Abstract
Pericytes are mural cells with contractile properties. Here, we provide evidence that microvascular pericytes modulate cerebral blood flow in response to neuronal activity (‘functional hyperemia'). Besides their role in neurovascular coupling, pericytes are responsive to brain damage. Cerebral ischemia is associated with constrictions and death of capillary pericytes, followed by fibrotic reorganization of the ischemic tissue. The data suggest that precapillary arterioles and capillaries are major sites of hemodynamic regulation in the brain.
Keywords: hemodynamic, neurovascular coupling, pericytes, scar formation, stroke
The localization of hyperemic responses in the cerebral cortex
Neuronal activity is associated with an increase in cerebral blood flow called ‘functional hyperemia'.1 The cortical vessels mediating this response are distributed in a stereotypical pattern in rodents and humans. Namely, surface arterioles form an interconnected mesh that gives rise to ‘penetrating arterioles', which dive perpendicularly into the cortex and branch radially to generate the capillary network. Notably, the location of these penetrating vessels does not match the distribution of functional neuronal units, even where the sizes of neuronal and vascular functional domains are approximately the same.2, 3 Although the hyperemic responses quickly spread beyond the areas of neuronal activation due to the recruitment of large pial vessels, the cortical vascular system nevertheless achieves hyperemic responses that initially match neuronal units. What enables these localized responses if their canonical effectors, namely pial and penetrating arterioles, are not in the position to mediate them? Is there potential for modulation of blood flow downstream of these large vessels? The rheology in capillaries is governed by interactions between squeezed red blood cells and the vessel wall, so that small diameter changes are likely to have a large impact on blood flow.1 A capillary site of blood flow control in the central nervous system would be capable of exerting an immediate and precise control of blood flow where the metabolic demand originates.4 These considerations have directed the attention to a relative newcomer to the neuroscience field with a talent for multitasking: the pericyte.
A multitude of mural cells
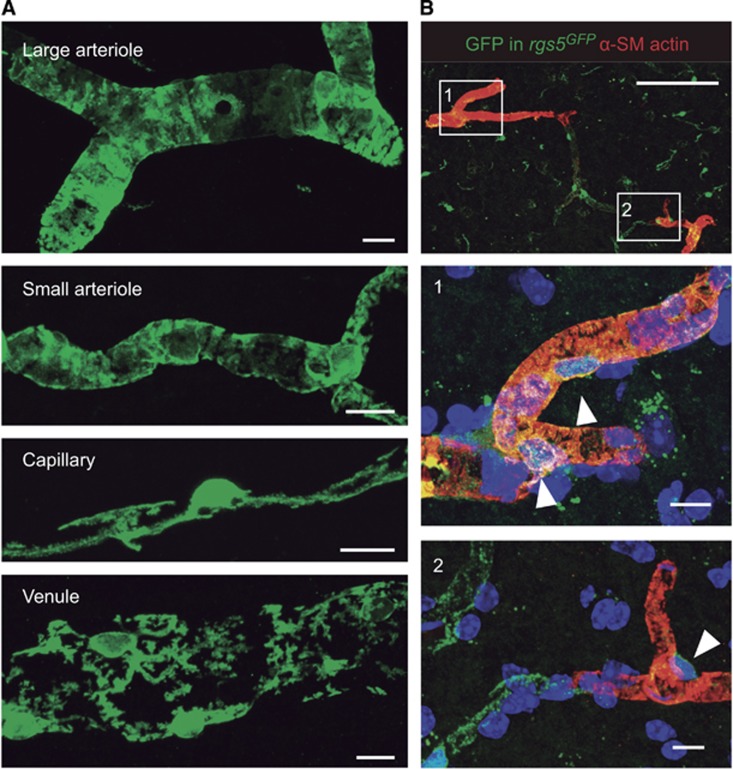
Pericytes are relatives of smooth muscle cells with a small fusiform cell soma and long processes that wrap around the vascular endothelium. They are tightly confined between sheets of the vascular basement membrane. Smooth muscle cells and pericytes compose a family of cells that populate all segments of the brain vasculature with a continuity of phenotypes (Figure 1A). The rod-shaped vascular smooth muscle cells in arterioles are replaced by more ramified cells in smaller branches. In these vascular segments, mural cell projections are circumferential and loaded with α-smooth muscle actin (Figure 1B). The mural cell processes completely cover the endothelial tube, indicative of their contractile function. In capillaries, where the main exchange of gas and metabolites across the blood–brain barrier occurs, pericytes extend longitudinal processes that do not completely cover the endothelial tube (Figures 1A and 1B). As a matter of fact, the absence of circumferential processes has been suggested to define ‘true' pericytes.5 In venules, mural cells display a mesh of cell processes (Figure 1A) that could modulate the transmigration of immune cells which occurs preferentially at this level.6 The degree of pericyte coverage of endothelial cells is inversely correlated with vascular permeability and the central nervous system has the highest pericyte density in the body.5 Pericytes are vital for the development and the maintenance of the blood–brain barrier as revealed by transgenic mouse models with partial pericyte deficiency.7, 8 They modulate the generation and regression of central nervous system capillaries to adapt to changes in oxygen availability.9
Figure 1.
Different mural cell phenotypes in the brain microcirculation. (A) In Sox10-iCreERT2 mice crossed to a green fluorescent protein (GFP) Cre-reporter strain, neural crest-derived vascular mural cells, including smooth muscle cells and pericytes, are genetically labeled with GFP.29 Large arterioles, including penetrating vessels, and their smaller branches show a complete coverage of the vessel lumen with robust circumferential mural cell processes. In contrast, capillary pericytes extend longitudinal processes that do not completely cover the endothelial tube. Pericytes in venules show a distinct stellate morphology. (B) In rgs5GFP mice, pericytes express GFP.30 Double staining for GFP (green) and α-smooth muscle (SM) actin (red) reveals the circumferential contractile machinery of pericytes (arrowheads) in penetrating vessels (1). An abrupt decrease in α-SM actin immunoreactivity is found in downstream vessels, including capillaries (2). Scale bars: 10 μm (A, B) middle and bottom panels), 100 μm (B, top panel).
Pericytes modulate cerebral blood flow during functional hyperemia
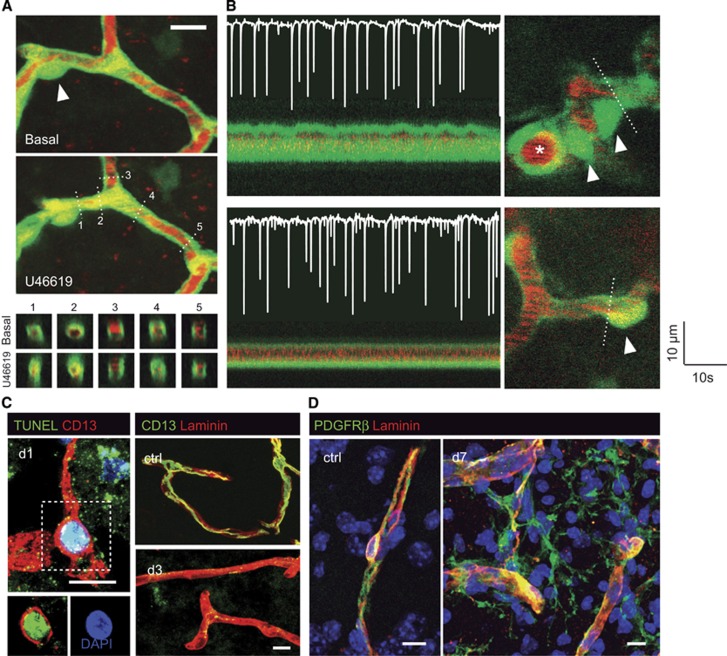
Contractile and blood flow-modulating properties have been ascribed to pericytes ever since their first descriptions by Eberth and Rouget more than a century ago. It is now well established that brain pericytes are contractile not only in culture, but also in isolated organ preparations.10, 11 Likewise, observations of vascular reactivity in response to neuronal activation have recently displaced the locus of vascular control from the immediately obvious sites at pial or penetrating arterioles to smaller downstream vessels, namely precapillary arterioles and capillaries invested with pericytes.11, 12 Using a pathologic model of functional hyperemia, we observed vascular reactivity in branches of the penetrating arterioles which we called ‘precapillary arterioles' by virtue of their higher red blood cell velocity (Figure 2B).11 In contrast, we failed to detect dilatations in capillaries with lower flow velocities (Figure 2B).11 Recently, Hall et al12 described functional responses in vessels termed capillaries by virtue of their lack of continuous coverage with mural cells using a more physiologic model of functional hyperemia. The responses to electrical whisker pad stimulation occurred more than a second earlier in these vessels than for the penetrating arterioles, suggesting that they did not just passively reflect a rise in upstream perfusion pressure. Also recently, Kornfield and Newman13 have observed that functional hyperemia in the retina was driven primarily by the active dilation of arterioles.13 Interestingly, blood flow in the retinal trilaminar vascular network was differentially regulated with the most pronounced active dilations occurring in intermediate layer capillaries. Altogether, the data suggest that pericytes in small vessels, rather than larger penetrating arterioles, are positioned at a privileged site to sense rises in neuronal activity, and effect blood flow changes.
Figure 2.
Contractility of the deep brain microcirculation. (A) Two-photon images showing the constriction of a capillary by the thromboxane A2 receptor agonist, U46619, in GFP transgenic mice in vivo.11 The plasma is labeled with TRITC (red). Note the collapse of the capillary segment in the vicinity of the pericyte body (arrowhead), while some blood flow is maintained in the capillary to the right. The bottom panels represent orthogonal reconstructions showing vessel cross-sections at the planes indicated by dashed lines. (B) Responses to neuronal activity in the first branches of a penetrating arteriole (top panel) and a downstream capillary (bottom panel). The images on the left trace the oscillations of vascular diameter observed at the planes indicated by dashed lines in the images on the right. Arrowheads point at pericyte bodies. The superimposed electrocorticogram recordings on the left show neuronal spikes elicited by the microinfusion of bicuculline into the cortex. Dilatations are present in the branches of the penetrating arteriole (top panel, marked with an asterisk), but not in capillaries (bottom panel). (C) Pericyte loss and stromal proliferation after cerebral ischemia.24 Double CD13+ TUNEL+ pericyte in the lesioned tissue 1 day after ischemia, indicative of DNA damage (left panel). Compared with nonischemic tissue, laminin-immunoreactive vascular segments lack CD13+ immunoreactivity (right panel). (D) PDGFRβ+ pericyte in nonischemic parenchyma (left panel). Proliferation of vessel-associated PDGFRβ+ stromal cells in the ischemic parenchyma 7 days after the insult (right panel). Scale bars: 10 μm.
The topological and functional measurements in recent publications3, 14 suggest that the vascular path between penetrating arterioles and ascending veins in the murine cortex could span between 10 and 15 vascular segments separated by branching points. The microvessels that responded to neuronal activity in recent publications were mainly located on the arteriolar side of the network.11, 12, 13 Irrespective of their categorization as capillaries or precapillary arterioles (denominations that lack general univocal definitions and are used inconsistently throughout the literature), there are other hints that suggest that the first branches downstream of the penetrating arterioles might be a privileged locus of blood flow regulation. Notably, these vessels carry higher flow rates than downstream capillaries. In a detailed in vivo two-photon study of the pattern of blood flow in cortical blood vessels in mice, velocities decreased about threefold over the first five branching orders downstream from penetrating arteries.14 After this number of divisions, the velocity of flow remained more or less constant at approximately 1 mm/s up to the tenth node, and also ten nodes upstream from the draining vein. This is congruent with blood flow models on the basis of network reconstructions of the marmoset cortex, where the calculated perfusion pressures decrease consecutively along the first four generations of vessels, reaching a plateau thereafter.15 The data suggest that a major decrease of the perfusion pressure, constitutive to the classical definition of resistance vessels, might extend somewhat downstream from the penetrating arterioles. Little is known about the spatial relationship between these first branches and the domains of neuronal functional units. Detailed reconstructions of the vascular network and neuronal units have dismissed a one-to-one relationship between penetrating arterioles and cortical barrels, but it would be interesting to see whether localized responses can be achieved through the concerted dilatation of these first branches of the penetrating vessels.
Functional heterogeneity of pericytes
Since we and others failed to obtain dilatory responses in capillaries to stimuli that caused dilatation in arterioles in vivo,11, 16 pericytes in different segments of the microcirculation may have different roles in hemodynamic regulation.
The expression of contractile proteins in mural cells diminishes with decreasing diameter in brain microvessels. In particular, α-smooth muscle actin, a protein central to vascular contractility, is reduced or not detectable in most brain capillaries (Figure 1B).13 Although this does not mean that capillary pericytes lack a contractile machinery (and indeed, the majority of pericytes across all vessel segments displayed diameter changes in response to in vitro stimulation11), it suggests that pericytes in different segments of the microcirculation may have different contractile properties.
Pericytes and endothelial cells are electrotonically coupled through gap channels,5 and contractile responses to electrical stimulation readily propagate between neighboring cells.10 In the ureteric microvascular network, calcium transients (which mediate long lasting tonic constrictions of pericytes in precapillary arterioles, but do not cause constrictions in capillaries) propagate across arterioles, capillaries, and venules.17 In the retina, electrotonic transmission is most efficient in capillaries.18 The spreading voltage dissipates where arterioles branch into capillaries, which could enable the integration of capillary inputs into proximal arteriolar branches. The electrotonic transmission appears to occur very efficiently between neighboring endothelial cells, but less so between endothelial cells and pericytes, or between neighboring pericytes.18 It therefore seems plausible that signals originating from pericytes in different capillary segments may be integrated and transmitted to upstream vessels by the endothelium. Pericytes and capillaries are also contacted by astrocytes, interneurons, and terminals from subcortical nuclei that affect cerebral perfusion.1, 19 Another possibility linking pericytes to the spatial control of blood flow are slow and stable changes in pericyte tone, which could affect the functional properties of the capillary network, and restrict the spread of functional hyperemia to match neuronal functional units (even if the geometry and topology of the network does not predict the boundaries, as discussed above).
Pericyte contribution to cerebral ischemia
After ischemia and reperfusion, a brief episode of hyperperfusion is followed by secondary hypoperfusion. Together with the increased metabolic needs of the peri-infarct region, the hypoperfusion contributes to the expansion of infarcted tissue beyond the boundaries of the initial perfusion deficit.20 Structural alterations of the ischemic capillary bed have been identified that could contribute to the so-called ‘no reflow phenomenon', including endothelial and astrocytic end-feet swelling.21 In addition to structural changes, functional disturbances of the capillaries may also contribute to no reflow and secondary hypoperfusion. In fact, transmission electron microscopic images of the cerebral microvasculature after ischemia revealed indentations compatible with constrictions of capillary pericytes.22 Peroxynitrite-mediated constriction of pericytes in ischemic lesions may impede the perfusion of the capillary bed even though proximal arteries are already reperfused.23 Notably, we observed that capillary pericytes rapidly die after middle cerebral artery occlusion in mice.24 These observations were later confirmed in rats.12
Multiple pathways may result in pericyte constriction and death after stroke. Reactive oxygen species cause translocation of myosin in pericytes, leading to constrictions in vitro.25 ATP and thromboxane A2 are released in the ischemic brain, and act as potent constrictors of pericytes.10, 11 Furthermore, an increase in cytosolic calcium is an intracellular signal for both apoptosis and increase in contractile tone. Contraction of the vessel wall can be maintained with very low energetic requirements (so-called ‘latch state').26 Thus, increased pericyte tone and loss of pericytes are not opposite terms, but may occur sequentially, leading to profound flow disturbances after stroke. However, constriction of pericytes during tissue damage could also represent an adaptive response related to hemostasis. Indeed, platelets release both ATP and thromboxane A2, which, as we have seen, potently constrict pericytes. Thus, pericyte constriction after stroke could help to shut down damaged microvessels, limit the breakdown of the blood–brain barrier and prevent edema, a life-threatening complication of stroke.
The demise of capillary pericytes after cerebral ischemia is associated with a fibrotic response that originates from a vascular niche and contributes to tissue remodeling.24 PDGFRβ+ stromal cells proliferate, detach from the neurovascular unit, and deposit extracellular matrix molecules in the ischemic mouse and human brain.24 The fibrotic response after stroke is reminiscent of PDGFRβ+ ‘type A' pericytes that reside abluminal to other mural cells, and give rise to scar-forming fibrotic cells that seal the injured spinal cord in GLAST-CreER mice.27 Similarly, collagen 1α1-expressing PDGFRβ+ perivascular fibroblasts were found to produce a fibrotic scar after contusive, nonpenetrating spinal cord injury.28
In conclusion, pericytes have emerged as important players in neurovascular coupling. The molecular and cellular mediators of central nervous system scarring are still being explored. Novel genetic and imaging tools will help to characterize the origin, fate, and function of the diverse mural cell populations in the healthy and diseased brain.
Acknowledgments
The authors thank Dr Guillem Genové and Drs Leda Dimou and Magdalena Götz for making available the rgs5GFP and Sox10-iCreERT2 mice, respectively.
The authors declare no conflict of interest.
Footnotes
This work was supported in part by grants from the German Research Foundation (TRR43 and FOR1336-2).
References
- 1Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Woolsey T, Rovainen C, Cox S, Henegar M, Liang G, Liu D et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex 1996; 6: 647–660. [DOI] [PubMed] [Google Scholar]
- 3Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci 2013; 16: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012; 32: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 6Proebstl D, Voisin M-B, Woodfin A, Whiteford J, D'Acquisto F, Jones GE et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 2012; 209: 1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 8Bell RD, Winkler EA, Sagare AP, Singh I, Larue B, Deane R et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal 2007; 9: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 10Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006; 443: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA 2010; 107: 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci 2014; 34: 11504–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Santisakultarm TP, Cornelius NR, Nishimura N, Schafer AI, Silver RT, Doerschuk PC et al. In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice. Am J Physiol Heart Circ Physiol 2012; 302: H1367–H1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Guibert R, Fonta C, Plouraboué F. Cerebral blood flow modeling in primate cortex. J Cereb Blood Flow Metab 2010; 30: 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Takano T, Tian G-F, Peng W, Lou N, Libionka W, Han X et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 2006; 9: 260–267. [DOI] [PubMed] [Google Scholar]
- 17Borysova L, Wray S, Eisner DA, Burdyga T. How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 2013; 54: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Zhang T, Wu DM, Xu G-Z, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol 2011; 589: 2383–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006; 100: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 20Hossmann KA, Lechtape-Gruter H. Blood flow and recovery of the cat brain after complete ischemia for 1 hour. Eur Neurol 1971; 6: 318–322. [DOI] [PubMed] [Google Scholar]
- 21Paljarvi L, Rehncrona S, Soderfeldt B, Olsson Y, Kalimo H. Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol 1983; 60: 232–240. [DOI] [PubMed] [Google Scholar]
- 22Takahashi A, Park HK, Melgar MA, Alcocer L, Pinto J, Lenzi T et al. Cerebral cortex blood flow and vascular smooth muscle contractility in a rat model of ischemia: a correlative laser Doppler flowmetric and scanning electron microscopic study. Acta Neuropathol 1997; 93: 354–368. [DOI] [PubMed] [Google Scholar]
- 23Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 24Fernández-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013; 33: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem 1999; 75: 118–129. [PubMed] [Google Scholar]
- 26Wingard CJ, Paul RJ, Murphy RA. Dependence of ATP consumption on cross-bridge phosphorylation in swine carotid smooth muscle. J Physiol 1994; 481: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science 2011; 333: 238–242. [DOI] [PubMed] [Google Scholar]
- 28Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 2013; 33: 13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Simon C, Lickert H, Götz M, Dimou L. Sox10-iCreERT2: a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis 2012; 50: 506–515. [DOI] [PubMed] [Google Scholar]
- 30Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, Genové G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol 2008; 28: 2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]