Abstract
Stem cell therapy has showed considerable potential in the treatment of stroke over the last decade. In order that these therapies may be optimized, the relative benefits of growth factor release, immunomodulation, and direct tissue replacement by therapeutic stem cells are widely under investigation. Fundamental to the progress of this research are effective imaging techniques that enable cell tracking in vivo. Direct analysis of the benefit of cell therapy includes the study of cell migration, localization, division and/or differentiation, and survival. This review explores the various imaging tools currently used in clinics and laboratories, addressing image resolution, long-term cell monitoring, imaging agents/isotopes, as well as safety and costs associated with each technique. Finally, burgeoning tracking techniques are discussed, with emphasis on multimodal imaging.
Keywords: animal models, brain imaging, brain ischemia, cell tracking, stem cells
Introduction
Disruption of blood flow to the brain during stroke is the third leading cause of death in the United States, with 800,000 new cases presenting each year, and an estimated annual cost of $60 billion.1 Early restoration of blood flow is crucial, yet at present, only clot-busting tissue plasminogen activator has FDA (Food and Drug Administration) approval for treatment in acute stroke. As of 2015, over 3,000 clinical trials have either been undertaken or are in progress (http://www.Strokecenter.Org/trials/clinicalstudies/list.2015). Potential therapeutic compounds have targeted different stages of stroke progression, including: excitatory cell death2, 3 and inflammation/ischemia-reperfusion injury,4 as well as trials aiming to reduce the risk of a subsequent stroke5—none has proven successful.
Conversely, preclinical studies and early-phase clinical trials indicate that the use of stem cells offers a promising reparative strategy for acute ischemic brain injury. A variety of stem/progenitor cell types are being investigated for their therapeutic potential, including: mesenchymal, hematopoietic, neural and adipose-derived progenitor cells, induced pluripotent stem cells and cell lines (see Liu et al6 for table of current trials). Although the precise mechanism underlying the benefit of stem cell transplantation remains to be determined, it now seems unlikely that these cells are able to replace lost neuronal circuitry directly in humans as initially proposed. In fact, they appear to function as microfactories for a protective cocktail of neurotrophins, growth factors, and other substances that induce restorative processes (e.g., angiogenesis, neurogenesis, and synaptogenesis), and anti-inflammatory mediators.7, 8 The ability of stem cells and their secretions to improve outcomes after stroke has been shown extensively through improvement in a variety of outcome parameters studied in animal models: decreased infarct volume and pro-inflammatory biomarker expression,9, 10 increased anti-inflammatory biomarker(s)11, 12 and growth factor expression,13 improved functional recovery,15, 16 inhibition of glial scar formation,17, 18, 19 and increased angiogenesis.7, 17 These outcomes offer convincing, yet relatively crude measures of the healing capacity of stem cells, as each provides only indirect evidence of beneficial stem cell activity.
Precise mechanistic understanding involves the study of cells in vivo, following administration to an animal, alongside complementary imaging of stroke pathology itself.20 Observing cell migration, localization, division and/or differentiation, cell survival and protein release or expression will enable optimization of a therapy that already shows considerable promise. Several questions require the use of a range of in vivo imaging technologies to provide an answer: do therapeutic stem cells end up in the brain? If not, can we engineer an environment to attract more there? What protective substances do they release? Are these substances different depending on the location of the cells? How long do transplanted cells survive and what do they turn into? Does this vary with respect to the source of therapeutic stem cells? Is one more effective than the other? Many of these aspects were reviewed in 2012 by Adamczak and Hoehn.21 In this review, we provide an update on significant advances and improvements in the field, as well as comprehensive selection guides for researchers and clinicians. Specifically, we discuss the recent advances in neuroimaging technologies (many of which we use in our laboratory to answer the above questions) that facilitate the study of these cellular activities in humans and animal models. We will also address image resolution, long-term cell monitoring, imaging agents/isotopes, as well as safety and costs associated with each technique. Finally, burgeoning tracking techniques are discussed, with emphasis on multimodal imaging.
Cell tracking technologies: overview
The first methods for cell tracking relied on analysis of the differential expression of surface markers on stem cells versus endogenous cells,22, 23 and therefore involved killing many animals to fulfill sufficient time points for a longitudinal study. The use of positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance (MR) imaging (MRI), and optical imaging minimizes the number of animals required for each study. More informative data are produced, as each animal can be used as its own control (i.e., prestroke and at various stages after stroke) in longitudinal experiments. Depending on the species used, behavioral improvements can be difficult to measure accurately and objectively, and small improvements are disguised if a single animal cannot be observed over days. However, the longitudinal, noninvasive nature of behavioral tests provides means for obtaining information that complements imaging well.
The scope of PET, SPECT, MRI, and optical imaging is discussed in the following sections. Facilities permitting, none of these is considered universally preferential. Each has benefits with respect to image resolution, long-term stem cell monitoring, sensitivity of imaging agent, duration of image acquisition, suitability for clinical use, and cost. Appropriate selection of a technique must consider the relative importance of each of these. For example, forms of bioluminescent optical imaging theoretically allow for stem cells to be imaged indefinitely (providing information about the dynamics of stem cells, such as location, migration, and proliferation), and can be performed at a relatively low cost, but due to the low penetration of light from bioluminescent cells,24 this imaging modality is constrained with respect to the depth of tissue that can be observed. Conversely, PET, SPECT, and MRI are able to detect stem cells deep within a subject, but either require the use of a radioactive isotope that may damage the functionality of cells (PET/SPECT),25 or typically have low sensitivity (MRI).26 Efforts are underway to overcome these restrictions (discussed in the sections below), through improved cell-labelling techniques and by combining modalities so that one may accommodate the shortfalls of another (multimodal imaging). The following sections describe the principles and practicalities of these methods clinically and preclinically and are summarized in Table 1.
Table 1. Imaging modalities currently available for tracking stem cells in stroke.
| Imaging modality | Principle | Nonclinical | Clinical | Equipment/labelling requirements | Long-term imaging potential | Resolution | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Positron emission tomography (PET) | ||||||||
| Direct labelling | Decaying radioisotope emits positrons which collide with neighboring electrons, two gamma rays subsequently released and detected. Cells labelled ex vivo with contrast agent (radioisotope) using lipophilic chelator48, 95 | ✓ | ✓ | PET scanner, positron-emitting radiotracer (e.g., 64Cu, 18F) | Dependent on half-life of radioisotope, which can range from 110 minutes (18F) to 12.7 hours (18F). Effectively <2 days | Small animals: >1 mm, clinic: >4 mm (aided by simultaneously acquired CT images)79, 92 | High sensitivity, able to image deep tissues | Expensive, particularly for longitudinal studies, requires radiotracer (hazardous and laborious to produce) with long half-life and low toxicity for long-term studies, requires accompanying CT |
| Indirect labelling | Detection as in 'PET direct labelling'. Cells genetically manipulated ex vivo to express reporter (receptor/transporter) for radioisotope. Contrast agent (radioisotope) is injected immediately before imaging48, 95 | ✓ | ✓ (Limited) | PET scanner, positron-emitting radiotracer (e.g., 64Cu, 18F) | Indefinite as contrast agent is administered immediately before imaging | High sensitivity, able to image deep tissues. No reliance on tracer with a long half-life as contrast agent is injected at the time of scanning. Cell numbers/viability not under-represented by decaying isotope | Expensive, particularly for longitudinal studies, requires radiotracer (hazardous), time-consuming protocol (pre-labelling of cells), requires accompanying CT, often even lower resolution that PET | |
| Single photon emission computed tomography (SPECT) | ||||||||
| Direct labelling | Gamma rays from radioisotope detected by camera. Cells labelled ex vivo with contrast agent (radioisotope) using lipophilic chelator48, 95 | ✓ | ✓ (Infrequent) | PET scanner, gamma-emitting radiotracer (e.g., 111In, 99mTc) | Dependent on half-life of radioisotope, which can range from 6 hours (99mTc) to 2.8 days (111In). Effectively a few days | Small animals: >0.4 mm, clinic: >8 mm (aided by simultaneously acquired CT images)48, 92 | Relatively high sensitivity, more than one target may be observed using >2 contrast agents | Expensive, particularly for longitudinal studies, requires radiotracer (hazardous and laborious to produce) with long half-life and low toxicity for long-term studies |
| Indirect labelling | Detection as in 'SPECT direct labelling'. Cells genetically manipulated ex vivo to express reporter (receptor/transporter) for contrast agent (radioisotope). Tracer is injected immediately before imaging48, 95 | ✓ | ✓ (Infrequent, limited) | PET scanner, gamma-emitting radiotracer (e.g., 111In, 99mTc) | Indefinite as contrast agent is administered immediately before imaging | No reliance on tracer with a long half-life and as tracer is injected at the time of scanning. Cell numbers/viability not under-represented by decaying isotope | Expensive, particularly for longitudinal studies, requires radiotracer (hazardous and laborious to produce), time-consuming protocol (prelabelling of cells) | |
| Computed tomography (CT) | ||||||||
| Detection of X-rays, cells can be labelled with nanoparticles for visualization | ✓ | ✓ | CT scanner, nanoparticles (e.g., Au) | <1 month93 | >50 μm | High resolution | Requires ionizing radiation | |
| Magnetic resonance imaging (MRI) | ||||||||
| In vivo labelling | Magnet allows detection of radio frequency of hydrogen atoms as they 'relax', contrast agents (paramagnetic nanoparticles/certain heavy metals) shorten relaxation time of hydrogen atoms, heightening signal. Targeted contrast agent infused i.v., internalized by target cells33 | ✓ | ✓ | MRI scanner, contrast agent (heavy metal e.g., Gd(III), paramagnetic nanoparticles e.g., SPIOs) | <1 month | Small animals: >24 μm, clinic: 300 μm85 | No radiation, very good tissue contrast, high resolution | Expensive, potentially long acquisition times, toxicity of contrast agent. Development of targeted contrast agent time-consuming (in vivo) or cells must be isolated and labelled (ex vivo) |
| Ex vivo labelling | Detection as in vivo labelling. Cells labelled prior to administration, contrast agent internalized by cells expressing reporter gene95 | ✓ | ✓ | MRI scanner, contrast agent (e.g., Gd(III), SPIOs or PFC+19 F)94 | <24 hours | |||
| Optical imaging | ||||||||
| Bioluminescence imaging (BLI) | Cells emit light dependent on luciferase reporter gene activity65, 66 | ✓ | ✗ | In vivo imaging system (e.g., IVIS®), reporter gene | <1 month | 1–20 mm (depends heavily on depth of tissue) | Cheap, simple, long-term, very fast image acquisition | Depth of tissue penetration and resolution are poor |
| Fluorescence imaging | Labelling with quantum dots and near-infrared dyes59 | ✓ | ✗ | In vivo imaging system (e.g., IVIS®), reporter gene, near-infrared dye or quantum dots | <1 month | >1 mm (depends heavily on depth of tissue, dye/nanoparticle used) | ||
Abbreviation: SPIONs, superparamagnetic iron oxide nanoparticles.
Magnetic resonance imaging: imaging with magnetism
The localization of therapeutic stem cells to an infarct after stroke is widely studied under the premise that successful transplantation relies heavily on targeted cell delivery. Magnetic resonance imaging has been used to confirm successful (or unsuccessful) cell migration,27, 28 to provide a quantitative assessment of various routes of administration (for example, intravenous versus intraarterial)29 and to identify potential safety concerns associated with each route.30 Whether one stem cell source if more suited than another to localize to an infarct is also of great interest. To date there have been very few studies directly comparing stem cells from various sources for stroke therapy,31 and none comparing their ability to localize at an infarct. Magnetic resonance imaging presents an exciting opportunity for conducting such research.
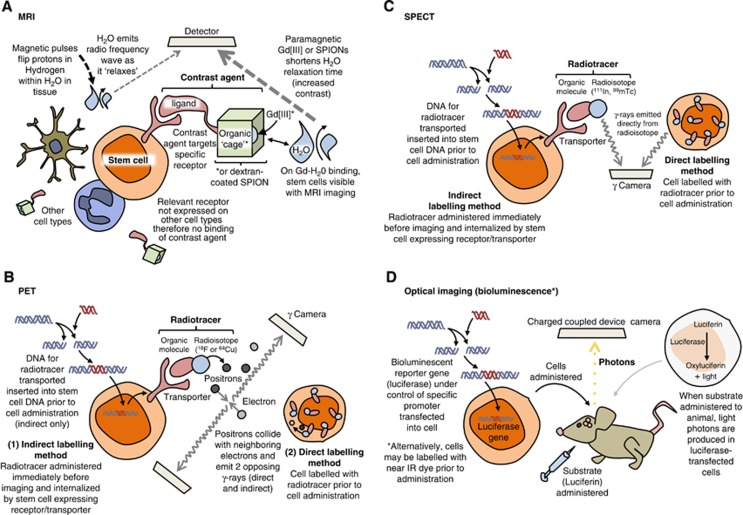
Classical MRI enables the observation of structural aspects of a subject with respect to the diffusion patterns of water through tissue. Images are acquired through the detection of radio frequency waves emitted from the hydrogen atoms within water, as the protons within hydrogen—acting as local magnets—are excited then ‘relax' following the application of a pulsed magnetic field to the subject (Figure 1A). In order for MRI to be useful in imaging at the cell and molecular level, this detection process must be enhanced through the application of either a contrast agent or superparamagnetic nanoparticles. These are either T1 or T2 agents depending on whether they affect the longitudinal (T1), or transverse (T2), relaxation times of water. Clinical MRI systems have magnetic field strengths ranging from 0.2 to 7 Tesla (T), the majority of machines functioning at 1.5 T,32 whereas small rodent systems operate using greater field strengths −4.7 to 11.7 T.33 4.7 T machines and above enable the acquisition of higher resolution images with lower cell detection limits, and are required to generate sufficient resolution to image detail of an ~25 g (versus ~80 kg) animal.
Figure 1.
Principles of stem cell labelling for different imaging modalities. (A) For magnetic resonance imaging (MRI), (B) for positron emission tomography (PET), (C) for single photo emission computed tomography (SPECT), and (D) for optical imaging. SPIONs, superparamagnetic iron oxide nanoparticles.
Recently, MRI has become highly useful in real-time, noninvasive imaging of stem cell activity.34 Gadolinium chelates and iron oxide particles are currently the best contrast agent candidates to label cells for MRI, because they are well tolerated when injected directly into the blood.35, 36, 37
Gadolinium (III) (Gd[III]) is a heavy metal widely used in humans38, 39 and experimentally40, 41 as an ionic base for T1 contrast agents. Gd(III) enhances image contrast—i.e., the variation in grayscale—due to its ability to act as strong local paramagnet and therefore increase the relaxation rate of surrounding water molecules. Paramagnetism, whereby a substance is attracted to a magnetic field, relies on the existence of unpaired (free) electrons, and the strength of that attraction is correlated to the number of free electrons. (Diamagnetism is the opposite, whereby a substance with no free electrons is repelled from a magnetic field.) The paramagnetic properties of Gd(III) are highly potent: free electrons are nearly 700 times stronger than protons (the source of paramagnetism in MR images related to water content) as local magnets, and Gd(III) has seven free electrons.42 The contrast of an image acquired with the use of Gd(III) is therefore enhanced approximately 5,000 times above what can be achieved without a contrast agent.42
Gd(III) is highly toxic as a solubilized-free ion43, 44 and so is chelated before use. The ability of Gd(III) chelates to enhance image contrast can be exploited further to observe specific cells, through modification of the chelate. A ligand that selectively binds to receptors on cells of interest may be covalently bound to the Gd(III) chelate, enhancing image contrast in the vicinity of the target cell/receptor (Figure 1A and Stasiuk et al45). In addition, Gd(III) chelates are able to label stem cells with the use of a transfection agent and have been used in research for several years,46 and more recently, Gd(III) ions have been used in nanoparticles to provide an even greater contrast enhancement.34, 47
Where Gd(III) is considered as a paramagnet, superparamagnets have a larger susceptibility to magnetization48 and so are excellent candidates for contrast enhancement in MR images.49 The most commonly used superparamagnets are superparamagnetic iron oxide nanoparticles (SPIONs).49 In addition to their ability to enhance T2 relaxation time of water, SPIONs have the benefit of being largely biologically compatible50 (they are safely removed by existing metabolic pathways in cells) and can be detected using light and electron microscopy.51, 52
Currently, many commercially available SPIONs are used in conjunction with a transfection agent, which may be toxic at certain levels either through cell death or prevention of cell division.53 As a result, there has been a drive over the last decade to produce alternative coatings that facilitate the administration of SPIONs to cells without the use of a transfection agent.49 Production of alternative coatings has also been driven by a more familiar magnetic trait of SPIONs: they have a tendency to clump together. Coating SPIONs with dextran or dextran derivatives is one method used to overcome this.55 (Other surfactant-like coatings for SPIONs include glycosaminoglycans, starch, polyethyleneglycol, siloxane, and poly-lactic acid.56).
Superparamagnetic iron platinum particles are precursors to iron-platinum-based nanoparticles currently synthesized for use in magnetic storage devices. These precursors exhibit magnetic moments superior to those of SPIONs, suggesting that they may form the basis of excellent contrast agents.57 Superparamagnetic iron platinum particles have yet to be used to label stem cells in stroke treatment, and have yet to undergo extensive toxicity studies, but have been used successfully in targeting prostate cancer cells with up to 13 times the specificity of commercially available SPIONs.58, 59
In addition to the development of more sensitive contrast agents and increased magnetic field strengths, increased resolution is being achieved through various means. Improving the system machinery by, for example, increasing the number of coil receiver channels or the magnetic field strength can increase signal strength.60 Longer acquisition times also increase resolution, although this may be hampered by the susceptibility of patients to claustrophobia, or the length of time tolerated by an animal in the machine.61 Should cost, time available for image acquisition, or movement of a subject be prohibitive, image-processing techniques can be applied postscanning. These usually rely on algorithmically combining several independent acquisitions of the same subject.
Magnetic resonance imaging is not without limitations: contrast agents can remain within injured tissue beyond the life span of the therapeutic cells, continuing to produce detectable signals and thus making discrimination between live and dead cells impossible.62, 63, 64 The use of indirect cell labelling methods (mentioned in the following section in conjunction with PET/SPECT) avoids this problem by requiring that a contrast agent be injected immediately before imaging. Where cells are directly labelled before administration, the metabolism/clearance of the contrast agent from tissue should be accounted for by assessing the retention of a contrast agent by transplanted viable, versus dead cells.65 Contrast agents may also be diluted due to cell division, especially when cells are rapidly dividing. However, novel transfection agents and indirect labelling methods (see below, PET and SPECT) aim to overcome the limitations of MRI stem cell tracking in stroke and other neurological disorders.
Positron emission tomography and SPECT: imaging with radioactive tracers
Both PET and SPECT require a radiotracer (a compound containing a radioactive isotope) to label the desired cell type in order for cells of interest to be detected by a scanner. In PET imaging, the tracer contains a radioactive isotope that emits positrons (the antiparticles of electrons), which cause emission of two or more divergent gamma rays (high frequency photons) on annihilation with surrounding electrons as little as 1 mm away (Figure 1Band Khalil et al66). These gamma rays are detected simultaneously by two opposing gamma cameras in a PET scanner as an indirect, high-resolution measure of the extent of cell localization or functional activity within a subject. Since PET images are created only where tracer is detected, scanning is often performed in conjunction with computed tomography (CT), which provides structural orientation for the PET images.
The key advantage of PET imaging over SPECT is increased sensitivity (by approximately two or three orders of magnitude). This is because SPECT imaging utilizes tracers that produce gamma rays directly rather than as a result of positron-electron annihilation (Figure 1C), which are detected by just a single gamma camera (although multiheaded cameras exist for accelerated acquisition of images). In both techniques, cameras rotate about a subject, recording gamma intensity every 3 to 6 degrees over each 360-degree rotation. Through this method, sequential tomographic 2D images are acquired through an axis of a subject, and constructed into a 3D interpretation of the recorded radioactivity. If radiolabelled stem cells are administered after stroke, these 3D acquisitions indicate the locations in which the tracer is most concentrated, and hence areas to which most cells have migrated. While physical aspects such as positron range and photon noncollinearity (the tendency of photons to deviate from annihilation at 180 degrees) limit PET resolution, SPECT resolution can be increased by improvements to equipment (for example, the beam-narrowing collimator).67 Technological advances are therefore being made to enhance image resolution in SPECT, such as pinhole SPECT and μSPECT systems (containing many pinholes), which are able to produce a spatial resolution of less than 1 mm,68, 69 and offer the possibility of small animal SPECT imaging.
To study stem cell migration in stroke using PET/SPECT, cells may be labelled directly or indirectly with a radiotracer. Direct labelling is the most common: a tracer is retained within a lipophilic chelator (an organic structure or ‘cage') that is able to enter cells of interest when applied ex vivo (Figure 1B). Once cells have been labelled, they can be administered to a subject. Labelling ex vivo in such a way does not distinguish between healthy and dying cells, as a radioisotope will decay (and therefore be detected) irrespective of the cellular environment. In addition, imaging over a long period of time means that decaying of the radioisotope may underrepresent the concentration of cells in a given area, or falsely indicate that the cells of interest are undergoing division. These two occurrences would be indistinguishable from each other: an isotope will inevitably produce a weakening signal relative to the volume of cells present over time, but the amount of tracer in each cell is divided after each cell division, and therefore also causes a weakened signal. Since it has been shown that both the number of cells administered and the timing of transplantation affect survival of transplanted cells,70 and some studies are focused entirely on improving cell survival (with the use of scaffolds/matrices for example, Zhong et al71), a means of discriminating between live and dead cells long term is highly desirable.
A better test of viability and cell concentration is through use of indirect labelling methods. These generally involve the genetic manipulation of cells ex vivo in order that they are able to produce an abundance of a particular protein involved in the uptake or accumulation of a tracer—a reporter protein.72, 73 The tracer itself is then administered a defined period of time before imaging. The inclusion of genes in such a way means that only living cells will be identified, as unviable cells will no longer undergo protein synthesis and therefore be unable to generate the essential machinery for tracer uptake. Unlike direct labelling of cells, gene transfer methods are also able to indicate expansion of transplanted cells—there is no dilution of a tracer as cells divide, because a parent cell will supply each daughter cell with the same genetic material it uses to aid tracer uptake.
While possible, isolating and genetically manipulating cells, to label them indirectly, is considerably laborious if the technique is to be viable for cell tracking in the clinic. The use of PET for long-term imaging of stem cells in humans would be highly valuable, however, and searches for positron-emitting radioisotopes that can compete with the longer half lives of SPECT radiotracers are underway. Zirconium-based tracer, 89Zr-oxinate4, for example, has recently been detected in cells up to 14 days after labelling and administration.74 These properties are comparable to Indium-111 (111In)-oxine, the gold-standard radiotracer for SPECT, which has been successfully used to monitor the migration and localization of CD34+ hematopoietic progenitor cells in rat models of myocardial infarction75 and stroke.76 In work by Brenner et al77 and elsewhere, however, 111In-oxine has been shown to affect the cellular integrity of hematopoietic progenitor cells. Reduced viability and proliferative capability reduce the potential for translation of this particular radiotracer, since the primary goal of therapeutic stem cells is that they are effective and not that they are of definitive whereabouts. 89Zr-oxinate4 has also been shown to reduce cell viability by about 20% during the labelling process, although no further reduction is seen 24 hours later. It is evident that the use of a radioisotope is highly likely to affect cell function and/or toxicity. Identifying the maximum safe dose of a radiotracer is therefore crucial when developing modalities requiring a radioisotope for the clinic; for example, 18F-fluoro-2-deoxy-D-glucose may used to label cells safely at concentrations up to 25 Bq/cell without compromising cellular function.78
Optical imaging
Optical imaging is used to improve the understanding of structural and functional components of stem cell therapy in preclinical stroke. Several fluorescent probes exist that are able to produce beautifully detailed images of stem cells within any tissue ex vivo. Recently, the use of fluorescent nano-diamonds (among other advanced probes) has even begun to overcome stalwart limitations of fluorescence imaging such as toxicity and background autofluorescence.79, 80
The density of brain tissue, however, has always been an obstacle with individual cell tracking in fluorescence-based imaging techniques in stroke. As a result, the use of traditional fluorescent markers such as green fluorescent protein to label cells before administration after stroke is severely limited by the short wavelengths of such probes, and subsequent poor tissue penetration. To detect cells using fluorescence alongside current in vivo imaging systems such as IVIS®, probes in the near infrared (NIR) spectrum may be used. These penetrate tissue well and allow tracking of labelled cells, particularly in small rodents. Lipophilic NIR dyes have been shown to be useful in identifying the location of large numbers of stem cells after administration, even in deep tissue such as the brain in a 6-hydroxydopamine-induced rat model of Parkinson's disease.81
Low tissue penetration of fluorescent labels (including NIR relative to other imaging modalities) makes optical imaging unfeasible for clinical use. Yet vastly improved acquisition time versus MRI and PET/SPECT, quick and simple cell-labelling protocols, and dyes that are long-lasting (1 month or more) with low toxicity and very good resolution (in particular with quantum dot-based fluorescent probes 82) form the basis of an informative, relatively cheap preclinical assessment of cell migration in vivo. The NIR-emitting quantum dots have been used successfully to visualize bone marrow stromal cells in traumatic brain injury in rats83 as well as in a focal model of stroke84 (the latter study observing NIR up to 8 weeks after stroke), indicating that NIR provides an excellent means for preclinical, long-term study of cell migration.
Bioluminescence imaging bares similar advantages and disadvantages to fluorescence imaging (it is quick and relatively cheap, but severely limited when imaging deeper tissue). Its use is also therefore confined to preclinical studies. Bioluminescence is light generated by intrinsic properties of certain bacteria, fireflies (Photinus pyralis) and the North American click beetles (Pyrophorus divergens) in nature.85, 86 These properties, involving chemiluminescent enzymatic (luciferase) reactions, have been applied to stem cells through genetic manipulation before cell administration (Figure 1D). While the light cannot be seen from inside an animal under normal conditions, light from both fluorescent probes and bioluminescence is detected by charged coupled device cameras cooled to around −150°C, rendering them highly light sensitive. Like fluorescence imaging, bioluminescence can be used to study cells long term: neural progenitor cells have been observed 21 days after stroke in both rat87 and mouse.88
Ultrasound is not currently widely used in cell tracking strategies due to very low resolution in the unmodified technique. High-resolution images, however, have been achieved after the inclusion of gold nanoparticles, which alter the optical properties of cells that would normally be indistinguishable through ultrasound.89 Interesting also is that—although not used in an observational capacity—ultrasound in conjunction with microbubbles has been used to enhance the delivery of therapeutic cells to injury sites.90
Multimodal imaging
As described above and in Table 1, imaging modalities have varied limits with respect to contrast/functional/spatial resolution, background interference, sensitivity, and specificity. Since no single technique is excellent through all these criteria, current imaging strategies make use of multiple modalities simultaneously, where facilities exist. Multimodal imaging integrates two or more techniques, exploiting the strengths and thereby overcoming limitations of each, and has been increasing in use rapidly over the last decade. Positron emission tomography/CT has been standard practice in the clinic for over a decade91 and is an excellent example of how imaging techniques can be combined effectively. The CT produces anatomic images that, along with MR images, are unparalleled in revealing structural detail. Positron emission tomography, and to a lesser extent SPECT, is highly indicative of functional activity. Overlaying simultaneously acquired PET and CT scans therefore enables the integration of structural and functional information, clarifying results, and reducing the likelihood of misinterpretation of data or misdiagnosis in the clinic. More recently, studies exploit the advantages of other techniques, combining MRI with SPECT imaging92 and PET with bioluminescence,93 among other examples. The huge advantage of these combinatorial approaches is rapidly becoming apparent to institutions, which are investing in imaging equipment with the capacity to execute more than one imaging modality simultaneously. The PET/MRI imaging has received particular attention for its potential in neuroimaging. While this is currently an expensive technique requiring specialized equipment (of which manufacturers suggest, ‘if you build it, they will come'.94), PET/MRI imaging was deemed feasible by Schlemmer et al,95 and provides a superb opportunity to combine anatomic, physiologic, functional, and metabolic information.96
Multimodal systems that have emerged using PET, SPECT, MRI, CT, and optical techniques in various combinations often require cells, which have been genetically manipulated to express a protein that has a binding site for not one, but two to three probes, each catering to a different modality—a double/triple-fusion protein. For example, using this approach, a protein has generated containing regions that report green or red fluorescent protein, luciferase and the HSV1-TK enzyme, enabling cells to be observed fluorescence, bioluminescence, and PET or SPECT.97
Translating cell tracking technologies to the clinic
In 2011, academic and industry leaders in stroke research gathered to consolidate the most recent advances and emerging data relating to ‘Stem Cells as an Emerging Paradigm in Stroke (STEPS3)'. In particular, barriers to successful translation of cell therapy from animal models to the clinic were discussed, and the need for excellent imaging tools highlighted as follows:
‘Use of imaging in clinical trials is strongly encouraged to provide as much information as possible to assess vascular/structural lesions, infarct size, cell viability, location, the success and safety of implantation, and inflammation. Imaging should also be used to monitor safety and recovery and, when possible, to investigate mechanisms of action and provide information on surrogate markers of treatment effect. Imaging measures might also be useful to help stratify patients at baseline.'98
Use of state of the art imaging in the translation of cell therapy is therefore extremely valuable. The use of small rodent models of stroke is highly useful in testing therapeutic strategies in a reproducible, relatively high-throughput mammalian model. Despite this, the small, lissencephalic rodent brain and cerebrovasculature are considerably different from those of a human, and their use in assessing the prospective efficacy of imaging technologies in the clinic is severely limited by the differences in machinery (for example: smaller aperture, higher resolution). Large animal stroke models are laborious and often complicated and expensive, but to some extent they bridge the gap between rodent models and humans. Canine,99 sheep,100 pig,101 and nonhuman primate102, 103 models therefore enable the viability of imaging modalities to be tested in a larger, more structurally complex brain, in larger, clinical scale (lower resolution) scanners, and as such their translational potential.
Testing imaging modalities in large as well as in small animal models will also enable the techniques to be used to assess the safety30 and efficacy of treatments in humans,104 targeted cell delivery,29 and even cell migration directly.105 Sequential imaging in particular may enable the detection of aberrant cell migration and/or proliferation.106, 107
Despite their advantages (cheap, high-throughput), optical/bioluminescent imaging systems are not suitable for translation into large animal models or the clinic, as they are severely limited by the low penetration of light through tissue of substantial thickness. Since they are not hampered by tissue depth, PET, SPECT, and MRI have significant potential for use in the clinic, and studies specifically investigating the translation of these modalities (radiotracer dose expansion108, for example) are underway. There is a clear trajectory toward the use of μPET and μSPECT systems in particular, where resolution of <0.5 mm can be achieved in rodent models, into large animal models and the clinic.68, 69
What should your lab be using?
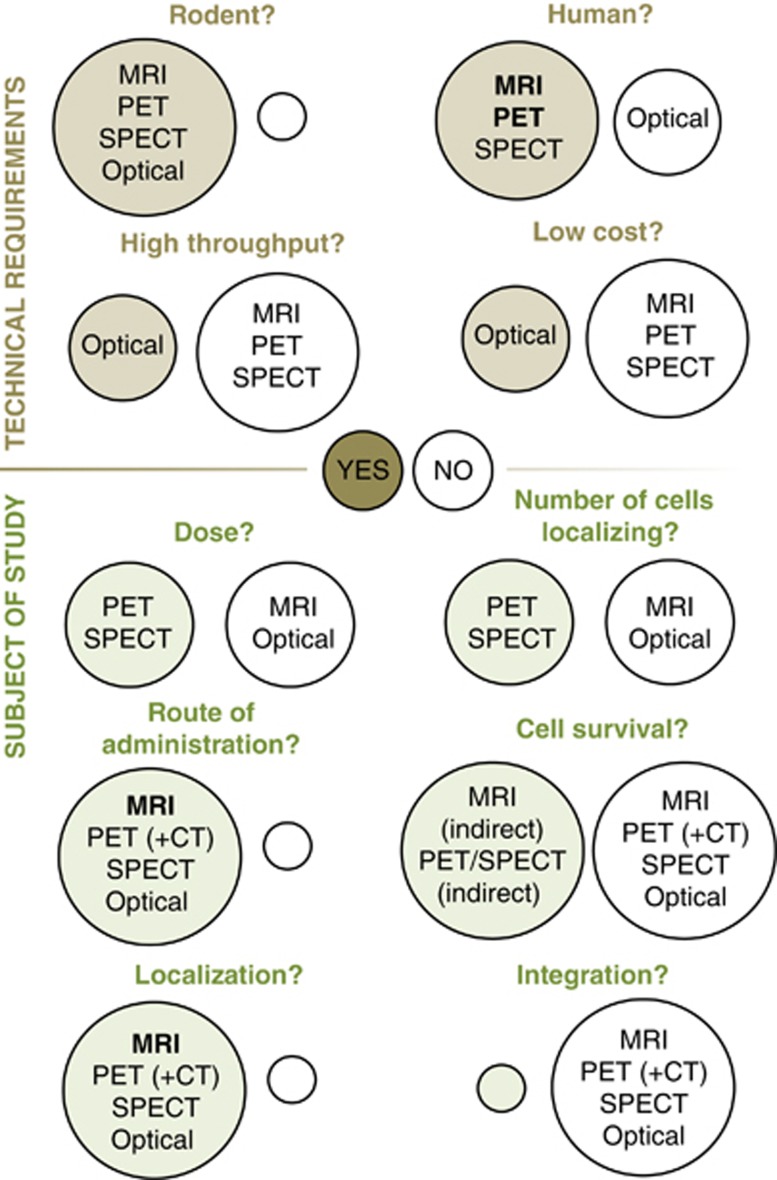
This is a complex question, as it dependent on a number of different factors. Table 1 summarizes the principles, requirements/facilities, advantages, and disadvantages (including cost, resolution, and sensitivity), and other aspects of imaging modalities covered in this review (see Figure 2 for comparison of images and Figure 3 for an ‘at-a-glance' guide to modality selection). Selection of imaging tools should be based on an assessment of the requirements of the study: are low cell numbers being used/is high sensitivity needed? Is morphological data/high spatial resolution needed? Is long-term/repeated imaging planned? Is the goal translation? Many imaging modalities are credited for their ability to be translated into the clinic—and rightly so. It should also be considered, however, that imaging protocols that are potentially detrimental to humans should not be overlooked, since they may be of use in preclinical studies: general priorities include keeping costs down in the laboratory and keeping safety high in the clinic; for example, while safety concerns with repeated doses of radiotracers may outweigh the advantages of long-term cell tracking in patients, the technique is extremely useful in the laboratory. Conversely, cost constraints in the laboratory may indicate the preferred use of optical imaging despite the low resolution in deeper tissues.
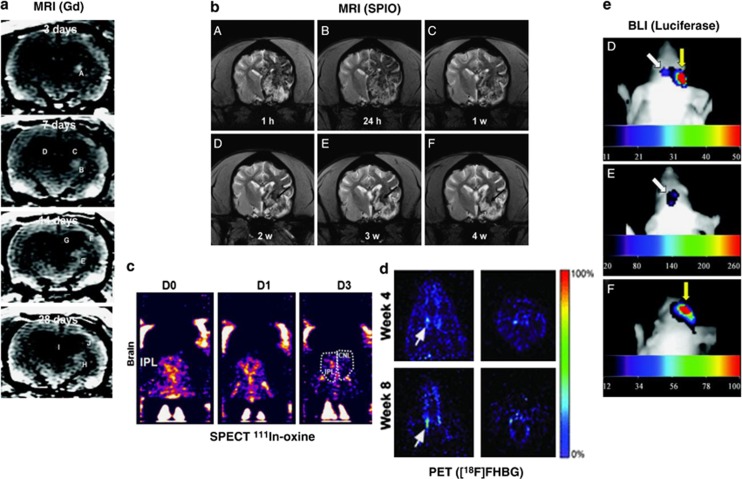
Figure 2.
Comparison of imaging techniques for transplanted therapeutic stem cells following preclinical middle cerebral artery occlusion. (A) Monitoring of Gadolinium (Gd)-labelled mesenchymal stem cells (MSCs) in rat brain using magnetic resonance imaging (MRI). White patch (arrows) shows migration of human MSCs toward the peri-infarct cortical area over 14 days.46 (B) Superparamagnetic iron oxide nanoparticle (SPIO)-labelled MSCs (area of hypointensity) viewed using magnetic resonance imaging (MRI), 1 hour to 4 weeks after transplantation into beagles.115 (C) Indium (111In)-oxine-labelled umbilical tissue-derived cells can be seen accumulating in the ipsilateral cortex (IPL. CNL=contralateral) 1 to 3 days after transplantation using single photo emission computed tomography (SPECT).76 (D) 9-(4-[18F]fluoro-3 hydroxymethylbutyl) guanine ([18F]FHBG)-labelled embryonic stem cell-derived neural stem cells (NSCs) viewed through positron emission tomography (PET) can be seen localizing in the striatal region of the forebrain.107 (E) Luciferase photon emission detected through bioluminescence imaging (BLI) 21 days after transplanting of neural progenitor cells (NPCs).88
Figure 3.
‘At-a-glance' guide to imaging modality selection. Brown/green circles=‘yes' (i.e., this modality is a viable option), white circles=‘no'. CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single photo emission computed tomography.
The majority of modalities currently fall short of being able to record true integration of transplanted cells into existing networks, and understanding this thoroughly—what cells differentiate into (if anything) and what they release—will be of great benefit. The resolution required for this is not inconceivable: while it has yet to integrate stem cell imaging, functional MRI offers the opportunity to observe changes in brain connectivity (structural and functional modifications) in astonishing detail after stroke.109, 110 In addition, the use of microoptical probes with in vivo multiphoton microscopy has now enabled the technique to be used to observe the processes of individual cells.111
Monitoring stem cell migration with high resolution will be immensely useful in refining cell therapies. So little is currently known about cells posttransplantation; whether cells end up in the brain may ultimately be of little significance to their ability to improve outcome in stroke (although their being dispersed in unknown regions of the recipient would certainly complicate imaging due to, for example, positioning of the coil in MRI). Indeed, while many studies show stem cell homing to the brain after stroke,19, 112 it has been shown that transplanted cells still improve recovery in rats treated with bone marrow stem cells after middle cerebral artery occlusion, despite the majority of these cells ending up in peripheral organs (including the lungs, spleen, and liver).113 Furthermore, where one route of administration is preferable to another for stem cells from a particular source, imaging will—and does—help identify this.114 Wherever therapeutic cells localize, tracking them in vivo will improve our knowledge of how these cells are of benefit. Understanding direct or indirect effects of these cells, whether they differentiate in the brain, resolves inflammation or releases growth factors; whether beneficial cells must migrate to an infarct or are able to act remotely is crucial in the refinement of a highly promising stroke therapy, and imaging promises to have an important role in this.
The authors declare no conflict of interest.
Footnotes
This work was funded by the Louisiana State University-Health Sciences Cardiovascular Center and the Louisiana State University-Health Sciences Center Malcolm Feist Cardiovascular Fellowship scheme.
References
- 1Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ et al. Heart disease and stroke statistics—2014 update: a report from the american heart association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Lyden P, Shuaib A, Ng K, Levin K, Atkinson RP, Rajput A et al. Clomethiazole acute stroke study in ischemic stroke (class-i): Final results. Stroke 2002; 33: 122–128. [DOI] [PubMed] [Google Scholar]
- 3Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive n-methyl-d-aspartate antagonist selfotel (cgs 19755) in the treatment of severe head injury: results of two phase iii clinical trials. The selfotel investigators. J Neurosurg 1999; 91: 737–743. [DOI] [PubMed] [Google Scholar]
- 4Enlimomab Acute Stroke Trial I. Use of anti-icam-1 therapy in ischemic stroke: results of the enlimomab acute stroke trial. Neurology 2001; 57: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 5Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (sportif iii): Randomised controlled trial. Lancet 2003; 362: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 6Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol 2014; 115: 92–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 2003; 92: 692–699. [DOI] [PubMed] [Google Scholar]
- 8Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev 2005; 14: 595–604. [DOI] [PubMed] [Google Scholar]
- 9Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 2013; 22: 977–991. [DOI] [PubMed] [Google Scholar]
- 10Shen CC, Lin CH, Yang YC, Chiao MT, Cheng WY, Ko JL. Intravenous implanted neural stem cells migrate to injury site, reduce infarct volume, and improve behavior after cerebral ischemia. Curr Neurovasc Res 2010; 7: 167–179. [DOI] [PubMed] [Google Scholar]
- 11Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of il-10 and tnf-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol 2009; 6: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by tgf-beta. Neurobiol Dis 2013; 58: 249–257. [DOI] [PubMed] [Google Scholar]
- 13Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 2011; 29: 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 2002; 59: 514–523. [DOI] [PubMed] [Google Scholar]
- 15Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 2013; 136: 3561–3577. [DOI] [PubMed] [Google Scholar]
- 16Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004; 35: 2390–2395. [DOI] [PubMed] [Google Scholar]
- 17Mora-Lee S, Sirerol-Piquer MS, Gutierrez-Perez M, Gomez-Pinedo U, Roobrouck VD, Lopez T et al. Therapeutic effects of hmapc and hmsc transplantation after stroke in mice. PLoS ONE 2012; 7: e43683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant 2012; 21: 1199–1211. [DOI] [PubMed] [Google Scholar]
- 19Hermann DM, Peruzzotti-Jametti L, Schlechter J, Bernstock JD, Doeppner TR, Pluchino S. Neural precursor cells in the ischemic brain - integration, cellular crosstalk, and consequences for stroke recovery. Front Cell Neurosci 2014; 8: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Aswendt M, Adamczak J, Tennstaedt A. A review of novel optical imaging strategies of the stroke pathology and stem cell therapy in stroke. Front Cell Neurosci 2014; 8: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Adamczak J, Hoehn M. In vivo imaging of cell transplants in experimental ischemia. Prog Brain Res 2012; 201: 55–78. [DOI] [PubMed] [Google Scholar]
- 22Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 1997; 94: 4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Garton HJL, Schoenwolf GC. Improving the efficacy of fluorescent labeling for histological tracking of cells in early mammalian and avian embryos. Anat Record 1996; 244: 112–117. [DOI] [PubMed] [Google Scholar]
- 24Close DM, Xu T, Sayler GS, Ripp S. In vivo bioluminescent imaging (BLI): Noninvasive visualization and interrogation of biological processes in living animals. Sensors 2011; 11: 180–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Gholamrezanezhad A, Mirpour S, Ardekani JM, Bagheri M, Alimoghadam K, Yarmand S et al. Cytotoxicity of 111in-oxine on mesenchymal stem cells: a time-dependent adverse effect. Nucl Med Commun 2009; 30: 210–216. [DOI] [PubMed] [Google Scholar]
- 26Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc Chem Res 2009; 42: 822–831. [DOI] [PubMed] [Google Scholar]
- 27Chang DJ, Oh SH, Lee N, Choi C, Jeon I, Kim HS et al. Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (enstem-a) migrate and improve brain functions in stroke-damaged rats. Exp Mol Med 2013; 45: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. NeuroImage 2004; 21: 311–317. [DOI] [PubMed] [Google Scholar]
- 29Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008; 39: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab 2013; 33: 921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011; 13: 675–685. [DOI] [PubMed] [Google Scholar]
- 32Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K et al. Magnetic field and tissue dependencies of human brain longitudinal 1h2o relaxation in vivo. Magn Reson Med 2007; 57: 308–318. [DOI] [PubMed] [Google Scholar]
- 33Denic A, Macura SI, Mishra P, Gamez JD, Rodriguez M, Pirko I. MRI in rodent models of brain disorders. Neurotherapeutics 2011; 8: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Berman SMC, Walczak P, Bulte JWM. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011; 3: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Dousset V, Tourdias T, Brochet B, Boiziau C, Petry KG. How to trace stem cells for MRI evaluation? J Neurol Sci 2008; 265: 122–126. [DOI] [PubMed] [Google Scholar]
- 36Dousset V, Brochet B, Deloire MSA, Lagoarde L, Barroso B, Caille JM et al. Mr imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. Am J Neuroradiol 2006; 27: 1000–1005. [PMC free article] [PubMed] [Google Scholar]
- 37Petry KG, Boiziau C, Dousset V, Brochet B. Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics 2007; 4: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Giraud M, Cho TH, Nighoghossian N, Maucort-Boulch D, Deiana G, Ostergaard L et al. Early blood brain barrier changes in acute ischemic stroke: a sequential MRI study. J Neuroimaging advance online publication, 20 February 2015; doi:10.1111/jon.12225 [e-pub ahead of print]. [DOI] [PubMed]
- 39Donahue MJ, Strother MK, Hendrikse J. Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke 2012; 43: 903–915. [DOI] [PubMed] [Google Scholar]
- 40Ramos-Cabrer P, Hoehn M. MRI stem cell tracking for therapy in experimental cerebral ischemia. Transl Stroke Res 2012; 3: 22–35. [DOI] [PubMed] [Google Scholar]
- 41Bezerra FJ, Brown M, Alarcon W, Karki K, Knight RA, Keenan KA et al. Rate and extent of leakage of a magnetic resonance contrast agent tend to be lower under isoflurane anesthesia in comparison to halothane in a rat model of embolic stroke. Neurol Res 2014; 36: 847–850. [DOI] [PubMed] [Google Scholar]
- 42Krishna MC, Devasahayam N, Cook JA, Subramanian S, Kuppusamy P, Mitchell JB. Electron paramagnetic resonance for small animal imaging applications. ILAR J 2001; 42: 209–218. [DOI] [PubMed] [Google Scholar]
- 43Penfield JG, Reilly RF. What nephrologists need to know about gadolinium. Nat Clin Pract Nephr 2007; 3: 654–668. [DOI] [PubMed] [Google Scholar]
- 44Wastie ML, Latief KH. Gadolinium: Named after finland's most famous chemist. Br J Radiol 2004; 77: 146–147. [DOI] [PubMed] [Google Scholar]
- 45Stasiuk GJ, Smith H, Wylezinska-Arridge M, Tremoleda JL, Trigg W, Luthra SK et al. Gd3+ cflflfk conjugate for MRI: a targeted contrast agent for fpr1 in inflammation. Chem Commun 2013; 49: 564–566. [DOI] [PubMed] [Google Scholar]
- 46Shyu WC, Chen CP, Lin SZ, Lee YJ, Li H. Efficient tracking of non-iron-labeled mesenchymal stem cells with serial MRI in chronic stroke rats. Stroke 2007; 38: 367–374. [DOI] [PubMed] [Google Scholar]
- 47Shen YY, Shao YZ, He HQ, Tan YP, Tian XM, Xie FK et al. Gadolinium(3+)-doped mesoporous silica nanoparticles as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo. Int J Nanomed 2013; 8: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Gillis P, Roch A, Brooks RA. Corrected equations for susceptibility-induced t2-shortening. J Magn Reson 1999; 137: 402–407. [DOI] [PubMed] [Google Scholar]
- 49Li L, Jiang W, Luo K, Song HM, Lan F, Wu Y et al. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013; 3: 595–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Li L, Jiang LL, Zeng Y, Liu G. Toxicity of superparamagnetic iron oxide nanoparticles: research strategies and implications for nanomedicine. Chinese Phys B 2013; 22: 127503; doi:10.1088/1674-1056/22/12/127503. [Google Scholar]
- 51Chen CCV, Ku MC, Jayaseema DM, Lai JS, Hueng DY, Chang C. Simple spion incubation as an efficient intracellular labeling method for tracking neural progenitor cells using MRI. PloS One 2013; 8: e56125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Alvarim LT, Nucci LP, Mamani JB, Marti LC, Aguiar MF, Silva HR et al. Therapeutics with spion-labeled stem cells for the main diseases related to brain aging: a systematic review. Int J Nanomed 2014; 9: 3749–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (spion). Nano Rev 2010; 1: 5358–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Ward RJ, Wilmet S, Legssyer R, Crichton RR. The influence of iron homoeostasis on macrophage function. Biochem Soc Trans 2002; 30: 762–765. [DOI] [PubMed] [Google Scholar]
- 55Hong RY, Feng B, Chen LL, Liu GH, Li HZ, Zheng Y et al. Synthesis, characterization and MRI application of dextran-coated fe3o4 magnetic nanoparticles. Biochem Eng J 2008; 42: 290–300. [Google Scholar]
- 56Laurent S, Mahmoudi M. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Int J Mol Epidemiol Genet 2011; 2: 367–390. [PMC free article] [PubMed] [Google Scholar]
- 57Taylor RM, Huber DL, Monson TC, Esch V, Sillerud LO. Structural and magnetic characterization of superparamagnetic iron platinum nanoparticle contrast agents for magnetic resonance imaging. J Vac Sci Technol B Nanotechnol Microelectron 2012; 30: 2C101–102C1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58Taylor RM, Sillerud LO. Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a psma-dependent manner. Int J Nanomed 2012; 7: 4341–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Taylor RM, Huber DL, Monson TC, Ali AMS, Bisoffi M, Sillerud LO. Multifunctional iron platinum stealth immunomicelles: targeted detection of human prostate cancer cells using both fluorescence and magnetic resonance imaging. J Nanopart Res 2011; 13: 4717–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Van Reeth E, Tham IWK, Tan CH, Poh CL. Super-resolution in magnetic resonance imaging: a review. Concept Magn Reson A 2012; 40A: 306–325. [Google Scholar]
- 61Enders J, Zimmermann E, Rief M, Martus P, Klingebiel R, Asbach P et al. Reduction of claustrophobia during magnetic resonance imaging: methods and design of the "claustro" randomized controlled trial. BMC Med Imaging 2011; 11: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011; 3: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Rice HE, Hsu EW, Sheng H, Evenson DA, Freemerman AJ, Safford KM et al. Superparamagnetic iron oxide labeling and transplantation of adipose-derived stem cells in middle cerebral artery occlusion-injured mice. AJR Am J Roentgenol 2007; 188: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 64Lee ES, Chan J, Shuter B, Tan LG, Chong MS, Ramachandra DL et al. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells 2009; 27: 1921–1931. [DOI] [PubMed] [Google Scholar]
- 65Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci USA 2007; 104: 10211–10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Khalil MM, Tremoleda JL, Bayomy TB, Gsell W. Molecular SPECT imaging: an overview. Int J Mol Imaging 2011; 2011: 796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Rahmim A, Zaidi H. Pet versus spect: strengths, limitations and challenges. Nucl Med Commun 2008; 29: 193–207. [DOI] [PubMed] [Google Scholar]
- 68van der Have F, Vastenhouw B, Ramakers RM, Branderhorst W, Krah JO, Ji C et al. U-SPECT-II: an ultra-high-resolution device for molecular small-animal imaging. J Nucl Med 2009; 50: 599–605. [DOI] [PubMed] [Google Scholar]
- 69Beekman FJ, van der Have F, Vastenhouw B, van der Linden AJ, van Rijk PP, Burbach JP et al. U-SPECT-I: a novel system for submillimeter-resolution tomography with radiolabeled molecules in mice. J Nucl Med 2005; 46: 1194–1200. [PubMed] [Google Scholar]
- 70Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab 2011; 31: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair 2010; 24: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol 2011; 8: 677–688. [DOI] [PubMed] [Google Scholar]
- 73Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK. Intravascular cell replacement therapy for stroke. Neurosurg Focus 2008; 24: E15. [DOI] [PubMed] [Google Scholar]
- 74Charoenphun P, Meszaros LK, Chuamsaamarkkee K, Sharif-Paghaleh E, Ballinger JR, Ferris TJ et al. [zr]oxinate for long-term in vivo cell tracking by positron emission tomography. Eur J Nucl Med Mol Imaging 2014; 42: 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U et al. 111in-labeled cd34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med 2004; 45: 512–518. [PubMed] [Google Scholar]
- 76Arbab AS, Thiffault C, Navia B, Victor SJ, Hong K, Zhang L et al. Tracking of in-111-labeled human umbilical tissue-derived cells (hutc) in a rat model of cerebral ischemia using spect imaging. BMC Med Imaging 2012; 12: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Nowak B, Weber C, Schober A, Zeiffer U, Liehn EA, von Hundelshausen P et al. Indium-111 oxine labelling affects the cellular integrity of haematopoietic progenitor cells. Eur J Nucl Med Mol Imaging 2007; 34: 715–721. [DOI] [PubMed] [Google Scholar]
- 78Elhami E, Goertzen AL, Xiang B, Deng J, Stillwell C, Mzengeza S et al. Viability and proliferation potential of adipose-derived stem cells following labeling with a positron-emitting radiotracer. Eur J Nucl Med Mol Imaging 2011; 38: 1323–1334. [DOI] [PubMed] [Google Scholar]
- 79Wu TJ, Tzeng YK, Chang WW, Cheng CA, Kuo Y, Chien CH et al. Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat Nanotechnol 2013; 8: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80Hsu TC, Liu KK, Chang HC, Hwang E, Chao JI. Labeling of neuronal differentiation and neuron cells with biocompatible fluorescent nanodiamonds. Sci Rep-Uk 2014; 4: 5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81Armentero MT, Bossolasco P, Cova L. Labeling and tracking of human mesenchymal stem cells using near-infrared technology. Methods Mol Biol 2013; 1052: 13–28. [DOI] [PubMed] [Google Scholar]
- 82Chen GC, Tian F, Zhang Y, Zhang YJ, Li CY, Wang QB. Tracking of transplanted human mesenchymal stem cells in living mice using near-infrared ag-2s quantum dots. Adv Funct Mater 2014; 24: 2481–2488. [Google Scholar]
- 83Osanai T, Kuroda S, Sugiyama T, Kawabori M, Ito M, Shichinohe H et al. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats—in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery 2012; 70: 435–444, discussion 444. [DOI] [PubMed] [Google Scholar]
- 84Sugiyama T, Kuroda S, Osanai T, Shichinohe H, Kuge Y, Ito M et al. Near-infrared fluorescence labeling allows noninvasive tracking of bone marrow stromal cells transplanted into rat infarct brain. Neurosurgery 2011; 68: 1036–1047. [DOI] [PubMed] [Google Scholar]
- 85Baldwin TO, Nicoli MZ, Becvar JE, Hastings JW. Bacterial luciferase. Binding of oxidized flavin mononucleotide. J Biol Chem 1975; 250: 2763–2768. [PubMed] [Google Scholar]
- 86Miloud T, Henrich C, Hammerling GJ. Quantitative comparison of click beetle and firefly luciferases for in vivo bioluminescence imaging. J Biomed Opt 2007; 12: 054018. [DOI] [PubMed] [Google Scholar]
- 87Bernau K, Lewis CM, Petelinsek AM, Benink HA, Zimprich CA, Meyerand ME et al. In vivo tracking of human neural progenitor cells in the rat brain using bioluminescence imaging. J Neurosci Methods 2014; 228: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke 2004; 35: 952–957. [DOI] [PubMed] [Google Scholar]
- 89Nam SY, Ricles LM, Suggs LJ, Emelianov SY. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS ONE 2012; 7: e37267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90Tong J, Ding J, Shen X, Chen L, Bian Y, Ma G et al. Mesenchymal stem cell transplantation enhancement in myocardial infarction rat model under ultrasound combined with nitric oxide microbubbles. PLoS ONE 2013; 8: e80186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91Townsend DW. Combined positron emission tomography-computed tomography: the historical perspective. Semin Ultrasound CT MR 2008; 29: 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92Hamamura MJ, Roeck WW, Ha S, Hugg J, Wagenaar DJ, Meier D et al. Simultaneous in vivo dynamic contrast-enhanced magnetic resonance and scintigraphic imaging. Phys Med Biol 2011; 56: N63–N69. [DOI] [PubMed] [Google Scholar]
- 93Wolfs E, Holvoet B, Gijsbers R, Casteels C, Roberts SJ, Struys T et al. Optimization of multimodal imaging of mesenchymal stem cells using the human sodium iodide symporter for pet and cerenkov luminescence imaging. PLoS ONE 2014; 9: e94833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Castelijns JA. Pet- MRI in the head and neck area: challenges and new directions. Eur Radiol 2011; 21: 2425–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95Schlemmer HPW, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R et al. Simultaneous mr/pet imaging of the human brain: feasibility study. Radiology 2008; 248: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 96Cho ZH, Son YD, Choi EJ, Kim HK, Kim JH, Lee SY et al. In-vivo human brain molecular imaging with a brain-dedicated pet/ MRI system. MAGME 2013; 26: 71–79. [DOI] [PubMed] [Google Scholar]
- 97Zhang HHsv1-tk/gfp/fluc. Molecular imaging and contrast agent database (MICAD). Bethesda, MD. 2004. [Google Scholar]
- 98Savitz SI, Cramer SC, Wechsler L, Aronowski J, Boltze J, Borlongan C et al. Stem cells as an emerging paradigm in stroke 3 enhancing the development of clinical trials. Stroke 2014; 45: 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99Spilberg G, Carniato SL, King RM, van der Bom IM, Mehra M, Walvick RP et al. Temporal evolution of susceptibility artifacts from coiled aneurysms on mr angiography: an in vivo canine study. AJNR Am J Neuroradiol 2012; 33: 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100Boltze J, Forschler A, Nitzsche B, Waldmin D, Hoffmann A, Boltze CM et al. Permanent middle cerebral artery occlusion in sheep: A novel large animal model of focal cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1951–1964. [DOI] [PubMed] [Google Scholar]
- 101Sakoh M, Rohl L, Gyldensted C, Gjedde A, Ostergaard L. Cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking after acute stroke in pigs: comparison with [(15)o]h(2)o positron emission tomography. Stroke 2000; 31: 1958–1964. [DOI] [PubMed] [Google Scholar]
- 102Zhang X, Tong F, Li CX, Yan Y, Kempf D, Nair G et al. Temporal evolution of ischemic lesions in nonhuman primates: a diffusion and perfusion MRI study. PLoS ONE 2015; 10: e0117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103Feng M, Zhu H, Zhu Z, Wei J, Lu S, Li Q et al. Serial 18f-fdg pet demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J Nucl Med 2011; 52: 90–97. [DOI] [PubMed] [Google Scholar]
- 104Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med 2011; 6: 45–52. [DOI] [PubMed] [Google Scholar]
- 105Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, Battistella V, Goldenberg RC, Kasai-Brunswick T et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp Neurol 2010; 221: 122–128. [DOI] [PubMed] [Google Scholar]
- 106Waerzeggers Y, Klein M, Miletic H, Himmelreich U, Li H, Monfared P et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol Imaging 2008; 7: 77–91. [PubMed] [Google Scholar]
- 107Daadi MM, Hu S, Klausner J, Li Z, Sofilos M, Sun G et al. Imaging neural stem cell graft-induced structural repair in stroke. Cell Transplant 2013; 22: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108Verschuer JD, Towson J, Eberl S, Katsifis A, Henderson D, Lam P et al. Radiation dosimetry of the translocator protein ligands [18f]pbr111 and [18f]pbr102. Nucl Med Biol 2012; 39: 742–753. [DOI] [PubMed] [Google Scholar]
- 109Westlake KP, Nagarajan SS. Functional connectivity in relation to motor performance and recovery after stroke. Front Syst Neurosci 2011; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110Li W, Li Y, Zhu W, Chen X. Changes in brain functional network connectivity after stroke. Neural Regen Res 2014; 9: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111Murray TA, Levene MJ. Singlet gradient index lens for deep in vivo multiphoton microscopy. J Biomed Opt 2012; 17: 021106. [DOI] [PubMed] [Google Scholar]
- 112van Velthoven CTJ, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res 2012; 71: 474–481. [DOI] [PubMed] [Google Scholar]
- 113Goldmacher GV, Nasser R, Lee DY, Yigit S, Rosenwasser R, Iacovitti L. Tracking transplanted bone marrow stem cells and their effects in the rat mcao stroke model. PLoS ONE 2013; 8: e60049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab 2010; 30: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW et al. In vivo mr imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS ONE 2013; 8: e54963. [DOI] [PMC free article] [PubMed] [Google Scholar]