Abstract
To aid in development of chronic stage treatments for sensorimotor deficits induced by ischemic stroke, we investigated the effects of GABA antagonism on brain structure and fine skilled reaching in a rat model of focal ischemia induced via cortical microinjections of endothelin-1 (ET-1). Beginning 7 days after stroke, animals were administered a gamma-aminobutyric acid (GABAA) inverse agonist, L-655,708, at a dose low enough to afford α5-GABAA receptor specificity. A week after stroke, the ischemic lesion comprised a small hypointense necrotic core (6±1 mm3) surrounded by a large (62±11 mm3) hyperintense perilesional region; the skilled reaching ability on the Montoya staircase test was decreased to 34%±2% of the animals' prestroke performance level. On L-655,708 treatment, animals showed a progressive decrease in total stroke volume (13±4 mm3 per week), with no change in animals receiving placebo. Concomitantly, treated animals' skilled reaching progressively improved by 9%±1% per week, so that after 2 weeks of treatment, these animals performed at 65%±6% of their baseline ability, which was 25%±11% better than animals given placebo. These data indicate beneficial effects of delayed, sustained low-dose GABAA antagonism on neuroanatomic injury and skilled reaching in the chronic stage of stroke recovery in an ET-1 rat model of focal ischemia.
Keywords: behavioral recovery, chronic treatment, GABA antagonism, magnetic resonance imaging, stroke rat modeling
Introduction
The majority of ischemic stroke patients (as much as 92% in some centers1) arrive at a care facility beyond the window of opportunity (i.e., 3 to 4.5 hours after the onset of symptoms) for acute treatment with tissue plasminogen activator, leaving rehabilitation as the only means to improve outcome.2 In the long term, most ischemic stroke patients suffer moderate to severe disabilities, and up to one-third require institutionalization.3 The need for restorative treatments that may be administered during the chronic stage (days to weeks after an ischemic event) is thus pressing.
It is now widely believed that the chronic stage recovery involves tissue remodeling—through the generation of new neurons and glia, axonal sprouting, and/or synaptogenesis4—in the periinfarct zone, a surviving meta-stable region of ‘at-risk' tissue that exhibits heterogeneity in both acute-stage perfusion deficit and long-term tissue fate.5 Indeed animal studies suggest that the period to support and/or enhance remapping of sensorimotor function within the periinfarct zone may be sufficiently long to enable effective treatment in the subacute and chronic stages.6, 7 Although neurons in the periinfarct zone had long been considered hyperexcitable,8 a recent study in a mouse photothrombic model of focal ischemia by Clarkson et al6 reports that the initial acute phase of hyperexcitability is followed (on the third day after photothrombosis) by abnormally high levels of tonic inhibition (proposed to be mediated by decreased extrasynaptic gamma-aminobutyric acid (GABAA) uptake) resulting in neuronal hypoexcitability. Clarkson et al show that infusing L-655,708, a GABAA-receptor inverse agonist, using ALZET-1002 mini pumps (Cupertino, CA, USA) 3 days post stroke improves sensorimotor function on the grid walking and cylinder tasks. By 7 days after stroke, animals treated with 400 μg/kg/day of L-655,708 have fewer foot faults on grid walking task (1.8 times the forelimb foot faults before stroke in the treated group versus 2.5 times in the control group) and lower affected-to-unaffected forelimb use difference on cylinder task (19% discrepancy in the treated group versus 35% discrepancy in the control group). Spurred by this report and in keeping with the recommendations of the Stroke Treatment Academic Industry Roundtable,9 we investigated the effects of L-655,708 on brain anatomy and forelimb skilled reaching over 3 weeks after focal ischemia induced by cortical microinjection of endothelin-1 (ET-1).
We used rats to evaluate the effects of L-655,708 and to enable the utilization of the Montoya skilled reaching task, which has been shown to be highly sensitive to sensorimotor injury while exhibiting much less spontaneous recovery than that seen with measures of spontaneous activity and neurologic test batteries.10 Cortical microinjection of ET-1 was performed to more faithfully recapitulate the kinetics of flow impairment observed in human stroke, and to produce a lesion comprised of a substantial periinfarct zone surrounding the necrotic core, as frequently observed in patients, where periinfarct region comprises up to 35% of the total volume of injury.11, 12, 13, 14, 15, 16 In contrast to Clarkson et al, we implanted the L-655,708 tablets subcutaneously and used a dosing regimen, following Atack et al,17 that results in a steady-state plasma drug concentration that is low enough to produce selective α5-subunit-containing GABAA receptor occupancy during the course of treatment. We thereby assessed drug effects following a clinically relevant delivery method at a dose that minimizes side effects by preserving receptor subtype specificity.17
Materials and Methods
Subjects
All experimental procedures in this study adhered to the guidelines specified by animal research: reporting in vivo experiments (ARRIVE) and were approved by the Animal Care Committee of the Sunnybrook Health Sciences Centre, in accordance with the Policies and Guidelines of the Canadian Council on Animal Care and the requirements of the Provincial statute of Ontario, Animals for Research Act as well those of the Federal Health of Animals Act.18 Forty-four adult male Sprague-Dawley rats (Charles River, Montreal, Canada) weighing 340±50 g (mean±s.d.) at the time of surgery were included in this study. Thirty-seven animals underwent stroke induction (by intracortical ET-1 injection), while the remaining 7 animals underwent sham stroke induction procedures (intracortical injection of vehicle, phosphate-buffered saline, PBS). Animals were housed in pairs on a 12-hour light/dark cycle. Administration of drug, magnetic resonance imaging (MRI) and behavior trials were performed during the light phase. Food and water were freely available except during behavioral test periods (14 consecutive days before stroke, and on days 4 to 6, 11 to 13, and 18 to 20) when food was restricted to 12 to 15 g per day. Throughout the study, the body weight of each animal was maintained at >90% of the free-feeding weight, see Supplementary Figure 1.
Stroke Induction
All animals underwent the same stroke induction or sham surgical procedure under isoflurane anesthesia (5% induction and 2% to 2.5% maintenance). Continuous physiologic monitoring was conducted throughout the procedure (Table 1) to ensure physiologic stability. An ischemic injury was induced via two intracortical microinjections of ET-1, into the forelimb area of the right primary sensorimotor cortex, as described in detail below.12 Sham surgery involved intracortical microinjection of PBS (vehicle) alone.
Table 1. Physiologic parameters during stroke induction and imaging.
| Temperature (°C) | Heart rate (bpm) | Breath rate (breaths per mimute) | O2 Saturation (%) | |
|---|---|---|---|---|
| Stroke induction | 37.5±0.2 | 319±20 | 45±5 | 98±1 |
| MRI sessions | 37.2±0.5 | 331±43 | 68±15 | 99±1 |
Abbreviations: bpm, beats per minute; MRI, magnetic resonance imaging.
Physiologic parameters during stroke induction with animals on 2% to 3% isoflurane, and during MRI with animals receiving a continuous intravenous infusion of propofol (45 mg/kg/h).
Animals were secured in a small animal stereotaxic apparatus (David KOPF Instruments, Tujunga, CA, USA). Under aseptic condition, a midline incision was made, and two burr holes (2 mm in diameter) were drilled (relative to bregma) at 0.0 mm anteroposterior (AR), −2.5 mm ML; and at 2.3 mm AP, −2.5 mm mediolateral (ML) over the right sensorimotor cortex using a high-speed micro drill (Foredom Electric, Bethel, CT, USA).12 A 10-μL Hamilton syringe (Franklin, MA, USA) was used to deliver 800 picomoles of ET-1 suspended in 4 μL of PBS, or PBS alone (sham surgery), at −2.3 mm dorsoventral (DV): 400 picomoles were delivered in 2 μL aliquots through each burr hole. After lowering the needle to −2.5 mm and retracting it to −2.3 mm DV, a 1-minute delay was allowed before injection began. A further 1-minute delay was kept between the delivery of each microliter, and a 2-minute delay preceded needle retraction. The injection was performed at a rate of 1 μL/min, for a total delivery time of 12 minutes (including four, 1-minute delays and two, 2-minute delays). Burr holes were closed with bone wax and the scalp was sutured over the skull. For analgesia, animals were given a subcutaneous dose of marcaine (0.2 mg/kg) suspended in PBS at the beginning and at the end of surgery.
Drug Treatment
Animals were divided in three groups: two groups with stroke (which received ET-1 microinjections) that were administered either L-655,708 treatment (first group) or a placebo (second group, treatment control), and sham-operated animals treated with L-655,708 (third group, surgical control). All animals underwent subcutaneous implantation of a tablet every 24 hours for 2 weeks beginning 1 week after stroke induction. Animals received tablets containing either 1.5 mg of L-655,708 (Tocris Bioscience, Minneapolis, MN, USA, cat. no. 1327) in 58.5 mg of high-viscosity hydroxypropyl methylcellulose (Sigma-Aldrich, CAS no. 9004-65-3), for the treated groups (one group with ET-1 induced injury and one group that underwent sham surgery); or 60 mg of high-viscosity hydroxypropyl methylcellulose for the placebo group (with ET-1 induced stroke).17 We thereby followed the dosing formulation that affords α5-subunit-containing GABAA-receptor specificity and clinically relevant dosing kinetics, as described by Atack et al,17 resulting in low, sustained drug concentration in the blood plasma (125 to 150 ng/ml) and brain tissue (50 to 60 ng/ml).17 Treatment group allocation of stroke animals was randomized.
Solid tablets were formulated in-house using a hand press. Animals were anesthetized with isoflurane (5% induction and 2% to 2.5% maintenance) for each tablet implantation procedure, which lasted <10 minutes. Under aseptic conditions, an incision was made in the skin overlying the fat pad on the neck of each animal and a tablet placed between the fat pad and the skin. The skin was then sutured and a subcutaneous dose of marcaine (0.1 mg/kg) suspended in PBS administered. During the course of treatment, three independent incisions were made for pill implantation. Each incision was used repeatedly by removing the stitches from the previous day and placing a new tablet within the pocket. To avoid excessive trauma to the tissue, each pocket was used for only five consecutive pill implantations. Animals were monitored daily; we observed no instances of infection or evidence of significant irritation.
Magnetic Resonance Imaging
All animals were imaged before surgery and at weekly intervals thereafter (on days 7, 14, and 21) on a 7 T Bruker BioSpec system (Ettlingen, Germany). Animals were briefly anesthetized with isoflurane (5% induction and 2% to 2.5% maintenance) for the placement of an intravenous catheter; isoflurane was discontinued as soon as the catheter was secured. Once responsive, a bolus (7.5 mg/kg) of propofol was administered intravenously and its continuous infusion (45 mg/kg/h) maintained while T2-weighted structural images were acquired. Continuous physiologic monitoring was conducted throughout all imaging sessions (Table 1) to ensure physiologic stability.
A birdcage body coil was used for signal excitation and a quadrature receive–only coil for signal reception. T2-weighted structural images were obtained with a rapid acquisition with relaxation enhancement sequence using a rapid acquisition with relaxation enhancement factor of 8, repetition time of 5,500 ms, echo time of 47 ms, and a matrix size of 128 × 256. Forty-five 0.5-mm-thick coronal slices with a nominal in-plane spatial resolution of 0.1 × 0.1 mm2 were obtained in 12 minutes.
Images were imported into the ImageMagick Display Studio LLC Command-Line tool for semiautomated segmentation.19 By using predetermined signal thresholds for two volumes of interest—necrotic core and perilesional zone—serial coronal images were segmented.20 Following previous work, voxels with signal >2 s.d. above the mean signal in the corresponding contralesional region of interest were classified as perilesional tissue; and voxels with signal 2 s.d. below the mean signal in the corresponding contralesional region of interest were classified as necrotic tissue.20, 21, 22, 23 Single voxel dilation and small hole (<6 voxels in three-dimensional diameter) filling were applied within slices and between neighboring slices. The volumes of necrotic core, perilesional tissue, and the total stroke volume (comprising necrotic and perilesional tissue) were computed at each time point for each animal. Animals in the sham surgery group (injected with PBS) showed no evidence of cortical damage (Supplementary Figure 2).
Behavioral Assessment
For behavioral assessment, each animal was placed in a staircase test box, which isolates the left from the right forelimb with a central platform. During each trial, three Noyes precision pellets (45 mg, Research Diets, New Brunswick, NJ, USA) were placed on each of the 14 steps, 7 on the right side and 7 on the left side of the box. Animals were allowed 15 minutes within the staircase box to retrieve and eat pellets. Whenever a pellet was dropped, it fell to the base of the box where it could not be retrieved. At the conclusion of each trial, the number of pellets successfully eaten with either the right or left forepaw was recorded.
To become proficient at the skilled reaching task, animals were trained for two trials per day (with a minimum of 3 hours between trials) for a period of 2 weeks before surgery. Baseline reaching ability was estimated as the average number of pellets successfully eaten during the final six consecutive trials (i.e., trials performed on the final 3 days of the 2-week training period).12, 23 Following earlier work,24 to reach baseline training criteria (before surgery), animals had to have eaten, on average across the final six training trials, at least 12 pellets with each forepaw and exhibit no evidence of handedness (defined as a discrepancy in the average fraction of pellets eaten of >20%).12 Three days after surgery, all animals were tested for a deficit in reaching ability twice daily for three consecutive days (on days 4, 5, and 6 after stroke) to estimate initial behavioral impairment (before treatment). Testing was repeated over the course of treatment, on days 11, 12, and 13, and on days 18, 19, and 20. There was thus ~24 hours between the final behavioral testing day and the corresponding weekly MRI session. Reaching ability was reported as the number of pellets successfully eaten on each side during all trials. All behavioral training, testing and analysis were conducted without knowledge of group allocation.
Histology
Animals were euthanized by isoflurane overdose. Brains were removed immediately and placed in 10% buffered formalin. Following full fixation (i.e., after at least 4 days in formalin), brains were processed using the Leica Biosystems (Heidelberg, Germany) automated tissue processor and mounted in paraffin wax using the Leica Biosystems embedding station. To sample the volume of injury for hematoxylin and eosin (H&E) staining, we collected 50 coronal sections, 4-μm thick at 40-μm intervals (between 0.0 and 2.3 AP). Stained sections were imaged with an SCN400 Leica Biosystems scanner and viewed using the Leica Image Viewer.
Statistical Analysis
Linear mixed-effects (lme) modeling and, for reference, analysis of variance were used to evaluate the effect of L-655,708 treatment on MRI regional volumes and forelimb reaching performance over the course of recovery using the lme function in the nlme package within the R software (Boston, MA, USA).25 As detailed in Laird and Ware,26 the lme produces robust restricted maximum likelihood estimates in the presence of unbalanced allocation of subjects by factor and provides a sensitive and considerably more general (when compared with repeated measures analysis of variance and its extensions) statistical framework for analyzing these data. The lme model was particularly well suited to the analysis of the current data because it recognizes the relationship between serial observations on the same subjects while allowing for unbalanced groups (here across surgical and treatment groups in addition to across MRI versus behavioral assays). The P-values reported here are generated using the t-test of the lme function and report on the significance of the interaction using the likelihood ratio test.25 We modeled each MR volumetric measure (necrotic core, perilesional, and total stroke) and skilled reaching performance as linear functions of treatment (L-655,708 or placebo) and time, expressed in days after surgery. Contrast within and between all the three groups at both 2 and 3 weeks after injury were considered. Absolute, as well as relative (to prestroke ability or volume of injury before treatment) changes were evaluated. Subjects were treated as random effects, thus accounting for across-subject variation. To facilitate comparison to existing literature, we have also computed the Pearson's product-moment correlation coefficient following averaging.
Results
Exclusion Criteria
Before stroke induction, one animal was identified as handed (with an average discrepancy in the number of pellets eaten between forepaws of >4 pellets) and excluded from the study. Of the remaining 44 animals, which underwent surgery: 7 received PBS injections (sham surgery controls), 9 either died during ET-1 injection or had to be killed because of excessive weight loss, evidence of persistent dehydration, or loss of the righting reflex within 48 hours after ET-1 injection. Two animals were excluded from all analysis after being identified as outliers in terms of the stroke volume at day 7 after ET-1 injection (one of them had a stroke volume approximately four times the mean stroke volume of the cohort, while the other had a stroke volume approximately five times less than the mean stroke volume of the cohort). Issues with MRI hardware (radiofrequency coil) precluded acquisition of MRI data in five animals. Finally, six animals did not reach the minimum training criterion for baseline reaching ability (average of 12 pellets eaten during the final six training sessions) and were excluded from the behavioral analysis. In total, 20 animals with stroke (N=10 treated with L-655,708 and N=10 given a placebo) were included in the structural MRI analysis of stroke lesion changes. Twenty-seven animals were included in the behavioral assessment: 11 with stroke, treated with L-655,708 (including 7 that had MRI); 7 that received PBS injections and were treated with L-655,708 (all with MRI); and N=9 with stroke that were given a placebo (including 8 that had MRI). There was no evidence of handedness in this group before stroke induction, with (mean pellets eaten with left forepaw−mean pellets eaten with right forepaw) ±s.e.m. being 0.98±0.3 pellets.
Structural Assessment
Representative images resulting from the semiautomated segmentation of structural MRI data are overlaid on the corresponding T2-weighted rapid acquisition with relaxation enhancement coronal images in Figure 1. The mean volumes of each region of injury, in the two treatment groups, are listed before and after treatment in Table 2. Seven days after stroke (before treatment), the average stroke volume, across all animals, of 68±11 mm3 was comprised of 6±1 mm3 of necrotic core and 62±11 mm3 of perilesional tissue, with no difference in necrotic core (P=0.5), perilesional (P=0.4), or total stroke (P=0.4) volume between L-655,708 and placebo-treated animals in either the structural or behavioral analysis cohorts with stroke.
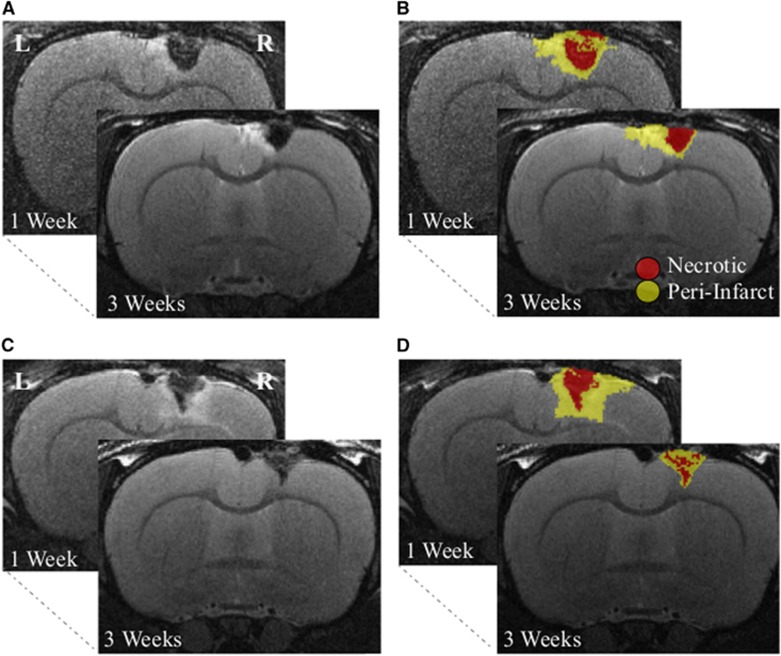
Figure 1.
Magnetic resonance imaging (MRI) data segmentation. Coronal T2-weighted MRI slices collected 7 and 14 days (i.e., before and after treatment) for an animal given a placebo (A and B) and an animal treated with L-655,708 (C and D) with semiautomated segmentation–based regions of interest (ROIs) overlaid on the right. Following previous work, voxels with signal >2 s.d. of the mean signal in the corresponding contralesional ROI were classified as perilesional tissue (yellow), and voxels with signal <2 s.d. of the mean signal in the corresponding contralesional ROI were classified as necrotic tissue (red).24, 25, 26, 27, 28
Table 2. Injury characterization.
|
7 Days (before treatment) |
21 Days (after treatment) |
||||
|---|---|---|---|---|---|
| Volume [mm3] | All subjects (N=20) | L-655,708 (N=10) | Placebo (N=10) | L-655,708 (N=10) | Placebo (N=10) |
| Total stroke | 68±11 | 77±19 | 58±12 | 52±18 | 60±18 |
| Necrotic core | 6±1 | 6±1 | 5±1 | 2.1±0.3 | 4±1 |
| Periinfarct | 62±11 | 71±19 | 53±11 | 50±18 | 56±18 |
| Skilled reaching | All subjects (N=20) | L-655,708 (N=11) | Placebo (N=9) | L-655,708 (N=11) | Placebo (N=9) |
| %Prestroke ability | 34±2 | 37±3 | 31±3 | 62±4 | 37±3 |
Sham-operated animals: 101%±5% at 7 and 107%±4% at 21 days after phosphate-buffered saline injection (N=7).
The regional volumes and skilled reaching performance before treatment (i.e., 1 week after stroke) and after 2 weeks of treatment (i.e., 3 weeks after stroke). The volumes of interest are quoted for all animals with stroke (N=21, column two); L-655,708-treated animals with stroke (N=11, columns three and five); and animals with stroke given a placebo (N=10, columns four and six). The total stroke volume comprises the necrotic core and the perilesional tissue. Skilled reaching is expressed in percentage of prestroke performance. Errors are in ±s.e.m.
Unlike placebo-treated animals, L-655,708-treated animals with stroke exhibited a progressive decrease in the volumes of injury, i.e., the perilesional zone, necrotic core, and total stroke. The cohort-wise volumes of injury at each imaging session are shown in Figure 2 along with the linear regression fits for each of the volume of interest across time (7 to 21 days after stroke). The slope of the volume of interest versus time regression was different between treatment groups, with P=0.03 for perilesional zone; P=0.01 for necrotic core; and P=0.01 for total stroke volume. In particular, each injury volume decreased with time in the L-655,708 treated but not in the placebo animals. The perilesional volume changed at a rate of −10.5±0.5 mm3 per week (P=0.007) in the L-655,708 animals versus 1±4 mm3 per week (P=0.7) in the placebo group; the necrotic core changed at a rate of −2.0±0.4 mm3 per week (P=0.00001) versus −0.4±0.4 mm3 per week (P=0.3) in the placebo group; and total stroke volume changed at a rate of −13±4 mm3 per week (P=0.002) versus 1±4 mm3 per week (P=0.8) in the placebo group.
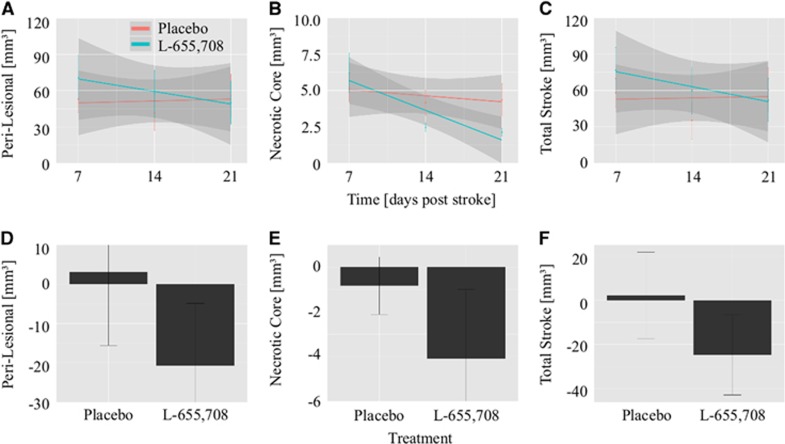
Figure 2.
Decrease in the volume of injury with treatment (mean±s.e.m.). Mean volumes within each treatment group and the regression lines corresponding to perilesional tissue (A), necrotic core (B), and total stroke volume (C) versus time for animals treated with L-655,708 (teal) and animals given a placebo (red). In animals treated with L-655,708, each volume of interest (VOI) decreased with treatment: perilesional (11±3 mm3 per week, P=0.007), necrotic core (2.0±0.4 mm3 per week, P=0.00001), and total stroke (13±4 mm3 per week, P=0.002). Animals given a placebo exhibited no change in any of the VOI over time (P>0.3). The slope for perilesional, necrotic, and total stroke volume was different between the two treatment groups (P=0.03, 0.01, and 0.01, respectively). The difference in each VOI between 7 and 21 days after stroke (before and after treatment) are plotted in D (perilesional), E (necrotic), and F (total stroke). The VOI change with treatment was significantly different between the two groups: this difference amounted to 24±11 mm3 (mean±s.e.m.) in perilesional zone (P=0.04); 3.3±1.5 mm3 in necrotic core (P=0.04); and 27±13 mm3 total stroke (P=0.03) from day 7 to day 21 after stroke.
We also contrasted, across the stroke animals between the two treatment groups, the changes in each volume of injury before versus after treatment. Animals treated with L-655,708 had a greater decrease in the volume of injury than animals given a placebo. Specifically, compared with the placebo-treated animals, the L-655,708 animals with stroke exhibited a 24±11 mm3 (mean±s.e.m.) greater reduction in perilesional volume (P=0.04); a 3.3±1.5 mm3 greater reduction in necrotic core volume (P=0.04), for a 27±12 mm3 greater decrease in total stroke volume (P=0.03). These treatment-dependent drops in the volumes of injury are shown in Figure 2D (perilesional zone), Figure 2E (necrotic core), and Figure 2F (total stroke). Animals that underwent sham stroke induction (PBS injection) showed no evidence of cortical damage at 7, 14, or 21 days after surgery and exhibited no evidence of structural changes with L-655,708 treatment (Supplementary Figure 2).
Behavioral Assessment
Before surgery, there was no difference in mean reaching ability between animals in the L-655,708-treated groups with (N=11) versus without (N=7) ET-1 injury versus the placebo group (N=9) with ET-1 injury. On average, animals consumed 16.3±0.6 pellets on the left side during the final six training sessions (animals with stroke in the L-655,708 treatment group consumed 15.1±0.4 pellets; animals with stroke given a placebo consumed 16.7±0.3 pellets, and animals in the sham surgery group consumed 17.6±0.8 pellets; i.e., animals with stroke consumed 72%±2% and 80%±2% of available pellets, respectively, while sham-operated animals consumed 84%±4% of available pellets). Seven days after surgery, before treatment, the mean reaching ability decreased similarly (P=0.8) in the L-655,708 and placebo groups with stroke, to 6±1 pellets (26%±2% of available pellets or 37%±4% of baseline ability) and 5±1 pellets (25%±3% of available pellets or 31%±4% of baseline ability), respectively. There was no change in the number or fraction of pellets retrieved by PBS-injected animals 7 days after sham surgery compared with their baseline performance (P=0.6). The skilled reaching performance levels of all groups before and after treatment (±s.d.) are listed in Supplementary Table 1.
Following earlier work, all behavioral scores were normalized, within each subject, to the subject's reaching success at baseline, i.e., before surgery. The affected (left) forepaw performance on the skilled reaching task is plotted as the percentage of presurgery ability (±s.e.m.) at 7 days after surgery (before treatment) and at 14 and 21 days after surgery (during and after treatment) for each group in Figure 3A. Superimposed is the regression of the reaching ability versus time in each cohort with the error in the fit shown as shading. In animals with stroke, the rate of reaching ability improvement was higher in the L-655,708 than in the placebo-treated animals (P=0.03). In particular, the reaching performance improved with time, by 9%±1% per week in the L-655,708 group (P=0.00001) versus 5%±1% per week in the placebo group (P=0.0006). By 14 days after stroke (after 7 days of treatment), L-655,708-treated animals consumed 23%±10% more pellets than the animals given the placebo (P=0.04), improving 17%±8% more than animals given a placebo (P=0.05). This difference increased to 25%±11% by 21 days after stroke (after 14 days of treatment; P=0.04), an improvement 19%±7% greater in the treated versus in the placebo-administered ET-1 injured animals (P=0.02). The reaching ability of sham-operated animals did not change over the 2 weeks of L-655,708 treatment (P=0.2). Figures 3B and 3C display the skilled reaching improvement in all groups at 14 and 21 days after stroke relative to their respective performance at 7 days after stroke.
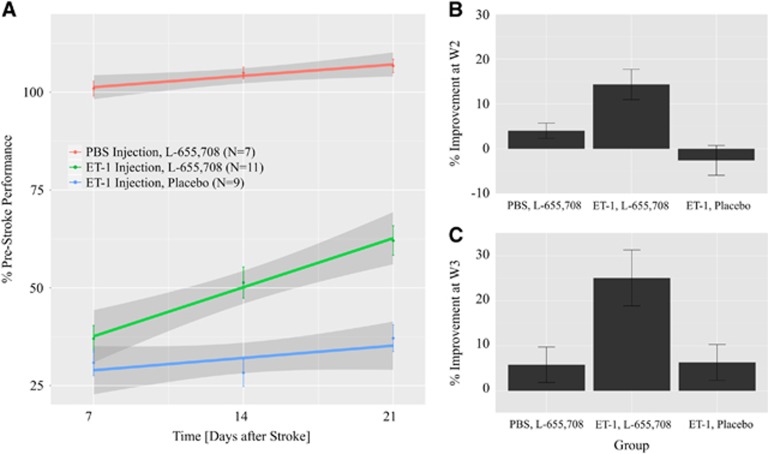
Figure 3.
Skilled reaching performance (mean±s.e.m.). (A) The affected forepaw reaching ability normalized subject wise to reaching ability before surgery (mean±s.e.m.) for all the three groups. For animals given intracortical microinjections of endothelin-1 (ET-1) and treated with L-655 708 (green), the slope was 9%±1% per week (P=0.00001); for animals induced with ET-1 and given a placebo (blue), the slope was 5%±1% per week (P=0.0006); for sham-operated animals treated with L-655,708 (red), there was no change in reaching ability before and after surgery (P=0.6), and, there was no time dependence of the reaching ability after surgery (P=0.2). The percentage of improvement was evaluated by taking the difference between performance at 14 (B) or 21 (C) days after surgery, respectively and 7 days after surgery. Animals with stroke that were treated with L-655,708 showed greater improvement than did animals with stroke given a placebo at 14 days (by 17%±8%, P=0.05) and at 21 days (by 19%±7%, P=0.02) after surgery. PBS, phosphate-buffered saline; W2, week 2 (14 days); W3, week 3 (21 days).
We next investigated whether behavioral recovery assessed with the Montoya staircase test related to the cortical changes observed on MRI in ET-1-injected animals. Figure 4 shows the regression of skilled reaching performance against perilesional (Figure 4A), necrotic (Figure 4B), and total stroke (Figure 4C) volumes for all animals for which both behavioral and MRI data were available (N=15). Skilled reaching performance decreased with increasing perilesional, necrotic, ventricular, and total stroke volume. Based on lme modeling, which obviates the need to average the six behavioral tests performed weekly, behavioral performance, normalized to prestroke performance, decreased (P=0.00001) at a rate of 0.24±0.06 per mm3 of perilesional volume, 3.2±0.6 per mm3 of necrotic volume, and 0.24±0.06 per mm3 of total stroke volume. For reference, the Pearson's product-moment coefficient (which necessitates averaging behavioral test results for weekly estimates) of normalized behavioral performance versus MRI volumes indicated a decrease in normalized behavioral performance with perilesional (P=0.0001, R=−0.6), ventricular (P=0.05, R=−0.3), and total stroke volume (P=0.0006, R=−0.5), and showed a trend toward behavioral performance decrease with necrotic volume (P=0.07, R=−0.3).
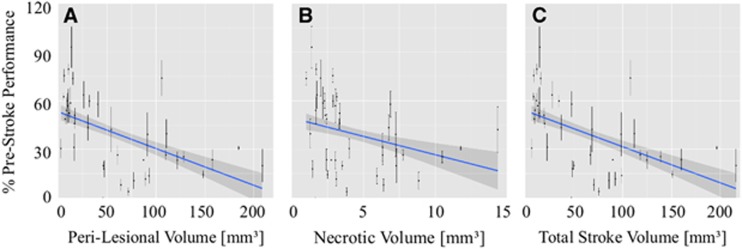
Figure 4.
Relationship between skilled reaching ability and volume of injury (mean±s.e.m.). The number of pellets eaten during each trial (normalized to performance before stroke) are plotted against the perilesional, necrotic, and total stroke volumes for each animal. From the linear mixed-effects (lme) modeling, across all animals, skilled reaching performance decreased with increasing volume of each region of injury. Reaching performance decreased by 0.24%±0.06% per mm3 of perilesional volume (A; P=0.00001), 3.2%±0.6% per mm3 of necrotic volume (B; P=0.00001), and 0.24%±0.06% per mm3 of total stroke volume (C; P=0.00001); W2, week 2 (14 days); W3, week 3 (21 days).
Histology
In agreement with the literature reports on ischemic stroke modeling via direct cortical injections of ET-1, H&E staining showed a narrow region devoid of any cells at the site of the ET-1 injection, surrounded by a region of tissue rarefaction, as illustrated in Figure 5. Glial scar formation in the perilesional region was apparent on immunohistochemical staining with glial fibrillary acidic protein and neuronal nuclei (Supplementary Figure 3).27 Close visual inspection of H&E sections and T2-weighted MRI data suggested that hypointense region of interest (necrotic core) on MRI corresponded to the cell-free region on H&E; whereas the surrounding, hyperintense region of interest exhibited tissue rarefaction on H&E, increased T2-weighted signal intensity likely resulting from accompanying edema and inflammation.27
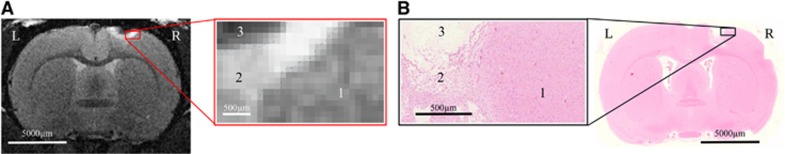
Figure 5.
Hematoxylin and eosin (H&E) staining. (A) One of 45 coronal T2-weighted magnetic resonance (MR) images collected in vivo 21 days after stroke induction in an animal given placebo, with the corresponding inset of the region of injury. (B) The corresponding H&E section and inset show differences in morphology of the three regions. Hypointense region of interest (ROI, necrotic core) on MRI corresponds to cell-free region on H&E; it is surrounded by a hyperintense ROI that exhibits tissue rarefaction and putative inflammatory cells (confirmed by immunohistochemical analysis; Supplementary Figure 3).
Discussion
Spurred by the Stroke Treatment Academic Industry Roundtable recommendations, in an ET-1 model of transient focal ischemia, we have examined a late time point treatment strategy with a dosing regimen that preserves receptor subunit–specific effects and used longitudinal MRI and a sensitive fine motor skilled reaching task to investigate recovery.9 We have found that sustained, low-dose treatment with L-655,708 progressively reduced volumes of necrotic core and perilesional tissue and improved fine motor reaching ability. Furthermore, behavioral improvement was inversely related to the necrotic, perilesional, and total stroke volume. These findings provide further evidence of the potential of sustained GABA antagonism as a late time point treatment strategy for ischemic stroke and call for further study of the underlying mechanisms of recovery to enable treatment optimization.
The translational obstacles encountered with numerous acutely administered neuroprotective treatment strategies that spurred revision of preclinical modeling also inspired great research interest in delayed, more accessible neurorestorative treatments.9, 28, 29, 30 The first studies to test neurorestorative strategies initiated treatment at a relatively conservative 4 to 24 hours after ischemia. Some of these studies showed reductions in lesion volume of ~20% to 45%31, 32, 33, 34 (similar to the present work), notably those using erythropoietin;31 granulocyte colony–stimulating factor;32 heparin-binding epidermal growth factor-like growth factor;33 or vascular endothelial growth factor.34 Mechanistically, these changes in lesion volumes have been attributed to: angiogenesis,32, 35 neurogenesis,32, 35 white matter reorganization,32 recruitment of autologous hematopoietic stem cells,33 and enhanced survival of ischemic tissue.34, 35 However, given the early initiation time (~24 hours) of these treatments, they probably effect downstream steps in cell death pathways comprising the acute injury process,34, 35 which are likely distinct from the processes underlying the effects of the present treatment initiated at 7 days after ischemic insult. The comparable effect sizes thus underscore the potential of late treatment with L-655,708 investigated presently.
To the best of our knowledge, there are only two investigations in murine models that report decreased lesion volume with treatment begun >24 hours after the onset of ischemia: human cord blood-derived CD34+ cells (after 48 hours)35 and erythropoietin (after 4 or 11 days).36 These treatments reduce lesion volume by ~30% to 80% as evaluated using an index of cortical thickness35 or histologic analysis.36 Mechanistically, these changes in lesion volume have been in turn been ascribed to: neovascularization,36 endogenous neurogenesis,36 and the attenuation of inflammatory responses.37 Although none of these are direct targets of L-655,708, they may well be downstream effects of this treatment: further work on the elucidation of L-655,708 elicited changes in neuronal functioning and other associated cellular processes is clearly warranted.
In the present work, the classification of the regions of injury into perilesional zone and necrotic core based on T2-weighted MRI signal intensity was buttressed qualitatively via examination of H&E slices, with T2-weighted hypointensity arising from regions devoid of cells on H&E; and T2-weighted hyperintensity resulting from edema and inflammation in the broadly defined surrounding region.28 In ongoing work, quantitative immunohistochemical analysis is being incorporated to examine changes in cellular populations underlying the ~30% reduction in volume of perilesional T2-weighted hyperintense tissue after treatment. The current data suggest that resolving edema and resolution/attenuation of inflammatory responses drive the normalization of perilesional T2 values in L-655,708-treated animals.38
In addition to the progressive reduction in necrotic core and perilesional tissue volume, L-655,708 treatment elicited skilled reaching ability improvement. Importantly, sensorimotor improvement on the Montoya staircase test is rare because of the specificity and difficulty of the task, underscoring the significance of the present observation of recovery to ~62% of baseline reaching ability after 2 weeks of treatment.12 Skilled reaching was tested because its impairment is very commonly observed clinically and because it has been shown to be very resistant to therapy both in patients and in animal models of injury.39 In particular, it has been previously shown that the Montoya staircase task is one of the most sensitive tests for detecting chronic deficits after sensorimotor ischemia and the most difficult test to show improvement on following an intervention after ischemic insult.39
To the best of our knowledge, enriched rehabilitation, which combines intensive daily reaching with the impaired forelimb and an enriched cage environment has hitherto been the only late time point therapeutic intervention (used alone or in combination) to result in sensorimotor improvement on the Montoya staircase test after ischemic injury.40 In two studies by Biernaskie and Corbett,40 after stereotaxic microinjection of ET-1 to the distal portion of the middle cerebral artery, recovery to ~50% of prestroke ability on the Montoya staircase test was shown by 40 days after ischemic insult with enriched rehabilitation therapy initiated within the first week after stroke. Jeffers et al24 in turn reported that after 2 weeks of epidermal growth factor and erythropoietin infusion into the ipsilateral ventricle and an additional 2 weeks of enriched rehabilitation therapy, animals recovered to ~55% of their prestroke performance; and further on, at 10 weeks after stroke, reached ~60% of their prestroke performance level. Of note, however, Biernaskie et al and Jeffers et al observed more severe deficits in skilled reaching ability 1 week after ischemic insult (~20% and ~25% of prestroke performance, respectively) than those observed in the present study (~34% of prestroke performance). On the whole, the present findings call for a comprehensive battery of behavioral tests in future work to thoroughly characterize behavioral effects of L-655,708 treatment.
In summary, the present findings on neuroanatomic changes in the perilesional tissue and skilled reaching improvement with L-655,708 treatment in the chronic stage after focal ischemic insult add to the growing body of work showing significant recovery on treatment initiated >24 hours after injury. The present data thus warrant further investigations into underlying mechanisms of recovery, including quantitative histologic characterization in addition to a comprehensive battery of behavioral tests.
Conclusion
To the best of our knowledge, the present work is the first to investigate L-655,708 efficacy in rats, use a systemic low-dose regimen a week after the ischemic insult, observe a decrease in lesion volume on MRI, an improvement in skilled reaching ability, and relate volume of injury to behavioral performance. The results thus provide evidence that treatment with L-655,708 in the chronic stage of stroke recovery may ameliorate some of the focal ischemia–induced injury in terms of both structural brain damage and skilled reaching deficit. Future work will investigate the mechanisms underlying structural remodeling and functional recovery using immunohistochemistry and functional MRI and if further gains in recovery can be achieved by combining L-655,708 with mild enriched rehabilitation.
Acknowledgments
The authors thank Drs Wendy Oakden and Paul Nagy for their assistance in data acquisition.
Author Contributions
EMRL was involved in MRI, behavior and histologic data acquisition and analysis. JC and LT contributed to behavioral data acquisition. RJ was involved in MRI technical support. MG and MB were responsible for histologic technical support. JM, DC, and GJS were involved in project supervision. BS was involved in project supervision and MRI and behavior analysis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- 1Statistics Canada. CANSIM Table 102-0529: deaths, by cause: diseases of the circulatory system. 2012, 2000–2006.
- 2Hill Buchan. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005; 172: 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Evenson KR, Rosamond WD, Morris DL. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology 2001; 20: 65–76. [DOI] [PubMed] [Google Scholar]
- 4Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab 2000; 20: 1149–1165. [DOI] [PubMed] [Google Scholar]
- 5Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol 2006; 16: 258–264. [DOI] [PubMed] [Google Scholar]
- 6Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2011; 468: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci 2009; 29: 1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Schiene K, Bruehl C, Zilles K, Qu M, Hagemann G, Kraemer M et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab 1996; 16: 906–914. [DOI] [PubMed] [Google Scholar]
- 9Khale MP, Bix GJ. Successfully climbing the ‘STAIRs': surmounting failed translation of experimental ischemic stroke treatments. Stroke Res Treat 2012; 2012: 374098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behavior. Nat Rev Neurosci 2009; 10: 861872. [DOI] [PubMed] [Google Scholar]
- 11Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab 2000; 20: 1276–1293. [DOI] [PubMed] [Google Scholar]
- 12Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol 2006; 201: 324–334. [DOI] [PubMed] [Google Scholar]
- 13Carmichael ST. Rodent models of focal stroke: size, mechanism and purpose. NeuroRx 2005; 2: 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Olsen TS, Lassen NA. A dynamic concept of middle cerebral artery occlusion and cerebral infarction in the acute state based on interpreting severe hyperemia as a sign of embolic migration. Stroke 1984; 15: 458–468. [DOI] [PubMed] [Google Scholar]
- 15Mohr JP, Gautier JC, Hier D, Stein RW. Middle cerebral artery. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, editors. Stroke, Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone; 1986; 1: 377–450. [Google Scholar]
- 16Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med 2001; 46: 827–830. [DOI] [PubMed] [Google Scholar]
- 17Atack JR, Pike A, Clarke A, Cook SM, Sohal B, McKernan RM et al. Rat pharmacokinetics and pharmacodynamics of a sustained release formulation of the GABAA alpha-5-selective compound L-655,708. Drug Metab Dispos 2006; 34: 887–893. [DOI] [PubMed] [Google Scholar]
- 18Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments—the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19ImageMagick Studio LLC2013, http://www.imagemagick.org/script/index.php. Copyright 1999–2013. Non-profit organization dedicated to making software imaging solutions freely available.
- 20Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 2003; 34: 2729–2735. [DOI] [PubMed] [Google Scholar]
- 21van der Zijden JP, van der Toorn A, van der Marel K, Dijhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol 2008; 212: 207–212. [DOI] [PubMed] [Google Scholar]
- 22Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Sprenger C, Wiedermann D et al. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 2006; 26: 38–47. [DOI] [PubMed] [Google Scholar]
- 23Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The ‘staircase test': a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods 1991; 36: 2–3. [DOI] [PubMed] [Google Scholar]
- 24Jeffers MS, Hoyles A, Morshead C, Corbett D. Epidermal growth factor and erythropoietin infusion accelerate functional recovery in combination with rehabilitation. Stroke 2014; 45: 1856–1858. [DOI] [PubMed] [Google Scholar]
- 25Baaven RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 2008; 59: 390–412. [Google Scholar]
- 26Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38: 963–974. [PubMed] [Google Scholar]
- 27Lively S, Moxon-Emre I, Schlichter CL. SC1/hevin and reactive gliosis after transient ischemic stroke in young and aged rats. J Neuropathol Exp Neurol 2011; 70: 913–929. [DOI] [PubMed] [Google Scholar]
- 28Grotta JC, Jacobs TP, Koroshetz WJ, Moskowitz MA. Stroke Program Reviews Group. An interim report. Stroke 2008; 39: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 29National Institutes of Neurological Disease and Stroke. Final Report of the Stroke Progress Review Group. 2012.
- 30Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci 2014; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Ding G, Jiang Q, Li L, Zhang L, Wang Y, Zhang ZG et al. Cerebral tissue repair and atrophy after embolic stroke in rat: a magnetic resonance imaging study of erythropoietin therapy. J Neurosci Res 2010; 88: 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS et al. Functional recovery of stroke rat induced by granulocyte colony-neurogenesis and angiogenesis and improves neurological function in rats. Circulation 2004; 110: 1847–1854. [DOI] [PubMed] [Google Scholar]
- 33Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 2004; 24: 399–408. [DOI] [PubMed] [Google Scholar]
- 34Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A et al. VEGF-induced neuro-protection, neurogenesis and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004; 114: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012; 33: 2681–2692. [DOI] [PubMed] [Google Scholar]
- 37Xiong Y, Zhu WZ, Zhang Q, Wang W. Observation of post-MCAO cortical inflammatory edema in rats by 7.0 Tesla MRI. J Huazhong Univ Sci Technolog Med Sci 2014; 34: 120–124. [DOI] [PubMed] [Google Scholar]
- 38Pomeroy V, Aglioti SM, Mark VW, McFarland D, Stinear C, Wolf SL et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair 2011; 25: 335–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci 2009; 29: 562–574. [DOI] [PubMed] [Google Scholar]
- 40Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth following focal ischemic injury. J Neurosci 2001; 21: 5272–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.