Abstract
We investigated the link between direct activation of inhibitory neurons, local neuronal activity, and hemodynamics. Direct optogenetic cortical stimulation in the sensorimotor cortex of transgenic mice expressing Channelrhodopsin-2 in GABAergic neurons (VGAT-ChR2) greatly attenuated spontaneous cortical spikes, but was sufficient to increase blood flow as measured with laser speckle contrast imaging. To determine whether the observed optogenetically evoked gamma aminobutyric acid (GABA)-neuron hemodynamic responses were dependent on ionotropic glutamatergic or GABAergic synaptic mechanisms, we paired optogenetic stimulation with application of antagonists to the cortex. Incubation of glutamatergic antagonists directly on the cortex (NBQX and MK-801) blocked cortical sensory evoked responses (as measured with electroencephalography and intrinsic optical signal imaging), but did not significantly attenuate optogenetically evoked hemodynamic responses. Significant light-evoked hemodynamic responses were still present after the addition of picrotoxin (GABA-A receptor antagonist) in the presence of the glutamatergic synaptic blockade. This activation of cortical inhibitory interneurons can mediate large changes in blood flow in a manner that is by and large not dependent on ionotropic glutamatergic or GABAergic synaptic transmission. This supports the hypothesis that activation of inhibitory neurons can increase local cerebral blood flow in a manner that is not entirely dependent on levels of net ongoing neuronal activity.
Keywords: interneurons, laser speckle, optical imaging, optogenetics, pharmacology, physiology
Introduction
Exploring how activity of specific cell types is coupled to alterations in local blood flow is fundamental to understanding the regulation of cerebral blood flow. This has become an issue of both interest and importance because many modalities of imaging use metabolic and vascular signals as proxies for neuronal activity.1 The application of optogenetics to the study of hemodynamics facilitates dissection of cell type-specific-mediated alterations in blood flow through stimulation of defined subpopulations of neurons. Here, we use VGAT-ChR2 line 8 mice2 to probe the capacity of cortical inhibitory interneurons to alter local cortical activity and blood flow in vivo. Gamma power is driven by fast spiking interneurons3 and is correlated to hemodynamic responses.4, 5, 6 Inhibitory neurons have a measureable metabolic load,7 can release vasoactive compounds,8 and have a substantial anatomic connection to the local microvasculature in the cortex, yet a causal link between their activation and alterations in blood flow has not yet been shown. This led us to hypothesize that direct stimulation of interneurons could increase blood flow. Although, in brain slice preparations, it has been shown that interneurons can have bidirectional control of vessel diameter;8 here, we reveal that optogenetic activation of GABAergic neurons can lead to a net increase in local blood flow. With extracellular recordings of spontaneous spiking, we show that increases in blood flow can occur while spontaneous activity of neurons is suppressed. Because pathologies, namely stroke, can produce immediate and prolonged deficits in both neurovascular coupling9 and the function of inhibitory circuits,10 we expect an increased importance will be placed on examining cell type-specific neurovascular coupling in disease states.
Materials and Methods
Animals and Surgery
Experimental procedures were performed under protocols approved by the University of British Columbia Animal Care Committee, conformed to the Canadian Council on Animal Care and ARRIVE guidelines. For acute experiments, 6 Jackson Laboratory (Bar Harbor, Maine, USA) Line 8 VGAT-ChR2-YFP BAC male and female 2 to 4-month-old animals weighing 20 to 30 g were used. Additionally, two ChR2 negative littermates and four B6.Cg-Tg(Thy1-YFPH)2Jrs/J mice of the same age and weight were used for control experiments. An additional mouse was used for confocal imaging to confirm expression of Channelrhodopsin-2 (Figure 1A). The animals were anesthetized with 5% isoflurane in air; this was reduced to 1% to 1.5% during surgery. Body temperature was maintained at 37±0.5 °C by a heating pad controlled by temperature feedback from a rectal probe. The mouse was secured in a custom-made head hold using cyanoacrylate and dental cement and a craniectomy was made over the right forelimb sensorimotor cortex. The dura was left intact.11 Agarose dissolved in HEPES-buffered artificial cerebral spinal fluid, pH 7.3, to 1.5% was placed over the surface of the cortex at 37 °C.12 The surface of the cortex was then covered with a No.1 glass coverslip. Before imaging, the brain was warmed to ~37 °C by circulating warm water through tubing attached to the custom-built, stainless steel headhold. In experiments with unit records, the brain was warmed with the same procedure but agarose was not applied to the cortex. During stimulation procedures, anesthesia was switched to a ketamine xylazine combination (100/10 mg/kg), because it conserves the neurovascular relationship;13 it was supplemented as required.14 Heart rate and pulse distension was monitored with MouseOx pulse oximeter (Star Life Sciences Corp, Oakmont, PA, USA) to monitor the stability of animal vitals during the experiment.
Figure 1.
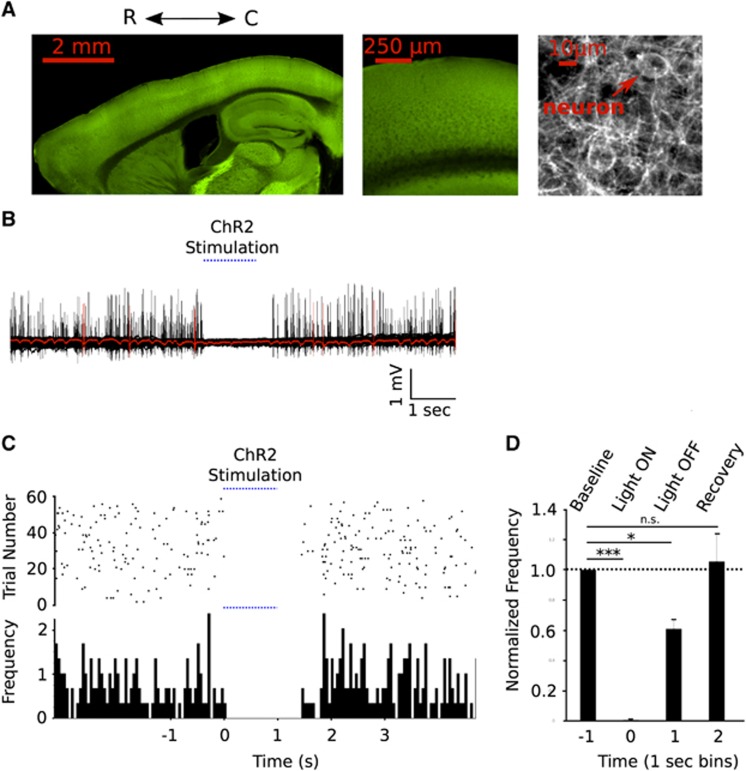
(A) Confocal microscopy was used to visualize emissions from YFP-ChR2 fusion protein. Left: Sagittal cross sections of a ChR2-VGAT mouse brain Middle: a view of the cortex highlighting laminar distribution of YFP-ChR2. Right: high power view showing dense network of labeled fibres and cell body seen in projection images from sensorimotor cortex. (B) Activation of cortical inhibitory interneurons silences spontaneous spiking activity. Sixty trials of spontaneous cortical recordings acquired with a glass electrode and interrupted by ChR2 stimulation (in one VGAT ChR2 mouse). A single trial is highlighted in red. (C) Raster plot of neuronal spikes over time in each trial and a quantification of identified spikes per second. (D) Normalized spikes per second from 1 second bins before, during, and for 2 seconds after stimulation (n=3). Error bars represent mean values±s.e.m.
In vivo Electrotrophysiology
For electroencephalography (EEG) recordings, a teflon-coated silver wire was placed on the lateral edge of the craniotomy on the surface of the cortex aligned with bregma. The reference electrode was placed subcutaneously in the nose. Signals were amplified (1000 × ) and filtered (0.1 to 1000 Hz) using a differential AC amplifier (Model 1700, A-M Systems, Sequim, WA, USA). Data were collected using Clampex 9.2 (Molecular Devices, Sunnyvale, CA, USA). Extracellular unit recordings were performed with a glass electrodes (1 to 1.3 MΩ impedance) inserted at a depth of 400 to 550 μm into the right sensorimotor cortex. The surface of the brain was identified through observing changes in impedance of the electrode while lowering the glass pipette with a manipulator (Model MP-225 Sutter Instruments, Novato, CA, USA). Recordings were amplified via a Molecular Devices MultiClamp 700B. A reference silver ball electrode was placed lateral to bregma. Electrophysiologic recordings were highpass filtered (270 Hz). Thresholds for spike detection in both the X (time) and Y (volts) axes were selected to be inclusive of as many spike as possible. Spikes were clustered with principal component analysis. We quantified the firing rate of spikes in the first cluster, where almost all units were grouped. Little or no spikes were detected during photostimulation, thus most neurons detected were unlikely to be optogenetically activated interneurons that are likely a smaller subset.
Optogenetic and Sensory Stimulation
A 473 nm laser (CrystaLaser BCL-473-050, Reno, NV, USA) collimated to a ~100 μm diameter was targeted to the cortex as previously described (Figure 2A).15 When imaging hemodynamic responses, photostimulation was delivered at an intensity of 2 mW at 100 Hz with each pulse having a 5 ms duration. Responses from varied durations of stimulation (1.0, 0.5, 0.1 seconds) were imaged. Electrical stimulation of the forepaw, delivered by a stimulus isolator (World Precision Instruments, Sarasota, FL, USA), through two 30-gauge needles, which were inserted subcutaneously into the left forepaw. Pulse trains at 100 Hz of 1 ms pulses (1 mA) were delivered for either 4 or 1 seconds durations while recording speckle imaging and intrinsic signal imaging. These stimulation durations were optimal for generating sensory-evoked hemodynamic responses with intrinsic signal16 or laser speckle imaging.14, 17 A single 1 ms pulse (2 mA) was sufficient to evoke a reliable response detectable by EEG.
Figure 2.
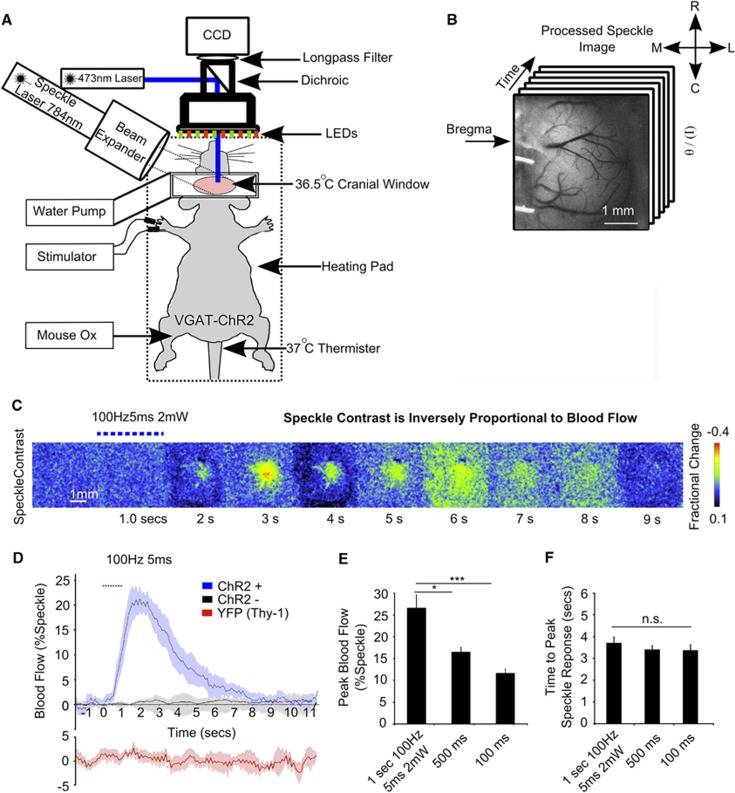
Cortical hemodynamic responses evoked with direct photostimulation of inhibitory interneurons. (A) Experimental arrangement for acute in vivo optogenetic stimulation experiments. (B) Variance-filtered speckle image, where darker tones represent higher velocity blood flow. (C) Montage of fractional change in speckle contrast from 1 second of stimulation (100 Hz, 5 ms, 2 mW) in a single VGAT-ChR2 mouse. (D) Evoked blood flow response (the inverse square of speckle contrast scaled between minimum and maximum) from 1 second of photostimluation (100 Hz, 5 ms, 2 mW) in VGAT-ChR2 mice (n=6), ChR2-negative littermates (n=2) and Thy-1 YFP mice (3 mW stimulation; n=4). Plots show mean values±s.e.m. (E) Peak blood flow and (F) latency to the peak response (n=6) from 100 Hz, 5 ms, 2 mW stimulation at three different durations (1.0; n=6, 0.5 n=6, 0.1; n=5 seconds).
Laser Speckle Contrast and Intrinsic Signal Imaging
The surface of the cortex was illuminated with a 784 nm, 32 mW laser (SNF-XXX-785S-35, StockerYale, Quebec, Quebec, Canada) with a 4 × beam expander (Edmund Optics, Barrington, NJ, USA) directed at the cortex at approximately a 30-degree angle. Light intensity was controlled with a polarizer. The flow of blood cells and resultant blurring in the interference patterns18, 19 was detected by a CCD camera (Dalsa 1M60, Waterloo, Ontario, Canada) and image acquisition was performed using EPIX XCAP software (v3.2) (Buffalo Grove, IL, USA). A total of 15 sweeps for each stimulation parameter consisting of 130 frames were acquired at 10 Hz with a 10 ms exposure time. Using Matlab (Mathworks, Natick, MA, USA), each 1024 × 1024 pixel image in the stack was variance-filtered spatially with a kernel size of 3 pixels. Blood flow measurements were estimated as the inverse square of speckle contrast values which were scaled between minimum and maximum flow rate (Figure 2B).20 Minimum flow was determined from contrast values measured from the mouse cortex after it was killed. For stimulation, a 473 nm laser beam (Crystalaser, focused to ~100 μm diameter, 5 ms pulses, 2 mW, 100 Hz, 0.1 to 1 second) was targeted to forelimb sensorimotor cortex.14 Light from the 473 nm laser was blocked from contaminating the speckle signal as detected by the camera by a 715 nm longpass filter (Edmund Optics, Barrington, NJ, USA). Regions of interest selected for assessment of speckle contrast over time were 1 mm2 and centered at the site where the laser was targeted on the cortex. Intrinsic optical signal imaging was conducted with 630 nm LEDs illuminating the cortex, while a CCD Dalsa 1M60 camera was used to measure changes in reflectance. These alterations are considered to be mediated by changes in blood volume and the ratio of oxy and de-oxy hemoglobin.16 Each run consisted of 20 trials and was obtained at 10 Hz with an exposure period of 100 ms. Reported values for intrinsic optical signal are percent fractional change in reflectance, relative to pre stimulus levels, from a 1 mm2 region of interest (centered over the peak response).
Pharmacology
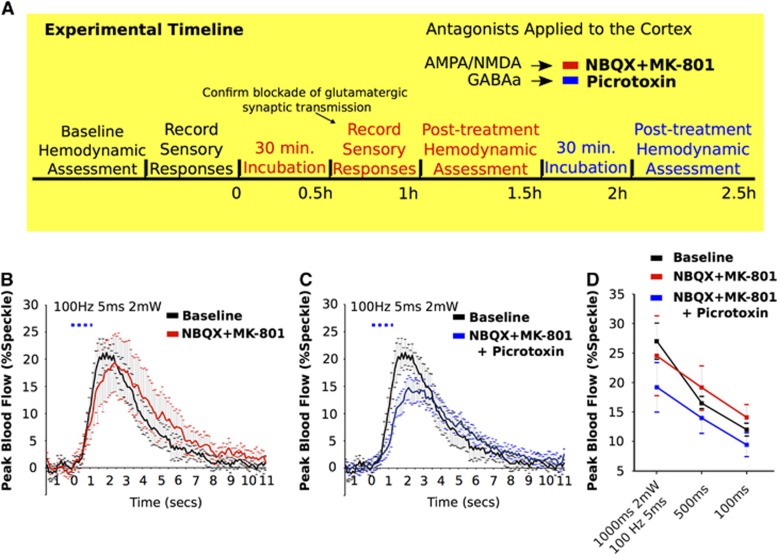
After baseline assessment of light-evoked hemodynamic responses, the agarose was removed from the surface of cortex and NBQX and MK-801 in a final concentration of 200 μM and 300 μM, respectively, was diluted in artificial cerebral spinal fluid and incubated directly on the cortex for 30 minutes. Agarose was then reapplied to the cortex at 37 °C, covered with a cover slip, and laser speckle contrast imaging was performed. This antagonist treatment significantly reduces spontaneous11 and sensory-evoked cortical activation.21, 22, 23 This process was repeated in the same animal for a second time with the incubation of picrotoxin (100 μM) (Figure 4A). We applied picrotoxin only in the presence of glutamatergic antagonists to prevent disinhibition of pyramidal neurons (and bursting) and isolate the direct inhibitory neuron-mediated hemodynamic response.
Statistics
To perform statistical testing, we used Graphpad Prism (GraphPad Software, La Jolla, CA, USA) and Igor Pro v.6.2 (Wavemetrics, Lake Oswego, OR, USA). Results from analyses of variance (ANOVA) and t-tests are reported as mean±s.e.m. Bonferroni post tests were used in combination with ANOVAs. In one-tailed student t-tests performed on data that were normalized to baseline, the hypothetical mean of 100% was used. Statistical significance: *P<0.05, **P<0.01, ***P<0.001.
Results
Optogenetic Activation of Cortical Inhibitory Interneurons Increases Local Blood Flow
Stimulation of cortical inhibitory interneurons (2 mW, 100 Hz, 5 ms) evoked a 26.5±3.0% increase in speckle signal, indicative of increases in local blood flow, in anesthetized VGAT ChR2 mice (Figures 2C and 2D). At shorter durations of stimulation, 0.5 and 0.1 seconds, increases in blood flow were 16.5±1.2 and 12.0±1.1%, respectively (Figures 2E and 2F). A one-way ANOVA (n=6) revealed a significant difference between the amplitude of hemodynamic responses from different durations of stimulation (P=0.0008). Comparisons within groups (Bonferroni tests) showed differences between 1 and 0.1 seconds (P<0.001), and 0.5 and 0.1 seconds (P<0.05) of stimulation. The time to peak amplitude of the evoked alteration in speckle signal from 1, 0.5, and 0.1 seconds of stimulation was 3.7±0.26 seconds, 3.4±0.17 seconds, and 3.3±0.26 seconds, respectively. No significant difference between these values was observed. Littermates of experimental animals that did not express ChR2 (n=2), even when photo-stimulated with the highest power and longest duration used in these experiments, did not evoke increases in blood velocity (as measured by laser speckle contrast imaging) (Figure 2D). Stimulation of Thy1-YFP animals (n=4; no ChR2 expression) with a higher power (3 mW) showed that YFP emissions and/or spurious light from the laser did not contribute to changes in laser speckle imaging signals (Figure 2D).
Confirmation of light activation of inhibitory neurons was assessed in a separate group of animals (n=3) in which unit activity recordings were performed during trials of 1 second of light stimulation (2 mW, 100 Hz, 5 ms) (Figures 1B and 1C). We assessed the difference in the number of spikes during and after stimulation relative to baseline. During the time Channelrhodopsin-2 was photoactivated, spiking was reduced to 5.7% of baseline (P<0.0001). In the second after stimulation, spiking partially recovered, increasing to a rate equivalent to 60.8% (P=0.0216) of baseline. It recovered to 105% (P=0.7941) of baseline in the subsequent second (Figure 1D).
Application of Ionotropic Glutamatergic Antagonists Blocks Sensory-Evoked Activity and Attenuates Hemodynamic Responses
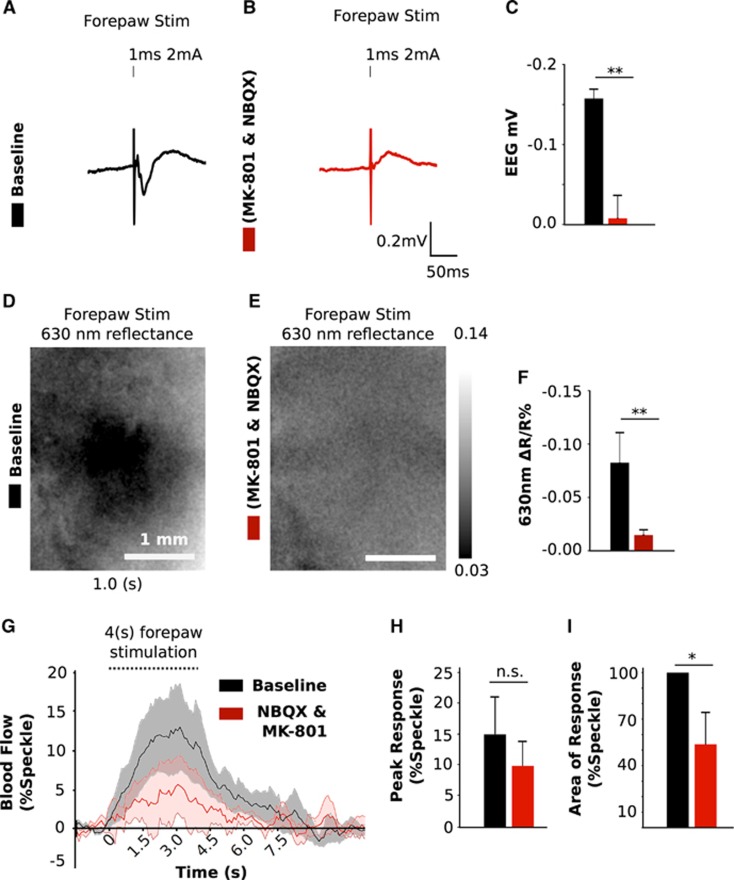
The effect of applying α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate receptor antagonists (NBQX; 200 μM and MK-801; 300 μM) directly to the cortex was assessed by measuring alterations in sensory-evoked responses detected with EEG as well as speckle contrast and intrinsic signal imaging (Figure 3). A single 1 ms (2 mA) pulse of stimulation delivered to the forepaw led to a 0.157±0.011 mV deflection which was blocked (0.007±0.02 mV) after application of the antagonists (P=0.006; n=4; Figures 3A and 3C). The initial dip of the hemodynamic dependent intrinsic signal from 1 second (100 Hz, 1 ms, 1 mA) of sensory stimulation (−0.085±0.028%) was also blocked (−0.007±0.005%) (P=0.002; n=5; Figures 3D and 3F). Speckle contrast imaging responses from 4 seconds of stimulation at the same intensity and frequency as intrinsic optical signal imaging led to a 15±5.2% increase in blood flow. This response was reduced to 9.8±3.1% with the application of ionotropic glutamatergic antagonists (n=6; Figures 3G and 3H). The area under the curve of these responses decreased to 51.2±23.0% of baseline (P=0.044; n=6; Figure 3I)
Figure 3.
Effect of glutamatergic antagonists on sensory responses. Example of the sensory-evoked response measured with electroencephalography (EEG) before (A) and after (B) application of glutamatergic antagonists. (C) Group data of peak EEG response (n=4). Average change of 630 nm reflectance after sensory stimulation from 1 second of activity captured before (D) and after (E) treatment (% change). (F) Peak 630 nm reflectance response (initial dip) after sensory stimulation of the forepaw from a 1 mm2 region of interest captured before and after application of glutamatergic antagonists (n=5). (G) Time course and (H) peak amplitude and area under the curve (I) of sensory-evoked hemodynamic responses from 4 seconds of stimulation before and after treatment (n=6). All values are mean values±s.e.m.
Optogenetically Evoked Increases in Blood Flow are Largely Independent of Ionotropic Glutamatergic Synaptic Transmission and are not Attenuated by Gamma Aminobutyric Acid (GABA)-A Receptor Antagonists
We sought to confirm that the optogenetically evoked increases in blood flow were not mediated indirectly via ionotropic glutamatergic synaptic mechanisms. After application of antagonists directly to the cortex (NBQX; 200 μM and MK-801; 300 μM), speckle responses evoked from optogenetic stimulation (1.0, 0.5, and 0.1 seconds of 100 Hz 5 ms 2 mW stimulation) remained fairly consistent (27.0±3.0%, 16.5±1.1%, and 12.0±1.0%) with pre-treatment stimulus-evoked responses (24.5±7.6%, 19.1±3.6%, 14.1±2.1%). No significant differences were found between the evoked responses elicited by the same stimulation duration (Figures 4B and 4D). After application of GABA-A antagonist these values were 19.2±4.2%, 13.9±2.6%, and 9.4±2.0% repeated measures ANOVA showed no significant differences in evoked blood flow as a consequence of either pharmacological manipulation. Two-way ANOVA revealed no significant interaction between stimulation duration and pharmacology treatment. Similarly, when examining the relative change of the area under the curve of these (light-evoked) responses, the pharmacological cocktail had no significant effect. One second of light stimulation yielded responses 108±24.5% and 94.0±35.2% compared with baseline after application of glutamatergic or glutamatergic and GABAergic antagonists combined.
Figure 4.
Glutamatergic and GABA-A receptor antagonists do not attenuate hemodynamic responses evoked by cortical optogenetic stimulation in VGAT-ChR2 mice. (A) Timeline for pharmacology experiments. Baseline hemodynamic responses were assessed before application of NBQX and MK801 to the brain. Post-treatment hemodynamic assessment began after a 30 minutes incubation period. Picrotoxin was directly applied to the cortex (which already has been incubated with glutamatergic antagonists) for 30 minutes before hemodynamic responses were assessed for a third time in the same animal. (B) Time course from optogenetically evoked increases in blood flow from 1.0 second stimulation, at baseline and after application of NBQX and MK-801 (B), and after incubation with Picrotoxin (C). (D) Alteration in peak blood velocity (laser speckle) at three stimulus durations (1.0, 0.5, and 0.1 seconds) from each condition (Baseline, glutamatergic antagonists, and after the addition of picrotoxin). All values are mean values±s.e.m.
Discussion
Activation of Cortical Inhibitory Neurons is Sufficient to Increase Local Blood Flow
The balance between activity originating from excitatory and inhibitory neurons can affect local blood flow in a region-specific manner. Empirically, this has been observed as a direct relationship between local neuronal activity and hemodynamic dependent imaging signals.24 However, under certain conditions, mismatches between local neurometabolic/neurovascular signals and neuronal activity have been observed.25, 26, 27 This may be due to a complex regulation of blood flow where both the cell type and transmitter released are a factor in shaping hemodynamic responses.28 Notably, interneurons have the capacity to decrease the activity of principal neurons,29 cells which are known to increase local blood flow.30 Additionally, interneurons can release compounds which exert bidirectional control over blood vessel diameter.8 The net effect from direct optogenetic activation of inhibitory neurons is an increase in local blood flow (Figures 2C and 2E); however, we cannot discount situations where predominant activation of subpopulation of interneurons31 responsible for constriction32 could decrease local blood flow. The observation that activation of inhibitory neurons can increase local blood flow is provoking; however, the magnitude and proportion that these neurons contribute to functional hyperemia31 while interacting with other cell types remains to be exacted.
The Nature of the Light-Evoked Cortical Inhibitory Neuron-Mediated Hemodynamic Response
VGAT-ChR2(H134R)-EYFP mice have a diffuse cortical expression of ChR2, with the channel found in all subtypes of cortical GABAergic interneurons.2 Photoactivation of ChR2 induces neuronal firing.2, 33, 34 We sought to investigate the polarity of the cerebral blood flow response as a consequence of activating inhibitory circuits (Figure 1). Because the majority of recorded cortical activity is dependent on ionotropic glutamatergic and GABAergic synaptic transmission,35 we took the approach of applying antagonists of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/N-methyl-D-aspartate and GABA-A receptors to test whether the observed light-evoked hemodynamic response would persist perhaps through alternate routes such as the release of vasoactive compounds,8, 36 interactions with metabotropic receptors on astrocytes,37 metabolic feedback,38, 39 and/or extracellular ion flux.40
In accordance with previous work,14, 15 applying glutamatergic receptor antagonists directly to the cortex blocked sensory-evoked responses as measured with intrinsic optical signal and cortical EEG recordings. The light-evoked hemodynamic response endured even in the presence of high concentrations of ionotropic glutamatergic antagonists (Figure 4); this suggests that secondary excitatory signaling was not mediating the light-evoked blood flow response within GABA neurons. The sensory-evoked hemodynamic response (laser speckle contrast) persisted at a lower level after application of glutamatergic antagonists to the cortex (Figures 3G and 3I). This suggests that non-ionotropic glutamatergic circuits known to mediate functional hyperemia41, 42 that interact with cortical interneurons8, 31 may in part drive sensory responses. Alternatively, the longer wavelength used in laser speckle as opposed to hemodynamic-dependent intrinsic signal imaging may offer a signal from deeper cortical layers which may be less affected by the diffusion of pharmacological agents applied to the surface of the cortex.
Experiments performed in hippocampal brain slices43 and in isolated canine vessels44 showed that GABA-A receptor agonists could dilate the vasculature. However, in vivo evidence from stimulation of excitatory circuits in the cerebellum indicate that high concentrations of GABA-A receptor antagonist do not effect basal or evoked cerebral blood flow.36, 45 Recently, low doses of GABA agonists have been found to increase blood flow in the mouse somatosensory cortex,46 this is perhaps through di-synaptic disinhibition of pyramidal neurons,47 or activation of astrocytic GABA-A-Rs,48 which can evoke calcium transients46, 49 and increase blood flow.38, 50 To investigate whether these mechanisms were responsible for the observed increases in blood flow, we assessed and observed light-evoked hemodynamic responses in VGAT-ChR2 mice after incubating the cortex with GABA-A, N-methyl-D-aspartate, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonists. Although GABA-A receptors may have an indirect role in mediating in sensory-evoked hyperemia in the cortex, under these experimental conditions, the ineffectiveness of GABA-A receptor antagonism in blocking the light-evoked hemodynamic response suggests that other interneuron-mediated pathways can contribute to elevations in brain blood flow. This may occur via interneuron release of factors such as vasoactive intestinal polypeptide or perhaps nitric oxide,8, 36, 51 which acts in the endothelium to relax smooth muscle52 of cortical vessels.53 Our data suggest that interneuron-mediated increases in blood flow can persist despite ionotropic GABA and glutamate receptor antagonism. Further study of mechanisms by which optogentically stimulated GABA neurons increase blood flow may be achieved from the application of antagonists to additional downstream factors. Here, we show that this response can persist despite our interference with ionotropic synaptic mechanisms. However, we do not rule out a role for GABA-A receptors in regulating functional hyperemia at the circuit level. We stress that these results are most relevant to the case of direct GABA neuron activation, and do not exclude roles for ionotropic GABA receptors in regulating network function and their associated hemodynamic responses. Furthermore, we have not implicitly tested the role of metabotropic GABA receptors.
Cortical Photostimulation of GABAergic Neurons Attenuates Spontaneous Spiking Activity
The use of optogenetics facilitates our ability to directly stimulate inhibitory neurons. Activation of inhibitory neurons can silence firing of principal neurons in barrel cortex.29 We show this phenomenon in the forelimb sensorimotor cortex (Figure 1) with stimulation parameters used here to increase local blood flow (Figures 2C and 2F). Stimulation of GABAergic neurons can both increase blood flow and inhibit spiking activity intrinsic to the brain conferring a situation where levels of cortical electrical activity and hemodynamics are dissociated. These results support the notion that sufficient activation of interneurons can increase blood flow irrespective of discharge in surrounding principal cells.1, 54 We show that this stimulation of interneurons attenuates local activity, whereas it can increase local blood flow. Future work may be directed towards understanding the degree to which the dissociation between the polarity of blood flow responses and neuronal activity occurs within activity intrinsic to the brain.
Conclusion
In summary, we find that direct optogenetic activation of cortical GABA neurons can increase local blood flow while suppressing spontaneous neuronal activity. The light-evoked hemodynamic response originating from activation of interneurons persisted despite antagonism of ionotropic glutamatergic and GABAergic receptors. Our data support a model whereby interneuron-mediated alterations in blood flow can be regulated by mechanisms that operate at least in part independently from the net state of ongoing neuronal activity. Although the contribution of interneurons to functional hyperemia remains unclear and experimentally challenging to address, we begin working towards a cell type-specific understanding of neurovascular coupling by showing that GABA neurons increase blood flow even without the contribution of excitable synaptic networks.
Acknowledgments
The authors thank Cindy Jiang for assistance with surgery and animal husbandry
Author Contributions
EA designed research, performed research, analyzed data, and wrote the paper. AWC and YX helped design the research. JL contributed tools for analysis and provided technical support. THM designed research, provided financial support, and assisted with writing.
The authors declare no conflict of interest.
Footnotes
This work was supported by a Canadian Institutes of Health Research (CIHR) Operating Grant MOP-12675, a Heart and Stroke Foundation of BC and Yukon grant in aid, and a Heart and Stroke Foundation of Canada grant in aid.
References
- 1Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci 2006; 29: 449–476. [DOI] [PubMed] [Google Scholar]
- 2Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 2011; 8: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459: 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 2005; 309: 948–951. [DOI] [PubMed] [Google Scholar]
- 5Sumiyoshi A, Suzuki H, Ogawa T, Riera JJ, Shimokawa H, Kawashima R. Coupling between gamma oscillation and fMRI signal in the rat somatosensory cortex: Its dependence on systemic physiological parameters. NeuroImage 2012; 60: 738–746. [DOI] [PubMed] [Google Scholar]
- 6Scheeringa R, Fries P, Petersson K-M, Oostenveld R, Grothe I, Norris DG et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron 2011; 69: 572–583. [DOI] [PubMed] [Google Scholar]
- 7Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol 1986; 245: 553–565. [DOI] [PubMed] [Google Scholar]
- 8Cauli B, Tong X-K, Rancillac A, Serluca N, Lambolez B, Rossier J et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 2004; 24: 8940–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Girouard H, Iadecola C. ‘Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006; 100: 328–335. [DOI] [PubMed] [Google Scholar]
- 10Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010; 468: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci 2008; 28: 1756–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Xie Y, Chen S, Anenberg E, Murphy TH. Resistance of optogenetically evoked motor function to global ischemia and reperfusion in mouse in vivo. J Cereb Blood Flow Metab 2013; 33: 1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S et al. The effect of different anesthetics on neurovascular coupling. NeuroImage 2010; 51: 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Scott NA, Murphy TH. Hemodynamic responses evoked by neuronal stimulation via channelrhodopsin-2 can be independent of intracortical glutamatergic synaptic transmission. PloS One 2012; 7: e29859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Ayling OGS, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods 2009; 6: 219–224. [DOI] [PubMed] [Google Scholar]
- 16Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci 2007; 27: 4572–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Royl G, Leithner C, Sellien H, Müller JP, Megow D, Offenhauser N et al. Functional imaging with laser speckle contrast analysis: vascular compartment analysis and correlation with laser Doppler flowmetry and somatosensory evoked potentials. Brain Res 2006; 1121: 95–103. [DOI] [PubMed] [Google Scholar]
- 18Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas 2001; 22: R35–R66. [DOI] [PubMed] [Google Scholar]
- 19Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab 2001; 21: 195–201. [DOI] [PubMed] [Google Scholar]
- 20Cheng H, Duong TQ. Simplified laser-speckle-imaging analysis method and its application to retinal blood flow imaging. Opt Lett 2007; 32: 2188–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Lim DH, Mohajerani MH, LeDue J, Boyd J, Chen S, Murphy TH. In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas. Front Neural Circuits 2012; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Chen S, Mohajerani MH, Xie Y, Murphy TH. Optogenetic analysis of neuronal excitability during global ischemia reveals selective deficits in sensory processing following reperfusion in mouse cortex. J Neurosci 2012; 32: 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Harrison TC, Ayling OG, Murphy TH. Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron 2012; 74: 397–409. [DOI] [PubMed] [Google Scholar]
- 24Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412: 150–157. [DOI] [PubMed] [Google Scholar]
- 25Devor A, Hillman EM, Tian P, Waeber C, Teng IC, Ruvinskaya L et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J Neurosci 2008; 28: 14347–14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Huo B-X, Smith JB, Drew PJ. Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J Neurosci 2014; 34: 10975–10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 2009; 457: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Kleinfeld D, Blinder P, Drew PJ, Driscoll JD, Muller A, Tsai PS et al. A guide to delineate the logic of neurovascular signaling in the brain. Front Neuroenergetics 2011; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT et al. Flow of cortical activity underlying a tactile decision in mice. Neuron 2014; 81: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E et al. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab 2009; 29: 976–986. [DOI] [PubMed] [Google Scholar]
- 32Urban A, Rancillac A, Martinez L, Rossier J. Deciphering the neuronal circuitry controlling local blood flow in the cerebral cortex with optogenetics in PV::Cre transgenic mice. Front Pharmacol 2012; 3: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 34Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100: 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 2012; 13: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Li J, Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology 1994; 33: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 37Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann K-A, Pozzan T et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 2003; 6: 43–50. [DOI] [PubMed] [Google Scholar]
- 38Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008; 456: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 2003; 299: 1070–1072. [DOI] [PubMed] [Google Scholar]
- 40Newman EA. REVIEW ▪: Regulation of extracellular K and pH by polarized ion fluxes in glial cells: the retinal Müller cell. Neuroscientist 1996; 2: 109–117. [Google Scholar]
- 41Toussay X, Basu K, Lacoste B, Hamel E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci Off J Soc Neurosci 2013; 33: 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Kocharyan A, Fernandes P, Tong X-K, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab 2008; 28: 221–231. [DOI] [PubMed] [Google Scholar]
- 43Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J Cereb Blood Flow Metab 1997; 17: 992–1003. [DOI] [PubMed] [Google Scholar]
- 44Edvinsson L, Krause DN. Pharmacological characterization of GABA receptors mediating vasodilation of verebral arteries in vitro. Brain Res 1979; 173: 89–97. [DOI] [PubMed] [Google Scholar]
- 45Akgören N, Dalgaard P, Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res 1996; 710: 204–214. [DOI] [PubMed] [Google Scholar]
- 46Jessen SB, Brazhe A, Lind BL, Mathiesen C, Thomsen K, Jensen K et al. GABAA receptor-mediated bidirectional control of synaptic activity, intracellular Ca2+, cerebral blood flow, and oxygen consumption in mouse somatosensory cortex in vivo. Cereb Cortex e-pub ahead of print 31 March 2014. [DOI] [PubMed]
- 47Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Lévesque M et al. Pyramidal neurons are ‘neurogenic hubs' in the neurovascular coupling response to whisker stimulation. J Neurosci 2011; 31: 9836–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Fraser DD, Mudrick-Donnon LA, MacVicar BA. Astrocytic GABA receptors. Glia 1994; 11: 83–93. [DOI] [PubMed] [Google Scholar]
- 49Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA 2013; 110: E4678–E4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci 2007; 27: 6268–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Perrenoud Q, Rossier J, Ferezou I, Geoffroy H, Gallopin T, Vitalis T et al. Activation of cortical 5-HT3 receptor-expressing interneurons induces NO mediated vasodilatations and NPY mediated vasoconstrictions. Front Neural Circuits 2012; 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 1983; 306: 174–176. [DOI] [PubMed] [Google Scholar]
- 53Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 2014; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Thomsen K, Offenhauser N, Lauritzen M. Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol 2004; 560: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]