Abstract
Statin therapy has been associated with improved cerebral blood flow (CBF) and decreased perihematoma edema in animal models of intracerebral hemorrhage (ICH). We aimed to assess the relationship between statin use and cerebral hemodynamics in ICH patients. A post hoc analysis of 73 ICH patients enrolled in the Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial (ICH ADAPT). Patients presenting <24 hours from ICH onset were randomized to a systolic blood pressure target <150 or <180 mm Hg with computed tomography perfusion imaging 2 hours after randomization. Cerebral blood flow maps were calculated. Hematoma and edema volumes were measured planimetrically. Regression models were used to assess the relationship between statin use, perihematoma edema and cerebral hemodynamics. Fourteen patients (19%) were taking statins at the time of ICH. Statin-treated patients had similar median (IQR Q25 to 75) hematoma volumes (21.1 (9.5 to 38.3) mL versus 14.5 (5.6 to 27.7) mL, P=0.25), but larger median (IQR Q25 to 75) perihematoma edema volumes (2.9 (1.7 to 9.0) mL versus 2.2 (0.8 to 3.5) mL, P=0.02) compared with nontreated patients. Perihematoma and ipsilateral hemispheric CBF were similar in both groups. A multivariate linear regression model revealed that statin use and hematoma volumes were independent predictors of acute edema volumes. Statin use does not affect CBF in ICH patients. Statin use, along with hematoma volume, are independently associated with increased perihematoma edema volume.
Keywords: intracerebral hemorrhage, perfusion imaging, perihematoma edema, statins
Introduction
The 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors or statins improve clinical outcomes in cardiovascular disease, primarily via their lipid-lowering properties, but may also have pleiotropic effects. One proposed mechanism is the effect of statin therapy on cerebral hemodynamics. Animal model studies have shown that parenteral statins given 2 weeks before ischemic stroke increased cerebral blood flow (CBF).1, 2, 3 However, evidence for these effects in humans is limited.4 In one study of 31 acute ischemic stroke patients imaged serially with perfusion-weighted magnetic resonance imaging at 4.5 and 6 hours, prior statin treatment was a predictor of relative reperfusion (measured as the difference in prolonged mean transit time (MTT) volumes at the two time points).5 In contrast, a PET study in 97 patients with carotid artery occlusion indicated no effect of prior statin use on either global or regional CBF.6
Statins may also affect perfusion in intracerebral hemorrhage (ICH). In an experimental ICH model, treatment with high-dose parenteral atorvastatin or simvastatin was associated with increased CBF, decreased edema, and lower blood-brain barrier permeability in the outer boundary zone of the hematoma on magnetic resonance imaging.7 There are no studies of the effect of statin use on the cerebral hemodynamics of patients with acute ICH.
Statin use in the acute phase of ICH is controversial. Post hoc analyses of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial8 and the Heart Protection Study,9 both showed an association between statin use and increased risk of hemorrhagic stroke. Conversely, a large prospective cohort study indicated statin use was associated with lower stroke severity and statin discontinuation was associated with worse outcomes in ICH patients.10 A neuroprotective role of statins in ICH, independent of baseline low-density lipoprotein (LDL) levels has also been proposed.11
Using data from a randomized trial of blood pressure (BP) lowering in acute ICH patients (Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial; ICH ADAPT),12 we assessed the effect of statin treatment on CBF and perihematoma edema volume. We tested the hypothesis that statin treatment before ICH is associated with increased CBF and decreased perihematoma edema volumes.
Materials and Methods
Patients and Clinical End Points
The ICH ADAPT was a multicenter, prospective, randomized, open-label, blinded endpoint (PROBE) study (clinicaltrials.gov NCT00963976). The study was reviewed and approved by local Human Research Ethics Boards at each of the participating trial sites (University of Alberta, University of Calgary, and University of Ottawa). The trial was conducted according to the International Conference of Harmonisation guidelines for Good Clinical Practice (ICH GCP). The protocol has been published previously.13 Briefly, ICH patients ⩾18 years of age presenting within 24 hours of symptom onset were randomized to a target systolic BP (SBP) of <150 or <180 mm Hg, followed by computed tomography perfusion (CTP) imaging 2 hours later. Exclusion criteria included evidence of secondary ICH, planned surgical resection, contraindications to BP reduction or indication for urgent reduction, or inability to undergo CTP imaging. Informed consent was obtained from each patient or an authorized representative, and human ethics committees at each site approved the study protocol. Intravenous antihypertensive agents (labetalol/hydralazine/enalapril) were used to achieve target SBPs within 1 hour of randomization, with serial BP measurements over 24 hours. NIH stroke scale (NIHSS) scores were recorded at baseline, 2 hours, 24 hours, and at 90 days. Modified Rankin scores and Barthel index scores were recorded at 30 and 90 days.
Image Acquisition
Patients underwent noncontrast CT (NCCT) scans at baseline, 2±1 and 24±3 hours after randomization. The NCCT scan protocol consisted of 18 to 20 five-mm slices through the whole brain with a 512 × 512 matrix (120 kvp, 300 mA per slice). CTP imaging was performed at 2 hours. A 38 to 80 mm thick section (slab thickness varied with scanner capabilities) was selected to assess perfusion, centered on the slice where the hematoma had the greatest diameter on the NCCT. CTP images were acquired with intravenous iodinated contrast (40 mL) given over 10 seconds with CT images acquired every 1 second for 50 seconds (80 kvp, 200 mA per image). All patients had a repeat NCCT scan at 24±3 hours.
Image Analysis
All images were postprocessed and measured centrally. Raw CTP source images were transferred to a PC workstation and analyzed using the PerfScape analysis package (PerfScape 2.0 CT Stoke Edition, Olea Medical, Marseilles, France). CTP maps were derived from the tissue time-density curve, based on the change in X-ray attenuation, which is linearly related to iodinated contrast concentration on a per-voxel basis over time. A single-value deconvolution algorithm was used to correct for the delay in contrast arrival and dispersion of the bolus, using an arterial input function defined by manually selected voxels from the anterior cerebral artery contralateral to the hematoma.14
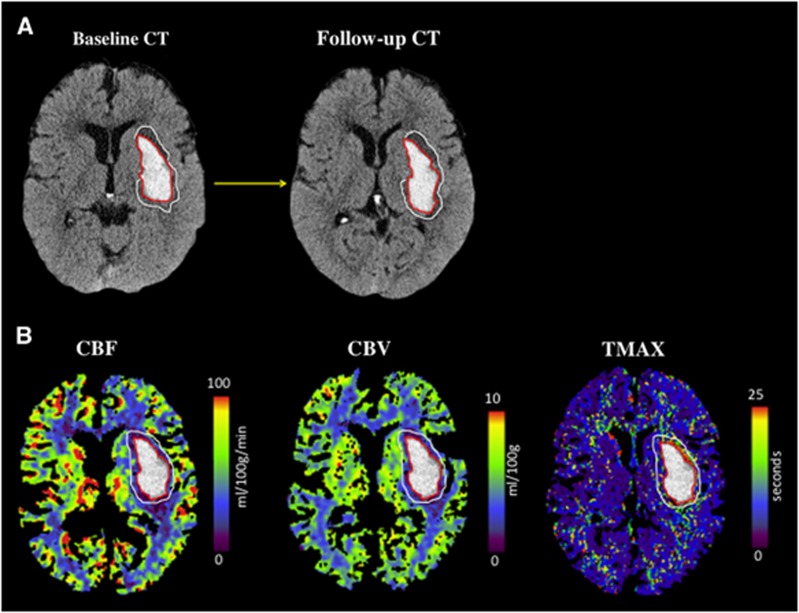
All hematoma, edema, and perfusion parameter volumes were measured using the Analyze 11.0 software suite (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA).15 Both hematoma and edema region of interest measurements were completed independently by two investigators (RM and BG) on NCCTs at all time points (baseline, 2, and 24 hours). Hematoma and total ICH (calculated as hematoma+intraventricular hemorrhage) volumes were measured using planimetric semiautomatic thresholding segmentation techniques, as previously described (Figure 1).16 Perihematoma edema regions were independently drawn manually by the two raters. A threshold of 5 to 23 Hounsfield units (HU) was subsequently applied to these regions of interest to provide an objective and standardized edema volume measurement.17 Relative edema volume (calculated as 5 to 23 HU edema volume/hematoma volume) was also measured, as previously described.18 The mean of volume measurements provided by each rater was subsequently used in statistical analyses. CBF, cerebral blood volume, and time to peak of the impulse response curve (TMAX) maps were calculated. Voxels containing blood vessels were removed using an intensity threshold function of CBF>100 mL/100 g/min or cerebral blood volume >8 mL/100 g.19 Regions of interest were drawn on all perfusion maps, using standard planimetric techniques. These consisted of a 1-cm region surrounding the hematoma, a homologous contralateral region, and both hemispheres, excluding ventricles. Relative perfusion measures were calculated as the ratio of absolute ipsilateral and contralateral values in each region.
Figure 1.
(A) Example of hematoma and perihematoma edema regions of interest (ROIs). The ROIs were drawn on the noncontrast computed tomography (CT) and transferred to perfusion maps. (B) Maps of cerebral blood flow (CBF), cerebral blood volume (CBV), and time to peak of the impulse response curve (TMAX) from an ICH ADAPT study patient randomized to a target systolic BP <150 mm Hg.
Statistical Analysis
All statistical analyses were performed using SPSS 20.0 (IBM SPSS Statistics 2012, Armonk, NY, USA). For binary analysis between baseline patient characteristics and imaging data between untreated and statin-treated use before ICH were analyzed using independent t-tests or Mann-Whitney tests (for parametric and nonparametric data, respectively). Pearson's chi-square or Fisher's exact tests were used to compare frequencies of categorical data. Correlations were assessed by Pearson's correlation and Spearman's correlation coefficients for parametric and nonparametric data, respectively. A multivariate linear regression model was used to assess independent predictors of perihematoma edema volumes.
Results
Patients
Seventy-five patients were enrolled and randomized in ICH ADAPT. Two patients were excluded because of lack of CTP data. A total of 73 patients were included in the final analysis. Mean time from symptom onset to randomization was 10.1±7.2 hours. Fourteen patients (19%) reported statin use before the index event. Baseline clinical characteristics between statin-treated and nontreated patients were similar except for higher rates of hypertension, acute coronary syndromes, and antihypertensive treatment in patients receiving statin therapy (Table 1). Mean serum LDL levels were lower in statin-treated patients (2.2±1.2 versus 3.1±0.7 mmol/L, respectively; P=0.002). Glasgow Coma Scale (GCS) and NIHSS scores were similar at baseline, 2, and 24 hours between groups. Mean hospital admission SBP was similar in statin-treated patients (174.2±13.1 mm Hg) than those without statins (185.2±30.9 mm Hg, P=0.099) as was the SBP at 2 hours (151.9±17 mm Hg versus 151.3±20, respectively; P=0.91).
Table 1. Baseline patient characteristics.
| Statins treated (n=14) | Nontreated (n=59) | P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (mean±s.d.) | 76.2±10.8 | 66.6±11.8 | 0.15 |
| Gender (n, % male) | 10 (71.4) | 44 (74.5) | 1 |
| GCS (median (IQR Q25–75)) | 15 (12–15) | 15 (13–15) | 0.68 |
| NIHSS (median (IQR Q25–75)) | 10.5 (5–19) | 10 (6.5–15.5) | 0.81 |
| Systolic BP, admission (mm Hg) | 174.2±21.5 | 185.2±23.7 | 0.099 |
| Systolic BP, 2 hours (mm Hg) | 151.9±17 | 151.9±20.0 | 0.91 |
| Medical history | |||
| Hypertension (n,%) | 13 (92.8) | 30 (66.1) | 0.005 |
| ICH | 0 | 5 (8.5) | 0.58 |
| Stroke | 3 (21.4) | 5 (8.5) | 0.18 |
| ACS | 5 (35.7) | 5 (8.4) | 0.008 |
| Diabetes | 5 (35.7) | 16 (35.6) | 0.52 |
| Medications used | |||
| Antihypertensives (n,%) | 10 (71.4) | 22 (37.3) | 0.03 |
| Antiplatelets | 2 (14.3) | 1 (1.7) | 0.09 |
| Anticoagulation (warfarin) | 1 (7.1) | 3 (5.1) | 1 |
| Lipid profile | |||
| Total cholesterol (mean±s.d.) | 4.2 (1.5) | 4.8 (1.0) | 0.07 |
| LDL (mean±s.d.) | 2.2 (1.2) | 3.1 (0.8) | 0.002 |
| HDL (mean±s.d.) | 1.5 (0.6) | 1.2 (0.6) | 0.88 |
| Triglycerides (mean±s.d.) | 1.2 (0.8) | 1.1 (0.8) | 0.2 |
Abbreviations: ACS, acute coronary syndrome; BP, blood pressure; GCS, Glasgow Coma Scale; HDL, high-density lipoprotein; ICH, intracerebral hemorrhage; IQR, interquartile range; LDL, low-density lipoprotein.
Hematoma and Perihematoma Edema Volumes
The median (IQR Q25 to 75) time from symptom onset to first CT was 7.8 (3.5 to 16.8) hours, and this did not differ between patients receiving statin therapy (10.1 (3.5 to 15.3)) hours and those who did not (7.4 (3.6 to 16.9) hours, P=0.72). The median (IQR Q25 to 75) hematoma volume for the entire cohort was 16.1 (6.3 to 29.0) mL. The majority of the hematomas (82%) were located in deep brain regions; 29% were lobar (Table 2). Median (IQR Q25 to 75) hematoma volumes in statin-treated patients (21.1 (9.5 to 38.3) mL) were similar to those in nontreated patients (14.5 (5.6 to 27.7) mL, P=0.25). Intraventricular hemorrhage extension was seen more frequently in patients treated with statins (9/14 (64.3%) versus 18/59 (30.5%), P=0.02).
Table 2. Imaging characteristics.
| Statin treated (n=14) | Nontreated (n=59) | P value | |
|---|---|---|---|
| Baseline CT scan | |||
| Hematoma location (n, %) | |||
| Lobar | 2 (14.3) | 12 (20.3) | 0.62 |
| Deep | 12 (85.7) | 46 (77.9) | 0.52 |
| Infratentorial | 0 | 1 | >0.99 |
| Hematoma volume, mL (median (IQR Q25–75)) | 21.1 (9.5–38.3) | 14.5 (5.6–27.7) | 0.25 |
| IVH (n, %) | 9 (64.3) | 18 (30.5) | 0.02 |
| IVH volume, mL (median (IQR Q25–75)) | 5.2 (1.0–12.0) | 3.8 (1.1–8.1) | 0.94 |
| Total ICH volume, mL (hematoma+IVH; median (IQR Q25–75)) | 27.1 (16.2–41.0) | 17.2 (7.4–28.2) | 0.13 |
| Perihematoma edema volume, mL (median (IQR Q25–75)) | 2.9 (1.7–9.0) | 2.2 (0.8–3.5) | 0.02 |
| Relative edema volume, mL (median (IQR Q25–75)) | 0.14 (0.07–0.24) | 0.17 (0.13–0.31) | 0.12 |
| 24-Hour follow-up CT scan | |||
| Total ICH volume, mL (hematoma+IVH; median (IQR Q25–75)) | 27.5 (16.2–40.0) | 17.1 (7.5–40.2) | 0.28 |
| Hematoma growth >6 mL (n, %) | 4 (28.6) | 12 (21.8) | 0.72 |
| Hematoma growth volume, mL (median (IQR Q25–75)) | 0.3 (0–9.0) | 0.7 (0–3.7) | 0.92 |
| Rate of hematoma growth (mL/h; median (IQR Q25–75)) | 0.02 (0–0.4) | 0.03 (0–0.2) | 0.84 |
| Perihematoma edema volume growth, mL (median (IQR Q25–75)) | 0.9 (−1.1 to 4.7) | 1.0 (0.5–3.4) | 0.60 |
| Relative edema volume, mL (median (IQR Q25–75)) | 0.3 (0.1–0.5) | 0.3 (0.1–0.4) | 0.87 |
Abbreviations: CT, computed tomography; ICH, intracerebral hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage.
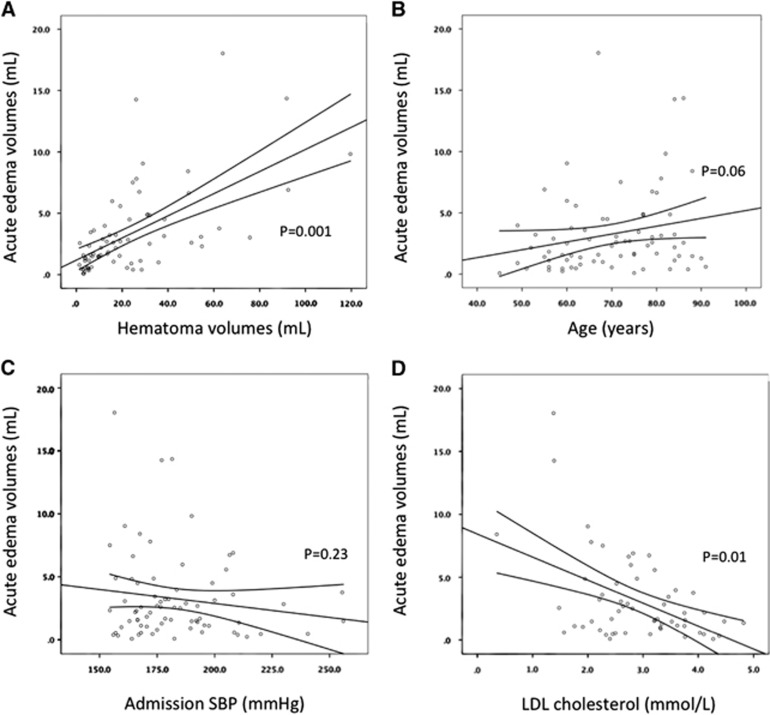
Baseline hematoma volumes were correlated with acute edema volumes (R=0.66, P<0.01; Figure 1). Acute median (IQR Q25 to 75) perihematoma edema volumes were larger in statin-treated patients (2.9 (1.7 to 9.0) mL than nontreated patients (2.2 (0.8 to 3.5) mL, P=0.02). At 24 hours, 16 patients experienced significant hematoma expansion (defined as >6 mL difference in volume on the repeat 24 hours NCCT). The frequency of hematoma expansion did not differ between groups (Table 2). The median (IQR Q25 to 75) rate of hematoma expansion in statin-treated patients (0.02 (0 to 0.4) mL/h) was similar to that in nontreated patients (0.03 (0 to 0.2) mL/h, P=0.84). The mean volume of edema growth at 24 hours was equally similar in both groups.
Cerebral Perfusion and Statin Use
The median (IQR Q25 to 75) time from symptom onset to CTP imaging was 9.9 (6 to 19.2) hours. Mean relative CBF in the perihematoma region was similar between statin-treated patients (0.86±0.1) and nontreated patients (0.85±0.1, P=0.61). Similarly, mean absolute perihematoma CBF did not differ between groups (37.8±15.1 mL/min/100 g in statin-treated patients and 38.9±11.1 mL/min/100 g in nontreated patients, P=0.52). Mean hemispheric CBF in statin-treated patients (41.9±13.8 mL/min/100 g) was not different than that in nontreated patients (42.1±9.7 mL/min/100 g, P=0.97). Statin use was not associated with any differences in perihematoma and hemispheric cerebral blood volume or Tmax values (Table 3).
Table 3. Computed tomography perfusion parameters and statin use.
| Statin treated (n=14) | Nontreated (n=59) | P value | |
|---|---|---|---|
| Absolute perihematoma CBF, mL/min/100 g (mean±s.d.) | 37.8±15.1 | 38.9±11.1 | 0.52 |
| Relative perihematoma CBF (mean±s.d.) | 0.86±0.1 | 0.85±0.1 | 0.61 |
| Absolute hemispheric CBF (mean±s.d.) | 41.9±13.7 | 42.1±9.7 | 0.97 |
| Relative hemispheric CBF (mean±s.d.) | 0.98±0.1 | 0.97±0.04 | 0.83 |
| Absolute perihematoma CBV, mL/100 g (mean±s.d.) | 3.7±0.71 | 3.6±0.6 | 0.53 |
| Relative perihematoma CBV (mean±s.d.) | 0.99±0.1 | 0.9±0.1 | 0.45 |
| Absolute perihematoma Tmax (seconds; mean±s.d.) | 5.8±2.1 | 6.2±2.9 | 0.97 |
| Relative perihematoma Tmax (mean±s.d.) | 1.7±0.5 | 1.6±0.4 | 0.93 |
Abbreviations: CBF, cerebral blood flow; CBV, cerebral blood volume.
Cerebral Perfusion and Serum Lipid Levels
Baseline serum lipid profiles were obtained in 56 (76%) patients at hospital admission. Seventeen patients did not have their lipid profile levels assessed acutely, most often because of early declaration of poor prognosis (13/17). Baseline median (IQR Q25 to 75) hematoma volumes were significantly larger (26.6 (13.7 to 39.5) mL) compared with those in patients whose lipid levels were tested (13.3 (5.4 to 26.8) mL, P=0.02). All perfusion parameters were similar in patients with and without lipid levels.
Serum LDL levels were inversely correlated with acute edema volumes (LDL: R=0.23, P=0.01; Figure 2). There was no relationship between serum LDL levels and perihematoma CBF (R=0.24, P=0.12), or hemispheric CBF (R=0.02, P=0.66). Serum LDL levels were not correlated with hematoma or intraventricular hemorrhage volumes.
Figure 2.
Univariate correlates of perihematoma edema volume. Correlations between acute perihematoma edema and hematoma volume (A), age (B), admission systolic blood pressure (SBP; C), and low-density lipoprotein (LDL) cholesterol (D). P values were calculated using Spearman's rank correlation tests.
Predictors of Acute Perihematoma Edema Volumes
A multivariate linear regression analysis adjusting for all variables with a univariate association with edema volumes (P value <0.10) was performed. A multiple linear regression model adjusted for age, admission SBP, hematoma volume, statin use, and serum LDL levels revealed that statin use and hematoma volumes were both independent predictors of acute perihematoma edema volumes, irrespective of admission SBP and LDL levels (Table 4).
Table 4. Multivariate linear regression model for predictors of acute edema volumes.
| β | SE | P value | 95% Confidence interval for β | |
|---|---|---|---|---|
| Age | 0.001 | 0.03 | 0.99 | −0.06 to 0.06 |
| Admission SBP | −0.10 | 0.14 | 0.50 | −0.04 to 0.02 |
| Acute hematoma volume | 0.09 | 0.02 | <0.001 | 0.05 to 0.12 |
| LDL | −0.86 | 0.43 | 0.051 | −1.7 to 0.01 |
| Statin use | 2.84 | 1.07 | 0.01 | 0.69 to 4.99 |
Abbreviations: LDL, low-density lipoprotein; SBP, systolic blood pressure.
Discussion
This is the first study of the effect of statin use on cerebral perfusion in humans. We found no relationship between statin use and CBF either in the perihematoma region or more globally. We did observe a direct correlation between statin use and perihematoma edema volumes.
Cerebral Perfusion and Statin Use
In an experimental rodent ICH model, statins were associated with increased cerebral perfusion, decreased edema volumes and upregulation of tight junction protein expression.6 We did not observe any relationship between statin use and CBF in the clinical setting of ICH patients. This may be related to the fact that in the experimental ICH study, high-dose parental statins likely resulted in elevated circulating drug levels than are seen clinically. Our patients were taking variable types and doses of statins, each with varying degrees of lipophilicity, affecting the ability to cross the blood-brain barrier. Atorvastatin and simvastatin possess higher lipophilic properties in comparison to rosuvastatin and pravastatin.20
Perihematoma Edema and Statin Use
Only one other study examined the effect of prior statin use on perihematoma edema volumes in ICH patients. A retrospective analysis of 125 ICH patients with CT scans performed within 12 hours from symptom onset indicated statin use was associated with decreased perihematoma volumes on initial CT scan images.21 We found precisely the opposite relationship. The discordant results may be related to differences in the method used to measure edema volume. Although the previous study also used planimetric measurements, these were completely subjective. We have shown previously that manual perihematoma measurement results in a high degree of interrater variability. Using the HU threshold method, as we did in the present study, significantly improves the reliability of the measurement and provides a more objective perihematoma edema volume.16
The mechanism(s) of perihematoma edema formation have been proposed to change over time,22 with hydrostatic pressure and clot retraction being responsible for edema formation in the first few hours after ICH, followed by coagulation and thrombin production in the subacute phase (first 2 days), and finally by late erythrocyte lysis and hemoglobin toxicity. Although serial imaging studies have shown that perihematoma edema growth may extend to ~14 days,23 maximal edema growth (75%) occurs within the first 24 hours from symptom onset.24 In our study, evaluation of edema growth was limited to analysis of two CT scans performed within the first 24 hours of ICH as part of the trial protocol. As a result, we could not study the effect of statins on edema growth in the subacute phase of ICH. However, given that a significant association was seen between statins and acute perihematoma edema volumes on the initial CT scan, with no difference between the two groups at 24 hours, it is possible that the maximum effect of statins on edema occurs acutely. This may be particularly true in our patients, where statins were all discontinued at the time of the ICH.
The pleiotropic effects of statins may play a role in perihematoma edema formation. Experimental studies have shown that statins display antiplatelet, antithrombotic, and fibrinolytic properties.25, 26, 27 In this context, one would expect that in the acute phase of ICH, statins would be associated with decreased clot retraction (because of interrupted platelet aggregation and activation) and therefore lower perihematoma edema volumes. We found precisely the opposite relationship between statin use and perihematoma edema volumes. This is most likely related to the trend to larger hematoma volumes in the statin-treated patients in our trial. It is well established that the most important predictor of edema volume is baseline hematoma volume.28 It is likely that the effect of baseline hematoma volume makes it impossible to detect any potential effects of statins on perihematoma edema volume in a relatively small trial.
Limitations
The small number of patients treated with statins in the ICH ADAPT trial limits the confidence with which we can conclude any relationship exists between clinical statin use and perihematoma edema formation. Similarly, we cannot entirely exclude an effect on perihematoma or global CBF. Furthermore, given the presence of an imbalance in the rates of preexisting hypertension between statin-treated and nontreated patients, we cannot rule out the possibility that antihypertensive therapy or cerebral vessel wall remodeling because of chronic hypertension may have modified the effect of statins on CBF. Nonetheless, this is the first investigation of this nature in ICH patients. This study is also larger than the only related investigation performed in ischemic stroke patients (n=31), in which a similar number of patients (n=12) were taking statins.5 Finally, the absolute CBF values calculated using the deconvolution algorithm may not be completely accurate. The relative values, however, did not reveal any differences in the pattern of CBF between statin- and nonstatin-treated patients.
Conclusion
Statin use does not affect CBF in patients with ICH. Statin use, along with hematoma volume, are independently associated with increased perihematoma edema volume. Although our results should be considered hypothesis generating only, the association between statin use and larger perihematoma edema volumes, whether mediated through larger ICH volumes and/or other mechanisms, is clinically relevant. If edema growth is indeed promoted by statin use, these drugs should be reconsidered after acute ICH. These findings should be explored in larger ICH trials, such as the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT 2).29
The authors declare no conflict of interest.
Footnotes
The ICH ADAPT trial was funded by grant-in-aid support from Alberta Innovates Health Solutions (G513000128) and the Heart and Stroke Foundation of Canada (G220170180). KB holds a Canada Research Chair in Cerebrovascular Disease, a Heart and Stroke Foundation of Alberta Professorship in Stroke Medicine, and a New Investigator Award from Alberta Innovates Health Solutions. AMD, DD, and MDH hold Heart and Stroke Foundation of Canada personnel awards. SC holds an Alberta Innovates Health Solutions New Investigator award. LCG and MPK are supported by clinical research fellowship bursaries from the Alberta Innovates Health Solutions. BG and RM were supported by Alberta Innovates Health Solutions studentships.
References
- 1Shabanzadeh AP, Shuaib A, Wang CX. Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res 2005; 1042: 1–5. [DOI] [PubMed] [Google Scholar]
- 2Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1998; 95: 8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Yamada M, Huang Z, Dalkara T, Endres M, Laufs U, Waeber C et al. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J Cereb Blood Flow Metab 2000; 20: 709–717. [DOI] [PubMed] [Google Scholar]
- 4Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab 2012; 32: 1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Ford AL, An H, D'Angelo G, Ponisio R, Bushard P, Vo KD et al. Preexisting statin use is associated with greater reperfusion in hyperacute ischemic stroke. Stroke 2011; 42: 1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Derdeyn CP, Carpenter DA, Videen TO, Grubb RL, Powers WJ. No effect of low-dose statins treatment on cerebral blood flow in humans with atherosclerotic cerebrovascular disease. J Cereb Blood Flow Metab 2007; 27: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Yang D, Knight RA, Han Y, Karki K, Zhang J, Chopp M et al. Statins protect the blood brain barrier acutely after experimental intracerebral hemorrhage. J Behav Brain Sci 2013; 3: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 9Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363: 757–767. [DOI] [PubMed] [Google Scholar]
- 10Dowlatshahi D, Demchuk AM, Fang J, Kapral MK, Sharma M, Smith EE. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke 2012; 43: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 11Biffi A, Devan WJ, Anderson CD, Ayres AM, Schwab K, Cortellini L et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology 2011; 76: 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB et al. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke 2013; 44: 620–626. [DOI] [PubMed] [Google Scholar]
- 13Butcher K, Jeerakathil T, Emery D, Dowlatshahi D, Hill MD, Sharma M et al. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial: ICH ADAPT. Int J Stroke 2010; 5: 227–233. [DOI] [PubMed] [Google Scholar]
- 14Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 2003; 50: 164–174. [DOI] [PubMed] [Google Scholar]
- 15Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph 13: 433–454. [DOI] [PubMed] [Google Scholar]
- 16McCourt R, Gould B, Gioia L, Kate M, Coutts SB, Dowlatshahi D et al. Cerebral perfusion and blood pressure do not affect perihematoma edema growth in acute intracerebral hemorrhage. Stroke 2014; 45: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 17Clasen RA, Huckman MS, Von Roenn KA, Pandolfi S, Laing I, Lobick JJ. A correlative study of computed tomography and histology in human and experimental vasogenic cerebral edema. J Comput Assist Tomogr 1981; 5: 313–327. [DOI] [PubMed] [Google Scholar]
- 18Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 2002; 33: 2636–2641. [DOI] [PubMed] [Google Scholar]
- 19Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006; 37: 1771–1777. [DOI] [PubMed] [Google Scholar]
- 20Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005; 19: 117–125. [DOI] [PubMed] [Google Scholar]
- 21Naval NS, Abdelhak TA, Urrunaga N, Zeballos P, Mirski MA, Carhuapoma JR. An association of prior statin use with decreased perihematomal edema. Neurocrit Care 2008; 8: 13–18. [DOI] [PubMed] [Google Scholar]
- 22Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006; 5: 53–63. [DOI] [PubMed] [Google Scholar]
- 23Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 2011; 42: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 2002; 33: 2631–2635. [DOI] [PubMed] [Google Scholar]
- 25Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J 2003; 24: 225–248. [DOI] [PubMed] [Google Scholar]
- 26Violi F, Calvieri C, Ferro D, Pignatelli P. Statins as antithrombotic drugs. Circulation 2013; 127: 251–257. [DOI] [PubMed] [Google Scholar]
- 27Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet 1996; 348: 1079–1082. [DOI] [PubMed] [Google Scholar]
- 28Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW et al. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology 2009; 73: 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368: 2355–2365. [DOI] [PubMed] [Google Scholar]