Abstract
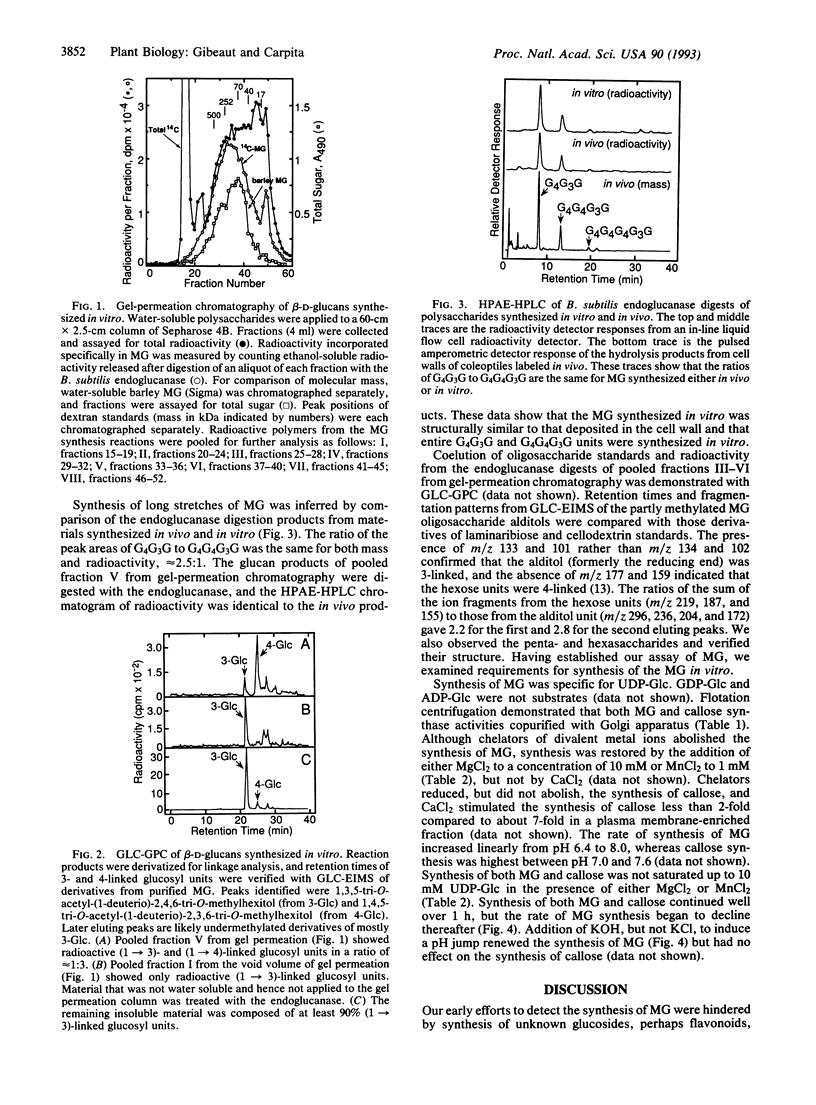
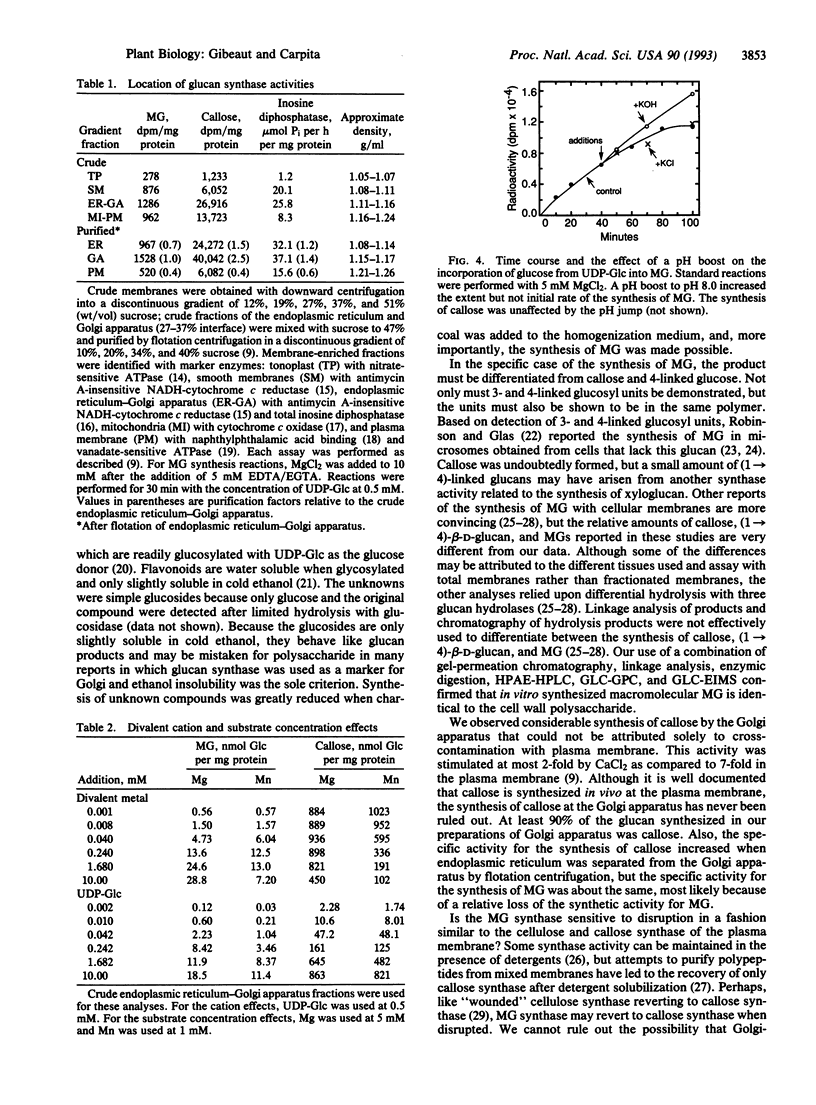
Membranes of the Golgi apparatus from maize (Zea mays L.) were used to synthesize in vitro the (1-->3), (1-->4)-beta-D-glucan (MG) that is unique to the cell wall of the Poaceae. The MG was about 250 kDa and was separated from a much larger (1-->3)-beta-D-glucan (callose) by gel-permeation chromatography. Diagnostic oligosaccharides, released by a sequence-dependent endoglucanase from Bacillus subtilis, were separated by HPLC and GLC. The trisaccharide beta-D-Glcp-(1-->4)-beta-D-Glcp-(1-->3)-D-Glc, the tetrasaccharide [beta-D-Glcp-(1-->4)]2-beta-D-Glcp-(1-->3)-D-Glc, and longer cellodextrin-(1-->3)-D-Glc oligosaccharides were synthesized in proportions similar to those found in purified MG. Activated charcoal added during homogenization enhanced synthesis of MG, presumably by removing inhibitory compounds. The Golgi apparatus was determined as the site of synthesis by a combination of downward and flotation centrifugations on sucrose step gradients. The rate of synthesis did not reach saturation at up to 10 mM UDP-Glc. Chelators completely abolished synthesis, but synthase activity was restored by addition of either MgCl2 or, to a lesser extent, MnCl2. Synthesis continued for well over 1 h; addition of KOH to raise the pH from 7.2 to 8.0 during the reaction increased the rate of synthesis, which indicates that a transmembrane pH gradient may facilitate synthesis of MG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Spencer D. B., Hamilton C. C., Smith S. F., Tietyen J., Bryant C. A., Oeltgen P. Oat-bran cereal lowers serum total and LDL cholesterol in hypercholesterolemic men. Am J Clin Nutr. 1990 Sep;52(3):495–499. doi: 10.1093/ajcn/52.3.495. [DOI] [PubMed] [Google Scholar]
- Anderson M. A., Stone B. A. A new substrate for investigating the specificity of beta-glucan hydrolases. FEBS Lett. 1975 Apr 1;52(2):202–207. doi: 10.1016/0014-5793(75)80806-4. [DOI] [PubMed] [Google Scholar]
- Ardies C. M., Lasker J. M., Bloswick B. P., Lieber C. S. Purification of NADPH:cytochrome c (cytochrome P-450) reductase from hamster liver microsomes by detergent extraction and affinity chromatography. Anal Biochem. 1987 Apr;162(1):39–46. doi: 10.1016/0003-2697(87)90008-x. [DOI] [PubMed] [Google Scholar]
- Bomhoff G. H., Spencer M. Optimum pH and ionic strength for the assay of cytochrome c oxidase from pea cotyledon mitochondria. Can J Biochem. 1977 Oct;55(10):1114–1117. doi: 10.1139/o77-165. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Protection of cellulose synthesis in detached cotton fibers by polyethylene glycol. Plant Physiol. 1980 Nov;66(5):911–916. doi: 10.1104/pp.66.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Kanabus J. Chemical structure of the cell walls of dwarf maize and changes mediated by gibberellin. Plant Physiol. 1988 Nov;88(3):671–678. doi: 10.1104/pp.88.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Mulligan J. A., Heyser J. W. Hemicelluloses of cell walls of a proso millet cell suspension culture. Plant Physiol. 1985 Oct;79(2):480–484. doi: 10.1104/pp.79.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Benziman M., Padan E. Requirement for a membrane potential for cellulose synthesis in intact cells of Acetobacter xylinum. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5282–5286. doi: 10.1073/pnas.79.17.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D. M., Carpita N. C. Tracing cell wall biogenesis in intact cells and plants : selective turnover and alteration of soluble and cell wall polysaccharides in grasses. Plant Physiol. 1991 Oct;97(2):551–561. doi: 10.1104/pp.97.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Factors Influencing beta-Glucan Synthesis by Particulate Enzymes from Suspension-Cultured Lolium multiflorum Endosperm Cells. Plant Physiol. 1982 Mar;69(3):632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Solubilization of beta-glucan synthases from the membranes of cultured ryegrass endosperm cells. Biochem J. 1982 Jun 1;203(3):629–636. doi: 10.1042/bj2030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Proton transport in isolated vacuoles from corn coleoptiles. Plant Physiol. 1985 May;78(1):104–109. doi: 10.1104/pp.78.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle P. J., Ng K. F., Johnson E., Hoogenraad N. J., Stone B. A. The beta-glucan synthase from Lolium multiflorum. Detergent solubilization, purification using monoclonal antibodies, and photoaffinity labeling with a novel photoreactive pyrimidine analogue of uridine 5'-diphosphoglucose. J Biol Chem. 1991 Nov 25;266(33):22569–22581. [PubMed] [Google Scholar]
- Smith M. M., Stone B. A. Beta-glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim Biophys Acta. 1973 Jun 20;313(1):72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]



