Abstract
Despite aggressive therapy, existing treatments offer poor prognosis for glioblastoma multiforme patients, in part due to poor penetration of most drugs across the blood–brain barrier (BBB). We propose a minimal-invasive combined treatment approach consisting of local BBB disruption in the tumor in parallel to systemic drug administration. Local BBB disruption is obtained by convection-enhanced delivery of a novel BBB disruption agent, enabling efficient/targeted delivery of the systemically administered drug by the tumors own vasculature. Various human serum albumin (HSA) analogs were synthesized and screened for BBB disruption efficacy in custom in vitro systems. The candidate analogs were then delivered into naïve rat brains by convection-enhanced delivery and screened for maximal BBB disruption and minimal brain toxicity. These studies found a noncationized/neutralized analog, ethylamine (EA)–HSA, to be the optimal BBB-opening agent. Immunocytochemical studies suggested that BBB disruption by EA–HSA may be explained by alterations in occludin expression. Finally, an efficacy study in rats bearing intracranial gliomas was performed. The rats were treated by convection-enhanced delivery of EA–HSA in parallel to systemic administration of Methotrexate, showing significant antineoplastic effects of the combined approached reflected in suppressed tumor growth and significantly (~x3) prolonged survival.
Keywords: blood–brain barrier, brain cancer, convection, glioma, tight junctions
Introduction
Although not highly prevalent, brain tumors are among the most lethal types of cancer. In the United States alone, more than 700,000 people are currently living with a primary brain or central nervous system (CNS) tumor diagnosis and it is reported that only 5% of diagnosed patients survive beyond 5 years (Parkin et al.1 and National Brain Tumor Society website). Malignant gliomas are the most common type of primary malignant brain tumor, accounting for 80% of patients and an annual incidence of 5.26 per 100,000 population.2 Prevalence studies estimate that 138,054 patients had a diagnosis of a primary malignant brain tumor in the United States in 2010.3
One of the major obstacles in treating brain tumors is the blood–brain barrier (BBB) that restricts the penetration of most drugs into the brain/tumor tissue. Consequently, attempts have been made to administer systemically high doses of chemotherapeutics to reach therapeutic concentrations in the brain, resulting in systemic toxicity and serious adverse effects.4 Although the BBB is compromised to some extent in malignant gliomas, making it more permeable for drugs, this disruption is not enough to enable therapeutic doses of systemically administered drugs to reach the tumor tissues. Moreover, the BBB in the infiltrating zone surrounding the tumor mass remains mostly intact restricting the penetration of drugs into these regions. For treatment to be effective, therapeutic drug doses should be delivered to the entire tumor and surrounding infiltrating zone, since survival of even a few cells may lead to cancer reoccurrence as typically takes place with high-grade gliomas.5
Cationized albumin was found to induce BBB disruption by absorptive-mediated transendocytosis6 when systemically administered in rats. Recently, we have confirmed these findings in an in vitro BBB-reflecting experimental model, composed of a brain capillary endothelial cell monolayer. Moreover, as this system permits studying brain capillary endothelial cell monolayer tightness and permeability, we found that reducing the transendothelial electrical resistance (TEER) to a large extent by either TAT peptide or cationized albumins significantly increased BBB penetration of impermeant chemotherapeutic agents.7 Systemically administered cationized albumins however are heavily taken by the liver and kidneys,8, 9 suggesting that alternative modes of administrations should be considered.
Convection-enhanced drug delivery (CED) is a novel approach for direct delivery of therapeutic agents into brain tumors, obtained by delivering continuous infusion of substances via intracranial catheters, leading to convective distribution within the tissue. This approach yields efficient drug distributions at high concentrations in brain tumors, orders of magnitude higher than those obtained by systemic administration.10 Despite the controversial results of initial clinical trials stemming from the complexity of this type of treatment, CED of therapeutic agents remains a promising strategy for treating malignant gliomas and is extensively studied. Multiple earlier stage trials have addressed only a fraction of the myriad of technical and technological issues accompanying this novel approach revealing its complexity. Development of CED has been further limited by the fact that both new technologies and novel therapeutic agents are being developed simultaneously.11 Despite the understanding that the efficacy of CED is determined by its ability to provide significant volumes of drug distribution, utilization of real-time imaging of the drug distribution has only recently been applied to clinical studies.12 The enormous potential of CED has been shown in these trials by depicting therapeutic concentrations distribution volumes in the range of 20 to 40 mL per implanted catheter.13, 14, 15, 16 After determination of the specific challenges of this approach, new trials are being planned/initiated with computerized planning of catheter location, real-time imaging, novel therapeutic agents, and novel catheters that are expected to provide more reliable drug distributions.11
Methotrexate (MTX), a folic acid analog widely used as a chemotherapeutic drug, was chosen in this study as an example of a potent antineoplastic agent which despite its effectiveness against glioma, has failed to provide clinical benefit due to its low brain penetrability.17
The goal of this study was to develop a combined approach, consisting of inducing significant local BBB disruption in the tumor and surrounding infiltration zone in parallel to systemic delivery of a chemotherapeutic agent. Since high-grade gliomas are highly vascularized tumors,18, 19 the presented approach uses the tumor's own vasculature for delivering the systemically administered drug to the target in the most natural way. In parallel, the BBB is disrupted efficiently and rapidly in the local vicinity of the tumor and infiltrating zone by local administration of the BBB-opening agent via CED.
Candidate BBB-opening agents were screened in vitro for BBB disruption efficacy. Those that were found efficient were delivered by CED into normal rat brain for further screening and optimization for maximum BBB opening with minimum brain toxicity. These studies determined the optimal BBB-opening agent to be a human serum albumin (HSA) analog, in which the carboxylates were neutralized with ethylamine (EA), which we termed EA–HSA. Finally, an efficacy study was performed in rats bearing intracranial gliomas, showing significant therapeutic benefits obtained by the administration of this analog intracranially by CED in parallel to systemically administrated MTX.
Materials and methods
Materials
Dulbecco's Modified Earl's medium (DMEM) was purchased from Gibco (Life Technologies, Carlsbad, CA, USA). Gentamicin, glutamine, new born calf serum, and penicillin/streptomycin were obtained from Biological Industries (Kibbutz Beit Haemek, Israel). Earl's Medium 199, Hoechst reagent, and hydrocortisone were purchased from Sigma (St Louis, MO, USA). For the immunocytochemistry, we used the following antibodies: mouse anti-occludin and zonula occludens-1 (ZO-1) (Zymed, Life Technologies). Cy and Alexa Fluor-conjugated secondary antibodies were acquired from Jackson Immunoresearch (West Grove, PA, USA) and Molecular Probes (Life Technologies), respectively, and used for immunocytochemistry. Alexa Fluor 488-conjugated phalloidin for the detection of F-actin was purchased from Molecular Probes. Unless otherwise mentioned, all other materials used were purchased from Sigma-Aldrich Israel Ltd (Rehovot, Israel).
Preparation of Cationized and Neutralized (Ethylamine) Human Serum Albumin
Human serum albumin (67 mg, 1 μmole) dissolved in 2.0 mL—containing 1 mol/L of 1.3 diaminopropane-2HCl, hexamethyldiamine-2HCl, Dicystamine-2HCl, argininamide-2HCl, or ethylamine-HCl. The pH was adjusted to pH 6.0±0.1. Solid EDC (100 mg, 526 μmoles) was then added, and the reaction was carried out with stirring for 4 hours at 25°C. The derivatives thus obtained were dialyzed against H2O for 2 days with several changes of H2O and lyophilized. In all analogs prepared by this procedure 45 to 85 carboxylate moieties of HSA (out of 99) were derivatized. This was quantitated by reacting an aliquot of each analog (~2 mg) with 1 mol/L glycinamide, excess EDC, in 8 mol/L urea. After dialysis, the additional glycine moieties were quantitated by amino-acid analyses after acid hydrolyses. The protein concentration was calculated according to alanine (62 residues) and valine (41 residues).
Media
Plating medium was composed of newborn calf serum (10%), L-glutamine (2 mmol/L), penicillin (100 units/mL), streptomycin (0.1 mg/mL), and gentamicin (0.1 mg/mL), all dissolved in Earl's Medium 199 (Sigma, St Louis, MO, USA). The assay medium consisted of L-glutamine (2 mmol/L), penicillin (100 units/mL), streptomycin (0.1 mg/mL), gentamicin (0.1 mg/mL), and hydrocortisone (550 nmol/L) in DMEM diluted 1:1 in Ham's F12 medium (Biological Industries).
Mono and Coculture In Vitro Blood–Brain Barrier Models and Transendothelial Electrical Resistance Measurements
Primary cultures of porcine brain endothelial cells (PBEC) were isolated from freshly collected porcine brains as described previously.20, 21 The purity of the culture was confirmed by specific staining for Von- Willebrand factor.22 Porcine brain endothelial cells were seeded at a density of 100,000 PBEC/cm2 on a microporous membrane of a Transwell insert (Corning Costar, Acton, MA, USA) placed into a 12-well plate. The cells were cultured in plating medium for up to 3 days until they reached confluence. The medium was replaced with a serum-free medium (assay medium) for an additional 24 to 48 hours and the integrity of this cellular barrier was determined by measuring TEER. This parameter reflects the impedance to the passage of small ions through the physiologic barrier and is recognized as one of the most accurate and sensitive measures of BBB integrity.23 A decrease in TEER reflects increased permeability and a loss of barrier function. Transendothelial electrical resistance of the filter insert was recorded using an Endohm chamber connected to an EVOM resistance meter (World Precision Inst., Inc., Sarasota, FL, USA). The TEER of each filter insert was calculated by subtracting the TEER of the microporous membrane without PBEC and is reported as Ωcm2. For testing the effects of the different compounds on TEER, they were diluted in assay medium at the desired concentrations, and added to the luminal (to mimic blood to brain passage) or abluminal (to mimic brain to blood passage) side of the inserts. The EA–HSA effect on TEER was also examined in a contact coculture BBB in vitro model (Supplementary Figure 1s, Supplementary Information). Glial cells were plated at the bottom of the Transwell insert membrane in addition to the PBEC seeded above as previously described.22 Contact coculture inserts exhibited an average of 30% increase in TEER in comparison with the mono culture BBB model.
Permeability of Methotrexate In Vitro
To evaluate the efficacy of MTX to permeate the BBB, we used the in vitro BBB model described above. Blood–brain barrier inserts were treated at the abluminal side with varying forms of cationized HSA and EA derivatized HSA serving as BBB-opening agents and assay medium serving as a control. Methotrexate was placed at the luminal side, and the amount that reached the abluminal side, in the presence and absence of a BBB-opening agent, was quantified by its absorption at 305 nm, using ɛ305=22,700. Permeability (Pe) values were calculated as previously described.22
Immunocytochemistry
Porcine brain endothelial cells were grown on Transwell inserts for several days until confluence was reached (TEER >300 Ωcm2). After 2 hours treatment with EA–HSA (abluminal side, 11.2 μmol/L) cells were fixed with ice-cold 4% paraformaldehyde for 10 minutes at 25°C and exposed to a blocking solution (20% horse serum/0.1% Triton/phosphate-buffered saline) for 2 hours. The PBEC were then incubated with mouse anti-occludin and rabbit anti ZO-1 antibodies at a 1:200 dilution, overnight at 4°C, washed with phosphate-buffered saline and stained with Cy3-labeled anti-rabbit or Alexa-Flour 488 anti-mouse secondary antibodies (1:200, 1 hour, room temperature). Nuclei were counterstained with Hoechst reagent for 20 seconds. After mounting (Aqua Poly/Mount), the inserts were observed and photographed using a BX43 Olympus fluorescent microscope with a DP73 Olympus camera (Olympus America Inc., Center Valley, PA, USA). Actin filaments were stained with Alexa Fluor 488-conjugated phalloidin (3 μL/insert, incubated together with the secondary antibody). Experiments were repeated at least three times.
Blood–Brain Barrier Disruption Evaluation in Normal Rat Brain—Experimental Outline
Several forms of cationized albumins and a neutralized analog (EA–HSA) of albumin were administered by CED into naïve rat brains under full anesthesia. The concentrations tested ranged from 10 μg/rat to 160 μg/rat and solvent used for the infusate varied. A list of the different derivatives, number of rats, and types of solvents are summarized in Supplementary Table 1 (Supplementary Information). The magnetic resonance imaging (MRI) contrast agent Gd-DOTA was administered intraperitoneally before CED (1 mmol/kg body weight) and BBB disruption was assessed by MRI shortly after administrating the BBB-opening agent by CED. A second MRI scan was performed on day 7 to assess possible tissue damage.
Convection Infusates for the Efficacy Study
To increase the distribution efficacy of the infusates, the viscosity of the solutions delivered by CED was increased by adding 10% sucrose.24 The following solutions were prepared: Solution #1—10% sucrose in saline, Solution #2—EA–HSA [HSA-(CONH-C2H5)85] at 0.5 mg/mL and 10% sucrose in saline.
Efficacy Study in a Rat Glioma Model—Experimental Outline
Glioma cells (rat CNS-1, 2 × 105 cells) were intracranially inoculated in three groups of rats (n=12 per group). All rats were scanned by contrast-enhanced MRI, 5 days after inoculation to determine the tumor volumes and were then divided into three groups each having similar tumor volume distributions. Immediately after the MRI the three groups were treated with (1) CED of Solution #1 and intraperitoneal (IP) saline, (2) CED of Solution #1 and IP MTX, and (3) CED of Solution #2 and IP MTX. Additional follow-up MRI scans were performed on days 2 and 7, respectively. Rats were also treated with IP MTX 2 and 4 days after the first MTX treatment (detailed experimental procedure in Table 1).
Table 1. Efficacy experiment design: groups, treatments, and time line.
| Group | Day -4 | Day 0a | Day 1 | Day 2b | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|---|---|
| Control | Tumor inoculation | Saline CED/Saline IP | Saline | Saline | Saline | Saline | Saline |
| MTX only | Tumor inoculation | Saline CED/MTX IP | FAc | MTXd | FA | MTX | FA |
| MTX+BBBe | Tumor inoculation | EA–HSA CED/MTX IP | FA | MTX | FA | MTX | FA |
Abbreviations: BBB, blood–brain barrier; EA, ethylamine; HSA, human serum albumin; IP, intraperitoneal; MRI, magnetic resonance imaging; MTX, Methotrexate.
Baseline tumor volume assessment and grouping.
MRI scanning for tumor volume assessments (follow-up MRI was conducted at day 7).
FA; folinic acid (leucovorin) injected at 8 mg/kg weight.
MTX (methotrexate) injected at 6 mg/kg weight.
MTX+BBB' group received EA–HSA intracranially by CED (convection enhanced delivery) and MTX systemically.
Animals and Ethics
The experiments were conducted according to the recommendations of the declarations of Helsinki and Tokyo and to the ARRIVE Guidelines for the use of experimental animals approved by the Animal Care Committees of Sheba Medical Center. Lewis male rats weighing 250 to 300 g (8 to 10 weeks, Harlan Biotech, Rehovot, Israel) were fed with Purina Chow and water available ad libitum. Ambient temperature was set to 22°C to 23°C with day/night light control.
Intracranial Tumor Inoculation
A midline scalp incision was made under general anesthesia to identify the bregma. A burr hole (1 mm) was then made on the right side, 3 mm anterior, and 2 mm lateral to the bregma. A 33-gauge needle attached to a 1000 μL syringe (Gastight; Hamilton, Reno, NV, USA) was placed 5.5 mm deep into the striatum. A pellet of 2x105 CNS-1 rat glioma cells precipitated in 10 μL phosphate-buffered saline buffer was infused into the striatum using a BASI syringe pump at a rate of 2 μL/min over a period of 5 minutes. The burr hole was then sealed with bone wax to avoid the tumors from growing out of the skull.
Convection-Enhanced Drug Delivery Procedure
A midline scalp incision was made under general anesthesia to identify the bregma. A burr hole (1 mm) was then made on the right side, 3 mm anterior, and 2 mm lateral to the bregma. For the tumor-bearing rats, the previously made burr hole was reopened. A 33-gauge needle attached to a 1,000-μL syringe was placed stereotactically 5.5 mm deep into the striatum. The infusion was carried out with a BASI syringe pump at a rate of 2 μL/min for a period of 20 minutes. The burr hole was then resealed with bone wax.
Magnetic Resonance Imaging Data Acquisition
Rats were scanned under general anesthesia using a clinical GE 1.5T MRI system (Optima MR450w, General Electric, Milwaukee, WI, USA) with a clinical phased array knee coil and the following sequences: contrast-enhanced T1-weighted MRI for depiction of BBB disruption and assessing tumor volumes, T2-weighted MRI for assessment of early and late toxicity and gradient-echo MRI for depiction of possible hemorrhages. All sequences were acquired with a field of view of 10 × 7 cm, 256x224 pixels and a slice thickness of 1 mm. T1-weighted MR images were acquired with a fast spin-echo sequences, bandwidth of 15.6 kHz, echo time of 16 ms, and repetition time of 494 ms. T2-weighted MR images were acquired with a fast spin-echo sequence, bandwidth of 20 kHz, echo time of 85 ms, and repetition time of 4,639 ms. Gradient-echo MR images were acquired with a flip angle of 15°, a bandwidth of 15.63 kHz, echo time of 15 ms, and repetition time of 300 ms.
Calculation of Blood–Brain Barrier Disruption Volumes and Tumor Growth Rates
The volume (in mm3) of BBB disruption or enhancing tumor volume was calculated from the contrast-enhanced T1-weighted MR images. Regions of interest were defined over the entire enhancing region for each slice (excluding the ventricles). The number of pixels in the regions of interest was then counted and multiplied by the volume of a single pixel. Tumor growth rates were calculated by dividing the tumor volumes at days 2 and 7 after treatment with the baseline tumor volumes measured at day 0.
Survival
The three groups of rats were monitored daily for survival and killed when they lost >20% of body weight and were unable to eat or drink.
Statistics
Statistics were calculated using Prism version 4.0 (GraphPad Software, CA, USA). Student's t-Test (two-tailed) was applied to detect t significant differences between two groups and one-way ANOVA with the Newman-Keuls posttest was applied to compare tumor growth rates in glioma-bearing rats. Kaplan-Meier survival curves were analyzed according to the Wilcoxon test. In all tests, P-value lower than 0.05 (P<0.05) was considered as statistically significant.
Results
Engineering a ‘Brain-Cancer Related' In Vitro Experimental System
The initial stage of this study was performed in an in vitro model, which reflects in vivo BBB integrity and penetration, or the lack of it, for a variety of substances.20 It resembles BBB in vivo in distinct criteria, including the efficacy of albumin after its cationization, to permeate this PBEC monolayer (PBEC-M).7 Moreover, recently we found that cationized albumins disrupt PBEC-M, to a level enabling the luminal to the albuminal penetration of impermeant chemotherapeutic agents to a great extent.7
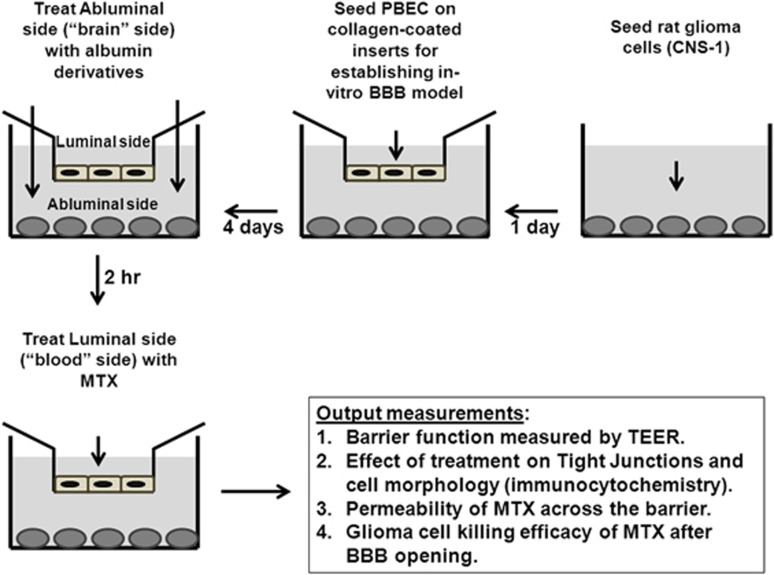
To fit with this study, we further modified this system to a model defined by us as the ‘brain-cancer-related-three component in vitro experimental system' (Figure 1) where the three components are the malignant glioma cells, that were plated at the abluminal (brain-like) side; MTX, that was added at the luminal (blood-like) side; and the BBB-opening agent, which if properly applied, permits the penetration of MTX at a desirable rate and at sufficient concentrations required to destruct the malignant gliomas, located at the brain side. All BBB-opening agents prepared by us were screened using this experimental system and optimized for determining the appropriate doses needed and time scales to be applied. Figure 1 shows the details of this system and its optional outcomes.
Figure 1.
Schematic description of the blood–brain barrier reflecting in vitro experimental system.
Screening Albumin Analogs for Inducing Blood–Brain Barrier Disruption In Vitro and In Vivo
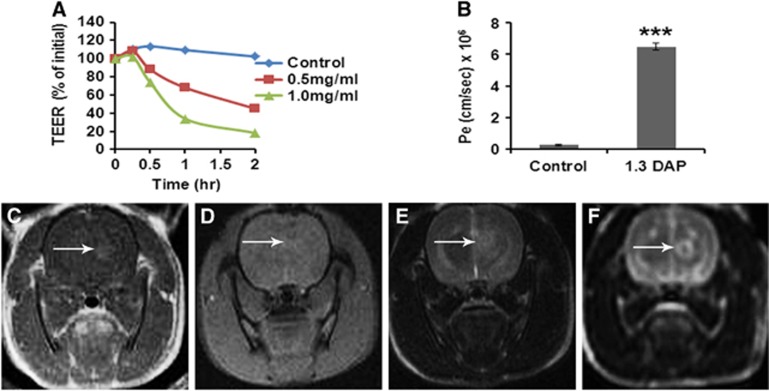
A family of cationized albumins was prepared and optimized in vitro for disrupting PBEC-M and in vivo for inducing maximum BBB disruption with minimum toxicity. In all cationized albumins, the carboxylate moieties (45 to 85 out of the 99 carboxylates of the protein) were turned into positively charged residues by the covalent linkage of 1.3-DAP (diaminopropane), 1.6 diaminohexane, dicystamine, or arginineamide. Most screened molecules disrupted the PBEC-M, allowing penetration of impermeable agents at a concentration range of 3 to 30 μmol/L. A representative experiment showing the time and concentration dependent decrease in TEER induced by 1.3 DAP cationized albumin and the permeability to MTX is shown in Figures 2A and 2B. Methotrexate per se did not decrease TEER values (not shown).
Figure 2.
In vitro and in vivo effects of 1.3 DAP cationized albumin on the blood–brain barrier (BBB). (A) In vitro barrier disruption as a function of time as reflected by the reduction in transendothelial electrical resistance (TEER) value (TEER at time 0>300 Ωcm2). (B) In vitro permeability (Pe) of methrotrexate (1 mmol/L) to the abluminal side in control (nontreated) porcine brain endothelial cells monolayer (PBEC-M) (left column) and in 1.3 DAP-treated PBEC-M (14 μmol/L, 2 hours at 37°C, right column). Data presented as the mean±s.e.m. values of three inserts per treatment. ***P<0.001 versus control. (C–F) Intracranial convection-enhanced drug delivery (CED) administration of 1.3 DAP cationized-HSA in naive rats. 1.3 DAP cationized-HSA at 40 μg/rat was infused into the rat brains. T1-weighted MR images acquired 30 minutes after treatment (C) are shown. Gradient-echo magnetic resonance (MR) image acquired immediately post treatment (D). T2-weighted images acquired immediately after treatment (E) and T2-weighted images acquired 7 days after treatment (F). The images reflect BBB disruption (C, indicated by arrows), lack of hemorrhages (D) and tissue damage (E, F). The T2-weighted MR images acquired immediately post CED (E) show enhancement in the treated region induced by the convective distribution of the infusate. HSA, human serum albumin.
All cationized albumins were subsequently studied in vivo, where they were administered by CED into naïve rat brains. Varying doses were used, all dissolved either in saline containing 30 mg/mL HSA or in saline with 10% sucrose for increased viscosity, which has been previously shown to provide efficient distributions in the brain.24 All screened cationized albumins were found unsatisfactory after testing them in vivo by CED due to either insufficient BBB opening and tissue toxicity. Examples of MR images acquired after CED of 40 μg/rat 1.3DAP cationized HSA are shown in Figures 2C and 2F.
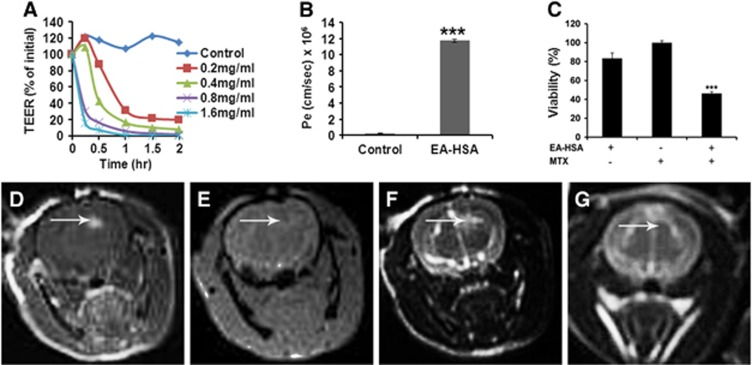
An exception was an analog of HSA in which the carboxylates were derivatized with EA, turning them into noncharged residues. This neutralized N-hexyl amidated albumin analog [HSA-CONH(C2H5)85], which we termed EA–HSA, was found suitable in all respects. It reduced TEER values of PBEC-M in a dose and time-dependent manner and yielded a Pe value for the penetration of MTX of 11.74±1.3 × 10−6 cm/s which is a 48-fold increase over control (Figures 3A and 3B). In addition, EA–HSA showed a similar pattern of dose-dependent decrease in TEER with slower kinetics attributed to the buffering capacity of the astrocytes when applied at the abluminal side in the contact coculture in vitro model (Supplementary Figure 1s, Supplementary Information).
Figure 3.
In vitro and in vivo effects of EA–HSA. (A) In vitro barrier disruption as a function of time as reflected by the reduction in transendothelial electrical resistance (TEER) value (TEER at time 0>300 Ωcm2). (B) In vitro permeability (Pe) of methrotrexate (1 mmol/L) to the abluminal side in control (nontreated) porcine brain endothelial cells monolayer (PBEC-M) (left column) and in EA–HSA-treated PBEC-M (14 μmol/L, 2 hours at 37°C, right column). Data presented as the mean±s.e.m. values of 3 to 5 inserts per treatment. ***P<0.001 versus control. (C) Reduced glioma (CNS-1 cells) viability at the ‘brain' side (Figure 1) after treatment of PBEC-M with EA–HSA and Methotrexate (MTX) at the ‘blood' side in the ‘brain cancer-related in vitro experimental system'. Data presented as the mean±s.e.m. values of 4 inserts per treatment. ***P<0.001 versus controls. (D–G) Intracranial convection-enhanced drug delivery (CED) administration of EA–HSA in naive rats. EA–HSA at 20 μg/rat was infused into the rats brains. T1-weighted MR images acquired 30 minutes after treatment (D) are shown. Gradient-echo MR image acquired immediately post treatment (E). T2-weighted images acquired immediately after treatment (F) and T2-weighted images acquired 7 days after treatment (G). The images reflect blood–brain barrier (BBB) disruption (D, indicated by arrows), lack of hemorrhages (E) and tissue damage (F) or the lack of it (G) after 1 week. The T2-weighted MR images acquired immediately post CED (F) show enhancement in the treated region induced by the convective distribution of the infusate. EA, ethylamine; HSA, human serum albumin; CNS, central nervous system.
The antineoplastic efficacy of this analog against glioma cells located at the brain side was validated in the ‘brain cancer-related' experimental system (Figures 1 and 3C). Therefore, EA–HSA at a concentration as low as 5.6 μmol/L, reduced TEER by 90% to 98% within a short period (Figure 3A) thus enabling MTX entry at a sufficient rate (Figure 3B) to yield 50% abluminal located glioma cell killing, within a period of 48 hours (Figure 3C).
Ethylamine–human serum albumin passed the in vivo screening as well. Twenty-nine rats were treated with concentrations ranging from 10 μg/rat to 40 μg/rat. Examples of MR images acquired after CED of EA–HSA at 20 μg/rat into normal rat brain are shown in Figures 3D and 3G. A significant BBB opening was depicted as bright enhancement on the T1-weighted MR images acquired 30 minutes after treatment (Figure 3D). Gradient-echo MR images showed no procedure-related hemorrhages (Figure 3E) and T2-weighted MR images acquired 7 days after treatment showed no tissue toxicity (Figure 3G). One week after CED, BBB was fully recovered as well (Supplementary Figure 2s, Supplementary Information).
Alterations in the Porcine Brain Endothelial Cells Monolayer after Treatment With Ethylamine–Human Serum Albumin
Figures 4 and 5 show typical results of our immunocytochemical studies, which aimed to identify alterations in tight junction (TJ)-related membrane protein(s). These studies were performed at a stage when TEER has been reduced by EA–HSA to a level permitting the paracellular passage of impermeable substances.
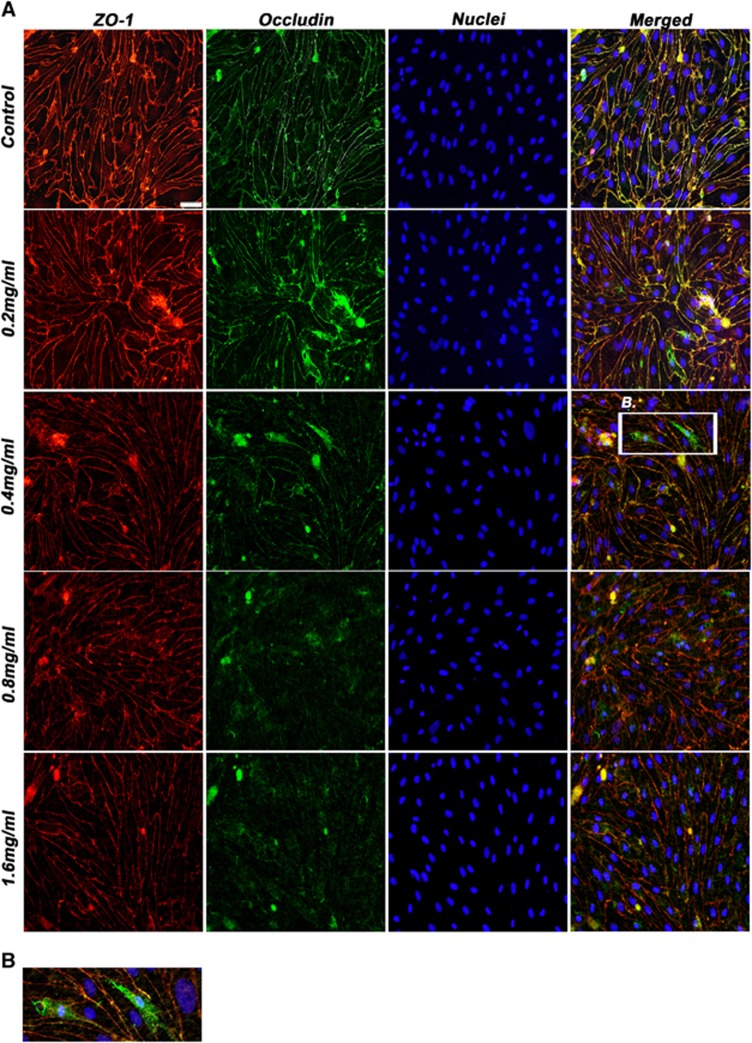
Figure 4.
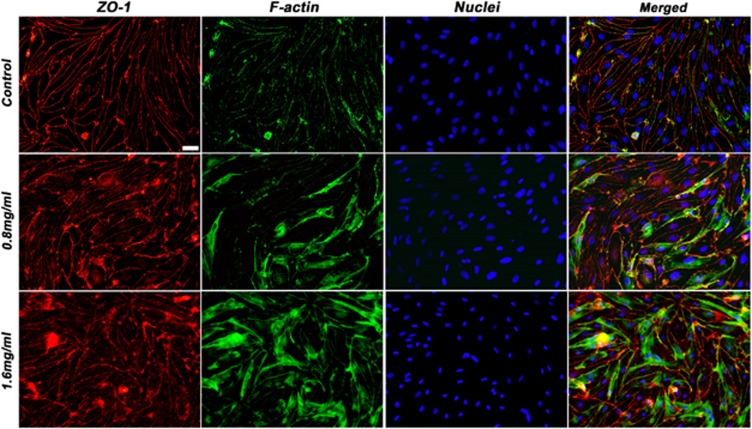
Expression of tight junction proteins after exposure to EA–HSA. (A) Porcine brain endothelial cells monolayer (PBEC-M) inserts were treated with increasing concentrations (shown from upper to lower panels) of EA–HSA at the abluminal side for 2 hours. Immunostaining of occludin and zonula occludens-1 (ZO-1) was then performed. Nuclei were stained with Hoechst reagent. Merged pictures are shown in the right column. (B) Zoomed region from the 0.4 mg/mL treated group showing the migration of occludin (in green) from the cell borders into the cytoplasm. Representative pictures are displayed from five different experiments. Bar 20 μm. EA, ethylamine; HSA, human serum albumin.
Figure 5.
Stress fibers formation after exposure to EA–HSA. Porcine brain endothelial cells monolayer (PBEC-M) inserts were treated with 11.2 μmol/L EA–HSA at the abluminal side for 2 hours. Immunostaining of zonula occludens-1 (ZO-1) and actin filaments was then performed. Nuclei were counterstained with Hoechst reagent. Representative pictures are displayed from four different experiments. Bar 20 μm. EA, ethylamine; HSA, human serum albumin.
Occludin and ZO-1 act as the membrane cell to cell connecting proteins and the assembly of the TJ proteins to the cytoskeleton, respectively.25, 26 Occludin is a major TJ protein responsible for the blockade of paracellular passage of molecules. Figure 4 shows that indeed the expression of this protein was significantly altered after incubation with EA–HSA while the expression pattern of ZO-1 was only slightly altered. Occludin has migrated from its location at the cell borders into the cytoplasm and degraded there (Figures 4A and 4B). zonula occludens-1, however, preserved its membrane location and showed minor alterations only at high concentrations of this BBB disrupting agent (Figure 4A).
Actin reorganization has an important role in the cells structural support and may also have an active role in the formation and maintenance of TJ expression and patterns of distribution.27 To examine whether actin fibers have a role in the process of EA–HSA-induced BBB opening, we treated PBEC-M at the abluminal side with increasing concentrations of EA–HSA. Cells were immunostained for ZO-1 and for fibrous actin. As shown in Figure 5, disorganization of actin filaments did take place to a certain extent. Thus, the observed reduced PBEC-M tightness and the passage of impermeable agents such as MTX (Figures 3A and 3B) appear to be manifested by disruption of occludin that is followed or accompanied by the reorganization of actin.
Overcoming Systemic Toxicity of Peripherally Administered Methotrexate in the Naive Rat Model
Initially, we have attempted to administer MTX per se at a dosage of 6 mg/kg body weight on days 0, 2, and 4, respectively (n=26 rats). This mode of administration was found inappropriate. Symptomatic toxicity, reflected by weight loss, diarrhea, mucositis, and shaggy fur, appeared within a short period after administration and this protocol was therefore abundant. This toxicity was fully avoided upon including folinic acid (leucovorin) into the scheme. Naive rats (n=3) treated by the same MTX protocol with the addition of folinic acid showed no toxicity as reflected by normal weight gain and no visible chemotherapy-dependent symptoms observed over a period of 2 weeks. The detailed MTX/folinic acid treatment protocol is summarized in Table 1.
Combined Convection-Enhanced Drug Delivery of Ethylamine–Human Serum Albumin and Methotrexate Therapy Suppressed Tumor Growth and Prolonged Survival in Rats Bearing Intracranial Glioma Tumors
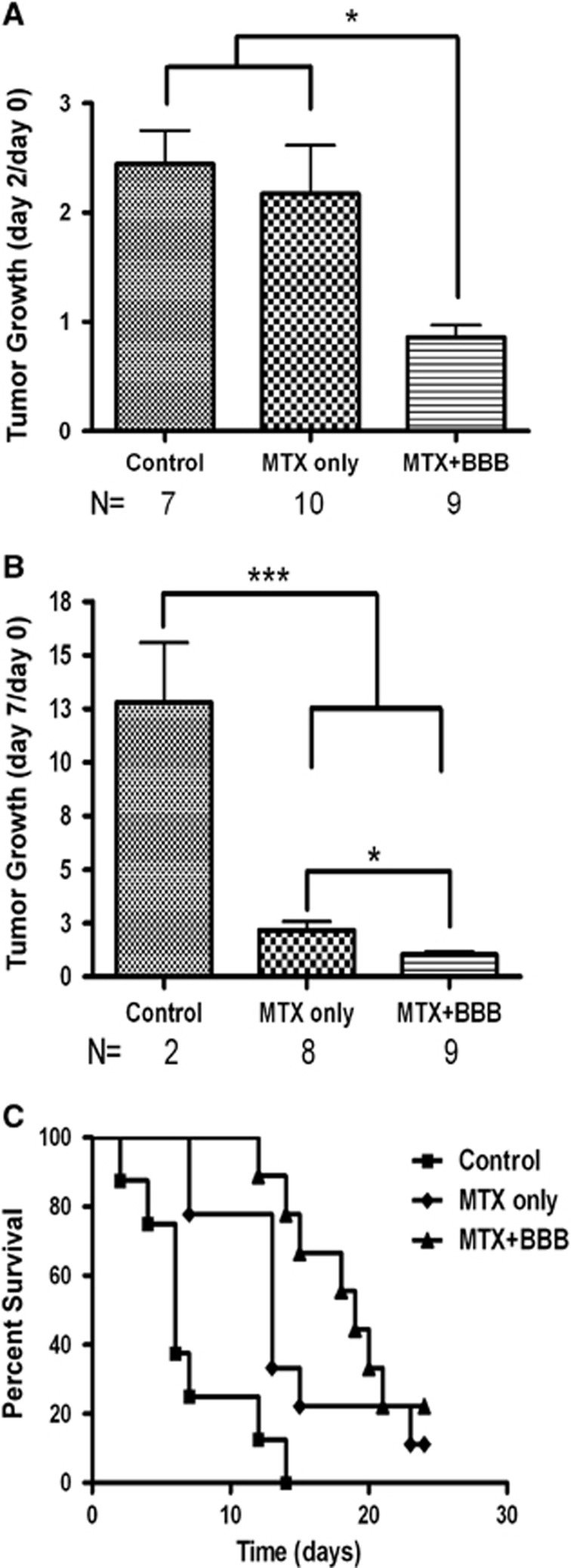
Figure 6 summarizes the outcome of the combined treatment in the glioma rat model. In this model, sham-treated rats showed increased average tumor volumes of 2.4±0.3 folds within the first 2 days and 12.8±2.8 folds within 1 week after CED. Systemically administered MTX alone did not facilitate significant effect in suppressing tumor growth rate in the first 2 days after treatment (mean values 2.2±0.4 and 2.5±0.3 for the MTX-treated and control groups, respectively, Figure 6A). Methotrexate alone however facilitated a significant effect between days 2 and 7 (mean values 2.2±0.4 and 12.8±1.5 in the MTX and the control groups, respectively, Figure 6B).The combined treatment of systemic MTX together with CED of EA–HSA fully suppressed tumor growth, leaving it nearly at the volume measured at day 0 (0.9±0.1 and 1.0±0.1 for day 2 and day 7, respectively). The effect of MTX per se (‘MTX only' group) on days 2 to 7 is of significant interest. It suggests that the BBB becomes leaky to the penetration of MTX when tumor volume is increased 2.5 times (above 86.1±13.6 mm3, day 2) or more in size in this rat glioma model. It also implies that no such blood to brain penetration of MTX would take place when the growth in tumor volume is fully suppressed (Figure 6B).
Figure 6.
Rates of tumor growth and survival after the combined EA–HSA–MTX therapy in the glioma rat model. Tumor growth rates (A, B) for the three treatment groups: Control (sham treated, n=7, left columns); MTX only (n=10, middle columns) and combined EA–HSA–MTX therapy (MTX+BBB, n=9, right columns) are shown. Tumor volumes were calculated from the T1-weighted magnetic resonance (MR) images and normalized to the tumor volumes at day 0. Data presented as the mean±s.e.m. values. Number of animals reduced in time as indicated in the figure. *P<0.05, ***P<0.001. (C) Kaplan-Meier curves of the three treatment groups. Animals were monitored up to 60 days after tumor innoculation. Long-rank test resulted in P<0.001 between all groups. BBB, blood–brain barrier; EA, ethylamine; HSA, human serum albumin; MTX, Methotrexate.
In agreement with the above findings, survival of the rats that received the combination therapy was significantly extended. Median survival times reached 5, 12, and 19 days for the control, MTX-treated, and the combination-treated groups, respectively (Figure 6C).
The CED administration of a neurtralized HSA analog without parallel systemic treatment of MTX showed no beneficial effects over control (Supplementary Results).
Leukocytes Transmigration Across Blood–Brain Barrier after Ethylamine–Human Serum Albumin Treatment
To study whether BBB disruption induced by EA–HSA allows for increased leukocyte migration over the BBB, thus potentially increasing the antineoplastic effects of the combined treatment, we studied such migration with/without EA–HSA treatment using our in vitro BBB model. The results (Supplementary Figure 3s, Supplementary Results) showed no significant difference in leukocyte migration with/without EA–HSA treatment, suggesting that BBB disruption induced by EA–HSA does not affect the migration.
Discussion
In spite of aggressive therapy, existing treatments offer poor prognosis for glioblastoma multiforme (GBM) patients. The reasons for limited therapeutic effects include tumor infiltration into the surrounding brain parenchyma as well as poor penetration of most therapeutic agents across the BBB. In addition, GBM cells are highly resistant to therapeutic apoptotic stimuli. However, they exhibit a paradoxical propensity for extensive cellular necrosis.28, 29 Due to the extreme adaptability of GBM cells, residual tumor, including the infiltrating zone surrounding the tumor, should be treated with high efficacy as well, to prevent sublethal hits of tumor cells leading to the growth of more malignant clonal cell populations.30
Blood–brain barrier disruption obtained by intracarotid and intraarterial injections of high concentrations of Mannitol has been studied extensively by the BBB disruption (BBBD) consortium initiated by Prof Neuwelt from Ohio State University.31 This type of therapy indeed showed promising results mainly in patients with CNS lymphoma but less in patients with GBM. We believe that the results of the BBBD consortium indeed show the potential of BBBD for the treatment of brain tumors. Still, a major limitation of BBBD by intracarotid/intraarterial administration of the BBB disruption agent may be that BBBD obtained by this method covers large regions of the vascular tree in the treated hemisphere with no specific targeting to the tumor region.32 We believe that the combined approached presented in our manuscript may overcome this limitation since targeting of the tumor and surrounding infiltrating zone is obtained by implanting the source of the BBB disruption agent within the tumor mass. Using this approach, the BBB disruption agent is efficiently delivered to the tumor mass and surrounding infiltrating zone with limited effects to more distant brain regions.
To overcome some of these challenges, we propose a minimal invasive combined treatment approach for GBM consisting of local disruption of the BBB in the tumor and infiltrating zone in parallel to systemic drug administration. Since gliomas are highly vascular tumor,18 we hypothesized that by applying the disrupting agent to the tumor mass, efficient BBB disruption will be induced in the tumor mass as well as in the infiltrating zone, thus enabling efficient delivery of the systemically administered therapeutic agent by the tumors own vasculature to the target regions. To obtain maximal BBB disruption at the vicinity of the tumor and surrounding infiltrating zone, we administered the EA–HSA by CED. Convection-enhanced drug delivery is advantageous both in allowing efficient delivery of high EA–HSA concentrations over large volumes of the target tissue and in fully avoiding systemic toxicity.
We hypothesize that the overall volume of BBB disruption should be larger than the volume of infusate distribution due to leakage of the BBB-opening agent into the tumor vascular. This way, the EA–HSA may be further carried by the tumor vasculature into the infiltrating zone, enabling efficient penetration of the systemically administered therapeutic agent into further regions of infiltrating tumor. To test this hypothesis, further studies are required.
Taking into account these considerations, the study was designed to determine an efficient and safe BBB-opening agent and establish the added value of the combined approach for the treatment of brain tumors. The study was carried out in four stages: (1) Chemical synthesis of a variety of candidate BBB opening albumin derivatives, (2) In vitro studies—applying in vitro experimental system for determining fast and efficient screening of the opening analogs without killing animals, (3) In vivo studies with naïve rats that were necessary for obtaining reproducible assessment of BBB disruption efficacy and toxicity to normal brain and for determining the optimal BBB opening analog, and (4) In vivo studies with rats bearing intracranial glioma—allowing assessment of the antineoplastic effects.
The chemotherapeutic agent a priory selected was MTX, a folic acid analog used as a chemotherapeutic drug. As folate receptors are over expressed on the cell membranes of many types of cancer cells, MTX is one of the most widely used drugs for the treatment of many forms of cancer, including tumors of the brain, breast, ovaries, and several leukemias. However, MTX is limited by its low solubility, dose-related toxicity, lack of selectivity, rapid diffusion throughout the body, short half-life in the bloodstream, drug resistance by target cells and low BBB penetration.17 The combined approach showed here may provide means for overcoming these common limitations thus taking advantage of potent drugs such as MTX for obtaining significant treatment effects in GBM patients.
To proceed to the animal efficacy experiment in vivo, it was necessary to reduce systemic toxicity induced by MTX. Folinic acid is a 5 formyl tetrahydrofolate derivative of folic acid. This molecule enters the folate pathway downstream to DHFR, enabling therefore the synthesis of purines and pyrimidines and the maintenance of other folate dependent metabolic pathways despite inhibition of the activity of DHFR by MTX.33 We found that combined administration of this antifolate antagonist, even at three times its maximal tolerated dose, with folinic acid (leucovorin) prevented MTX toxicity.
The results of the efficacy study in the rat glioma model showed that despite the rapidly growing tumors in the control group, the combined therapy completely suppressed tumor growth and significantly prolonged survival by nearly a factor of 3 compared with control. Interestingly, systemically administered MTX seemed to show no therapeutic effect in the first 2 days after treatment (no significant change in tumor growth versus control) while the combined approach showed significant antitumor effects (complete arrest in tumor growth). Later on, 7 days after treatment, systemically administered MTX did show therapeutic benefits, although still significantly lower than those obtained by the combined approach. This delayed effect of MTX may be explained by increased BBB disruption as the tumor matures or by an accumulated effect on the tumor vasculature induced by repeated treatments with MTX. It also suggests that in the group receiving the combination therapy, where the tumor failed to grow, MTX entry was highly dependent on the BBB-opening efficacy of EA–HSA. To improve treatment efficacy, it may be possible to repeat the CED treatment (using the same implanted catheter) more than once.
From a cellular mechanistic point of view, the mode of action by which EA–HSA induces BBB opening was investigated in a set of immunocytochemical studies. We have shown that out of the several transmembrane and cytosolic TJ proteins responsible for connecting neighboring endothelial cells to each other, occludin expression was particularly altered upon incubation of PBEC-M with EA–HSA. Zonula occludens-1 is a scaffolding protein responsible for the linkage between the intracellular actin cytoskeleton and the outer membrane TJ's proteins (claudin-5, occludin). This interaction is postulated to provide additional rigidity to the structures and allow for rapid alterations in barrier integrity in response to a variety of stimuli.34 One can assume that the relatively unchanged ZO-1 may be explained by the cells attempt to maintain BBB functionality under the stress induced by the EA–HSA, also manifested by the formation of stress fibers. It should be mentioned however that increased leakiness of the BBB is not always correlated with visible changes in major structural component of TJ.35 Although occludin trafficking away from TJ's complexes was found to be a sensitive, early, and reliable sign for TJ opening and BBB disruption in osmotically-affected rat BBB,36 Youakim et al.37 described BBB disruption by interferon γ that was ZO-1 dependent while occludin expression remained intact. Claudin-5 was the only TJ protein that presented a decreased expression levels while occludin and ZO-1 remained intact after exposure of endothelial cells to anthrax.38 Thus, the exact mechanism leading to BBB opening depends on the stress/compound imposed. Our in vitro data, i.e., the reduction and redistribution of occluding from cell-cell borders and the TEER reduction (which reflects the paracellular passage of ions) which occurred in a similar pattern in both mono and coculture BBB in vitro systems, strongly suggest that EA–HSA-induced BBB disruption is a paracellular mechanism in nature. From the rat MRI study, we learned that EA–HSA-induced BBB disruption is a transient phenomenon in vivo. In our model, the BBB returned back to its native-impermeable state within a short period after a single challenge with this HSA analog, most likely due to denovo synthesis of occludin that was disoriented in this process.
An important aspect of disrupting the BBB is the possible induction or enhancement of an immune response, which may contribute significantly to the antineoplastic effects of the treatment. It has been previously shown that significant BBB disruption, observed after acute traumas such as head trauma or stroke, was accompanied by immune cells that were recruited to the inflamed/damaged area inducing significant immune response (NK, neutrophils, monocytes, and other types of leukocytes. For example: stroke;39 and traumatic brain injury40). Our observations that leukocytes migration across the in vitro BBB was not enhanced after EA–HSA treatment (Supplementary Figure 3s) and that rats treated by CED of a neutralized-HSA BBB-opening agent with no systemic chemotherapy did not show therapeutic benefits over untreated rats (Supplementary Results) suggest that BBB disruption induced by EA–HSA does not enhance immune system antitumor effects observed in this study.
In summary, the results of this study present a new compound, EA–HSA, for inducing efficient, transient and safe local BBB disruption that may be explained by alterations in occludin expression. In addition, the study showed the feasibility of using this analog in a combined minimal-invasive treatment approach for inducing significant treatment effects reflected by tumor growth arrest and prolonged survival in a rat glioma model.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by a joint Weizmann Institute of Science—Sheba Medical Center grant for Biomedical Research and by the Kimmelman Center for Biomolecular Structure and Assembly research program, Weizmann Institute of Science.
Supplementary Material
References
- 1Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94: 153–156. [DOI] [PubMed] [Google Scholar]
- 2Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 2012; 14: v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 2010; 12: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Dhanikula RS, Argaw A, Bouchard JF, Hildgen P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharm 2008; 5: 105–116. [DOI] [PubMed] [Google Scholar]
- 5Kostarelos K, Emfietzoglou D, Papakostas A, Yang WH, Ballangrud A, Sgouros G. Binding and interstitial penetration of liposomes within avascular tumor spheroids. Int J Cancer 2004; 112: 713–721. [DOI] [PubMed] [Google Scholar]
- 6Pardridge WM, Triguero D, Buciak J, Yang J. Evaluation of cationized rat albumin as a potential blood-brain barrier drug transport vector. J Pharmacol Exp Ther 1990; 255: 893–899. [PubMed] [Google Scholar]
- 7Cooper I, Sasson K, Teichberg VI, Schnaider-Beeri M, Fridkin M, Shechter Y. Peptide derived from HIV-1 TAT protein destabilizes a monolayer of endothelial cells in an in vitro model of the blood-brain barrier and allows permeation of high molecular weight proteins. J Biol Chem 2012; 287: 44676–44683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Bergmann P, Kacenelenbogen R, Vizet A. Plasma clearance, tissue distribution and catabolism of cationized albumins with increasing isoelectric points in the rat. Clin Sci (Lond) 1984; 67: 35–43. [DOI] [PubMed] [Google Scholar]
- 9Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev 2001; 46: 247–279. [DOI] [PubMed] [Google Scholar]
- 10Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 1994; 91: 2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Vogelbaum MA, Iannotti CA. Convection-enhanced delivery of therapeutic agents into the brain. Handb Clin Neurol 2012; 104: 355–362. [DOI] [PubMed] [Google Scholar]
- 12Mehta AI, Choi BD, Raghavan R, Brady M, Friedman AH, Bigner DD et al. Imaging of convection enhanced delivery of toxins in humans. Toxins (Basel) 2011; 3: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Sampson JH, Akabani G, Friedman AH, Bigner D, Kunwar S, Berger MS et al. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus 2006; 20: E14. [DOI] [PubMed] [Google Scholar]
- 14Tanner PG, Holtmannspotter M, Tonn JC, Goldbrunner R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery 2007; 61: E880–E882, discussion E882. [DOI] [PubMed] [Google Scholar]
- 15Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T. Regression of recurrent glioblastoma infiltrating the brainstem after convection-enhanced delivery of nimustine hydrochloride. J Neurosurg Pediatr 2011; 7: 522–526. [DOI] [PubMed] [Google Scholar]
- 16Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 2004; 100: 472–479. [DOI] [PubMed] [Google Scholar]
- 17Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 2003; 49: 92–104. [DOI] [PubMed] [Google Scholar]
- 18Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol 2006; 32: 532–538. [DOI] [PubMed] [Google Scholar]
- 19Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996; 77: 362–372. [DOI] [PubMed] [Google Scholar]
- 20Cooper I, Cohen-Kashi Malina K, Cagnotto A, Bazzoni G, Salmona M, Teichberg VI. Interactions of the prion peptide (PrP 106-126) with brain capillary endothelial cells: coordinated cell killing and remodeling of intercellular junctions. J Neurochem 2011; 116: 467–475. [DOI] [PubMed] [Google Scholar]
- 21Franke H, Galla H, Beuckmann CT. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res Brain Res Protoc 2000; 5: 248–256. [DOI] [PubMed] [Google Scholar]
- 22Cohen-Kashi Malina K, Cooper I, Teichberg VI. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res 2009; 1284: 12–21. [DOI] [PubMed] [Google Scholar]
- 23Rutten MJ, Hoover RL, Karnovsky MJ. Electrical resistance and macromolecular permeability of brain endothelial monolayer cultures. Brain Res 1987; 425: 301–310. [DOI] [PubMed] [Google Scholar]
- 24Mardor Y, Rahav O, Zauberman Y, Lidar Z, Ocherashvilli A, Daniels D et al. Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res 2005; 65: 6858–6863. [DOI] [PubMed] [Google Scholar]
- 25Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol 1998; 14: 89–109. [DOI] [PubMed] [Google Scholar]
- 26Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 27Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev 2005; 50: 7–13. [DOI] [PubMed] [Google Scholar]
- 28Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery 2002; 51: 2–12, discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 29Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest 2004; 84: 397–405. [DOI] [PubMed] [Google Scholar]
- 30Nieto-Sampedro M, Valle-Argos B, Gomez-Nicola D, Fernandez-Mayoralas A, Nieto-Diaz M. Inhibitors of glioma growth that reveal the tumour to the immune system. Clin Med Insights Oncol 2011; 5: 265–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol 2014; 71: 203–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Zylber-Katz E, Gomori JM, Schwartz A, Lossos A, Bokstein F, Siegal T. Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin Pharmacol Ther 2000; 67: 631–641. [DOI] [PubMed] [Google Scholar]
- 33Goldman ID, Matherly LH. Biochemical factors in the selectivity of leucovorin rescue: selective inhibition of leucovorin reactivation of dihydrofolate reductase and leucovorin utilization in purine and pyrimidine biosynthesis by methotrexate and dihydrofolate polyglutamates. NCI Monogr 1987; 5: 17–26. [PubMed] [Google Scholar]
- 34Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 2008; 1123: 134–145. [DOI] [PubMed] [Google Scholar]
- 35Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W et al. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res 2004; 315: 157–166. [DOI] [PubMed] [Google Scholar]
- 36Dobrogowska DH, Vorbrodt AW. Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood-brain barrier. J Mol Histol 2004; 35: 529–539. [DOI] [PubMed] [Google Scholar]
- 37Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999; 276: G1279–G1288. [DOI] [PubMed] [Google Scholar]
- 38D'Agnillo F, Williams MC, Moayeri M, Warfel JM. Anthrax lethal toxin downregulates claudin-5 expression in human endothelial tight junctions. PLoS One 2013; 8: e62576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Hammond MD, Ambler WG, Ai Y, Sansing LH. alpha4 integrin is a regulator of leukocyte recruitment after experimental intracerebral hemorrhage. Stroke 2014; 45: 2485–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Al Nimer F, Lindblom R, Strom M, Guerreiro-Cacais AO, Parsa R, Aeinehband S et al. Strain influences on inflammatory pathway activation, cell infiltration and complement cascade after traumatic brain injury in the rat. Brain Behav Immun 2013; 27: 109–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.