Abstract
Interventional studies suggest that changes in physical fitness affect brain function and structure. We studied the influence of high intensity physical exercise on hippocampal volume and metabolism in 17 young healthy male adults during a 6-week exercise program compared with matched controls. We further aimed to relate these changes to hypothesized changes in exercised-induced brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α). We show profound improvement of physical fitness in most subjects and a positive correlation between the degree of fitness improvement and increased BDNF levels. We unexpectedly observed an average volume decrease of about 2%, which was restricted to right hippocampal subfields CA2/3, subiculum, and dentate gyrus and which correlated with fitness improvement and increased BDNF levels negatively. This result indicates that mainly those subjects who did not benefit from the exercise program show decreased hippocampal volume, reduced BDNF levels, and increased TNF-α concentrations. While spectroscopy results do not indicate any neuronal loss (unchanged N-acetylaspartate levels) decreased glutamate-glutamine levels were observed in the right anterior hippocampus in the exercise group only. Responder characteristics need to be studied in more detail. Our results point to an important role of the inflammatory response after exercise on changes in hippocampal structure.
Keywords: brain morphometry, brain structures, hippocampus, inflammation, metabolism, MR spectroscopy, physical activity
Introduction
The degree of an individual's physical fitness influences not only bodily well-being and life-expectancy but also overall mental health and cognition. Many studies in rodents point to adult hippocampal neurogenesis as one important exercise-associated mechanism related to improved learning abilities.1 Pereira et al2 provide evidence for a specific link between neurogenesis and increased regional cerebral blood volume in the dentate gyrus (DG) of the hippocampus in mice. A comparable selective effect of a 12-week aerobic physical training program on regional cerebral blood volume in the DG has also been observed in humans.2 The relationship between cardiorespiratory fitness, hippocampal volume, and cognitive performance has been proven in cross-sectional studies of children3 and older adults.4 Similarly, a controlled longitudinal study in older adults reveals an increase in hippocampal volume after 1 year of aerobic exercise training, in contrast to a volume decline in a control group (CG) that performed stretching exercises.5
This interesting relationship between physical exercise and brain structure (especially in the hippocampus) raises many questions with respect to the underlying mechanisms, dose–response relationships, therapeutic implications, and interpersonal and exercise characteristics. Four principal hypotheses have been proposed to explain the underlying mechanisms: the cardiovascular, neurotrophic, immunologic, and neuroendocrine signaling hypothesis. Cardiovascular mechanisms in response to physical exercise mainly comprise exercise capacity, increased rates of heart rate recovery and variability, improved blood rheology and hemodynamics, while neurotrophic mechanisms include the increase in peripheral and central growth factor concentrations. Here, the increase in exercise induced brain-derived neurotrophic factor (BDNF) is one of the key mechanisms. Most studies suggest that both acute and enduring aerobic exercise lead to BDNF concentration elevations.6 Brain-derived neurotrophic factor, a neurotrophin, is widely expressed in the brain, and influences neuronal survival, differentiation, axonal path-finding, regulation of dendritic trafficking to postsynaptic densities,7 protection against neuronal death in the hippocampus,8 and induction and maintenance of late-phase long-term potentiation.9 Thus, BDNF is critically important to the cellular and subcellular processes that underlie learning and memory in healthy subjects and aging-related diseases. Immunologic mechanisms account for the fact that sustained physical activity contributes to overall enhancement of immune functioning and anti-inflammatory processes. For instance, Kohut et al10 and others describe significant reductions in plasma levels of interleukin 6 (IL-6), interleukin 8 (IL-8), C-reactive protein, and tumor necrosis factor (TNF-α) in response to regular exercise, thereby linking physical activity to anti-inflammatory processes in the central nervous system.11 This relation is particularly interesting for many neurobiologic processes with an assumed increase of cerebral inflammation that underlies cognitive dysfunction, aging, or neurodegeneration. However, the link between exercise and the immune system extends well beyond classic chemotactic functioning and suggests neuromodulatory and neurotransmitter-like effects in the brain.
Taken together, these findings provide strong evidence for a relationship between the level of cardiorespiratory fitness and the extent of neuroplasticity, in particular in the hippocampus. However, there is still a need to better understand the sequence of events, time frames, and responder characteristics in a controlled experimental setting. For instance, most previous studies have investigated sedentary, middle-aged, or older adults, and applied a variety of exercise and intensities for rather long training periods. In contrast, there is only limited evidence that the reported neurophysiologic changes also occur in young healthy adults after short-term intense physical training regimes.2 In clinical settings, however, the latter is of particular importance, as the durations of hospital stays commonly allow only for short-term training periods.
This controlled longitudinal magnetic resonance imaging (MRI) study was designed to investigate the impact of a short-term intense aerobic exercise program on hippocampal structures of young male adults. Proton magnetic resonance spectroscopy (1H-MRS) of the hippocampus was performed to elucidate putatively altered metabolic processes behind the structural changes. In particular, we were interested in changes of N-acetylaspartate (NAA) and glutamate/glutamine (Glx) as indicators of neuronal integrity and activity. On the basis of previous studies, we expected to find an increased hippocampal volume as well as changes in the hippocampal glutamate/glutamine and NAA compositions. Furthermore, we aimed to relate exercise-induced alterations in BDNF, IL-6, and TNF-α to potential changes in brain structure. We expected to find a significant relationship between increased hippocampal volume and improved fitness levels, as well as an increase in BDNF levels after regular exercise.
Materials and methods
Subjects
A total of 34 male students were recruited from the local university to participate in the present study for a total period of 8 weeks, which included a pre- and post-training testing 1 week before and after a 6-week controlled training period (see Figure 1). Seventeen subjects (age: 25.0±3.3 year, body mass index: 23.8±2.1 kg/m2, maximal oxygen uptake (VO2max)=45.9±4.7 mL/kg per minute, maximal power output (Pmax)=295±23.4 W) were assigned to the experimental condition. Seventeen subjects, matched for age, gender, BMI and VO2max, were assigned to the control condition (age: 23.7±1.7 year, body mass index: 23.8±2.1 kg/m2, VO2max=45.0±6.4 mL/kg per minute, Pmax=318±41.2 W). The participants had no present or past history of psychiatric, neurologic, or other clinically significant disorders as assessed by using the short form of the structured diagnostic interview for DSM-IV psychiatric disorders12 as well as a semi-structured interview and a thorough clinical investigation. All participants were right-handed according to the modified version of the Annett's handedness inventory.13 Informed written consent was obtained in accordance with the protocols approved by the ethics committee of the Jena University Hospital in accordance with the ethical guidelines of the Helsinki Declaration of 1975 (and as revised in 1983) before conducting the study. Each subject received 50 Euros as compensation for participation in the study.
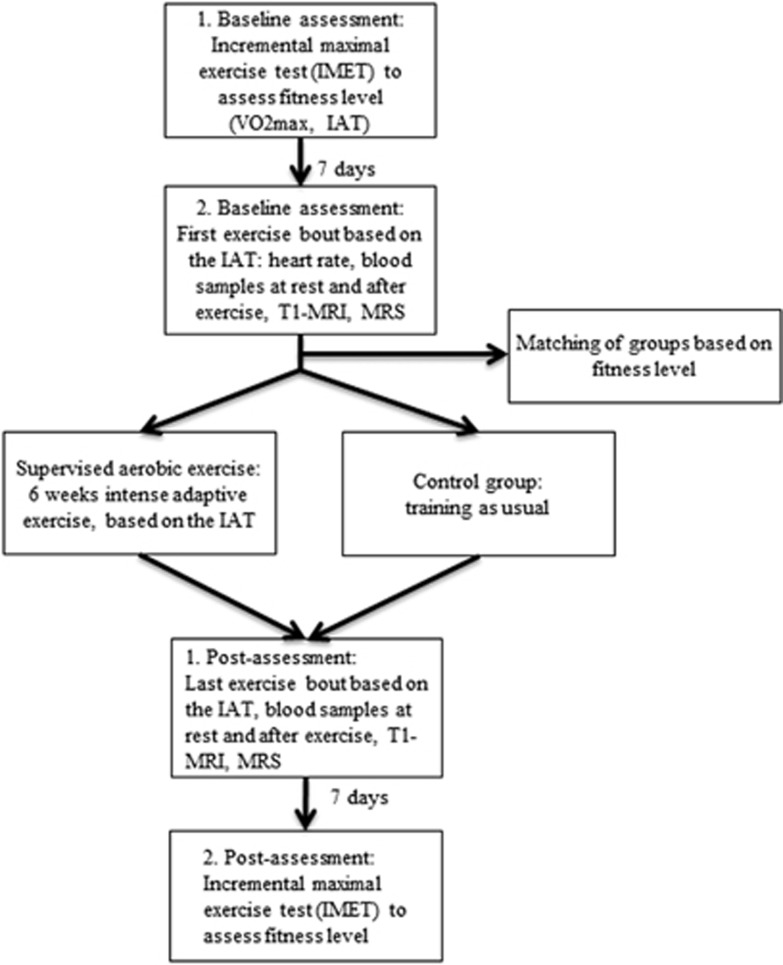
Figure 1.
Flow chart of the study. IAT, individual anaerobic threshold; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
Physical Training
Participants in the experimental group received a supervised exercise program for 6 weeks, 3 days per week. All subjects performed the laboratory training on the same electronically braked cycle ergometer (Ergometrics 900, Ergoline, Bitz, Germany). Each training session lasted 60 minutes, including standard warm-up (5 minutes) and cool-down (5 minutes) periods. To account for individual differences and to avoid fixed percentages of maximum oxygen uptake (VO2max), the training intensity was prescribed according to the range between the individual aerobic and anaerobic thresholds (IAT), which were determined before the training by using an incremental maximal exercise test (IMET, see below). The individualized intensity of the training sessions corresponded to a power output of 85% of the interval between the individual aerobic threshold and IAT (equivalent to 77±9% VO2max). After 3 weeks of training, the intensity was again adjusted to the participants' physical capacity. The heart rate of the members of both groups was assessed during the first and the last training sessions.
Incremental Maximal Exercise Test
The assessment of fitness parameters during the IMET was performed 1 week before and 1 week after completion of the training intervention on an electronically braked cycle ergometer (SRM System, Schoberer Radmesstechnik, Jülich, Germany). The incremental bicycle protocol started at 50 W after 5-minute resting period and was increased by 50 W every 3 minutes to volitional exhaustion. The maximum lactate levels, maximum heart rate, and the respiratory exchange ratio (RER) were assessed. Breath-by-breath gas exchange measurement data (Ganshorn, Medizin Electronic GmbH, Niederlauer, Germany) were transferred to Microsoft Excel for further analysis. The VO2 data were time averaged using 10-second intervals to examine the VO2max. The highest VO2 over three intervals was regarded as VO2max. The maximum achieved workload that was sustained for 3 minutes during the test was defined as Pmax. When participants were not able to cycle to the end of the last 3-minute interval, Pmax was linearly interpolated based on the proportion of the time completed during the terminal stage.
Capillary whole-blood samples were collected for lactate measurements (Enzymatic-Amperometric Measuring System, Eppendorf, Hamburg, Germany) at 1, 3, 5, 7, and 10 minutes after cessation of exercise. From the lactate-power output plot, the individual aerobic threshold and IAT were determined. While the individual aerobic threshold represents the first increase in the blood lactate concentration above the resting state, the IAT describes the maximum lactate steady state and corresponds to an exercise intensity above which a continuous increase in blood lactate is unavoidable. The IAT was calculated according to the method described by Stegmann et al.14 The degree of effort exerted by the participants was determined using the standardized subjective exhaustion Borg 6-to-20 scale.
Rigorous criteria were applied for successful IMET accomplishment: (1) VO2 levelling off, defined as an increase in oxygen uptake during the last 60 seconds of the test of <100 mL/min, (2) the attainment of maximal RER (RERmax)⩾1.15, (3) maximum blood lactate concentration ⩾8 mmol/L, (4) maximum heart rate⩾90% of the age-predicted maximum heart rate, and (5) a Borg rating of perceived exertion >18. To accomplish the IMET successfully, six subjects had to repeat the test.
Blood Parameter Analysis
Before and after the 6-week exercise intervention, venous blood samples were obtained from each participant in the exercise group (EG) immediately before (baseline) and after (exercise) the training session.
To allow comparison with the experimental group, subjects of the CG had to perform two separate training sessions corresponding to the 85% power output of the interval between the individual aerobic threshold and IAT. Blood samples were centrifuged and frozen (−80 °C). To quantify serum BDNF, inflammation parameters TNF-α and IL-6 enzyme-linked immunosorbant assays (ELISAs) were used (Human BDNF Quantikine ELISA, R&D Systems, Minneapolis, MN, USA; Human TNF-α Quantikine HS ELISA, R&D Systems, Minneapolis, MN, USA; IL-6 reagents, Access Immunoassay Systems Beckman-Coulter, Krefeld, Germany) according to the manufacturer's instructions to quantify serum BDNF, inflammation parameters TNF-α and IL-6 serum. In addition, changes in packed cell volume were analyzed to control for dehydration effects on BDNF values.
Blood samples of two subjects in each group could not be used for the final analysis due to scheduling conflicts or errors in blood storage.
Magnetic Resonance Imaging Parameters
Brain structural scans were acquired from all participants before and after the 6-week exercise intervention at 3 T using a whole-body scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany) equipped with a 12-channel head matrix coil and a 3D MP-RAGE sequence with 192 contiguous sagittal slices of 1 mm thickness (repetition time 2,300 ms; echo time 3 ms; flip angle 9° matrix size 256 × 256; isotropic voxel dimensions of 1 × 1 × 1 mm3). To minimize potential influence of dehydration on brain volume, a 24-hour safety gap between the last workout and MRI scanning was established.
Magnetic Resonance Imaging: Freesurfer Longitudinal Data Processing
FreeSurfer (http://surfer.nmr.mgh.harvard.edu) provides a reliable and automatic pipeline to perform longitudinal studies, in particular with the new 5.3 version15 which was used in the present study. The longitudinal scheme is designed to estimate brain morphometric measurements that are unbiased with respect to any time point. FreeSurfer's longitudinal workflow consists of four stages: (1) processing of all time points individually using the cross-sectional workflow, (2) creation of a within-subject template from the cross-sectional data of each time point, which is unbiased with respect to the time points, (3) using information from the within-subject template to initialize several of the algorithms during the longitudinal processing, and (4) comparison of differences between time points. The longitudinal processing included the main steps: resampling to the unbiased template voxel space, an affine registration of the within-subject template image to the Talairach space, and brainmask creation. A subsequent multidimensional nonlinear volumetric alignment to the Talairach space is initialized with the transformation parameters from the within-subject template, significantly improving reliability in several brain structures. Subsequently, each voxel is automatically assigned to one of about 40 neuroanatomic labels. For the subcortical segmentation, a fused segmentation is created for each time point by an intensity-based probabilistic voting scheme.15 The volumes of the hippocampus and the total intracranial volume (ICV) were extracted. The ICV was used to test for differences in individual brain size differences and to correct single volumes in case of such differences. To investigate differences in specific hippocampal regions, FreeSurfer also provides segmentation methods of hippocampal subfields.16 The definition of the hippocampal subfield includes the DG, the CA fields, the subiculum/parasubiculum, and the fimbria. The segmentation procedure employed by FreeSurfer is based on assigning a neuroanatomic label to each voxel in an MRI volume which, in turn, is labeled based on probabilistic information estimated automatically from a manually labeled training set and a process of completely automated parcellation of the brain cortex and subcortical structures.
Magnetic Resonance Imaging: Voxel-Based Morphometry Longitudinal Data Processing
To ensure the validity of the FreeSurfer results, the differences in the hippocampal subregions calculated with FreeSurfer were compared with those obtained using voxel-based morphometry (VBM). The preprocessing and statistical analyses were performed by applying a longitudinal data processing batch within the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) as implemented in SPM8. First, individual T1-weighted images were aligned to a T1-weighted template in MNI space to bring them into a common reference frame regarding translation and rotation. A mean image was calculated from these realigned images, followed by a first realignment of raw data by using this mean image as a reference. At this stage, individual images were bias-corrected to account for signal inhomogeneities. The resulting mean image was segmented into gray matter (GM), white matter, and cerebrospinal fluid. The VBM8 segmentation procedure contains partial volume estimation to account for mixed voxels with two different tissue types. The algorithm is based on an adaptive maximum a posteriori approach with subsequent application of a hidden Markov random field model. This accounts for intensity inhomogeneities and other local variations of intensity. The segmented GM was then normalized using DARTEL. The resulting normalization parameters were applied to the single images, which had been realigned and bias-corrected. Afterwards, these images were segmented and a second realignment followed. Smoothing of GM segments was performed using a 6-mm FWHM Gaussian kernel and entered the two-way ANOVA with the first factor GROUP (EG vs CG) and the second factor TIME (before and after exercise). The comparisons were masked with the hippocampus mask image using WFU pickatlas (http://fmri.wfubmc.edu/software/pickatlas) and thresholded at a family-wise error -corrected voxel-level significance of P<0.05.
Magnetic Resonance Spectroscopy Data Analysis
Multivoxel 1H-MR spectroscopic data were collected with a 12-mm thick transverse chemical shift imaging (CSI) slice oriented parallel to and covering the left and right hippocampus (PRESS localization, TE/TR=30/2,000 ms, VoIPRESS,AP × LR: 84 × 70 × 12 mm3, acquisition weighted k-space encoding of 112 × 112 mm2 FoV with 16 × 16 phase encoding steps, nominal voxel size VAP × LR × FH: 7 × 7 × 12 mm3) (Supplementary Figure 1). Zero-, first-, and second-order shim gradients were adjusted with an automatic B0-field 3D mapping technique, followed by manual fine-tuning of the first-order shim gradients. Signal contaminations from adjacent regions were suppressed by means of eight outer volume suppression slabs around the PRESS volume of interest. Overall, 16 and 1 averages were acquired with and without water suppression, respectively. To reduce chemical shift displacements for Glx resonances, the transmitter RF was shifted by −2.3 p.p.m. relative to the water resonance in the water suppressed scans. All spectra showed sufficient signal-to-noise ratio (range: 8 to 15, as estimated for NAA peak at 2 p.p.m.), which was reflected in adequate quantification accuracy of metabolite intensities (Cramer Rao Lower Bounds below 20% for Glx and NAA intensity).
Before spatial 2D Fourier transformation, the CSI k-space data were multiplied with a 2D Hamming kernel to reduce intervoxel contaminations in the CSI grid. Proton spectra were quantified with the LCModel (V6.2, http://s-provencher.com/pages/lcmodel.shtml), using a vendor-provided basis data set of 15 in vitro model spectra of different metabolites. We focused on glutamate+glutamine (Glx) and NAA intensities, which were normalized to creatine (Cr) ratios and presented as Glx/Cr and NAA/Cr ratios. Additionally, the 0.65-p.p.m. chemical shift of Cr relative to the Glx resonances leads to the PRESS volume displacements between Glx and Cr (up to 1.5 mm), which may be reflected in artificially increased Glx/Cr ratios especially in voxels located close to the PRESS volume edges (Supplementary Figure 1). Therefore, these voxels were not taken into account in the subsequent statistical analyses.
Statistical Analyses of Fitness Parameter and Correlation Analyses
All statistical analyses were performed with SPSS V22.0 (IBM Corp, Armonk, NY, USA). Before group comparisons were made, all dependent variables were tested and found to be normally distributed. The effects of the intense physical training on other fitness parameters as well as the volume of the hippocampus and its specific subfields were examined using the ANOVA design with a between-subject factor GROUP (EG vs CG) and a within-subject factor TIME (before vs after intervention). Pearson correlations were calculated between post-pre differences of the Z-transformed parameters of interest, i.e., volumes of the hippocampal subfields, IAT, BDNF, and TNF-α. The standardized post-pre differences (before and after the intervention) of the latter two blood parameters were computed using the BDNF and TNF-α concentrations of the first and the last exercise session (see exercise values Table 1), while controlling for variation in the baseline values.
Table 1. Blood and exercise parameters obtained in the study.
| Parameters | Unit | Control group, mean±SD | Exercise group, mean±SD |
|---|---|---|---|
| Inflammatory and stress parameters | |||
| Baseline: IL-6T0 | pg/mL | 0.48±0.32 | 0.55±0.61 |
| Baseline: IL-6T1 | pg/mL | 0.97±1.27 | 0.41±0.35 |
| Exercise: IL-6T0 | pg/mL | 3.50±1.48 | 3.59±1.87 |
| Exercise:IL-6T1 | pg/mL | 3.99±2.58 | 2.78±1.49 |
| Baseline: TNFαT0 | pg/mL | 1.42±0.72 | 1.18±0.79 |
| Baseline: TNFαT1 | pg/mL | 1.25±0.80 | 1.27±0.83 |
| Exercise: TNFαT0 | pg/mL | 1.96±0.64 | 1.76±0.90 |
| Exercise: TNFαT1 | pg/mL | 1.72±0.81 | 2.26±1.12 |
| Baseline: BDNFT0 | ng/mL | 8.1±3.6 | 12.4±5.8 |
| Baseline: BDNFT1 | ng/mL | 8.1±2.8 | 10.1±4.2 |
| Exercise: BDNFT0 | ng/mL | 12.3±3.9 | 16.7±7.7 |
| Exercise: BDNFT1 | ng/mL | 14.8±5.9 | 11.1±4.4 |
| Peak values obtained during graded exercise test | |||
| PT0 | W | 318±41 | 295±23 |
| PT1 | W | 316±43 | 329±20 |
| RERT0 | – | 1.27±0.04 | 1.25±0.05 |
| RERT1 | – | 1.25±0.06 | 1.24±0.07 |
| LactateT0 | mmol/L | 11.2±2.9 | 12.2±2.0 |
| LactateT1 | mmol/L | 10.4±2.7 | 13.2±1.6 |
| fT0 | breaths/min | 47±7 | 48±9 |
| fT1 | breaths/min | 47±9 | 49±7 |
| Borg 6-20 scale T0 | – | 19.0±0.8 | 19.2±0.8 |
| Borg 6-20 scale T1 | – | 18.9±0.8 | 19.3±0.7 |
BDNF, brain-derived neurotrophic factor; Borg Scale, Rating of Perceived Exertion Scale with a number range from 6 to 20; f, respiratory frequency; IL-6, interleukin-6; P, power output; PIAT, power output at the individual anaerobic threshold; RER, respiratory exchange ratio; TNF-α, tumor necrosis factor-alpha; T0, data obtained before training intervention; T1, data obtained after training intervention; VO2, oxygen uptake.
Results
Changes in Fitness Parameter
The calculated ANOVAs revealed a significant GROUP (EG vs CG) × TIME (before vs after intervention) interaction for maximum workload Pmax [F=80.3, P<0.001], IAT [F=11.0, P=0.002] and also a trend for VO2max [F=3.4, P=0.07]. There were no significant main effects of GROUP, but significant main effects of TIME for Pmax [F=71.0, P<0.001], IAT [F=43.7, P<0.001], and for VO2max [F=13.3, P=0.001].
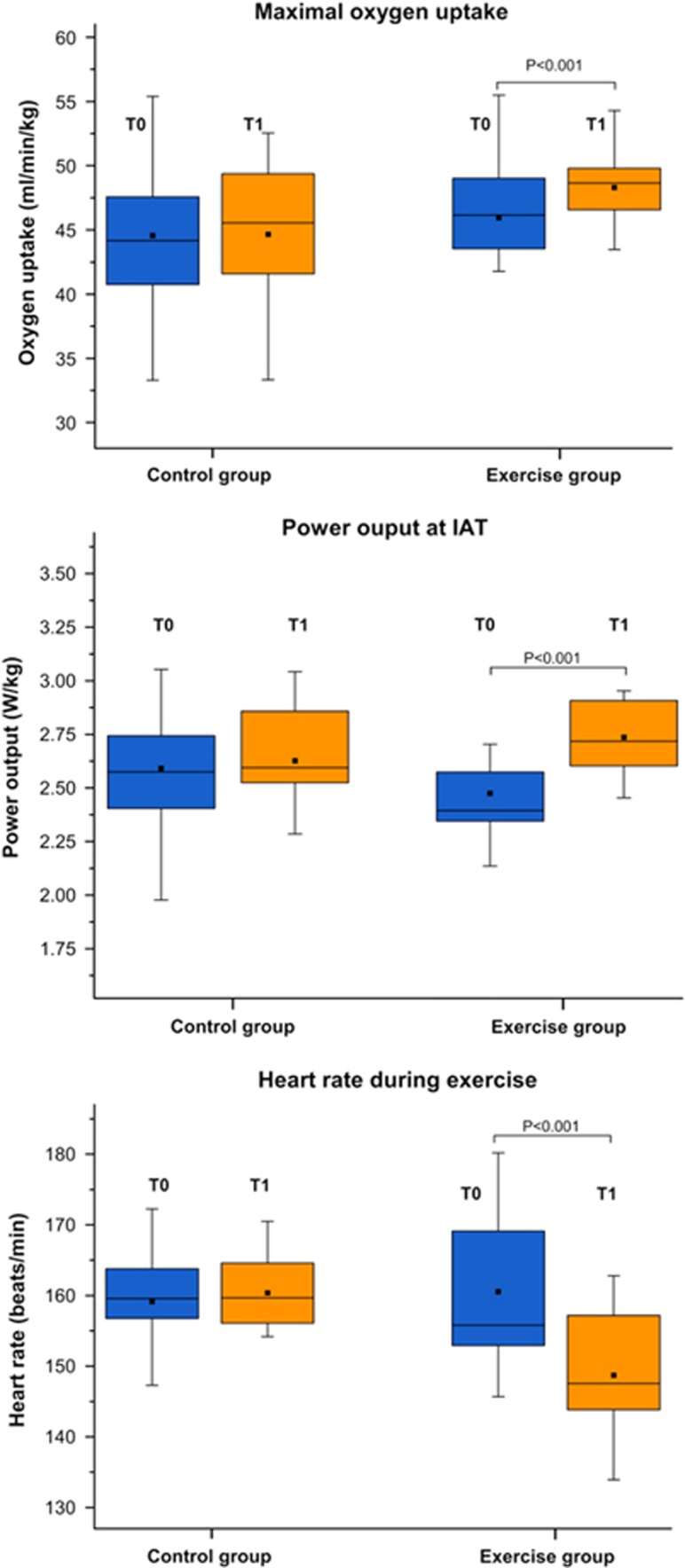
As displayed in Figure 2, in the post hoc t-tests we observed substantial increases in aerobic fitness in the EG as indicated by increases in the workload at the IAT by 11.4% (P<0.001), the maximum workload Pmax by 11.2% (P<0.001) and for VO2max by 4.7% (P<0.001).
Figure 2.
The box plots illustrate pre-post changes in the maximal oxygen uptake (VO2max), in the power output (W/kg) at the individual anaerobic threshold (IAT) and in heart rate during exercise.
Furthermore, there was a highly significant decrease in the average heart rate during exercise in the EG by 7.5% (P<0.001) but no significant changes in the CG. This apparent group difference was confirmed by a significant GROUP × TIME interaction [F=16.6, P<0.001].
Pre-post Changes in Brain-Derived Neurotrophic Factor, Interleukin-6, and Tumor Necrosis Factor-α
At rest (baseline concentration)
No significant main effects of TIME or GROUP × TIME interactions were detected in the serum level of BDNF, IL-6, and TNF-α at rest. A significant main effect of GROUP was observed only for the BDNF level [F=5.6, P=0.03]. Post hoc t-tests indicated a higher BDNF level in the EG than in the CG before the physical training intervention (P=0.02). No significant baseline differences were detected for IL-6 and TNF-α level (Table 1) neither before nor after the intervention.
Immediately after exercise
The exercise-induced serum levels of BDNF, IL-6, and TNF-α were controlled for variations in baseline levels using a linear regression approach. In this way, the standardized residuals were included in the two-way ANOVA and were also used for the correlation analysis.
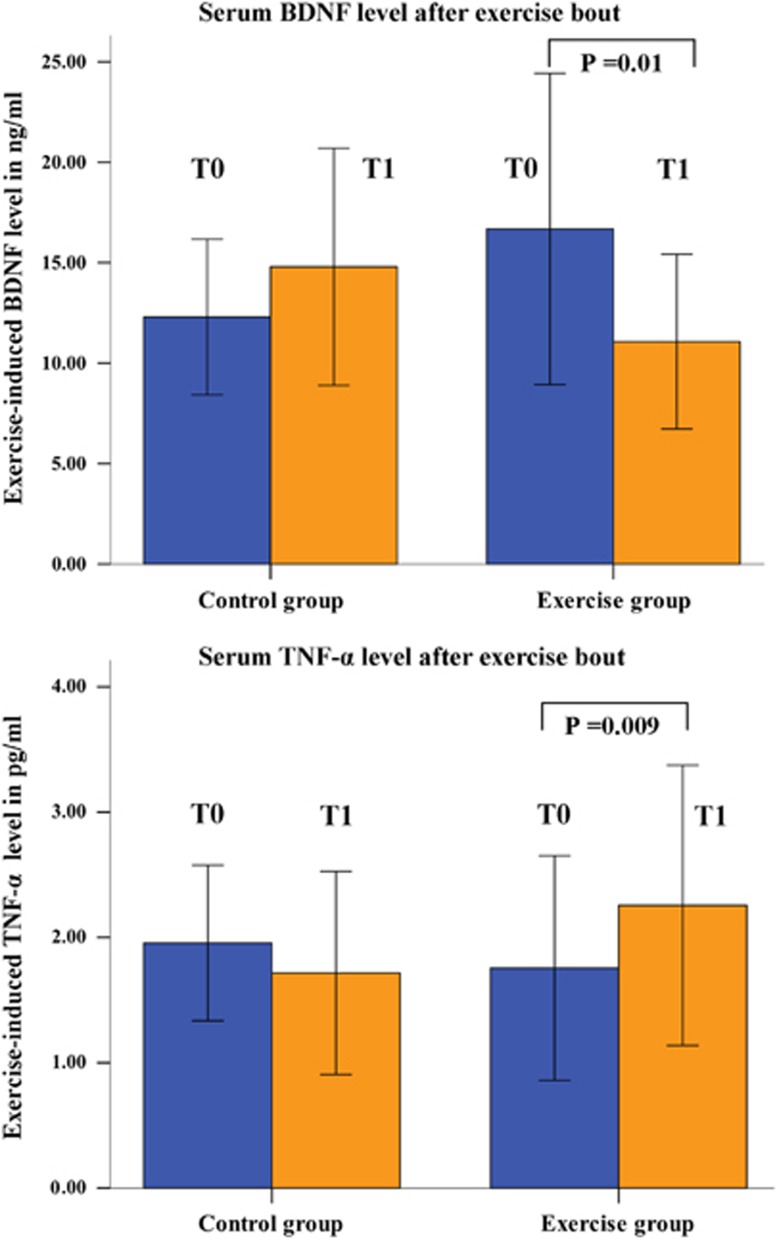
A significant GROUP × TIME interaction [F=8.5, P=0.007] was observed for the exercise-induced BDNF serum concentration. As illustrated in Figure 3, post hoc t-tests indicated a significantly decreased exercise-induced BDNF concentration after intervention in the EG (P=0.01), but nonsignificant changes in the CG (Figure 3, Table 1). The main effects of GROUP and TIME were not significant. Changes in exercise-induced BDNF levels were positively correlated with changes in the workload at the IAT in the EG (r=0.57, P=0.03), but not in the CG (r=0.11, P=n.s.).
Figure 3.
Change in the exercise-induced (as measured immediately after exercise bout) serum brain-derived neurotrophic factor (BDNF) and tumor necrosis factor alpha (TNF-α) concentration (T0 before intervention; T1 after intervention).
For the exercise-induced IL-6 levels, neither significant main effects nor an interaction effect was detected (Table 1).
A highly significant GROUP × TIME interaction [F=13.6, P=0.001] was observed for the exercise-induced levels of TNF-α. Post hoc t-tests indicated a significant increase in the exercise-induced TNF-α concentration after the intervention in the EG (P<0.009) and nonsignificant changes in the CG (Figure 3, Table 1). The main effects of GROUP and TIME were insignificant.
A trend for negative correlation was observed in the EG between the post-pre difference of the standardized, baseline corrected, exercise-induced TNF-α serum level and the standardized post-pre difference in the volume of CA2/3 (r=−0.48, P=0.068).
Magnetic Resonance Imaging Results
Volumetric comparison using Freesurfer
At baseline, the groups did not differ significantly in their total ICV (t=−0.73, P=0.47). We therefore did not correct the extracted subcortical volumes for ICV. When comparing the total hippocampus volume, we observed neither a significant group difference before physical exercise (both hemispheres) nor a significant GROUP × TIME interaction at the significance level of 0.05.
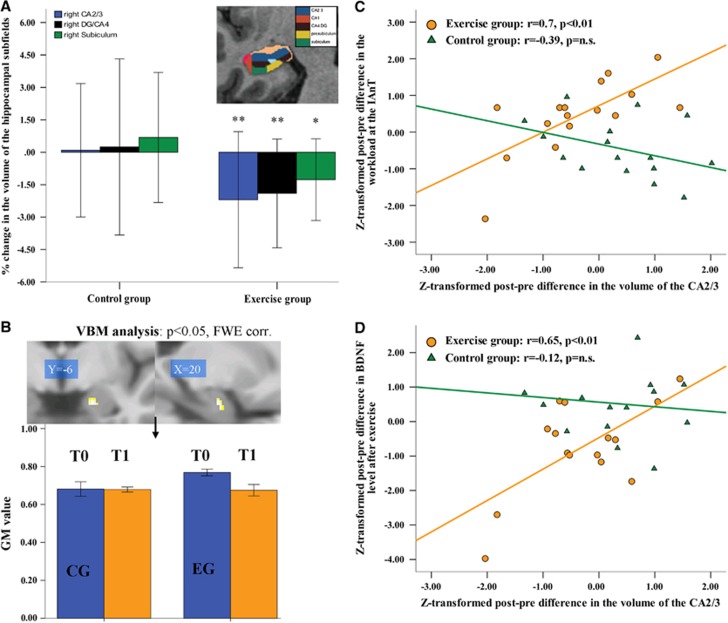
However, when comparing the hippocampal subfields, a significant GROUP × TIME interaction was observed in the right-sided subfields CA2/3 [F=5.0, P=0.032] and the subiculum [F=4.8, P=0.032]. A trend of significant GROUP × TIME interaction was also detected in the right DG/CA4 [F=3.3, P=0.078]. As illustrated in Figure 4A, post hoc t-tests indicated that the EG showed a significant decrease in the volume of the right CA2/3 of 2.2% (P=0.01), subiculum of 1.3% (P=0.01), and DG/CA4 of 1.9% (P=0.005). There were no significant changes in these subfield volumes in the CG.
Figure 4.
(A) Changes in the volumes of the hippocampal subfields CA2/3, dentate gyrus (DG/CA4), and subiculum on the right side as determined by Freesurfer longitudinal data processing (*p≤0.05, **p≤0.01); (B) a significant GROUP × TIME interaction (post-hoc t-test) in the right anterior hippocampus (family-wise error corrected at the voxel and cluster levels, masked for the right hippocampus) as revealed by voxel-based morphometry (VBM). The bar graph illustrates changes in gray matter (GM) volume (T0 before intervention; T1 after intervention); (C) correlations analysis between Z-transformed post-pre differences in the CA2/3 volume and Z-transformed post-pre differences in the power output at the individual anaerobic threshold (exercise group=orange dots, control group (CG)=green triangles); (D) correlation analysis between Z-transformed post-pre differences in the CA2/3 volume and Z-transformed post-pre differences in the BDNF serum concentration as measured immediately after exercise bout (in the exercise group=orange dots; CG=green triangles).
Other subfields, i.e., CA1, hippocampal fissure, fimbria, and presubiculum on the right and left sides, showed no significant differences in their pre-post volume changes between both groups. The GROUP × TIME interactions remained significant after controlling for the ICV.
Voxel-based morphometry analysis
Using the VBM longitudinal data analysis, we were able to confirm the result of the hippocampal subfield analysis and observed a significant GROUP × TIME interaction (post hoc t-test) in the right anterior hippocampus (x=20, y=−6, z=−23, t=4.13, cluster size=8, family-wise error corrected at the voxel and cluster levels, masked for the right hippocampus), indicating a GM decrease in the training group (EG) after 6 weeks of physical training (Figure 4B). Decreased GM volume correlated positively with the volume decrease in the CA2/3 subfield in the EG (r=0.62, P=0.008).
Correlational Analysis
As illustrated in Figure 4C, in the EG the standardized post-pre volume differences of CA2/3 correlated positively and significantly with the standardized post-pre differences in the workload at the IAT (r=0.703, P=0.002). This relationship was not significant in the CG (r=−0.391, P=0.13). The difference in the correlation coefficients was significant (z=3.28, P<0.001).
We further observed a significant positive correlation between the post-pre differences of the standardized, baseline corrected, exercise-induced BDNF serum levels and the standardized post-pre volume differences of CA2/3 (r=0.648, P=0.009), indicating a positive association between reduced BDNF concentration and decreased CA2/3 volume (Figure 4D). In contrast, no significant correlation was observed in the CG (r=−0.118, P=0.676). The difference in the correlation coefficients was again significant (z=2.18, P=0.029).
Magnetic Resonance Spectroscopy Results
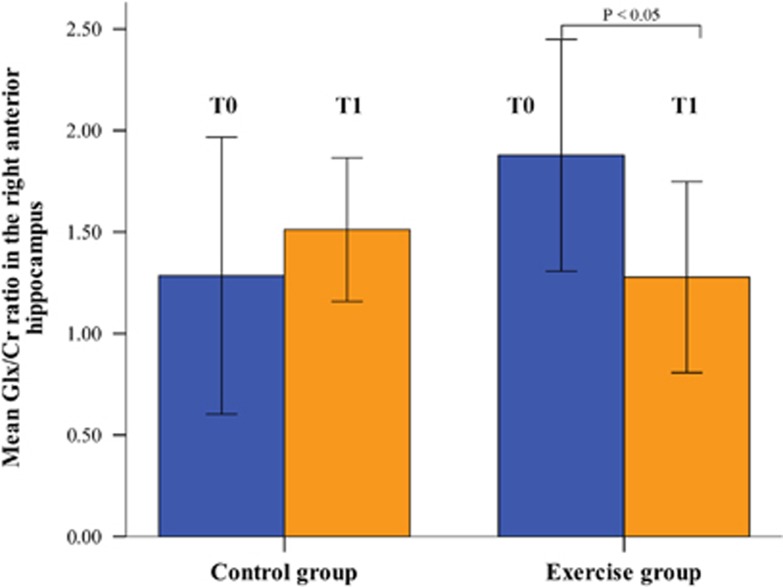
Due to limited allotment of measurement time, MRS data could only be acquired for 12 subjects in the CG and 13 subjects in the EG. A comparison of Glx/Cr ratios in the right anterior hippocampus revealed a significant GROUP × TIME interaction [F=6.3, P=0.02], but no significant main effects of GROUP or TIME. Post hoc t-tests revealed that this significant interaction was mainly due to a significant decrease (P=0.02) in the Glx/Cr ratio in the EG group after intervention (Figure 5).
Figure 5.
Change in the glutamate+glutamine to creatine (Glx:Cr) ratio measured with magnetic resonance spectroscopy in the right anterior hippocampus (T0 before intervention; T1 after intervention).
This exercise effect was not correlated with volume changes in the right anterior hippocampus. In the left anterior hippocampus, no significant findings were observed for Glx/Cr. In case of NAA/Cr, no significant effects were found neither in the right nor in the left anterior hippocampus.
Discussion
The present study shows robust effects of a short-term intense aerobic exercise program on the physical capacity of young male healthy participants. A marked improvement of about 11% was observed for the target parameter workload at the IAT. Increases of VO2max (about 5%) and Pmax (about 11%) as well as reduced heart rates during exercise underline the robust effects of such a program on cardiovascular fitness.
Concerning brain structural changes the physical exercise intervention resulted in a volume reduction of specific, right-sided hippocampal subregions, encompassing the DG, the adjacent CA2/3, and the subiculum. Additional longitudinal VBM analysis confirmed this unexpected finding and furthermore narrowed the anatomic location to the anterior part of the right hippocampus. Of interest, glutamate was significantly decreased after exercise intervention in the same region. Furthermore, exercise-induced changes of BDNF levels significantly correlated with structural findings.
Structural Results
Observational studies in adolescents and older adults suggest that subjects with higher amounts of physical activity and increased levels of fitness show better cognitive functioning and reduced brain volume atrophy compared with less fit age-matched individuals.17 Furthermore, interventional studies have reported a volume increase in the hippocampus as a beneficial result of physical exercise5 in contrast to our findings here. However, the study by Erickson et al5 reported—despite the overall increase—a wide range of volumetric changes in the hippocampus, indicating both increases and decreases of the hippocampal volume in participants after long-lasting aerobic exercise. In line with this scattered pattern, Brickman et al18 failed to observe an improvement in the hippocampal rCBF in older adults and Falkai et al19 found no exercise induced structural changes in patients with schizophrenia or controls.
In contrast to most published studies investigating older age groups, our participants were young male and active students. This factor might have an impact on our findings. The results of our study reveal decreased hippocampal volume after the intervention. However, beside the different direction of average volumetric changes, there are nevertheless striking similarities between our study and the results presented by Erickson et al.5 First, both studies clearly point to the influence of aerobic exercise on the anterior portion of the hippocampus. Using hippocampal subfield segmentation and VBM, we were able to localize the observed changes to the right anterior portion of the DG, the adjacent CA2/3 regions, and the subiculum. Second, we corroborated the close relationship between aerobic fitness, BDNF levels, and hippocampal volume. We show here a positive association between improvements of the target parameter workload at the IAT, increased BDNF levels and increases in right anterior hippocampal volume. Recently, Biedermann et al20 performed a multiple regression analysis to find out the best predictor for hippocampal GM changes in structural MRI after wheel running in mice. The authors showed that the marker for newborn neurons was the best factor in explaining the variation in GM volume. This study may support our observation of the positive association between volume changes in the hippocampus and the BDNF level, which is an important neuplasticity-promoting factor.
Building on the wide range of volumetric changes in the hippocampus shown by Erickson et al5 we now speculate that individual responder characteristics might influence this relationship between physical capacity and hippocampal volume. For instance, Erickson et al21 suggested that critical age periods, characterized by steep loss in brain volume, might predispose to an influence of physical exercise on the former. Here, young male subjects with average fitness levels completed a short-term (6 weeks) intense physical exercise regime. Young adulthood, however, is characterized by relatively stable brain structure, which might consequently be less responsive to physical exercise. Our observed volume decrease of about 2% on a group level points to the possibility that mainly those subjects who did not benefit from the exercise program demonstrated hippocampal volume decrease and reduced BDNF levels as depicted in Figure 4D.
Thomas et al22 have illustrated the approximate percentage of the components of cerebral GM based on several histologic studies. The authors showed that over 50% of GM is composed of the neuropil, while cell bodies account for less than 20%, and the vasculature accounts for no more than 5%. Thus, the observed volume decrease in the EG is unlikely to result from a loss of neurons (which would then be indicated by the decrease of NAA) or changes in the vasculature. The present findings originate more likely from potential changes in the glial number and morphology (which also impacts the glutamate/glutamine turnover), changes in the dendritic branching and synaptogenesis or changes in fiber organization.23
Furthermore, it has been suggested that brain glycogen of astrocytes decreases with prolonged exhaustive exercise that induces hypoglycemia.24 Matsui et al25 described a decrease in glycogen levels in the rat hippocampus by 43%. One might speculate that nonresponder to our intense and prolonged physical exercise program differ with respect to the glucose metabolism and might therefore show marked depletion of brain glycogen storages. This process is accompanied by diffusion of water from the intracellular to the extracellular space due to the high water binding capacity of glycogen and thereby influencing cell volume of astrocytes.
Exercise effects on BDNF, IL-6, and TNF-α concentration
We observed unchanged baseline BDNF serum concentrations as well as decreased exercise-induced BDNF elevations in the EG. Although the sources and the regulation mechanism of the acute exercise-induced response of BDNF remain to be determined, most studies pinpoint to a transient increase in response to exercise that returns to baseline during the exercise recovery period.26 Our results are at variance to findings by Zoladz et al27 and Griffin et al28 who showed that the acute exercise-induced elevation of BDNF is augmented after prolonged aerobic exercise training periods. However, Schiffer et al29 describe no influence of aerobic training on resting BDNF levels in healthy sport students and most studies did not observe an influence of strength training on BDNF concentrations.6 We speculate that acute and chronic changes of BDNF concentrations depend on age, the type of exercise, and the individual level of physical fitness before training.
The correlation analysis suggests that the lack of exercise-induced BDNF increase after 6 weeks might be associated with a nonresponse to physical exercise in some subjects. Since peripheral BDNF can cross the blood–brain barrier while influencing molecular signaling, dendritic arborization, and incorporation of new neural progenitor cells into the neural architecture, decreased hippocampal BDNF levels might be related to decreased volume in specific hippocampal subregions.9
Our results raise important questions in terms of BDNF regulation and the possibility of additional individual factors contributing to the relationship between exercise and brain structure. Unfortunately, physical exercise is a rather ‘dirty' intervention, influencing countless physiologic and biochemical circuits in various parts of the body. In particular, effects of acute and chronic exercise on unspecific immune functions are thought to contribute to anti-inflammatory effects.30 Interleukin-6, mainly produced in the skeletal muscle, modulates the expression of other cytokines, and is the most frequently investigated cytokine in exercise research.31 For instance, an 8,000-fold increase in plasma IL-6 was observed after a 246-km ‘Spartathlon' race, which returned to normal levels after 48 h.32 This illustrates that the IL-6 response is sensitive to exercise intensity and duration.
In the present study, we did not observe any significant changes in exercise-induced IL-6 levels. Only a slight decrease was detected after the training program, which did not reach the level of significance. Again, this result may be due to the fact that we included only healthy and relatively fit young students in our study in contrast to other studies that focused on sedentary participants.
Interestingly, we observed a significant increase in TNF-α concentrations after our 6-week training intervention. This is at variance with previous reports that mainly involved elderly sedentary subjects,31 and is possibly due to the short duration of our intervention study. Nevertheless, we observed a trend toward a significant correlation between changes in TNF-α concentration and changes in the CA2/3 volume in the EG. This possible association has not been reported before and might indicate that subjects showing decreased CA2/3 volumes are characterized by higher pro-inflammatory TNF-α levels after exercise. Interestingly, this inflammatory marker might influence neurogenesis in the hippocampus.33 Our observation warrants further investigation since there exists substantial evidence that pro-inflammatory mechanisms may have an important role in psychiatric diseases and in neurodegenerative processes.
Spectroscopic Results
Using the spatially resolved CSI (1H-CSI) we were able to determine the concentration of NAA in the anterior hippocampus. Since the experiments were performed at 3 T, it was difficult to disentangle the peaks of glutamate and glutamine revealing similar chemical shift values. Therefore, we determined an intensity value of Glx, which comprises the intensities of both metabolites. No significant changes were detected for the NAA level, which is considered to be a marker for assessing neuronal loss in a variety of adult brain diseases. Thus, this finding provides further support for our interpretation that the observed volume changes are not a consequence of a neuronal loss in the right hippocampus, but rather result from potential changes in the gliogenesis and/or fiber organization. This assumption is to some extent further supported by the decreased Glx levels in the right anterior hippocampus that were only observed in the EG. Astroglia are actively involved in the uptake, metabolism, and recycling of glutamate.34 The glutamate–glutamine cycle between neurons and glia is a major metabolic pathway that reflects the synaptic release of glutamate. Even if no significant association was found between Glx levels and volume changes, changes of the glutamate metabolism might be linked indirectly to the observed structural changes, in particular those of glial morphology.
However, Biedermann et al35 found in a mouse study an increased right hippocampal volume after 6 to 8 weeks of voluntary wheel running, which was accompanied by decreased absolute glutamate as well as Glx levels in the right hippocampus. Thus, Biedermann et al35 and our results show volume changes and glutamatergic metabolism in the right hippocampus. Despite differences in the direction of volumetric changes, the results of both studies point to changes of the right hippocampus. Furthermore, with regard to the asymmetric hippocampal glutamate and volume change in the present study, a right-greater-than-left asymmetry in the hippocampal volume in right-handed healthy subjects was reported not only on the macroscopic level36 but also on the microscopic level. Kawakami et al37 observed an asymmetric synaptic distribution of N-methyl-d-aspartate receptor between the left and right adult mouse hippocampus. Shinohara et al38 reported that hippocampal CA1 synapses, which receive neuronal input from the right CA3 pyramidal cells are larger and have markedly higher expression level from glutamate receptor GluR1 than those which receive input from the left CA3. Thus, we can only speculate that this lateralized molecular pattern might have contributed to our findings. However, further multimodal studies in mouse and in humans are required to investigate asymmetric changes after exercise.39, 40
Some limitations need to be addressed. We have not specifically controlled for nutritional state, which may possibly influence physical fitness or the relation between physical fitness and hippocampal volume. Spectroscopic results are limited by the smaller number of examined participants. Furthermore, a change in the denominator (i.e., Cr) cannot be completely ruled out, having thereby a potential impact on the Glx/Cr and the NAA/Cr ratios. We also reduced the influence of dehydration due to a 24-hour safety gap between the last workout and MRI scanning. In addition, changes of packed cell volume were analyzed to control for dehydration effects on BDNF values, which were not significant between groups. Moreover, the initial IMET was performed twice to adjust for a learning bias between the baseline and final fitness assessment.
In conclusion, our study reveals robust effects of intense, controlled 6-week exercise training on the physical fitness in young male participants. Striking similarities to previous studies were observed in terms of associations between physical fitness, BDNF levels, and hippocampus volumes. The finding of an overall decreased volume of the hippocampus after exercise differed from previous findings, but may be explained by differential effects on the diverse parts of the hippocampus and by interindividual differences in characteristics predisposing to training effects.
Spectroscopic results underlined the functional implications of training effects on the hippocampal findings. We suggest that in addition to the duration and intensity of training, individual response characteristics, such as age, gender, genetic background, or the inflammatory response, may have a role for the overall influence on brain structure and thus ultimately on brain function. This knowledge is highly useful to design training interventions for patients with psychiatric or neurodegenerative disorders.
Acknowledgments
The authors would like to thank Mrs Dorschner for technical support.
Author Contributions
GW contributed to study idea and enrollment, VBM analysis, writing of the manuscript. MH contributed to training of participants and design of exercise intervention. FC contributed to VBM and Freesurfer analysis, statistical analysis. AS contributed to analysis of heart rate changes and study assistance in MRI department. FB contributed to study assistance in MRI department, recruiting and training of participants. CS contributed to clinical assessment of participants and recruiting participants. SS contributed to MRS analysis and figure design, review of manuscript. AG contributed to MRS analysis and figure design, review of manuscript. CP contributed to design of exercise study, inflammatory marker analysis, review of manuscript. HG contributed to supervision of the study, exercise intervention plan, inflammatory marker analysis. JRR contributed to supervision of MRS part of the study, reviewing manuscript. K-JB is a study coordinator and contributed to writing the manuscript, coordination of contributors.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- 1van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005; 25: 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 2007; 104: 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res 2010; 1358: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009; 19: 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports 2014; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- 7Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett 2007; 581: 2047–2054. [DOI] [PubMed] [Google Scholar]
- 8Pringle AK, Sundstrom LE, Wilde GJ, Williams LR, Iannotti F. Brain-derived neurotrophic factor, but not neurotrophin-3, prevents ischaemia-induced neuronal cell death in organotypic rat hippocampal slice cultures. Neurosci Lett 1996; 211: 203–206. [DOI] [PubMed] [Google Scholar]
- 9Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007; 30: 464–472. [DOI] [PubMed] [Google Scholar]
- 10Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun 2006; 20: 201–209. [DOI] [PubMed] [Google Scholar]
- 11Flynn MG, McFarlin BK, Markofski MM. The anti-inflammatory actions of exercise training. Am J Lifestyle Med 2007; 1: 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 (Suppl 20): 22–33, quiz 34-57. [PubMed] [Google Scholar]
- 13Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex 1975; 11: 230–238. [DOI] [PubMed] [Google Scholar]
- 14Stegmann H, Kindermann W, Schnabel A. Lactate kinetics and individual anaerobic threshold. Int J Sports Med 1981; 2: 160–165. [DOI] [PubMed] [Google Scholar]
- 15Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus 2009; 19: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 2008; 9: 58–65. [DOI] [PubMed] [Google Scholar]
- 18Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 2014; 17: 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Falkai P, Malchow B, Wobrock T, Gruber O, Schmitt A, Honer WG et al. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci 2013; 263: 469–473. [DOI] [PubMed] [Google Scholar]
- 20Biedermann SV, Fuss J, Steinle J, Auer MK, Dormann C, Falfan-Melgoza C et al. The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct Funct e-pub ahead of print 01 January 2014; doi:10.1007/s00429-014-0976-5. [DOI] [PubMed]
- 21Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging 2014; 35: S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol 2012; 3: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 2012; 15: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 1994; 91: 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Matsui T, Soya S, Okamoto M, Ichitani Y, Kawanaka K, Soya H. Brain glycogen decreases during prolonged exercise. J Physiol 2011; 589: 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Nofuji Y, Suwa M, Sasaki H, Ichimiya A, Nishichi R, Kumagai S. Different circulating brain-derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J Sports Sci Med 2012; 11: 83–88. [PMC free article] [PubMed] [Google Scholar]
- 27Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008; 59: 119–132. [PubMed] [Google Scholar]
- 28Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav 2011; 104: 934–941. [DOI] [PubMed] [Google Scholar]
- 29Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res 2009; 41: 250–254. [DOI] [PubMed] [Google Scholar]
- 30Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol 2009; 587: 5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005; 98: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 32Margeli A, Skenderi K, Tsironi M, Hantzi E, Matalas AL, Vrettou C et al. Dramatic elevations of interleukin-6 and acute-phase reactants in athletes participating in the ultradistance foot race spartathlon: severe systemic inflammation and lipid and lipoprotein changes in protracted exercise. J Clin Endocrinol Metab 2005; 90: 3914–3918. [DOI] [PubMed] [Google Scholar]
- 33Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res 2005; 80: 789–797. [DOI] [PubMed] [Google Scholar]
- 34Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 2002; 22: 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T et al. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage 2012; 61: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 36Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol 2001; 22: 1342–1345. [PMC free article] [PubMed] [Google Scholar]
- 37Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science 2003; 300: 990–994. [DOI] [PubMed] [Google Scholar]
- 38Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc Natl Acad Sci USA 2008; 105: 19498–19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Farhang S, Barar J, Fakhari A, Mesgariabbasi M, Khani S, Omidi Y et al. Asymmetrical expression of BDNF and NTRK3 genes in frontoparietal cortex of stress-resilient rats in an animal model of depression. Synapse 2014; 68: 387–393. [DOI] [PubMed] [Google Scholar]
- 40Dunham JS, Deakin JF, Miyajima F, Payton A, Toro CT. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J Psychiatr Res 2009; 43: 1175–1184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.