Abstract
Recent studies have shown that blood glutamate grabbing is an effective strategy to reduce the excitotoxic effect of extracellular glutamate released during ischemic brain injury. The purpose of the study was to investigate the effect of two of the most efficient blood glutamate grabbers (oxaloacetate and recombinant glutamate oxaloacetate transaminase 1: rGOT1) in a rat model of intracerebral hemorrhage (ICH). Intracerebral hemorrhage was produced by injecting collagenase into the basal ganglia. Three treatment groups were developed: a control group treated with saline, a group treated with oxaloacetate, and a final group treated with human rGOT1. Treatments were given 1 hour after hemorrhage. Hematoma volume (analyzed by magnetic resonance imaging (MRI)), neurologic deficit, and blood glutamate and GOT levels were quantified over a period of 14 days after surgery. The results observed showed that the treatments used induced a significant reduction of blood glutamate levels; however, they did not reduce the hematoma, nor did they improve the neurologic deficit. In the present experimental study, we have shown that this novel therapeutic strategy is not effective in case of ICH pathology. More importantly, these findings suggest that blood glutamate grabbers are a safe treatment modality that can be given in cases of suspected ischemic stroke without previous neuroimaging.
Keywords: blood glutamate grabbing, GOT, intracerebral hemorrhage, oxaloacetate, protection
Introduction
Intracerebral hemorrhage (ICH) represents approximately 15% of all strokes; moreover, the incidence of ICH is expected to grow, given the increase in the use of anticoagulants and aging of the population.1 It is a type of acute stroke characterized by extravasation of blood into the brain parenchyma and formation of hematoma. Hematoma produces primary damage because of the toxic effect of thrombin, a mass effect due to the extravasated blood that compresses the surrounding brain tissue; sometimes, this damage is also enhanced by rebleeding. Primary damage is followed by a secondary damage mainly characterized by edema formation, tissular ischemia, and glutamate excitotoxicity.2
Depending on the underlying cause of bleeding, ICH originates from the spontaneous rupture of small arteries or arterioles damaged because of two major factors: hypertensive arteriolosclerosis and amyloid angiopathy, which accounts for 78% to 88% of cases. Other cases of ICH occur in a minority of patients in association with coagulopathy, brain tumors, aneurysms, vascular anomalies, and thrombolytic treatment of ischemic stroke (reviewed in Wang and Dore1). Treatment for ICH is primarily supportive, and the clinical outcome is poor with potential extensive burden for the caretakers. Early administration of hemostatic agents, meticulous blood pressure control, and early surgical evacuation and catheter hematoma aspiration have also been used to limit hematoma expansion.3 Therefore, to improve the clinical outcome of ICH and better understand its pathogenesis, induced brain injury is in high demand.1
Several lines of evidence suggest that glutamate is involved in the secondary damage of ICH. Thus, transient elevation of the extracellular concentration of glutamate in the perihematomal region was shown in animal models of ICH induced by means of injection of autologous blood or collagenase into the basal ganglia.4 We have also previously described in clinical observational studies that high levels of blood glutamate were associated with the outcome of ICH.5, 6 In addition, the effect of memantine, a blocker of the N-methyl-D-aspartate (NMDA) glutamate receptor, was investigated in an ICH collagenase-injection rat model as a protective drug. Administration of memantine, after induction of ICH, reduced hemorrhagic volume, apoptotic cell death, and neutrophil infiltration, decreased the number of microglia/macrophages in the periphery of hematoma, and improved the neurologic deficit,7, 8, 9, 10, 11, 12 confirming the critical role of glutamate in this pathology.
Nowadays, the concept of blood glutamate grabbing has been presented as a novel and attractive protecting strategy to reduce the excitotoxic effect of extracellular glutamate in the brain. This mechanism is based on the administration of oxaloacetate or recombinant glutamate oxaloacetate transaminase 1 (rGOT1), which leads to a metabolization and reduction of glutamate in blood and a subsequent lowering of glutamate in the cerebral parenchyma (see Campos et al13 and Teichberg et al14 for review). The protective efficacy of this strategy has been widely shown by independent laboratories in different types of ischemic animal models,15, 16, 17, 18, 19, 20 and it has also been tested in other pathologies associated with brain glutamate increase, such as traumatic brain injury,21, 22 subarachnoid hemorrhage,23, 24 or glioma,25 with successful results. However, the use of blood glutamate grabbers has never been tested in an ICH model.
Due to the critical role of glutamate in the ICH outcome, the aim of this study was to determinate whether blood glutamate reduction by oxaloacetate or rGOT1 can reduce the hemorrhagic damage in a rat model of ICH induced through the intraparenchymal injection of collagenase.
Materials and methods
Animals
The animal experiments were conducted in accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimentation and the current ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). Experiments were approved by the Galician Network Committee for Ethics Research following the Spanish and European Union (EU) rules (86/609/CEE, 2003/65/CE, 2010/63/EU, RD 1201/2005 and RD53/2013).
Male Sprague-Dawley rats weighing between 300 and 350 g (10 to 12 weeks old) were used for this study. Rats were given water and were fed ad libitum. Anesthesia was induced with sevoflurane (5% to 6% for induction and 3% to 4% for maintenance) evaporated in an oxygen–air mixture (30%:70%). Throughout the experimental period, rectal temperature was maintained at 37±0.5°C by means of an electronic thermostat-controlled warming blanket. Body temperature was maintained until animals completely recovered from anesthesia and displayed normal motor activity.
Surgical Procedures
For the ICH model, we used one of the most common ICH models that involve the intraparenchymal injection of bacterial collagenase. Collagenase disrupts the basal lamina of blood vessels, which leads to the leaking of blood into the surrounding tissue.26
To perform the model, rats were placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA) under sevoflurane anesthesia. A 1-cm-long midline incision was made in the scalp, beginning midway between the eyes and terminating behind the lambda. A cotton swab was used to clear away the soft tissue covering the skull. A Hamilton syringe (Hamilton, Reno, NV, USA; 10 μL) was filled with 1.5 μL of collagenase type VII (sterile-filtered, high purity; Sigma-Aldrich, St Louis, MO, USA) dissolved in saline (0.2 U/μL). The syringe was mounted onto the injection pump and the needle was positioned directly over the bregma. The x, y, and z axis coordinates were all set to zero. The needle was then positioned at the entry point, +0.6 mm anterior and −2.9 mm lateral of the bregma to the right. A small cranial burr hole was drilled through the skull at the entry point. The needle was slowly inserted into the basal ganglia to a depth of 5.5 mm below the surface of the skull, and a volume of 1 μL collagenase was injected at a rate of 0.1 μL/min over 10 minutes. The needle was left in place for 10 minutes and then removed at a rate of 1 mm/min to prevent the reflux of collagenase and blood. The burr hole was filled with bone wax (Ethicon, Somerville, NJ, USA), and the scalp incision was closed. Sham control rats were injected with an equal volume of saline. The rats were placed in an animal box after surgery for recovering in a warm place with access to food.
Experimental Groups
To know the hematoma growth profile of the ICH model, the hemorrhagic volumes were determined by magnetic resonance imaging (MRI) at 1, 3, 5, and 8 hours and at 1, 3, 7, and 14 days after collagenase injection in a group of four animals.
Once the growth of hematoma was characterized in the ICH model, the protective effect of two blood glutamate grabbers was tested, oxaloacetate and human rGOT1 (human rGOT1 was provided by Professor David Mirelman from Weizmann Institute, Israel).
For this purpose, three ICH experimental groups were developed: (1) the ICH control group treated with saline (0.9% of NaCl) (n=12); (2) the ICH group treated with an effective dose of oxaloacetate 3.5 mg/100 g (n=8); and (3) the ICH group treated with an effective dose of rGOT1 12.88 μg/100 g (n=8). Sample size was based on expected variances: α=0.05 (confidence level: 95%) and β=0.20 (power: 80%). Effective doses of treatments were selected based on our previous experience.15, 17 Treatments and saline were administered intravenously (jugular vein) as a bolus injection, adjusting the dose to a final volume injection of 1 mL for all animals. Treatments were administered 1 hour after brain collagenase injection. Researchers were blinded to treatments, and treatments were randomized.
Based on the hematoma growth profile study, we selected the critical time points of the hemorrhage growth, and volumes were determined in the three experimental groups at 1 hour (before treatment administration), 3 hours, 24 hours and 7 and 14 days after hemorrhage induction. Animals without hemorrhage, with small hemorrhage, or with hemorrhage located far from the basal ganglia were excluded from the study before treatment administration.
Neurologic deficit was analyzed by means of Bederson and Wahl scales in basal conditions (1 day before surgery) and 5 hours, 24 hours and 7 and 14 days after hemorrhage onset.
Blood glutamate levels and blood GOT activity were determined before surgery (basal conditions) and at 1 hour and 3 hours after collagenase injection.
Hemorrhage Volume Analysis
Lesion size and hematoma growth were assessed by means of MRI. The MRI studies were conducted on a 9.4-T horizontal bore magnet (BrukerBioSpin, Ettligen, Germany) with 20 cm wide actively shielded gradient coils (440 mT/m). Radiofrequency transmission was achieved with a birdcage volume resonator; signal was detected using a four-element surface coil, positioned over the head of the animal, which was fixed with a teeth bar, earplugs, and adhesive tape. Transmission and reception coils were actively decoupled from each other. Gradient-echo pilot scans were performed at the beginning of each imaging session for accurate positioning of the animal inside the magnet bore.
T2-weighted images were acquired using a RARE-VTR sequence with the following acquisition parameters: echo time=9.5 ms, 8 echos, rare factor=2, repetition time=3 seconds, number of averages=2, field-of-view=19.2 × 19.2 mm2, image matrix=192 × 192 (isotropic in-plane resolution of 0.1 mm/pixel × 0.1 mm/pixel), and 18 consecutive slices of 0.5 mm thickness.
T2*-weighted images were acquired using a MGE sequence with 8 echos, first echo time=3.13 ms, echo epacing=3.38 ms, repetition time=1.4 seconds, number of averages=2, and the same geometry parameters as that of T2-weighted images (field-of-view, image matrix, number of slices and thickness).
Magnetic Resonance Imaging Analysis
All images were processed using ImageJ (RasbandWS, ImageJ, NIH, http://rsb.info.nih.gov/ij). Hematoma size was manually measured in T2-weighted images. Researchers were blinded to image analysis.
Determination of Blood Glutamate Concentration
Blood samples were collected from the tail vein, centrifuged at 4°C 3,000 g for 7 minutes, and serum was immediately frozen and stored at −80°C until glutamate analysis. Serum levels of glutamate were determined by high-performance liquid chromatography and a Waters AccQ•Tag kit following the manufacturer's technical specifications (Waters, Milford, MA, USA). Blood glutamate levels were determined before surgery and 1 hour (before treatment administration) and 3 hours after hemorrhage. Researchers were blinded to glutamate analysis.
Serum Glutamate Oxaloacetate Transaminase Activity Analysis
Blood GOT activity was determined by means of the Reflotron GOT (AST) test following the manufacturer's technical specifications (Roche, Basel, Switzerland). Blood GOT levels were determined before surgery and at 1 hour (before treatment administration) and 3 hours after hemorrhage. Researchers were blinded to GOT analysis.
Behavioral Tests
Evaluation of neurologic damage and motor deficits was measured by two complementary tests, Bederson and Wahl scales.27, 28 Observers were blinded to the group analyzed.
In brief, the Bederson scale is a global neurologic assessment that was developed to measure neurologic impairments after stroke. Tests include forelimb flexion, resistance to lateral push, and circling behavior. A grading scale of 0 to 8 is used to assess behavioral deficits after cerebral damage.27 Zero value represents an animal without neurologic deficit and 8 represents an animal with high neurologic deficit.
In the Wahl scale, neurologic status was rated for the following items: equilibrium: on a horizontal bar and an inclined plane; grasping reflex in which observation of the four paws allowed for separate scoring of the contralateral and ipsilateral (i.e., occlusion) sides; righting reflexes: ‘head tilted', ‘on back', and ‘dropped' placing reactions: visual and ‘leg hanging' in which observation of the four paws allowed for separate scoring of the contralateral and ipsilateral sides; and motility: spontaneous and circling behavior.28 In this test, a grading scale of 0 to 8 was used to assess behavioral deficits: 0 value represents an animal with high neurologic deficit, and 8 represents an animal without neurologic deficit.
Behavioral tests were performed during the light cycle of animal housing, with environmental conditions consistently maintained across examinations. Neurologic tests were performed 1 day before surgery and 5 hours, 24 hours, 7 days, and 14 days after onset of the hemorrhagic lesion. Researchers were blinded to behavioral analysis.
Statistical Analysis
Results were expressed as mean±s.e.m. Behavioral data were indicated as median±s.e.m. Statistical analyses were performed using one-way ANOVA followed by a Bonferroni post hoc analysis to determine differences between groups and differences with respect to basal levels. A P-value <0.05 was considered statistically significant. The statistical analysis was conducted using PASW Statistics 18 for Mac (SPSS Inc., Chicago, CA, USA). Researchers were blinded to statistical analysis.
Results
Hematoma Growth Profile in Intracerebral Hemorrhage Model
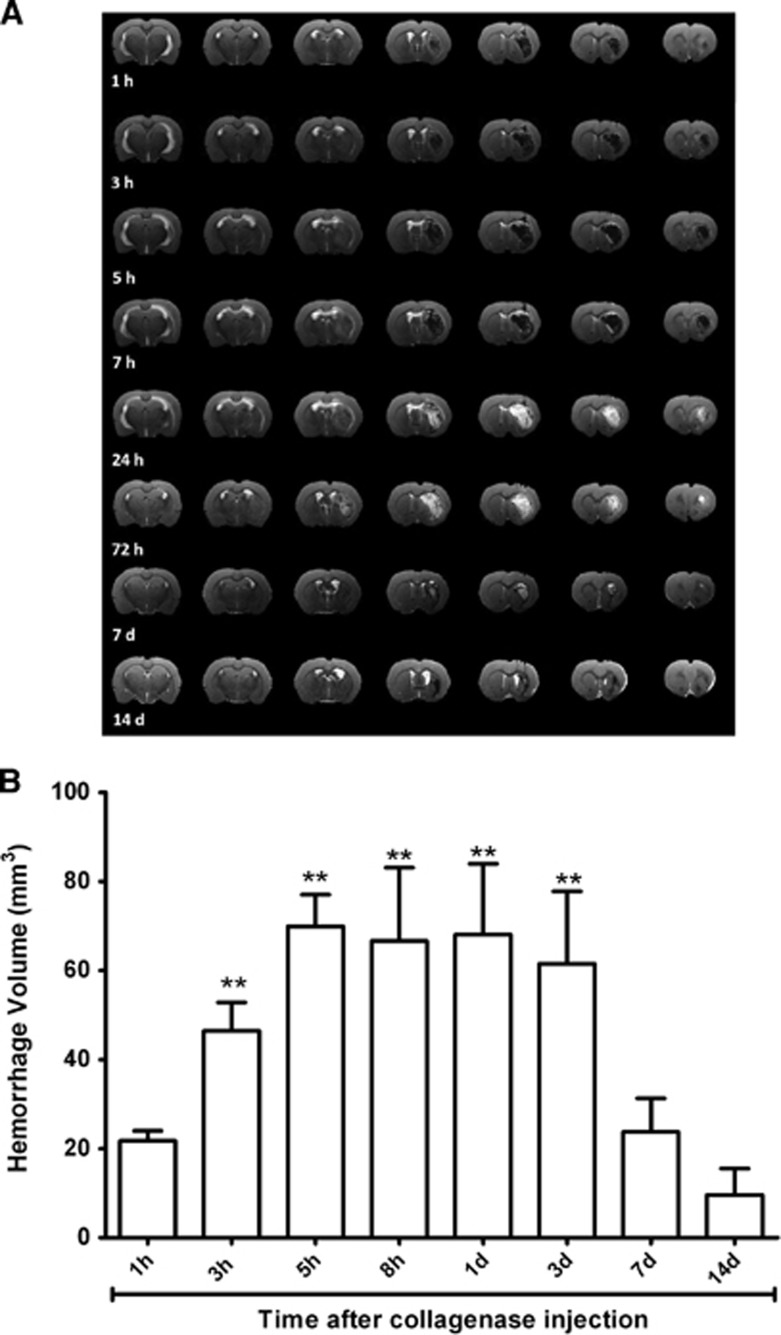
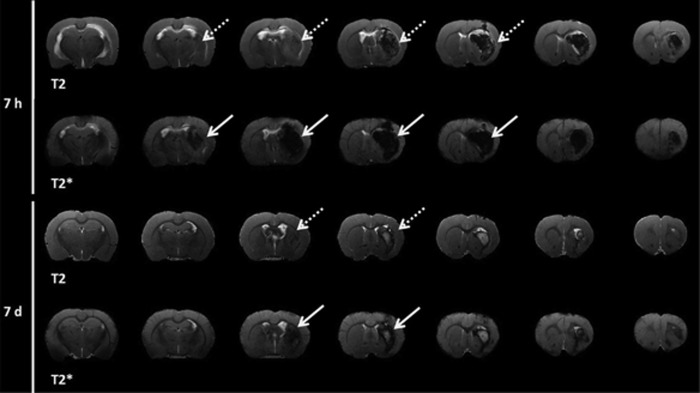
To determine the evolution of hematoma growth in the ICH model induced through intraparenchymal injection of collagenase, hematoma volume was determined at different time points over a 14-day follow-up period. Figure 1 shows that collagenase injection induces an immediate (1 hour after enzyme injection) hemorrhagic lesion of 21.74±2.20 mm3. Maximum hematoma volume was achieved at 5 hours (69.94±7.12±16.50 mm3) and volume stabilized 3 days later. No significant differences were observed in hematoma volume between 5 hours and 3 days (69.94±7.12 versus 61.53±16.26 mm3, P>0.05). After 3 days, hematoma reduced until 23.72±7.60 mm3 at day 7 (P<0.05 with respect to the third day), followed by a hemorrhagic residual volume at day 14 (9.65±5.87 mm3). Because of the blooming effect in T2*-weighted images due to bleeding, the MRI analysis of hematoma was performed by measuring the extension of the lesion in T2-weighted images (Figure 2).
Figure 1.
Evolution of hematoma growth in the intracerebral hemorrhage (ICH) model induced through intraparenchymal injection of collagenase. (A) Hemorrhagic volume analysis was determined by means of T2-weighted magnetic resonance imaging (MRI). (B) Hemorrhagic volume was determined at 1, 3, 5, and 8 hours and 1, 3, 7, and 14 days after collagenase injection (n=4). Data are shown as mean±s.e.m. Statistical evaluation of the results was performed using one-way ANOVA to determine differences with respect to initial damage. **P<0.01 with respect to hemorrhagic volume at 1 hour after collagenase injection.
Figure 2.
Analysis of hemorrhagic lesion at 7 hours and 7 days after hemorrhage onset by means of T2 and T2*-weighted magnetic resonance imaging (MRI). Blooming effect enhances the hypointensity of T2* images (indicated as continous white arrow) in relation to T2 images (indicated as discontinous white arrow). T2-weighted MRI images were chosen to determine the hemorrhagic volume.
Based on the hematoma growth profile, we selected 1 hour (before treatment), 3 hours, 24 hours, 7 days and 14 days as representative time points of the hematoma evolution to determine whether the treatments tested had any effect on the hemorrhagic damage.
The sham group did not show a hemorrhagic lesion, further than the track injection damage caused by the needle (data not shown).
Effect of Blood Glutamate Grabbers on Hemorrhagic Volume
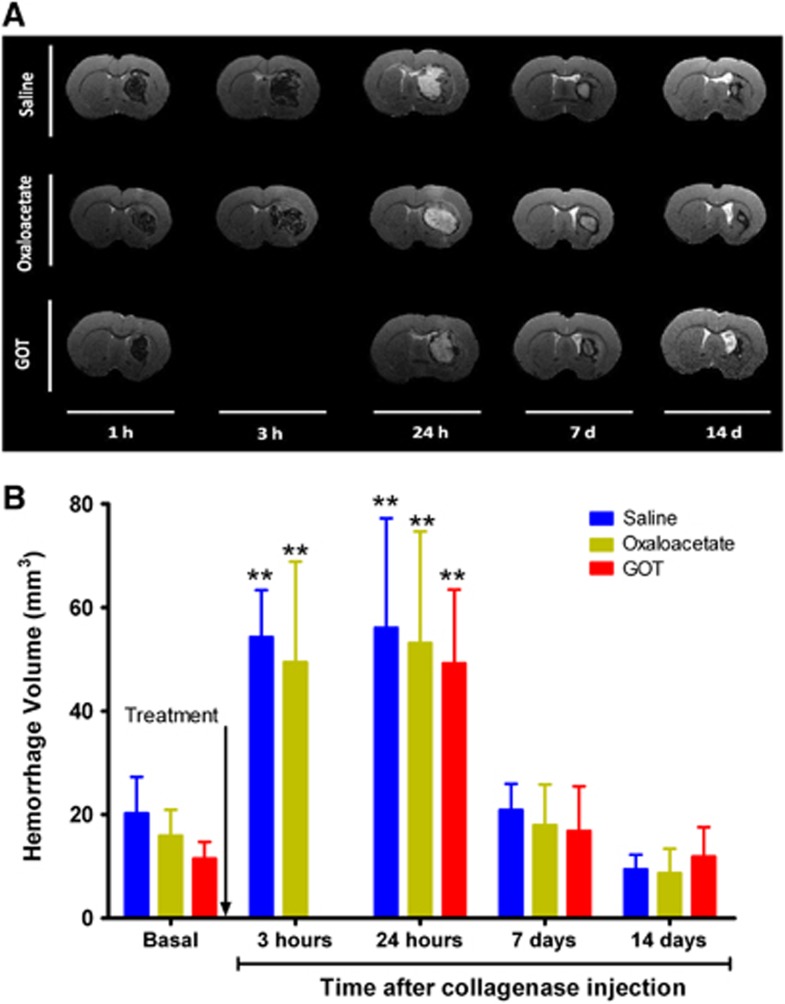
To determine the effect of the reduction of blood glutamate levels on hematoma growth, we used two glutamate grabbers, oxaloacetate and rGOT1, at the same effective dose previously tested in our stroke models (3.5 mg/100 g and rGOT1 12.88 μg/100 g, respectively). Saline administration was used in the control group. Analysis of hematoma growth in the control group over 14 days showed the same profile as that of animals whose hematoma evolution had been previously determined. The hemorrhagic volume increased until 24 hours after surgery and decreased 6 days later, with a residual hemorrhagic volume at 14 days (Figure 3). Administration of oxaloacetate 3.5 mg/100 g at 1 hour after hemorrhage onset did not modify the hematoma growth curve profile with respect to the control group (P>0.05). To validate these results, rGOT1 was proved in a second set of animals. Because the maximum increase in hemorrhage was achieved at 24 hours, in this group, analysis at 3 hours was not measured. Administration of rGOT1 confirmed the previous results seen with oxaloacetate, non-effect on hemorrhagic growth. Hemorrhagic volume measured 1 hour after enzyme injection (before treatment administration) was similar in all groups (P>0.05), which indicates that all treated animals were subjected to the same hemorrhagic damage. Four animals were excluded before treatment administration: three animals due to small hemorrhage (volumes<10 mm3) and one because hemorrhage was not located in the basal ganglia. Only one animal died 3 days after treatment administration (oxaloacetate). One extra animal was included in the oxaloacetate group to complete a total number of n=8. Excluded animals were not recorded in any of the groups after treatment administration (saline, oxaloacetate, or rGOT).
Figure 3.
Effect of blood glutamate grabbers on hemorrhagic volume. (A) Analysis of hemorrhagic volume was determined by means of T2-weighled magnetic resonance imaging (MRI). (B) Saline treatments (n=12), oxaloacetate 3.5 mg/100 g (n=8), and recombinant glutamate oxaloacetate transaminase 1 (rGOT1) 12.88 μg/100 g (n=8) were administered 1 hour after hemorrhage onset (indicated by the arrow). Hemorrhagic volume was analyzed 1 hour (before treatment administration), 3 hours, 24 hours and 7 and 14 days after hemorrhage induction. In the rGOT1-treated group (used to validate oxaloacetate results) T2 imaging at 3 hours was not performed. Data are shown as mean±s.e.m. **P<0.01 with respect to basal damage. One animal died after 3 days in the oxaloacetate group. One extra animal was included in this group to complete a total number of n=8. Excluded animals were not recorded after treatment administration.
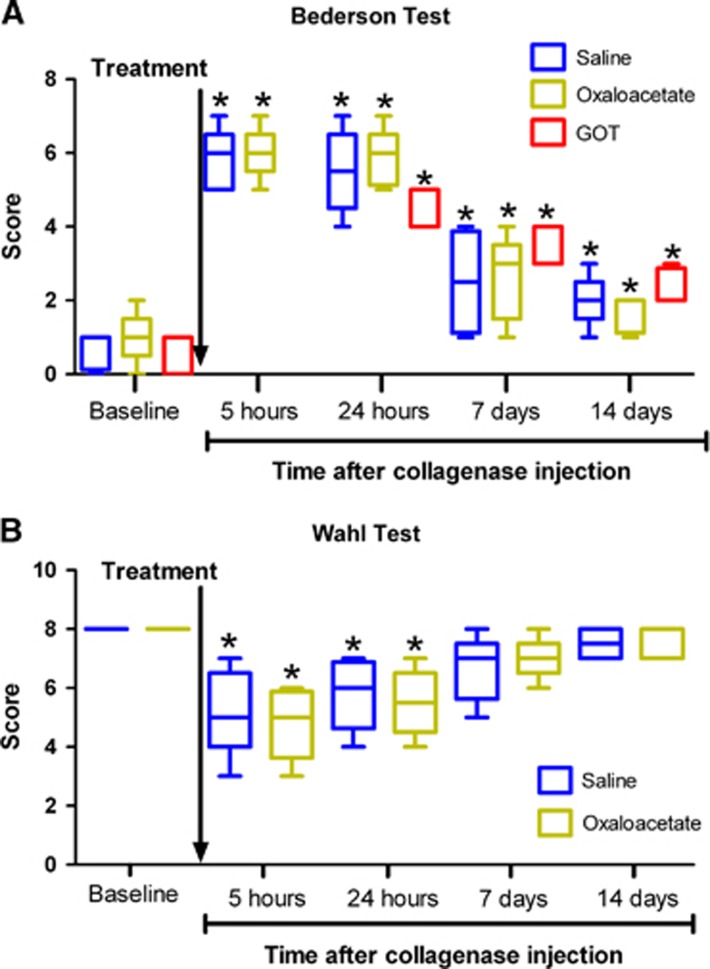
Effect of Blood Glutamate Grabbers on Neurologic Deficit Caused by Intracerebral Hemorrhage
To determine whether the administration of blood glutamate grabbers could induce any effect on the neurologic deficit caused by the hemorrhagic lesion, the Bederson scale and the Wahl test were performed at 1 day before surgery and at 5 hours, 24 hours, 7 days and 14 days after appearance of the hemorrhagic lesion. Neurologic evolution in the control group showed that the hemorrhagic lesion caused a significant deficit (P<0.05) 5 hours after brain lesion and persisted at least for a further 24 hours, as seen in Figures 4A and 4B. Neurologic deficit recovery appears at day 7; however, at day 14 there still existed a significant deficit as detected by the Bederson scale (Figure 4A), possibly caused by the residual hemorrhagic lesion (Figure 3). Of note, in both behavioral analyses, neurologic deficit progression matched the hematoma growth evolution. In line with the hematoma volume results, oxaloacetate treatment did not alter either of the behavioral analyses in comparison with the control group (P>0.05). Again, to confirm the oxalocatate results on neurologic deficit, the Bederson scale was performed in the rGOT group in basal conditions and 24 hours, 7 days, and 24 days after hemorrhage onset (the analysis after 5 hours was not included). Results showed that, similar to oxalocatate, rGOT also did not improve the hemorrhagic neurologic deficit (P>0.05).
Figure 4.
Effect of blood glutamate grabbers on neurologic damage caused by intracerebral hemorrhage (ICH). Saline treatments (n=12), oxaloacetate 3.5 μg/100 g (n=8), and recombinant glutamate oxaloacetate transaminase 1 (rGOT1) 12.88 μg/100 g (n=8) were administered 1 hour after hemorrhage onset (indicated by the arrow). Neurologic deficit was evaluated by means of the Bederson scale (A) and the Wahl test (B). Neurologic tests were performed 1 day before surgery and 5 hours, 24 hours, 7 days, and 14 days after appearance of the hemorrhagic lesion. In the rGOT1-treated group (used to validate oxaloacetate results) Bederson test at 5 hours was not performed and the Wahl test was not included in the study. Data are shown as mean±s.e.m. Statistical evaluation of the results was performed using one-way ANOVA followed by a Bonferroni post hoc analysis to determine differences between groups and differences with respect to basal levels. *P<0.05 with respect to baseline values.
Analysis of Blood Glutamate and Glutamate Oxaloacetate Transaminase Levels in Intracerebral Hemorrhage-Treated Animals
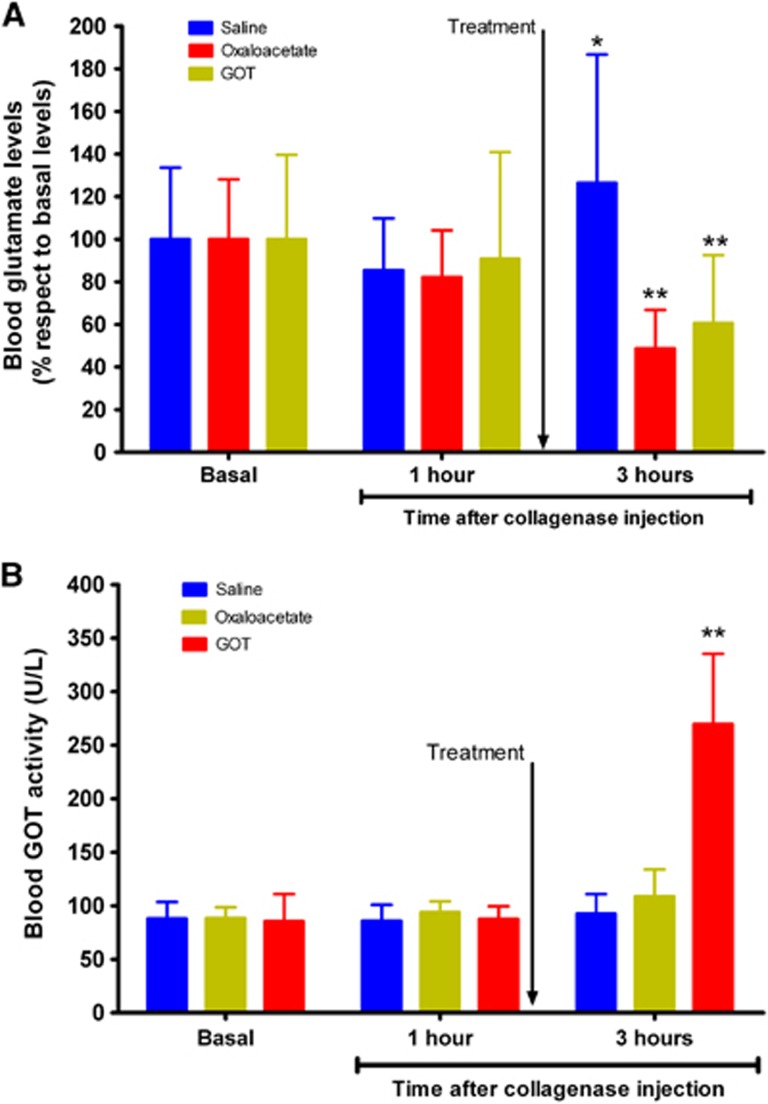
To determine whether both treatments were able to induce a significant reduction in blood glutamate levels, the levels were measured before surgery, to know the basal concentration, 1 hour after hemorrhage (before treatment administration), and 2 hours later. Figure 5A shows that, while in the control group appears a significant (P<0.05) increase of blood glutamate 3 hours after hemorrhage onset, in the treated groups it was observed a significant (P<0.05) reduction of glutamate respect to the basal levels. In line with our previous ischemic studies,15, 17 these results therefore confirm the efficacy of the treatments in decreasing the blood glutamate concentration.
Figure 5.
Analysis of blood glutamate and glutamate oxaloacetate transaminase (GOT) levels in intracerebral hemorrhage (ICH)-treated animals. Saline treatments (n=12), oxaloacetate 3.5 mg/100 g (n=8), and recombinant glutamate oxaloacetate transaminase 1 (rGOT1) 12.88 μg/100 g (n=8) were administered 1 hour after hemorrhage onset (indicated by the arrow). Blood glutamate (A) and GOT (B) levels were determined before surgery and 1 hour (before treatment administration) after hemorrhage. Data are shown as mean±s.e.m. *P<0.05, **P<0.01 compared with basal levels. Statistical evaluation of the results was performed using one-way ANOVA followed by a Bonferroni post hoc analysis to determine differences between groups and differences with respect to basal levels. *P<0.05 and **P<0.01 with respect to basal levels.
The GOT levels in blood were measured in all groups, before surgery (basal levels), 1 hour after hemorrhage (before treatment administration), and 2 hours later. In the control and oxaloacetate groups, hemorrhagic damage did not alter the blood GOT levels with respect to basal conditions (average: 90±5 U/L). In the rGOT1 group, treatment administration induced a significant (P<0.05) increase in GOT activity 2 hours after administration (269.62±65.67 U/L) with respect to the basal state (85.58±25.24 U/L). These data, in agreement with our previous reports,17 confirm that a dose of rGOT1 12.88 μg/100 g induces a significant increase in endogenous blood GOT levels.
Discussion
Intracerebral hemorrhage is understood as a dynamic and serial process in which early hematoma is followed by a combination of molecular pathways such as glutamate excitotoxicity, inflammation, brain–blood barrier breakdown, and development of vasogenic edema, which contribute to the growth of the hemorrhagic lesion.9, 29 The critical role of glutamate in ICH outcome has led to the use of different types of glutamate antagonists (mainly NMDA antagonist) as potential treatments against hematoma lesions after hemorrhage. In this regard, drugs such as MK801 or memantine have showed positive results in animal models of ICH when hematoma volume and brain edema are analyzed.8, 11, 12 However, the continuous failure, as well as the adverse effects observed with these drugs in stroke clinical trials, has made other alternatives different from glutamate antagonists preferable for reducing the effect of glutamate excitotoxicity.30
Mainly focused on ischemic stroke pathology, we have described that a reduction in blood glutamate concentration can induce a lowering of brain glutamate with significant protective effects after ischemic insults.14, 15, 17, 31 This novel mechanism has also been validated for other independent laboratories with similar results.16, 32, 33
However, despite the beneficial effect observed in ischemic stroke models and other neurologic disorder animal models, in this study, administration of two of the most efficient blood glutamate grabbers used (oxaloacetate or rGOT1) was not able to reduce hematoma lesion or improve the neurologic outcome in animals subjected to an ICH. Reduction of blood glutamate levels showed that both drugs had indeed an effect on lowering blood and subsequently brain glutamate levels.
It could be tentative to speculate that brain glutamate levels were not evaluated in this study and therefore we cannot guarantee that brain glutamate was reduced in our ICH model after treatment administration. In previous experimental studies, again in the stroke model, to confirm that the protective effect observed was mediated throughout a decrease in brain glutamate, we analyzed cerebral glutamate levels by means of noninvasive magnetic resonance spectroscopy in the infarct region. Spectroscopic analysis revealed that the increase in brain glutamate seen in control animals after cerebral artery occlusion was significantly reduced in animals treated with both treatments, oxaloacetate15 and rGOT1.17 This finding provided the evidence on the efficacy of both drugs to act on the brain glutamate. However, brain glutamate determination could not be performed by magnetic resonance spectroscopy in the ICH model because of the presence of blood in the hemorrhagic lesion, which produces inhomogeneity effects that interfere with glutamate peaks in the spectroscopy analysis. Therefore, the present results seem to suggest that blood glutamate grabbing is an efficient strategy in ischemic stroke, but not a therapeutic option against ICH.
Although the mechanisms are different, the protective effect reported previously regarding glutamate antagonists in ICH models is not contradictory with our results. Glutamate antagonists (such as MK801 or memantine) induce a block of NMDA receptors, which leads to an inhibition of NMDA receptor signaling and multiple downstream molecular pathways involved in hemorrhage pathology.34 In case of blood glutamate grabbers, they have a systemic effect based on the reduction of glutamate levels but they do not act on glutamate receptors like antagonists do; therefore, it is possible that complete inhibition of several molecular pathways involved in NMDA receptor signaling is needed to achieve significant protective effect. By contrast, the fact that glutamate grabbers do not interact with neuronal ionotropic glutamate receptors also avoids problems of toxicity described for glutamate antagonists.
However, hematoma expansion is considered one of the main physiologic complications associated with poor outcome in ICH.29 Although the precise mechanisms involved in the deleterious effect of early hematoma growth during the acute phase are also poorly understood, matrix metalloproteinase overexpression, breakdown of the brain–blood barrier, and intracranial pressure are proposed as important processes associated with early hematoma damage higher than other secondary mechanisms such as glutamate toxicity.29, 35 In fact, glutamate release is transiently accumulated in the perihematoma region due to hematoma expansion, and minimal invasive surgery for removal of intracerebral hematoma and pressure are able to reduce the glutamate content significantly.36 In this line, we dare to hypothesize that glutamate release could be considered an epiphenomenon of ICH, involved in neuronal damage, although with a less important role than other molecular mechanisms, such as brain–blood barrier breakdown or intracranial pressure. This hypothesis is also in agreement with a recent report from our group35 wherein we described in a clinical observational study that the association between hyperthermia and poor outcome in ICH seems to be mainly mediated by active matrix metalloproteinase-9 (a biomarker of brain–blood barrier breakdown) and the baseline neurologic deficit, but not by an increase in glutamate levels as occurs in ischemic stroke. In brief, the fact that glutamate could act as a secondary mechanism of the ICH could explain, in part, the non-effect of glutamate grabbers observed in our study as well.
In addition, we have suggested that, in ischemic stroke, blood glutamate grabbers could be administrated immediately as an ambulatory treatment in case of suspected ischemic stroke, without previous computerized tomography scan, because adverse effects in case of hemorrhagic stroke could be discarded.17 Therefore, the lack of effect of blood glutamate grabbers in the ICH herein described confirms our previous indications and guarantees the safety of these drugs.
In conclusion, despite the well-demonstrated beneficial effect of blood glutamate grabbers against glutamate excitotoxicity, in the present experimental study we have shown that this novel therapeutic strategy seems to be ineffective in case of ICH pathology. However, these findings allow us to suggest that blood glutamate grabbers can be given as early as possible, without neuroimaging, in cases of suspected stroke without risk in case of hemorrhagic stroke.
The authors declare no conflict of interest.
Footnotes
This study has been partially supported by grants from Instituto de Salud Carlos III (PI13/00292 and PI11/00909), Spanish Research Network on Cerebrovascular Diseases RETICS-INVICTUS (RD12/0014), Xunta de Galicia (Consellería Educación GRC2014/027) and by the European Union program FEDER. Furthermore, FC (CP14/00154) and TS (CP12/03121) are recipients of a research contract from Miguel Servet Program of Instituto de Salud Carlos III. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007; 27: 894–908. [DOI] [PubMed] [Google Scholar]
- 2Katsuki H. Exploring neuroprotective drug therapies for intracerebral hemorrhage. J Pharmacol Sci 2010; 114: 366–378. [DOI] [PubMed] [Google Scholar]
- 3Belur PK, Chang JJ, He S, Emanuel BA, Mack WJ. Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurg Focus 2013; 34: E9. [DOI] [PubMed] [Google Scholar]
- 4Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med 2003; 31: 1482–1489. [DOI] [PubMed] [Google Scholar]
- 5Leira R, Castellanos M, Alvarez-Sabin J, Diez-Tejedor E, Davalos A, Castillo J. Headache in cerebral hemorrhage is associated with inflammatory markers and higher residual cavity. Headache 2005; 45: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 6Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva Y et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 2002; 58: 624–629. [DOI] [PubMed] [Google Scholar]
- 7Lee ST, Chu K, Jung KH, Kim J, Kim EH, Kim SJ et al. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. J Cereb Blood Flow Metab 2006; 26: 536–544. [DOI] [PubMed] [Google Scholar]
- 8Montagne A, Hebert M, Jullienne A, Lesept F, Le Behot A, Louessard M et al. Memantine improves safety of thrombolysis for stroke. Stroke 2012; 43: 2774–2781. [DOI] [PubMed] [Google Scholar]
- 9Sharp F, Liu DZ, Zhan X, Ander BP. Intracerebral hemorrhage injury mechanisms: glutamate neurotoxicity, thrombin, and src. Acta Neurochir Suppl 2008; 105: 43–46. [DOI] [PubMed] [Google Scholar]
- 10Lapchak PA, Araujo DM. Advances in hemorrhagic stroke therapy: conventional and novel approaches. Expert Opin Emerg Drugs 2007; 12: 389–406. [DOI] [PubMed] [Google Scholar]
- 11Sinn DI, Lee ST, Chu K, Jung KH, Song EC, Kim JM et al. Combined neuroprotective effects of celecoxib and memantine in experimental intracerebral hemorrhage. Neurosci Lett 2007; 411: 238–242. [DOI] [PubMed] [Google Scholar]
- 12Thiex R, Weis J, Krings T, Barreiro S, Yakisikli-Alemi F, Gilsbach JM et al. Addition of intravenous n-methyl-d-aspartate receptor antagonists to local fibrinolytic therapy for the optimal treatment of experimental intracerebral hemorrhages. J Neurosurg 2007; 106: 314–320. [DOI] [PubMed] [Google Scholar]
- 13Campos F, Sobrino T, Ramos-Cabrer P, Castillo J. Oxaloacetate: a novel neuroprotective for acute ischemic stroke. Int J Biochem Cell Biol 2012; 44: 262–265. [DOI] [PubMed] [Google Scholar]
- 14Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 2009; 158: 301–308. [DOI] [PubMed] [Google Scholar]
- 15Campos F, Sobrino T, Ramos-Cabrer P, Argibay B, Agulla J, Perez-Mato M et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab 2011; 31: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Knapp L, Gellert L, Kocsis K, Kis Z, Farkas T, Vecsei L et al. Neuroprotective effect of oxaloacetate in a focal brain ischemic model in the rat. Cell Mol Neurobiol 2014; 35: 17–22. [DOI] [PubMed] [Google Scholar]
- 17Perez-Mato M, Ramos-Cabrer P, Sobrino T, Blanco M, Ruban A, Mirelman D et al. Human recombinant glutamate oxaloacetate transaminase 1 (got1) supplemented with oxaloacetate induces a protective effect after cerebral ischemia. Cell Death Dis 2014; 5: e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Rink C, Gnyawali S, Peterson L, Khanna S. Oxygen-inducible glutamate oxaloacetate transaminase as protective switch transforming neurotoxic glutamate to metabolic fuel during acute ischemic stroke. Antioxid Redox Signal 2011; 14: 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Teichberg VI. Got to rid the body of excess glutamate. J Cereb Blood Flow Metab 2011; 31: 1376–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Nagy D, Marosi M, Kis Z, Farkas T, Rakos G, Vecsei L et al. Oxaloacetate decreases the infarct size and attenuates the reduction in evoked responses after photothrombotic focal ischemia in the rat cortex. Cell Mol Neurobiol 2009; 29: 827–835. [DOI] [PubMed] [Google Scholar]
- 21Boyko M, Gruenbaum SE, Gruenbaum BF, Shapira Y, Zlotnik A. Brain to blood glutamate scavenging as a novel therapeutic modality: a review. J Neural Transm 2014; 121: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Zlotnik A, Sinelnikov I, Gruenbaum BF, Gruenbaum SE, Dubilet M, Dubilet E et al. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology 2012; 116: 73–83. [DOI] [PubMed] [Google Scholar]
- 23Boyko M, Melamed I, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A et al. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics 2012; 9: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Boyko M, Stepensky D, Gruenbaum BF, Gruenbaum SE, Melamed I, Ohayon S et al. Pharmacokinetics of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase and their blood glutamate-lowering activity in naive rats. Neurochem Res 2012; 37: 2198–2205. [DOI] [PubMed] [Google Scholar]
- 25Ruban A, Berkutzki T, Cooper I, Mohar B, Teichberg VI. Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas. Invest New Drugs 2012; 30: 2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke 2010; 41: S95–S98. [DOI] [PubMed] [Google Scholar]
- 27Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med 2010; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Wahl F, Allix M, Plotkine M, Boulu RG. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 1992; 23: 267–272. [DOI] [PubMed] [Google Scholar]
- 29Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol 2012; 11: 101–118. [DOI] [PubMed] [Google Scholar]
- 30Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke 2009; 40: S111–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem 2003; 87: 119–126. [DOI] [PubMed] [Google Scholar]
- 32Khanna S, Briggs Z, Rink C. Inducible glutamate oxaloacetate transaminase as a therapeutic target against ischemic stroke. Antioxid Redox Signal 2014; 22: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Li Y, Hou X, Qi Q, Wang L, Luo L, Yang S et al. Scavenging of blood glutamate for enhancing brain-to-blood glutamate efflux. Mol Med Rep 2014; 9: 305–310. [DOI] [PubMed] [Google Scholar]
- 34Gaberel T, Macrez R, Gauberti M, Montagne A, Hebert M, Petersen KU et al. Immunotherapy blocking the tissue plasminogen activator-dependent activation of n-methyl-d-aspartate glutamate receptors improves hemorrhagic stroke outcome. Neuropharmacology 2013; 67: 267–271. [DOI] [PubMed] [Google Scholar]
- 35Campos F, Sobrino T, Vieites-Prado A, Perez-Mato M, Rodriguez-Yañez M, Blanco M et al. Hyperthermia in human ischemic and hemorrhagic stroke: similar outcome, different mechanisms. PLoS ONE 2013; 8: e78429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Wu G, Li C, Wang L, Mao Y, Hong Z. Minimally invasive procedures for evacuation of intracerebral hemorrhage reduces perihematomal glutamate content, blood-brain barrier permeability and brain edema in rabbits. Neurocrit Care 2011; 14: 118–126. [DOI] [PubMed] [Google Scholar]