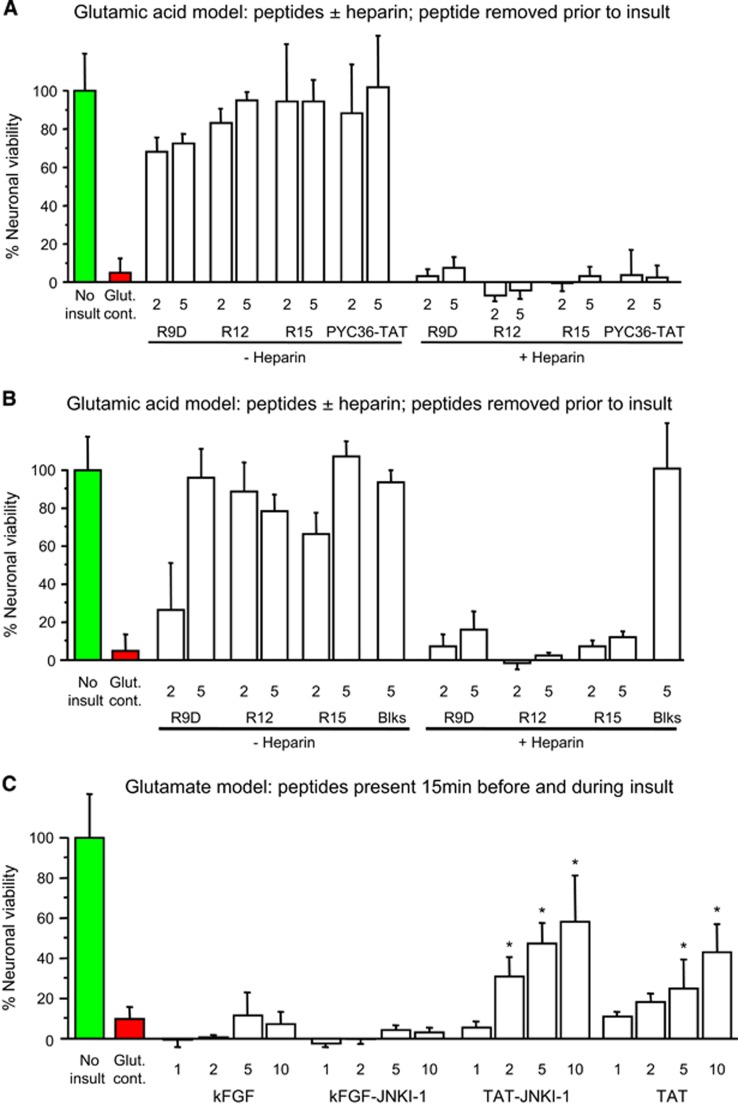
Given the effects of poly-arginine peptides, we next examined the effects of six arginine-rich peptides (R9/X7/R9, NCXBP3, XIP, BEN0254, BEN0540, BEN1079) that contain both arginine and other amino acid residues, and found that they were also neuroprotective in the glutamic acid model (Figure 1B; Figures 2A and 2B). To determine whether cationic charge because of arginine residues was important for neuroprotection, we examined the effects of R9 fused to the negatively charged poly-glutamic acid-9 peptide (E9: R9/E9; charge neutral at pH 7), and poly-lysine-10 peptide (K10, net charge +10) and showed that both R9/E9 and K10 displayed only minimal neuroprotection (Figures 2C and 2D). Similarly, a modified TAT peptide (PTD-4: 3 of 11 amino acids arginine; net charge +3) that is reported12 to have 33-fold greater transduction efficiency than TAT was also shown to display minimal neuroprotection (Figure 3C). Interestingly, although JNKI-1-TATD (net charge +11) is neuroprotective in the glutamic acid model,3 TAT-NR2B9c (net charge +7) was not neuroprotective, even at high concentrations (20 μmol/L; Figure 2D). This peptide, however, was modestly neuroprotective in a milder excitotoxic model caused by NMDA (Figure 2E). These findings indicated that arginine residues combined with peptide charge are the main structural elements determining peptide neuroprotective efficacy.
Figure 2.
Glutamic acid (and NMDA) excitotoxicity model; dose response experiments for arginine-rich and control peptides. (A–D) Peptides present in neuronal cultures for 15 minutes before and during the 5-minute glutamic acid exposure. (E) Peptides present in neuronal cultures for 15 minutes before and during the 5-minute NMDA exposure; note increased neuronal survival in NMDA control because of milder insult. Neuronal viability measured 20 to 24 hours after glutamic acid or NMDA exposure. Concentration of peptide in μmol/L. MTS data were expressed as percentage neuronal viability with no insult control taken as 100% viability (mean±s.d.; n=4; *P<0.05). MTS, 3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt; NMDA, N-methyl-d-aspartate.
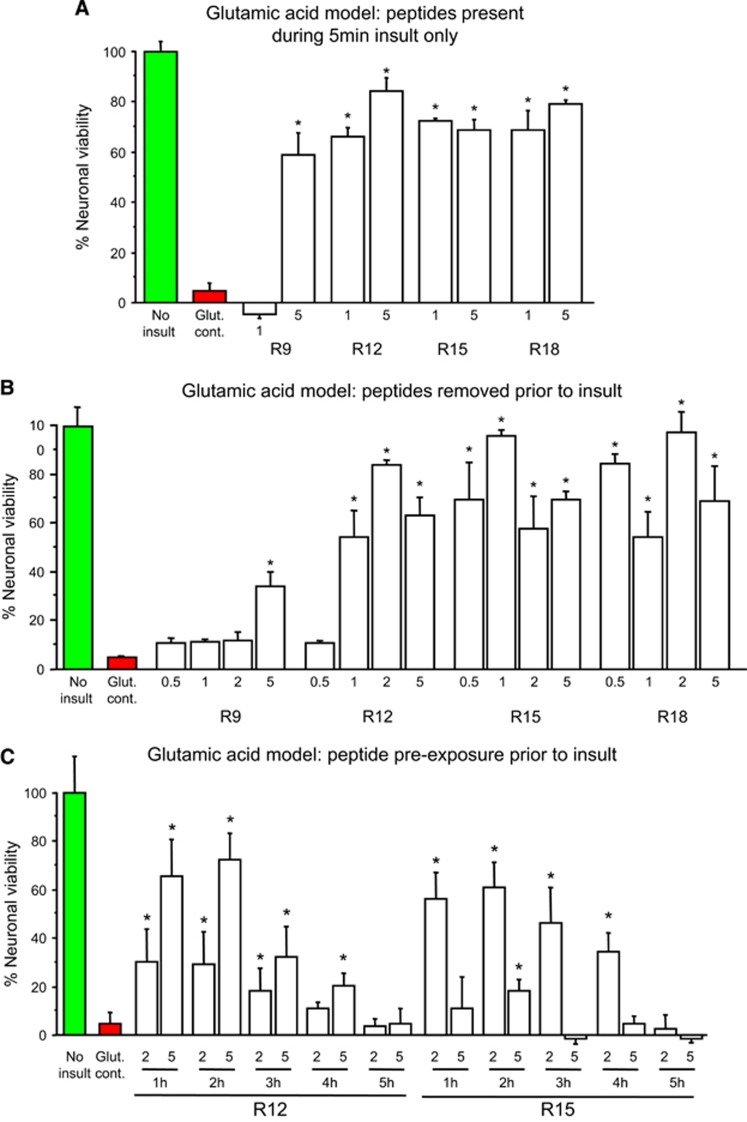
Figure 3.
Glutamic acid excitotoxicity model; poly-arginine peptide treatment before or during glutamic acid exposure. (A) Peptides present in neuronal cultures for 10 minutes only before glutamic acid exposure. (B) Peptides present in neuronal cultures only during the 5-minute glutamic acid exposure. (C) Peptides present in neuronal cultures for 10 minutes only at 1 to 5 hours before glutamic acid exposure. Neuronal viability measured 20 to 24 hours after glutamic acid exposure. Concentration of peptide in μmol/L. MTS data were expressed as percentage neuronal viability with no insult control taken as 100% viability (mean±s.d.; n=4; *P<0.05). MTS, 3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt.
Effects of Poly-Arginine Peptide Pre-Treatment on Cultured Neurons Exposed to Glutamic Acid Excitotoxicity
The observation that R9 to R18 poly-arginine peptides are neuroprotective when present both before and during a glutamic acid insult (Figure 1), at least at low concentrations, raises the question of whether pre-exposure is a prerequisite for neuroprotection. Therefore, we next tested the neuroprotective efficacy of R9, R12, R15, and R18 when added to neuronal cultures only before (10 minutes treatment) or during 5 minutes of glutamic acid exposure. We observed that in both treatment paradigms, peptides were neuroprotective and that the level of neuroprotection was peptide and dose-dependent (Figures 3A and 3B). We next tested if an extended time interval between peptide treatment (10-minute treatment) and glutamic acid insult would be neuroprotective. Experiments involving prolonged intervals (1 to 5 hours) between R12 or R15 peptide treatment of neuronal cultures and glutamic acid insult, revealed that a 1 to 4 hours pre-exposure interval was protective, and that the effect was peptide, time, and dose-dependent (Figure 3C).
The Neuroprotective Mechanism of Action of Peptides in Cells Exposed to Glutamic Acid Excitotoxicity
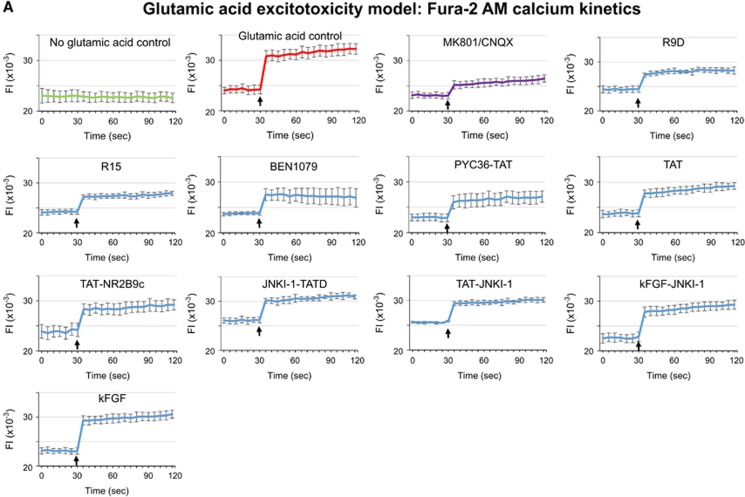
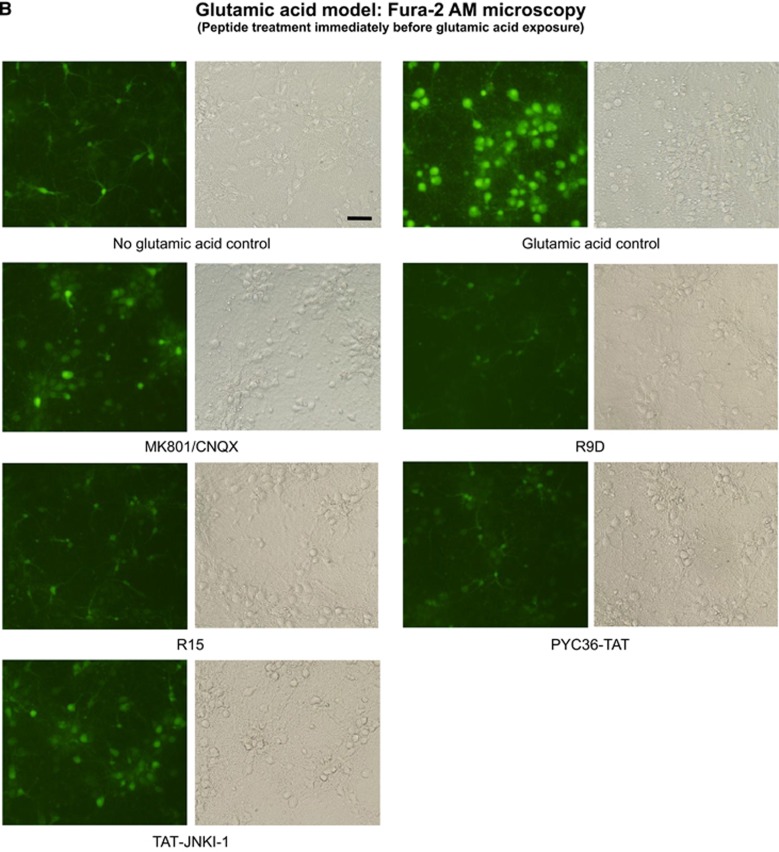
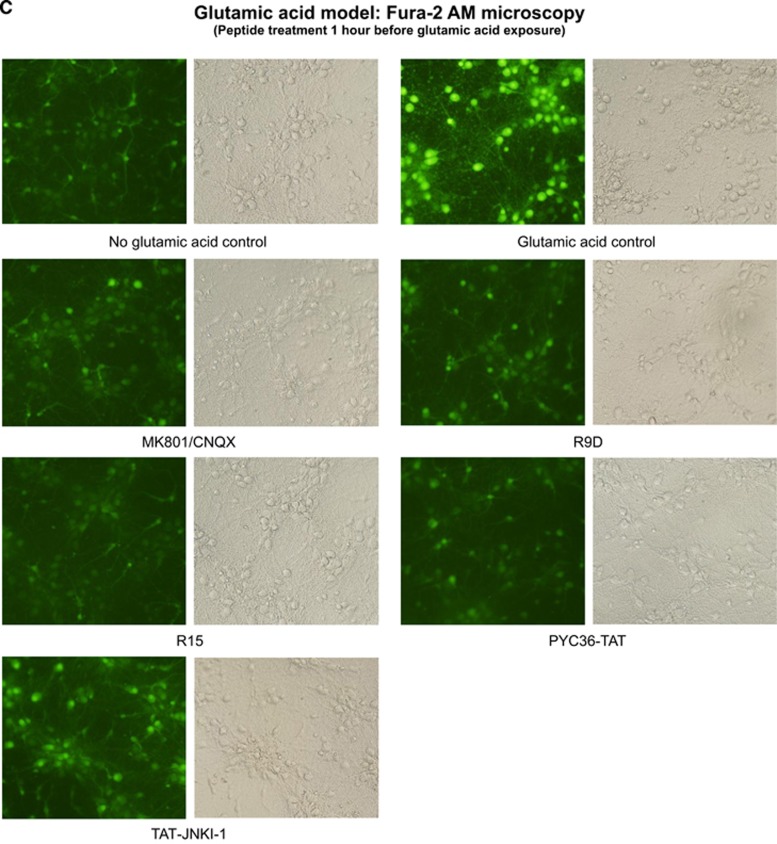
To investigate the mechanism of action of neuroprotective peptides, we compared the effects relative to controls (no peptide) of R9D (D-isoform peptide), R15, BEN1079, PYC36-TAT, TAT, TAT-NR2B9c, and JNKI-1-TATD on intracellular calcium levels follow glutamic acid exposure. All peptides to varying degrees were shown to reduce intracellular neuronal calcium levels when administered immediately before and/or 1 hour before glutamic acid exposure (Figures 4A–C). To determine whether cell surface heparan sulfate proteoglycan-mediated endocytosis is important for peptide neuroprotection,13 we pre-incubated peptides (R12, R15, R9D, PYC36-TAT) with heparin before treatment of neuronal cultures and showed that this completely eliminated peptide neuroprotective efficacy in the glutamic acid model (Figure 5A). Similarly, the presence of heparin in neuronal cultures before and during peptide treatment (R12, R15, R9D), abolished peptide neuroprotection after glutamic acid insult, while glutamate receptor blockers (MK801/CNQX) were still effective (Figure 5B).
Figure 4.
Intracellular calcium assessment using Fura-2 AM after glutamic acid exposure in neuronal cultures. (A) Fluorescent Fura-2 AM tracers; fluorescence intensity (FI) of neuronal cultures 30 seconds before and after the addition (arrow) of glutamic acid (100 μmol/L final concentration). Peptides (5 μmol/L) or glutamate receptor blockers (5 μmol/L MK801/5 μmol/L CNQX) were added to neuronal cultures for 10 minutes and removed (time=0) before glutamic acid addition. Values are mean±s.e.; n=3. (B and C) Representative images of Fura-2 AM fluorescence in neuronal cultures treated with peptides (5 μmol/L) or glutamate receptor blockers (5 μmol/L MK801/5 μmol/L CNQX) for 10 minutes immediately before or 1 hour before glutamic acid exposure (100 μmol/L). Images captured at 5 to 10 minutes after glutamic acid exposure.
Figure 5.
Glutamic acid excitotoxicity model. (A) Peptides incubated±heparin (20 IU/mL) for 5 minutes at room temperature before being added to neuronal cultures for 10 minutes only, and then removed before glutamic acid exposure. Neuronal viability measured 24 hours after glutamic acid. (B) Neuronal cultures incubated±heparin (40 IU/mL) for 5 minutes at 37 °C before the addition of peptides or glutamate receptor blockers (Blks: 5 μmol/L MK801/5 μmol/L CNQX) for 10 minutes only, and then removed before glutamic acid exposure. Neuronal viability measured 24 hours after glutamic acid. (C) Neuroprotective efficacy of JNKI-1 peptide fused to the kFGF or TAT CPPs. Peptides present in neuronal cultures for 15 minutes before and during the 5-minute glutamic acid exposure. Neuronal viability measured 12 hours after glutamic acid. Concentration of peptide in μmol/L. MTS data were expressed as percentage neuronal viability with no insult control taken as 100% viability (mean±s.d.; n=4; *P<0.05). CPP, cell-penetrating peptide; kFGF, Kaposi fibroblast growth factor; MTS, 3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt.
To establish whether the JNKI-1 peptide is neuroprotective when fused to a non-cationic, non-arginine containing CPP in the glutamic acid model, we assessed JNKI-1 fused to the kFGF peptide (kFGF-JNKI-1). The kFGF peptide (also known as MTS; membrane-translocating sequence) is derived from the hydrophobic region (h-region) of the signal sequence of Kaposi fibroblast growth factor (kFGF or FGF-4). Importantly, intracellular uptake of kFGF does not rely on endocytosis, and is thought to occur by direct translocation through the plasma membrane lipid bilayer.14 Importantly, neither the kFGF-JNKI-1 or kFGF peptides were neuroprotective, whereas the control peptides TAT-JNKI-1 and TAT were neuroprotective (Figure 5C). In line with these results, neither kFGF-JNKI-1 or kFGF significantly reduced glutamic acid-induced intracellular neuronal calcium levels (Figure 4C).
Neuroprotective Effects of Peptides in Cultured Neurons Exposed to Oxygen-Glucose Deprivation and In Vivo After Permanent MCAO
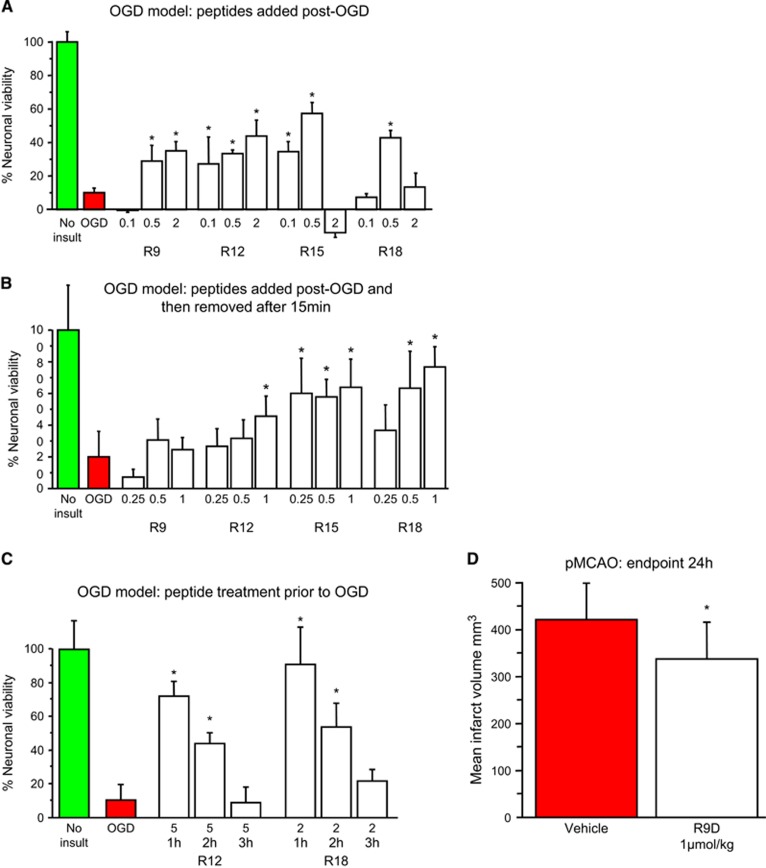
To corroborate the neuroprotective findings obtained in the glutamic acid model, and to explore the broader applicability of poly-arginine peptides, we examined their effects in another in vitro model of stroke (exposure of cultured neurons exposed to oxygen-glucose deprivation; OGD). For these experiments lower peptide concentrations were assessed, as in previous studies we have observed that high peptide concentrations can be ineffective after OGD.15 Peptides R9, R12, R15, and R18 were shown to be neuroprotective when added to neuronal cultures immediately after OGD (Figure 6A). Interestingly, adding R2, R15, or R18 to neuronal cultures immediately after OGD was neuroprotective, even when the peptides were removed (by medium replacement) after 15 minutes (Figure 6B). Similarly, exposure of neurons to peptides R12 or R18 for only 10 minutes 1 or 2 hours before OGD was neuroprotective (Figure 6C), with efficacy decreasing with increasing pre-exposure time. To explore the relevance of our in vitro findings to ischemic stroke, we intravenously administered R9D at a single dose 30 minutes after permanent MCAO in rats. When assessed 24 hours after stroke, we observed that treatment with R9D resulted in a statistically significant 20% reduction in infarct volume (Figure 6D).
Figure 6.
Oxygen-glucose deprivation (OGD) and permanent MCAO models. (A) Peptides added to neuronal cultures immediately after OGD. (B) Peptides added to neuronal cultures immediately after OGD and removed after 15 minutes. (C) Peptides present in neuronal cultures only for 10 minutes at 1 to 3 hours before OGD. Neuronal viability measured 20 to 24 hours after OGD. Concentration of peptide in μmol/L. MTS data were expressed as percentage neuronal viability with no insult control taken as 100% viability (mean±s.d.; n=4–6; *P<0.05). (D) Neuroprotective effects of the R9D peptide in permanent middle cerebral artery occlusion (MCAO) stroke model when administered intravenously 30 minutes after occlusion. Peptide dose was 1 μmol/kg (600 μL: intravneous) and infarct assessment was at 24 hours after MCAO (mean±s.d.; n=12; *P<0.05). Animal treatments were randomized and all procedures were performed masked to treatment, and physiologic measurements did not differ between the treatment groups (data not shown). MTS, 3-(4,5,dimethyliazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt.
Discussion
The study has made a number of novel findings that have implications for our understanding of the neuroprotective mechanism of action of arginine-rich CPPs with or without fusion to a neuroprotective cargo peptide. We have demonstrated that poly-arginine peptide neuroprotective potency increases with increasing length. Moreover, poly-arginine peptides are effective even when given several hours before an in vitro insult and, equally in the case of R9D, are effective in vivo at reducing ischemic brain injury in the rat when administered intravenously after permanent MCAO. In addition, several other arginine-rich peptides were shown to be neuroprotective, highlighting the importance of arginine residues in the process of neuroprotection. Regarding the mechanism of neuroprotection, we showed that the poly-arginine (R9D, R15), arginine-rich (BEN1079, TAT), and TAT-fused (JNKI-1-TATD, TAT-JNKI-1, PYC36-TAT, TAT-NR2B9c) peptides reduce neuronal intracellular calcium levels after glutamic acid exposure. Recently, the TAT-NR2Bct neuroprotective peptide has been shown to attenuate neuronal intracellular calcium influx after NMDA exposure, despite targeting the intracellular protein DAPK1.16
Our findings that the neuroprotective efficacy of arginine-rich peptides is attenuated by heparin is consistent with heparan sulfate receptor-mediated endocytic uptake being essential for neuroprotection. In support of this hypothesis, a non-arginine, non-endocytic CPP (kFGF) alone or when fused to the JNKI-1 peptide was shown to be ineffective at reducing neuronal intracellular calcium levels and neuronal cell death after glutamic acid exposure. Taken together, although other mechanisms cannot be ruled out, our findings indicate that peptide suppression of excitotoxic calcium influx is at least one mechanism underlying neuroprotection, which we have hypothesized occurs as a consequence of peptide-induced endocytic internalization of calcium ion channels and transporters. To this end, the neuroprotective peptide TAT-CBD3 has been shown to induce internalization of the NMDA NR2B subunit and the sodium calcium exchanger (NCX) proteins,17, 18 and the TAT-Src peptide internalization of NR2B.19 Although TAT-CBD3 blocks collapsing response mediator protein 2 (CRMP2) binding to the N-type voltage-gated calcium channel protein (CaV2.2), and TAT-Scr inhibits Src tyrosine protein kinase phosphorylating NR2B, it is also likely that the reduced surface expression of NR2B and NCX has occurred as a result of endocytosis during neuronal uptake of these TAT-fused peptides.
As noted in our previous study,1 the number of arginine residues and peptide net charge are important factors determining the neuroprotective efficacy. Arginine, along with lysine (K) and histidine (H; weakly charged) are the only positively charged amino acids, whereas glutamic acid (E) and aspartic acid (D) are the only negatively charged amino acids. The significance of arginine in terms of charge and chemistry (guanidine head-group) for neuroprotection is demonstrated by the finding that the charge-neutral E9/R9 peptide and the poly-lysine K10 peptide provide only minimal neuroprotection in the glutamic acid excitotoxicity model. Our data also show that for maximum neuroprotection, approximately 15 arginine residues are required. In this regard, peptides R18 and R9/X7/R9 (19 of 25 amino acids arginine) are no more potent than R15 (on the basis of efficacy at 0.1 and 0.5 μmol/L). Moreover, neuroprotective potency appears to correlate with poly-arginine peptide transduction efficiency,20 a feature that is likely to be directly related to the peptide's endocytosis inducing properties. In this regard, on the basis of our hypothesis, a peptide's endocytic properties are likely to determine its ability to reduce excitotoxic calcium influx, and its toxicity at high concentrations.21
It is also probable that cationic charge provided by lysine residues within arginine-rich peptides contributes to endosomal-mediated peptide transduction and neuroprotective efficacy. Evidence suggests that cationic peptide charge provided by arginine and lysine residues facilitates electrostatic attraction with negatively charged cell surface heparan sulfate proteoglycans.22, 23, 24 Consequent peptide hydrophobic interactions with proteoglycans mediated predominantly by arginine residues facilitates heparan sulfate clustering and endocytosis.22, 23, 24 Other amino acids or the sequence of amino acids may decrease or increase peptide neuroprotective efficacy as demonstrated for peptides fused to TAT as shown in our earlier studies. For example TAT as opposed to PYC71-TAT, and PYC36-TATD as opposed to PYC36scrambled-TATD, decreased peptide efficacy,3 whereas TATD in contrast to AM8-TATD increased peptide efficacy.2 Interestingly, alanine (A) which is commonly used as an amino acid substitute to generate negative control peptides in neuroprotection studies, has been shown to significantly impede peptide-proteoglycan binding,24 whereas tryptophan (W) residues within basic peptides can also promote proteoglycan binding and endocytosis.25, 26
There is a growing body of evidence that arginine-rich peptides (R6;27 R4W2;28 TAT-NR2Bct;16 TAT-Src;19 TAT-CBD3;17, 18, 29 TAT-3.2-III-IV30) can interfere with cell surface ion channels and transporters (NMDAR;16, 17, 18, 19, 27 VR1;28 CaV2.2;17, 18, 29 NCX;17 CaV3.330), and most likely other plasma membrane receptors. We have hypothesized that this occurs during peptide endocytosis resulting in the internalization of cell surface structures. In this regard, TAT-Src19 and TAT-CBD318 peptide-induced internalization of NR2B, and TAT-CBD317-induced internalization of NCX have been directly demonstrated. Similarly, TAT, penetratin, and R9 have been shown to induce endocytosis of TNF receptors and/or the EGF receptor in HeLa cells.31 Furthermore, and as mentioned above, the TAT-NR2Bct peptide has been shown to attenuate neuronal intracellular calcium influx after NMDA exposure.16 In the setting of ischemia, reduced levels of cell surface ion channels would reduce the excitotoxic influx of calcium and other ions, and the associated downstream pathologic pathways (e.g., activation of calpain, JNK and nitric oxide synthase). In addition, peptide-induced endocytic internalization of non-ion channel receptors such as FasR, TNFR and AQP4 on neuronal and non-neuronal plasma membranes may also be beneficial after brain ischemia. Conversely, reduced levels of potentially neuroprotective receptors and transporters (e.g., Trk and EAAT) or prolonged suppression of ion channels (e.g., NMDA) may be detrimental. Importantly, about the later point, it appears that based on the in vitro neuroprotective findings, the effects of arginine-rich peptides can be relatively brief, indicating restoration of cell surface receptor activity.
Peptide-induced endosomal receptor internalization may also explain the immediate and transient nature of the neuroprotection, and be an important mechanism of action. For example, endosomal-mediated internalization of receptors can occur within minutes,32 whereas restoration of cell surface receptor expression relies on endosomal receptor recycling33 and/or the synthesis and assembly of new receptors, a process that takes considerably more time. The neuroprotective mechanism we propose is consistent with neuronal endocytic activity4, 34, 35 associated with negatively charged cell surface-sulphated proteoglycans,36 which can promote arginine-rich CPP endosomal transduction.4, 22, 35 In addition, as mentioned previously, the escape of cargo peptides and/or proteins from endosomes is considered a highly inefficient process,9, 10 and as a consequence, endocytic cargoes are unlikely to have a significant impact within the cytoplasm. In this regard, the escape of neuroprotective peptides fused to CPPs is rarely, if ever directly, confirmed in neuroprotection studies.
Conclusion
Our findings indicate that for neuroprotective peptides, arginine (and to a lesser extent lysine and possibly tryptophan) residues in carrier peptides (e.g., R9, TAT, penetratin) and/or in cargo peptides are the critical structural components for neuroprotection and strongly suggest that poly-arginine and arginine-rich peptides may represent an exciting new class of receptor-neuromodulating agents, which could be developed into neuroprotective drugs in their own right for the treatment of a range of neurologic conditions. In this regard, although it is clear peptide arginine content is important, it is also apparent that other amino acid residues can influence peptide behavior, a feature which could be utilized to target and modulate the activity of specific neuronal and non-neuronal receptors within the CNS for the development of therapies of other neurologic disorders (e.g., pain, epilepsy, multiple sclerosis).
Acknowledgments
The authors acknowledge Norman Palmer for providing assistance with the preparation of the manuscript.
Footnotes
This study in part was supported by the Department of Neurosurgery, Sir Charles Gairdner Hospital and by a Neurotrauma Research Program of Western Australia.
RMH is the Chief Executive Officer for Phylogica Ltd Pty. KH is a Senior Scientist working for Phylogica.
References
- 1Meloni BP, Craig AJ, Milech N, Hopkins RM, Watt PM, Knuckey NW. The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell Mol Neurobiol 2014; 34: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Meade AJ, Meloni BP, Mastaglia FL, Watt PM, Knuckey NW. AP-1 inhibitory peptides attenuate in vitro cortical neuronal cell death induced by kainic acid. Brain Res 2010; 1360: 8–16. [DOI] [PubMed] [Google Scholar]
- 3Meade AJ, Meloni BP, Cross J, Bakker AJ, Fear MW, Mastaglia FL et al. AP-1 inhibitory peptides are neuroprotective following acute glutamate excitotoxicity in primary cortical neuronal cultures. J Neurochem 2010; 112: 258–270. [DOI] [PubMed] [Google Scholar]
- 4Vaslin A, Rummel C, Clarke PG. Unconjugated TAT carrier peptide protects against excitotoxicity. Neurotox Res 2009; 15: 123–126. [DOI] [PubMed] [Google Scholar]
- 5Xu W, Zhou M, Baudry M. Neuroprotection by cell permeable TAT-mGluR1 peptide in ischemia: synergy between carrier and cargo sequences. Neuroscientist 2008; 14: 409–414. [DOI] [PubMed] [Google Scholar]
- 6Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 2002; 298: 846–850. [DOI] [PubMed] [Google Scholar]
- 7Ortolano F, Colombo A, Zanier ER, Sclip A, Longhi L, Perego C et al. c-Jun N-terminal kinase pathway activation in human and experimental cerebral contusion. J Neuropathol Exp Neurol 2009; 68: 964–971. [DOI] [PubMed] [Google Scholar]
- 8Zhou M, Xu W, Liao G, Bi X, Baudry M. Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp Neurol 2009; 218: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Appelbaum JS, LaRochelle JR, Smith BA, Balkin DM, Holub JM, Schepartz A. Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm. Chem Biol 2012; 19: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Qian Z, LaRochelle JR, Jiang B, Lian W, Hard RL, Selner NG et al. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry 2014; 53: 4034–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Campbell K, Meloni BP, Knuckey NW. Combined magnesium and mild hypothermia (35 degrees C) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 hours. Brain Res 2008; 1230: 258–264. [DOI] [PubMed] [Google Scholar]
- 12Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res 2001; 61: 474–477. [PubMed] [Google Scholar]
- 13Vaslin A, Puyal J, Clarke PG. Excitotoxicity-induced endocytosis confers drug targeting in cerebral ischemia. Ann Neurol 2009; 65: 337–347. [DOI] [PubMed] [Google Scholar]
- 14Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem 1995; 270: 14255–14258. [DOI] [PubMed] [Google Scholar]
- 15Craig AJ, Meloni BP, Watt PM, Knuckey NW. Attenuation of neuronal death by peptide inhibitors of AP-1 activation in acute and delayed in vitro ischaemia (oxygen/glucose deprivation) models. Int J Pept Res Ther 2011; 17: 1–6. [Google Scholar]
- 16Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 2010; 140: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Brustovetsky T, Pellman JJ, Yang XF, Khanna R, Brustovetsky N. Collapsin response mediator protein 2 (CRMP2) interacts with N-methyl-d-aspartate (NMDA) receptor and Na+/Ca2+ exchanger and regulates their functional activity. J Biol Chem 2014; 289: 7470–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM et al. Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2). J Biol Chem 2011; 286: 37778–37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Sinai L, Duffy S, Roder JC. Src inhibition reduces NR2B surface expression and synaptic plasticity in the amygdala. Learn Mem 2010; 17: 364–371. [DOI] [PubMed] [Google Scholar]
- 20Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res 2000; 56: 318–325. [DOI] [PubMed] [Google Scholar]
- 21Cardozo AK, Buchillier V, Mathieu M, Chen J, Ortis F, Ladrière L et al. Cell-permeable peptides induce dose- and length-dependent cytotoxic effects. Biochim Biophys Acta 2007; 1768: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 22Amand HL, Rydberg HA, Fornander LH, Lincoln P, Nordén B, Esbjörner EK. Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim Biophys Acta 2012; 1818: 2669–2678. [DOI] [PubMed] [Google Scholar]
- 23Wallbrecher R, Verdurmen WP, Schmidt S, Bovee-Geurts PH, Broecker F, Reinhardt A et al. The stoichiometry of peptide-heparan sulfate binding as a determinant of uptake efficiency of cell-penetrating peptides. Cell Mol Life Sci 2014; 71: 2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Yang J, Tsutsumi H, Furuta T, Sakurai M, Mihara H. Interaction of amphiphilic α-helical cell-penetrating peptides with heparan sulphate. Org Biomol Chem 2014; 12: 4673–4681. [DOI] [PubMed] [Google Scholar]
- 25Rydberg HA, Matson M, Amand HL, Esbjörner EK, Nordén B. Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry 2012; 51: 5531–5539. [DOI] [PubMed] [Google Scholar]
- 26Bechara C, Pallerla M, Zaltsman Y, Burlina F, Alves ID, Lequin O et al. Tryptophan within basic peptide sequences triggers glycosaminoglycan-dependent endocytosis. FASEB J 2013; 27: 738–749. [DOI] [PubMed] [Google Scholar]
- 27Ferrer-Montiel AV, Merino JM, Blondelle SE, Perez-Payà E, Houghten RA, Montal M. Selected peptides targeted to the NMDA receptor channel protect neurons from excitotoxic death. Nat Biotechnol 1998; 16: 286–291. [DOI] [PubMed] [Google Scholar]
- 28Planells-Cases R, Aracil A, Merino JM, Gallar J, Pérez-Payá E, Belmonte C et al. Arginine-rich peptides are blockers of VR-1 channels with analgesic activity. FEBS Lett 2000; 481: 131–136. [DOI] [PubMed] [Google Scholar]
- 29Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat Med 2011; 17: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30García-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V et al. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron 2014; 83: 1144–1158. [DOI] [PubMed] [Google Scholar]
- 31Fotin-Mleczek M, Welte S, Mader O, Duchardt F, Fischer R, Hufnagel H et al. Cationic cell-penetrating peptides interfere with TNF signalling by induction of TNF receptor internalization. J Cell Sci 2005; 118: 3339–3351. [DOI] [PubMed] [Google Scholar]
- 32Yashunsky V, Shimron S, Lirtsman V, Weiss AM, Melamed-Book N, Golosovsky M et al. Real-time monitoring of transferrin-induced endocytic vesicle formation by mid-infrared surface plasmon resonance. Biophys J 2009; 97: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5: 121–132. [DOI] [PubMed] [Google Scholar]
- 34Parton RG, Dotti CG. Cell biology of neuronal endocytosis. J Neurosci Res 1993; 36: 1–9. [DOI] [PubMed] [Google Scholar]
- 35Vaslin A, Naegele-Tollardo S, Puyal J, Clarke PG. Excitotoxicity-induced endocytosis mediates neuroprotection by TAT-peptide-linked JNK inhibitor. J Neurochem 2011; 119: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 36Litwack ED, Stipp CS, Kumbasar A, Lander AD. Neuronal expression of glypican, a cell-surface glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan, in the adult rat nervous system. J Neurosci 1995; 14: 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 2003; 9: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 38He Z, Petesch N, Voges K, Röben W, Philipson KD. Identification of important amino acid residues of the Na+-Ca2+ exchanger inhibitory peptide, XIP. J Membr Biol 1997; 156: 149–156. [DOI] [PubMed] [Google Scholar]