Abstract
Exercise is a uniquely effective and pluripotent medicine against several noncommunicable diseases of westernised lifestyles, including protection against neurodegenerative disorders. High-intensity interval exercise training (HIT) is emerging as an effective alternative to current health-related exercise guidelines. Compared with traditional moderate-intensity continuous exercise training, HIT confers equivalent if not indeed superior metabolic, cardiac, and systemic vascular adaptation. Consequently, HIT is being promoted as a more time-efficient and practical approach to optimize health thereby reducing the burden of disease associated with physical inactivity. However, no studies to date have examined the impact of HIT on the cerebrovasculature and corresponding implications for cognitive function. This review critiques the implications of HIT for cerebrovascular function, with a focus on the mechanisms and translational impact for patient health and well-being. It also introduces similarly novel interventions currently under investigation as alternative means of accelerating exercise-induced cerebrovascular adaptation. We highlight a need for studies of the mechanisms and thereby also the optimal dose-response strategies to guide exercise prescription, and for studies to explore alternative approaches to optimize exercise outcomes in brain-related health and disease prevention. From a clinical perspective, interventions that selectively target the aging brain have the potential to prevent stroke and associated neurovascular diseases.
Keywords: aging, brain health, cerebrovascular conditioning strategies, cognition, dementia, high-intensity interval exercise training
Introduction
Strenuous physical activity (e.g., exercise) is the most accessible, effective, pluripotent, and safe intervention to improve and maintain health, as well as treat most modern chronic diseases.1, 2, 3, 4 Evidence from randomized controlled trials indicates that exercise is as effective as drug interventions in terms of mortality benefits in the secondary prevention of coronary heart disease, treatment of heart failure and prevention of diabetes, and is more beneficial than drug treatment in stroke rehabilitation.5 Thus, exercise has a significant role to play in both the prevention and treatment of disease. However, despite its clear benefits, more than one-third of the global adult population, and four-fifths of adolescents, fail to meet current public health guidelines for physical activity6, 7 (i.e., ⩾30 minutes of moderate-intensity exercise on at least 5 days of the week (⩾150 min/week), or 20 minutes of vigorous-intensity aerobic exercise training on at least 3 days of the week (⩾75 min/week)).8, 9 Inactivity appears more prevalent in higher income countries (e.g., 80% of British and 90% of American adults10, 11), particularly among the less wealthy, who also comprise the majority of these populations.12, 13 Global health statistics highlight ‘physical inactivity' as a top 10 risk factor for poor health,14 associated with an increased risk of premature cardiovascular and cerebrovascular mortality.15, 16, 17, 18 Therefore, to better harness its health benefits, we need to more effectively establish the underlying mechanisms, and therefore the role of each exercise parameter (intensity, frequency, mode, and duration) in optimizing health and well-being. This knowledge will inform exercise prescription guidelines and allow exploration of alternative approaches to access the health benefits that exercise provides for both healthy and diseased populations.
The benefits of exercise for the brain are becoming increasingly evident but remain poorly understood. Regular exercise promotes angiogenesis, neurogenesis, and synaptic plasticity,19, 20, 21 which translate into improved and more efficient cerebral perfusion and metabolism.22, 23 Such neural and vascular adaptations contribute to the maintenance of cognitive function, which declines during aging and more markedly in dementia.3, 24, 25, 26 However, the mechanisms that underpin the neuroprotective benefits of exercise remain to be established, and thus so does the rationalization of exercise parameters. Optimizing exercise to target the aging brain has the potential to prevent stroke and associated neurovascular diseases including dementia, thus reducing the global economic burden associated with the aging population. This is critical given that the societal cost of dementia was estimated at >$600 billion globally in 2010, and in the United Kingdom the cost of dementia alone almost matched the combined costs of cancer, heart disease, and stroke.27 Urgent implementation of effective countermeasures is critical to fully prepare for the challenges of the world's changing demographics and to create an equitable, affordable, and sustainable aging society for the future. Since there are no curative treatments currently available, major efforts need to focus on prevention, with emphasis on modifiable risk factors such as engagement in physical activity.
Conceptual Focus
In this review, we critically address to what extent high-intensity interval exercise training (HIT) may improve cerebrovascular function, with a focus on the mechanisms and translational impact for patient health and well-being. We begin by highlighting the potential mechanisms by which exercise can improve brain function. Next, we review evidence to illustrate the effectiveness of HIT in healthy and clinical populations associated with impaired brain function. We then discuss the potential danger that HIT may pose to the brain, and how current understanding of cerebral blood flow (CBF) regulation could be used to limit potential risk and inform novel conditioning approaches that target the brain. Finally, we introduce novel interventions that are under investigation as alternative means of accelerating exercise-induced cerebrovascular adaptation, and suggest avenues for future research.
Exercise and the functional regulation of cerebral blood flow
The regulation of CBF involves complex interactions between brain metabolic and neuronal activity, blood pressure, partial pressure of arterial carbon dioxide (PaCO2), cardiac output, and, perhaps sympathetic nervous system activity28 (see review by Ogoh and Ainslie29). Exercise affects all of these factors and their interactions.29 Traditionally, CBF during exercise was thought to be unchanged from rest;30, 31 however, more recent studies utilizing technologies with greater temporal resolution (e.g., transcranial Doppler and magnetic resonance imaging) have showed that global CBF increases with exercise intensity up to ~70% of maximal aerobic power (i.e., V̇O2max),32, 33, 34, 35 although region-specific increases only to brain areas associated with locomotion have also been suggested.36, 37 This elevation in CBF is mediated via elevations in cerebral metabolic and neuronal activity,38, 39 blood-borne molecular factors (e.g., nitric oxide (NO), vascular endothelial growth factor (VEGF)) and PaCO2;40, 41, 42 the latter two likely to have a global effect.
Increased blood flow elevates mechanical shear stress within blood vessels, which has a beneficial effect on the endothelium via Akt- (protein kinase B) dependent expression of endothelial nitric oxide synthase, NO generation, and complementary improvement of antioxidant defences (reviewed in Bolduc et al37). The increase in vascular NO bioavailability is considered as a key factor in the maintenance of cerebrovascular function and optimal regulation of CBF. While in humans much of this premise has been inferred from studying shear stress-mediated improvement in endothelial function of the systemic vasculature (e.g., via flow-mediated dilation of the brachial artery),43 extrapolating this to the cerebrovasculature seems reasonable, although with some caveats specific to high-intensity exercise as will be discussed below. Evidence from animal-based and cell-culture studies provides strong support for shear stress-mediated adaptation of the cerebrovasculature (see review by Bolduc et al37). Further, Padilla et al44 have proposed that alternative signals (i.e., circumferential stretch (cyclic strain), circulating humoral factors) to chronic exercise may act independently or synergistically with shear forces in the modulation of systemic endothelial adaptations in noncontracting tissues (e.g., the cerebrovasculature). Nevertheless, the role of different exercise parameters—and thus blood flow rate/profile—on cerebrovascular endothelium has not been studied. In the systemic vasculature of humans, however, an exercise intensity-dependent response is evident acutely;45 severe HIT46 as well as moderate-intensity continuous exercise training (MICT)47 can improve flow-mediated dilation, and MICT increases arterial compliance whereas resistance exercise reduces it.47, 48 Whether such effects translate to the cerebrovasculature is, however, complicated by other effects of intense exercise (see below).
Another key component of exercise is the increased neural activation associated with generating movement. While elevated neuronal activity will increase perfusion to meet metabolic demand (i.e., neurovascular coupling)49 and thus have an influence in shear stress-mediated adaptation, exercise also activates the expression of genes associated with neuroplasticity and stimulates neurogenesis.50, 51 These processes may thus represent a primordial constituent in the positive relationship between exercise and brain health. Different exercise parameters may influence the rate and magnitude of the neural activation, which in turn may alter the vascular response and potentially the signalling stimulus for adaptation (vascular and neural).
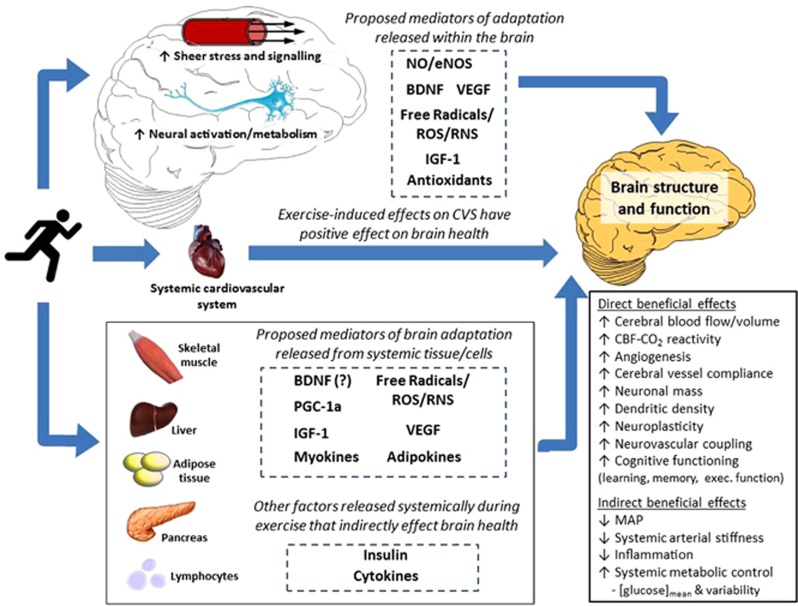
Understanding the cellular and molecular basis of exercise-induced neuroprotection is vital for optimizing exercise to improve brain health. Research to date has revealed several key exercise-induced mediators of neurogenesis, synaptic plasticity, and brain angiogenesis (e.g., brain-derived neurotrophic factor (BDNF), VEGF, insulin-like growth factor 1 (IGF-1)), along with their gene-level and humoral modulators (e.g., tropomyosin receptor kinase B, protein kinase C, GluR5, synapsin I, fibronectin type III domain containing 5, irisin; for recent reviews, see Voss et al.51and Phillips et al.52). Figure 1 illustrates such proposed local and humoral mediators of exercise-induced adaptation of brain structure and function. Much of the evidence for these cellular and molecular pathways necessarily comes from animal work, thus translation to the human remains speculative. Nevertheless, the role of exercise intensity has received very little attention even in these models, let alone in humans.
Figure 1.
Schematic summarizing proposed mechanisms by which exercise training may alter brain structure and function as well as lower the risk of brain-related dysfunction and disease via alterations in systemic function. BDNF, brain-derived neurotrophic factor; CBF, cerebral blood flow; CO2, carbon dioxide; eNOS, endothelial nitric oxide synthase; IGF-1, insulin-like growth factor 1; MAP, mean arterial blood pressure; NO, nitric oxide; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator; ROS, reactive oxygen species; RNS, reactive nitrogen species; VEGF, vascular endothelial growth factor.
Exercise perturbs redox homeostasis transiently within cells and tissues. While exercise-induced formation of free radicals and reactive oxygen (ROS) and nitrogen (RNS) species was originally suggested to cause structural tissue damage, recent evidence has shown that in physiologically controlled, albeit undefined concentrations, they serve as critical signalling molecules that mediate adaptation.53, 54 Radical species upregulate antioxidant enzymes55 and increase neurotropic factors, such as BDNF, VEGF, and IGF-1.56, 57 The Janus Face of exercise-induced oxidative-nitrosative-inflammatory stress reflects a fundamental concept known as hormesis:58 a toxicological term characterizing a biphasic dose-response encompassing a low-dose stimulation or beneficial effect and a high-dose inhibitory or toxic effect;59 thus quantifying the impact of each exercise parameter on radical species may be a crucial step in determining the best exercise strategy for optimizing brain structure and function. Accordingly, people with higher baseline oxidative-nitrosative-inflammatory stress (e.g., older or diseased) might benefit from a different prescription of exercise with respect to this mediator of (mal)adaptation.
While this review is focused on the effects of exercise on the brain, an important point to be made is that exercise confers systemic metabolic and immunomodulatory benefits. Indeed, hyperglycemia and diabetes are important risk factors for dementia,60 and systemic low-grade chronic inflammation is evident in populations with mild cognitive impairment and Alzheimer's disease.61 Numerous exercise training studies, including those using models of HIT (discussed next), have shown the efficacy of exercise as a tool to lower blood glucose levels, improve insulin sensitivity, and overall glycemic control, as well as reduce neuro-inflammation (see Figure 1).
High-Intensity Interval Exercise Training; An Emerging Paradigm
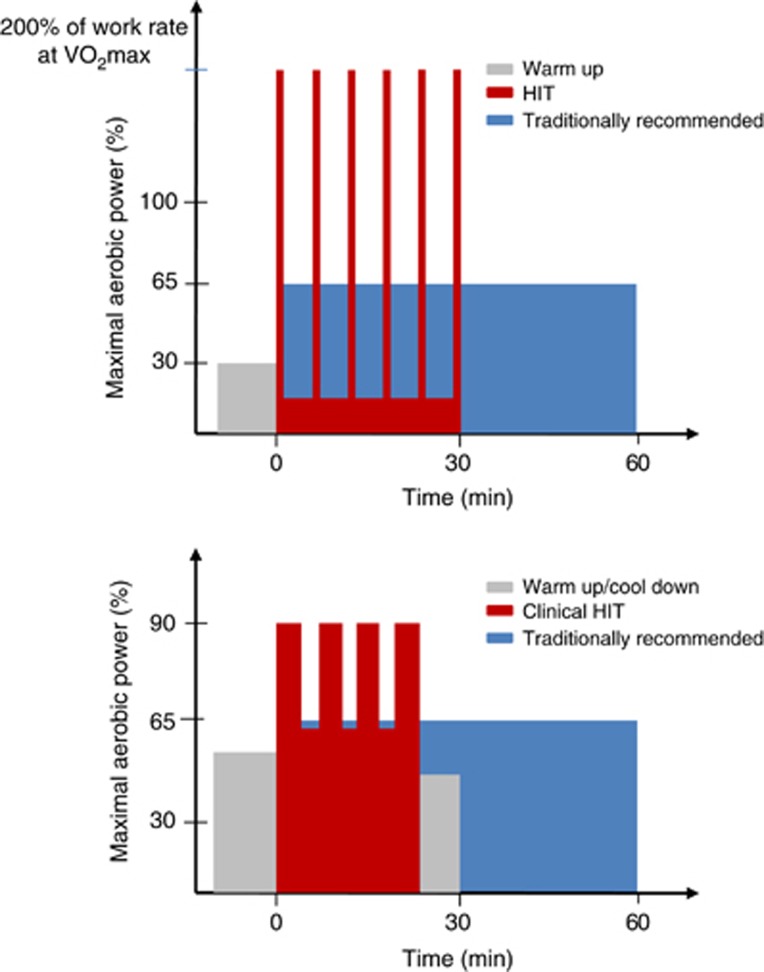
There is a burgeoning interest in HIT as an alternative means of improving health, motivated in part by the need to combat the perceived and frequently reported ‘lack of time' barrier associated with traditional exercise guidelines, which promote MICT.62, 63 There are various forms of HIT,64, 65 but it generally involves repeated bouts of relatively brief intermittent exercise, often performed at an intensity close to (~85% to 95%) or beyond maximal aerobic power.66, 67 Two examples of the HIT profile are illustrated in Figure 2.
Figure 2.
Top panel: Schematic comparing high-intensity interval exercise training (HIT) against traditional exercise guidelines recommended by leading health agencies (e.g., World Health Organization8 and American College of Sports Medicine9). The HIT protocol illustrated here consists of 6 × 30-second all-out cycling efforts separated by 4.5 minutes of active recovery at very low intensity (e.g., 30 W). This HIT protocol is typically performed three times per week, while exercise training under the traditional model consists of continuous moderate intensity cycling at 65% of maximal aerobic power (V̇O2max) for 60 minutes, five times per week. Weekly training volume is ~90% lower and time commitment ~one-third for HIT versus that of traditional aerobic exercise training.69 Bottom panel: Schematic comparing a ‘clinical HIT' protocol against traditional exercise guidelines. This HIT protocol consists of 4-minute intervals of exercise at 85% to 95% of heart rate maximum, separated by 3-minute low-intensity active recovery. A recent meta-analysis of studies utilizing this clinical model of HIT in patients with lifestyle-induced chronic cardiometabolic disease revealed that HIT induced greater reductions in blood pressure, improved blood glucose control, and increased aerobic capacity to a greater extent than did exercise conducted according to traditional guidelines; furthermore, no increase in adverse events was reported with HIT.77
Compared with traditional MICT, emerging evidence indicates that HIT provides equivalent if not indeed superior metabolic, cardiac, and systemic vascular adaptations, thereby supporting more time-efficient approaches to optimize metabolic and cardiovascular health (e.g.;64, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 see Figure 3). Such studies have provided mechanistic support for the epidemiologic observations that intensity of exercise appears more important than its duration in preventing cardiovascular disease.78, 79 High-intensity interval exercise training has also been shown to be more effective than traditional exercise interventions for cardiac function in various diseases for which there was major concern regarding its safety and appropriateness.64, 72 The evidence to date in the cardiac rehabilitation setting indicates a low risk for acute adverse cardiovascular events during HIT, albeit perhaps ~5 times higher than that observed during MICT (1 event per 23 182 hours of HIT exercise versus 1 event per 129 456 hours of MICT80). Further, a recent meta-analysis of HIT studies in patients with lifestyle-induced chronic cardiometabolic disease (coronary artery disease, heart failure, hypertension, metabolic syndrome, and obesity) reported no adverse events related to the exercise training, and revealed that HIT provided almost twice the improvement in cardiorespiratory fitness (i.e., V̇O2max)—a strong predictor of mortality17—compared with MICT (19.4% versus 10.3% increase in maximal rate of oxygen consumption; i.e., V̇O2max).77 Further, one study in hypertensive patients included within this meta-analysis reported that 12 weeks of HIT lowered blood pressure by more than twice that achieved with MICT (ambulatory 24-hour systolic blood pressure down 12 versus 4.5 mm Hg, and diastolic blood pressure down 8 versus 3.5 mm Hg). This study is noteworthy as hypertension is the single most important risk factor for stroke. However, before exercise guidelines are rewritten to make shorter bouts of higher intensity exercise a more convenient and arguably more effective option for healthy and diseased populations to consider, what are the corresponding implications for brain health? Research on the impact and potential benefits of HIT on the cerebrovasculature and corresponding implications for cognitive function is notably absent (e.g., no studies have examined even just the effects of HIT on CBF), which is surprising given the importance of brain structure and function in health and disease. Moreover, HIT may present ‘unique' dangers for the brain in the short term that warrant clinical consideration.
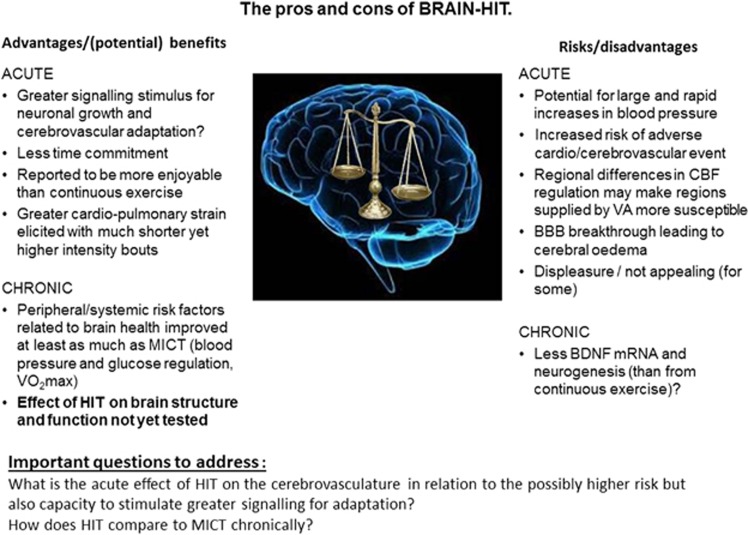
Figure 3.
The pros and cons of BRAIN-HIT. BBB; brain–blood barrier; BDNF, brain-derived neurotrophic factor; CBF, cerebral blood flow; HIT, high-intensity interval exercise training; MICT, moderate intensity continuous exercise training; VA, vertebral arteries; VV̇O2 max, maximal aerobic power.
Does High-Intensity Interval Exercise Training Pose a Danger to the Brain?
Exercise is not risk free; elevated exercise intensity in unscreened and potentially ‘at risk' populations carries an increased risk acutely, particularly in sedentary adults.81, 82 As mentioned above, this is a large proportion of the population in many countries. Furthermore, while a functional diagnostic 12-lead electrocardiogram exercise stress test exists to screen for cardiovascular abnormalities, an equivalent, universally-accepted screening process for the cerebrovasculature is lacking; thus, the development and clinical implementation of a brain-specific test could further optimize both health and safety for the brain before undertaking any exercise training program, let alone HIT.
The potential dangers of HIT to the brain are not trivial since high-intensity exercise has the capacity to elicit rapid and potentially damaging increases in systemic blood pressure that may be transmitted to the brain.83, 84 Unless countered by the neuroprotective influences of sympathetic activation or cerebral autoregulation (CA; see later), this potentially increases the risk of hyperperfusion injury predisposing to stroke or blood–brain barrier (BBB) breakthrough.85 This risk has been well publicized in the media in the United Kingdom recently, due to a high-profile clinical case of a BBC journalist claiming that the stroke suffered was caused by ‘HIT' while exercising on a rowing ergometer.86, 87 While certainty about cause-and-effect is difficult to establish, a clinical case study such as this highlights the potential safety issues associated with the HIT paradigm for the brain.
Without evidence examining the effects of HIT on the brain, any answer to the question of safety can be supported only by case studies and speculation. Ironically, the metabolic and cardiovascular efficacy of HIT was implied from studies with animals and healthy individuals mostly before being tested in metabolic- and cardiovascular-diseased populations, whereas patients with brain-related pathology have already begun using HIT-based protocols despite the lack of evidence of its cerebrovascular efficacy; e.g., in stroke rehabilitation (reviewed in Boyne et al.65) and Parkinson's disease.88 While no adverse events have been reported in studies assessing HIT for stroke rehabilitation (no events in 294 recorded HIT exercise hours among 41 patients with stroke; see Boyle et al.65), there are too few studies to make firm conclusions supporting the clinical implementation of HIT for stroke rehabilitation, let alone other brain-related pathologies. Nevertheless, the functional benefits (e.g., improved gait speed, stride length and cadence) reported by such studies are encouraging—some of which show greater improvement than traditional MICT protocols.89 Further, given the relationship between heart disease and stroke (heart disease in ~75% of patients who suffer a stroke90), the implementation of HIT in cardiovascular disease populations means that patients with potentially undiagnosed cerebrovascular pathology may already be benefiting from HIT, if indeed it is beneficial for cerebrovascular function.
In summary, the obvious and exciting positive effect(s) that HIT has shown for metabolic and cardiovascular adaptations has led to promotion of this exercise strategy among the general population and use in some clinical populations. While in general, any form of physical activity should be encouraged, it seems premature to promote HIT for better whole-body health without clear and comprehensive supporting evidence, particularly given the lack of research focused toward cerebrovascular adaptation. Nevertheless, while there is limited information documenting the impact of HIT on acute and chronic brain health, given the clear metabolic and cardiovascular benefits shown in healthy and diseased populations, abandoning or delaying the implementation of such a potentially effective strategy to optimize health is equally misplaced. Indeed, even with the potential elevated risk of an acute cardiovascular or cerebrovascular event, the health gains and cost savings in the longer term arguably outweigh potential risks, at least on a population-wide scale. Figure 3 presents a summary of the ‘pros and cons' of HIT for the brain. Notwithstanding the absence of clear evidence examining the impacts of HIT on the brain at the moment, current knowledge of CBF regulation can be applied to help optimize strategies for cerebrovascular adaptation and brain safety during training.
Optimizing Cerebrovascular Adaptation and Safety for High-Intensity Interval Exercise Training
In the context of HIT, it is important to note that the response of the cerebrovasculature to exercise is different from that of the peripheral vasculature.91 While increased perfusion during high-intensity exercise is one likely mediator of improved systemic vascular function (as detailed above), exercising above ~70% V̇O2max induces a hyperventilation-induced hypocapnia and subsequent cerebral vasoconstriction, reducing CBF toward resting values.32, 33, 34 This constrictive effect may serve as a neuroprotective response to prevent BBB disruption and hyperperfusion injury, and has been associated with improved CBF regulation during changes in blood pressure (i.e., autoregulation).92, 93, 94 However, whether this vasoconstriction is sufficient to counteract the increased cerebral perfusion pressure induced from HIT is not known. However, assuming that shear stress is necessary for the majority of adaptive cerebrovascular phenomena during exercise, and that vasoconstriction occurs at high exercise intensities sufficient to lower CBF toward baseline levels, then this shear stress may not occur, which may constrain vascular adaptation at high intensities. Therefore, perhaps limiting this vasoconstrictive effect via controlled breathing or clamping of end-tidal PCO2 strategies could optimize this stimulus-response effect; but would seem an inappropriate option for individuals with elevated risk of a hyperperfusion event, particularly given the link with impaired CA and increased PaCO2.93, 94, 95 Further, chronic exposure to elevated PaCO2 may alter cerebrovascular-CO2 responsiveness, a notion suggested by Thomas et al.96 to explain their observed blunted CBF-CO2 responsiveness in a group of life-long exercisers (i.e., Masters athletes).
It is important to note that this constrictive effect triggered by hyperventilation-induced hypocapnia during higher exercise intensities may differ depending on the brain region. Sato et al.97 identified that flow in the posterior circulation (measured via the vertebral artery (VA)) increased further at 80% than at 60% V̇O2max, whereas flow in the anterior circulation (measured via internal carotid artery (ICA) flow and middle cerebral artery velocity (MCAv)) decreased toward resting values, as has been reported previously.32 Regional differences in cerebrovascular reactivity to CO2 between the anterior and posterior cerebral circulations could partly explain this differential response, with CBF-CO2 reactivity during hypercapnia reportedly blunted in the posterior circulation (i.e., VA and posterior cerebral artery velocity) compared with the anterior circulation (ICA and/or MCAv);98, 99 although this is not a universal finding.100 Thus, the acute and chronic effect of exercise-induced changes in PaCO2 on regional CBF during and after HIT requires future research.
The effect of the systemic circulation on CBF is also important to consider.83, 91 CA requires ~5 seconds to initiate its protective influence;101 therefore, explosive HIT protocols could expose the cerebrovasculature to potentially damaging increases in perfusion pressure, particularly in diseased populations with impaired autoregulation (e.g., diabetes,102 hypertension,103 and Alzheimer's disease104). Furthermore, intense exercise can increase BBB permeability subsequent to a free radical-mediated impairment in CA, rendering the brain more susceptible to overperfusion and extracellular (vasogenic) edema; which has been shown to occur even in response to a more graduated incremental exercise test to exhaustion.85
The direct effect of sympathetic nervous activity (SNA) on the cerebrovasculature remains controversial28, 105, 106 and is further complicated in humans by the common inference of global SNA measures reflecting cerebral effects (e.g., via microneurography of SNA in the peroneal nerve versus noradrenaline spillover measurements107). In fact, studies in sheep have indicated an inverse relationship between global and cerebral SNA;108, 109 thus, increases in muscle SNA may be reflected in a lowering of cerebral SNA.110 Nevertheless, HIT will presumably elicit large increases in cerebral SNA that may act to restrict rapid increases in CBF as a consequence of the surge in blood pressure associated with high-intensity exercise.111 The asymmetrical CBF changes in response to acute hypertension compared with acute hypotension have been attributed, in part,112 to a SNA-mediated role in CBF regulation.108, 113, 114 Such a role may be vital in limiting hyperperfusion injury during HIT before CA is effective, particularly in populations with autonomic dysfunction who display impaired CBF regulation.115 With this knowledge at hand, a graduated increase in exercise intensity over the first 10 seconds may limit this hyperperfusion risk by ‘priming' the cerebrovasculature. Indeed, the HIT exercise used in the stroke rehabilitation studies reviewed by Boyne et al.65 used an incremental ramp during the initial 1 to 2 minutes of the bout. Whether the prolonged nature of this increase in intensity influences the adaptive processes, notably mechanical shear stress or consequent signalling, is unknown. Regional differences in CA have also been reported,116, 117 with that of the VA being lower than the ICA and MCA (reviewed in Ainslie and Brassard118). Therefore, impaired buffering of blood pressure during high-intensity exercise may further explain why flow is elevated in the posterior circulation during high-intensity exercise. Regions of the brain directly supplied by the VAs (e.g., midbrain and cerebellum) may therefore be more susceptible to hyperperfusion injury during HIT.
Sato et al.97 also showed that the high intensity-induced reduction in ICA flow was correlated to both the relative hypocapnia and the increased flow in the external carotid artery.97 This redistribution to the external carotid artery was suggested to illustrate that brain and head thermoregulation have higher priority for CBF regulation during heavy exercise, which is in contrast with what happens at rest during passive heating, where no regional differences are apparent and the reduction in anterior flow is mediated by reductions in end-tidal PCO2 and unrelated to increased external carotid artery flow.119 From a teleological perspective, this thermoregulatory mediated response may also serve as a neuroprotective mechanism during HIT, diverting a proportion of the elevated blood flow away from the cerebral tissue, thus attenuating the increased cerebral perfusion pressure and risk of injury to the brain. Clearly this may also have consequences for oxygen and nutrient delivery to the neurons supplied by the ICA; however, the interconnectedness between the posterior and anterior cerebral circulations via the Circle of Willis may mean that some of this reduction in ICA flow is compensated for by the VA.97 Whether such differential responses occur during short HIT bouts is unknown (Sato et al. used 5-minute steady-state intensities up to 80% V̇O2 max, whereas HIT typically involves intensities of 80% to 200% V̇O2 max imposed rapidly); however, light exercise or mild passive heating before HIT would seem advantageous to prime cutaneous blood flow to reduce the risk of brain hyperperfusion injury. Nevertheless, while potentially having a lower risk of hyperperfusion injury, the lower absolute flow could attenuate shear stress-induced adaptation (see above). In summary, improving our understanding of regional differences in CBF regulation may help improve safety during HIT.
One more factor that can influence CBF during intense exercise is the Valsalva maneuver (VM), which initially raises blood pressure and CBF120 (identified as phase I of the maneuver) and thus raises the risk of an adverse event; hence the clinical recommendation to avoid VMs during resistance training.121 Paradoxically, however, the VM may also help protect against hyperperfusion injury when large abrupt increases in blood pressure are expected (e.g., ‘super HIT exercise' for very short (~5 to 10 seconds) durations), as recently demonstrated for CBF velocity responses during load-dependent increases in blood pressure during weightlifting exercise122 and with graded VM alone.123 Though hyperperfusion may be constrained while performing the VM (phase I), presumably via elevated intracranial pressure ‘clamping' the cerebral vessels, releasing the VM elicits cerebral hypoperfusion and increases the risk of fainting, particularly if upright.124 Alternatively, this transient hypoperfusion and resultant hypoxemia may stimulate further adaptation via activation of oxygen-sensitive genes orchestrated by hypoxia-inducible factor 1-α that serve to salvage cerebral oxygen transport. Therefore the many factors affecting CBF during intense exercise are complex for both acute risks and adaptive stimuli.
Finally, avoiding certain exercise modes for HIT may limit the risk of cerebrovascular injury. For example, rowing elicits stroke-by-stroke fluctuations in blood pressure and CBF,125 and the biomechanical motion of rowing provokes changes in respiratory mechanics including timing of breaths during the stroke,126 which has been shown to uncouple the CBF response from the normal hyperventilation-induced hypocapnic lowering of CBF above 70% V̇O2max; CBF velocity in the MCA increased further during a 30-second sprint performance despite the lowering of the end-tidal PCO2.127 Therefore, during rowing, cerebral perfusion is elevated in the context of rapid and repeated fluctuations in perfusion pressure, potentially increasing the risk of hyperperfusion injury.
Optimizing Cerebrovascular Adaptation Through Brain-Targeted Training Interventions
Can interventions be tailored to maximize cerebral perfusion and optimize adaptation? In this final section, we consider strategies and discuss emerging interventions with the capacity to maximize cerebrovascular adaptation.
Elevations in shear stress can be stimulated by simple maneuvers such as repeated squat-to-stand maneuvers, which result in oscillating blood flow within a safer (i.e., lower) autoregulatory range of blood pressure as well as potentially stimulating a mechanical stress-mediated adaptive response for the cerebrovascular endothelium (see above). Recently this concept has been illustrated via water immersion, where CBF increases in a similar magnitude to that obtained during land-based exercise,128 and more so when water immersion was combined with exercise.129 How this then translates to longer-term effects (i.e., after a training intervention) has not yet been reported, but the combined strategy may result in greater improvements in cerebrovascular function than previously reported from a standard land- and aerobic-based training study.130 Such a training strategy may be a powerful adjunct to increase the shear-stress stimulus for clinical populations with impaired mobility as a consequence of injury (e.g., hip fracture) or large body mass (e.g., obese).
The neurovascular coupling relation within the brain means that particular movements can stimulate region-specific increases in CBF, that when combined could optimize global CBF (if performed at an appropriate intensity); e.g., combining handgrip exercise with lower-limb cycling. This neurovascular effect need not be restricted to motor output for various muscles, with somatosensory input potentially contributing to even greater elevations in CBF (particularly from those areas with greater representation in the cortex; e.g., face and hands). Consistent with this concept, a recent Cochrane Review has argued that a combined multimodal (aerobic and motor) intervention may compound cerebrovascular adaptation and improve cognitive function.131 Using such an approach, Vaughan et al.132 showed medium-to-large improvements in cognitive function, relative to the smaller effects reported from previously-employed single or bimodality exercise interventions.
This larger-scale activation type of approach makes intuitive sense given that motor fitness training requires additional sustained mental effort and the corresponding cognitive load is inherently greater. This is likely to engage higher-order cognitive processes that activate the prefrontal cortex, an area of the brain that is especially vulnerable to early declines associated with aging and inactivity.133 Whether such improvements are coupled with increased CBF and vascular reactivity are unknown. A higher CBF and vascular reactivity outcome seems tenable given the established links between habitual physical activity, improved cognition, and increased availability of neurotrophins and growth factors in the brain that facilitate neurogenesis, synaptic plasticity, and angiogenesis,134, 135 which as outlined above, may underpin the improved brain vascular function.136 Further, recent findings in both young137 and older138 adults indicate that vascular reactivity may mediate the positive exercise-cognition relation. Interestingly, in the context of this review, a dose-response relationship between exercise intensity and cognitive function has been reported,139, 140 which provides some rationale to test the hypothesis that HIT may prove an optimal strategy to improve cognitive function.
Thus, a different approach may be necessary when ‘training the brain' to optimize vessel adaptation and improve function. There is an urgent need to develop optimal and appropriate interventions that will target key mechanistic pathways linked to improved vascular and brain function; interventions that could supplement or indeed replace current exercise-based conditioning practices for whole-body health. High-intensity interval exercise training may prove one such strategy though the safety concerns and its mechanistic basis requires a great deal more scientific and clinical support (see Figure 3). While we have suggested some alternatives here, this list is by no means exhaustive of potential alternative or adjunct conditioning strategies. Other possibilities include: heat and hypoxia conditioning, repetitive brain stimulation (e.g., via transcranial magnetic stimulation), as well as combined nutritional and exercise-based protocols (e.g., flavonoid containing products). Future research is required to test the efficacy and effectiveness of such approaches on brain health (and whole-body health).
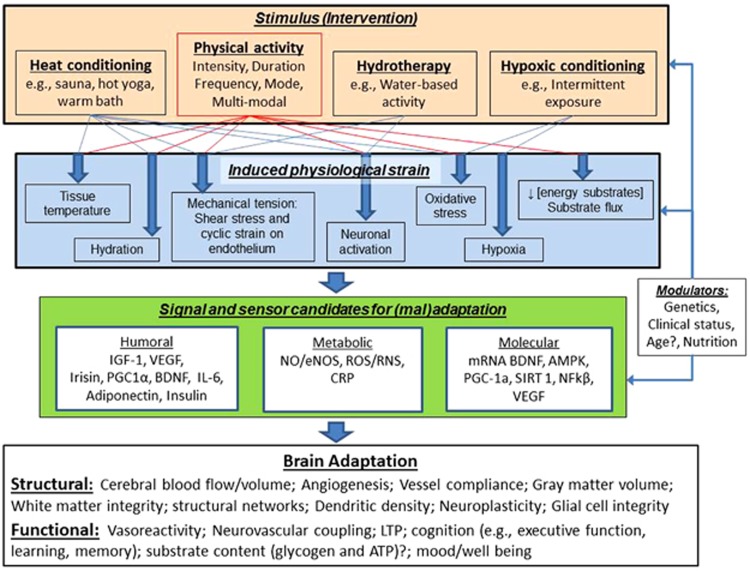
Figure 4 illustrates how exercise and other conditioning strategies may lead to beneficial adaptation of brain structure and function. A vital concept highlighted within this figure is the link between the stress imposed by an intervention and the physiologic strain that it induces. Specifically, it is the resultant strain from the stimulus (or combination of stimuli) that mediates the release of humoral and metabolic signals from various cells, tissues, and organs (see Figure 1) that then drives the molecular sensing and transcription that underpins the exercise-induced adaptation of brain structure and function. Research is needed to identify the optimal dose and combination of stimuli, as well as the processes that mediate the adaptation for such interventions. We have suggested some possible candidates that may be involved in this process based on cell culture and animal-based studies (mentioned above), as well as human-based work examining the mechanistic underpinning of vascular adaptation in the systemic circulation. Work is required to establish whether these are the pathways through which exercise and other conditioning strategies bring about positive adaptation for brain structure and function, as well as determining which intervention or combination of stimuli bring about optimal changes in brain health and which may be different depending on age, clinical status, and/or genetics. Clearly, this is a difficult but important field for future research, which may produce some unusual lifestyle interventions for optimizing brain health.
Figure 4.
This figure illustrates the potential pathways through which the components of physical activity (intensity, duration, mode, and frequency) as well as other conditioning strategies may lead to beneficial adaptation of brain structure and function. Note that the interventions/stimuli can be applied individually or in combination, and the induced physiologic strain and integration of humoral, metabolic, and molecular signalling, sensoring and transcription may be modulated by individual characteristics (age, sex, and clinical status) and/or other factors (e.g., nutritional supplements) to influence the nature of brain structure and function adaptation. AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; CRP, C-reactive protein; eNOS, endothelial nitric oxide synthase; IGF-1, insulin-like growth factor 1; IL-6, Interleukin 6; LTP, Long-term potentiation; NFkβ, nuclear factor kappa B; NO, nitric oxide; PGC-1α, Peroxisome proliferator-activated receptor-γ coactivator; RNS, reactive nitrogen species; ROS, reactive oxygen species; SIRT 1, Sirtuin 1; VEGF, vascular endothelial growth factor.
Conclusions and Recommendations for Future Research
High-intensity interval exercise training for metabolic and cardiovascular health appears generally comparable if not indeed superior to the gains incurred after traditional low-to-moderate intensity continuous exercise interventions. However, to what extent HIT impacts cerebrovascular function acutely or adaptively and the corresponding implications for cognitive function remain unknown. Research is urgently needed to address this imbalance and provide clinical practitioners with objective, evidence-based recommendations to promote safe practice and effective conditioning strategies to optimize brain health. Ideally, this evidence would come in the form of randomized controlled trials examining the effectiveness of HIT versus MICT in the short term. Overall, research is required to establish the optimal exercise strategy for the brain as well as the usefulness of alternative and adjunct strategies that might mediate beneficial adaptation, and the underlying mechanisms involved, to optimize brain function across the lifespan. Optimizing interventions that target key mechanistic pathways linked to improved vascular and brain function could ultimately protect against and/or treat cognitive decline and brain vasculature-related neurodegenerative diseases, and thus reduce the looming global economic burden that is projected to cost billions in the years to come.
The authors declare no conflict of interest.
Footnotes
Author Contributions
SJEL, JDC, PB, and DMB all contributed to the drafting of the manuscript and made critical revisions thereof. All authors have read and approved the final manuscript.
Patrice Brassard is a Junior 1 Research Scholar of the Fonds de recherche du Québec – Santé (FRQS).
References
- 1Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16: 3–63. [DOI] [PubMed] [Google Scholar]
- 2Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37: 1583–1633. [DOI] [PubMed] [Google Scholar]
- 3Lange-Asschenfeldt C, Kojda G. Alzheimer's disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp Gerontol 2008; 43: 499–504. [DOI] [PubMed] [Google Scholar]
- 4Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. New Engl J Med 2002; 347: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 5Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ 2013; 347: f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012; 380: 247–257. [DOI] [PubMed] [Google Scholar]
- 7WHOPrevalence of Insufficient Physical Activity. World Health Organization: Geneva. 2012. [Google Scholar]
- 8WHOGlobal Recommendations on Physical Activity for Health. WHO Press, World Health Organization: Geneva. 2012. [Google Scholar]
- 9Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 10Farrell L, Hollingsworth B, Propper C, Shields MA. The Socioeconomic Gradient in Physical Inactivity in England. Centre for Market and Public Organisation, University of Bristol: Bristol. 2013. [Google Scholar]
- 11Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S. adults: compliance with the physical activity guidelines for Americans. Am J Prev Med 2011; 40: 454–461. [DOI] [PubMed] [Google Scholar]
- 12Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am J Clin Nutr 2006; 83: 139–145. [DOI] [PubMed] [Google Scholar]
- 13Wilson D, Kirtland K, Ainsworth B, Addy C. Socioeconomic status and perceptions of access and safety for physical activity. Ann Behav Med 2004; 28: 20–28. [DOI] [PubMed] [Google Scholar]
- 14Murray CJL, Lopez AD. Measuring the global burden of disease. New Engl J Med 2013; 369: 448–457. [DOI] [PubMed] [Google Scholar]
- 15Paffenbarger RS, Hyde R, Wing AL, Hsieh C-C. Physical activity, all-cause mortality, and longevity of college alumni. New Engl J Med 1986; 314: 605–613. [DOI] [PubMed] [Google Scholar]
- 16Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996; 276: 205–210. [PubMed] [Google Scholar]
- 18Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011; 378: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 19Vaynman S, Gomez-Pinilla F. Revenge of the "Sit": How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res 2006; 84: 699–715. [DOI] [PubMed] [Google Scholar]
- 20Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging 2002; 23: 941–955. [DOI] [PubMed] [Google Scholar]
- 21Isaacs KR, Anderson TJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 1992; 12: 110–119. [DOI] [PubMed] [Google Scholar]
- 22Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006; 61: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 23Pantano P, Baron J, Lebrun-Grandie P, Duquesnoy N, Bousser M, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984; 15: 635–641. [DOI] [PubMed] [Google Scholar]
- 24Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol 2010; 30: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology 2006; 67: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 26Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochim Biophys Acta 2012; 1822: 474–481. [DOI] [PubMed] [Google Scholar]
- 27Wimo A, Jönsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement 2013; 9: 1–11.e3. [DOI] [PubMed] [Google Scholar]
- 28ter Laan M, van Dijk JMC, Elting JWJ, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 2013; 111: 361–367. [DOI] [PubMed] [Google Scholar]
- 29Ogoh S, Ainslie PN. Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev 2009; 37: 123–129. [DOI] [PubMed] [Google Scholar]
- 30Scheinberg P, Blackburn LI, Rich M, Saslaw M. Effects of vigorous physical exercise on cerebral circulation and metabolism. Am J Med 1954; 16: 549–554. [DOI] [PubMed] [Google Scholar]
- 31Zobl EG, Talmers FN, Christensen RC, Baer LJ. Effect of exercise on the cerebral circulation and metabolism. J Appl Physiol 1965; 20: 1289–1293. [Google Scholar]
- 32Moraine JJ, Lamotte M, Berre J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 1993; 67: 35–38. [DOI] [PubMed] [Google Scholar]
- 33Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 2008; 294: H164–H171. [DOI] [PubMed] [Google Scholar]
- 34Smith KJ, Wong LE, Eves ND, Koelwyn GJ, Smirl JD, Willie CK et al. Regional cerebral blood flow distribution during exercise: Influence of oxygen. Respir Physiol Neurobiol 2012; 184: 97–105. [DOI] [PubMed] [Google Scholar]
- 35Brugniaux JV, Marley CJ, Hodson DA, New KJ, Bailey DM. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J Cereb Blood Flow Metab 2014; 34: 1873–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Faraci FM. Protecting the brain With eNOS: run for your life. Circ Res 2006; 99: 1029–1030. [DOI] [PubMed] [Google Scholar]
- 37Bolduc V, Thorin-Trescases N, Thorin E. Endothelium-dependent control of cerebrovascular functions through age: exercise for healthy cerebrovascular aging. Am J Physiol Heart Circ Physiol 2013; 305: H620–H633. [DOI] [PubMed] [Google Scholar]
- 38Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 2008; 104: 306–314. [DOI] [PubMed] [Google Scholar]
- 39Brassard P, Seifert T, Wissenberg M, Jensen PM, Hansen CK, Secher NH. Phenylephrine decreases frontal lobe oxygenation at rest but not during moderately intense exercise. J Appl Physiol 2010; 108: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 40Olin JT, Dimmen AC, Subudhi AW, Roach RC. Cerebral blood flow and oxygenation at maximal exercise: The effect of clamping carbon dioxide. Respir Physiol Neurobiol 2010; 175: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Sato K, Moriyama M, Sadamoto T. Influence of central command on cerebral blood flow at the onset of exercise in women. Exp Physiol 2009; 94: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 42Sato K, Sadamoto T, Ueda-Sasahara C, Shibuya K, Shimizu-Okuyama S, Osada T et al. Central command and the increase in middle cerebral artery blood flow velocity during static arm exercise in women. Exp Physiol 2009; 94: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 43Tinken TM, Thijssen DHJ, Hopkins N, Black MA, Dawson EA, Minson CT et al. Impact of shear rate modulation on vascular function in humans. Hypertension 2009; 54: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology 2011; 26: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DH et al. Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med 2013; 34: 409–414. [DOI] [PubMed] [Google Scholar]
- 46Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 2008; 295: R236–R242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Seals DR, Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 2014; 117: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K et al. Unfavorable effects of resistance training on central arterial compliance. Circulation 2004; 110: 2858–2863. [DOI] [PubMed] [Google Scholar]
- 49Bélanger M, Allaman I, Magistretti Pierre J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metabolism 2011; 14: 724–738. [DOI] [PubMed] [Google Scholar]
- 50Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002; 25: 295–301. [DOI] [PubMed] [Google Scholar]
- 51Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 2013; 17: 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain a closer look at trophic factor signaling. Front Cell Neurosci 2014; 8: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the Real Polypill. Physiology 2013; 28: 330–358. [DOI] [PubMed] [Google Scholar]
- 54McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev 2002; 1: 79–93. [DOI] [PubMed] [Google Scholar]
- 55Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 2008; 44: 126–131. [DOI] [PubMed] [Google Scholar]
- 56Elfving B, Christensen T, Ratner C, Wienecke J, Klein AB. Transient activation of mTOR following forced treadmill exercise in rats. Synapse 2013; 67: 620–625. [DOI] [PubMed] [Google Scholar]
- 57Gligoroska JP, Manchevska S. The effect of physical activity on cognition - physiological mechanisms. Mater Sociomed 2012; 24: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Radak Z, Chung H, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 2005; 6: 71–75. [DOI] [PubMed] [Google Scholar]
- 59Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol Appl Pharmacol 2007; 222: 122–128. [DOI] [PubMed] [Google Scholar]
- 60Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: Dementia and Risk Reduction, an Analysis of Protective and Modifiable Factors, Alzheimer's Disease International: London 2014.
- 61Lunnon K, Ibrahim Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M et al. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer's disease blood. J Alzheimers Dis 2012; 30: 685–710. [DOI] [PubMed] [Google Scholar]
- 62Gaesser GA, Angadi SS. High-intensity interval training for health and fitness: can less be more? J Appl Physiol 2011; 111: 1540–1541. [DOI] [PubMed] [Google Scholar]
- 63Reichert FF, Barros AJD, Domingues MR, Hallal PC. The role of perceived personal barriers to engagement in leisure-time physical activity. Am J Public Health 2007; 97: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42: 587–605. [DOI] [PubMed] [Google Scholar]
- 65Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. High-intensity interval training in stroke rehabilitation. Top Stroke Rehabil 2013; 20: 317–330. [DOI] [PubMed] [Google Scholar]
- 66Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev 2008; 36: 58–63. [DOI] [PubMed] [Google Scholar]
- 67Fox EL, Bartels RL, Billings CE, Mathews DK, Bason R, Webb WM. Intensity and distance of interval training programs and changes in aerobic power. Med Sci Sports 1973; 5: 18–22. [PubMed] [Google Scholar]
- 68Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 2010; 588: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 2008; 586: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Currie KD, Dubberley JB, McKelvie RS, MacDonald MJ. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc 2013; 45: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 71Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 72Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev 2009; 37: 139–146. [DOI] [PubMed] [Google Scholar]
- 73Francois ME, Baldi JC, Manning PJ, Lucas SJE, Hawley JA, Williams MJA et al. ‘Exercise snacks' before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 2014; 57: 1437–1445. [DOI] [PubMed] [Google Scholar]
- 74Kessler H, Sisson S, Short K. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 2012; 42: 489–509. [DOI] [PubMed] [Google Scholar]
- 75Lalande S, Okazaki K, Yamazaki T, Nose H, Joyner MJ, Johnson BD. Effects of interval walking on physical fitness in middle-aged individuals. J Prim Care Community Health 2010; 1: 104–110. [DOI] [PubMed] [Google Scholar]
- 76Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol 2012; 19: 151–160. [DOI] [PubMed] [Google Scholar]
- 77Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 2014; 48: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 78Wisløff U, Nilsen TIL, Drøyvold WB, Mørkved S, Slørdahl SA, Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? ‘The HUNT study, Norway'. Eur J Cardiovasc Prev Rehabil 2006; 13: 798–804. [DOI] [PubMed] [Google Scholar]
- 79Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002; 288: 1994–2000. [DOI] [PubMed] [Google Scholar]
- 80Rognmo Ø, Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012; 126: 1436–1440. [DOI] [PubMed] [Google Scholar]
- 81Thompson PD, Buchner D, Piña IL, Balady GJ, Williams MA, Marcus BH et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107: 3109–3116. [DOI] [PubMed] [Google Scholar]
- 82Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion — protection against triggering by regular exertion. New Engl J Med 1993; 329: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 83Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Lucas SJE, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 2010; 55: 698–705. [DOI] [PubMed] [Google Scholar]
- 85Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE et al. Exercise-induced oxidative–nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood–brain barrier leakage. Exp Physiol 2011; 96: 1196–1207. [DOI] [PubMed] [Google Scholar]
- 86BBC. Andrew Marr says he's lucky to be alive after stroke. BBC News 2013. 14 April 2013; Secthttp://www.bbc.co.uk/news/uk-22141372.
- 87Bee P, Waters J After Andrew Marr blames his stroke on overdoing it on the rowing machine at 53, how risky is high-intensity exercise for the over-fifties? Daily Mail 2013 15 April 2013;Sect. http://www.dailymail.co.uk/health/article-2309565/After-Andrew-Marr-blames-stroke-overdoing-rowing-machine-53-risky-high-intensity-exercise-fifties.html#ixzz30IzsD8ZF.
- 88Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson's disease. J Appl Physiol (1985) 2014; 116: 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Lau KW, Mak MK. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J Rehabil Medicine 2011; 43: 709–713. [DOI] [PubMed] [Google Scholar]
- 90Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part 1: Classification and prevalence. Arch Phys Med Rehabil 1993; 74: 752–760. [DOI] [PubMed] [Google Scholar]
- 91Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 2009; 107: 1370–1380. [DOI] [PubMed] [Google Scholar]
- 92Brassard P, Kim Y-S, van Lieshout J, Secher NH, Rosenmeier JB. Endotoxemia reduces cerebral perfusion but enhances dynamic cerebrovascular autoregulation at reduced arterial carbon dioxide tension*. Crit Care Med 2012; 40: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 93Maggio P, Salinet ASM, Panerai RB, Robinson TG. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J Appl Physiol 2013; 115: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 1989; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 95Perry BG, Lucas SJE, Thomas KN, Cochrane DJ, Mündel T. The effect of hypercapnia on static cerebral autoregulation. Physiol Rep 2014; 2: pii: e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 2013; 38: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 2011; 589: 2847–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T et al. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 2012; 590: 3277–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99Skow RJ, MacKay CM, Tymko MM, Willie CK, Smith KJ, Ainslie PN et al. Differential cerebrovascular CO2 reactivity in anterior and posterior cerebral circulations. Respir Physiol Neurobiol 2013; 189: 76–86. [DOI] [PubMed] [Google Scholar]
- 100Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 1998; 274: H233–H241. [DOI] [PubMed] [Google Scholar]
- 102Kim Y-S, Immink RV, Stok WJ, Karemaker JM, Secher NH, van lieshout JJ. Dynamic cerebral autoregulatory capacity is affected early in Type 2 diabetes. Clin Sci 2008; 115: 255–262. [DOI] [PubMed] [Google Scholar]
- 103Immink RV, van den Born B-JH, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 2004; 110: 2241–2245. [DOI] [PubMed] [Google Scholar]
- 104den Abeelen AS, Lagro J, van Beek AH, Claassen JA. Impaired cerebral autoregulation and vasomotor reactivity in sporadic Alzheimer's disease. Curr Alzheimer Res. 2014; 11: 11–17. [DOI] [PubMed] [Google Scholar]
- 105Strandgaard S, Sigurdsson ST. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol 2008; 105: 1366–1367. [DOI] [PubMed] [Google Scholar]
- 106van Lieshout JJ, Secher NH. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol 2008; 105: 1364–1366. [DOI] [PubMed] [Google Scholar]
- 107Mitchell DA, Lambert G, Secher NH, Raven PB, van Lieshout J, Esler MD. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 2009; 587: 2589–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 109Cassaglia PA, Griffiths RI, Walker AM. Cerebral sympathetic nerve activity has a major regulatory role in the cerebral circulation in REM sleep. J Appl Physiol 2009; 106: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 110Ainslie PN. Have a safe night: intimate protection against cerebral hyperperfusion during REM sleep. J Appl Physiol 2009; 106: 1031–1033. [DOI] [PubMed] [Google Scholar]
- 111Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 1991; 19: 313–349. [PubMed] [Google Scholar]
- 112Numan T, Bain AR, Hoiland RL, Smirl JD, Lewis NC, Ainslie PN. Static autoregulation in humans: a review and reanalysis. Med Eng Phys 2014; 36: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 113Tzeng Y-C, Willie CK, Atkinson G, Lucas SJE, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 2010; 56: 268–273. [DOI] [PubMed] [Google Scholar]
- 114Perry BG, Schlader ZJ, Raman A, Cochrane DJ, Lucas SJE, Mundel T. Middle cerebral artery blood flow velocity in response to lower body positive pressure. Clin Physiol Funct Imaging 2013; 33: 483–488. [DOI] [PubMed] [Google Scholar]
- 115Ogawa M, Fukuyama H, Harada K, Kimura J. Cerebral blood flow and metabolism in multiple system atrophy of the Shy-Drager syndrome type: A PET study. J Neurol Sci 1998; 158: 173–179. [DOI] [PubMed] [Google Scholar]
- 116Haubrich C, Wendt A, Diehl RR, Klotzsch C. Dynamic autoregulation testing in the posterior cerebral artery. Stroke 2004; 35: 848–852. [DOI] [PubMed] [Google Scholar]
- 117Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 2012; 97: 1272–1280. [DOI] [PubMed] [Google Scholar]
- 118Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Reports 2014; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119Bain AR, Smith KJ, Lewis NC, Foster GE, Wildfong KW, Willie CK et al. Regional changes in brain blood flow during severe passive hyperthermia: effects of PaCO2 and extracranial blood flow. J Appl Physiol (1985) 2013; 115: 653–659. [DOI] [PubMed] [Google Scholar]
- 120Tiecks FP, Lam AM, Matta BF, Strebel S, Douville C, Newell DW. Effects of the Valsalva Maneuver on cerebral circulation in healthy adults: a Transcranial Doppler Study. Stroke 1995; 26: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 121Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B et al. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation 2000; 101: 828–833. [DOI] [PubMed] [Google Scholar]
- 122Perry BG, Schlader ZJ, Barnes MJ, Cochrane DJ, Lucas SJE, Mündel T. Hemodynamic response to upright resistance exercise: effect of load and repetition. Med Sci Sports Exerc 2014; 46: 479–487. [DOI] [PubMed] [Google Scholar]
- 123Perry BG, Mündel T, Cochrane DJ, Cotter JD, Lucas SJE. The cerebrovascular response to graded Valsalva maneuvers while standing. Physiol Rep 2014; 2: e00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124Pott F, van Lieshout JJ, Ide K, Madsen P, Secher NH. Middle cerebral artery blood velocity during a Valsalva maneuver in the standing position. J Appl Physiol 2000; 88: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 125Pott F, Knudsen L, Nowak M, Nielsen HB, Hanel B, Secher NH. Middle cerebral artery blood velocity during rowing. Acta Physiol Scand 1997; 160: 251–255. [DOI] [PubMed] [Google Scholar]
- 126Szal SE, Schoene RB. Ventilatory response to rowing and cycling in elite oarswomen. J Appl Physiol 1989; 67: 264–269. [DOI] [PubMed] [Google Scholar]
- 127Faull OK, Cotter JD, Lucas SJE. Cerebrovascular responses during rowing performance: Do circadian rhythms explain a difference between morning and afternoon performance? Scand J Med Sci Sports 2014. doi:10.1111/sms.12273. [DOI] [PubMed]
- 128Carter HH, Spence AL, Pugh CJ, Ainslie PN, Naylor LH, Green DJ. Cardiovascular responses to water immersion in humans: impact on cerebral perfusion. Am J Physiol Regul Integr Comp Physiol 2014; 306: R636–R640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129Pugh CJA, Sprung VS, Ono K, Spence AL, Thijssen DHJ, Carter HH et al. The effect of water immersion during exercise on cerebral blood flow. Med Sci Sports Exerc 2015; 47: 299–306. [DOI] [PubMed] [Google Scholar]
- 130Murrell C, Cotter J, Thomas K, Lucas SE, Williams MA, Ainslie P. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: Effect of age and 12-week exercise training. Age 2013; 35: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev, advance online publication, 16 July 2008;(3):CD005381; doi:10.1002/14651858.CD005381.pub3. [DOI] [PubMed]
- 132Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing 2014; 43: 623–629. [DOI] [PubMed] [Google Scholar]
- 133Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the lifespan. J Appl Physiol 2011; 111: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134Ratey JJ, Loehr JE. The positive impact of physical activity on cognition during adulthood: a review of underlying mechanisms, evidence and recommendations. Rev Neurosci 2011; 22: 171–185. [DOI] [PubMed] [Google Scholar]
- 135Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 2010; 298: R372–R377. [DOI] [PubMed] [Google Scholar]
- 136Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007; 30: 464–472. [DOI] [PubMed] [Google Scholar]
- 137Guiney H, Lucas SJE, Cotter JD, Machado L. Evidence cerebral blood-flow regulation mediates exercise-cognition links in healthy young adults. Neuropsychology 2015; 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 138Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 2010; 31: 2047–2057. [DOI] [PubMed] [Google Scholar]
- 139Angevaren M, Vanhees L, Wendel-Vos W, Verhaar HJJ, Aufdemkampe G, Aleman A et al. Intensity, but not duration, of physical activities is related to cognitive function. Eur J Cardiovasc Prev Rehabil 2007; 14: 825–830. [DOI] [PubMed] [Google Scholar]
- 140Brown BM, Peiffer JJ, Sohrabi HR, Mondal A, Gupta VB, Rainey-Smith SR et al. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry 2012; 2: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]