Abstract
Benefit of endovascular recanalization beyond established treatment time windows likely exists in select stroke patients. However, there is currently no imaging model that predicts infarction adjusting for elapsed time between the pathologic snapshot of admission imaging until endovascular recanalization. We trained and cross validated a multivariate generalized linear model (GLM) that uses computer tomography perfusion and clinical data to quantify patient-specific dynamic change of tissue infarction depending on degree and time of recanalization. Multicenter data of 161 patients with proximal anterior circulation occlusion undergoing endovascular therapy were included. Multivariate voxelwise infarct probability was calculated within the GLM. The effect of increasing time to treatment and degree of recanalization on voxelwise infarction was calculated in each patient. Tissue benefit of successful relative to unsuccessful recanalization was shown up to 15 hours after onset in individual patients and decreased nonlinearly with time. On average, the relative reduction of infarct volume at the treatment interval of 5 hours was 53% and this salvage effect decreased by 5% units per hour to <5% after 10 additional hours to treatment. Treatment time-adjusted multivariate prediction of infarction by perfusion and clinical status may identify patients who benefit from extended time to recanalization therapy.
Keywords: acute stroke, computed tomography perfusion, endovascular therapy, mathematical modeling, time
Introduction
Interventional treatment of acute stroke patients with proximal artery occlusion of the anterior circulation remains a major focus of current endovascular trials.1 With technical advancements of mechanical thrombectomy, higher and more consistent rates of recanalization are achieved compared with intravenous (IV) therapy alone. However, endovascular treatment has not unequivocally translated into a measureable patient benefit in randomized controlled trials.2, 3, 4, 5, 6, 7, 8 There are multiple factors that influence the benefit of endovascular treatment and successful recanalization on a macrovascular level may not always result in brain saving reperfusion on the capillary-tissue level.
Benefit of treatment is likely to be highly dependent on time and degree of recanalization6, 9, 10, 11 and it can be assumed that the favorable effect of successful recanalization on tissue outcome decreases with increasing time intervals to treatment. Recanalization-dependent benefit beyond established windows of 4.5 hours for IV recombinant tissue plasminogen activator (rt-PA) or 6 hours for mechanical recanalization likely exists in select patients. However, there is currently no imaging model that estimates tissue infarction adjusting for elapsed time between the snapshot of ischemic pathophysiology at admission imaging until endovascular treatment.
Imaging-based criteria for therapy rely on quantifying the size of infarct core in relation to the tissue at risk that may be spared from infarction if successful recanalization occurs. To separate voxels into the theoretical construct of core, salvageable penumbra, and benign oligemia, a perfect imaging model would precisely predict two tissue fates depending on recanalization status: infarct after permanent occlusion versus infarct after successful recanalization. The volumetric difference between these tissue fates is the therapeutic target, the tissue at risk or penumbra accessible to endovascular therapy.
The traditional approach of quantifying recanalization-dependent tissue fate is based on imaging thresholds of MR-apparent diffusion coefficient and MR- or computed tomography (CT)-perfusion (CTP) parameters maps that were established with disregard of variable imaging time and treatment time. However, there is reason to believe that tissue susceptibility to ischemia, i.e., a threshold that defines infarct, may depend on the time interval until recanalization. Optimal parameter thresholds have been empirically determined in CTP for core and penumbra using image data stratified by recanalization status. In recanalized patients, thresholds were determined that define the core (equivalent to infarct in follow-up imaging) based on the idealized assumption of immediate and complete recanalization at the time of imaging. In patients with permanent occlusion, thresholds were determined that define the penumbra (equivalent to infarct in follow-up imaging after subtraction of core threshold as determined on patients with recanalization).12 Thus, establishing a parameter threshold that defines the core lesion at the time of imaging ideally requires reference voxel data of follow-up infarct lesions from instantaneous and complete recanalization at the time of imaging. However, such data are practically not available since recanalization occurs after imaging with variable time intervals.
The predictive power of conventional univariate thresholding of a single imaging parameter is further limited by disregard of multiple additional factors that contribute to voxel-specific infarct risk. These factors include the combined effect of different perfusion parameters per voxel, variable tissue-specific susceptibility to ischemia, degree of clustering of low perfusion voxels, voxel location with respect to the anatomy of the affected vessel territory, and a priori infarct risk by type of occlusion.13, 14, 15, 16, 17, 18 Furthermore, thresholding does not integrate infarct risk imposed by variables on a patient level such as severity of symptoms at admission, age, and sex.5, 9, 19
To combine the independent contributing effect of multiple variables on voxelwise infarct probability, multivariate image analysis has been explored including logistic regression or generalized linear model (GLM).14, 20, 21 A multivariate imaging model that contains variables of timing and recanalization status opens the opportunity for dynamic estimation of infarction and salvageable tissue in the context of elapsing time and recanalization status after endovascular treatment. This is particularly important in light of evidence that growth of infarct lesions is heterogeneous and likely occurs beyond generally applied time windows for treatment in a large minority of patients.22, 23, 24 Quantifying salvageable tissue accounting for evolving infarct growth between imaging and treatment requires stroke imaging models that estimate tissue outcome adjusting for treatment time. Few studies employed mathematical dynamic models to simulate stroke evolution but none used medical data or have been assessed using imaging or clinical outcome.20
The purpose of this study was (1) to construct and validate a multivariate CTP-based imaging model for voxelwise infarct probability by incorporating multiple contributors of infarct risk including recanalization status and time to treatment based on a large empirical multicenter data set, (2) to examine how patient-specific multivariate voxelwise infarct risk is related to degree of recanalization in the context of variable time intervals to treatment. We hypothesize that there are patient-specific multivariate constellations of brain perfusion and clinical status where a benefit of successful recanalization on tissue outcome exists at extended time intervals to treatment.
Materials and methods
Patients
This multicenter study pooled anonymized data from prospectively collected stroke registries of four academic primary stroke centers (9-2008 to 12-2012). The study was performed with ethical review board approval (Ethik-Kommission der Ärztekammer Hamburg) and followed the Helsinki guidelines for Human experiments. Patients were selected consecutively based on a priori defined inclusion criteria: (1) first ever acute ischemic stroke with known time of onset and National Institutes of Health Stroke Scale (NIHSS) score above 3, (2) complete admission multimodal CT imaging protocol including CT-angiography (CTA) and CTP with follow-up imaging at 48 hours to 7 days, (3) occlusion of the (M1 segment) of the middle cerebral artery (MCA) or intracranial carotid artery confirmed by CTA, (4) endovascular therapy in general anesthesia with known time and degree of recanalization, (5) time from symptom onset to admission imaging within a maximum of 7 hours. Because the purpose was to model ischemic tissue outcome only, patients with significant parenchymal hemorrhage on follow-up imaging were a priori excluded (substantial space-occupying intracerebral hemorrhage associated with death or clinical deterioration of at least 4 points by NIHSS).25
Endovascular recanalization by thrombolysis in cerebral infarction (TICI) score and time of treatment (i.e., time of recanalization or last attempt) in DSA was recorded. Recanalization was dichotomized as successful (TICI 2b-3) versus unsuccessful (TICI 0-2a) defined as any result less than near-complete arterial reperfusion on DSA specific for poor outcome.26, 27 Baseline characteristics included age, sex, admission NIHSS score, clinical outcome by modified Rankin Scale (mRS), and stroke etiology.
Imaging
All patients received a comprehensive stroke imaging protocol at admission with standard native CT, CTA, and dynamic time resolved perfusion CT performed in equal order on 64 or 128 dual slice scanners (Siemens Definition AS+ Siemens Definition Flash, Siemens Healthcare, Forchheim, Germany; Philips Brilliance 64, Philips Medical Systems, Eindhoven, Netherlands). CT: 120 kV, 280 to 320 mA, 5.0 mm slice reconstruction; CTA: 100 to 120 kV, 260 to 300 mA, 1.0 mm slice reconstruction, 5 mm MIP reconstruction with 1 mm increment; CTP: 80 kV, 200 to 250 mA, 5 mm slice reconstruction (max. 10 mm), slice sampling rate 1.50 seconds (min. 1.33 seconds), scan time 45 seconds (max. 60 seconds), biphasic injection with 30 mL (max. 40 mL) of highly iodinated contrast medium with 350 mg iodine/mL (max. 400 mg/mL) injected with at least 4 mL/s (max. 6 mL/s) followed by 30 mL NaCl chaser bolus. All perfusion data sets were inspected for quality and excluded in case of severe motion artefacts.
Perfusion Postprocessing
All perfusion raw data were processed in a central core-lab on a workstation dedicated for perfusion analysis (Syngo mmwp VE52A with VPCT-Neuro; Siemens Healthcare, Forchheim, Germany) with motion correction and low band temporal noise removal. Nonparenchymal voxels corresponding to bone, vasculature, calcification, and cerebrospinal fluid were automatically excluded by time resolved intensity thresholding. Perfusion parameter maps were calculated based on a deconvolution model by least mean squares fitting.28 Voxelwise parenchymal time attenuation curves were deconvolved with a mean arterial input function measured in early arterialized voxels of the middle and anterior cerebral artery of the nonischemic side (on the level where arterial branches cross the plane perpendicular); venous outflow and reference for maximum enhancement was measured in the superior sagittal sinus. Voxels of arterial and venous reference vessels were detected automatically with supervision. Perfusion data sets with erratic or incomplete arterial and venous attenuation time curves and incomplete coverage of the ischemic MCA territory were excluded. All calculated perfusion maps were reformatted to 5 mm slice thickness with nearest neighbor interpolation so that quantitative image gray values remained unchanged.
Perfusion Image Variables of Infarct Risk
Quantitative perfusion maps were obtained for cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time (MTT), and time to drain (TTD), which describes the mean start of outflow out of the voxel.28 In temporal parameter maps (MTT and TTD) brain voxel with very low CBV and undetectable attenuation levels of bolus arrival could not be assigned a discrete time value exhibiting a theoretical indefinite delay. However, these voxels are likely characterizing tissue with high infarct probability as they are preferably located within the core lesion. The perfusion software marked these voxels with a negative unitless marker value (−900), which was used to segment all voxels of indeterminate delay in a separate binary map to be included in multivariate analysis (INDET) (Supplementary Material).
Spatial Image Variables of Infarct Risk
Besides perfusion parameters, we considered novel voxel-specific spatial features likely to influence infarct probability based on tissue-dependent ischemic vulnerability, anatomy of arterial supply, and spatial clustering of low perfusion (Supplementary Material).
Infarct risk imposed by reduced perfusion depends on gray-matter (GM) and white-matter content due to heterogeneous tissue-specific susceptibility to ischemia.13, 18 We coded voxelwise percentage of GM content within each CTP image as a variable for infarct risk using a population-based probabilistic GM tissue map in MNI stereotaxic standard space from 600 normal brain MRI.29 A precise deformation model was obtained between the baseline time average of each patient's CTP data set and a custom nonlinear averaged CT template in standard space using FMRIB Software Library v5.0 (Analysis Group, FMRIB, Oxford, UK). The voxel-specific map of GM content in standard space was then transformed to each individual CTP image (GMmap).29
The regional probability of final infarct is a function of anatomic arterial supply.15, 17 The location of low perfusion voxels in relation to the vascular territory of the affected artery may specify infarct risk in a multivariate model. We added an empirical map encoding the average a priori voxelwise infarct probability associated with proximal MCA occlusion. The map was created using a separate data set of 112 consecutive infarct lesions due to CTA confirmed MCA mainstem occlusions.30 Infarct lesions were segmented and coregistered to standard space to calculate average voxelwise infarct distribution (Analyze 11.0, AnalyzeDirect, Overland Park, KS, USA). As described above a deformation model was used to transform the map of a priori MCA territorial infarct probability from standard space to each CTP image (ProbMCA).
The degree of spatial clustering of low perfusion voxels is likely an independent contributor of infarct risk. It is conceivable that infarct probability of a low perfusion voxel is increased if it is located within a cluster of ischemic neighbors opposed to randomly distributed within normal perfused tissue. In each patient, low perfusion clustering was calculated and added as a variable to the model. The degree of low perfusion clustering within the MCA territory was expressed by the cumulative extent of low CBV voxels (CBVcluster) and TTD voxels (TTDcluster) weighted over the MCA territorial map as derived above (Supplementary Material).
Global Variables of Infarct Risk
We added global factors of infarct risk to voxel feature space. Therapy-specific variables included time from onset to admission imaging (TOtoTA), time from onset to treatment (TOtoRECA), and dichotomized recanalization status (RECA-0: TICI 0-2a, RECA-1: TICI 2b-3). Because recanalization status was known exactly by DSA in every patient, the presence or abscence of IV bridging therapy was not added as a separate variable to the model.
Patient-specific variables included admission NIHSS score, age, and sex. Each variable was coded in a separate map covering all CTP brain voxels.
Response Variable
Final tissue outcome was defined as voxelwise binary response variable (1=infarct, 0=no infarct). To classify voxels as infarct and calculate infarct volume, infarct lesions on follow-up CT between 48 hours and 7 days were manually segmented with semiautomated edge detection followed by rigid registration to the baseline time average of the CTP data set with nearest neighbor interpolation (Analyze 11.0, AnalyzeDirect). Manual segmentations were performed by trained raters blinded to clinical patient data in a separate imaging corelab not affiliated with and independent of the centers contributing the stroke imaging data.
The goal was a pragmatic approach with high consistency of method of defining final infarct lesions accross multiple centers. Follow-up lesions were chosen to be segmented on CT imaging only to avoid center bias by modality. For the chosen time intervall of follow-up imaging, brain shift may occur due to increased water content per voxel. We chose not to use follow-up imaging at later times because it was not available for all patients and to avoid a bias of different degrees of brain shift occuring at earlier and later stages of the infarct final lesion.
Generalized Linear Model for Prediction of Infarct Volume
We employed a GLM approach for voxelwise prediction of infarct probability as described previously.14, 31 The GLM converts the multivariate constellation for each individual voxel to one statistical parameter of voxel-wise infarct probability. In a GLM, the mean expected value E(I) of the response variable I follows some exponential family of probability distribution. A linear combination η of explanatory variables Xk with intercept β0 and coefficients βk predicts E(I) through a link function g.
 |
Because the response variable I for tissue outcome in each voxel was defined by binary status of infarction, the probability distribution of E(I) was binomial and a logit link function was used within a logistic regression model.
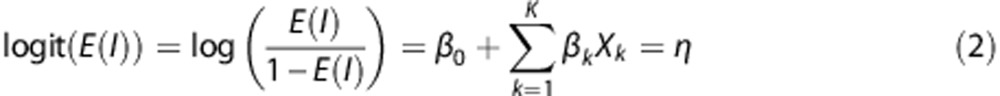 |
The expected value of the response variable I for the linear combination of explanatory variables in η is then the probability of infarction per voxel i.
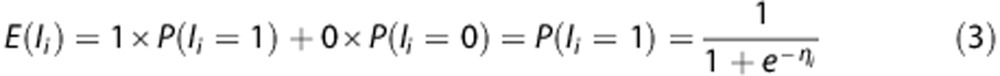 |
Infarct probability per voxel was used to calculate the total predicted infarct volume per brain, which is directly related to the mean expected value. The total infarct volume can be expressed as the product between the total number of voxels n within brain and mean observed infarction per voxel μ (=the number of infarcted voxels per total number of brain voxels). Because the mean observed infarction per voxel μ within brain (=sample space) is equivalent to the mean expected value or mean predicted probability of infarction per voxel P̂(I=1) after model fit, the total predicted infarct volume is calculated by summation of all infarct probabilities of every voxel i obtained within the GLM.
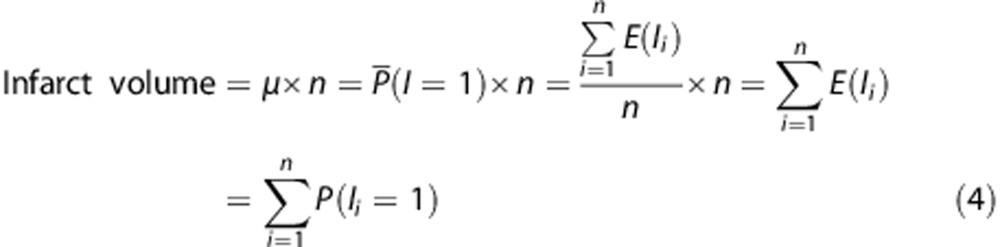 |
The following input variables as described above were added to the GLM to test their independent contribution to voxelwise infarct probability and total infarct volume: CBV, CBF, MTT, TTD, INDET, GMmap, ProbMCA, CBVcluster, TTDcluster, NIHSS, Age, Male, TOtoTA, TOtoRECA, and RECA. An interaction term between time until treatment and recanalization status (TOtoRECA × RECA) was added to the GLM to test the effect of treatment time on infarct risk with changing quality of recanalization.
Statistical Analysis
The parameter maps of input variables and the response variable of all patients were converted to a voxelwise data matrix (Matlab R2014a, The MathWorks, Natick, MA, USA). The GLM coefficients were calculated in R (v3.02, R Development Core Team) using the iterative maximum likelihood estimation method, and tested for robustness with leave-one-out cross validation for unbiased estimate of the prediction error.32 Thus, infarct prediction was validated in each single patient by leave-one-out cross validation using the coefficients obtained from the remaining patients as training set. Performance of multivariate infarct probability for correct classification of infarct voxels was tested by the area under the receiver operating characteristic curve. Mean cross-validated coefficients from each patient were used for infarct volume prediction in the final model. Accuracy of predicted infarct volumes with respect to the observed was assessed by the root mean square error  . A bootstrap estimate of the 95% confidence interval (CI) of the RMSE was calculated from 100,000 random samples.32
. A bootstrap estimate of the 95% confidence interval (CI) of the RMSE was calculated from 100,000 random samples.32
Stratified patients (successful versus unsuccessful recanalization) were compared using unpaired t-test (normal distribution) and Mann–Whitney U rank-sum test (non-normal distribution) for quantitative continuous or discrete variables and Fisher's exact test for qualitative categorical variables, respectively. Correlation analyses were performed using Pearson correlation coefficient. The level of significance was defined as a two-tailed P<0.05. Continuous variables are shown as mean and standard deviation (s.d.) or as median and interquartile range (IQR), discrete variables are reported as counts (n) and percentages (%).
We tested the mean effect of recanalization with increasing time intervals to treatment on voxelwise infarct probability and subsequently on absolute infarct volume independent of multivariate patient heterogeneity. To accomplish this, infarct probability specific to each patient was calculated for every voxel within the GLM with increasing TOtoRECA for each possible recanalization status, RECA-0 and RECA-1, while holding all other variables constant. The benefit of successful versus poor recanalization was defined as the percent reduction of infarct volume expected with RECA-0 versus RECA-1 (VolumeRECA-0−VolumeRECA-1)/VolumeRECA-0.
To estimate the adjusted odds ratio (OR) of a potential clinical benefit from successful recanalization, the total number of patients with predicted infarct size below and above 50 mL were calculated for each recanalization status with increasing treatment interval. A final infarct volume of 50 mL has been suggested as an optimal cut off for indicating the shift from poor to good clinical outcome defined by dichotomized mRS (0 to 2 versus 3 to 6) in major anterior circulation strokes.33 The OR of expected final infarct volume below 50 mL for RECA-1 versus RECA-0 was calculated with increasing time intervals up to 26 hours.
Results
Data of 161 consecutive patients with endovascular treatment for acute proximal MCA occlusion were analyzed. Median time from symptom onset to CTP imaging and treatment was 1.9 hours (IQR=1.4 to 2.9) and 4.6 hours (IQR=3.8 to 5.8), respectively. Median final infarct volume was 41.4 mL (IQR=11.5 to 109.7). In 93 patients (58%), TICI 2b-3 recanalization was achieved with significantly lower final infarct volume and discharge mRS than patients with TICI 0-2a (Table 1). By CBVcluster, the median percentage of the MCA territory affected by relative CBV reduction was higher in TICI 0-2a versus TICI 2b-3 patients (7.9%, IQR=0 to 19.0 versus 1.6%, IQR=0 to 12.4, P=0.05).
Table 1. Baseline characteristics and therapy of 161 patients.
| Baseline characteristics | All patients | TICI 2b-3 | TICI 0-2a | P |
|---|---|---|---|---|
| Subjects, n (%) | 161 (100.0) | 93 (57.8) | 68 (42.2) | |
| Age, years, mean (s.d.) | 69.0 (14.3) | 68.5 (14.4) | 69.6 (14.3) | 0.64 |
| Male sex, n (%) | 72 (44.7) | 42 (45.6) | 30 (44.1) | 1.00 |
| Admission NIHSS, median (IQR) | 16 (12–18) | 15 (11–18) | 16 (13–19) | 0.03 |
| Discharge mRS, median (IQR) | 4 (1–5) | 2 (0–4) | 4 (1–5) | <0.01 |
| Vessel occlusion | ||||
| MCA main stem, n (%) | 138 (86) | 86 (92.5) | 52 (76.5) | <0.01 |
| Carotid-T, n (%) | 23 (14) | 7 (7.5) | 16 (23.5) | <0.01 |
| Etiology | ||||
| Atherothrombotic, n (%) | 16 (9.9) | 8 (8.6) | 8(11.8) | 0.60 |
| Cardioembolic, n (%) | 103 (64.0) | 64 (68.8) | 39(57.4) | 0.13 |
| Undetermined etiology, n (%) | 36 (22.4) | 18 (19.4) | 18(26.5) | 0.13 |
| Other etiology, n (%) | 6 (3.7) | 3 (3.2) | 3(4.4) | 0.70 |
| IV bridging, n (%) | 126 (78.0) | 76 (81.2) | 50(73.5) | 0.14 |
| IA treatment, n (%) | 161 (100.0) | |||
| Mechanical only, n (%) | 85 (52.8) | 52 (55.9) | 33 (48.5) | 0.42 |
| Thrombolysis and mechanical, n (%) | 32 (19.9) | 17 (18.3) | 15 (22.1) | 0.56 |
| Thrombolysis only, n (%) | 44 (27.3) | 24 (25.8) | 20 (29.4) | 0.72 |
| Time from onset to | ||||
| Admission imaging, hours, mean (s.d.) | 2.3 (1.5) | 2.4 (1.5) | 2.2 (1.5) | 0.49 |
| Recanalization, hours, mean (s.d.) | 5.0 (1.7) | 5.0 (1.6) | 5.0 (1.8) | 0.72 |
| Final tissue outcome | ||||
| Infarct, mL, median (IQR) | 41.4 (11.5–109.7) | 31.6 (12.0–92.5) | 147.0 (59.1–228.3) | <0.001 |
| Infarct, % hemisphere, median (IQR) | 14.5 (3.8–34.3) | 6.4 (2.4–18.7) | 29.7 (11.9–46.1) | <0.001 |
IA, intraarterial; IQR, interquartile range; IV, intravenous; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; s.d., standard deviation; TICI, thrombolysis in cerebral infarction score.
Trained GLM coefficients in η after leave-one-out cross validation are listed in Table 2. All tested variables were retained in the model. According to negative GLM coefficient for recanalization status (βRECA=−1.81), odds for voxelwise infarction decreased by 83.6% with successful recanalization (TICI 0-2a versus TICI 2b-3) holding all other variables constant. According to positive coefficients for time interval to treatment (βTOtoRECA=0.174 and βTOtoRECA × RECA=0.113), odds for infarction increased by 18.9% for every additional hour until TICI 0-2a recanalization, and by 33.2% for every additional hour until TICI 2b-3 recanalization.
Table 2. Coefficients of input variables in η for calculating infarct probability and volume.
| Input variables | Coefficients (s.e.) |
|---|---|
| CBV (mL/100 mL) | −0.0235 (−0.0019) |
| CBVcluster | −2.6866 (−0.2117) |
| CBF (mL/100 mL/min) | −0.0068 (−0.0005) |
| MTT (s) | −0.0217 (−0.0017) |
| TTD (s) | 0.0604 (0.0048) |
| TTDcluster | 0.5654 (0.0446) |
| INDET | 0.4025 (0.0317) |
| GMmap (%) | −0.0041 (−0.0003) |
| ProbMCA (%) | 0.1532 (0.0121) |
| NIHSS | 0.0555 (0.0044) |
| Age (years) | −0.0203 (−0.0016) |
| Male | 0.0659 (0.0052) |
| TOtoTA (hours) | −0.0819 (−0.0065) |
| TOtoRECA (hours) | 0.1737 (0.0137) |
| RECA | −1.8067 (−0.1424) |
| TOtoRECA × RECA (h) | 0.1129 (0.0089) |
| Intercept | −0.2855 (−0.0225) |
CBF, cerebral blood flow; CBV, cerebral blood volume; GM, gray matter; INDET, indeterminate perfusion delay; MCA, middle cerebral artery; MTT, mean transit time; NIHSS, National Institutes of Health Stroke Scale; ProbMCA, a priori territorial infarct probability of middle cerebral artery; RECA, recanalization status; s.e., standard error; TOtoTA, time from onset to admission imaging; TOtoRECA, time from onset to treatment; TTD, time to drain.
After ROC curve analysis of GLM derived infarct probability the area under the ROC curve for correct classification of voxels as infarct was 0.85±0.07. The observed and predicted mean infarct volume across the entire cohort was 68.2±79.4 and 68.2±58.5 mL, respectively, with mean time to admission imaging (TOtoTA) of 2.3±1.6 hours and mean time to recanalization of 5.0±1.7 hours. The Pearson correlation coefficient between observed and predicted volumes was 0.844 (P<0.0001). The mean error (RMSE) of predicted infarct volumes was 38.0 mL (CI=27.5 to 47.7).
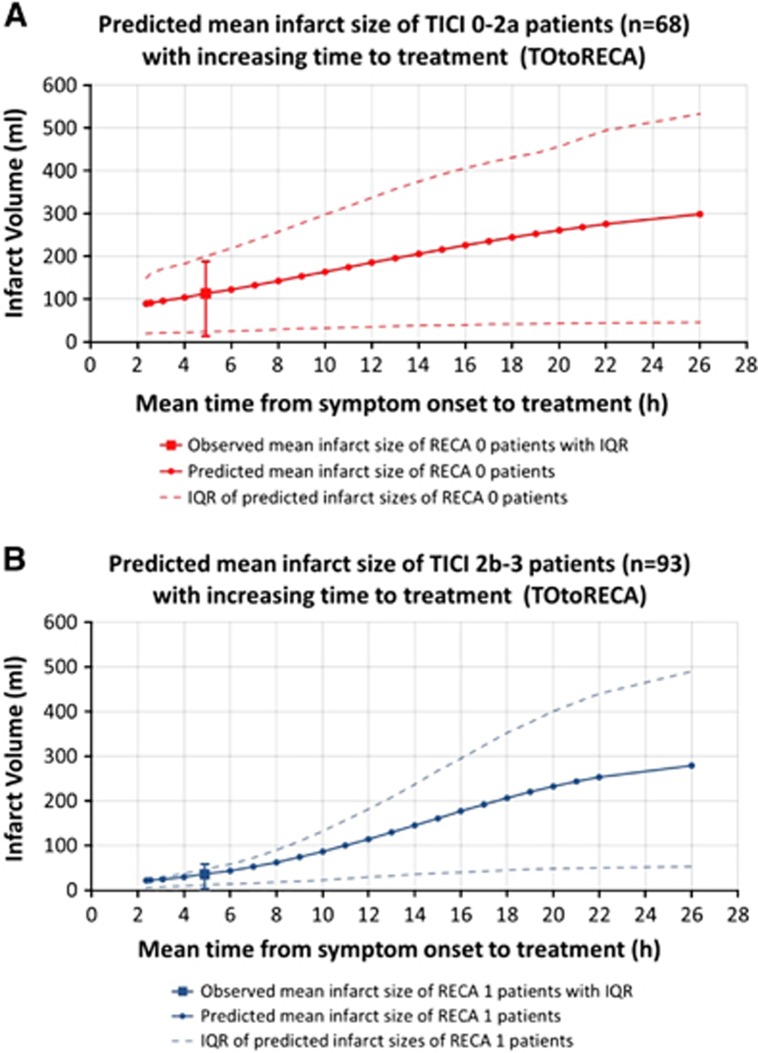
We tested the mean effect of recanalization and time interval to treatment (RECA and TOtoRECA) on tissue outcome adjusting for all other included variables within the GLM. Figure 1 shows an illustrative case where multifactorial voxelwise infarct probability was predicted for different time intervals to treatment by changing the variable TOtoRECA only. The mean predicted infarct size with increasing TOtoRECA was calculated across 68 patients with TICI 0-2a and 93 patients with TICI 2b-3 recanalization. In TICI 0-2a patients, the mean observed and predicted infarct size for the mean observed treatment interval (5.0 hours) was 112.8±137.2 and 112.5±101.8 mL, respectively. With increasing treatment interval, the predicted infarct volume increased to 122.2, 132.2, 142.5, and 153.0 mL at mean 6.0, 7.0, 8.0, and 9.0 hours TOtoRECA, respectively (Figure 2A). In TICI 2b-3 patients, the mean observed and predicted infarct size for the mean observed treatment interval (5.0 hours) was 35.7±61.54 and 35.9±38.8 mL, respectively. The predicted infarct volume increased to 43.6, 52.5, 62.6, and 74.0 mL at 6.0, 7.0, 8.0, and 9.0 hours, respectively (Figure 2B). In both groups, initially faster increase was followed by a tail of asymptotic growth tapering off beyond 22 hours.
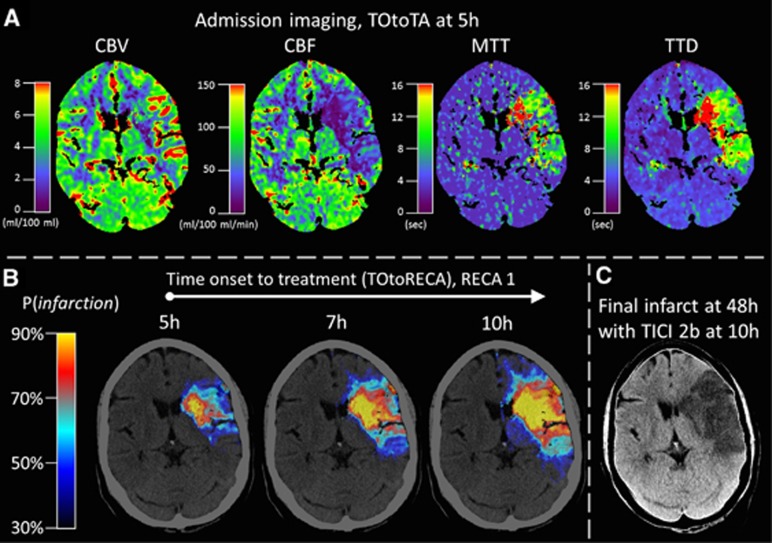
Figure 1.
Illustrative case of dynamic infarct prediction with increasing time to treatment. (A) Admission imaging showing four perfusion parameter maps that are included in the generalized linear model (GLM) for infarct prediction. The observed time of onset to admission imaging (TOtoTA) was 5 hours. (B) Multifactorial voxelwise infarct probability was predicted for different time intervals to successful recanalization by changing the variable time from onset to treatment (TOtoRECA) within the GLM. Increasing time to treatment resulted in a growing volume of voxels with high infarct probability. (C) The observed final infarct after late recanalization (TICI 2b) at 10 hours corresponds to the pattern of high infarct probability calculated with TOtoRECA=10 hours and RECA=1 within the GLM. CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; RECA, recanalization status; TTD, time to drain.
Figure 2.
Infarct volume with increasing time from onset to treatment (TOtoRECA) was calculated in (A) 68 patients with TICI 0-2a and (B) 93 patients with TICI 2b-3 recanalization. At the mean observed treatment interval of 5.0 hours, the predicted mean infarct size in each group was equal to the observed mean infarct size. With increasing treatment interval (TOtoRECA), the predicted mean infarct volume increased within the generalized linear model (GLM). IQR, interquartile range; RECA, recanalization status by TICI (thromboloysis in cerebral infarction) score.
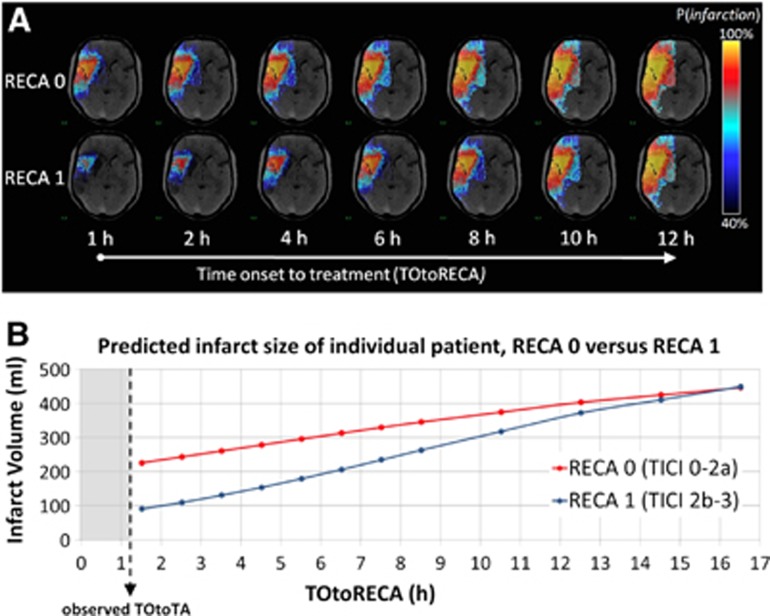
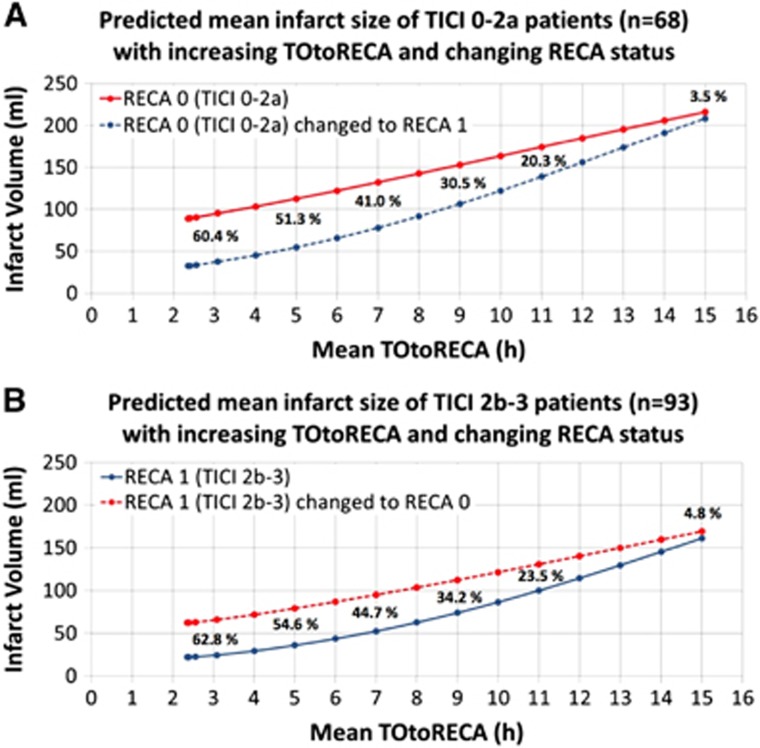
The time-dependent benefit of successful recanalization defined as percent reduction of infarct volume expected with RECA-0 versus RECA-1 was calculated in each individual patient of both patient groups (Figure 3). In TICI 2b-3 patients, the mean infarct volume of 35.9 mL at the mean treatment interval of 5.0 hours increased to 79.2 mL when changing RECA status to ‘0'. Thus, the mean benefit of recanalization in the TICI 2b-3 patient group was a volume difference of 54.6% at 5.0 hours. Analogously, in TICI 0-2a patients, the mean infarct volume of 112.5 mL at the mean treatment interval of 5.0 hours decreased to 54.8 mL when changing RECA status to ‘1'. Thus, the benefit of recanalization in TICI 0-2a patients was a volume difference of 51.3% at 5.0 hours. In both patient groups, the percent difference of infarct volume between RECA status ‘0' and ‘1' decreased to <5% after 15 hours. Figure 4 shows the time curves of predicted mean infarct volumes for TICI 0-2a (A) and TICI 2b-3 (B) patients compared with the time curves of infarct volumes after respective change in recanalization status.
Figure 3.
(A) Infarct probability and (B) infarct volume of an individual patient based on generalized linear model (GLM) prediction for successful (RECA-1) and unsuccessful (RECA-0) recanalization with increasing treatment intervals. The benefit of successful recanalization expressed as the percent reduction of infarct volume for RECA-0 versus RECA-1 decreased to <5% after 12 hours for the individual patient shown. TOtoRECA, time to recanalization; RECA, recanalization status by TICI (thromboloysis in cerebral infarction) score.
Figure 4.
Time curves of mean infarct volumes based on generalized linear model (GLM) prediction in patients with observed (A) TICI 0-2a and (B) TICI 2b-3 recanalization compared with infarct volumes when changing respective recanalization status within the model. Percentages indicate the relative volumetric difference (tissue benefit) of infarct volume RECA-0 versus RECA-1. The percent difference of infarct volume between RECA status ‘0' and ‘1' decreased from approximately 61% at 3 hours to <5% at 15 hours. RECA, recanalization.
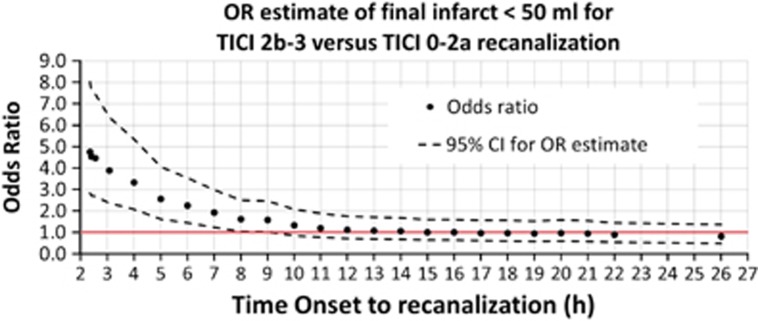
The OR of a potential clinical benefit based on an expected final infarct volume below 50 mL for RECA-1 versus RECA-0 was calculated with increasing time intervals across all 161 included patients (Figure 5). The OR (lower CI) was above 1.0 up to 9 hours onset to treatment.
Figure 5.
Odds ratio (OR) of expected final infarct volume below 50 mL among 161 included patients for RECA-1 (TICI 2b-3) versus RECA-0 (TICI 0-2a) adjusted for generalized linear model (GLM) variables (age, sex, NIHSS score, time to imaging, and perfusion status). Upper and lower 95% confidence interval (CI) calculated as 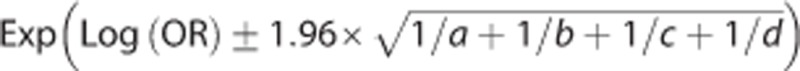 . RECA, recanalization.
. RECA, recanalization.
Discussion
The aim of this study was to determine the effect of the time interval to treatment and degree of recanalization on final infarct volume within the multifactorial constellation of variables that define an individual stroke patient and differentially contribute to infarct probability on the individual brain voxel level. Multivariate imaging approaches to predict infarct size have focused primarily on perfusion parameters only without adjusting for varying time intervals to recanalization.20 We expanded a GLM-based method to derive and validate the first multivariate brain perfusion imaging model that predicts voxelwise tissue fate within the dynamic context of time and degree of recanalization.
For any degree of recanalization and time to treatment, the trained and cross-validated GLM calculates a brain map of infarct probability based on the individual constellation of multiple CT perfusion parameters adjusting for tissue type, spatial clustering of ischemia, anatomy of vessel territory, severity of symptoms, age, sex, and time to imaging. Total infarct volume was calculated from cumulative voxelwise infarct probability based on the concept of the expected value in probabilistic statistical analysis. This threshold-free approach to determine infarct volume has several advantages. First, it does not rely on parameter cutoff values that binarize voxel values with loss of statistical information and that are subjected to variable definitions after receiver operating characteristic curve analysis. Second, it is rater independent and does not rely on manual segmentation. Third, the intrinsically exact model fit to the mean expected value ensures that, despite discrete prediction errors for individual patients, the mean predicted infarct volume of the study population is always equal to the mean observed infarct volume after model training. The mean observed and predicted infarct volume (68.2±79.4 and 68.2±58.5 mL, respectively) of the entire cohort was within the expected range of the final volume of typical large vessel supratentorial strokes (54 mL, range 19 to 100 mL).34 The higher observed and predicted mean infarct volume in TICI 0-2a (112.8±137.17 and 112.5±101.78 mL, respectively) and lower volume in TICI 2b-3 patients (35.7±61.54 and 35.9±38.8 mL, respectively) suggests a benefit of successful recanalization. However, these absolute differences of mean infarct volume do not directly translate to a true benefit of recanalization because a direct comparison of the two groups requires adjustment for heterogeneous variables such as brain perfusion status, clinical status, and other factors that contribute to tissue outcome.
Within the GLM, the incremental effect of increasing time intervals to treatment on tissue outcome was determined in the two distinct patient groups with TICI 0-2a and TICI 2b-3 recanalization adjusting for all other included variables. According to independent coefficients for time to treatment, delaying TICI 2b-3 recanalization increased voxelwise odds for infarction almost twice as fast than delaying TICI 0-2a recanalization. From voxelwise time curves of infarct probability, we determined the dynamic change of infarct size against time to treatment. Infarct volumes for shorter treatment intervals were lower in TICI 2b-3 patients but increased faster approaching the higher infarct volume of TICI 0-2a patients at longer treatment intervals. Dynamic progression of lesion volume over time is not well characterized in human stroke, logarithmic and sigmoidal growth patterns have been shown in various experimental animal stroke models depending on sampling resolution and range of time interval.35, 36 Both recanalization groups showed initially faster infarct volume increase at shorter treatment intervals up to 18 hours followed by a tail of asymptotic growth tapering off beyond 22 hours. This was above a reported estimated range for the average duration of nonlacunar lesion evolution for supratentorial strokes (10 hours, range 6 to 18 hours)34 but corresponded to the range of dynamic time curves of penumbra in human and animal studies.23, 36 The proportion of patients showing a substantial penumbral pattern after onset has been reported to decrease within the first 18 hours to 50%. Thereafter, the rate of decrease markedly slowed until 24 hours.23 In rat models, considerable infarct growth after MCA occlusion persisted until 22 hours at which 18F-FMISO binding in PET was attenuated corresponding to obliterated penumbral volume.36 Thus, evidence suggests that a large minority of patients exhibit ongoing penumbral ischemia at extended time intervals with probable effects on neuronal recovery.
We investigated the time-dependent benefit of successful recanalization, i.e., time-dependent penumbral salvage, within the multivariate data for each patient. The expected percent reduction of infarct volume after successful recanalization with respect to infarct volume after unsuccessful recanalization was calculated in both patient groups vice versa. The mean infarct volume of patients with observed TICI 0-2a and observed TICI 2b-3 recanalization was compared with outcome after respective change in recanalization status within the model. The relative reduction of infarct volume at the observed mean treatment interval of 5 hours was approximately equal in both patient groups, between 51% and 55%. This salvage effect of successful versus unsuccessful recanalization was reduced by 5% units per hour to <5% after 10 additional hours to treatment and obliterated at a mean interval of 15 hours. It is important to note that the mean benefit of recanalization for extended time intervals across the study population was based on individual multivariate cross-validated predictions. The prediction of treatment time-dependent dynamics of infarct volume for successful versus unsuccessful recanalization and the absolute volumetric tissue salvage were unique to the multivariate constellation of voxelwise parameters in each individual patient. In a clinical setting, patient-specific infarct prediction would be important to account for individual variability.
To estimate the time-dependent clinical benefit of successful recanalization across our representative study cohort, the total number of patients with good and poor outcome was calculated for each recanalization status assuming that an absolute final infarct volume of 50 mL optimally differentiates mRS 0-2 from 3 to 6.33 The OR (lower CI) of good outcome (OR of infarct below 50 mL) for successful versus unsuccessful recanalization was higher and remained longer above 1.0 (up to 9 hours to treatment) than the reported OR of good clinical outcome for rt-PA IV thrombolysis versus control (up to 4.5 hours).10, 25 It is conceivable that a treatment effect is less robust in rt-PA trials lacking patient stratification by occlusion type where low recanalization rates were likely in patients with proximal occlusions, whereas spontaneous recanalization was likely in controls with peripheral branch occlusions. In line with recent data from randomized trials of intraarterial treatment for acute ischemic stroke, our model suggests that the direct treatment effect of successful versus unsuccessful recanalization in a focused patient cohort stratified by CTA confirmed the presence of proximal artery occlusion may be statistically robust for extended time periods (adjusted OR or rate ratio for mRS 0-2 at an approximate mean recanalization time of 5 hours: 2.16 MR CLEAN, 1.7 ESCAPE, 4.2 EXTEND-IA, 2.55 GLM model).6, 7, 8 The model estimated OR for good clinical outcome may be lower because it is based on predicted infarct only and a bias may result from additional probability of occuring parenchymal hemorrhage.
The multivariate imaging model has limitations. In an ideal model scenario of permanent and complete vessel occlusion (i.e., failed treatment), calculated final infarct volumes should be constant regardless of treatment time. According to this scenario, GLM coefficients for TOtoRECA would be significant for an interaction effect with successful recanalization (RECA-1) only. However, the calculated coefficients for TOtoRECA also showed some time-dependent treatment effect for unsuccessful recanalization (RECA-0). Despite failed recanalization, predicted infarct lesion volumes increased with longer treatment intervals, although at any treatment time the absolute infarct volume was higher and increased less dynamically than with successful recanalization.
There are plausible reasons why predicted infarct volume for unsuccessful recanalization may show some time dependence. First, patient data cannot be dichotomized with respect to treatment success in an ideal scenario of ‘no treatment with complete occlusion' versus ‘complete recanalization ad integrum'. Instead, dichotomized recanalization status represented the group average of ‘near complete' (TICI2b-3) versus ‘less than near complete' (TICI 0-2a) recanalization and some treatment effect may be expected in TICI 0-2a patients. The important clinical implication is that even though TICI 0-2a recanalization usually translates to poor clinical outcome, a measureable benefit on the tissue level may still be predicted in some patients when lesion size is intermediate and shifts to a smaller size with early treatment, which may be relevant for good clinical outcome.
Second, even in cases of permanent complete vessel occlusion, the time interval to treatment may be predictive of infarct risk due to endogenous and external interaction effects. All patients received endovascular treatment and it can be assumed that prolonged procedure times with higher difficulty of endovascular access and intervention are associated with severe general vascular disease imposing global risk on infarct outcome. Furthermore, the interaction of later treatment times with longer initiation and duration of general anesthesia may contribute to global infarct risk and subsequently higher final infarct volume. Monitoring during anesthesia during prolonged procedure times is an opportunity to tap further data of potentially confounding variables of outcome until recanalization that were not included in the model, such as heart rate, blood pressure, cardiac index, oxygen extraction index by near infrared spectroscopy, or bispectral index.
A major benefit of the GLM is that it converts the cumulative effect of multiple factors on voxelwise tissue outcome into one single image of infarct probability, a concept that has been employed before to examine the physiologic heterogeneity of infarction risk in separate patients with and without IV rt-PA treatment.14 Because we included variables of treatment time and recanalization status in the GLM, it was possible to directly visualize probability of tissue outcome for different treatment scenarios over time. The model showed a diminishing effect of tissue salvage for successful versus unsuccessful recanalization with increasing time intervals to treatment. The dynamic quantification of tissue outcome holds out the prospect of triaging patients by expected time and quality of treatment on an individual basis. This may improve multiparametric imaging models using CTP that have been used to define infarct core and favorable penumbral pattern at the time of admission imaging with disregard of expected treatment time and that so far have failed to select patients who benefit from endovascular treatment over IV rt-PA alone.21, 37 It must be noted, however, that GLM coefficients derived in this study may be applicable only to the specific algorithm used for calculating perfusion parameters maps since different algorithms and vendor specific software are known to produce different perfusion values with different predictive power.38 Furthermore, model coefficients are tailored to the data of our study population (endovascular-treated patients with major strokes due to proximal occlusion in the anterior circulation) and may not apply to a stroke populations defined by different inclusion criteria and treatment modes.
We chose a multicenter study design including the largest sample size (n=161) to date that has been used for training of a multiparametric imaging model. Multivariate prediction requires large numbers of patients to fully characterize the true empirical variability of each included variable. Avoiding the risk of overfitting is essential, especially in high dimensional patient data used in model training.39 The majority of published multiparametric imaging models for lesion prediction are based on single center data with fewer than 30 mostly mixed cases of major and minor strokes.20 We argue that less than 30 cases is below the required sample size needed to stabilize voxelwise risk prediction for a general stroke population particularly when using cases that are not stratified by vascular occlusion, which in itself is a predictor of early lesion growth.40 Wu et al14 discussed that their GLM approach with 11 patients in the training set was likely not sufficient to adequately characterize heterogeneity in human stroke populations. To reduce heterogeneity while maintaining clinical relevance, our study included patients with major anterior circulation strokes only amendable to endovascular treatment. Nonetheless, predicted infarct volumes may be distorted where the GLM extrapolates the effect of time to treatment beyond the range of available patient training data. Also, the model predicts infarct only in brain covered by CTP imaging, thus infarct volume estimation should only be used in brain perfusion CTP covering the entire extend of ischemia.
The presented GLM was based on a rigorous data mining approach on the patient and voxel level. Variables were sampled in a total of 72,585,741 brain voxels producing a multidimensional data matrix of 1,233,957,597 data points. Besides multiple perfusion parameters, the GLM contained several novel spatial covariates that have been implicated to improve prediction of infarct probability but none have been jointly used in multivariate imaging models so far.
First, voxelwise infarct risk imposed by reduced perfusion is variable depending on white-matter and GM content.13, 18 In a preliminary study, multivariate infarct prediction was improved in an anatomically-weighted GLM using tissue-specific probability maps.41 Our model adjusted for tissue-specific infarct risk using a population-based probabilistic tissue map of voxelwise GM content.29
Second, to further improve predictive precision, we incorporated a priori anatomic information of the MCA territory encoding the empircal distribution of infarct lesions after MCA occlusion.15, 30 Probabilistic voxel information by vessel territory has not been used in a multivariate imaging model before. In our model, the variable ProbMCA improved prediction considerably by suppressing information of low perfusion voxels not associated with the MCA territory.
Finally, the degree of spatial correlation of ischemic voxels has been suggested as a method to pinpoint ischemic clustering with higher risk of infarction. Preliminary model extension of GLM by covariates defining spatial correlation showed improvement in prediction accuracy.42 In our study, two covariates that correlated with the degree of ischemic clustering within the MCA territory in CBV and TTD perfusion maps were included and coefficients significantly contributed to GLM infarct prediction.
Summary
We established a multivariate CTP-based imaging model to calculate dynamic change of voxelwise infarct probability and predict infarct volume related to degree and time of recanalization in stroke patients with anterior circulation proximal occlusion. Based on a cross-validated GLM, volume of final brain infarct was calculated in individual patients using multiple independent variables of infarct risk on the tissue-, patient-, and treatment level.
The benefit of successful recanalization on infarct volume decreased nonlinearly with time. A relevant reduction of lesion size by successful relative to unsuccessful recanalization can be expected on average up to 15 hours after onset. The model suggests that benefit on clinical outcome based on infarct size can be expected, on average, up to 9 hours after onset.
This is the first multivariate imaging model that predicts voxelwise brain infarction adjusting for the time interval between imaging and endovascular recanalization. Time-adjusted multivariate prediction of tissue salvage allows patient-specific visualization of tissue outcome with increasing time to treatment and may improve selecting patients who benefit from extended time to vessel recanalization.
AK—UNRELATED to article.
Research collaboration agreement: Siemens Healthcare.
JF—UNRELATED to article.
Consultancy: Codman, Stryker, MicroVention;
Grants/Grants Pending: Codman, Stryker, MicroVention;
Payment for Lectures (including service on Speakers Bureaus): Penumbra, Philips, Covidien;
Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Covidien.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author contributions
All authors have contributed substantially to the article and approved the version to be published. AK contributed to study design, image analysis, statistical analysis, and drafting the article and revising it critically. FF contributed to study design, image processing, statistical analysis, and drafting the article and revising it critically. NDF contributed to image processing, analysis interpretiation, and drafting the article and revising it critically. JM, WH, BE, MK, MP, and SL contributed to acquisition of data and drafting the article and revising it critically. GT and JF contributed to analysis and interpretation, and drafting the article and revising it critically.
Supplementary Material
References
- 1Fiehler J, Söderman M, Turjman F, White PM, Bakke SJ, Mangiafico S et al. Future trials of endovascular mechanical recanalisation therapy in acute ischemic stroke patients—a position paper endorsed by ESMINT and ESNR: part II: methodology of future trials. Neuroradiology 2012; 54: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 2Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 3Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008; 39: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 5Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2014; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 8Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 9Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009; 73: 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Hacke W, Donnan G, Fieschi C, Kaste M, Kummer von R, Broderick JP et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768–774. [DOI] [PubMed] [Google Scholar]
- 11Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000; 55: 1649–1655. [DOI] [PubMed] [Google Scholar]
- 12Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979–985. [DOI] [PubMed] [Google Scholar]
- 13Murphy BDB, Fox AJA, Lee DHD, Sahlas DJD, Black SES, Hogan MJM et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 2008; 247: 818–825. [DOI] [PubMed] [Google Scholar]
- 14Wu O, Christensen S, Hjort N, Dijkhuizen RM, Kucinski T, Fiehler J et al. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using MRI. Brain 2006; 129: 2384–2393. [DOI] [PubMed] [Google Scholar]
- 15Phan TG, Donnan GA, Wright PM, Reutens DC. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke 2005; 36: 986–991. [DOI] [PubMed] [Google Scholar]
- 16Siemonsen S, Forkert ND, Hansen A, Kemmling A, Thomalla G, Fiehler J. Spatial distribution of perfusion abnormality in acute MCA occlusion is associated with likelihood of later recanalization. J Cereb Blood Flow Metab 2014; 34: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Cheng B, Golsari A, Fiehler J, Rosenkranz M, Gerloff C, Thomalla G. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J Cereb Blood Flow Metab 2011; 31: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab 2005; 25: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 19Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013; 136: 3554–3560. [DOI] [PubMed] [Google Scholar]
- 20Rekik I, Allassonnière S, Carpenter TK, Wardlaw JM. Medical image analysis methods in MR/CT-imaged acute-subacute ischemic stroke lesion: segmentation, prediction and insights into dynamic evolution simulation models. A critical appraisal. Neuroimage Clin 2012; 1: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Kidwell CS, Wintermark M, de Silva DA, Schaewe TJ, Jahan R, Starkman S et al. Multiparametric MRI and CT models of infarct core and favorable penumbral imaging patterns in acute ischemic stroke. Stroke 2012; 44: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Finitsis S, Kemmling A, Havemeister S, Thomalla G, Fiehler J, Brekenfeld C. Stability of ischemic core volume during the initial hours of acute large vessel ischemic stroke in a subgroup of mechanically revascularized patients. Neuroradiology 2014; 56: 325–332. [DOI] [PubMed] [Google Scholar]
- 23Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke 1999; 30: 2043–2052. [DOI] [PubMed] [Google Scholar]
- 24Markus R, Reutens DC, Kazui S, Read S, Wright P, Pearce DC et al. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain 2004; 127: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 25Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 26Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke 2013; 44: 2509–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Jayaraman MV, Grossberg JA, Meisel KM, Shaikhouni A, Silver B. The clinical and radiographic importance of distinguishing partial from near-complete reperfusion following intra-arterial stroke therapy. Am J Neuroradiol 2013; 34: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Abels B, Klotz E, Tomandl BF, Kloska SP, Lell MM. Perfusion CT in acute ischemic stroke: a qualitative and quantitative comparison of deconvolution and maximum slope approach. Am J Neuroradiol 2010; 31: 1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Kemmling A, Wersching H, Berger K, Knecht S, Groden C, Nölte I. Decomposing the Hounsfield unit: probabilistic segmentation of brain tissue in computed tomography. Clin Neuroradiol 2012; 22: 79–91. [DOI] [PubMed] [Google Scholar]
- 30Kemmling A, Kamalian S, Souza L, Lev MH, Fiehler J, Forkert N. Abstract TP38: improved infarct prediction after acute ischemic stroke: a priori infarct probability defined by site of vessel occlusion can be combined with infarct likelihood-ratios based on Ct perfusion imaging. Stroke 2013; 44: ATP38. [Google Scholar]
- 31Wu O, Koroshetz WJ, Østergaard L, Buonanno FS, Copen WA, Gonzalez RG et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke 2001; 32: 933–942. [DOI] [PubMed] [Google Scholar]
- 32Efron B. Estimating the error rate of a prediction rule: improvement on cross-validation. J Am Stat Assoc 1983; 78: 316–331. [Google Scholar]
- 33Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012; 43: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 34Saver JL. Time is brain—quantified. Stroke 2006; 37: 263–266. [DOI] [PubMed] [Google Scholar]
- 35Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke 2000; 31: 1965–1972, discussion 1972–3. [DOI] [PubMed] [Google Scholar]
- 36Saita K, Chen M, Spratt NJ, Porritt MJ, Liberatore GT, Read SJ et al. Imaging the ischemic penumbra with 18F-fluoromisonidazole in a rat model of ischemic stroke. Stroke 2004; 35: 975–980. [DOI] [PubMed] [Google Scholar]
- 37Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Kudo K, Sasaki M, Yamada K, Momoshima S, Utsunomiya H, Shirato H et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 2010; 254: 200–209. [DOI] [PubMed] [Google Scholar]
- 39Subramanian J, Simon R. Overfitting in prediction models—is it a problem only in high dimensions? Contemp Clin Trials 2013; 36: 636–641. [DOI] [PubMed] [Google Scholar]
- 40Fiehler J, Knudsen K, Thomalla G, Goebell E, Rosenkranz M, Weiller C et al. Vascular occlusion sites determine differences in lesion growth from early apparent diffusion coefficient lesion to final infarct. AJNR Am J Neuroradiol 2005; 26: 1056–1061. [PMC free article] [PubMed] [Google Scholar]
- 41Wu O, Christensen S, Rosa-Neto P, Hjort N, Rodell A, Dijkhuizen RM et al. Anatomy as a parameter in multiparametic MRI-based predictive algorithms. Proc Intl Soc Mag Reson Med 2004; 11: 1392. [Google Scholar]
- 42Nguyen V, Pien H, Menenzes N, Lopez C, Melinosky C, Wu O et al. Stroke tissue outcome prediction using a spatially-correlated model. Prog Proc PPIC 2008; 8: 238–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.