Abstract
Atrial fibrillation (AF) increases the risk and severity of thromboembolic stroke. Generally, antithrombotic agents increase the hemorrhagic risk of thromboembolic stroke. However, significant reductions in thromboembolism and intracerebral hemorrhage have been shown with the antithrombin dabigatran compared with warfarin. As thrombin has been implicated in microvessel injury during cerebral ischemia, we hypothesized that dabigatran decreases the risk of intracerebral hemorrhage by direct inhibition of the thrombin-mediated increase in cerebral endothelial cell permeability. Primary murine brain endothelial cells (mBECs) were exposed to murine thrombin before measuring permeability to 4-kDa fluorescein isothiocyanate-dextran. Thrombin increased mBEC permeability in a concentration-dependent manner, without significant endothelial cell death. Pretreatment of mBECs with dabigatran completely abrogated the effect of thrombin on permeability. Neither the expressions of the endothelial cell β1-integrins nor the tight junction protein claudin-5 were affected by thrombin exposure. Oxygen-glucose deprivation (OGD) also increased permeability; this effect was abrogated by treatment with dabigatran, as was the additive effect of thrombin and OGD on permeability. Taken together, these results indicate that dabigatran could contribute to a lower risk of intracerebral hemorrhage during embolism-associated ischemia from AF by protection of the microvessel permeability barrier from local thrombin challenge.
Keywords: astrocyte, dabigatran, endothelial cell, microvessel, permeability barrier, α-thrombin
Introduction
Nonvalvular atrial fibrillation (AF) increases the risk and severity of thromboembolic stroke. Prevention of thrombus formation with warfarin, whereas decreasing ischemic stroke risk, is associated with increased hemorrhagic risk. The RE-LY (randomized evaluation of long-term anticoagulant therapy) trial, comparing warfarin with the direct thrombin inhibitor dabigatran, showed that dabigatran was not inferior to warfarin in efficacy (reduction of thromboembolic events), and that for prophylaxis of nonvalvular AF, there was a significant reduction in the incidence of intracerebral hemorrhage with dabigatran compared with warfarin.1, 2 Although it has been suggested that the observed hemorrhagic risk reduction with dabigatran is the consequence of a higher hemorrhagic risk of warfarin, which targets multiple coagulation factors,3 this does not explain the confinement of the risk reduction to the cerebral vasculature. This could be explained by a difference in microvascular hemostatic responses in the central nervous system (CNS) under conditions of focal ischemia. Rosenberg and Aird4 proposed that hemostatic responses in the CNS are unique among vascular beds;5 although the contributions that microvessel beds make to regulating hemostasis in the human CNS are not completely understood,6, 7 particularly in the setting of focal ischemia.8, 9
The unique structure of cerebral microvessels is related to their functions in regulating both vascular permeability and hemostasis within the CNS. First, brain capillaries consist of a non-fenestrated endothelium, basal lamina matrix, and surrounding astrocyte end-feet attached to the basal lamina. This intact endothelium–matrix–astrocyte assembly constitutes the ‘blood–brain' permeability barrier.10 The integrity of the permeability barrier depends on the interendothelial tight junctions (TJs), interactions between endothelial cells and astrocytes, and adhesion of endothelial cells and astrocytes to the basal lamina matrix.10, 11 Second, the cerebral hemispheres contain significant quantities of tissue factor (TF), the major initiator of extrinsic coagulation, which is broadly distributed in the gray matter and surrounds noncapillary microvessels.12 del Zoppo et al12 showed that quantities of TF in the resting cerebral gray matter of the non-human primate are in significant excess of that found in the white matter. The presence of TF has been associated with astrocytes, based on TF transcripts in the glia limitans.13 It is unknown whether TF has a role in brain functions other than hemostasis. However, the recently discovered ability of thrombin to modulate synaptic plasticity suggests that this may be the case.14 Third, CNS microvessels are functionally and structurally connected to the neurons, they serve as parts of the ‘neurovascular unit,' through which neurons can control microvessel diameter and regional flow with signals via intervening astrocytes.15
Thrombin can be acutely generated in the cerebral microvessel wall during focal ischemia as indicated by fibrin deposition within the plasma column and vessel wall (see Figure 5 in del Zoppo et al16). Thrombin has pleiotropic effects including the cleavage of fibrinogen, activation of factors V, VIII, XI, and XIII, the activation of resting platelets, the activation of protein C (in the presence of thrombomodulin), modulation of endogenous fibrinolysis, and activation of inflammation. In addition to its role in the coagulation cascade, thrombin can induce vascular permeability.17 Further, thrombin has been implicated as a mediator of blood–brain barrier permeability in a number of different model systems.18, 19, 20, 21 α-Thrombin has been shown to degrade laminin, fibronectin, and collagens.22, 23
After the onset of focal cerebral ischemia, rapid alterations in brain microvessels occur, including decreased matrix adhesion receptor expression and matrix proteins, and increased permeability.24, 25 The interaction of endothelial cell β1-integrins with the extracellular matrix (ECM) stabilizes expression of the TJ protein claudin-5 and maintains permeability barrier integrity, such that direct interference with this interaction is sufficient to increase permeability.11 Focal ischemia is associated with acute loss of β1-integrins from brain microvessels.26 However, a causal relationship between the loss of matrix adhesion receptors in focal ischemia and increased microvessel permeability has not been established.
As thrombin has been implicated in vascular injury during ischemia,16 we hypothesized that dabigatran could decrease the risk of intracerebral hemorrhage by direct inhibition of thrombin-induced permeability in part via the protection of β1-integrin. This study focuses on the role of the endothelial component of brain microvessels in the effects of thrombin and dabigatran on the permeability barrier under the conditions of normoxia and experimental ischemia, as from an embolic insult.
Materials and Methods
Institutional Approvals
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Washington and conform to the standards set by the National Institutes of Health.
Reagents
Purified murine α-thrombin was obtained from Hematologic Technologies (Essex Junction, VT, USA). Murine α-thrombin was produced by the methods of Lundblad et al27 with the modifications described by Nesheim.28 Dabigatran was a gift from Boehringer-Ingelheim GmbH (Ingelheim am Rhein, Germany). Mouse anti-claudin-5 was obtained from Invitrogen(Camarillo, CA, USA), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody was obtained from Vector Laboratories (Burlingame, CA, USA). A phycoerythrin-conjugated antibody against β1-integrin (clone HM β1) was obtained from BioLegend (San Diego, CA, USA). All other agents were obtained from Sigma (St Louis, MO, USA), unless otherwise noted. bEnd.3 cells (Mus musculus) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) as ATCC CRL-2299, and were used for condition setting where appropriate.
Brain Endothelial Cell and Astrocyte Cultures
Primary brain microvessel endothelial cells (mBECs) were obtained from 2- to 3-month-old male C57 BL/6 mice (Jackson Laboratories, Sacramento, CA, USA) and prepared as described previously.11 Brains were removed, cleaned of meninges and external blood vessels, finely minced, and dissociated in a solution containing 20 U/mL papain and 250 U/mL DNase I type IV in MEM-HEPES (minimum essential medium-4-2-hydroxyethyl-1-piperazineethanesulfonic acid) for 1 hour. The dissociated brain tissue was triturated, added to a 15 mL tube containing 22% bovine serum albumin (final concentration) and centrifuged at 1000 g for 20 minutes. The isolated cells were then washed and resuspended in endothelial cell growth media consisting of Hams F12, supplemented with 10% fetal bovine serum, heparin, ascorbic acid, l-glutamine, and endothelial cell growth supplement. The mBECs in endothelial cell growth media were then added to collagen I-coated six-well plates, and incubated at 37 °C under 5% CO2.
Endothelial cell growth media were changed the next day and the cultures were treated with puromycin (3 μg/mL) for a total of 3 days. The endothelial cell growth culture media were then replaced every 3 days thereafter. Murine brain endothelial cell were seeded onto the top surface of collagen IV-coated 24-well inserts (Greiner bio-one, Kremsmünster, Austria), without or with astrocytes seeded on the bottom surface, on day 9 or 10, and maintained for at least 48 hours before dabigatran/thrombin exposure, and/or oxygen-glucose deprivation (OGD), and permeability experiments. Alternatively, cells were seeded onto collagen IV-coated 6-well inserts for flow cytometry experiments, or onto collagen IV-coated 24-well plates for cell viability assays.
bEnd.3 cells (an immortalized murine endothelial cell line) were cultured as per the manufacturer's protocols in T75 flasks with Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal calf serum, glutamine, and antibiotics before passage to inserts as described above.
Astrocytes were cultured from the brains of neonatal (1 to 2 days old) mouse pups (Charles River, Raleigh, NC, USA) using established techniques.29 Briefly, cerebral hemispheres were dissected from the brainstem and cerebellum, meninges, and choroid plexuses removed, and the remaining tissue minced and digested in media containing papain and DNAse. Cells were pelleted, resuspended in growth media (DMEM supplemented with fetal bovine serum, glutamine, and antibiotics) and grown to confluence in poly-d-lysine-coated flasks. Microglial cells and oligodendrocytes were removed by shaking before passage. Astrocytes typically reached confluence in 9 to 12 days, and were used in P1 only. For coculture with endothelial cells, astrocytes were passaged to the bottom surface of collagen IV-coated 24-well inserts.
Flow Cytometry
Murine brain endothelial cells were mechanically removed from the inserts, washed, blocked in 5% normal goat serum/phosphate-buffered saline, and incubated with phycoerythrin-conjugated anti-β1-integrin antibody for 1 hour on ice. Cells were permeabilized and incubated with mouse anti-claudin-5 monoclonal antibody followed by FITC-conjugated anti-mouse IgG, and then washed and suspended in 2% formaldehyde for flow cytometry analysis, as described previously.11 The fluorescence intensity of the labeled cells was analyzed with a Becton-Dickinson FACScan (BD Biosciences), with 8,000 to 10,000 events recorded for each condition, in triplicate.
Cell Viability Assay
Cells were washed with phosphate-buffered saline, incubated with propidium iodide (PI, 33 μg/ml) for 5 minutes, and washed again before fixing with 2% paraformaldehyde. Propidium iodide-positive cells were counted on a fluorescent microscope with a x10 objective. Results are reported as the percentage of positive cells within a 0.371 mm2 grid at x200.
Permeability Measurements
The permeability of monolayers and cocultures were measured as described previously.11 Cells were washed with phosphate-buffered saline and placed in serum-free, phenol red-free DMEM. Fluorescein isothiocyanate-dextrans (4-, 40-, or 150-kDa sizes) were added to the upper chamber (1 mg/ml, final concentration) and 50 μL samples were collected from the lower chamber at 0, 5, 10, 20, 40, and 60 minutes. The volume of each sample removed was replaced with fresh media at each time point. A 50 μl aliquot from the upper chamber was collected at the end of the experiment. Fluorescence intensity was measured (excitation 485 nm, emission 510 nm) on a fluorescent plate reader, with standard curves for FITC-dextran run in parallel. The apparent permeability coefficient (Papp, cm/s) of each well was calculated by:
where dM/dt is the cumulative amount of FITC-dextran in the lower chamber per unit time (mmol/s) corrected for dilution because of sampling, A is the surface area of the insert membrane (0.33 cm2), and C is the measured concentration (mmol/mL) in the upper chamber.
Oxygen-Glucose Deprivation
Oxygen-glucose deprivation was performed under conditions previously set in our laboratory.29, 30, 31 Serum-containing media were removed from the cell cultures by washing two times with phosphate-buffered saline before adding serum-free high-glucose medium (4.5 g/L, DMEM containing 4 mmol/L l-glutamine, penicillin, and streptomycin, supplemented with N1 medium) for normoxic controls, or low-glucose medium (1 g/L, supplemented DMEM) for OGD. Cultures containing low-glucose medium were placed in a hypoxia chamber flushed with 95% N2 and 5% CO2 for 1 hour, and then sealed for the duration of the experiment. O2 levels decreased to 0.1% to 0.4% at 4 hours, and were maintained throughout the experiment (18 hours). Normoxic controls were maintained in serum-free media under standard incubator conditions in parallel.
It was noted that endothelial cell monolayers (bEnd.3 and primary cell) showed no morphologic alterations after exposure to thrombin, dabigatran, or the combination thrombin with dabigatran under the conditions of the experiments (see Results and Supplementary Figures).
Statistical Analysis
All data are presented as mean±s.d. with the numbers of replicates as indicated. Individual sample allocations were known by the principal author only. Normality of data sets was tested by the D'Agostino and Pearson's test. Means were compared by one-way analysis of variance (ANOVA), and significance set at P<0.05. All analyses were carried out using GraphPad Prism v. 5.04 (La Jolla, San Diego, CA, USA).
Results
Thrombin Increases Murine Brain Endothelial Cell Permeability Without Endothelial Cell Toxicity
Preliminary experiments to define the conditions for the studies with mBECs were performed with bEnd.3 cells exposed to media without or with murine α-thrombin (10 U/mL) for 1 hour. Permeability was then tested with 4-, 40-, 70-, or 150-kDa FITC-dextrans. Thrombin significantly increased the permeability of bEnd.3 cell monolayers to 4-kDa FITC-dextran only (data not shown; see Supplementary Figure 1).
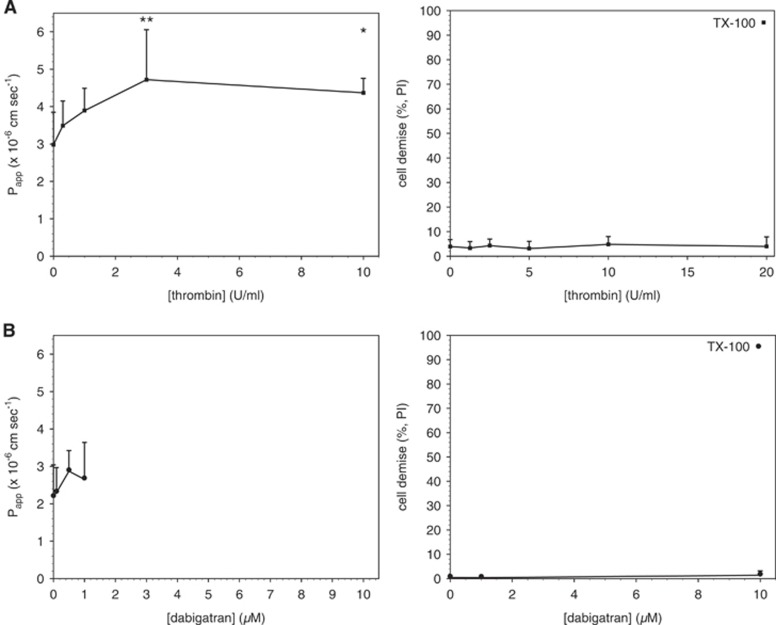
Primary mBECs were then exposed to thrombin (0 to 10 U/ml) in serum-free media for 1 hour before measurement of permeability. Thrombin had a concentration-dependent effect on mBEC permeability to 4-kDa FITC-dextran (Figure 1A), as reported previously.21 However, thrombin concentrations up to 20 U/mL did not cause a significant increase in PI uptake (Figure 1A), indicating that the permeability changes observed were not because of cell death. Baseline transendothelial electrical resistance measurements were made in a subset of endothelial cell monolayers and reflected those reported previously.11
Figure 1.
Acute exposure to thrombin increases brain endothelial cell (mBEC) permeability without cell toxicity. Primary mBEC grown to confluence were exposed to thrombin (A) for 1 hour or dabigatran (B) for 24 hours in serum-free media before incubation with propidium iodide (PI) or measurement of permeability to 4-kDa fluorescein isothiocyanate (FITC)-dextran. (A) Thrombin had a concentration-dependent effect on permeability (closed squares, left panel). Acute exposure to thrombin had no effect on brain endothelial cell viability (closed squares, right panel). TX-100=cells exposed to the permeabilizing agent Triton X-100 (positive control). (B) Dabigatran did not significantly increase permeability to 4-kDa FITC-dextran (closed circles, left panel). Dabigatran had no effect on cell viability (closed circles, right panel). Cell viability data shown are averages from four fields, 30 to 55 cells per field counted. Permeability data are pooled from two (A) and three (B) independent experiments; n=3 per condition per experiment for a total of n=6 or 9 inserts per condition, respectively. All data are presented at mean±s.d. Significance was determined by one-way analysis of variance (ANOVA) with a Bonferroni multiple comparison test. *P<0.05 and **P<0.01 versus vehicle controls.
Dabigatran Is Not Toxic to Murine Brain Endothelial Cell and Does Not Affect Permeability at Therapeutic Concentrations
Murine brain endothelial cells were exposed to dabigatran (0 to 1 μmol/L) in serum-free media for 24 hours before the measurement of permeability to 4-kDa FITC- dextran. Dabigatran alone did not affect permeability, and did not cause a significant change in PI uptake up to 10 μmol/L (Figure 1B), a concentration ~20 times higher than the plasma concentration of dabigatran typically achieved in patients.32
Thrombin Does Not Affect Endothelial Expression of β1-Integrin or Claudin-5
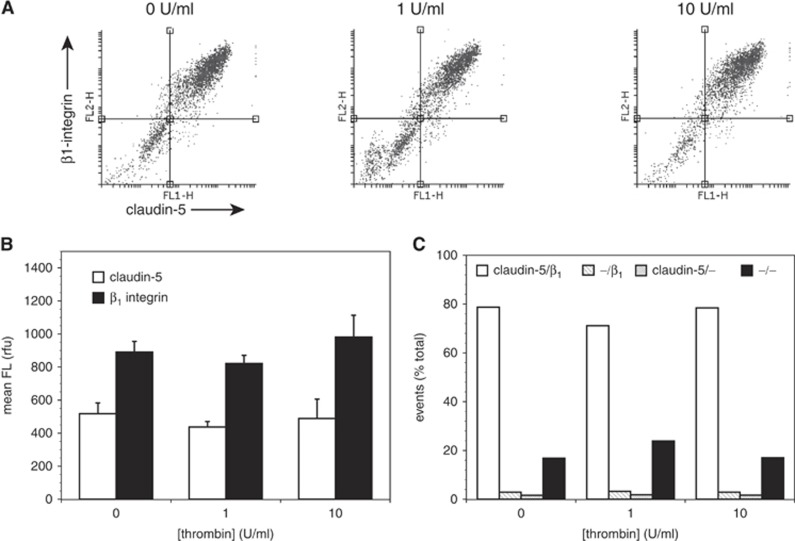
Exposure of mBEC to α-thrombin (1 or 10 U/mL) for 1 hour had no effect on the mean fluorescence intensity of cells labeled for β1-integrin and claudin-5 (Figures 2A and 2B) nor on the distribution of cells expressing each antigen (Figure 2C and Supplementary Figure 2). No effect on the morphology of the endothelial cells was observed under any condition.
Figure 2.
Acute thrombin has no effect on murine brain endothelial cell (mBEC) expression of claudin-5 or β1-integrin. Primary mouse brain endothelial cells were exposed to thrombin (0 to 10 U/mL) for 1 hour before labeling for claudin-5 and β1-integrin and analysis by flow cytometry. (A) Representative scatter plots of each condition. (B) Mean fluorescence (FL) intensities on each channel for claudin-5 (open bars) and β1-integrin (closed bars). (C) Quadrant distributions of cells in scatter plots as designated for claudin-5 and β1-integrin expression. No concentration dependence of claudin-5 and β1-integrin expression was observed. Data are mean±s.d; n=3 in this experiment; this experiment has been performed two times with identical results.
Dabigatran Blocks the Effects of Thrombin and Oxygen-Glucose Deprivation on Murine Brain Endothelial Cell Permeability
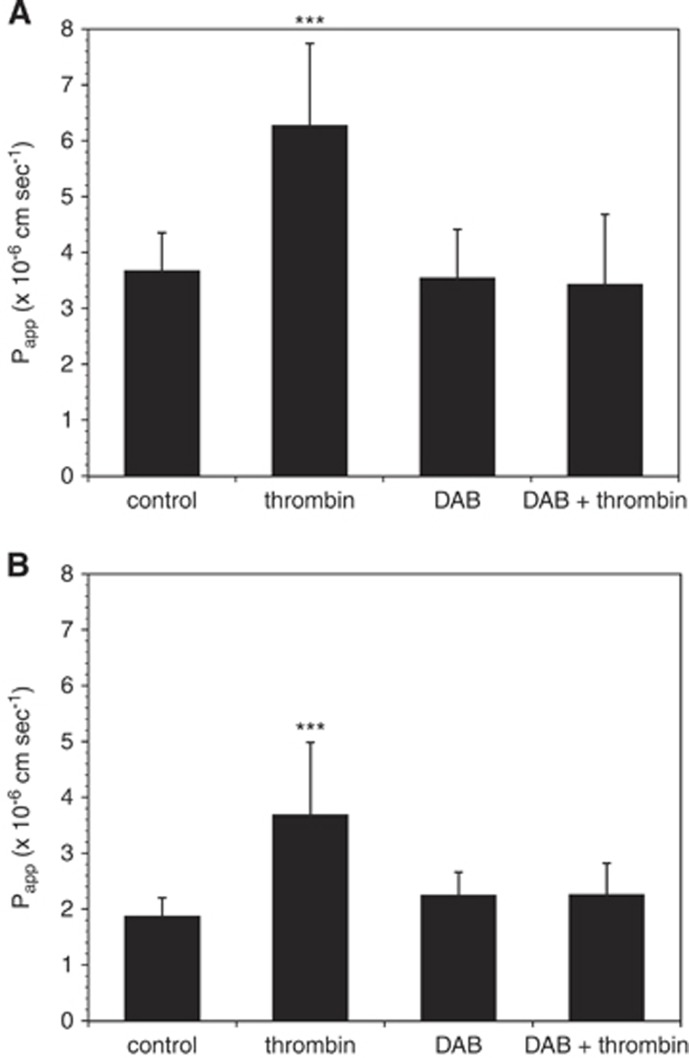
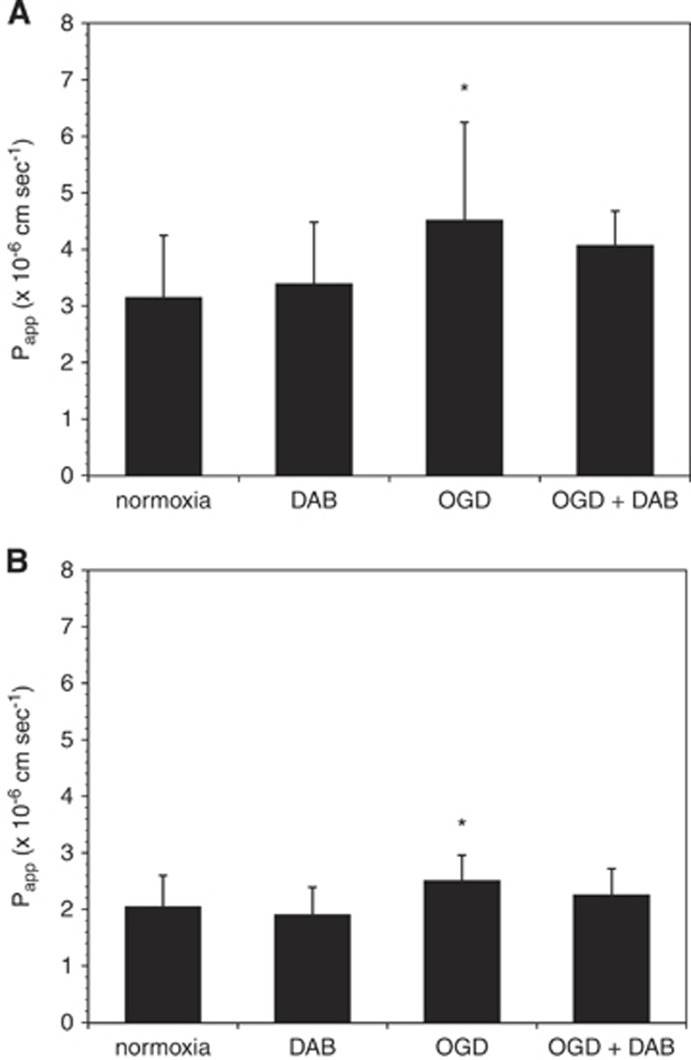
Preincubation of mBEC with dabigatran (500 nmol/L) for 24 hours before thrombin (10 U/mL, 1 hour) exposure completely blocked the effect of thrombin on mBEC permeability in both mBEC monolayers (Figure 3A) and in mBEC cocultured with astrocytes (Figure 3B). Furthermore, the addition of 500 nmol/L dabigatran to the media of cells exposed to OGD attenuated the effect of OGD on mBEC permeability (Figure 4).
Figure 3.
Dabigatran (DAB) inhibits the effect of thrombin on murine brain endothelial cell (mBEC) permeability. (A) Incubation with dabigatran (500 nmol/L) for 24 hours before thrombin exposure (1 hour, 10 U/mL) abolishes the effect of thrombin on permeability (shown are pooled data from two separate experiments in primary brain endothelial cells, n=7 in total). (B) These findings were confirmed in endothelial cells cocultured with astrocytes. Shown are pooled data from four separate experiments (n=12 in total). Significance was determined by one-way analysis of variance (ANOVA) with a Bonferroni post hoc test. ***P<0.001 versus control.
Figure 4.
Dabigatran (DAB) abrogates the effect of oxygen-glucose deprivation (OGD) on murine brain endothelial cell (mBEC) permeability. Incubation with dabigatran (500 nmol/L) for 24 hours before and during 18 hours OGD abrogates the effect of OGD on permeability on primary brain endothelial cells grown alone (A) and in coculture with astrocytes (B). Data are pooled from three separate experiments in each condition (n=12 to 15 in total). Significance was determined by one-way analysis of variance (ANOVA) with a Bonferroni post hoc test. *P<0.05 versus normoxia.
Dabigatran Attenuates the Effect of Thrombin on Permeability in the Setting of Oxygen-Glucose Deprivation
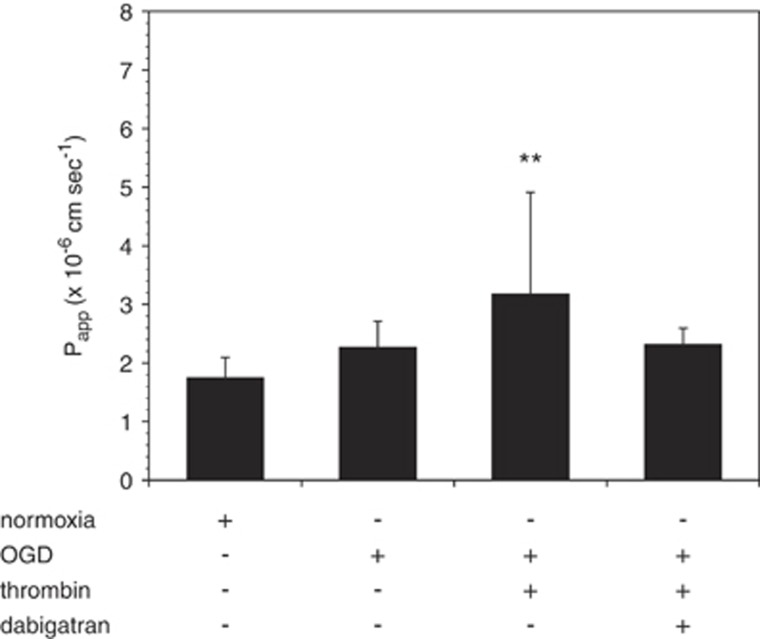
Murine brain endothelial cell cocultured with astrocytes were exposed to OGD for 18 hours, followed by thrombin (10 U/mL, 1 hour) to model the generation and extravasation of thrombin after an ischemic event in vivo. Dabigatran attenuated the additive effect that α-thrombin produced with OGD on permeability of the endothelial cell–astrocyte complex when the complex was preincubated with dabigatran (Figure 5).
Figure 5.
Dabigatran abrogates the effect of thrombin after oxygen-glucose deprivation (OGD). Addition of thrombin (10 U/mL, 1 h) to murine brain endothelial cell (mBEC) cocultured with astrocytes immediately after OGD (18 h) augments the effect of OGD on permeability. This additive effect is blocked by dabigatran (500 nmol/L). Data are pooled from three separate experiments (n=9 in total). **P<0.05 versus normoxia.
Discussion
Focal cerebral ischemia induces fibrin formation within microvessels in the territory-at-risk (ischemic regions) and is accompanied by increased vascular permeability that causes edema formation and hemorrhage.8, 16 Hemorrhagic transformation in the CNS is a common accompaniment of ischemic strokes, being part of the natural history, and anticoagulation is associated with an increased risk and severity of cerebral hemorrhage.33 In a high-quality non-human primate model of middle cerebral artery occlusion, we have shown that fibrin-containing occlusions obstruct the microvasculature, and that fibrin deposits in the microvessel wall and the perivascular space occur acutely, within 60 to 120 minutes of ischemia onset.8 This shows the acute local generation of thrombin at the abluminal interface or within the perivascular space of microvessels within the ischemic territory. Furthermore, thrombin has been implicated in barrier damage after experimental ischemia in the rodent.
In this study, we show that α-thrombin increases the permeability of primary mBEC, alone or in coculture with astrocytes, to 4-kDa FITC-dextran and that the direct thrombin inhibitor dabigatran at a clinically relevant concentration completely reverses the increased permeability (Figure 3). Previous studies investigating the effect of thrombin on the blood–brain permeability barrier have shown altered barrier function in other in vitro model systems, including immortalized mBEC (bEnd.3),21 immortalized human BEC (hCMEC/D3),20 and (unpurified) rat primary BEC,19 as well as after intracerebral injection of thrombin into rat brain.18 To our knowledge, this is the first report of thrombin-induced barrier permeability in primary mBEC (both alone and in coculture with primary astrocytes), as well as the first such study to use purified α-thrombin in a species-consistent system. The α-thrombin used here was derived by methods originally used for the purification and characterization of human α-thrombin.34 The combination of primary cells with species-matched thrombin minimizes the possibility that any observations made can be attributed to alteration of brain endothelial cell genotype or physiology with multiple passages, innate inflammatory responses of the cells, contaminants, or species differences between the reagent and the target cells. Furthermore, blockade of the α-thrombin-mediated changes in the permeability barrier was generated by the clinically relevant specific inhibitor dabigatran.
The mechanism by which thrombin leads to increased endothelial cell permeability is not yet known. Thrombin can exert effects on brain endothelial cells via its interaction with the protease-activated receptors (PAR)-1 and -3,35 and Src kinase signaling has been directly linked to thrombin-induced damage to the permeability barrier in vivo.18 Investigations of thrombin-induced brain tissue damage have implicated the involvement of multiple signaling pathways.36 Furthermore, thrombin is associated with the induction of an ‘inflammatory phenotype' in brain endothelial cells.20 Finally, the effects of thrombin on astrocytes37 together with the role of astrocytes in microvessel barrier integrity38, 39 suggest that in vivo thrombin may exert its effects on the permeability barrier in part through astrocytes. Here, coculture with astrocytes increased the permeability barrier features of the endothelium as described previously.39 Dabigatran also reversed the increased permeability caused by acute exposure to α-thrombin in the presence of astrocytes (Figure 3B).
Another potential mechanism lies in the serine protease activity of thrombin. There is sparse information concerning the impact of thrombin on ECM proteins.22, 23, 40, 41 Among its cell-activating properties, thrombin has been shown to stimulate collagen IV synthesis from mesangial cells in culture, and to stimulate the polarized secretion of ECM components.40, 41 Liotta et al22 have shown that α-thrombin selectively degrades laminin and fibronectin, and has the ability to degrade collagens in a temperature-sensitive manner.23 We therefore hypothesized that stabilization of TJ protein expression by β1-integrin might be impaired after exposure to thrombin, given the putative proteolytic activity of thrombin on the ECM.22 Although we observed no change in β1-integrin or claudin-5 expression using flow cytometry (Figure 2), nor any alteration of the continuous staining of claudin-5 in brain endothelial cells using immunofluorescence (Supplementary Figure 2), it remains possible that alterations in other TJ proteins are involved. Alternatively, increased permeability to 4-kDa FITC-dextrans via a transcellular mechanism cannot be ruled out based on the present data, and recent studies suggest that transcellular mechanisms dominate in increased microvessel permeability early after an ischemic event.42 In short, dabigatran inhibits the increase in primary cerebral endothelial cell permeability to 4-kDa dextran caused by α-thrombin, but this effect does not appear to involve stabilization of the TJ complex by preservation of β1-integrin.
Another potential explanation for the observation of increased permeability caused by α-thrombin is suggested by receptor studies. Studies with bEnd.3 cells, as an in vitro model of the permeability barrier, have suggested that thrombin increases permeability to sucrose in a concentration-dependent manner via TRPV channels, which increase intracellular Ca2+ ([Ca2+]i).21 The similar increase in permeability to 4-kDa dextrans indicates that murine α-thrombin initiates increased permeability by increasing [Ca+2]i rapidly. Thrombin appears to achieve the increases in [Ca+2]i from stores by activation of the G-protein-coupled proteinase-activated receptor-1.43 The specificity of dabigatran for the serine active site of thrombin, then, indicates that the effects of α-thrombin to increase cerebral endothelial cell permeability require this active site. Additionally, thrombin can elicit disorganization of VE-cadherins and thereby loss of intact interendothelial adherens junctions.44, 45
In these experiments OGD itself resulted in increased permeability that was partly reversed by dabigatran. Although these experiments were strictly serum free, the possibility that trace amounts of prothrombin and other coagulation factors adherent to the endothelial cell surface could generate thrombin locally cannot be ruled out. Tissue factor can appear on cultured endothelial cells under specific circumstances.46 However, in bEnd.3 cells, we have observed that fetal bovine serum detectably increases endothelial cell permeability, and that this effect is not inhibited by dabigatran (Y Izawa, unpublished observations). This suggests that thrombin is not present in sufficient amounts in fetal bovine serum to account for the observed change in permeability in response to serum, or that dabigatran interacts differently with bovine thrombin. Alternatively, these observations could reflect the tightening of the barrier in response to serum-free media previously reported,47 and indicate that factors other than thrombin are involved.
The magnitude of the effect of α-thrombin on permeability exceeds that of OGD in this system in all experiments performed. Furthermore, we found that α-thrombin and OGD additively increased endothelial cell permeability, and that dabigatran attenuates this effect to the level of OGD alone. Hence, under conditions of experimental ischemia, where thrombin is generated within the microvasculature, it is expected that the thrombin component of increased cerebral microvessel permeability can be abrogated by the antithrombin dabigatran, or other antithrombins. The action of OGD to modestly increase the Papp of the layer is left unexplained at this time. However, the observation has some relevance to the clinical situation, and to other injury settings. Potential players in this reversible OGD-permeability effect include (1) adsorbed thrombin (our first choice, and the simplest to explain), (2) endothelial cell surface receptor(s) (e.g., thrombomodulin), (3) the presence of plasma proteins in caveolae, (4) exposure of TF on the cell surface(s), or (5) cell heterogeneity. There appears no reported relationship between thrombin action and the fate of caveolin, so far. Or rather this remains unexplored. However, the reorganization of the cytoskeleton appears to be mediated by proteinase-activated receptor-1 through rafts, and not caveolae.48 There is little known about TF expression in in vitro murine endothelial cell culture systems (originally described for human arterial endothelial cells by Drake et al46) with regard to any contribution to OGD-related downstream effects on the permeability of those monolayers. It was noted that TF expression on endothelium in vivo is unusual, and is not seen in human or non-human primate brain microvascular endothelium.12, 49, 50 The work of Brown et al21 suggest that individual cells within an endothelial cell monolayer (in that case bEnd.3 cells) are affected differentially by thrombin, suggesting that heterogeneity of response might also be a feature of OGD insults.21 Heterogeneity of function by endothelial cells is recognized in other systems,4 and is certainly likely to operate in the cultures here. The PI studies did not identify cell demise; however, heterogeneity of the α-thrombin effects on these primary endothelial cells seems likely.
We also noted that this in vitro setting lacks the contributions of pericytes, plasma constituents, coagulation factor activation, platelets, and leukocytes, which in intact brain are likely to have roles in microvascular permeability barrier function under focal ischemia as we have shown in the non-human primate. In addition, other aspects of hemostasis within the CNS not captured by any experimental work, including ours, are the contributions of plasma, flow dynamics, the vessel wall, the local tissue environment, and a vasculature that communicates with the systemic circulation.
There is no data regarding direct effects of a selective antithrombin on cerebral microvessel endothelial behavior during or after experimental ischemia in vitro. However, a recent report showed no effect of dabigatran, at concentrations used in the studies here, on proteinase-activated receptor-1-mediated proliferation of fibroblasts or expression of smooth muscle α-actin from baseline in the absence of thrombin.51 Accessory studies confirmed the absence of any effect on fibroblast function of dabigatran alone. Those studies suggest that dabigatran has no direct effect on surface proteinase-activated receptor-1 receptor-mediated events. Control studies here confirm this impression for endothelium. The observation here (Figure 4), while intriguing requires a larger effect to be able to dissect by biochemical techniques.
In the clinic, increased barrier permeability (as visualized by Gd-DTPA hyperintensities on a contrast-weighted magnetic resonance imaging) is predictive of hemorrhagic transformation in ischemic stroke patients.25 This suggests that barrier permeability may precede the development of hemorrhage. If so, inhibition of any thrombin-dependent increase in the endothelial cell-related permeability during focal ischemia by dabigatran could contribute to the reduced risk of hemorrhage associated with this agent. It is unclear whether inhibition of other steps in extrinsic coagulation (e.g. factor Xa) might have a similar effect, as compatible studies would require the presence of plasma, which is technically not feasible.
In conclusion, we have shown that dabigatran exerts a protective effect on an in vitro model of blood–brain barrier permeability in the setting of acute thrombin exposure under normoxia and during experimental ischemia (OGD). The protective effect does not appear to involve any alteration in the endothelial cell β1-integrin expression (under normoxia). Thus, the ability to target thrombin with a specific, direct inhibitor may present an opportunity to preserve barrier function in the setting of stroke and decrease the risk of subsequent hemorrhage. This is related to the proteolytic activity of thrombin on endothelial cell permeability barrier fidelity; however, the exact signaling mechanisms involved remain unknown. Other microvessel components (e.g. ECM) that participate in maintaining the permeability barrier in the CNS may have roles in the hemorrhagic risk reduction. Future studies will address these possibilities.
Acknowledgments
The authors thank Ms GI Berg for her able assistance in the preparation of this manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This investigator-initiated work was supported in part by a research grant from Boehringer Ingleheim GmbH, and by research grants NS 053716 and NS 038710 from the NIH (to GJdZ). The support of the Uehara Foundation (to YI) is gratefully acknowledged. The laboratory also received funds from Mr and Mrs A Gonsalves during this work, which we gratefully acknowledge.
Supplementary Material
References
- 1Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 2Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007; 100: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 3Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011; 123: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 4Rosenberg RD, Aird WC. Vascular bed-specific hemostasis and hypercoagulable states. N Engl J Med 1999; 340: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 5Tran ND, Schreiber SS, Fisher M. Astrocyte regulation of endothelial tissue plasminogen activator in a blood-brain barrier model. J Cereb Blood Flow Metab 1998; 18: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 6Fisher MJ. Brain regulation of thrombosis and hemostasis: from theory to practice. Stroke 2013; 44: 3275–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7del Zoppo GJ, Izawa Y, Hawkins BT. Hemostasis and alterations of the central nervous system. Semin Thromb Hemost 2013; 39: 856–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke 1994; 25: 1847–1853. [DOI] [PubMed] [Google Scholar]
- 9Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke 2001; 32: 1341–1348. [DOI] [PubMed] [Google Scholar]
- 10Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–185. [DOI] [PubMed] [Google Scholar]
- 11Osada T, Gu Y-H, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M et al. Interendothelial claudin-5 expression depends upon cerebral endothelial cell–matrix adhesion by β1-integrins. J Cereb Blood Flow Metab 2011; 31: 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12del Zoppo GJ, Yu JQ, Copeland BR, Thomas WS, Schneiderman J, Morrissey JH. Tissue factor localization in non-human primate cerebral tissue. Thromb Haemost 1992; 68: 642–647. [PubMed] [Google Scholar]
- 13Eddleston M, de la Torre JC, Oldstone MB, Loskutoff DJ, Edgington TS, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system. A role for astrocytes in cerebral hemostasis. J Clin Invest 1993; 92: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Maggio N, Itsekson Z, Dominissini D, Blatt I, Amariglio N, Rechavi G et al. Thrombin regulation of synaptic plasticity: Implications for physiology and pathology. Exp Neurol 2013; 247: 595–604. [DOI] [PubMed] [Google Scholar]
- 15Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 2007; 10: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 16del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 17Siller-Matula JM, Schwameis M, Blann A, Mannhalter C, Jilma B. Thrombin as a multi-functional enzyme. Focus on in vitro and in vivo effects. Thromb Haemost 2011; 106: 1020–1033. [DOI] [PubMed] [Google Scholar]
- 18Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W et al. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol 2010; 67: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Guan JX, Sun SG, Cao XB, Chen ZB, Tong ET. Effect of thrombin on blood brain barrier permeability and its mechanism. Chin Med J (Engl) 2004; 117: 1677–1681. [PubMed] [Google Scholar]
- 20Alabanza LM, Bynoe MS. Thrombin induces an inflammatory phenotype in a human brain endothelial cell line. J Neuroimmunol 2012; 245: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Brown RC, Wu L, Hicks K, O'Neil RG. Regulation of blood–brain barrier permeability by transient receptor potential type C and type V calcium-permeable channels. Microcirculation 2008; 15: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Goldfarb RH, Liotta LA. Thrombin cleavage of extracellular matrix proteins. Ann NY Acad Sci 1986; 485: 288–292. [DOI] [PubMed] [Google Scholar]
- 23Liotta LA, Goldfarb RH, Terranova VP. Cleavage of laminin by thrombin and plasmin: Alpha-thrombin selectively cleaves the beta chain of laminin. Thromb Res 1981; 21: 663–673. [DOI] [PubMed] [Google Scholar]
- 24del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Koziol JA. Vascular matrix adhesion and the blood-brain barrier. Biochem Soc Trans 2006; 34: 1261–1266. [DOI] [PubMed] [Google Scholar]
- 25Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 2004; 35: 2659–2661. [DOI] [PubMed] [Google Scholar]
- 26Tagaya M, Haring H-P, Stuiver I, Wagner S, Abumiya T, Lucero J et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab 2001; 21: 835–846. [DOI] [PubMed] [Google Scholar]
- 27Lundblad RL, Kingdon HS, Mann KG. Thrombin. Methods Enzymol 1976; 45: 156–176. [DOI] [PubMed] [Google Scholar]
- 28Nesheim ME. A simple rate law that describes the kinetics of the heparin- catalysed reaction between antithrombin III and thrombin. J Biol Chem 1983; 258: 14708–14717. [PubMed] [Google Scholar]
- 29Hawkins BT, Gu YH, Izawa Y, del Zoppo GJ. Disruption of dystroglycan–laminin interactions modulates water uptake by astrocytes. Brain Res 2013; 1503: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke 2008; 39: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab 2008; 28: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Okada Y, Yamaguchi T, Minematsu K, Miyashita T, Sawada T, Sadoshima S et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989; 20: 598–603. [DOI] [PubMed] [Google Scholar]
- 34Fenton JW, Fasco MJ, Stackrow AB. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem 1977; 252: 3587–3598. [PubMed] [Google Scholar]
- 35Bartha K, Domotor E, Lanza F, Adam-Vizi V, Machovich R. Identification of thrombin receptors in rat brain capillary endothelial cells. J Cereb Blood Flow Metab 2000; 20: 175–182. [DOI] [PubMed] [Google Scholar]
- 36Fujimoto S, Katsuki H, Kume T, Akaike A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis 2006; 22: 130–142. [DOI] [PubMed] [Google Scholar]
- 37Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Medicine 2001; 7: 59–64. [DOI] [PubMed] [Google Scholar]
- 38Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 39Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 1987; 325: 253–257. [DOI] [PubMed] [Google Scholar]
- 40Kaizuka M, Yamabe H, Osawa H, Okumura K, Fujimoto N. Thrombin stimulates synthesis of type IV collagen and tissue inhibitor of metalloproteinases-1 by cultured human mesangial cells. J Am Soc Nephrol 1999; 10: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 41Papadimitriou E, Manolopoulos VG, Hayman GT, Maragoudakis ME, Unsworth BR, Fenton JW et al. Thrombin modulates vectorial secretion of extracellular matrix proteins in cultured endothelial cells. Am J Physiol 1997; 272: C1112–C1122. [DOI] [PubMed] [Google Scholar]
- 42Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron 2014; 82: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 2006; 13: 693–708. [DOI] [PubMed] [Google Scholar]
- 44Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol 1996; 16: 488–496. [DOI] [PubMed] [Google Scholar]
- 45Kim YV, Di Cello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am J Physiol Cell Physiol 2004; 286: C31–C42. [DOI] [PubMed] [Google Scholar]
- 46Drake TA, Hannani K, Fei H, Lavi S, Berliner JA. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol 1991; 138: 601–607. [PMC free article] [PubMed] [Google Scholar]
- 47Nitz T, Eisenblatter T, Psathaki K, Galla HJ. Serum-derived factors weaken the barrier properties of cultured porcine brain capillary endothelial cells in vitro. Brain Res 2003; 981: 30–40. [DOI] [PubMed] [Google Scholar]
- 48Carlile-Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol 2007; 293: H366–H375. [DOI] [PubMed] [Google Scholar]
- 49Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: Implications for disorders of hemostasis and thrombosis. Am J Pathol 1989; 134: 1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 50Drake TA, Cheng J, Chang A, Taylor FB, Jr. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 1993; 142: 1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 51Bogatkevich GS, Ludwicka-Bradley A, Silver RM. Dabigatran, a direct thrombin inhibitor, demonstrates antifibrotic effects on lung fibroblasts. Arthritis Rheum 2009; 60: 3455–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.