Abstract
The ketogenic diet (KD) is an effective alternative treatment for refractory epilepsy in children, but the mechanisms by which it reduces seizures are poorly understood. To investigate how the KD modifies brain metabolism, we infused control (CT) and 7-day KD rats with either [1-13C]glucose (Glc) or [2,4-13C2]β-hydroxybutyrate (β-HB). Specific enrichments of amino acids (AAs) measured by 1H- and 13C-NMR in total brain perchloric acid extracts were similar between CT and KD rats after [1-13C]Glc infusion whereas they were higher in KD rats after [2,4-13C2]β-HB infusion. This suggests better metabolic efficiency of ketone body utilization on the KD. The relative rapid metabolic adaptation to the KD included (1) 11%-higher brain γ-amino butyric acid (GABA)/glutamate (Glu) ratio versus CT, (2) liver accumulation of the ketogenic branched-chain AAs (BCAAs) leucine (Leu) and isoleucine (ILeu), which were never detected in CT, and (3) higher brain Leu and ILeu contents. Since Glu and GABA are excitatory and inhibitory neurotransmitters, respectively, higher brain GABA/Glu ratio could contribute to the mechanism by which the KD reduces seizures in epilepsy. Increased BCAA on the KD may also contribute to better seizure control.
Keywords: astrocyte, 13C-β-hydroxybutyrate, 13C-glucose, epilepsy, ketogenic diet, neuron, NMR spectroscopy
Introduction
Glucose (Glc) normally supplies almost all the energy required by the adult brain. When blood Glc decreases over a period of more than a few hours, ketone bodies (KB; β-hydroxybutyrate (β-HB), acetoacetate (AcetoAc), and acetone) start to replace Glc and become the main brain energy substrate during prolonged fasting or starvation.1 Under conditions of Glc deficit, low plasma insulin increases lipolysis from adipose tissue and fatty acid β-oxidation, raising the amount of acetyl-CoA (Ac-CoA) in liver mitochondria thereby stimulating ketogenesis.2
Ketone bodies are physiologically important for the brain in the neonatal period, during which they are not only an essential energy substrate for brain development, but are also a key substrate for brain lipid and amino-acid synthesis.3 They are transported into the brain by monocarboxylic acid transporters in endothelial cells of the blood–brain barrier.4 Brain KB uptake is regulated by both plasma KB and monocarboxylic acid transporter expression level.5 Fasting increases plasma KB, but another way to increase and sustain plasma KB and monocarboxylic acid transporter expression at the blood–brain barrier is to follow a very high fat, low carbohydrate, and low protein ‘ketogenic' diet (KD). It was observed almost a century ago that fasting was able to reduce, or even treat, epileptic seizures.6 Fasting is still currently used as an initial step preceding the KD and leads to more rapid seizure reduction in children7 and young adults.8
It is of interest to know the impact of the KD on brain fuel utilization. Some studies report that the KD decreases brain Glc uptake9, 10 while others have shown that it increases brain Glc transporter expression11 or 18F-fluorodeoxyglucose uptake using positron emission tomography.5, 12
The primary aim of the present study was to investigate the short-term effects of the KD on brain metabolism, using mainly 13C-NMR spectroscopy, a technique that allows the study of metabolic pathways by following the fate of 13C-labeled substrates. A secondary aim was to evaluate the impact of the KD on liver metabolites, to have an integrative metabolic view. Results on liver branched-chain amino acids (AAs) (BCAAs) were relevant to interpret the effects of the KD in the brain.
Materials and Methods
Animals
Male Sprague-Dawley rats weighting 160 g (4 weeks old, corresponding to puberty) were purchased from Janvier Labs (Le Genest Saint Isle, France) and housed in a room with controlled temperature (21 to 23 °C) and a 12/12 hour normal light/dark cycle (lights on at 08:00 am). The rats were acclimatized for 6 days and then fed a control diet (CT; reference 101091, Safe, Augy, France; n=12 rats) or a high fat KD (KD; reference 180478; n=12 rats) for 7 days. Diet composition was as previously described.5 The ratio of fat/protein+carbohydrate (weight/weight) was 3.5/1.0 for the KD and 1.0/12.5 for the CT. The 3.5/1.0 ratio for the KD was close to the most common ratio used clinically to treat refractory epilepsy in children.7 Seven days on a high fat KD is sufficient to induce mild ketosis in rats (plasma ketones<1 mmol/L).13
The KD group was fasted for 48 hours before starting the KD to deplete hepatic glycogen stores and favor ketogenesis, as recommended during clinical use of the KD.7, 14 Body weight and food intake were monitored every day at 09:00 am. Tail vein whole blood β-HB was measured every other day using test strips and a hand-held device (Precision Xtra, Abbott Laboratories, Alameda, CA, USA). At the end of the 7-day feeding period, the CT and KD groups were each separated into two subgroups for the substrate infusion: [1-13C]Glc or [2,4-13C2]β-HB (n=6/subgroup). The experimental protocol was approved by the Institutional Animal Research Ethics Review Board and met the guidelines of the French governmental agency (In accordance with ARRIVE: personal authorization no. 3310003, project authorization no. 5012029-A delivered from the ethical committee C2EA 50, http://cache.media.enseignementsup-recherche.gouv.fr/file/utilisation_des_animaux_fins_scientifiques/22/1/comiteethiqueea17_juin2013_257221.pdf).
13C-Substrate Infusion
At the end of the 7-day feeding period, the rats were anesthetized with an intraperitoneal injection of chloral hydrate (8%, 0.5 mL/100 g body weight). [1-13C]Glc (D-glucose) or [2,4-13C2]β-HB (sodium D-3-hydroxybutyrate) (Cambridge Isotope Laboratories, Andover, MA, USA) was then infused into the tail vein over a total period of 60 minutes. The [1-13C]Glc infusion followed our previous 20 steps, time-decreasing exponential rate protocol, going from 15 to 1.23 mL/h during the first 25 minutes, after which the rate was kept unchanged for the last 35 minutes.15 Total Glc administered was 2.19 mmol/h per 200 g body weight. [2,4-13C2]β-HB was infused according to a three-step protocol:9 (1) 0 to 5 minutes at 80 μL/min per 200 g body weight, (2) 5 to 10 minutes at 40 μL/min per 200 g body weight, and (3) 10 to 60 minutes at 16 μL/min per 200 g body weight. Total β-HB infused was 2.1 mmol/h per 200 g body weight (stock solution concentration=1.5 mol/L). These infusion protocols aimed to attain constant 13C-substrate concentrations in the blood as demonstrated previously.9, 15 At the end of the 60-minute infusion period and while the rats were still anesthetized, the abdomen was immediately opened sufficiently to collect 2 mL of blood from the inferior vena cava into heparinized syringes. Blood samples were centrifuged (1,500 g; 10 minutes) within 15 minutes and plasma was kept at −80°C until analyzed. Immediately after blood sampling, rats were rapidly euthanized by focused brain microwaves (5 KW, 1 second; Sacron 8000, Sairem, Neyron, France), which completely prevents postmortem metabolic changes in the brain.16 The dome of the skull was cut open with a microcircular saw and the brain rapidly removed and plunged into liquid nitrogen. Brains were kept at −80°C until the metabolites were extracted. The liver was also removed, freeze-clamped in liquid nitrogen and kept at −80°C until the NMR analysis.
Blood gazes and pH were controlled during the 1-hour anesthesia on a separate group of four animals (i-STAT, Abbott Laboratories, using EG7+ cartridges). Partial oxygen pressure (pO2), partial dioxide carbon pressure (pCO2), and pH were similar at the beginning and the end of the infusion, when the brain and the liver were removed (t=0: pO2=5.5±0.01 kPa, pCO2=5.8±0.01 kPa and pH=7.34±0.03; t=1 hour: pO2=5.6±0.01 kPa, pCO2=5.9±0.01 kPa and pH=7.32±0.02). Body temperature was monitored and maintained at 37°C during the infusions. Respiratory rate was also monitored (Small animal monitoring & gating system model 1025, SAII, Stony Brook, NY, USA).
Brain Perchloric Acid Extracts
Water-soluble metabolites of the whole brain were extracted into perchloric acid as previously described15 with the following modifications: after homogenization, the suspension was centrifuged at 3,000 g for 10 minutes. The supernatant was neutralized with KOH, and centrifuged to eliminate perchlorate salts. Samples were then lyophilized. Before NMR spectroscopy, lyophilysates were dissolved in 50 μL of D2O and ethylene glycol (1 mmol/L in D2O; 2 μL) was added as an external reference.
High Resolution Magic Angle Spinning NMR Spectroscopy
High resolution magic angle spinning NMR spectroscopy was performed on an 11.7-Tesla spectrometer (DPX 500 MHz; Bruker Biospin, Wissembourg, France). For brain, 50 μL of each perchloric acid extract containing 2 μL of ethylene glycol (1 mmol/L in D2O) was analyzed. For liver, around 20 mg of unextracted whole tissue was weighed and directly placed in the high resolution magic angle spinning rotor in the presence of 20 μL of fumarate (disodium salt, 50 mmol/L in D2O), which was added as an external reference.17
1H-NMR spectra were acquired with a 90° pulse angle (adjusted for each sample), relaxation time of 8 seconds, spectrum width of 10 parts per million (p.p.m.), acquisition time of 3.28 seconds, 128 scans, and 32 K memory size. The water signal was suppressed by homonuclear presaturation. The specific enrichment (% 13C) of glucose carbon 1 (Glc C1 SEnr) was determined from the ratio of the integral of the Glc satellite peaks resulting from heteronuclear spin coupling to the sum of the integrals of satellite and central peaks. Brain content of γ-amino butyric acid (GABA) relative to Glu and of glutamine (Gln) relative to N-acetylaspartate was measured. Brain and liver content of the two BCAA isoleucine (ILeu) and leucine (Leu) was also determined because they can be metabolized into ketogenic acids. In this study relative to the mechanistic effects of a KD, it was appropriate to evaluate their content, not only in the brain (relative to ethylene glycol) but also in the liver (relative to fumarate).
1H-decoupled 13C-NMR spectra were acquired with a 90° pulse angle, relaxation time of 20 seconds, 200 p.p.m. spectrum width, 1.31 seconds acquisition time, ~5,500 scans, and 64 K memory size. Relative enrichment of Glu C2/C3, Gln C2/C3, and Glu C4/Gln C4 was measured from these spectra.
1H-observed/13C-edited NMR spectra were acquired as previously described.15 The method included a first scan consisting of a standard spin-echo acquisition in which 1H coupled to all carbons (12C and 13C) was visible, and a second scan corresponding to a 13C inversion pulse, in which only 1H coupled to 13C was visible. Brain metabolite 13C enrichments were determined from the ratio of the integral of a resonance on the edited 13C-1H spectrum to its integral in the standard spin-echo spectrum.15
Peaks were integrated using GSim software (open source: http://sourceforge.net/projects/gsim/files/latest/download). For liver NMR analysis, a Carr-Purcell-Meiboom-Gill sequence was applied to eliminate the macromolecule contribution to the baseline. The sequence parameters were as previously described.17
Plasma Metabolites
Plasma samples were analyzed using a clinical chemistry analyzer (Olympus AU2700, BCO, Villepinte, France) with kits for Glc (BC OSR6221, Beckman Coulter, Brea, CA, USA), β-HB (Ranbut, Randox Laboratories, Crumlin, UK), lactate (Lac) (BC OSR6193), free fatty acids (FFA; FA115, Randox Laboratories), and triglycerides (BC OSR6118). Insulin levels were assessed by ELISA (Diasorin, Antony, France). These assays were performed in the medical laboratories of Bordeaux Hospital.
Statistical Analysis
Statistical analyses were performed using Prism 6 (GraphPad, La Jolla, CA, USA). Comparisons between the CT and KD groups and between the [1-13C]Glc and [2,4-13C2]β-HB groups were performed using the Mann-Whitney test. Correlations were established by Spearman's test. Two-tailed P<0.05 was considered as statistically significant.
Results
Weight Gain, Food Intake, and Plasma Measurements
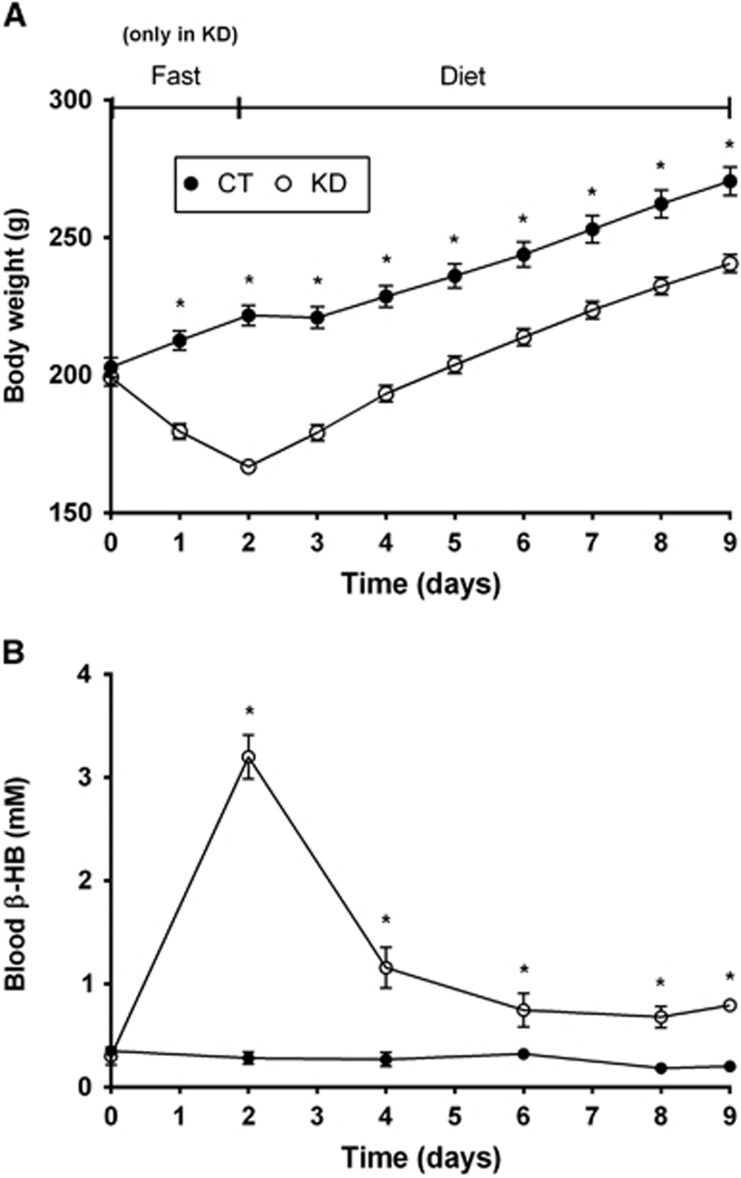
As expected, the initial fasting step used to deplete hepatic glycogen stores and favor ketogenesis led to a transitory decrease in body weight in the KD group (Figure 1A). However, immediately after the reintroduction of food in KD group, the slopes of the growth curves were similar between CT and KD rats (7.2 and 6.9 g/day; respectively). In the KD group, blood β-HB reached a mean value of 3.2 mmol/L after 48 h fasting and decreased thereafter to ~1 mmol/L during the KD (Figure 1B). In CT rats, blood β-HB was consistently around 0.3 mmol/L. Just before labeled substrate infusion, KD rats had fourfold higher blood β-HB than CT rats (0.8 and 0.2 mmol/L; respectively).
Figure 1.
Body weight (A) and blood β-hydroxybutyrate (β-HB; B) of rats fed the control diet (CT, •) or the ketogenic diet (KD, ○) for 7 days. The 48-hour fasting was conducted only in the KD group. Values are mean±s.e.m. (n=12/point); *P<0.05.
At the end of the 1-hour [1-13C]Glc infusion, the KD rats had 84% higher plasma Glc compared with the CT rats (Table 1). After the [2,4-13C2]β-HB infusion, there was no statistical difference in plasma β-HB between CT and KD rats. The KD rats had lower plasma insulin compared with CT rats (Table 1); this difference was partially masked by the [1-13C]Glc infusion, inducing insulin secretion. Plasma Lac and FFA were not statistically different between the four groups (Table 1).
Table 1. Plasma metabolite concentrations (mean±s.d.) in control (CT) or ketogenic diet (KD) rats at the end of [1-13C]glucose ([1-13C]Glc) or [2,4-13C2]β-hydroxybutyrate ([2,4-13C2]β-HB) infusion.
|
[1-13C]Glc |
[2,4-13C2]β-HB |
|||
|---|---|---|---|---|
| CT (n=6) | KD (n=5) | CT (n=6) | KD (n=6) | |
| Glucose (mmol/L) | 13.9±3.2 | 25.6±5.6a | 10.0±3.3b | 11.4±1.1c |
| β-HB (μmol/L) | 94±85 | 103±50 | 5,000±1543b | 3,066±2,086c |
| Insulin (μU/mL) | 119±70 | 77±50 | 81±29 | 38±17a,c |
| Lactate (mmol/L) | 3.9±1.6 | 3.9±2.1 | 4.8±0.6 | 4.5±1.1 |
| Free fatty acid (mmol/L) | 0.2±0.2 | 0.2±0.1 | 0.2±0.1 | 0.2±0.2 |
| Triglyceride (mmol/L) | 0.8±0.5 | 0.5±0.2 | 0.7±0.2 | 0.4±0.1a |
Significantly different between CT and KD rats in the same infusion group (P⩽0.05).
Significantly different between CT rats infused with [1-13C]Glc or [2,4-13C2]β-HB (P⩽0.05).
Significantly different between KD rats infused with [1-13C]Glc or [2,4-13C2]β-HB (P⩽0.05).
13C-Specific Enrichment in Brain Metabolites
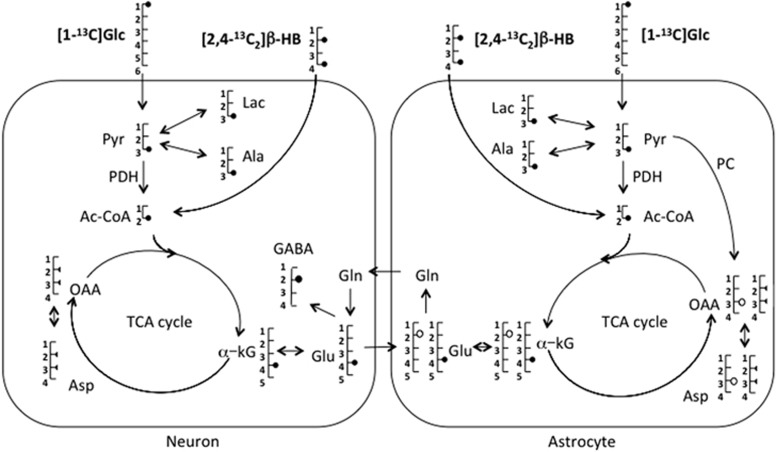
The incorporation and the fate of 13C during the first turn of the tricarboxylic acid (TCA) cycle after infusion of [1-13C]Glc or [2,4-13C2]β-HB are illustrated in Figure 2. The 13C SEnr, i.e., the % of 13C from the infused substrates incorporated into a carbon position in a given metabolite, was measured for Glc C1, GABA C2, C3 of lactate, alanine, and aspartate (Lac C3, Ala C3, and Asp C3, respectively), and β-HB C4, Glu C4, and Gln C4 (Table 2).
Figure 2.
Schematic representation of metabolite labeling from [1-13C]glucose (Glc) or [2,4-13C2]β-hydroxybutyrate (β-HB) in neurons and astrocytes. In astrocytes, 13C-labeled pyruvate C3 (Pyr) follows either the pyruvate dehydrogenase (PDH; •) or the pyruvate carboxylase (PC; ○) pathway, but only the PDH pathway in neurons. This scheme is limited to the first turn of the tricarboxylic acid (TCA) cycle. In neurons, half of the molecules of oxaloacetate (OAA) and aspartate (Asp) are labeled on C2 and the other half on C3 (half symbol). α-KG, α-ketoglutarate; Ala, alanine; Ac-CoA, acetyl-CoA; Glu, glutamate; Gln, glutamine; GABA, γ-aminobutyric acid; Lac, lactate.
Table 2. Brain metabolite 13C-specific enrichments in rats on the control diet (CT) or the ketogenic diet (KD) 1 hour after infusion of [1-13C]glucose ([1-13C]Glc) or [2,4-13C2]β-hydroxybutyrate ([2,4-13C2]β-HB).
|
[1-13C]Glca |
[2,4-13C2]β-HB |
|||
|---|---|---|---|---|
| CT | KD | CT | KD | |
| Glc C1 | 47.3±7.2 | 54.7±6.2b | ND | ND |
| β-HB C4 | ND | ND | 86.1±2.9 | 84.4±1.8 |
| Lac C3 | 15.6±3.3 | 20.6±2.3b | 3.2±0.4c | 4.2±0.5b,d |
| Ala C3 | 16.2±3.1 | 19.2±1.8 | 3.5±0.3c | 4.4±0.5b,d |
| Glu C4 | 13.9±2.3 | 14.8±1.1 | 19.6±4.8c | 29.8±7.3b |
| Gln C4 | 10.7±1.6 | 11.8±1.7 | 18.5±4.3 | 28.5±7.8b |
| GABA C2 | 12.7±1.9 | 13.0±1.4 | 15.9±3.0c | 25.0±8.1b |
| Asp C3 | 11.5±2.1 | 12.2±0.9 | 13.4±2.9c | 17.4±3.2b,d |
Ala, alanine; Asp, aspartate; GABA, γ-amino butyric acid; Glc, glucose; Glu, glutatmate; Lac, lactate.
Units are percent enrichment (mean±s.d.; n=6/group).
To statistically compare metabolite labeling after [1-13C]Glc and [2,4-13C2]β-HB infusion, values after [1-13C]Glc were doubled from the actual values shown so as to take into account the isotope dilution (one 13C-labeled carbon on [1-13C]Glc versus two on [2,4-13C2]β-HB).
Significantly different between CT and KD rats in the same infusion group (P⩽0.05).
Significantly different between CT rats infused with [1-13C]Glc or [2,4-13C2]β-HB (P⩽0.05).
Significantly different between KD rats infused with [1-13C]Glc or [2,4-13C2]β-HB (P⩽0.05).
After [1-13C]Glc infusion, Glc C1 SEnr was significantly higher in KD compared with CT rats and was positively correlated with glycaemia, in both groups (r=0.87). Lac C3 SEnr was also higher in KD compared with CT rats, whereas the SEnr of brain Ala, Glu, Gln, or Asp did not differ statistically between the two groups (Table 2).
After [2,4-13C2]β-HB infusion, SEnr of all measured metabolites (except for the substrate, β-HB C4) was higher in KD compared with CT rats (Table 2): Lac C3 (+31%), Ala C3 (+26%), Glu C4 (+52%), Gln C4 (+54%), GABA C2 (+57%), and Asp C3 (+30%). Incorporation of 13C into C4 of AcetoAc (30.4 p.p.m.) did not differ between CT and KD groups.
In CT rats and compared with [2,4-13C2]β-HB infusion, [1-13C]Glc infusion led to significantly higher 13C SEnr in all brain metabolites except for Gln C4 (Table 2, keeping in mind the isotopic dilution between [1-13C]Glc, containing one 13C and [2,4-13C2]β-HB, containing two 13C). In KD rats infused with [1-13C]Glc, only Asp C3 SEnr was significantly higher (+40%) compared with KD rats infused with [2,4-13C2]β-HB. In KD rats, Lac C3 and Ala C3 SEnr were sevenfold to eightfold higher after [1-13C]Glc infusion versus [2,4-13C2]β-HB infusion, reflecting the different metabolic entry points into the TCA cycle between [1-13C]Glc, which leads 13C labeling at C3 of pyruvate, versus [2,4-13C2]β-HB, which bypasses pyruvate and labels C2 of Ac-CoA.
Brain γ-Amino Butyric Acid, Glutamate, and Glutamine Content
The GABA/Glu ratio was 11% higher in KD rats compared with CT rats (0.21±0.03 versus 0.19±0.02, respectively, P<0.05), without any influence of the infused substrate. On the contrary, Gln/N-acetylaspartate ratio was dependent on the infused substrate. After [1-13C]Glc infusion, this ratio was 0.81±0.08 and 0.80±0.08 in CT and KD rats, respectively, whereas after [2,4-13C2]β-HB infusion, it was twofold higher in KD than in CT rats (1.39±0.17 versus 0.70±0.07, respectively).
Brain Glutamate and Glutamine C2, C3, and C4 Relative Enrichments
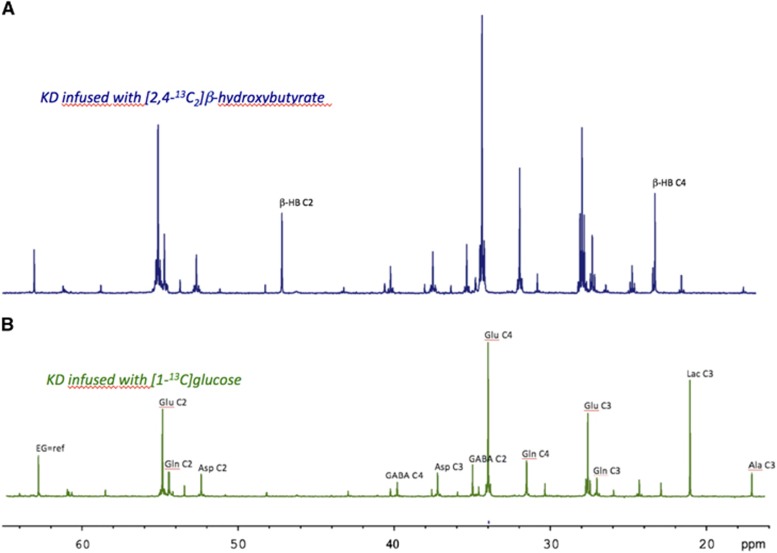
13C incorporation from the two 13C-labeled substrates into different carbons of Glu and Gln was measured from 13C spectra (Figure 3). For both substrates, there was no difference in Glu or Gln labeling linked to the CT diet or KD, so the two dietary groups were combined for statistical analysis (Table 3). After [1-13C]Glc infusion, the Gln C2/C3 ratio was 32% higher than Glu C2/C3 ratio (1.21 versus 0.92, respectively). A Gln C2/C3 ratio of >1 reflects pyruvate carboxylase (PC; EC 6.4.1.1) activity, which is present only in astrocytes and which leads to higher 13C incorporation in C2 compared with C3 of Gln.18 Higher incorporation of 13C at C2 compared with C3 was not observed for Glu (mainly present in neurons, which do not possess PC). After [2,4-13C2]β-HB infusion, both Gln C2/C3 and Glu C2/C3 were close to 1 and did not differ significantly, reflecting once again the different metabolic fates of [1-13C]Glc and [2,4-13C2]β-HB. Glu C4/Gln C4 was 29% higher in [1-13C]Glc-infused rats compared with [2,4-13C2]β-HB-infused rats.
Figure 3.
Typical 13C-NMR spectra of whole brain perchloric acid extracts from rats on the ketogenic diet (KD) infused with either [1-13C]Glc (spectrum B) or [2,4-13C2]β-HB (spectrum A). These spectra were used to determine brain glutamate (Glu) and glutamine (Gln) C2, C3, and C4 13C enrichments. Spectra were normalized to the protein contents using the ethylene glycol peak (EG; external reference).
Table 3. Brain glutamate (Glu) and glutamine (Gln) 13C relative enrichments (mean±s.d.) 1 hour after infusion of [1-13C]glucose ([1-13C]Glc) or [2,4-13C2]β-hydroxybutyrate ([2,4-13C2]β-HB).
| [1-13C]Glc | [2,4-13C2]β-HB | |
|---|---|---|
| Glu C2/C3 | 0.92±0.16 | 0.91±0.14 |
| Gln C2/C3 | 1.21±0.19a | 1.03±0.15 |
| Glu C4/Gln C4 | 3.47±0.43 | 2.70±0.39b |
Rats on the control diet and the ketogenic diet were combined since the values were not significantly different (n=12/group).
Significant difference between Glu C2/C3 and Gln C2/C3 (P⩽0.05).
Significant difference between rats infused with [1-13C]Glc or [2,4-13C2]β-HB (P⩽0.05).
Branched-Chain Amino Acids
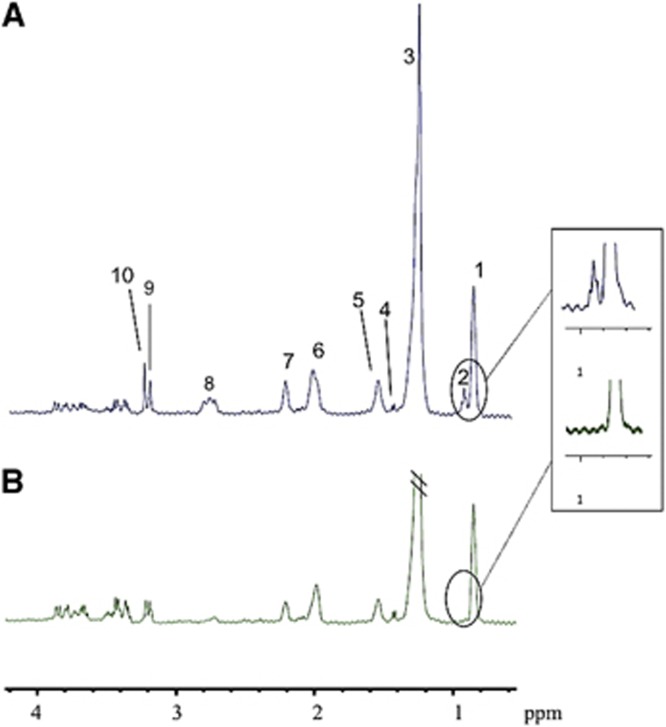
In 1H-NMR spectra of brain extracts, Leu and ILeu were 44% higher in KD rats compared with CT rats (ratio relative to external reference: 0.108±0.015 versus 0.075±0.009, respectively), a difference that was independent of the nature of the substrate infused. Liver 1H-NMR spectra systematically revealed a peak representing free Leu and ILeu in KD rats (0.92 p.p.m.) that was never present in CT rats (Figure 4). As in the brain, the presence of these ketogenic BCAA in liver was independent of the substrate infused, pointing once again to a specific effect of the KD.
Figure 4.
Typical 1H-NMR spectra of a liver sample from a ketogenic diet (KD) rat (A) and a control (CT) rat (B). The peak assignments are as follows: (1) CH3-(CH2)n- (0.87 parts per million (ppm)); (2) leucine+isoleucine (0.92 ppm); (3) CH3-(CH2)n- (1.26 ppm); (4) alanine+lysine (1.42 ppm); (5) -CH2-CH2-C=O (1.55 ppm); (6) CH=CH-CH2-CH2- (1.99 ppm); (7) -CH2-CH2-C=O (2.2 ppm); (8) CH=CH-CH2-CH=CH (2.80 ppm); (9) choline+phosphocholine (3.19 ppm); (10) betaine (3.24 ppm). Fumarate (disodium salt) was used as an external reference to calibrate the spectra (singlet at 6.5 ppm, outside the window).
Discussion
We determined that a 7-day KD was sufficient to observe modification of both brain and liver metabolism. In rats on the KD, the main results were (1) a better efficiency of brain KB utilization, (2) a 11% increase in GABA/Glu ratio, and (3) liver accumulation of Leu and ILeu. Our results suggest that in rats on the KD, the liver is contributing not only FFA but also BCAA toward ketogenesis and altered brain intermediary metabolism.
Brain Glucose Metabolism
After [1-13C]Glc infusion, the 16% higher 13C SEnr at brain Glc C1 in the KD rats (Table 2) confirms a previous report19 and could potentially be explained by higher brain Glc uptake in the KD group. This would agree with our previous positron emission tomography studies in which brain 18F-fluorodeoxyglucose uptake was significantly higher in KD rats.5, 12 Higher brain Glc uptake on the KD could potentially be due to overexpression of Glc transporter 1 (GLUT1).11, 20 In the present study, the rats were on the KD for 7 days, which is sufficient to upregulate GLUT1;21 indeed, this upregulation is rapid and can be observed as soon as 18 hours after intense exercise.22 If GLUT1 expression increases on the KD, then one might question whether increased glycemia achieved during our [1-13C]Glc infusion (1 hour) might be sufficient to modify brain GLUT1 expression. It has been shown that, even though hyperglycemia (2 weeks, glycemia >20 mmol/L), GLUT1 expression was not affected.4 On the contrary, cerebral GLUT1 expression increases during hypoglycemia and hypoinsulinemia,21 which could lead to increase brain Glc uptake in KD rats during [1-13C]Glc infusion.
After [1-13C]Glc infusion, SEnr at Ala C3 and Lac C3 was higher in the KD compared with the CT group (Table 2). This difference may be partially explained by the SEnr of the precursor, Glc C1, which was higher both in the plasma (data not shown) and in the brain of KD rats. Indeed, in the present study, brain Glc C1 SEnr was proportional to plasma Glc after [1-13C]Glc infusion. Higher plasma Glc in response to [1-13C]Glc infusion in KD rats was previously reported23 and may be related to lower plasma insulin in these rats. However, there was a 15% increase in brain Glc C1 SEnr in KD compared with CT rats, whereas Lac C3 SEnr showed a 32% increase. Therefore, the higher SEnr at Lac C3 could potentially also be due to an increase in glycolysis. From these SEnrs, it can be estimated that 65.8% of labeled brain Lac was derived from [1-13C]Glc in the CT group (Glc C1 enriched at 47.3% can give rise to a maximum of 23.7% (47.3/2) of enriched Lac C3). However, in the KD group, 86.9% of labeled Lac in the brain was derived from [1-13C]Glc, suggesting that brain glycolysis was enhanced on the KD. In the present study, rats were euthanized with focused microwaves, which is the only technique that prevents postmortem Lac production by the brain.16 Therefore, Lac reported here (labeled or not) was derived strictly from premortem brain aerobic glycolysis, a phenomenon occurring predominantly in astrocytes.16, 24 Higher aerobic glycolytic flux in the KD group agrees with previous reports showing that glycolytic enzymes are upregulated in the hippocampus of rats on a KD for 3 weeks.25 Our results indicate that this increase is a relatively early phenomenon after starting the KD. Glc conversion to Lac was also shown to increase during starvation in rats,26 a ketogenic situation metabolically analogous to the KD.
Although brain uptake of 18F-fluorodeoxyglucose was previously shown to be higher in KD rats,5, 12 in our study, CT and KD rats had similar brain 13C enrichments of Glu C4, Gln C4, GABA C2, and Asp C3 after [1-13C]Glc infusion. This implies that the KD may modify brain Glc uptake but appears to have little or no effect on the capacity of the brain to use Glc for amino-acid synthesis. This distinction between brain Glc uptake and metabolism is compatible with the fact that the entry of Glc into the brain is not the limiting step in its utilization.27, 28 Our results differ somewhat from a study reporting lower 13C enrichment in brain Glu in ketotic mice infused with 13C-Glc.19 However, in this latter study, the experimental conditions were quite different from the present 13C-NMR work, since Yudkoff et al. analyzed samples obtained 30 minutes after intraperitoneal administration of 13C-Glc in mice under a KD for 4 days without previous fasting. It must be noted that 13C-NMR spectroscopy is a low-sensitive technique, which needs high amount of 13C-labeled substrate to follow carbon metabolism. We chose [1-13C]Glc and [2,4-13C2]β-HB concentrations that are commonly used in 13C-NMR studies.
In the present study, the relative enrichment of Gln C2/C3 after [1-13C]Glc infusion was ~1.2, reflecting PC activity, which is present only in astrocytes.29 Astrocyte PC activity is responsible for the higher 13C labeling of Gln C2 than C3 when [1-13C]Glc is the substrate. Indeed in astrocytes after [1-13C]Glc infusion, 13C labeling of oxaloacetate C3 generated from 13C-labeled pyruvate through the anaplerotic pathway leads to the synthesis of α-ketoglutarate (α-KG) 13C-labeled on C2 (Figure 2). Therefore, the labeled Gln synthesized from α-KG via Glu has higher 13C enrichment at C2 than at C3. On the contrary, the relative enrichment of Glu C2/C3 after [1-13C]Glc infusion was <1, reflecting the absence of PC activity in neurons, which have the biggest pool of Glu in the brain. No difference in Gln C2/C3 relative enrichment was found between CT and KD rats, indicating no detectable effect of the KD on PC activity. Higher apparent PC activity in rats on a KD was however observed by Melo et al.,23 but in that study, the rats were on the KD for 21 days compared with 7 days in the present work. These disparities suggest that care must be taken when comparing different studies since the level and duration of ketosis and the dietary status (fed or fasted) may influence the adaptive metabolic changes.
Brain β-Hydroxybutyrate Metabolism
Compared with CT rats, KD rats infused with [2,4-13C2]β-HB had higher SEnr in all brain AAs measured (Table 2). At 29.8%, the SEnr in Glu C4 was the highest and very similar to the 27% reported elsewhere for a similar study design.9 Higher brain AA SEnr in KD compared with CT rats indicates that the 7-day KD probably led to metabolic adaptation such that KB are used more efficiently as both a brain fuel and an anabolic substrate. Three to four weeks on a KD upregulate expression of genes in the hippocampus linked to mitochondrial metabolic pathways,30 including mitochondrial biogenesis,25 long-chain acyl CoA synthase 2, and several subunits of mitochondrial ATP synthase, which is essential for β-oxidation (R-COOH+ATP+CoA-SH → R-CO-S-CoA+AMP +PPi).
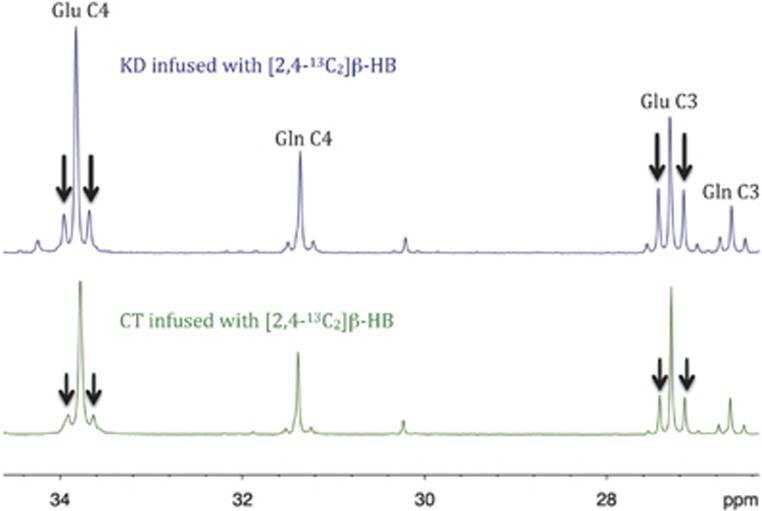
Since Glu is in rapid equilibrium with α-KG, the incorporation of 13C into different carbon positions of Glu (and Gln thereafter) essentially reflects 13C enrichment in α-KG, and thus gives information on TCA cycle activity and oxidative metabolism.31 In the present study, Glu homonuclear couplings were increased after [2,4-13C2]β-HB infusion in the KD group compared with the CT group (Figure 5). This homonuclear coupling (multiplets instead of a singlet) appears when 13C enrichment is on several neighboring carbons and indicates that the 13C-enriched Glu was produced after several turns of the TCA cycle, leading to the 13C enrichment of C 4, 2, and 3. Greater homonuclear coupling (higher satellite peaks) means that more Glu is turning into the TCA cycle, so our results show that the KD increases TCA cycle activity compared with the CT. No such difference was observed after [1-13C]Glc infusion (data not shown), indicating once again that the KD increased the efficiency of [2,4-13C2]β-HB utilization for the brain's oxidative metabolic needs.
Figure 5.
Typical 13C-NMR spectra of whole brain perchloric acid extracts from [2,4-13C2]β-HB-infused rats fed the control diet (CT; lower spectrum) or the ketogenic diet (KD; upper spectrum). Higher glutamate (Glu) and glutamine (Gln) homonuclear coupling (multiplet peaks; ↓) was observed for KD rats, which is an indication that tricarboxylic acid cycle activity was higher in the KD group.
Comparison of Brain Glucose and β-Hydroxybutyrate Metabolism
Higher 13C enrichments in brain metabolites (Table 2) in CT rats infused with [1-13C]Glc compared with [2,4-13C2]β-HB confirm that in normal, non-ketonemic conditions, the brain preferentially uses Glc as an oxidative substrate rather than KB.
Asp C3 SEnr was 40% higher in KD rats infused with [1-13C]Glc than in those infused with [2,4-13C2]β-HB (12.2 × 2=24.4% after [1-13C]Glc compared with 17.4% after [2,4-13C2]β-HB), supporting the proposal by Yudkoff et al.19 that KB reduce Asp production and favor Glu and GABA synthesis. The Glu C4/Gln C4 relative enrichment in our CT rats infused with [1-13C]Glc was 3.63, a result similar to the 3.9 reported elsewhere for CT rats infused with [1-13C]Glc.23 This ratio was lower when [2,4-13C2]β-HB was infused (2.70), an effect also reported elsewhere.32 This lower ratio of Glu C4/Gln C4 relative enrichment suggests either that β-HB may be a more predominant astrocytic substrate (since Gln is mainly localized in astrocytes32, 33) or, as recently shown,34 that Glu can also be synthetized from another unlabeled carbon source (in addition to [2,4-13C2]β-HB), such as glucose or astrocytic Lac, which leads to an isotopic dilution of Glu C4.
Brain γ-Amino Butyric Acid and Relationship with Liver Metabolism
In [2,4-13C2]β-HB-infused rats, the ratio of brain GABA/Glu was 16% higher in the KD group than in the CT group, a result supporting the hypothesis that the anticonvulsant effect of the KD may be due at least in part to higher brain GABA, an inhibitory neurotransmitter,33, 35, 36 compared with Glu, which is an excitatory neurotransmitter. A previous in vitro study showing that the addition of AcetoAc or β-HB protected neurons in culture against Glu cytotoxicity37 supports our present results.
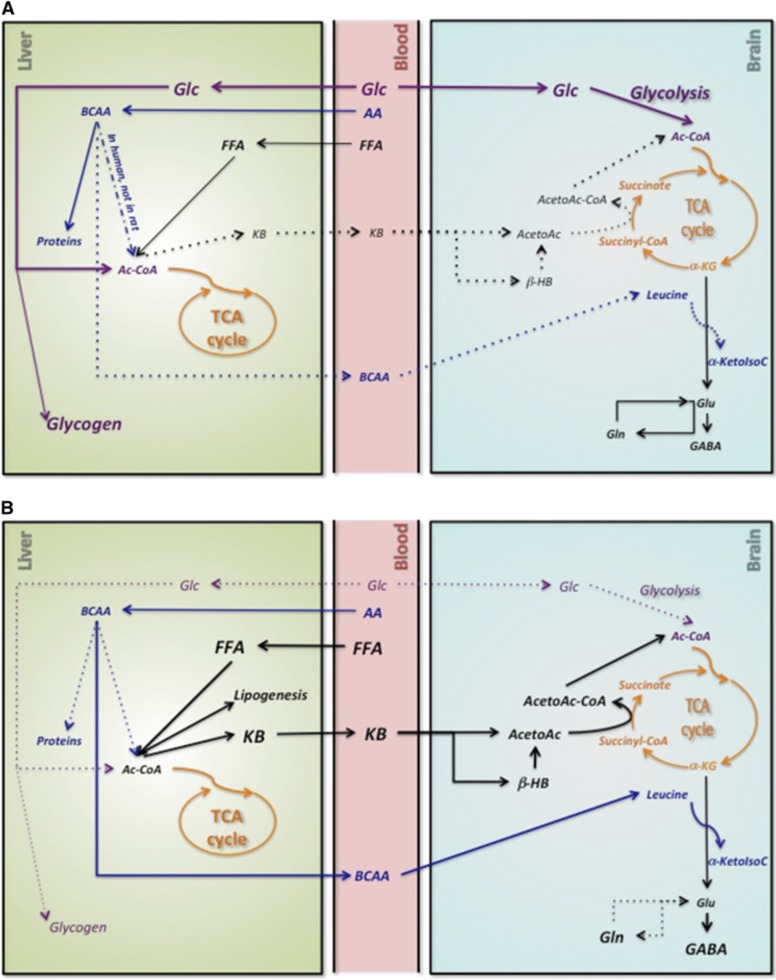
We show here for the first time that, within 7 days, the KD had increased ketogenic BCAAs in the liver (Figure 4) and brain, especially Leu and ILeu. The nature of the infused substrates had no significant impact on this observation. Branched-chain amino acids are transaminated by BCAA aminotransferase to produce branched-chain alpha-keto acids, which are catalyzed by alpha-keto acids dehydrogenase to form Ac-CoA and succinyl-CoA. The increase in liver BCAA exclusively on the KD could in part be the result of metabolic competition between BCAA and FFA, which are also important ketogenic precursors. Based on our present data and on results from the Yudkoff's group, we propose that the KD induces certain metabolic modifications (Figure 6). The KD increases the influx of FFA into the liver, which may to some extent impede the catabolism of BCAA (Figure 6), hence increasing the liver BCAA content. However, unlike in humans, BCAA aminotransferase activity is low to undetectable in rat liver.38 Therefore, this metabolic competition probably occurs in other organs, where BCAA aminotransferase activity is present, leading to a general decrease in BCAA catabolism and higher BCAA in organs and blood. Higher plasma BCAA (nearing 800 nmol/L) has been reported in children with epilepsy on the KD.39 Higher liver BCAA may also be due to lower insulinemia in KD rats and therefore lower protein synthesis (Figure 6B). Since BCAA cross the blood–brain barrier (a phenomenon accompanied by counter-transport of Gln leading to an efflux of Gln from the brain40), they may contribute to the improved control of seizures while on the KD.39 Branched-chain amino acid may be carriers of ammonia nitrogen between neurons and astrocytes and precursors for GABA synthesis in GABAergic neurons.41 Indeed, the administration of 15N-Leu to KD mice leads to 15N-labeled GABA.35 A pilot study showed the effectiveness of BCAA as an adjunct therapy to the KD in children with refractory epilepsy,42 thereby strengthening our hypothesis that higher BCAA on the KD may raise brain GABA. In the present study, the increase in brain Gln content relative to N-acetylaspartate in KD rats supports this interpretation and may in part arise due to the competition between Leu and Gln as precursors for Glu (Figure 6B).
Figure 6.
Proposed adaptation of liver and brain metabolisms to a ketogenic diet (KD). (A) On a control diet, glucose (Glc) is used as the main energy substrate for the brain and γ-aminobutyric acid (GABA) is synthetized from glutamate (Glu). In the liver, acetyl-CoA (Ac-CoA) is synthetized from both Glc and free fatty acids (FFAs). (B) On a KD diet, increased plasma FFAs are converted into Ac-CoA in the liver. Ac-CoA is thus mainly produced from FFA, decreasing the use of all other substrates to provide Ac-CoA. α-KG, α-ketoglutarate; α-KetoIsoC, α-ketoisocaproic acid; β-HB, β-hydroxybutyrate; AA, amino acids; AcetoAc, acetoacetate; AcetoAc-CoA, acetoacetyl-CoA; BCAA, branched chain amino acids; Gln, glutamine; KB, ketone bodies.
Conclusions
We show that a 7-day KD leads to relatively rapid metabolic remodeling in the brain during which KB are metabolized more efficiently. Branched-chain amino acids may be directly involved in increasing the ratio of GABA/Glu in brain, so ketones are not necessarily the only mediators of the neuroprotective effect of the KD.
Acknowledgments
The authors thank Mélanie Fortier, Gérard Raffard, Eric Bezançon, Véronique Bouchaud, Samir Mesli and Stéphane Sanchez for generous support and technical assistance.
The authors declare no conflict of interest. This study was supported by a public grant from the French “Agence Nationale de la Recherche” within the context of the Investments for the Future Program, referenced ANR-10-LABX-57 and named TRAIL.
Footnotes
This study was financially supported by the Fonds de recherche du Québec-Santé, Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, Canada Research Chairs Secretariat and Université de Sherbrooke Research Chair (SCC), CFQCU program of the FQRNT, INAF and the FRQS Research Center on Aging, Université de Sherbrooke. Anne-Karine Bouzier-Sore was supported by a public grant from the French ‘Agence Nationale de la Recherche' within the context of the Investments for the Future Program, referenced ANR-10-LABX-57 and named TRAIL.
References
- 1Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF, Jr.. Brain metabolism during fasting. J Clin Invest 1967; 46: 1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 243–251. [DOI] [PubMed] [Google Scholar]
- 3Webber RJ, Edmond J. Utilization of L(+)-3-hydroxybutyrate, D(-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J Biol Chem 1977; 252: 5222–5226. [PubMed] [Google Scholar]
- 4Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F et al. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 1999; 72: 238–247. [DOI] [PubMed] [Google Scholar]
- 5Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R et al. Mild experimental ketosis increases brain uptake of 11C-acetoacetate and 18F-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr Neurosci 2011; 14: 51–58. [DOI] [PubMed] [Google Scholar]
- 6Wilder RM. The effect of ketonemia on the course of epilepsy. Mayo Clin Bull 1921, 307–308.
- 7Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics 2009; 6: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Coppola G, Veggiotti P, Cusmai R, Bertoli S, Cardinali S, Dionisi-Vici C et al. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Res 2002; 48: 221–227. [DOI] [PubMed] [Google Scholar]
- 9Jiang L, Mason GF, Rothman DL, de Graaf RA, Behar KL. Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J Cereb Blood Flow Metab 2011; 31: 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Zhang Y, Kuang Y, Xu K, Harris D, Lee Z, LaManna J et al. Ketosis proportionately spares glucose utilization in brain. J Cereb Blood Flow Metab 2013; 33: 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab 2007; 292: E1607–E1615. [DOI] [PubMed] [Google Scholar]
- 12Roy M, Nugent S, Tremblay-Mercier J, Tremblay S, Courchesne-Loyer A, Beaudoin JF et al. The ketogenic diet increases brain glucose and ketone uptake in aged rats: a dual tracer PET and volumetric MRI study. Brain Res 2012; 1488: 14–23. [DOI] [PubMed] [Google Scholar]
- 13Taha AY, Ryan MA, Cunnane SC. Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism 2005; 54: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 14Zhou H, Zhang T, Bogdani M, Oseid E, Parazzoli S, Vantyghem MC et al. Intrahepatic glucose flux as a mechanism for defective intrahepatic islet alpha-cell response to hypoglycemia. Diabetes 2008; 57: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 15Bouzier AK, Thiaudiere E, Biran M, Rouland R, Canioni P, Merle M. The metabolism of [3-(13)C]lactate in the rat brain is specific of a pyruvate carboxylase-deprived compartment. J Neurochem 2000; 75: 480–486. [DOI] [PubMed] [Google Scholar]
- 16Sampol D, Ostrofet E, Jobin ML, Raffard G, Sanchez S, Bouchaud V et al. Glucose and lactate metabolism in the awake and stimulated rat: a (13)C-NMR study. Front Neuroenergetics 2013; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Roy M, Hennebelle M, St-Pierre V, Courchesne-Loyer A, Fortier M, Bouzier-Sore AK et al. Long-term calorie restriction has minimal impact on brain metabolite and fatty acid profiles in aged rats on a Western-style diet. Neurochem Int 2013; 63: 450–457. [DOI] [PubMed] [Google Scholar]
- 18Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 1985; 329: 364–367. [DOI] [PubMed] [Google Scholar]
- 19Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B et al. Response of brain amino acid metabolism to ketosis. Neurochem Int 2005; 47: 119–128. [DOI] [PubMed] [Google Scholar]
- 20Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int 2001; 38: 519–527. [DOI] [PubMed] [Google Scholar]
- 21Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 1995; 44: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 22Takimoto M, Hamada T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J Appl Physiol 2014; 116: 1238–1250. [DOI] [PubMed] [Google Scholar]
- 23Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int 2006; 48: 498–507. [DOI] [PubMed] [Google Scholar]
- 24Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab 2012; 32: 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 2006; 60: 223–235. [DOI] [PubMed] [Google Scholar]
- 26Hawkins RA. Uptake of ketone bodies by rat brain in vivo. Biochem J 1971; 121: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 2011; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev 1983; 63: 1481–1535. [DOI] [PubMed] [Google Scholar]
- 29Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science 1977; 195: 1356–1358. [DOI] [PubMed] [Google Scholar]
- 30Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM et al. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res 2004; 129: 80–87. [DOI] [PubMed] [Google Scholar]
- 31Mason GF, Rothman DL, Behar KL, Shulman RG. NMR determination of the TCA cycle rate and alpha- ketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab 1992; 12: 434–447. [DOI] [PubMed] [Google Scholar]
- 32Kunnecke B, Cerdan S, Seelig J. Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed 1993; 6: 264–277. [DOI] [PubMed] [Google Scholar]
- 33Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia 2008; 49: 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Chowdhury GM, Jiang L, Rothman DL, Behar KL. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J Cereb Blood Flow Metab 2014; 34: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, amino acid metabolism, and seizure control. J Neurosci Res 2001; 66: 931–940. [DOI] [PubMed] [Google Scholar]
- 36Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 277–285. [DOI] [PubMed] [Google Scholar]
- 37Noh HS, Hah YS, Nilufar R, Han J, Bong JH, Kang SS et al. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res 2006; 83: 702–709. [DOI] [PubMed] [Google Scholar]
- 38Torres N, Vargas C, Hernandez-Pando R, Orozco H, Hutson SM, Tovar AR. Ontogeny and subcellular localization of rat liver mitochondrial branched chain amino-acid aminotransferase. Eur J Biochem 2001; 268: 6132–6139. [DOI] [PubMed] [Google Scholar]
- 39Jirapinyo P, Kankirawatana P, Densupsoontorn N, Thamonsiri N, Wongarn R. High plasma branched-chain amino acids:aromatic amino acids ratio in children on the ketogenic diet: a mechanism in controlling epilepsy. J Med Assoc Thai 2004; 87: 432–437. [PubMed] [Google Scholar]
- 40Yudkoff M. Brain metabolism of branched-chain amino acids. Glia 1997; 21: 92–98. [DOI] [PubMed] [Google Scholar]
- 41Bak LK, Waagepetersen HS, Sorensen M, Ott P, Vilstrup H, Keiding S et al. Role of branched chain amino acids in cerebral ammonia homeostasis related to hepatic encephalopathy. Metab Brain Dis 2013; 28: 209–215. [DOI] [PubMed] [Google Scholar]
- 42Evangeliou A, Spilioti M, Doulioglou V, Kalaidopoulou P, Ilias A, Skarpalezou A et al. Branched chain amino acids as adjunctive therapy to ketogenic diet in epilepsy: pilot study and hypothesis. J Child Neurol 2009; 24: 1268–1272. [DOI] [PubMed] [Google Scholar]