Abstract
Pro-inflammatory cytokines contribute to hypoxic–ischemic brain injury. Blood–brain barrier (BBB) dysfunction represents an important component of hypoxic–ischemic brain injury in the fetus. Hypoxic–ischemic injury could accentuate systemic cytokine transfer across the fetal BBB. There has been considerable conjecture suggesting that systemic cytokines could cross the BBB during the perinatal period. Nonetheless, evidence to support this contention is sparse. We hypothesized that ischemia–reperfusion increases the transfer of systemic interleukin-1β (IL-1β) across the BBB in the fetus. Ovine fetuses at 127 days of gestation were studied 4 hours after 30 minutes of bilateral carotid artery occlusion and compared with a nonischemic group. Recombinant ovine IL-1β protein was expressed from an IL-1β pGEX-2 T vector in E. coli BL-21 cells and purified. The BBB function was quantified in 12 brain regions using a blood-to-brain transfer constant with intravenous 125I-radiolabeled IL-1β (125I-IL-1β). Interleukin-1β crossed the intact BBB in nonischemic fetuses. Blood-to-brain transport of 125I-IL-1β was higher (P<0.05) across brain regions in fetuses exposed to ischemia–reperfusion than nonischemic fetuses. We conclude that systemic IL-1β crosses the intact fetal BBB, and that ischemia–reperfusion increases transfer of this cytokine across the fetal BBB. Therefore, altered BBB function after hypoxia–ischemia facilitates entry of systemic cytokines into the brain of the fetus.
Keywords: blood–brain barrier, brain, cytokines, fetus, hypoxia–ischemia, sheep
Introduction
Hypoxic–ischemic brain injury is one of the major causes of neurologic abnormalities originating in the perinatal period.1 The majority of hypoxic–ischemic episodes begin before birth frequently as antepartum events.2 These episodes can predispose to long-term disabilities such as cerebral palsy, mental retardation, and seizures.1
Pro-inflammatory cytokines have been previously shown to have a critical role in hypoxic–ischemic brain injury and could cause and/or exacerbate damage to the fetal and neonatal brain after a variety of insults.3, 4, 5, 6 Cerebral ischemia initiates an inflammatory cascade in the brain that is associated with increases in pro-inflammatory cytokines including tumor necrosis factor-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6).7 Increases in systemic, vascular, and endogenous parenchymal pro-inflammatory cytokines could exacerbate perinatal brain damage.3 Interleukin-1β is one of the key pro-inflammatory cytokines that contributes to acute hypoxic–ischemic injury in the developing brain.5 Furthermore, we have recently shown that IL-1β protein expression increases in the cerebral cortices of the ovine fetus after ischemia and reperfusion.8 However, it is not clear whether increases in cytokines represent de-novo cytokine production within the brain parenchyma, increases in vascular endothelial production or potentially influx of cytokines from the systemic circulation across the blood–brain barrier (BBB) or a combination of these factors. A better understanding of some of the mechanism(s) underlying increases in cytokines after brain ischemia and reperfusion could provide valuable insights to develop strategies that could attenuate ischemia–reperfusion related neuro-inflammatory brain injury.
The BBB consists of a complex cellular system of highly specialized endothelial cells bound together by intercellular tight junctions.9 The cerebrovascular endothelial cells of the BBB maintain the homeostasis of the central nervous system by limiting the entry of substances that could alter neuronal function.9 We have previously reported decreases in BBB permeability from 60% of fetal development up to maturity in adult sheep,10 and that a variety of perinatal insults and therapies modify BBB function in the ovine fetus.11 Furthermore, we have recently reported that BBB dysfunction represents a previously unappreciated major component of hypoxic–ischemic brain injury in the fetus.12 In addition, we found that the largest increase in BBB permeability measured with a nonspecific molecule (α-aminoisobutyric acid (AIB)) was identified 4 hours after the onset of ischemia.12
The previous work in adult rodents has suggested that pro-inflammatory cytokines can cross the BBB by saturable transport systems.13, 14, 15, 16 We have recently generated and further purified ovine IL-1β protein,17, 18, 19 and shown that this ovine IL-1β protein can cross the adult mouse BBB by a saturable transport mechanism.16 Moreover, we have also reported that the pro-inflammatory cytokine IL-1β contributes to BBB dysfunction after ischemic injury in the fetus.19
Pro-inflammatory cytokines produced in the systemic circulation have been postulated to cross the BBB in the fetus and neonate.4 It has long been suggested that pro-inflammatory cytokines could gain access to the fetal brain after being produced during maternal intrauterine infection or inflammation.4 In order for systemically produced inflammatory substances to reach the fetal brain, they would need to cross the fetal BBB or alternatively alter its permeability.4 Although there is a plethora of clinical evidence in support of the contention that pro-inflammatory cytokines could penetrate the immature BBB, there is a paucity of experimental evidence to show that systemic cytokines actually can cross the fetal BBB.20, 21, 22
In the current study, we used the translational preclinical fetal sheep model of ischemia and reperfusion23 along with radiolabeled IL-1β to quantify the influx of this pro-inflammatory cytokine across the BBB in the fetus. The development of the sheep brain has similarities to the human fetal and premature brain with reference to completion of neurogenesis, white-matter development, and onset of cerebral sulcation.23, 24 In addition, the maturation of ovine brain at 127 days of gestation has similarities to those of the near term human infant.23, 24 Moreover, procedures such as systemic intravenous infusions of radiolabeled IL-1β and quantitative kinetic studies of the fetal BBB are not readily achievable in fetuses and neonates of small animals such as rodents. Given the above considerations, we tested the hypothesis that systemic IL-1β crosses the intact BBB and that ischemia increases the transfer of IL-1β across the BBB in the ovine fetus.
Materials and methods
The current study was conducted after approval of the Institutional Animal Care and Use Committees of The Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island, and in accordance with the National Institutes of Health Guidelines for the use of experimental animals, and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines.
Animal Preparation, Study Groups, and Experimental Study Design
Surgery was performed on 12 mixed breed ewes at 116 to 121 days of gestation. As previously described in detail,12, 23 catheters were placed into a brachial vein for isotope administration and the thoracic aorta for blood sampling, heart rate, and blood pressure monitoring in the fetuses. An amniotic fluid catheter was placed for pressure monitoring as a referent for fetal arterial pressures.
After exposure of the fetal carotid arteries, the lingual arteries and vertebral-occipital anastomoses were ligated to restrict noncerebral and vertebral blood flow.23 Two inflatable vascular occluders (3 mm, In Vivo Metric, Healdsburg, CA, USA) were placed around each carotid artery along with ultrasonic flow probes (Transonic Systems Inc., Ithaca, NY, USA).12 To measure the electrocorticogram (ECoG) of the fetus, two pairs of screws (Small Parts, Inc., Miami Lakes, FL, USA) were placed onto the dura with a reference electrode sewn to the scalp23 and connected with insulated wires (Alpha Wire Co., Elizabeth, NJ, USA) to a recorder (ADInstruments, Colorado Springs, CO, USA). A femoral artery catheter was also placed in the ewes.
After recovery from surgery at 125 to 128 days of gestation, the sheep was randomly assigned to a nonischemic sham control group (n=6) or ischemic group (n=6) in which the fetal carotid arteries were occluded for 30 minutes followed by 4 hours of reperfusion. We selected the 4-h period of reperfusion because we have previously shown that the largest increase in permeability measured with a non-specific small inert neutral hydrophilic amino acid (AIB) occurred 4 hours after ischemia.12 Full-term gestation of the sheep used in our study is 148 to 150 days. Consequently, the ovine fetuses in this study were at approximately 85% of the full-term sheep gestation.
After baseline measurements of pH, blood gases, plasma lactate concentrations, heart rates, arterial blood pressures, carotid arterial blood flow, and ECoG recordings, ischemia was induced by inflating the carotid occluders for 30 minutes in the ischemic group.12 After 30 minutes, the occluders were deflated and reperfusion continued for 4 hours. In the sham control nonischemic group, the occluders were not inflated. Measurements of pH, blood gases, plasma lactic acid concentrations, heart rate, arterial blood pressures, carotid arterial blood flow, and ECoG recordings were also obtained at 15 minutes, 1, 2, and 4 hours after the termination of ischemia.
Analytic Methods
Interleukin-1β protein production
As we have previously described,18 we generated and purified recombinant ovine IL-1β protein encoded by pGEX-2 T vectors (Commonwealth Scientific and Industrial Research Organization (CSIRO) Industries, Clayton, South Victoria, Australia) in E. coli BL-21 cells based on methods by Frangioni and Neel25 with some additional modifications. The IL-1β protein was further purified on DEAE-CIM (Diethylaminoethyl-Convective Interaction Media) anion-exchange monolithic resins (BIASeparations, Villach, Pöttelsdorf, Austria). Most of the contaminants remained tightly bound to the DEAE column, whereas the IL-1β proteins were collected from the unbound fraction. An additional separation of the high molecular weight contaminants was performed on a TSKgel G3000SW size exclusion chromatographic column (Tosoh Bioscience, King of Prussia, PA, USA).
The reactivity of IL-1β protein was confirmed by western immunoblot by probing polyvinylidene diflouride membranes (0.2 micron, Bio-Rad Laboratories, Hercules, CA, USA) membranes with primary rabbit polyclonal anti-IL-β antibody (Lifespan Biosciences, Seattle, WA, USA) at a dilution of 1:5,000 and goat anti-rabbit (Alpha Diagnostic, San Antonio, TX, USA) horseradish peroxidase-conjugated secondary antibody at a dilution of 1:10,000. Figure 1 illustrates a western immunoblot of the IL-1β protein before and after the size-exclusion chromatographic purification procedures. As we have previously reported, the IL-1β protein contained a 37-kDa protein band18 but this contaminant protein was successfully removed after the additional size-exclusion chromatography procedure. After the final purification, there was a single 17-kDa protein band suggesting a high degree of purity. We confirmed that after these two chromatographic procedures the purity of the IL-1β proteins was greater than 95%.
Figure 1.
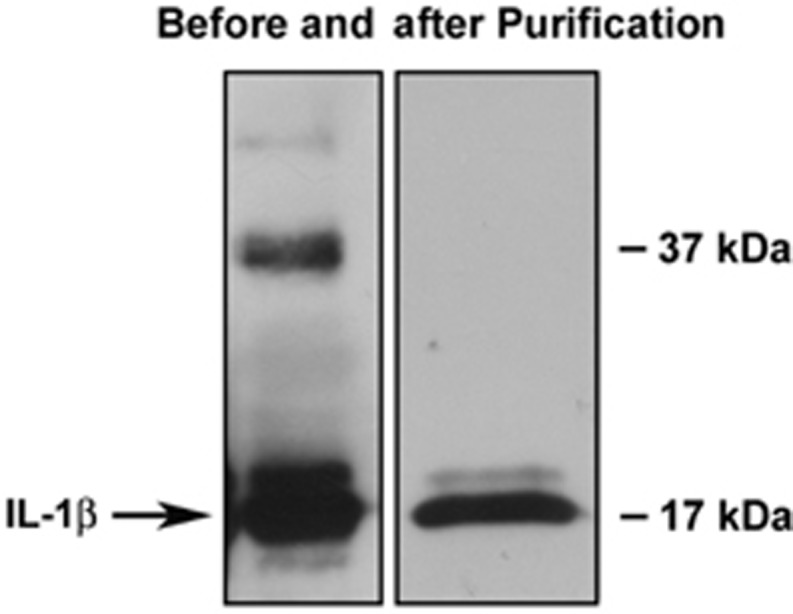
Western immunoblot showing the interleukin-1β (IL-1β) ovine protein before and after the additional purification by size-exclusion chromatographic column described in Materials and methods.
Radiolabeling of the interleukin-1β ovine protein
On the day of the study, the pure IL-1β protein was radioactively labeled with iodine-125 (125I-IL-1β) using the chloramine-T method as previously described.26 One μCi of 125I-, 5 μg of IL-1β protein, and 10 μL of 1 mg/mL chloramine-T (Sigma-Aldrich, St Louis, MO, USA) were combined and vigorously mixed. After 60 seconds, the reaction was stopped with 10 μL of 10 mg/mL sodium metabisulfite (Sigma, St Louis, MO, USA). The iodinated protein was separated from the free iodine on a PD-10 desalting column (GE Healthcare, Newark, NJ, USA). An acid precipitation assay as previously described26, 27 was used to calculate the percent of the 125I-radiolabel that was tightly bound to the purified ovine IL-1β protein. Ten microliters of the iodinated IL-1β protein was added to 500 μL of 1% bovine serum albumin (Sigma-Aldrich) in lactated Ringer's solution (Sigma-Aldrich) in a glass tube, and then 500 μL of 30% trichloroacetic acid (Sigma-Aldrich) was added. The above constituents were mixed and then centrifuged. The resulting supernatant and pellet were separated and counted individually in a gamma counter (Wallac Wizard 1470–010 Gamma Counter, Long Island Scientific Service Corp., Port Jefferson, NY, USA). The efficiency of iodine-125 radioactive labeling of IL-1β protein was calculated as the percent of the labeled iodinated IL-1β protein contained in the acid precipitated protein pellet and was calculated according to the following equation: Efficiency of radioactive labeling (percent acid precipitation)=Pellet CPM/(Pellet CPM+Supernatant CPM) × 100. The radioactive labeling efficiency, e.g., percent of acid precipitation, exceeded 97% in both the sham control and ischemia groups.
Blood-to-brain transfer constant (Ki) measured with 125I-labeled interleukin-1β ovine protein
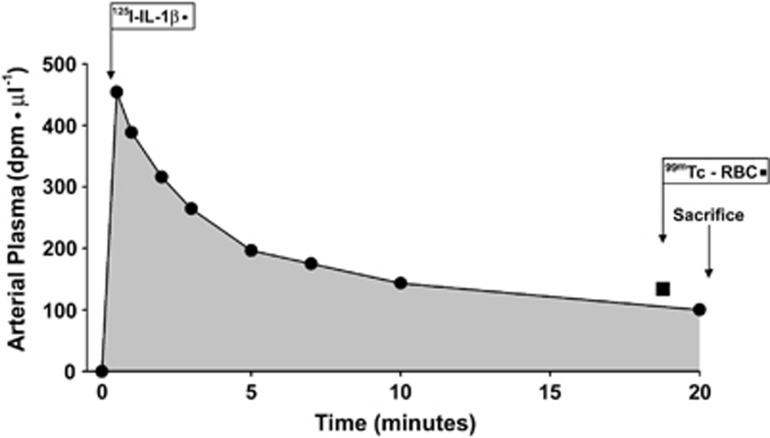
After 4 hours of reperfusion, the blood-to-brain transfer constant was measured with the 125I-labeled IL-1β ovine protein as we have previously described for α-[14C]-aminoisobutyric acid with a few modifications.10, 12 In all, 150 to 200 μCi of 125I-IL-1β was rapidly injected intravenously and the arterial plasma isotope concentrations obtained at fixed times sequentially before and after the 125I-IL-1β injection until 20 minutes of study as shown in Figure 2. Iodination of IL-1β by the method described above results in biologic activity for IL-1β of 80%.28 Therefore, the tracer 125I-IL-1β can be metabolized or degraded in tissues. Consequently, we measured the blood-to-brain transfer constants over 20 minutes in the current study to achieve a balance between limiting the amount of biodegradation of the IL-1β protein and capturing the entire area under the curve for the integral equation. Figure 2 illustrates a representative study showing the arterial plasma counts in dpm/μL plotted against study time in minutes.
Figure 2.
Representative fetal arterial plasma radioactivity profile of 125I-radiolabeled IL-1β (125I-IL-1β) in dpm/μL plotted against study time in minutes. 99mTc-red blood cell (RBC) is shown by the black square. 125I-IL-1β was given intravenously to the fetus at study time zero. IL-1β, interleukin-1β.
At the end of the studies, the ewe and the fetus were killed with phenobarbital (100 to 200 mg/kg). The brain was quickly removed, and the fetus and brain weighed. The brain was dissected into the following regions: cerebral cortex, white matter, caudate nucleus, hippocampus, cerebellum, thalamus, superior colliculus, inferior colliculus, pons, medulla, spinal cord, and pituitary gland.
Tissue and plasma samples for the Ki measurements were processed as previously described in detail.10, 12 Brain vascular volume was determined by giving technetium-99m (99mTc) radioactively labeled red blood cells to the fetus 2 minutes before the termination of the studies as we have described and as shown in Figure 2 by the square.12 Knowledge of the plasma concentration profile and tracer concentration in the brain parenchyma permits accurate calculation of the blood-to-brain transfer constant:10
 |
where Abr is the amount of tracer that crossed the BBB from blood to brain during the tracer study (dpm/g), and cp is the tracer concentration in plasma (dpm/μL) at the time t (minutes). Abr is obtained by correcting the total amount of isotope measured in the tissue Am (dpm/g) for the residual part remaining in the brain vasculature space, which is measured by 99mTc-labeled red blood cells. Thus, Abr=Am−Vpcp, where Vp is the blood volume of brain tissue (μL/g) and cp is the concentration of tracer in the terminal plasma sample (dpm/g). Vp=A†m/c†p, where A†m and c†p have the same definitions as Am and cp above except that they apply to the 99mTc-labeled red blood cells.10, 12
Physiologic determinations
As previously described,12 fetal pH, arterial blood gases, heart rate, arterial blood pressure (MABP) hematocrit, and lactate values were measured at baseline, and sequentially after the end of ischemia. Electrocorticogram and carotid arterial blood flow were obtained at baseline, and during and after ischemia. Hematocrit was measured by the microhematocrit method and plasma lactate on a glucose/lactate analyzer (YSI 2300, STAT, Yellow Springs, OH, USA). Carotid arterial blood flow was measured by flow probes (Transonic System Inc) connected to a PowerLab Data Acquisition System to confirm that blood flow approached zero during carotid occlusion. Carotid arterial blood flow was calculated as a sum of left and right carotid arterial blood flow averaged over 5 minutes at each measurement time.
Electrocorticogram processing
As previously described,12 the ECoG was recorded on a PowerLab Data Acquisition System (ADInstruments). The ECoG files were visually inspected by a board certified epileptologist (JG), who was not aware of the group designation. The recorded ECoG signals were digitized (16 bits, 1,000 Hz) and the signal processing was performed with MATLAB (Mathworks, Natick, MA, USA). The average duration of the ECoG segments for different study periods was 15 minutes and it did not differ between the two groups.12 The grand average power spectral densities (PSDs) were used to determine the frequency ranges with the largest differences among the study periods with a discriminatory frequency band of 10 to 100 Hz found to be optimal for the analysis.12 The power was calculated for 10- second ECoG segments from 10 to 100 Hz. A 10-sececond window was selected for accurate power analysis estimates and to eliminate potential artifacts. A fifth order Butterworth band pass filter of 10 to 100 Hz and a 60 Hz notch filter were applied to each window. The window mean was subtracted and variance of the de-meaned windows used as the window power as the power of a sample with a zero mean is equal to the sample variance.29 The power of each segment was calculated as an average power for the windows within the segment that did not contain artifactual discontinuities. The power of the segments was normalized to a zero mean and unit standard deviation for each fetus to account for offset power differences among the fetuses. The baseline power for each group was subtracted from the power for the rest of the segments before analysis.
Statistical Analysis
Electrocorticogram results are expressed as median±standard error of the mean (s.e.m.) because the ECoG values in the ischemic group were not normally distributed. The Mann–Whitney U-test was used to compare the ECoG recordings from the ischemia study period to the normalized baseline ECoG values. All other results are expressed as mean±s.e.m. Two-way repeated measures analysis of variance (ANOVA) was used to compare physiologic, biochemical, carotid blood flow, and regional BBB permeability between ischemic and nonischemic fetal groups. If a significant difference (P<0.05) was found by ANOVA, then the two groups were further compared by the Fischer least-significant difference test. The two group Student's T-test was also used to compare mean values from the sham control and ischemic groups. A value of P<0.05 was considered as statistically significant.
Results
The gestational age (127±1 and 127±1 days), brain weights (41.3±3.3 and 37.8±3.3 g), and body weights (3.1±0.6 and 2.9±0.5 kg) of the sham control and ischemic group did not differ. All fetuses were from singleton pregnancies except for one twin pregnancy in the ischemic group.
The baseline pH, blood gas, heart rate, and mean arterial blood pressure values of the fetal sheep were within the physiologic range for our laboratory and did not differ between the sham control and ischemic study groups. Fetal arterial pH, PO2 and PCO2, heart rate, MABP, lactate, and hematocrit values during the study are summarized in Table 1.
Table 1. Fetal arterial pH, blood gases, base excess, heart rate, mean arterial blood pressure, hematocrit, and lactate values by study group.
| Group | Baseline | 0.25 | 1 | 2 | 4 | 4.25 |
|---|---|---|---|---|---|---|
| pH | ||||||
| Sham control | 7.35±0.01 | 7.34±0.01 | 7.33±0.01 | 7.32±0.01 | 7.32±0.01 | 7.32±0.01 |
| Ischemic | 7.34±0.01 | 7.33±0.01 | 7.31±0.01 | 7.31±0.01 | 7.31±0.01 | 7.31±0.01 |
| Arterial PO2 (mm Hg) | ||||||
| Sham control | 21±1 | 22±1 | 21±1 | 21±1 | 20±1 | 19±1 |
| Ischemic | 20±1 | 21±1 | 21±1 | 21±1 | 20±2 | 21±1 |
| Arterial PCO2 (mm Hg) | ||||||
| Sham control | 50±2 | 50±1 | 50±2 | 53±1 | 54±2 | 54±2 |
| Ischemic | 50±1 | 51±1 | 50±2 | 50±2 | 53±2 | 53±2 |
| Arterial base excess (mEq/L) | ||||||
| Sham control | 0.9±1 | 0.2±1 | −0.2±1 | 0.1±1 | 0.7±1 | 0.2±1 |
| Ischemic | 0.1±1 | −0.3±1 | −1.7±1 | −1.8±1 | −0.7±1 | −0.8±1 |
| Heart rate (beats/min) | ||||||
| Sham control | 178±4 | 169±5 | 163±6 | 170±5 | 173±13 | 189±12 |
| I/R-4 | 177±6 | 163±7 | 170±5 | 166±7 | 171±9 | 183±7 |
| MABP (mm Hg) | ||||||
| Sham control | 46±2 | 47±1 | 45±1 | 46±2 | 40±5 | 50±5 |
| I/R-4 | 45±2 | 42±2 | 42±1 | 45±3 | 37±2 | 45±3 |
| Hematocrit (%) | ||||||
| Sham control | 28±2 | ND | ND | ND | 29±2 | 29±1 |
| I/R-4 | 33±1 | ND | ND | ND | 32±1 | 33±2 |
| Lactate (mg/dL) | ||||||
| Sham control | 14±1 | ND | ND | ND | 18±3 | 19±3 |
| I/R-4 | 14±1 | ND | ND | ND | 15±2 | 15±2 |
MABP, mean arterial blood pressure; ND, not determined. Values are mean±standard error (mean±s.e.m.); sham control fetuses n=6, ischemic fetuses n=6; arterial PO2 and PCO2 are oxygen and carbon dioxide pressures, respectively.
Electrocorticogram and Carotid Arterial Blood Flow
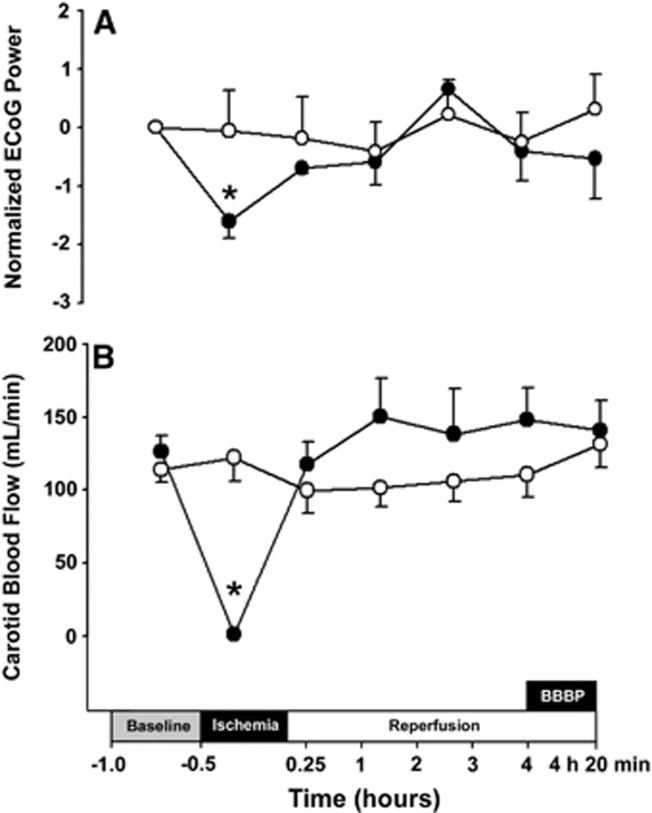
The ECoG and carotid arterial blood flow values were measured to ascertain that the ischemic exposure was adequate in the ischemic group.12 Thirty minutes of bilateral carotid artery occlusion resulted in a significant attenuation (P<0.01) of the grand average power spectral density (PSD-ECoG) signals compared with the baseline values, whereas the values did not differ (P=0.38) from baseline in the sham control group (Figure 3A). Visual inspection of the ECoG signals (JG) showed that attenuation in the ischemic group was greater than 50% of the baseline amplitude during ischemia, whereas the sham control fetuses did not exhibit attenuation in amplitude during sham control treatment (data not shown). After ischemia, the ECoG values returned to the baseline range and did not differ between ischemic and sham control groups (Figure 3A). Carotid artery occlusion resulted in immediate reductions (P<0.01) in the carotid arterial blood flow values, which approached zero within seconds after the onset of ischemia and remained in this range for the 30-minute duration of occlusion (Figure 3B). After the termination of occlusion, the carotid arterial blood flow values did not differ from the baseline values. Carotid blood flow remained similar to the baseline values in the sham control group.
Figure 3.
Changes in electrocorticogram (ECoG) and carotid blood flow during the study. ECoG (A) and carotid blood flow (B) values for the nonischemic (open circles, n=6) and ischemic–reperfusion (closed circle, n=6) plotted against study time in hours. The study design is reproduced on the x axis. The box indicating ‘BBBP' shows the time of the blood–brain barrier permeability study. ECoG (A) Power spectral densities, PSD-ECoG plotted as the difference from the individually averaged baseline ECoG values. Values are shown as median±s.e.m. for ECoG and mean±s.e.m. for carotid arterial blood flow, *P<0.05 ischemia versus baseline within the ischemic group.
Blood–Brain Barrier Permeability (Ki) Measurements
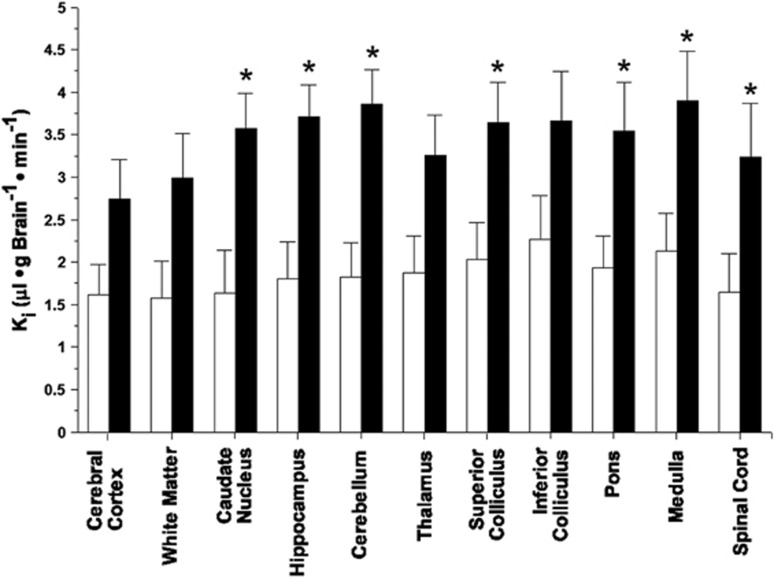
The Ki values showed that the iodinated IL-1β protein crossed the BBB in the nonischemic sham control fetal sheep (Figure 4). The Ki values measured with the iodinated IL-1β protein were significantly higher (P<0.03) across the brain regions of the ischemic compared with the sham control fetal sheep. The Ki values measured with the iodinated IL-1β protein exhibited significant regional heterogeneity within the fetuses in the sham control (P<0.01) and ischemic (P<0.01) groups. Moreover, the magnitude of the ischemia-related increases in the Ki values was not uniform across the brain regions, suggesting that there was a significant regional heterogeneity in the response to ischemia–reperfusion among the different brain regions.
Figure 4.
Blood-to-brain transfer constants (Ki) in the nonischemic (open bars, n=6) and ischemic–reperfusion (closed bars, n=6), groups plotted for the brain regions on the x axis. Values are mean±s.e.m. *P<0.05 versus nonischemic group.
As we have previously reported, the pituitary gland is an intracranial structure that does not have an effective BBB.10 The Ki values of the pituitary gland measured with the iodinated IL-1β protein were substantially higher compared with those of the other brain regions (15.75±5.6 and 42.34±7.29 μL/g Brain/min in the sham control and ischemic groups, respectively). The substantially higher Ki values in the pituitary gland compared with the other brain regions further confirm the validity of the Ki values measured with iodinated IL-1β protein in the brain regions that are known to possess tight BBB properties. The Ki values of the pituitary gland were also higher in the group exposed to ischemia–reperfusion than in the sham control group (P<0.05) suggesting that even though the pituitary gland does not possess a tight BBB, the permeability measured with IL-1β protein increases after ischemic–reperfusion injury.
Discussion
The BBB is increasingly recognized to have a critical role in a variety of central nervous system disorders in the adult particularly as related to neurodegenerative diseases.30 However, there is less information regarding the importance of abnormalities in blood–brain function related to disorders in perinatal medicine. We have previously shown that several perinatal conditions along with maternal and neonatal therapies can modify BBB function in the fetal and neonatal brain.11, 12, 19 In addition, maternal exposure to inflammatory conditions may impair the integrity of the BBB in the fetal cerebellum.31
Pro-inflammatory cytokines including IL-1β are known to cause and accentuate parenchymal brain injury in fetuses and neonates.32 There is a substantial amount of clinical evidence in support of the contention that elevated circulating systemic pro-inflammatory cytokines can be associated with the development of brain damage in the neonate.4, 6 Conditions such as systemic inflammation in the fetus and neonate, sepsis, necrotizing enterocolitis, and mechanical ventilation in the neonate, which are associated with elevations in systemic pro-inflammatory cytokines, are also associated with later brain injury in premature infants.20, 21, 22 In addition, we have recently shown that BBB dysfunction represents an important component of ischemic–reperfusion related brain injury in the fetus,12 and that the pro-inflammatory cytokine IL-1β contributes to this barrier dysfunction.19 Nonetheless, although it has been suggested that systemic cytokines might gain access to the fetal brain by crossing the BBB,4 there is a paucity of direct experimental evidence to support this concept and no quantitative data measuring BBB permeability with cytokines in the fetal or neonatal brain. Therefore, the objectives of the current study were to determine the ability of systemic circulating IL-1β to cross the intact BBB under homeostatic conditions in the normal fetus, and to determine whether ischemia accentuates the transfer of IL-1β across the BBB in the fetus.
There are three main findings in our study. First, systemic IL-1β crosses the BBB in control nonischemic ovine fetuses at 85% of gestation. Second, ischemia followed by 4 hours of reperfusion accentuated the transfer of IL-1β across the BBB. Third, BBB permeability measured with iodinated IL-1β protein exhibits heterogeneity among brain regions under control conditions and after exposure to ischemia–reperfusion.
Consistent with our previous reports,12, 19 carotid occlusion resulted in near-complete cessation of carotid arterial blood flow and attenuation in the fetal ECoG amplitude confirming the efficacy of the ischemic insult. In addition, carotid blood flow and ECoG values after ischemia did not differ from baseline values, confirming that reperfusion was effective after release of the occlusion. Consistent with our previous findings, brain ischemia and reperfusion did not have a major impact on the systemic hemodynamic and biochemical homeostasis in the fetus.12, 19
The findings of our study show that systemically administered IL-1β is able to cross the BBB in significant amounts to reach the brain during normal homeostatic conditions in the fetus. This finding suggests that conditions such as perinatal inflammation that result in increases in systemic pro-inflammatory cytokines could facilitate cytokines to cross the intact BBB and cause brain injury before birth even under nonischemic conditions.6, 20, 22 Several aspects of our study deserve comment. We used recombinantly expressed, pure ovine IL-1β protein to study blood-to-brain transport in fetal sheep. Therefore, the radiolabeled protein used to measure BBB permeability in the ovine fetus was species specific. In addition, the radiolabeling procedures used in the current study have been widely used for a number of compounds in various species and have repeatedly been shown to yield consistent reproducible results.14, 26 Moreover, we have previously shown that this ovine protein was able to cross the mouse BBB by a saturable transport mechanism.16 Taken together, our findings suggest that systemically produced IL-1β can in fact cross the immature BBB and potentially injure or accentuate injury to the brain.4
We also have previously showed increases in BBB permeability measured with AIB (molecular weight 103 daltons) suggesting impaired integrity of the BBB after exposure of the ovine fetus to ischemia for 30 minutes and reperfusion for 4 hours.12 In the present study, the same ischemic–reperfusion exposure accentuated IL-1β entry into the brain. Our findings can be interpreted that ischemic–reperfusion related injury to the cerebral vascular endothelium particularly in an inflammatory setting could accentuate the transfer of circulating cytokines across the fetal and potentially neonatal BBB.4, 31 Consequently, increased pro-inflammatory cytokine transport across the BBB could potentially further exacerbate ischemia-mediated damage to the fetal or neonatal brain parenchyma.4, 31 We cannot discern from our studies the precise nature of the cellular or molecular consequences of the increased IL-1β transport across the fetal BBB. However, previous work suggests that intracerebral injections of IL-1β result in astrogliosis, increases in caspase 3 activity, apoptosis, reductions in the number of developing oligodendrocytes, along with reduced myelin basic protein staining in neonatal rats.32 Hypoxia also upregulates astrocytic IL-1β in periventricular white matter, which induces receptor-mediated apoptosis of oligodendrocytes, resulting in periventricular white-matter hypomyelination.33 Moreover, inhibiting lipopolysaccharide stimulated IL-1β with an IL-1 receptor antagonist attenuates the lipopolysaccharide-induced white-matter injury further substantiating that L-1β may have an important role in white-matter injury in the neonate.34 In a model of systemic pre-exposure to lipopolysaccharide before hypoxic–ischemic injury, an increased IL-1β response also appeared to be responsible for neuronal injury.35 Taken together with the present findings, hypoxia–ischemia related increased transport of IL-1β across the BBB in the fetus could accentuate to injury of the cellular components of white matter and exacerbate hypoxic–ischemic neuronal injury.
Even though we cannot be certain of the morphologic changes in the BBB that predisposed to the hypoxia–ischemia related increases in IL-1β transport across the barrier, the BBB is a complex structure comprised of an array of tight junction proteins as well as astrocyte end-feet processes, pericytes, and basement membranes, all of which contribute to the integrity of the BBB.30 Although hypoxia–ischemia can affect all components of the neurovascular unit including the astrocytes, pericytes, basement membranes, and matrix metalloproteinases, potentially contributing to the increased permeability of cytokines, we have recently shown that ischemia–reperfusion results in changes in the expression of some tight junction proteins, which are associated with increases in barrier permeability in the fetus.12, 36 Therefore, changes in the molecular composition of tight junction proteins as well as changes in other components of the neurovascular unit could have contributed to the ischemia-related increases in IL-1β transport across the BBB in the fetus.12, 36
Although, thus far, we have only investigated the ability of IL-1β to cross the fetal BBB, it is likely that cytokines such as tumor necrosis factor-α, IL-6, and others could also cross the fetal barrier, based on the work by Banks et al in adult rodents.13, 14, 15, 37, 38 Moreover, we have already shown that the highly specific ovine IL-6 protein that we have generated can cross the BBB of the adult mouse.16, 18 However, comparative quantitative aspects of transfer of other cytokines await future investigation. We would very much like to examine the ability of radiolabeled ovine IL-6 to cross the fetal BBB in future studies. In addition, it is likely that hypoxia–ischemia could also accentuate this process based on our recent and current findings with IL-1β.12 Consistent with our findings showing that a pro-inflammatory cytokine can cross the fetal BBB, a recent study compared the hypothalamic transcriptomic response between fetal exposure to transient hypoxia and brachiocephalic occlusion, and found that responses between the two stimuli differed markedly.39 The differentially regulated genes in the hypothalamus between the hypoxic exposure and brachiocephalic occlusion insults could suggest that there was a humoral agent released into the fetal circulation during hypoxia that could cross the fetal BBB to stimulate or augment the transcriptomic responses in the fetal hypothalamus.39
Our current work deserves comparison with our previous findings.12 However, the two studies cannot be directly compared because of differences in methodology between the current study and our previous work with AIB.12 Nonetheless, it is possible to compare the ratios of the ischemic–reperfusion to the nonischemic Ki values measured with AIB12 and the iodinated IL-1β in the current work. After ischemia and 4 hours of reperfusion, the Ki values measured with AIB increased by 1.3 to 2.4 fold12 and those measured with the iodinated IL-1β by 1.6 to 2.6 fold in the current study. These findings suggest that the ischemia–reperfusion related relative increases in BBB permeability were relatively similar when measured with both compounds.
The current findings are also consistent with the previous work showing that there is a proportionately greater propensity for cytokines to cross the BBB relative to their size because of the saturable transport systems.14, 40 Although the pathways by which cytokines affect the central nervous system may be multiple, at least in adult subjects, direct penetration of the BBB is important.14, 37, 40 Moreover, the biologic importance of cytokine transport across the BBB has been established because memory impairment induced by IL-1α depends directly on the ability of this cytokine to cross the BBB.40 In addition, there are several lines of evidence to support the contention intact cytokines cross the BBB: (1) previous rigorous analysis by Banks et al in several manuscripts established that a number of cytokines cross the adult BBB undegraded;13, 14, 26, 27, 37, 40, 41, 42 (2) free iodine does not cross the BBB well because of a brain-to-blood efflux system for iodine;43 (3) we have previously shown that brain levels of cytokines are decreased when antibodies against that specific cytokine were infused into the blood;19, 44 and (4) transport of the labeled ovine cytokines across the BBB is inhibited by unlabeled murine cytokine.16 This latter finding is particularly important, as unlabeled cytokines would not be expected to inhibit brain uptake of free iodine.
The previous work has also shown that IL-1β is transported across cultured cerebromicrovascular endothelial cells via a temperature-sensitive, microtubule-dependent mechanism, potentially by type II IL-1 receptors.45 We have found in fetal sheep in vivo that IL-1β penetrates the BBB in significant amounts to reach the fetal brain under control conditions and that ischemia–reperfusion increases the transfer across the BBB. The increased IL-1β entry into the brain that we observed could reflect increased paracellular transport across damaged tight junctions.12 Alternatively, the increased ischemia–reperfusion related entry of IL-1β into the brain could also have occurred via increased transport across the cerebromicrovascular endothelial cells via increased receptor mediated transcytosis upregulation resulting in increased penetration into IL-1β the fetal brain.45
We have previously shown that the endothelium of pituitary gland is highly permeable compared with all other brain regions in ovine fetuses, lambs, and adults.10 Consistent with these findings, the Ki values of the pituitary gland measured with the iodinated IL-1β protein were also higher than all other brain regions. In addition, consistent with our previous findings with AIB,12 permeability of the pituitary gland increased after ischemia, when measured with the radiolabeled IL-1β suggesting that after ischemia cytokines can also penetrate the pituitary gland and potentially could alter its function.
Comparable to our previous findings showing heterogeneity of barrier permeability when blood–brain permeability was measured with AIB under physiologic and pathophysiologic conditions,11, 12 the various brain regions exhibited heterogeneity in barrier permeability when measured with the iodinated IL-1β under both the nonischemic and ischemic–reperfusion related conditions. Consistent with the present findings, work in adults has shown that transport rates differ among cytokines, among brain regions, under physiologic circumstances, and with disease states suggesting that there are cytokine-specific regulatory mechanisms and region-specific effects.41 Potential mechanisms for regional differences in barrier permeability could relate to regional differences in tight junction density, differences in the molecular biology of the tight junction,46 and differences in regional brain function that influence BBB cytokine transport.42
In summary, we conclude that in the ovine fetal brain at 85% of gestation, systemic IL-1β crosses the intact BBB and that ischemia with reperfusion for 4 hours facilitates the entry of systemic cytokines into the brain parenchyma, where they could potentially accentuate brain damage. We speculate that reducing ischemia-related increases in IL-1β protein transport across the BBB could potentially represent a promising therapeutic strategy to reduce brain injury in the fetus and the neonate. In this regard, we have recently shown that systemic infusions of anti-IL-1β monoclonal antibodies result in penetration of the monoclonal antibodies into the fetal brain, and that the monoclonal antibodies have biologic effects that include reducing the ischemia-related endogenous upregulation of IL-1β protein and the ischemia-related increases in BBB permeability measured with a nonspecific inert molecule (AIB) in the fetal brain.18
Acknowledgments
We gratefully acknowledge the gift of the ovine IL-1 β pGEX-2 T vector and mouse monoclonal cell lines from which we produced the monoclonal antibodies against ovine IL-1β from Commonwealth Scientific and Industrial Research Organization (CSIRO), Livestock Industries, Victoria, Australia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
GBS contributed to study design, performed the surgical preparation of the fetal sheep, conducted the study, analyzed and interpreted the data, and wrote the first draft of the manuscript. XC assisted with the surgical preparation of the animals, contributed to data collection, analyzed and interpreted the data, and reviewed the manuscript. JZ assisted with the surgical preparation of the animals, contributed to data collection and analysis, and reviewed the manuscript. Y-PL purified the IL-1β protein, contributed to the development of the iodination methodology, and revised the manuscript. EEC contributed to data collection and revised the manuscript. OM analyzed and interpreted ECoG measurements of the fetal sheep, and reviewed the manuscript. WGB developed the software to analyze and interpret ECoG measurements in the fetal sheep, and reviewed the manuscript. JG reviewed the ECoG tracings for accuracy of the tracings, and reviewed the manuscript. JP provided the methodology, assisted with the production of the IL-1β protein, and revised the manuscript. WAB contributed to conceptual development of the BBB study, provided the methodology for the iodination procedures and the BBB studies, assisted with the interpretation of the data, and revised the manuscript. BSS developed the concept and study design, interpreted the data, revised the manuscript, and gave approval for the final version of the manuscript. All authors have made substantive contributions to this study and/or manuscript, and all have reviewed the final paper before its submission.
The authors declare no conflict of interest.
Footnotes
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R01-HD-057100, by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 RR018728 and P20GM103537, and by a postdoctoral fellowship award (JZ) from the American Heart Association under grant number 13POST16860015.
References
- 1Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997; 100: 1004–1014. [DOI] [PubMed] [Google Scholar]
- 2Low JA, Robertson DM, Simpson LL. Temporal relationships of neuropathologic conditions caused by perinatal asphyxia. Am J Obstet Gynecol 1989; 160: 608–614. [DOI] [PubMed] [Google Scholar]
- 3Kinney HC, Back SA. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol 1998; 5: 180–189. [DOI] [PubMed] [Google Scholar]
- 4Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997; 42: 1–8. [DOI] [PubMed] [Google Scholar]
- 5Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke 1995; 26: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 6Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr 2011; 158:(897–903): e891–e895. [DOI] [PubMed] [Google Scholar]
- 7Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal 2008; 8: 1119–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Sadowska GB, Threlkeld SW, Flangini A, Sharma S, Stonestreet BS. Ontogeny and the effects of in utero brain ischemia on interleukin-1beta and interleukin-6 protein expression in ovine cerebral cortex and white matter. Int J Dev Neurosci 2012; 30: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 10Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol 1996; 271: R1594–R1601. [DOI] [PubMed] [Google Scholar]
- 11Stonestreet BS, Sadowska GB, Leeman J, Hanumara RC, Petersson KH, Patlak CS. Effects of acute hyperosmolality on blood-brain barrier function in ovine fetuses and lambs. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1031–R1039. [DOI] [PubMed] [Google Scholar]
- 12Chen X, Threlkeld SW, Cummings EE, Juan I, Makeyev O, Besio WG et al. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience 2012; 226: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett 1994; 179: 53–56. [DOI] [PubMed] [Google Scholar]
- 14Banks WA, Kastin AJ, Ehrensing CA. Blood-borne interleukin-1 alpha is transported across the endothelial blood-spinal cord barrier of mice. J Physiol 1994; 479: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 1993; 47: 169–176. [DOI] [PubMed] [Google Scholar]
- 16Threlkeld SW, Lynch JL, Lynch KM, Sadowska GB, Banks WA, Stonestreet BS. Ovine proinflammatory cytokines cross the murine blood-brain barrier by a common saturable transport mechanism. Neuroimmunomodulation 2010; 17: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest 1997; 100: 2648–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Chen X, Threlkeld SW, Cummings EE, Sadowska GB, Lim YP, Padbury JF et al. In-vitro validation of cytokine neutralizing antibodies by testing with ovine mononuclear splenocytes. J Comp Pathol 2013; 148: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Chen X, Sadowska GB, Zhang J, Kim JE, Cummings EE, Bodge CA et al. Neutralizing anti-interleukin-1beta antibodies modulate fetal blood-brain barrier function after ischemia. Neurobiol Dis 2015; 73: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004; 292: 2357–2365. [DOI] [PubMed] [Google Scholar]
- 21Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr 2005; 146: 798–804. [DOI] [PubMed] [Google Scholar]
- 22Bose CL, Laughon MM, Allred EN, O'Shea TM, Van Marter LJ, Ehrenkranz RA et al. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine 2013; 61: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997; 99: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol 2006; 21: 582–589. [DOI] [PubMed] [Google Scholar]
- 25Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem 1993; 210: 179–187. [DOI] [PubMed] [Google Scholar]
- 26Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 1991; 259: 988–996. [PubMed] [Google Scholar]
- 27Banks WA, Goulet M, Rusche JR, Niehoff ML, Boismenu R. Differential transport of a secretin analog across the blood-brain and blood-cerebrospinal fluid barriers of the mouse. J Pharmacol Exp Ther 2002; 302: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 28Dower SK, Kronheim SR, Hopp TP, Cantrell M, Deeley M, Gillis S et al. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature 1986; 324: 266–268. [DOI] [PubMed] [Google Scholar]
- 29Shiavi R. Random signals, linear systems, and power spectra In: Introduction to Applied Statistical Signal Analysis: Guide to Biomedical and Electrical Engineering Applications. Academic Press, Elseiver, Inc.: San Diego, CA. 2007. pp 201–205. [Google Scholar]
- 30Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia 2012; 53 (Suppl 6): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Hutton LC, Castillo-Melendez M, Walker DW. Uteroplacental inflammation results in blood brain barrier breakdown, increased activated caspase 3 and lipid peroxidation in the late gestation ovine fetal cerebellum. Dev Neurosci 2007; 29: 341–354. [DOI] [PubMed] [Google Scholar]
- 32Cai Z, Lin S, Pang Y, Rhodes PG. Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res 2004; 56: 377–384. [DOI] [PubMed] [Google Scholar]
- 33Deng Y, Xie D, Fang M, Zhu G, Chen C, Zeng H et al. Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS One 2014; 9: e87420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res 2003; 975: 37–47. [DOI] [PubMed] [Google Scholar]
- 35Savard A, Lavoie K, Brochu ME, Grbic D, Lepage M, Gris D et al. Involvement of neuronal IL-1beta in acquired brain lesions in a rat model of neonatal encephalopathy. J Neuroinflammation 2013; 10: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–185. [DOI] [PubMed] [Google Scholar]
- 37Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995; 2: 241–248. [DOI] [PubMed] [Google Scholar]
- 38Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol 1997; 146: 367–373. [DOI] [PubMed] [Google Scholar]
- 39Wood CE, Rabaglino MB, Richards E, Denslow N, Zarate MA, Chang EI et al. Transcriptomics of the fetal hypothalamic response to brachiocephalic occlusion and estradiol treatment. Physiol Genomics 2014; 46: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1alpha impairs memory processing in mice: dependence on blood-brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther 2001; 299: 536–541. [PubMed] [Google Scholar]
- 41Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 2005; 11: 973–984. [DOI] [PubMed] [Google Scholar]
- 42Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab 2000; 278: E1158–E1165. [DOI] [PubMed] [Google Scholar]
- 43Davson H, Segal MB. The secretion of the cerebrospinal fluid In: Physiology of CSF and Blood-Brain Barrier. CRC Press: New York. 1996, 199–255. [Google Scholar]
- 44Zhang J, Sadowska GB, Chen X, Park SY, Kim JE, Bodge CA et al. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J 2015; 29: 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 2009; 156: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 2000; 279: G250–G254. [DOI] [PubMed] [Google Scholar]