Abstract
The relationship between peri-infarct depolarizations (PIDs) and infarction was investigated in a model of preconditioning by cortical freeze lesions (cryogenic lesions, CL) in the Spontaneously Hypertensive Rat. Small (< 5 mm3) lesions produced 24 hours before permanent focal ischemia were protective, without impacting baseline cerebral blood flow (CBF) and metabolism. Prior CL reduced infarct volume, associated with improved penumbral CBF as previously showed for ischemic preconditioning. The brief initial procedure avoided sham effects on infarct volume after subsequent occlusion under brief anesthesia. However, under prolonged isoflurane anesthesia for perfusion monitoring both sham and CL rats showed reduced PID incidence relative to naive animals. This anesthesia effect could be eliminated by using α-chloralose during perfusion imaging. As an additional methodological concern, blood glucose was frequently elevated at the time of the second surgery, reflecting buprenorphine-induced pica and other undefined mechanisms. Even modest hyperglycemia (>10 mmol/L) reduced PID incidence. In normoglycemic animals CL preconditioning reduced PID number by 50%, demonstrating associated effects on PID incidence, penumbral perfusion, and infarct progression. Hyperglycemia suppressed PIDs without affecting the relationship between CBF and infarction. This suggests that the primary effect of preconditioning is to improve penumbral perfusion, which in turn impacts PID incidence and infarct size.
Keywords: cold lesion, focal ischemia, hyperglycemia, peri-infarct depolarization, preconditioning
Introduction
Peri-infarct depolarizations (PIDs) are well recognized in experimental and clinical stroke,1, 2, 3 and propagated depolarizations also occur in brain after hemorrhage and trauma.4 Available evidence suggests that such events contribute to pathology by both increasing metabolic demand and limiting perfusion.5 The latter effect appears to involve a transition from vasodilatory to vasoconstrictive responses to depolarization under pathologic conditions,6, 7, 8 and steal may also contribute in the context of focal ischemia.5, 9 The PID number is usually too variable to predict outcome for an individual animal, but infarct size and depolarization parameters are correlated in most studies of experimental stroke.10 The cumulative duration of depolarization, dependent largely on position in the perfusion gradient, is the primary determinant of local tissue fate,11, 12, 13 but depolarization number can still be relevant. Numerous interventions simultaneously attenuate PID incidence and reduce infarct size,14, 15, 16 whereas superimposing additional depolarizations exacerbates injury.17 Most importantly, stepwise infarct expansions can occur in association with the propagation of individual PIDs.5, 13, 18
Conditioning paradigms constitute robust but mechanistically heterogeneous interventions to modulate outcome in experimental stroke. Preconditioning protection in the Spontaneously Hypertensive Rat (SHR) is associated with early recovery of penumbral cerebral blood flow (CBF).19, 20 Improved CBF has also been reported in other preconditioning models,21, 22 suggesting that perfusion effects are more general contributors to protection than initially appreciated. In view of the recognized effects of PID propagation on penumbral CBF, an impact of preconditioning on such events could be mechanistically relevant.
It has been challenging to produce conditioning models with the quantitative reliability necessary for mechanistic studies. Ischemic preconditioning in the SHR has two components, a modest effect associated with the sham procedure and likely arising due to prior anesthesia exposure, and a robust component more evident in animals subjected to preconditioning occlusions.20 Both components are also found in autoradiographic assessments of ischemic territory volume at 3 hours after occlusion.20 A retrospective evaluation of the sections from that analysis determined that more robust protection was associated with the presence of small prior lesions, sometimes remote from the site of surgical manipulation.23 This component of ischemic preconditioning therefore seems analogous to that produced by intentional cortical lesions.24
In the current study, a preconditioning model based on cortical freeze lesions (cryogenic lesions, CL) was optimized in the SHR. Since cortical lesions can impact baseline CBF,23, 24 as well as glucose metabolism,25, 26 the impact of lesion size on CBF and preconditioning efficacy was compared. Hyperglycemic effects secondary to buprenorphine analgesia were identified and minimized by adjustments in animal husbandry. In addition, it was found essential to use α-chloralose for anesthesia during PID monitoring to avoid confounding effects of prior isoflurane exposure. The optimized model was then used to determine the impact of CL preconditioning on PID incidence. Preliminary results have been reported.27
Materials and methods
Experimental Animals and Study Design
Male SHR (approximately 3 months of age, weighing 250 to 300 g, n=215) were obtained from Harlan Laboratories (Indianapolis, IN, USA) or Charles River Laboratories (Wilmington, MA, USA). All experiments were approved by the Institutional Animal Care and Use Committee, University of Tennessee Health Science Center, and were conducted according to United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. The general study design involved an initial interval of anesthesia to produce a preconditioning cortical freeze lesion (CL), or the corresponding sham procedure. On the following day both Sham and CL rats were subjected to permanent focal ischemia, as were additional animals in a Naive comparison group. The pretreatment study design precluded formal randomization, but each component of the experiment involved multiple shipments of animals over the course of many weeks, with allocations among groups distributed over time. The same individual performed all experimental procedures and was therefore aware of group designation at the time of occlusion surgery. However, any derived samples or images were thereafter identified only numerically.
In Study 1, cortical infarct volumes were measured at 24 hours after occlusion for groups in which all procedures had been performed under anesthesia with either halothane (Naive, n=6; Sham, n=9; CL, n=9) or isoflurane (Naive, n=9; Sham, n=10; CL, n=10). In Study 2, acute ischemic territory volumes (CBF⩽30 mL/100 g per minute) were assessed autoradiographically at 15 minutes (Naive, n=2; CL, n=2) and 3 hours (Naive, n=5; Sham, n=4; CL, n=5), following procedures performed under isoflurane anesthesia. In Study 3, rats received cortical lesions alone and were evaluated the following day for asymmetries in CBF (n=25). In Study 4, PID-associated CBF responses were monitored by speckle contrast perfusion imaging during the initial 4 hours of occlusion, under anesthesia with either isoflurane (Naive, n=5; Sham, n=5; CL, n=6) or α-chloralose (Naive, n=8; Sham, n=9; CL, n=7). The above group sizes were based on prior experience establishing that 5 to 6 animals per group were sufficient to detect preconditioning effects after permanent occlusions in the model.20 Fewer animals were used in confirmatory autoradiographic CBF studies, whereas more animals were devoted to novel end points related to perfusion imaging. Additional animals were used in preliminary studies to develop and troubleshoot experimental procedures. These primarily involved comparison of preconditioning efficacy for cortical lesions at different locations (n=48), and adjustment of animal husbandry to minimize hyperglycemia during the second surgical procedure (n=21).
Animals were excluded from the study due to unexplained mortality (n=2), local hemorrhage indicative of surgical trauma at the site of occlusion (n=5), or if there was evidence of subcortical infarction (n=7). Rats were also excluded from the grouped data of studies 1, 2, and 4 if preconditioning lesions injured subcortical white matter (n=6), or if blood glucose levels at the time of occlusion surgery exceeded 10 mmol/L (Sham, n=7; CL, n=10), but these animals did contribute to studies evaluating effects of lesion size and hyperglycemia.
Preconditioning Lesions
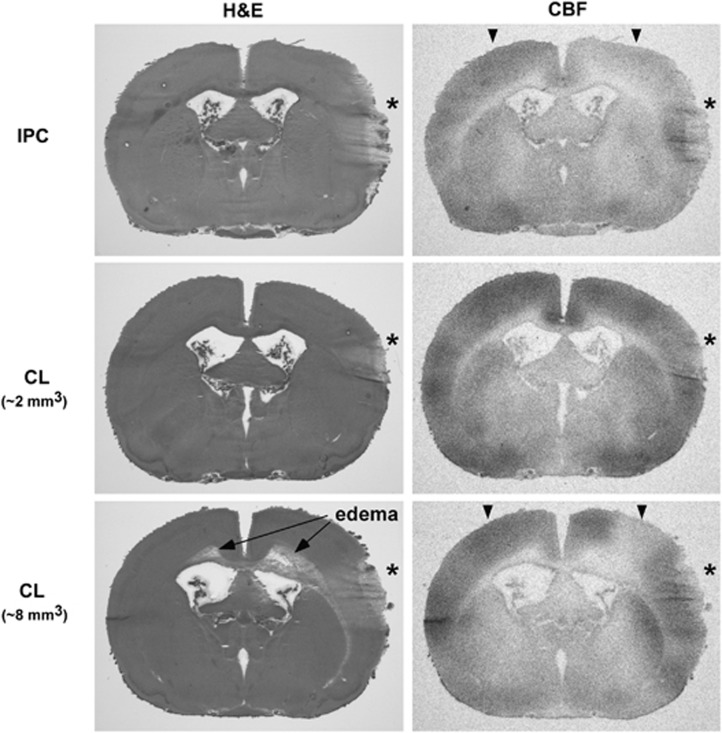
The rationale for a lesion-based preconditioning model is founded in the observation that a subset of animals experiencing surgery to produce preconditioning middle cerebral artery (MCA) occlusions exhibited incidental cortical injury, frequently associated with reductions in ipsilateral CBF.23 An example of a relatively large such lesion and its impact on perfusion are illustrated in Figure 1 (upper panels). For the current study, intentional CL replaced these comparatively rare, unpredictable events.
Figure 1.
Cortical lesions incidental to preconditioning surgery or after freeze injury, and their effects on cerebral blood flow (CBF). Hematoxylin-eosin stained sections illustrate regions of cortical damage (*) 24 hours after middle cerebral artery (MCA) manipulation for ischemic preconditioning (IPC) or after intentional cryogenic lesions (CL). Larger lesions (>5 mm3) resulted in subcortical white-matter edema, sometimes propagating contralaterally (long arrows). CBF autoradiograms of the same sections illustrate local hyperemia, but more generalized CBF reduction after surgical injury or larger CL (compare signal intensities at the corresponding ipsilateral and contralateral locations, arrowheads).
Animals were anesthetized and ventilated with 1% to 2% halothane or isoflurane in 70% N2, 30% O2. Body temperature was monitored with a rectal probe and maintained at 37°C via a feedback-controlled heating pad and infrared lamp until complete recovery from anesthesia. The skull was exposed and a 3-mm diameter window was thinned over temporal MCA-territory cortex until brain surface vasculature was uniformly visible, during which time temperature was maintained with a thermostated saline drip (TC-324B, Warner Instruments Inc., Hamden, CT, USA). A 2-mm diameter stainless steel probe cooled in liquid nitrogen was applied to the thinned skull for 10 seconds. Sham animals were subjected to identical surgery without probe application. Incisions were closed with surgical suture and swabbed with povidone/iodine, and animals received subcutaneous buprenorphine (0.05 mg/kg). Anesthesia was discontinued, animals were weaned from the respirator and allowed to recover consciousness and thermal equilibrium, and were returned to their cages. Total anesthesia duration averaged 20 minutes.
The position selected for the preconditioning lesion was typically 3 mm ventral and 1 mm caudal to the site of MCA occlusion, corresponding to approximate stereotaxic coordinates 7 mm lateral, 1 mm caudal to bregma (Figure 1, middle panels). Preliminary studies examined the efficacy of placement at other locations, including frontal cortex, as used in previous studies involving hypertonic lesions.24 Less robust effects were observed at such sites, and lesion and infarct borders sometimes overlapped due to the large infarct size in the SHR, complicating quantitative measurements. When placed at the selected position the region of preconditioning damage always remained within the margins of the eventual infarct. Freezing times were varied in preliminary studies to produce a range of lesion sizes. Small lesions were restricted to cortex and had no impact on CBF remote from the lesion site (Figure 1, middle panels). Large lesions (>5 mm3) were associated with subcortical white-matter edema, propagating contralaterally in severe cases and produced widespread reductions in ipsilateral baseline perfusion (Figure 1, lower panels). Since white-matter involvement was readily detected in the frozen sections used for either CBF autoradiography or infarct assessment, brains exhibiting such edema could be excluded from analysis in preconditioning studies.
Post-Preconditioning Husbandry
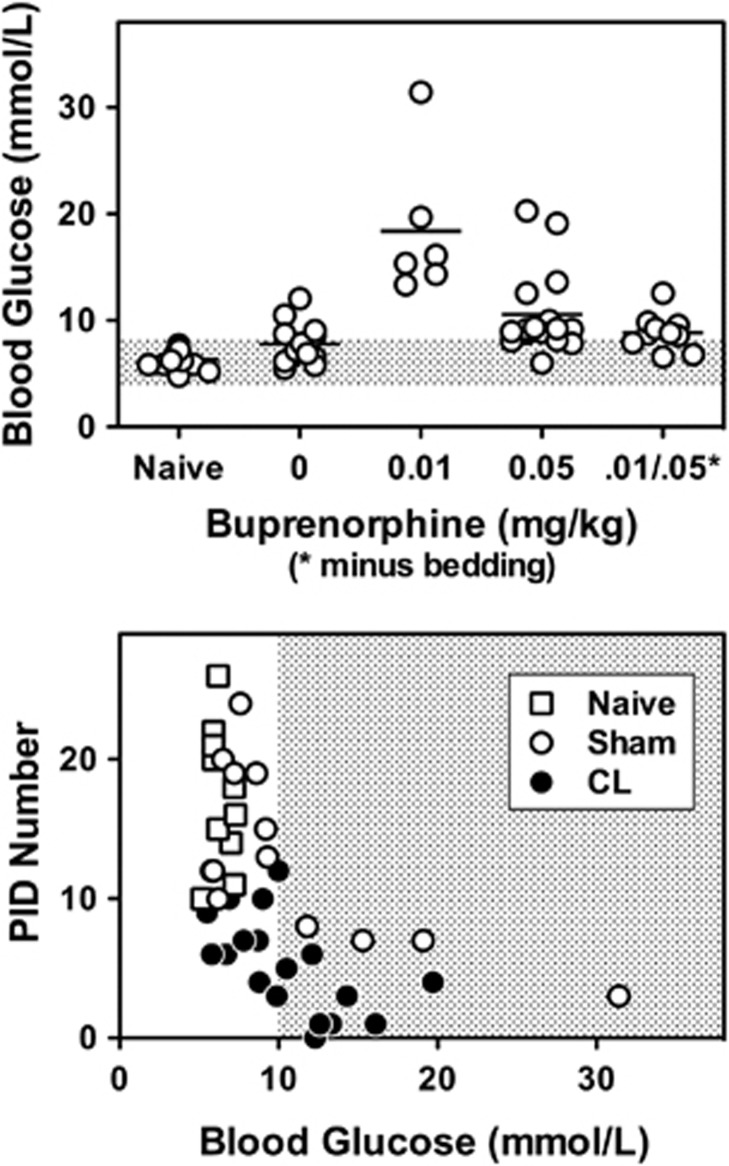
Preconditioning lesions were produced in fed animals, but rats of all experimental groups were fasted overnight before MCA occlusion. In naïve animals, this procedure successfully avoided recognized hyperglycemic effects of isoflurane. However, in preliminary studies it emerged that both Sham and CL groups tended to show elevated glucose levels during the second interval of isoflurane anesthesia, even when fasted (Figure 2, upper panel). The most prominent component of this effect was associated with buprenorphine analgesia, and occurred secondary to a previously described pica behavior that resulted in bedding consumption and gastrointestinal distress.28 This was attenuated by housing animals overnight in wire-bottom cages, which restored glucose levels to those observed in the absence of buprenorphine. Nevertheless, modest hyperglycemia could occur in previously operated animals, independent of analgesia effects. It has long been recognized that profound hyperglycemia can block the occurrence of PIDs during focal ischemia,2 and more recent results showed that hyperglycemia both raises the stimulus threshold for generating cortical spreading depression and reduces depolarization frequency.29 Rats with blood glucose levels greater than 10 mmol/L were excluded from the current studies, based on observations that PID incidence declined above this threshold in both Sham and CL animals (Figure 2, lower panel).
Figure 2.
Hyperglycemia after prior surgical procedures and its impact on peri-infarct depolarization (PID) incidence. Upper panel: In naïve rats, blood glucose was adequately maintained by an overnight fast before isoflurane anesthesia. However, glucose levels tended to be higher in animals that had experienced anesthesia and surgery the previous day (pooled data from Sham and cryogenic lesion (CL) groups). Overt hyperglycemia was observed when such animals received buprenorphine. This was attenuated in rats housed in wire-bottom cages after the initial surgery to prevent bedding consumption, but glucose levels remained elevated relative to the naive range (shaded bar), as also observed in the absence of buprenorphine. Lower panel: PID number decreased with increasing glucose levels in both Sham and CL groups. A threshold of 10 mmol/L was chosen for inclusion in the study to minimize confounding effects of hyperglycemia on PID incidence.
Permanent Focal Ischemia
Focal brain ischemia was produced by tandem occlusion of the right MCA and ipsilateral common carotid artery (CCA), essentially as previously described.20, 30 Animals were anesthetized and ventilated, and temperature was controlled as described above. A tail artery cannula (PE-50) was placed to monitor blood pressure and for periodic sampling to measure blood gases, pH, and glucose level (Supplementary Tables S1 and S2, Supplementary Data). The right CCA was exposed and cauterized between two ligations. The right MCA was exposed at the level of the rhinal fissure by dissecting the temporalis and masseter muscles and drilling a 2-mm burr hole through the temporal-squamous bone. The MCA was snared with a micromanipulator-controlled wire hook, raised approximately 1 mm, and cauterized to produce permanent focal brain ischemia.
Incisions were closed with suture and swabbed with povidone/iodine. For survival studies of infarct volume and autoradiographic CBF, animals routinely received subcutaneous buprenorphine (0.05 mg/kg). Anesthesia was discontinued, animals were weaned from the ventilator and allowed to recover consciousness and thermal equilibrium, and were returned to their cages.
Histologic Analysis
Animals were decapitated under halothane or isoflurane anesthesia 24 hours after MCA occlusion. The brains were rapidly dissected, frozen in hexane at −40°C, and stored at −70°C. Coronal sections (20 μm) were cut in a cryostat at −20°C and collected at 1 mm intervals through the extent of the MCA territory. Slides were briefly fixed in 95% ethanol and stained with hematoxylin and eosin, after which calibrated images were collected (NIH Image). The pale infarct area of each section was summed across all sections to determine the infarct volume in cubic millimeters. Edema volume was calculated by the difference in cortical volume between ischemic and contralateral hemispheres, and subtracted from the total infarct volume to yield the corrected infarct volume.
Autoradiographic Cerebral Blood Flow Measurement
Cerebral blood flow was evaluated by an indicator-fractionation technique using 4-iodo-[N-methyl-14C]antipyrine (Perkin-Elmer Life Sciences, Boston, MA, USA) as the diffusible tracer, as previously described.20 Femoral artery and vein cannulae were placed during the course of surgery to produce MCA occlusion, or in a separate surgical procedure approximately 3 hours before tracer infusion in animals that were not subjected to ischemia. Blood flow measurements were performed in awake animals under brief restraint. Isotope was injected as an intravenous bolus (15 μCi in 0.4 mL saline) while arterial blood was withdrawn into a syringe containing heparinized saline at a constant rate of 1 mL/min using a programmable pump (SP230iw, World Precision Instruments, Sarasota, FL, USA). The animals were decapitated approximately 6 seconds after isotope injection, and the pump was simultaneously stopped. Brains were rapidly removed and frozen in hexane at −40°C, and stored at −70°C. Sampled blood was transferred with saline rinses to a preweighed tube and reweighed to determine the total volume, assuming a density of 1.05 g/mL. A sample (20 μL) was decolorized with 0.2 mL H2O2, mixed with 7 mL of scintillation fluid (Ultima Gold, Packard Instrument Company, Meriden, CT, USA), and radioactivity was determined by scintillation counting. Coronal brain sections (20 μm) were cut serially at −20°C and collected at 0.5 mm intervals throughout the extent of the MCA territory. These were thaw mounted on slides and exposed to Kodak Biomax MR film for 7 days, together with 14C standards (Amersham Biosciences, Piscataway, NJ, USA), after which calibrated images were captured and stored using NIH Image. For the stroke studies, cortical areas with CBF below 30 mL/100 g per minute, previously identified as a threshold that reliably correlated with infarction in this model,20 were integrated across all sections to derive the volume of ischemic territory. Lesion effects on baseline perfusion were assessed by determining CBF in regions of ipsilateral dorsal cortex rostral and caudal to the lesion site, as well as the corresponding contralateral locations, and an average ratio was determined for each animal.
Perfusion Imaging
Real-time imaging of the distribution of perfusion deficits, as well as of the CBF response to PID, was accomplished by speckle contrast perfusion imaging using a commercially available instrument package (Full-field Laser Perfusion Imaging system, Moor Instruments Inc., Wilmington, DE, USA), as previously described.5 Rats were anesthetized with isoflurane, ventilated, and prepared for occlusion as described above. In addition, the dorsal surface of skull was exposed and thinned bilaterally until surface vasculature was uniformly visible. During this procedure, isoflurane levels were transiently increased to approximately 3%, reducing blood pressure to 90 to 100 mm Hg, and minimizing bleeding. A stream of warmed saline was delivered to maintain a surface temperature of 37°C. Images were collected over a field of 152 × 113 pixels at a sampling rate of 25 Hz, exposure time of 4 ms, and time constant of 1.0 second. Regions of interest were continuously recorded at three ipsilateral and two contralateral locations, avoiding major blood vessels. Full field images were saved at 30-second intervals. After 1 hour of baseline recording the arteries were occluded, and the animal was repositioned and monitored for an additional 4 hours. Some rats were maintained under isoflurane anesthesia, whereas others were transitioned to α-chloralose after occlusion and repositioning (intravenously 50 mg/kg during 5 minutes, then 30 mg/kg per hour beginning at 1 hour), administered as the 2-hydroxypropyl-β-cyclodextrin complex (Sigma-Aldrich, St Louis, MO, USA).
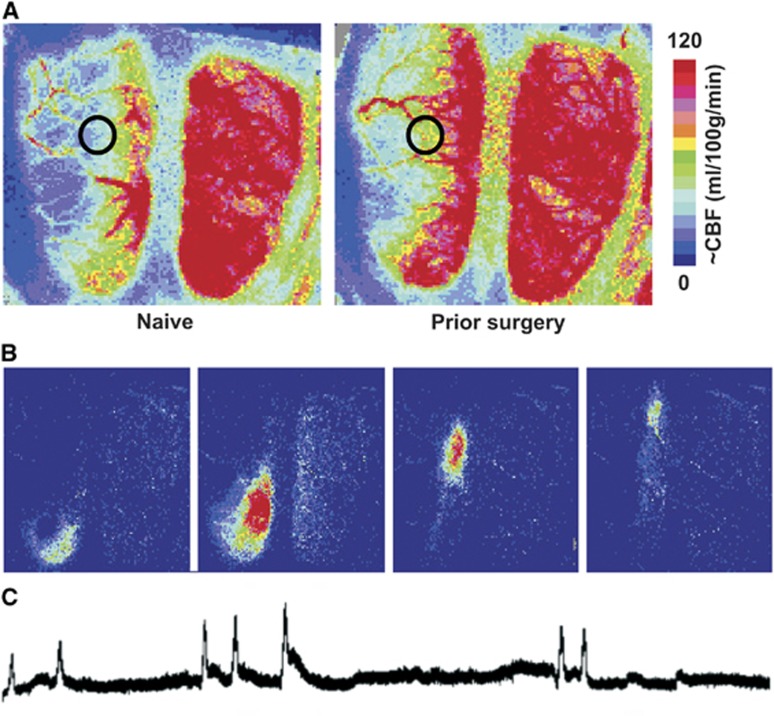
Stored images were used to derive quantitative regional CBF estimates in a region of interest centered 2.5 mm lateral, 3 to 4 mm caudal to bregma, at the medial edge of MCA territory (Figure 3, panel A). Rostro-caudal position was adjusted to avoid major blood vessels. This region had been identified in a previous study to reach flow values near the threshold for infarction, and CBF was calculated using an empirical calibration also established in that study.5 Peri-infarct depolarizations were identified in the recorded traces as transient hyperemic events restricted to ipsilateral cortex, each verified as a propagating perfusion response in sequential stored images (Figure 3, panels B and C).
Figure 3.
Perfusion imaging of penumbral cerebral blood flow (CBF) and peri-infarct depolarization (PID)-associated hyperemic transients. (A) Distribution of CBF deficits during occlusion. Representative speckle contrast perfusion images are shown for a naive (left) and previously sham-operated animal (right), both maintained under isoflurane anesthesia during focal ischemia. Circles identify regions of interest at the margin of the middle cerebral artery (MCA) territory used for quantitative comparisons, at a location having flow values in naive animals near the perfusion threshold for infarction. Scale bar shows calibration with autoradiographic CBF derived in a previous study.5 (B) PID-associated hyperemia. Difference images obtained at 1-minute intervals illustrate the rostro-caudal migration of a PID-associated flow transient at the margin of the ischemic territory. (C) PID recording. Representative trace illustrates a series of PID-associated hyperemic events during MCA occlusion.
Statistical Analyses
Group values are stated as mean±standard deviation (s.d.), and values for individual animals are illustrated in all figures. Comparisons of infarct volumes, ischemic territory volumes, and physiologic parameters among Naïve, Sham, and PC groups used Analysis of Variance followed by Scheffe' F-test. Analyses were implemented in StatView 5.0 (SAS Institute, Inc., Cary, NC, USA) or GraphPad Prism 5.0c (GraphPad Software, San Diego, CA, USA), with P<0.05 considered as statistically significant.
Results
Lesion-Induced Preconditioning
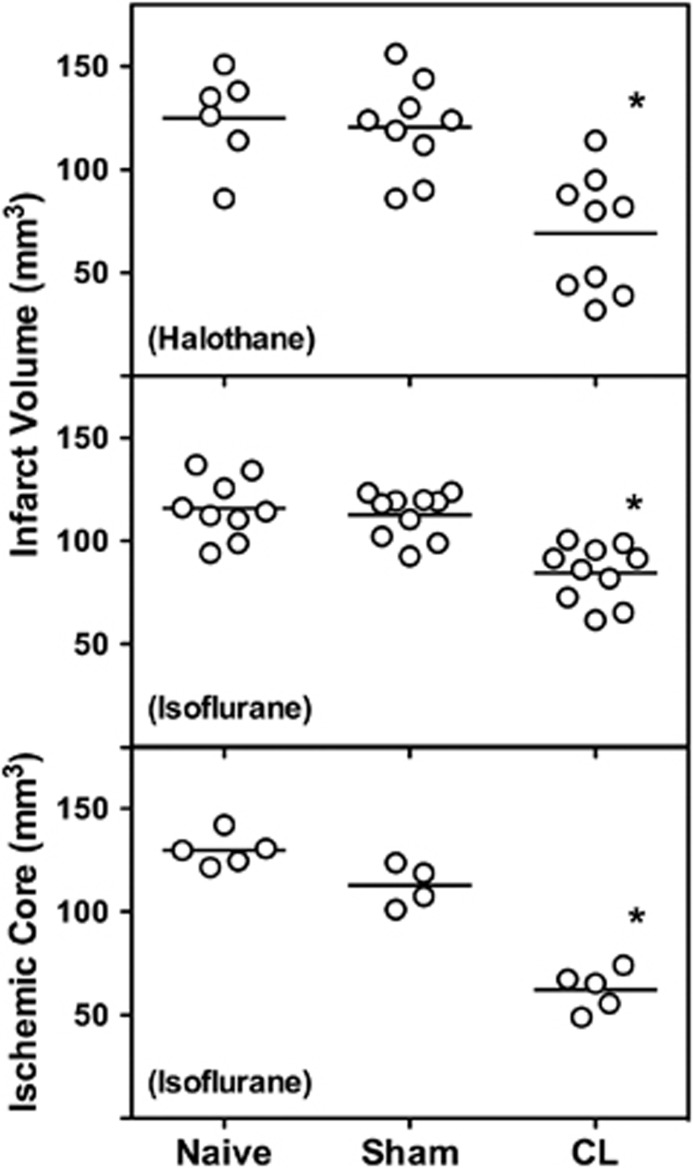
The model was developed first with halothane as the anesthetic, and these initial results were then replicated under isoflurane anesthesia. Independent of anesthetic agent, prior cortical lesions reduced final infarct volume after subsequent permanent MCA/CCA occlusion, without a significant impact of sham anesthesia exposure and surgical manipulation (Figure 4, upper panels). Protection was associated with a corresponding early recovery of collateral perfusion after recovery from anesthesia. Whereas Naive and CL rats showed comparable CBF deficit volumes at 15 minutes after MCA occlusion (Naive, 95 to 129 mm3; CL, 110 to 115 mm3), ischemic territory at 3 hours varied in parallel with group differences in final infarct size (Figure 4, lower panel).
Figure 4.
Preconditioning effects of cortical lesions. Edema-corrected infarct volumes at 24 hours after ischemia onset were reduced in rats subjected to prior cryogenic lesions (CL), with no effect of sham procedures. Comparable results were obtained under halothane and isoflurane anesthesia. Volumetric assessment of the ischemic territory (cerebral blood flow (CBF)⩽30 mL/100 g per minute) at 3 hours after occlusion, using an autoradiographic [14C]-iodoantipyrine method, predicted final histopathologic outcomes in the respective groups. *P⩽0.05 versus Naive groups.
Lesion Size and Baseline Effects
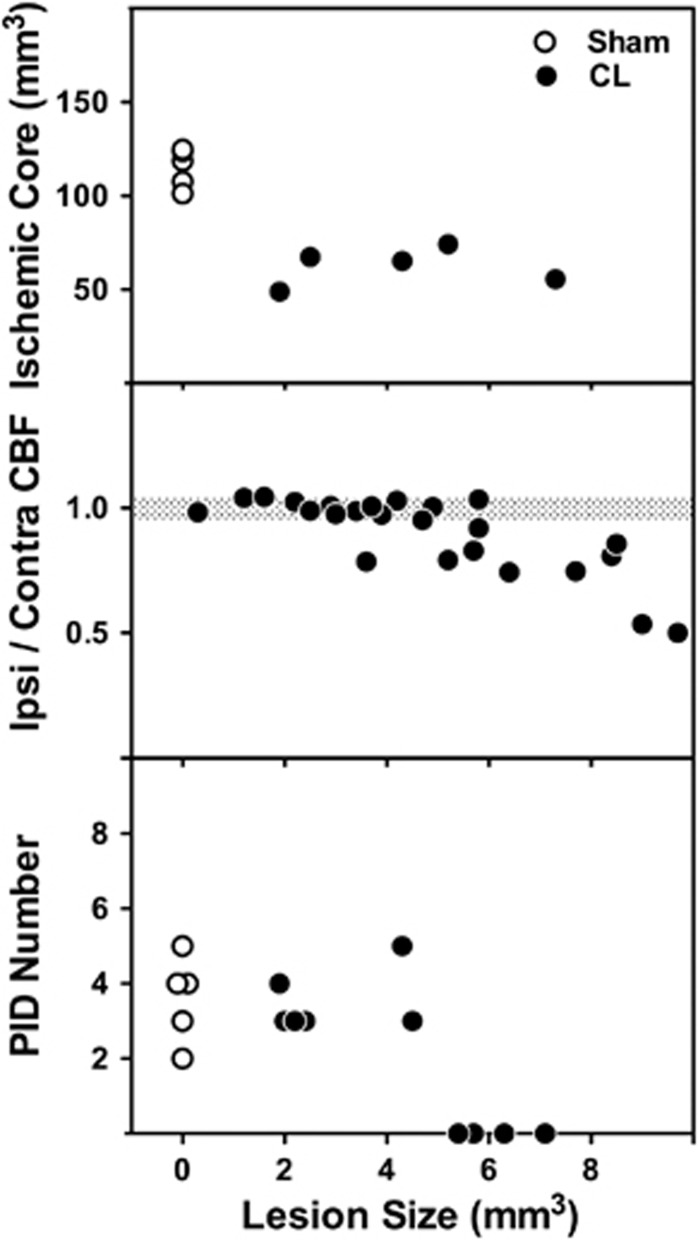
Since CBF changes predicted infarct distribution in advance of its clear histologic demarcation, this permitted an assessment of their dependence on size of the prior lesion. Preconditioning efficacy, as indicated by the volume of perfusion deficit at 3 hours after occlusion, was independent of lesion size over the range examined (Figure 5, upper panel). Robust protection was evident in rats with 2 to 5 mm3 lesions that remained below the threshold for a consistent impact on baseline CBF (Figure 5, middle panel). Large lesions that impacted baseline CBF also eliminated PIDs when monitored under isoflurane anesthesia (Figure 5, lower panel). Since all animals exhibiting perfusion asymmetry and gross PID suppression showed white-matter edema these would have been excluded from the preconditioning studies. Observed protection was therefore independent of changes in baseline perfusion.
Figure 5.
Impact of lesion size on preconditioning efficacy, baseline perfusion, and peri-infarct depolarization (PID) incidence. Upper panel: The volume of the preconditioning lesion was determined for animals that provided ischemic territory data of Figure 4. Protection was observed for lesions as small as 2 mm3 and did not vary with lesion size. Note that animals exhibiting white-matter edema were excluded from this analysis (see Figure 1). Middle panel: Freezing conditions were varied to produce cold lesions of varying size, and cerebral blood flow (CBF) in ipsilateral and contralateral cortex was compared 24 hours later. Reductions in ipsilateral CBF became prominent for lesions larger than 5 mm3. Such asymmetry was invariably associated with edema in subcortical white matter. Lower panel: Large lesions resulting in white-matter edema, which would have reduced baseline perfusion, also eliminated the occurrence of PIDs during subsequent occlusion. When monitored under isoflurane anesthesia, animals with small lesions that would have been protective exhibit PID incidence comparable to the sham group. CL, cryogenic lesions.
Peri-Infarct Depolarization Incidence, Perfusion, and Effects of Anesthesia and Hyperglycemia
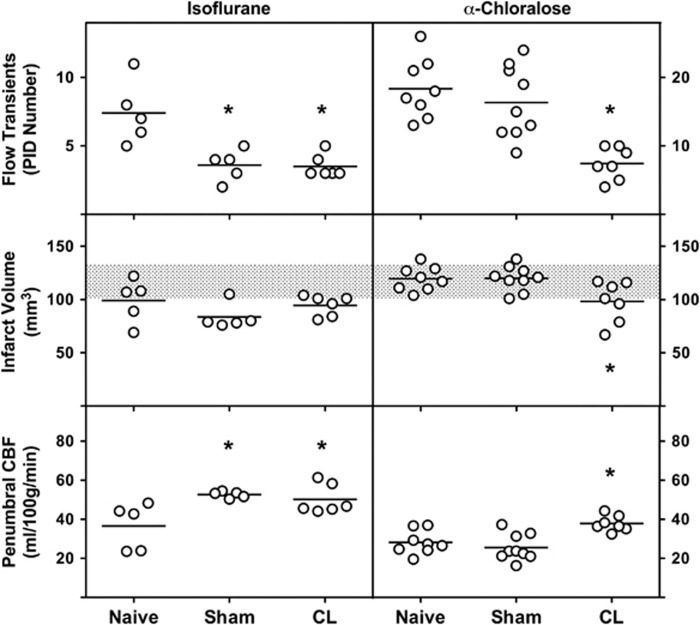
The impact of preconditioning CL on PID incidence and early infarct expansion differed markedly with the anesthetic in use during occlusion (Figure 6). Animals maintained under continuous isoflurane anesthesia during 4 hours after MCA/CCA occlusion exhibited comparable 50% reductions in the number of PID-associated hyperemic events in both Sham and CL groups, relative to Naïve animals. Infarct volumes at the end of the recording period also tended to be smaller in Sham and CL groups although these differences did not achieve statistical significance. Animals in which perfusion imaging was performed under α-chloralose anesthesia showed an approximate doubling in PID incidence compared with isoflurane anesthesia in the Naïve state, and showed no effect of Sham manipulations, but a significant reduction in PID number was observed in the CL preconditioned group. Naïve and Sham groups showed identical infarct volumes at the end of the 4 hours recording session under α-chloralose anesthesia, equivalent to the final infarct volume observed 24 hours after occlusion, whereas CL animals exhibited significantly smaller infarcts. Thus, conditions of sustained α-chloralose anesthesia allow reproduction of the selective lesion-induced preconditioning protection observed in the awake state, and permit identification of an associated reduction in PID incidence.
Figure 6.
Effect of preconditioning on peri-infarct depolarization (PID) incidence, acute infarct progression, and penumbral perfusion, and the impact of anesthesia. Rats subjected to cryogenic lesion (CL) or Sham procedures exhibited comparable reductions in the incidence of PID-associated flow transients during 4 hours of focal ischemia when maintained under isoflurane anesthesia. Infarct size also tended to be reduced in both groups relative to naive animals but this did not achieve statistical significance. When monitored under α-chloralose anesthesia, PID incidence in naive animals doubled relative to that observed under isoflurane. PID number under α-chloralose was unaffected by the prior sham procedure, but was markedly reduced by CL preconditioning. Naive and Sham groups achieved infarct volumes after 4 hours of α-chloralose anesthesia comparable to final 24 hour infarct volume in the model (shaded bars, ±1 s.d.), and significant protection was observed after CL preconditioning. Penumbral cerebral blood flow (CBF) estimates were systematically higher under isoflurane anesthesia. When monitored under isoflurane anesthesia both Sham and CL groups exhibited significantly increased CBF relative to Naive animals, whereas under α-chloralose only CL animals showed increased perfusion. *P⩽0.05 versus Naive.
The variations in PID incidence were paralleled by differences in penumbral CBF (Figure 6). There was a systematic decrease in overall perfusion after the transition from isoflurane to α-chloralose (not shown), after which the distribution of ischemic territory remained essentially constant throughout the duration of recording under a given anesthesia condition. Cerebral blood flow at the margin of the MCA territory was consistently higher in both Sham and CL groups under isoflurane anesthesia, whereas this increase was restricted to the CL group under α-chloralose.
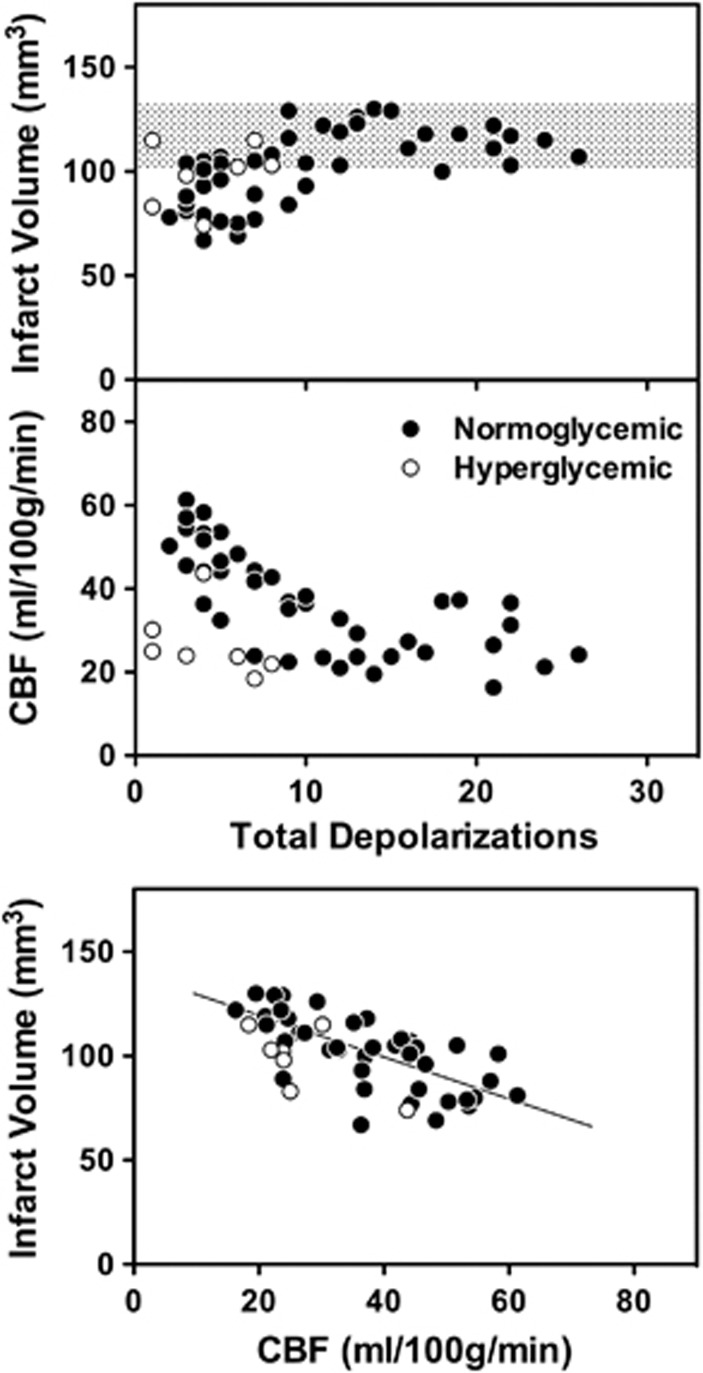
Pooled data for all normoglycemic animals imaged in these studies define consistent relationships among PID number, CBF, and infarct volume (Figure 7), and inclusion of limited data available from animals with elevated blood glucose (3 Sham, 4 CL, under α-chloralose anesthesia) shows an uncoupling effect of hyperglycemia. Consistent maximum infarct volumes were observed over a wide range of PID number (Figure 7, upper panel). Although only weakly predictive of outcome for individual animals, decreasing PID incidence in rats preconditioned by prior CL or anesthesia was associated with smaller infarcts below a threshold of approximately 10 PIDs. There was no clear impact of hyperglycemia on this relationship, although infarct volumes tended to be higher for a given number of PIDs. In contrast, whereas decreasing PID number was invariably associated with better-maintained penumbral perfusion in normoglycemic animals (Figure 7, middle panel), perfusion deficits were pronounced in hyperglycemic rats that nevertheless exhibited few PIDs. Infarct volume was inversely correlated with regional CBF in distal MCA territory, and this relationship was comparable for normoglycemic and hyperglycemic rats (Figure 7, lower panel). Together, these results indicate that the parallel reductions in PID number and infarct volume are secondary to improvements in penumbral perfusion after anesthesia or lesion preconditioning. In contrast, hyperglycemic PID suppression in the absence of increased perfusion is of limited benefit.
Figure 7.
Peri-infarct depolarization (PID) incidence, infarct volume, regional cerebral blood flow (CBF), and the effect of hyperglycemia. Correlations are shown for individual animals that contributed to the data of Figure 6, with inclusion of additional data from hyperglycemic animals. Group designations are omitted for clarity. Upper panel: There was a general correlation between PID incidence and infarct volume below a threshold of approximately 10 PIDs, above which infarct size was constant over a wide range of higher PID incidence. (Shaded bar, naive 24 hour infarct volume±1 s.d.) Middle panel: Reduced PID incidence was associated with improved perfusion of distal middle cerebral artery (MCA) territory in normoglycemic animals, whereas hyperglycemia decreased PID number independent of penumbral perfusion. Lower panel: Penumbral perfusion was inversely correlated with infarct volume, with little effect of blood glucose on the relationship. (Line indicates best fit for normoglycemic animals.)
Discussion
These studies established a model of preconditioning by cryogenic cortical lesions that permits discrimination between components of protection due to focal injury versus prior anesthesia exposure, which jointly contribute to ischemic preconditioning in the SHR.20, 23 Under conditions of transient anesthesia for routine occlusions the brief prior surgical procedure itself had no impact on the volumes of ischemic territory and subsequent infarction. However, there was a marked effect of prior sham manipulations to reduce PID incidence during a second prolonged anesthesia interval required for perfusion imaging. When this confound was avoided by the use of α-chloralose anesthesia an association of CL preconditioning with reduced PID incidence emerged. It is suggested that both derive from a primary effect of the preconditioning procedure on perfusion. A number of considerations influenced the development of the model and impact the interpretation of the results.
Rationale for a Lesion-induced Preconditioning Model
Previous studies showed a bimodal distribution of protection after brief focal ischemia in the SHR,20 and suggested that this reflected interacting effects of anesthesia exposure and incidental cortical lesions.23 Although rarely emphasized, some degree of tissue injury is frequently noted after most preconditioning insults.31 Intentional hypertonic lesions have been reported to precondition cerebral cortex,24 and to account for the protection attributed to cortical spreading depression induced by KCl application.32 Whereas propagating depolarizations may be essential for hippocampal preconditioning,33 local injury produced by NaCl without associated depolarizations appears sufficient to provide cortical protection after subsequent focal ischemia.24 Cryogenic lesioning is an established method for producing discrete cortical injury that has been widely applied to the study of brain pathophysiology.34 Controlled lesions can be produced through thinned skull in the rat, minimizing the invasiveness of the procedure and the duration of anesthesia required.
Impacts on Baseline Cerebral Blood Flow and Metabolism and the Effect of Lesion Size
As observed for ischemic preconditioning20 the distribution of the perfusion deficit at 3 hours provided an early index of protection efficacy (Figure 4), making it possible to measure the size and distribution of previously produced lesions in the same sections used for CBF autoradiography. Importantly, preconditioning effects could be shown for small lesions below the threshold for impacting baseline CBF (Figure 5). This establishes that the cortical protection described in the current study is not secondary to ongoing reductions in baseline activity and metabolic demand.
An early examination of CL effects on CBF and metabolism identified decreases in glucose utilization but not CBF,25 whereas in our hands CBF was clearly a sensitive parameter (Figure 5). Subsequent independent evaluations of hypertonic lesions have found them to impact both glucose utilization26 and CBF24 for at least 3 days after the insults. It seems likely that perfusion and metabolism remain coupled after such insults, and that unrecognized variations in lesion size or position could account for their reported dissociation in the previous CL study. For example, acute CBF decreases after cortical electrode penetration recovered within 6 hours.35 Comparable effects of microdialysis probe placement were more persistent, but showed considerable recovery by 24 hours,36 and a subsequent description of CSD preconditioning using KCl microdialysis reported the presence of small histologic lesions around the site of probe placement but no effect on baseline CBF at 3 days.37 Thus, the recovery time course may be sensitive to lesion size. Metabolic or perfusion effects have been shown for lesion positions frontal24 or caudal25, 26 to that utilized here, in all cases medial to those of the present study, and lesion site could conceivably impact the absolute size threshold for such effects.
The complete elimination of PIDs in animals with large lesions was striking (Figure 5). These had been intentionally produced in a few preliminary experiments to examine the impact of lesion size, in which all perfusion imaging had been performed under isoflurane anesthesia. No large lesions were generated in the subsequent preconditioning study under α-chloralose, so it is unknown whether such a profound effect would be observed under conditions that favor increased PID incidence. Whether the white-matter edema associated with large lesions could have any direct role in PID attenuation remains unclear.
Peri-Infarct Depolarizations, Blood Glucose, and Anesthesia/analgesia Management
The impact of anesthesia on PIDs has been recognized for some time. Volatile anesthetics such as halothane and isoflurane reduce PID incidence relative to that observed under α-chloralose,5, 38 and the latter may be considered as the anesthetic of choice for such studies.39 However, effects on PIDs specifically related to repeated use of volatile anesthetics have not been previously described.
Hyperglycemia is a recognized complication of isoflurane anesthesia that can usually be mitigated by a prior overnight fast. Nevertheless, glucose levels tended to be elevated when previously fasted animals were reanesthetized for a second surgical procedure, and profound hyperglycemia became evident with the introduction of postoperative buprenorphine analgesia (Figure 2). The latter was apparently associated with the stress of bedding consumption (pica) during the intended overnight fast, a behavior previously described after administration of this agent to rats.28 Clearly, it is essential to consider alternative analgesic agents for future studies of this type. However, modest blood glucose elevation was observed even without postoperative analgesia (Figure 2), so its potential impact must be examined in the context of any studies involving repeated surgical procedures. The effect of hyperglycemia to reduce spreading depression or PID incidence during ischemia is well known,2, 29 although PID sensitivity to modest elevations in blood glucose does not appear to be widely recognized. In the specific context of hyperglycemic ischemia such an effect could be mediated by the associated decrease in tissue pH, which is known to increase the depolarization threshold.40
Previous CBF autoradiography studies in awake animals documented a delayed recovery of penumbral perfusion between 1.5 hours and 3 hours after occlusion in animals preconditioned by focal ischemia.20 The same phenomenon was observed here after CL preconditioning (3-hour data in Figure 4). The previous report had also shown recovery of penumbral CBF during laser Doppler imaging in preconditioned rats maintained under halothane anesthesia after occlusion.20 However, a sham-operated group had not been included in that evaluation, and the present results now suggest that even a brief sham procedure is sufficient to improve perfusion in animals when they are maintained under the same volatile anesthetic during imaging (Figure 6). Preliminary experiments indicate that intubation and anesthesia exposure alone can replicate the impact of sham surgery (not shown), suggesting that the PID reduction constitutes an anesthetic preconditioning phenomenon. Importantly, these effects of prior isoflurane exposure are lost when animals are maintained under α-chloralose anesthesia after occlusion (Figure 6). The present results therefore further suggest that such preconditioning does not represent a direct effect on stroke pathophysiology, which might be expected to be transportable across anesthesia conditions. Rather, prior isoflurane exposure affects the impact of a subsequent exposure to the same agent.
The mechanisms underlying this differential response during a second isoflurane exposure remain unclear. As a practical matter, the comparatively brief isoflurane anesthesia interval needed for lesion production appeared to have minimal impact when rats were allowed to rapidly recover from anesthesia after subsequent occlusion surgery (Figure 4). Its effects on PID number and perfusion became prominent when anesthesia was maintained for perfusion imaging (Figure 6). Such anesthetic preconditioning effects might therefore be predicted to have their greatest impact on infarct volume under experimental conditions that involve prolonged anesthesia during an occlusion, as for example in many transient ischemia models. It is possible that the relatively long interval of deep isoflurane anesthesia required for skull preparation further increases the potential for interactions in the context of the current studies.
Peri-Infarct Depolarization Number, Infarct Size, and the Impact of Hyperglycemia
Although the present results show associated effects of CL preconditioning on PID incidence and infarct volume, they do not unambiguously define the cause-effect relationship underlying this result. Consistently large infarcts (representing Naive and Sham animals under α-chloralose anesthesia) were observed over a wide range of PID incidence (Figure 7, upper panel). Therefore, PID number is not an important determinant of infarct size for animals exhibiting already maximum MCA territory involvement, although it may well impact functional or histologic end points not evaluated in these studies. Correlated decreases in infarct size and PID number are observed in animals exhibiting graded infarcts (those maintained under isoflurane anesthesia, or CL preconditioned rats under α-chloralose), but these are also associated with attenuation of the underlying perfusion deficit (Figure 7, middle panel). Smaller ischemic territories have shorter perimeters and smaller penumbra volumes, reducing the probability of triggering PIDs. The decrease in PID incidence after preconditioning could therefore be as much a consequence as a cause of the protection observed.
It should be noted that although hyperglycemic animals were excluded from the initial group analyses, their inclusion would not have greatly impacted the results. For example, CL animals still exhibited fewer PIDs than did Sham animals with comparable blood glucose levels (Figure 2), and still had smaller infarcts (not shown). Hyperglycemia therefore does not alter CL effects, but rather superimposes further reductions in PID incidence. Since blood glucose elevations were viewed primarily as technical issues to be resolved, only a few such animals were fully evaluated. However, the finding that PID incidence could be uncoupled from perfusion and infarction (Figure 7) weakens the case for a causal link between PID reduction and preconditioning protection.
As a final comment, although hyperglycemia is variably reported to worsen outcome in experimental stroke, the absence of an effect on infarct volume in the current study is consistent with previous observations in the SHR.41 A recent study also found no effect in fructose-fed stroke-prone SHR under conditions that increased infarct size in the normotensive Wistar-Kyoto strain,42 although the initial rate of lesion expansion was increased by modest hyperglycemia in both. The limited vascular collaterals of the SHR, and the already large infarcts resulting from permanent MCA occlusion in this strain, reduce the likelihood of detecting an exacerbating effect of hyperglycemia.
Conclusions
These results confirm that small cortical lesions can account for a major component of the protection often attributed to focal ischemic preconditioning. This is largely independent of changes in baseline perfusion, although the latter might also be expected to contribute additionally to the effect in case of larger lesions. Clearly, even small lesions will never be relevant as intentional interventions for brain protection, but could conceivably occur in patients experiencing repeated ischemic insults, and such a model should prove useful to further investigate mechanisms modulating insult severity during subsequent stroke. Both PID incidence and perfusion are impacted by repeated isoflurane anesthesia, requiring use of α-chloralose to replicate the parallel effects on these end points observed in awake animals. Peri-infarct depolarization number and infarct volume appear to be linked via their common dependence on penumbral CBF. Hyperglycemia uncouples this relationship, reducing PID number independent of effects on infarct size.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author Contributions
LZ performed all surgery, conducted perfusion imaging, prepared sections, and performed initial data analysis. TN designed experiments, prepared and administered isotope for quantitative CBF measurement, performed final data analysis, prepared figures and wrote the manuscript.
This work was supported by the Ganey Fund, Department of Neurology, University of Tennessee Health Science Center.
Supplementary Material
References
- 1Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischemia in baboon cerebral cortex. J Neurol Sci 1977; 32: 305–321. [DOI] [PubMed] [Google Scholar]
- 2Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab 1986; 6: 607–615. [DOI] [PubMed] [Google Scholar]
- 3Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus R-I et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol 2008; 63: 720–728. [DOI] [PubMed] [Google Scholar]
- 4Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011; 31: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Takeda Y, Zhao L, Jacewicz M, Pulsinelli W, Nowak TS, Jr. Metabolic and perfusion responses to peri-infarct depolarization during focal ischemia in the Spontaneously Hypertensive Rat: dominant contribution of sporadic CBF decrements to infarct expansion. J Cereb Blood Flow Metab 2011; 31: 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab 1998; 18: 978–990. [DOI] [PubMed] [Google Scholar]
- 7Sukhotinsky I, Dilekoz E, Moskowitz MA, Ayata C. Hypoxia and hypotension transform the blood flow response to cortical spreading depression from hyperemia into hypoperfusion in the rat. J Cereb Blood Flow Metab 2008; 28: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 8Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009; 132: 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Pinard E, Nallet H, MacKenzie ET, Seylaz J, Roussel S. Penumbral microcirculatory changes associated with peri-infarct depolarizations in the rat. Stroke 2002; 33: 606–612. [DOI] [PubMed] [Google Scholar]
- 10Mies G, Iijima T, Hossman K-A. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport 1993; 4: 709–711. [DOI] [PubMed] [Google Scholar]
- 11Mies G. Blood flow dependent duration of cortical depolarizations in the periphery of focal ischemia of rat brain. Neurosci Lett 1997; 221: 165–168. [DOI] [PubMed] [Google Scholar]
- 12Dijkhuizen RM, Beekwilder JP, van der Worp HB, van der Sprenkel JWB, Tulleken KAF, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res 1999; 840: 194–205. [DOI] [PubMed] [Google Scholar]
- 13Higuchi T, Takeda Y, Hashimoto M, Nagano O, Hirakawa M. Dynamic changes in cortical NADH fluorescence and direct current potential in rat focal ischemia: relationship between propagation of recurrent depolarization and growth of the ischemic core. J Cereb Blood Flow Metab 2002; 22: 71–79. [DOI] [PubMed] [Google Scholar]
- 14Iijima T, Mies G, Hossmann K-A. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK-801: effect on volume of ischemic injury. J Cereb Blood Flow Metab 1992; 12: 727–733. [DOI] [PubMed] [Google Scholar]
- 15Mies G, Kohno K, Hossman K-A. Prevention of periinfarct direct current shifts with glutamate antagonist NBQX following occlusion of the middle cerebral artery in the rat. J Cereb Blood Flow Metab 1994; 14: 802–807. [DOI] [PubMed] [Google Scholar]
- 16Golanov EV, Reis DJ. Neuroprotective electrical stimulation of cerebellar fastigial nucleus attenuates expression of periinfarction depolarizing waves (PIDs) and inhibits cortical spreading depression. Brain Res 1999; 818: 304–315. [DOI] [PubMed] [Google Scholar]
- 17Back T, Ginsberg MD, Dietrich WD, Watson BD. Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab 1996; 16: 202–213. [DOI] [PubMed] [Google Scholar]
- 18Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab 2006; 26: 1018–1030. [DOI] [PubMed] [Google Scholar]
- 19Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg 2005; 103: 715–723. [DOI] [PubMed] [Google Scholar]
- 20Zhao L, Nowak TS, Jr. CBF changes associated with focal ischemic preconditioning in the Spontaneously Hypertensive Rat. J Cereb Blood Flow Metab 2006; 26: 1128–1140. [DOI] [PubMed] [Google Scholar]
- 21Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab 2007; 27: 928–938. [DOI] [PubMed] [Google Scholar]
- 22Hoyte LC, Papadakis M, Barber PA, Buchan AM. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res 2006; 1121: 231–237. [DOI] [PubMed] [Google Scholar]
- 23Zhao L, Nowak TS, Jr. (Abstract) Cortical lesions that suppress resting cerebral blood flow and metabolism are required for robust neuroprotection by focal ischemic preconditioning in the Spontaneously Hypertensive Rat Program No. 680.4. 2006 Neuroscience Meeting Planner Society for Neuroscience: Atlanta, GA. 2006. Online. [Google Scholar]
- 24Muramatsu H, Karikó K, Welsh FA. Induction of tolerance to focal ischemia in rat brain: dissociation between cortical lesioning and spreading depression. J Cereb Blood Flow Metab 2004; 24: 1167–1171. [DOI] [PubMed] [Google Scholar]
- 25Pappius HM. Local cerebral glucose utilization in thermally traumatized rat brain. Ann Neurol 1981; 9: 484–491. [DOI] [PubMed] [Google Scholar]
- 26Kawahara N, Ruetzler CA, Mies G, Klatzo I. Cortical spreading depression increases protein synthesis and upregulates basic fibroblast growth factor. Exp Neurol 1999; 158: 27–36. [DOI] [PubMed] [Google Scholar]
- 27Nowak TS, Jr, Zhao L. Conditioning studies in focal cerebral ischemia: model selection, physiological monitoring, and other methodological issues. In: Gidday JM, Pérez-Pinzón MA, Zhang J (eds) Innate tolerance in the CNS: translational neuroprotection by pre- and post-conditioning. Springer: New York. 2012. pp 269–289. [Google Scholar]
- 28Clark JAJ, Myers PH, Goelz MF, Thigpen JE, Forsythe DB. Pica behavior associated with buprenorphine administration in the rat. Lab Anim Sci 1997; 47: 300–303. [PubMed] [Google Scholar]
- 29Hoffmann U, Sukhotinsky I, Eikermann-Haerter K, Ayata C. Glucose modulation of spreading depression susceptibility. J Cereb Blood Flow Metab 2013; 33: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: Methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab 1988; 8: 474–485. [DOI] [PubMed] [Google Scholar]
- 31Sommer C. Ischemic preconditioning: postischemic structural changes in brain. J Neuropathol Exp Neurol 2008; 67: 85–92. [DOI] [PubMed] [Google Scholar]
- 32Otori T, Greenberg JH, Welsh FA. Cortical spreading depression causes a long-lasting decrease in cerebral blood flow and induces tolerance to permanent focal ischemia in rat brain. J Cereb Blood Flow Metab 2003; 23: 43–50. [DOI] [PubMed] [Google Scholar]
- 33Kawahara N, Ruetzler C, Klatzo I. Protective effect of spreading depression against neuronal damage following cardiac arrest cerebral ischemia. Neurol Res 1995; 17: 9–16. [DOI] [PubMed] [Google Scholar]
- 34Murakami K, Kondo T, Yang G, Chen SF, Morita-Fujimura Y, Chan PH. Cold injury in mice: a model to study mechanisms of brain edema and neuronal apoptosis. Prog Neurobiol 1999; 57: 289–299. [DOI] [PubMed] [Google Scholar]
- 35Tomida S, Wagner HG, Klatzo I, Nowak TS, Jr. Effect of acute electrode placement on regional CBF in the gerbil: a comparison of blood flow measured by hydrogen clearance, [3H]nicotine, and [14C]iodoantipyrine techniques. J Cereb Blood Flow Metab 1989; 9: 79–86. [DOI] [PubMed] [Google Scholar]
- 36Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. J Neurochem 1987; 49: 729–734. [DOI] [PubMed] [Google Scholar]
- 37Matsushima K, Hogan MJ, Hakim AM. Cortical spreading depression protects against subsequent focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1996; 16: 221–226. [DOI] [PubMed] [Google Scholar]
- 38Saito R, Graf R, Hübel K, Taguchi J, Rosner G, Fujita T et al. Halothane, but not α-chloralose, blocks potassium-evoked cortical spreading depression in cats. Brain Res 1995; 699: 109–115. [DOI] [PubMed] [Google Scholar]
- 39Luckl J, Keating J, Greenberg JH. Alpha-chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: a comparative study. Brain Res 2008; 1191: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Tong CK, Chesler M. Modulation of spreading depression by changes in extracellular pH. J Neurophysiol 2000; 84: 2449–2457. [DOI] [PubMed] [Google Scholar]
- 41Slivka AP. Hypertension and hyperglycemia in experimental stroke. Brain Res 1991; 562: 66–70. [DOI] [PubMed] [Google Scholar]
- 42Tarr D, Graham D, Roy LA, Holmes WM, McCabe C, Macrae M et al. Hyperglycemia accelerates aparent diffusion coefficient-defined lesion growth after focal cerebral ischemia in rats with and without features of metabolic syndrome. J Cereb Blood Flow Metab 2013; 33: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.