Abstract
Background
3-Iodothyronamine (3-T1AM), a signaling molecule with structural similarities to thyroid hormones, induces numerous physiological responses including reversible body temperature decline. One target of 3-T1AM is the trace amine-associated receptor 1 (TAAR1), which is a member of the rhodopsin-like family of G protein-coupled receptors (GPCRs). Interestingly, the effects of 3-T1AM remain detectable in TAAR1 knockout mice, suggesting further targets for 3-T1AM such as adrenergic receptors. Therefore, we evaluated whether β-adrenergic receptor 1 (ADRB1) and 2 (ADRB2) signaling is affected by 3-T1AM in HEK293 cells and in human conjunctival epithelial cells (IOBA-NHC), where these receptors are highly expressed endogenously.
Methods
A label-free EPIC system for prescreening the 3-T1AM-induced effects on ADRB1 and ADRB2 in transfected HEK293 cells was used. In addition, ADRB1 and ADRB2 activation was analyzed using a cyclic AMP assay and a MAPK reporter gene assay. Finally, fluorescence Ca2+ imaging was utilized to delineate 3-T1AM-induced Ca2+ signaling.
Results
3-T1AM (10−5−10−10M) enhanced isoprenaline-induced ADRB2-mediated Gs signaling but not that of ADRB1-mediated signaling. MAPK signaling remained unaffected for both receptors. In IOBA-NHC cells, norepinephrine-induced Ca2+ influxes were blocked by the nonselective ADRB blocker timolol (10 µM), indicating that ADRBs are most likely linked with Ca2+ channels. Notably, timolol was also found to block 3-T1AM (10−5M)-induced Ca2+ influx.
Conclusions
The presented data support that 3-T1AM directly modulates β-adrenergic receptor signaling. The relationship between 3-T1AM and β-adrenergic signaling also reveals a potential therapeutic value for suppressing Ca2+ channel-mediated inflammation processes, occurring in eye diseases such as conjunctivitis.
Key Words: β-Adrenergic receptors, Thyronamine, Signaling, Calcium homeostasis, Calcium channel, Human conjunctiva
Introduction
3-Iodothyronamine (3-T1AM) is an amine with structural similarities to thyroid hormones [1]. 3-T1AM modifies several important physiological parameters in rodents (for reviews see Zucchi et al. [2] and Piehl et al. [3]).
In vitro, the receptor target of 3-T1AM was assigned to the trace amine-associated receptor 1 (TAAR1) [4]. Interaction of 3-T1AM with TAAR1 activates the Gs/adenylyl cyclase signaling pathways [4]. Paradoxically, several 3-T1AM-induced physiological effects still persist in mTaar1 knockout mice, suggesting further receptor targets in vivo. In this context, previous studies using turkey erythrocytes indirectly suggested β-adrenergic receptors as potential targets for thyronamines [3,5]. Furthermore, several findings indicate that 3-T1AM binds with high affinity to the locus coeruleus [6].
3-T1AM has been proposed to be a multitarget ligand (reviewed by Zucchi et al. [2]), interacting also with a member of nonselective Ca2+ entry channels such as the transient receptor potential melastatin channel 8 (TRPM8), which is also known as menthol receptor [7]. In the eye, functional expression of TRPM8 and other thermosensitive TRP isoforms such as the TRP vanilloid 1 channel (capsaicin receptor) have been identified in corneal neurons as well as in corneal endothelial cells [8,9]. Both are temperature-sensitive TRP channels occurring in corneal tissue layers and cells [10]. Interestingly, TRPV1, in connection with a putative modulation by thyronamines via G protein-coupled receptors (GPCRs), may be a potential drug target in reducing inflammatory symptoms in eye and conjunctiva diseases. It is known that TRPV1 can be stimulated during exposure to the same hypertonic conditions identified in tear samples obtained from dry eye patients or patients with conjunctivitis [11,12].
The present study was undertaken to elucidate a potential role of β-adrenergic receptor 1 (ADRB1) and 2 (ADRB2) as new targets for 3-T1AM. We also explored a putative link between 3-T1AM actions on Ca2+ channels and these adrenergic receptors, which are able to specifically modulate the aforementioned TRP channels. The human conjunctival epithelial cell line (IOBA-NHC) was utilized due to the increased expression of β-adrenergic receptors, which has also been observed in the conjunctiva and eye [13,14,15].
Materials and Methods
Cloning of Adrenergic Receptors
All full-length β-adrenergic receptors were cloned from genomic human DNA into the eukaryotic expression vector pcDps. The β1− (ADRB1, NM_000684.2) and β2-adrenergic receptors (ADRB2, NM_000024.5) were N-terminally tagged with a hemagglutinin (YPYDVPDYA) epitope. Plasmids were sequenced and verified with BigDye-terminator sequencing (PerkinElmer Inc., Waltham, Mass., USA) using an automatic sequencer (ABI 3710xl; Applied Biosystems, Foster City, Calif., USA).
Cell Culture and Transient Transfection
For the cyclic AMP (cAMP) assay and MAPK characterization, HEK293 cells were cultured in MEM Earle's media containing L-glutamine supplemented with 5% FBS and nonessential amino acids (Biochrom AG, Berlin, Germany) in a humidified 5% CO2 incubator at 37°C. For functional assays, transfections were performed as described previously [16]. The IOBA-NHC cell line was used as a cell model for human conjunctival epithelial cells. Cells were grown in DMEM/HAMs F12 1:1 supplemented with 10% FBS, 1 μg/ml insulin, 5 μg/ml hydrocortisone and antibiotics in a humidified 5% CO2 incubator at 37°C [17].
Dynamic Mass Redistribution
HEK293 cells (500,000 cells/well) were seeded in poly-L-lysine-coated 50-ml cell culture flasks. Twenty-four hours after seeding, cells were transiently transfected using Lipofectamine™ 2000 (Life Technologies). On the next day, cells were detached using Versene solution (Life Technologies, Darmstadt, Germany) and were transferred into fibronectin-coated 384-well plates (15,000 cells/well) and incubated for 24 h at 37°C with 5% CO2. For receptor activation, a high-throughput dynamic mass redistribution (DMR) technology (Corning® Epic® system) was used [18] to determine direct effects of 3-T1AM (Santa Cruz Biotechnology Inc., Dallas, Tex., USA; final concentration of DMSO 0.1%) on ADRB1 and ADRB2 following costimulation with isoprenaline (ISOP, Sigma Aldrich, St. Louis, Mo., USA, dissolved in H2O).
cAMP Assay
Gs signaling was determined by measuring cAMP in transiently transfected HEK293 cells. For control purposes, we transfected cells with empty vector. All experiments were performed in comparison to ADRB1 and ADRB2 transfection. 3-T1AM was dissolved in DMSO and used in a concentration of 0.1% for 10−5M 3-T1AM, which did not affect cAMP accumulation. Stimulation was performed 48 h after transfection as described previously [19]. Cells were incubated for 40 min without ligand or with 3-T1AM (final concentration of DMSO 0.1%), ISOP or norepinephrine (NorEpi; Sigma Aldrich, St. Louis, Mo., USA; both dissolved in H2O), or were costimulated with ISOP, or NorEpi and 3-T1AM, or NorEpi with 3,3′,5-triiodothyronine (T3; Sigma Aldrich; dissolved in HCl final concentration of 0.01%). Intracellular cAMP accumulation was determined as previously described [19].
MAPK Activation Assay
MAPK activation was measured using a luciferase reporter gene assay (SRE-luc) (Promega, Fitchburg, Wis., USA). HEK293 cells were cotransfected with serum response element (SRE) plasmid DNA (pGL4.33), a reporter construct containing an SRE and the firefly luciferase reporter gene, and either receptor or empty vector plasmid DNA (mock). Two days after transfection, cells were incubated for 6 h with the respective substances (ISOP, NorEpi or 3-T1AM) in supplement-free MEM at 37°C with 5% CO2. Reactions were terminated by aspirating the media. Cells were lysed using 1× passive lysis buffer (Promega). Measurement was conducted with automatic luciferase substrate injection of 40 µl in a black 96-well plate using a Berthold Microplate Reader (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany).
Fluorescence Calcium Measurements
The intracellular Ca2+ concentration in IOBA-NHC cells was measured as previously reported [17,19]. In brief, IOBA-NHC cells were loaded with 1 μM fura-2/AM for 30-40 min at 37°C. Fluorescence measurements were performed with a microscope (Olympus BW50WI) at room temperature using a Ringer-like solution containing 150 mM NaCl, 6 mM CsCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM glucose (pH 7.4) (∼300 mOsM). In addition, a digital-imaging system (TILL-Photonics, Munich, Germany) and TIDA software were used (HEKA-Electronics, Lamprecht, Germany). Fura-2 fluorescence was alternately excited at wavelengths of 340 and 380 nm and emission was measured at 510 nm. The fluorescence ratio (f340 nm/f380 nm) is a relative index of changes in [Ca2+]i[20]. Results are presented as mean traces of f340/f380 ± SEM. Measurements lasted 10 min.
Statistics
Data are shown as means ± SEM of independent experiments as indicated in the respective figure legends. For statistical analysis, one-way ANOVA was performed with Dunnett's multiple comparisons test with a threshold of statistical significance of p ≤ 0.05. Student's t test was used if the values were normally distributed in accordance with the Gaussian distribution (normality test). GraphPad Prism 6.0 (GraphPad software, San Diego, Calif., USA) was chosen for data analysis. SigmaPlot software version 12.5 (Systat, San Jose, Calif., USA) was used for the creation of particular diagrams.
Results
ADRB1 and ADRB2 Are Targets of 3-T1AM
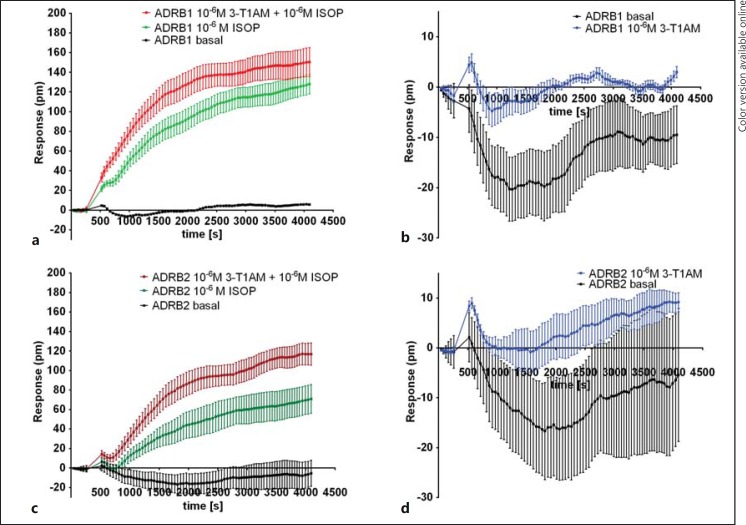
Using DMR technology, we prescreened the potential effects of 3-T1AM alone and in combination with ISOP on ADRB1 and ADRB2 signaling. A robust change in DMR following stimulation with 10−6M ISOP for ADRB1 (fig. 1a) and ADRB2 (fig. 1b) in comparison to nonstimulated cells was registered. We also investigated the direct effect of 10−5M 3-T1AM on ADRB1 (fig. 1c) and ADRB2 (fig. 1d). The effect of 3-T1AM on both receptors was small but statistically significant at several time points in comparison to nonstimulated cells (one-way ANOVA with a Dunnett's multiple comparisons test, p < 0.05). In addition, the modulatory effect of 3-T1AM in the presence of 10−6M ISOP was examined (p < 0.05; fig. 1a, b), with a shift in DMR for both receptors demonstrated in comparison to ISOP stimulation alone.
Fig. 1.
EPIC technology to identify 3-T1AM effects on ADRB1 and ADRB2 signaling. HEK293 cells were transiently transfected with ADRB1 or ADRB2, and their response to ISOP and 3-T1AM alone or in costimulation was measured using the label-free dynamic mass distribution assay (EPIC technology). a Signaling of ADRB1 followed by application of 10−6M ISOP with coincubation of 10−6M ISOP and 10−6M 3-T1AM in comparison to untreated cells (basal) was measured. b 3-T1AM stimulation in comparison to basal level of ADRB1 was measured. c The basal level of ADRB2 compared to 10−6M ISOP or in coincubation with 10−6M ISOP and 10−6M 3-T1AM is shown. d Signaling was measured in cells expressing ADRB2 following incubation with 10−6M 3-T1AM in comparison to untreated cells. Data are represented as means ± SEM of mock-corrected response over time. Original data are shown as one representative experiment of four independent experiments performed in three technical replicates.
3-T1AM Alone Does Not Activate Gs and MAPK at ADRB1 and ADRB2
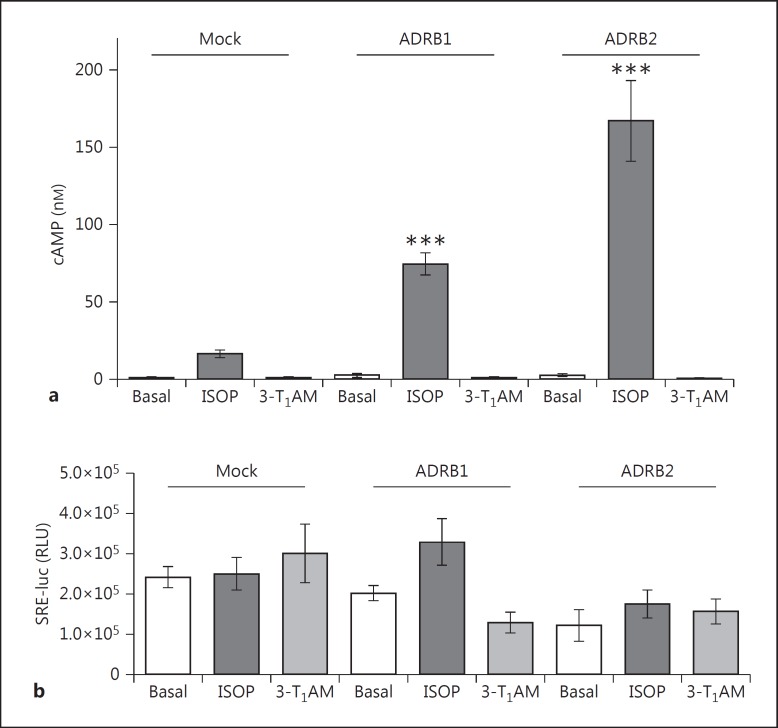
Stimulation of ADRB1 and ADRB2 with 10−6M ISOP resulted in a robust increase of ADRB1- and ADRB2- mediated Gs signaling (fig. 2a). However, a high 3-T1AM concentration of 10−5M was not sufficient to activate these receptors (fig. 2a). Therefore, the observed effect in DMR was not compatible with Gs activation.
Fig. 2.
3-T1AM supplementation shows no classic signaling effects on ADRB1 and ADRB2. a HEK293 cells transfected with mock, ADRB1 or ADRB2 were left unstimulated or treated with 10−6M ISOP or 10−5M 3-T1AM, followed by measurement of cAMP levels. b Cells were cotransfected with mock, ADRB1 or ADRB2, and with SRE-luc, a reporter construct for MAPK activation. Relative light units (RLU) were then measured. Data were obtained from 3-4 independent experiments measured in at least triplicate and are means ± SEM. A one-way ANOVA against ISOP-stimulated mock was used for statistical analysis. *** p ≤ 0.001.
To test whether the observed signal in DMR was due to MAPK activation, stimulation of 10−6M ISOP or 10−5M 3-T1AM was performed. A slight increase in MAPK signaling for ADRB1 with ISOP was observed, which was not found to be statistically significant (fig. 2b). In addition, no increase of MAPK signaling following 3-T1AM stimulation was observed in comparison to basal levels (fig. 2b).
3-T1AM Modulates ISOP-Induced cAMP Signaling of ADRB2
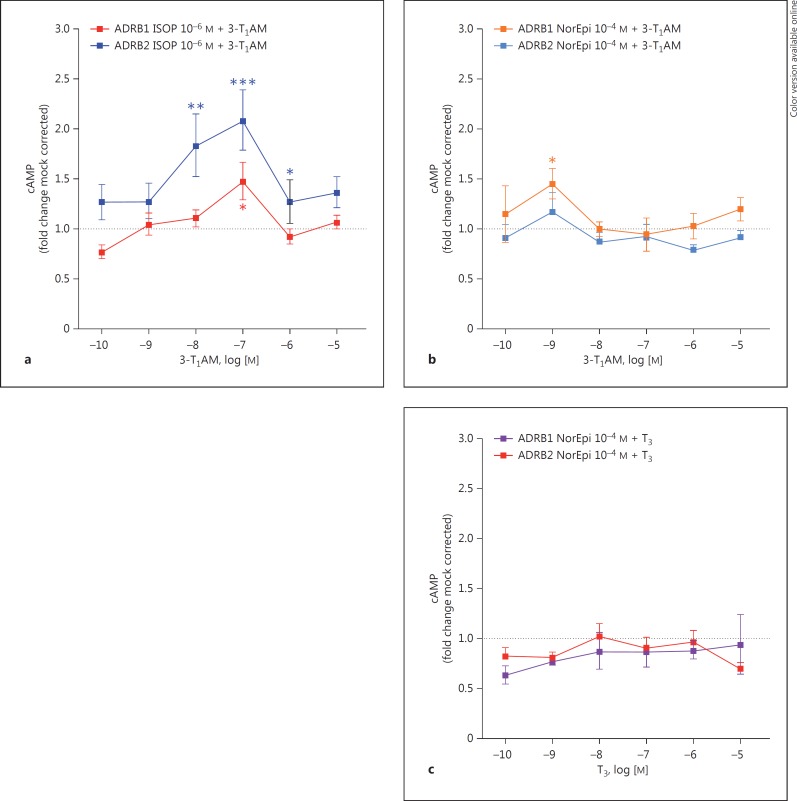
To determine a modulatory effect of 3-T1AM on ADRB1 and ADRB2 on ISOP-induced signaling, we performed costimulation studies with a constant concentration of ISOP (10−6M) and increasing concentrations of 3-T1AM (10−10-10−5M) in Gs signaling (fig. 3).
Fig. 3.
3-T1AM modulates ISOP-induced cAMP signaling in ADRB2. a HEK293 cells transiently expressing ADRB1 or ADRB2 were costimulated with a concentration of 10−6M ISOP and increasing concentrations of 3-T1AM (10−10-10−5M), followed by measurement of cAMP levels. b Following stimulation of cells with 10−4M NorEpi and challenging with increasing concentrations of 3-T1AM (10−10-10−5M), cAMP levels were measured. c Using a concentration of 10−4M NorEpi with increasing concentrations of T3 served as a noninterfering substance in the cAMP assay at ADRB1 and ADRB2. Data are represented as means ± SEM of 4-6 independent assays performed in triplicate as mock-corrected fold change compared to the cAMP value of the respective constantly incubated substance (set as 1). Statistical significance was determined by one-way ANOVA; * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
Coincubation of ISOP and increasing concentrations of 3-T1AM resulted in a modest effect for ADRB1, particularly at a concentration of 10−7M 3-T1AM (fig. 3a). The formation of cAMP at an ISOP concentration of 10−6M was normalized to 1 and the fold change in cAMP formation induced by 3-T1AM was indicated. A significant modulatory effect for ADRB2 signaling with a maximal enhancement of Gs signaling of twofold over mock-corrected 10−6M ISOP signaling (fig. 3a) was demonstrated. Incubation of 3-T1AM on mock-transfected cells did not result in cAMP formation (data not shown).
To further analyze whether this effect is reproducible with the endogenous ligand NorEpi, we performed costimulation studies with a concentration of 10−4M NorEpi and increasing concentrations of 3-T1AM (10−10−10 −5M; fig. 3b). For the endogenous ligand, which has a lower affinity at ADRB1 and ADRB2 in comparison to ISOP [21], the modulatory effects were much smaller (fig. 3b). Only a stimulatory effect for costimulation with 10−9M 3-T1AM was observed.
To rule out that the modulation of signaling is an artificial result of costimulation, we repeated the experiments by costimulation of 10−4M NorEpi and increasing concentrations of T3 (10−10-10−5M). Only for stimulation of ADRB1 with 3-T1AM (at a concentration of 1 nM) was a significant difference compared to stimulation with NorEpi alone observed (fig. 3c).
3-T1AM-Induces Ca2+ Influx in Human Conjunctival Epithelial Cells
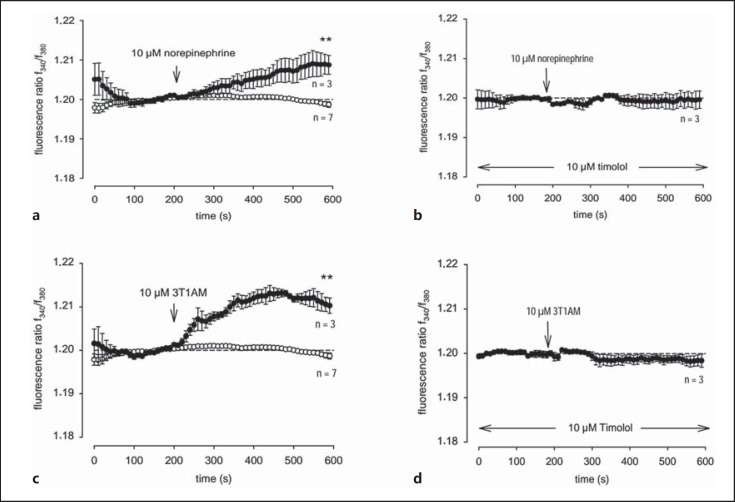
To ascertain whether endogenously expressed adrenergic receptors respond to 3-T1AM, we investigated IOBA-NHC cells [22] since β-adrenergic receptors are highly expressed in eye layers and cells [14,23,24]. It is also known that there is an association between voltage-dependent Ca2+ channels and adrenergic receptors [25]. In addition, there is also an association between adrenergic receptors and nonvoltage-dependent Ca2+ channels, such as the aforementioned TRP channels which are expressed in IOBA-NHC cells [17]. As shown in figure 4a and b, a NorEpi-induced Ca2+ influx was blocked by the nonselective β-adrenergic receptor antagonist timolol. More specifically, stimulation with 10−6M NorEpi increased the fluorescence ratio f340 nm/f380 nm from 1.200 ± 0.001 to 1.209 ± 0.002 (p ≤ 0.05, n = 3) at 600 s (fig. 4a), whereas the NorEpi-induced Ca2+ increase was clearly suppressed in the presence of 10−6M timolol to 1.200 ± 0.02 (p ≤ 0.05; n = 3; at 600 s; fig. 4b). Similarly, extracellular application of 10−6M 3-T1AM increased the fluorescence ratio from 1.199 ± 0.001 to 1.210 ± 0.002 (p < 0.01; n = 3; at 600 s) at 600 s (fig. 4c), which was also blocked by 10−6M timolol (1.199 ± 0.002; p ≤ 0.01; n = 3; at 600 s; fig. 4d). The effect of 3-T1AM in DMR might therefore be due to an effect of endogenously expressed TRP channels and ADRB1 or ADRB2.
Fig. 4.
NorEpi and 3-T1AM-induced Ca2+ entry suppressed by the nonselective ADRB blocker timolol. Changes in cytosolic free Ca2+ are depicted as the ratio of the fluorescence induced by the excitation wavelength at 340 and 380 nm. Reagents were added to nontransfected IOBA-NHC cells at the time points indicated by the arrows. a Cells were stimulated with 10−6M NorEpi (filled circles) and Ca2+ influxes were measured (n = 3) in comparison to basal levels (n = 7, open circles). b Cells were incubated with 10−6M NorEpi in the presence of 10−6M timolol (n = 3). c 10−6M 3-T1AM (filled circles) were added to the cells, and Ca2+ levels were measured (n = 3). d Cells were coincubated with 10−6M 3-T1AM and 10−6M timolol (n = 3). Data are represented as means ± SEM of 3-7 experiments. Ca2+ baselines were recorded as controls (n = 7).
Discussion
3-T1AM Targets ADRB2 and Is Involved in Regulation of Calcium Influx
3-T1AM is an endogenous thyroid hormone derivative that activates TAAR1 (reviewed by Piehl et al. [3]). It has been suggested that 3-T1AM is a multitarget ligand and affects further GPCRs or interacts with non-GPCR proteins [2]. In this study, DMR, cAMP assay and a MAPK activation assay were used to ascertain whether 3-T1AM-induced cellular signaling is linked with ADRB1 and ADRB2. Furthermore, modulation of Ca2+ signaling by 3-T1AM was evaluated using an IOBA-NHC cell line which has been described as a cell model for the investigation of human conjunctiva [22], also in an electrophysiological context [12,17].
Our studies revealed that 3-T1AM enhances Gs-mediated signaling of ISOP-stimulated ADRB2 as a modulator. This finding might be reasoned by positive allosteric effects, which means that binding of 3-T1AM enhances binding and/or agonistic signaling properties (maximum of capacity) of ISOP. This may be explained by two different scenarios. In the case of so-called off-target effects, dimers constituting two interacting receptor protomers would mutually influence one another, and binding of 3-T1AM at one protomer increases the capacity for ISOP-induced signaling at the second protomer. Several examples for such a signaling (or binding) modification in GPCR dimers have been reported (for review see Smith and Milligan [26]) and ADRB2 is known to constitute dimeric arrangements [27]. In the second potential scenario of ‘on-target’ allosterism, 3-T1AM would bind at an allosteric receptor site and improve, by spatial rearrangements of the monomeric receptor conformation, the signaling capacity for ISOP bound in the orthosteric binding site. Such a principle scenario of two different ligand-binding sites at a GPCR monomer has been previously suggested, for example at the lutropin receptor [28].
Interestingly, the observed effect of signaling inhibition at higher 3-T1AM concentrations compared to lower concentrations (fig. 3) would be compatible with a two-binding site model for 3-T1AM (low and high affinity), whereby one site would have a stimulating effect and the second an inhibitory influence. Such mechanisms have been described for the M3 muscarinic acetylcholine receptor [29] or the β1-adrenergic receptor [30].
It can be also hypothesized that the effect of a decreased Gs-mediated cAMP accumulation at increasing 3-T1AM concentrations in costimulation experiments with ISOP or NorEpi (constant concentration) should be related to the activation of Gi. This would inhibit Gs-mediated signaling. It is known that β-adrenergic receptors are promiscuous for different G protein subtypes and that their differentiated activation can be dependent on the ligand variant or concentration [31].
Furthermore, NorEpi as well as 3-T1AM increased intracellular Ca2+ levels in IOBA-NHC cells, which could be blocked by the nonselective adrenergic receptor blocker timolol (fig. 4). This prompts the suggestion that 3-T1AM is connected with Ca2+-permeable channels or intracellular store depletion processes in connection with adrenergic receptors, which was also reported in cardiomyocytes [32].
Since regulation of body temperature is more or less conducted by temperature-sensitive receptors, we postulate that 3-T1AM somehow activates cold ‘receptors’ such as the aforementioned menthol receptor TRPM8. In previous studies using the same cell line, we could delineate that the 3-T1AM-induced Ca2+ effect was due to TRPM8 activation since the specific TRPM8 blocker BCTC abolished the 3-T1AM effect [7]. Interestingly, timolol was observed to have the same effect, suggesting that 3-T1AM binds to ADRBs, in particular ADRB2, which in turn activates Ca2+ channels such as TRPM8. A first assumption might be that 3-T1AM effects are directly mediated via protein interactions between the GPCR and TRP channel (reviewed by Veldhuis et al. [33]), or indirectly via the β/γ-subunits of Gi/o[34], which can also be activated by the β-adrenergic receptors [31]. This hypothesis is supported by our observations in costimulation experiments (fig. 3), which might show activation of Gi at higher 3-T1AM concentrations combined with constant ISOP or NorEpi levels (and therefore inhibition of Gs signaling). In consequence, the direct 3-T1AM-mediated Gi-induced signaling should be investigated in future studies.
Potential Role of 3-T1AM in Conjunctival Epithelial Cells
The established human conjunctival IOBA-NHC cell line was used as a relevant model of ocular surface cell biology as well as for investigation of electrophysiological investigation of TRP channels [10,17]. Notably, an association between direct interaction of 3-T1AM with TRPM8 and suppression of TRPV1 activity could be demonstrated in this cell line [7]. The results of the present study further indicate that the multitarget 3-T1AM is a modulator of ADRB2 and mediates cellular and Ca2+ signaling. At this point, 3-T1AM appears to play a potential role in both TRPM8- and TRPV1-mediated Ca2+ regulation and linked biological processes.
Conclusions
We demonstrate that 3-T1AM-enhances ISOP-induced ADRB2 signaling via the Gs/adenylyl cyclase pathway. This suggests that ADRB2 is a GPCR target for 3-T1AM and that 3-T1AM might also have implications for tissues expressing ADRB2 such as the lung, skeletal muscles and skin [24]. In addition, there is evidence that ADRB is linked with Ca2+ channels in human conjunctival epithelial cells. This conclusion is warranted since the nonselective ADRB blocker timolol had an inhibitory effect on both NorEpi- and 3-T1AM-induced Ca2+ increases. In summary, 3-T1AM is a multitarget ligand and modulates ADRB-mediated signaling in human conjunctival epithelial cells. Therefore, thyronamines, in connection with ADRB, may provide novel compounds for suppressing Ca2+ channels such as TRPV1 since its activation is associated with inflammation processes occurring in conjunctivitis or dry eye syndrome. Our findings shed new light on the previously proposed complex spectrum of 3-T1AM action [35] and point to a diversified functional profile of 3-T1AM.
Disclosure Statement
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG): Graduate College 1208 (Hormonal Regulation of Energy Metabolism, Body Weight and Growth) TP1 and TP3, KL2334/2-1 and DFG Priority Program SPP1629 Thyroid Trans Act BI 893/5-1, Me 1706/13-1, KO 922/16-1 and 922/17-1, STA 1265/1-1. Human conjunctival epithelial cells were a generous gift from the laboratory of Yolanda Diebold (University Institute of Applied Ophthalmobiology, University of Valladolid, Valladolid, Spain) and kindly provided within a collaboration by Friedrich Paulsen (Institute of Anatomy II, University of Erlangen-Nuremberg, Germany).
References
- 1.Scanlan TS. Minireview: 3-iodothyronamine (T1AM): a new player on the thyroid endocrine team? Endocrinology. 2009;150:1108–1111. doi: 10.1210/en.2008-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucchi R, Accorroni A, Chiellini G. Update on 3-iodothyronamine and its neurological and metabolic actions. Front Physiol. 2014;5:402. doi: 10.3389/fphys.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piehl S, Hoefig CS, Scanlan TS, Kohrle J. Thyronamines – past, present, and future. Endocr Rev. 2011;32:64–80. doi: 10.1210/er.2009-0040. [DOI] [PubMed] [Google Scholar]
- 4.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 5.Cody V, Meyer T, Dohler KD, Hesch RD, Rokos H, Marko M. Molecular structure and biochemical activity of 3,5,3′-triiodothyronamine. Endocr Res. 1984;10:91–99. doi: 10.3109/07435808409035410. [DOI] [PubMed] [Google Scholar]
- 6.Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–14551. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khajavi N, Reinach PS, Slavi N, Skrzypski M, Lucius A, Strauss O, Kohrle J, Mergler S. Thyronamine induces TRPM8 channel activation in human conjunctival epithelial cells. Cell Signal. 2015;27:315–325. doi: 10.1016/j.cellsig.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Mergler S, Mertens C, Valtink M, Reinach PS, Szekely VC, Slavi N, Garreis F, Abdelmessih S, Turker E, Fels G, Pleyer U. Functional significance of thermosensitive transient receptor potential melastatin channel 8 (TRPM8) expression in immortalized human corneal endothelial cells. Exp Eye Res. 2013;116:337–349. doi: 10.1016/j.exer.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 10.Mergler S, Valtink M, Takayoshi S, Okada Y, Miyajima M, Saika S, Reinach PS. Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells. Ophthalmic Res. 2014;52:151–159. doi: 10.1159/000365334. [DOI] [PubMed] [Google Scholar]
- 11.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khajavi N, Reinach PS, Skrzypski M, Lude A, Mergler S. L-Carnitine reduces in human conjunctival epithelial cells hypertonic-induced shrinkage through interacting with TRPV1 channels. Cell Physiol Biochem. 2014;34:790–803. doi: 10.1159/000363043. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo T, Cynader MS. Localization of α2-adrenergic receptors in the human eye. Ophthalmic Res. 1992;24:213–219. doi: 10.1159/000267170. [DOI] [PubMed] [Google Scholar]
- 14.Messina Baas O, Pacheco Cuellar G, Toral-Lopez J, Lara Huerta SF, Gonzalez-Huerta LM, Urueta-Cuellar H, Rivera-Vega MR, Babayan-Mena I, Cuevas-Covarrubias SA. ADRB1 and ADBR2 gene polymorphisms and the ocular hypotensive response to topical betaxolol in healthy Mexican subjects. Curr Eye Res. 2014;39:1076–1080. doi: 10.3109/02713683.2014.900807. [DOI] [PubMed] [Google Scholar]
- 15.Neufeld AH, Zawistowski KA, Page ED, Bromberg BB. Influences on the density of β-adrenergic receptors in the cornea and iris – ciliary body of the rabbit. Invest Ophthalmol Vis Sci. 1978;17:1069–1075. [PubMed] [Google Scholar]
- 16.Mühlhaus J, Dinter J, Nürnberg D, Rehders M, Depke M, Golchert J, Homuth G, Yi CX, Morin S, Köhrle J, Brix K, Tschop M, Kleinau G, Biebermann H. Analysis of human TAAR8 and murine Taar8b mediated signaling pathways and expression profile. Int J Mol Sci. 2014;15:20638–20655. doi: 10.3390/ijms151120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mergler S, Garreis F, Sahlmuller M, Lyras EM, Reinach PS, Dwarakanath A, Paulsen F, Pleyer U. Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem Cell Biol. 2012;137:743–761. doi: 10.1007/s00418-012-0924-5. [DOI] [PubMed] [Google Scholar]
- 18.Schröder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, Müller A, Blättermann S, Mohr-Andrä M, Zahn S, Wenzel J, Smith NJ, Gomeza J, Drewke C, Milligan G, Mohr K, Kostenis E. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol. 2010;28:943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- 19.Piechowski CL, Rediger A, Lagemann C, Mühlhaus J, Muller A, Pratzka J, Tarnow P, Grüters A, Krude H, Kleinau G, Biebermann H. Inhibition of melanocortin-4 receptor dimerization by substitutions in intracellular loop 2. J Mol Endocrinol. 2013;51:109–118. doi: 10.1530/JME-13-0061. [DOI] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21.Frielle T, Daniel KW, Caron MG, Lefkowitz RJ. Structural basis of β-adrenergic receptor subtype specificity studied with chimeric β1/β2-adrenergic receptors. Proc Natl Acad Sci USA. 1988;85:9494–9498. doi: 10.1073/pnas.85.24.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, Siemasko KF, Stern ME. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–4274. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- 23.Enriquez de Salamanca A, Siemasko KF, Diebold Y, Calonge M, Gao J, Juarez-Campo M, Stern ME. Expression of muscarinic and adrenergic receptors in normal human conjunctival epithelium. Invest Ophthalmol Vis Sci. 2005;46:504–513. doi: 10.1167/iovs.04-0665. [DOI] [PubMed] [Google Scholar]
- 24.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bavencoffe A, Gkika D, Kondratskyi A, Beck B, Borowiec AS, Bidaux G, Busserolles J, Eschalier A, Shuba Y, Skryma R, Prevarskaya N. The transient receptor potential channel TRPM8 is inhibited via the alpha 2a adrenoreceptor signaling pathway. J Biol Chem. 2010;285:9410–9419. doi: 10.1074/jbc.M109.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartania N, Appelbe S, Pediani JD, Milligan G. Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric β2-adrenoceptor. Cell Signal. 2007;19:1928–1938. doi: 10.1016/j.cellsig.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Heitman LH, Kleinau G, Brussee J, Krause G, Ijzerman AP. Determination of different putative allosteric binding pockets at the lutropin receptor by using diverse drug-like low molecular weight ligands. Mol Cell Endocrinol. 2012;351:326–336. doi: 10.1016/j.mce.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Thor D, Schulz A, Hermsdorf T, Schöneberg T. Generation of an agonistic binding site for blockers of the M3 muscarinic acetylcholine receptor. Biochem J. 2008;412:103–112. doi: 10.1042/bj20071366. [DOI] [PubMed] [Google Scholar]
- 30.Baker JG, Hill SJ. Multiple GPCR conformations and signalling pathways: implications for antagonist affinity estimates. Trends Pharmacol Sci. 2007;28:374–381. doi: 10.1016/j.tips.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzel-Seifert K, Seifert R. Molecular analysis of β2-adrenoceptor coupling to Gs-, Gi-, and Gq-proteins. Mol Pharmacol. 2000;58:954–966. doi: 10.1124/mol.58.5.954. [DOI] [PubMed] [Google Scholar]
- 32.Ghelardoni S, Suffredini S, Frascarelli S, Brogioni S, Chiellini G, Ronca-Testoni S, Grandy DK, Scanlan TS, Cerbai E, Zucchi R. Modulation of cardiac ionic homeostasis by 3-iodothyronamine. J Cell Mol Med. 2009;13:3082–3090. doi: 10.1111/j.1582-4934.2009.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev. 2015;67:36–73. doi: 10.1124/pr.114.009555. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaumer L, Perez-Reyes E, Bertrand P, Gudermann T, Wei XY, Kim H, Castellano A, Codina J. Molecular diversity and function of G proteins and calcium channels. Biol Reprod. 1991;44:207–224. doi: 10.1095/biolreprod44.2.207. [DOI] [PubMed] [Google Scholar]
- 35.Gompf HS, Greenberg JH, Aston-Jones G, Ianculescu AG, Scanlan TS, Dratman MB. 3-Monoiodothyronamine: the rationale for its action as an endogenous adrenergic-blocking neuromodulator. Brain Res. 2010;1351:130–140. doi: 10.1016/j.brainres.2010.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]