Abstract
Prospective studies on magnetic resonance imaging (MRI)-guided systemic thrombolysis >4.5 hours after stroke onset did not reach their primary end points. It was discussed and observed in post hoc data re-assessment that this was partly because of limited MRI accuracy to measure critical hypoperfusion. We report the first cases of simultaneous [15O]H2O-positron emission tomography (PET)/MRI in stroke patients and an ovine model. Discrepancies between simultaneously obtained PET and MRI readouts were observed that might explain the above current limitations of stroke MRI. By offering highly complementary information, [15O]H2O-PET/MRI might help to identify critically hypoperfused tissue resulting in an improved patient stratification in thrombolysis trials.
Keywords: Mismatch, penumbra, PET/MRI, stroke, thrombolysis
Introduction
Current clinical stroke research aims at selecting ischemic stroke patients who may benefit from systemic thrombolysis beyond the standard therapeutic window of 4.5 hours.1 For that purpose, the concept of salvageable brain tissue with critical cerebral blood flow (CBF) reduction has been transferred from positron emission tomography (PET)2 to magnetic resonance imaging (MRI)3 to allow therapeutic decisions within routine stroke management. Nowadays, brain tissue with perfusion values below established thresholds, but preserved diffusion in MRI is considered as tissue at risk (MR ‘mismatch' concept).3 However, selecting stroke patients on the basis of this MR ‘mismatch' concept did not improve clinical outcome after systemic thrombolysis3, 4, 5 and even for endovascular treatment, conclusive evidence for this MR-based selection strategy remains to be provided.6 The optimal way to derive quantitative CBF values or time-based variables out of perfusion-weighted (PW) MRI that best describe the ischemic penumbra is still a matter of debate. Technical challenges and a lack of standardization3 in acquiring PW MR data have contributed to this situation. As a consequence, a more precise target profile to delineate perfusion deficits on MRI was recently proposed for future stroke trials.7
PET with its ability to detect radiotracers at high sensitivity provides highly accurate quantification of pathophysiologic parameters. Cross-calibration of PW MRI against [15O]H2O PET, an established technique to noninvasively quantify CBF,8 may help to address the above-mentioned problem. Comparison of PW MRI versus [15O]H2O PET data in acute stroke has so far only been possible on the basis of sequential data acquisition. As acute stroke is a highly dynamic pathophysiologic situation, the significant time delay between both modalities possibly affected accuracy in previous research.
We report results from a series of investigations using for the first time simultaneous [15O]H2O PET/MR brain imaging in acute stroke. We aimed at providing proof of concept for simultaneous PET/MRI both in patients with acute stroke as well as in a recently established ovine stroke model.9 We were able to directly relate CBF estimates from PW MRI to absolute measurements from PET and could exemplify how the same PW MRI threshold can either correctly classify benign oligemia in some patients or fail to do so in other cases.
Materials and Methods
A detailed description of the Materials and Methods is available in the Online Supplementary Methods. Ten patients with symptoms of stroke and contraindication against systemic thrombolysis prospectively underwent simultaneous [15O]H2O PET/MRI. The local ethics committee of the University of Leipzig approved the study and waived requirement for informed consent. The study was performed in accordance with the Declaration of Helsinki. Apart from [15O]H2O PET/MRI, instead of MR-only examination, basic stroke care remained unchanged. The degree of perfusion disturbance was evaluated immediately on-site by an experienced neuroradiologist using standard non-quantitative perfusion-weighted imaging (PWI) maps (syngo.MR NeuroPerfusion, Siemens Health Care, Erlangen, Germany, not shown). Quantitative maps of cerebral perfusion were calculated post hoc for both PET and MRI. Briefly, the dynamic [15O]H2O PET data together with the arterial input functions were used for standard full kinetic modeling using a one-tissue-compartment model, which resulted in parametric CBF maps (perfusion[PET]). In parallel, PWI maps (TTPdelay, Tmax, and CBF) were obtained from the motion-corrected EPI data (perfusion[MRI]) by a model-independent, standard singular value decomposition algorithm10 using the Perfusion Mismatch Analyzer software (Acute Stroke Imaging Standardization Group ASIST, Iwate Medical University Japan). Areas of restricted diffusion were subtracted from perfusion[MRI] and perfusion[PET]. Post hoc disturbances in perfusion[PET], perfusion[MRI], and the initial non-quantitative clinical MR reports were evaluated visually. Further, the presence, location, and extent of a final cerebral infarction were identified on fluid-attenuated inversion recovery (T2 FLAIR) MRI follow-up 5 days after stroke symptom onset.
Likewise, three male merino sheep were examined either 4.5 hours, 27 hours, or 14 days after permanent middle cerebral artery occlusion (pMCAO).9 No follow-up imaging was available in the animal studies. The animal experiments were conducted in accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimentation and the current ARRIVE guidelines. The animal experiments were approved by the local animal welfare authorities (Directorate Leipzig, Germany).
Results
Ten patients were prospectively imaged in this proof of concept trial. Supplementary Table 1 provides patient characteristics and imaging findings. Standard stroke MRI data acquisition started 43±11 minutes after the patients' informed consent. [15O]H2O for simultaneous CBF PET and PW MRI data acquisition was available 29±18 minutes later. Total PET/MRI scanning time was 61±16 minutes.
According to visual inspection, there was good correspondence between perfusion[PET] and perfusion[MRI] in four patients. In two patients, perfusion[MRI] deficits were detected, which were not evidenced by perfusion[PET], but could correctly be classified as non-critical hypoperfusion with a Tmax >6 seconds. In two patients, unilateral discrete hypoperfusion according to perfusion[PET] was not mirrored in perfusion[MRI]. In one patient, critical hypoperfusion was observed in both perfusion[PET] and (less severe) perfusion[MRI]. In this case, no infarct growth was seen over time because of spontaneous recanalization of the obstructed MCA. Severe head motion prohibited perfusion[MRI] analysis in one patient, whereas perfusion[PET] did not reveal a perfusion deficit in this case. A typical patient with an MCA infarction is presented in Figure 1. In this case, the final infarction on FLAIR follow-up was almost equally well predicted by perfusion[PET] and perfusion[MRI] (choosing a Tmax >6 seconds as threshold). A case of transient diffusion restriction in which reversibility was correctly predicted by both perfusion[MRI] and perfusion[PET] is illustrated in Supplementary Figure 1.
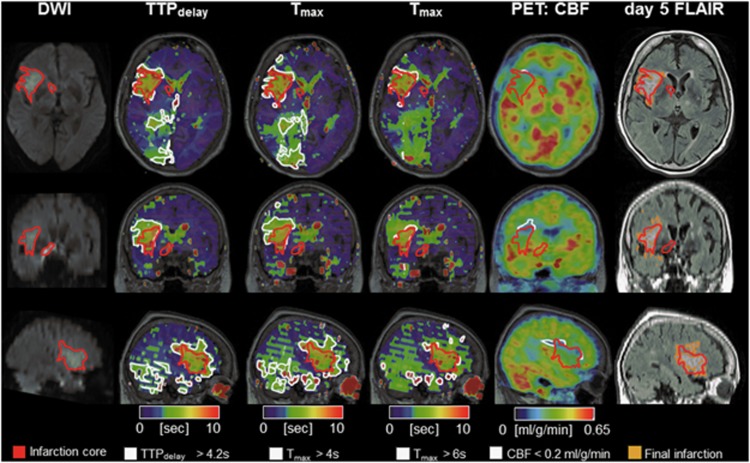
Figure 1.
[15O]H2O PET/MRI in a typical human MCA infarction. A 78-year-old, right-handed woman with left hemiparesis and dysarthria upon waking up. Diffusion-weighted MRI showed a disturbance in the right MCA territory (red-bordered area, 22 mL). Perfusion[MRI] flow delays in the right PCA territory did not result in a perfusion restriction according to PET and may thus indicate sufficient collateral flow secondary to the MCA occlusion. Perfusion[MRI] indicated a penumbral volume (white-bordered areas) of 42 mL for Tmax >6 seconds. According to perfusion[PET] (17 mL, white-bordered area) a smaller penumbral volume was predicted that corresponded better with the follow-up infarct size according to T2 FLAIR (infarct growth of 7.5 mL) on day 5. Note: a slight change to time-based variables (TTPdelay >4.2 seconds, Tmax >4 seconds; see Supplementary Methods) leads to misclassification and overestimation of the penumbra (79 mL and 121 mL, respectively). MCA, middle cerebral artery; MRI, magnetic resonance imaging; PCA, posterior cerebral artery; PET, positron emission tomography.
In addition, three merino sheep with pMCAO underwent simultaneous [15O]H2O PET/MRI. Perfusion[PET] and perfusion[MRI] image data 4.5 hours after pMCAO in a sheep are provided in Figure 2. Here, a considerable difference between the PET penumbra and the MR penumbra was observed, and the application of alternative PW MR thresholds did not lead to perfusion[MRI] maps similar to the perfusion[PET] maps.
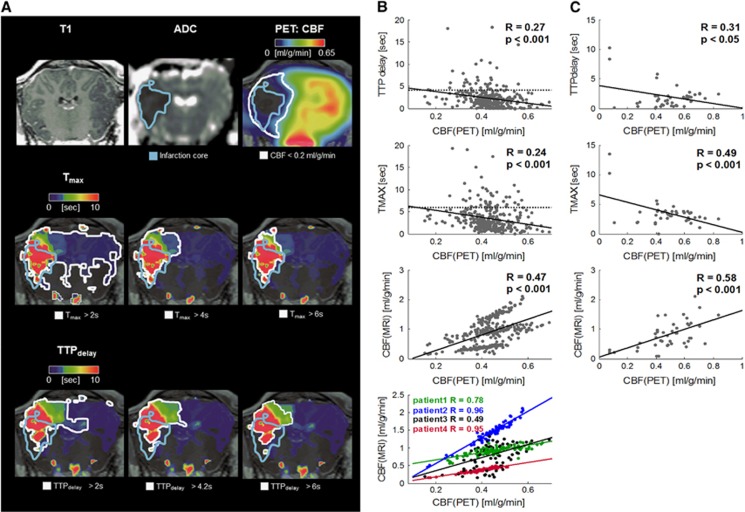
Figure 2.
(A) [15O]H2O PET/MRI 4.5 hours after middle cerebral artery occlusion in sheep. ADC imaging indicated a respective disturbance (blue-bordered area). Applying several thresholds for perfusion[MRI] could not reveal the same extent of the critically hypoperfused brain tissue as defined by perfusion[PET]. MR hypoperfusion either extended to the contralateral hemisphere or did not account for the penumbral part below the infarction core. According to perfusion[PET], penumbral volume was 9.8 mL (white-bordered area in upper row). Perfusion[MRI] yielded penumbral volumes of 27.2/6.3/5.4 mL (Tmax=2/4/6 seconds) or 14.2/5.0/4.5 mL (TTPdelay=2/4.2/6 seconds; white-bordered areas in lower rows). Note: susceptibility artefacts in perfusion[MRI], leading to slight spatial distortions especially close to the surface of the upper part of the sheep brain, were not corrected and may have affected correct ROI overlap and the resulting penumbral volumes. (B and C) Pooled regression analysis of regional values of perfusion[MRI] (TTPdelay (top) Tmax (second row) and CBF (third row)) and the reference method perfusion[PET]. The data were obtained in four human stroke patients (B) on the basis of 83 brain volumes of interest defined by an anatomic standard atlas, and in stroke sheep (C) on the basis of 15 manually defined brain volumes of interest. The dotted lines in the top and middle rows of B represent respective MR penumbra thresholds applied in this study. In humans and sheep, a moderate-to-strong linear relation between pooled TTPdelay/Tmax/CBF and the PET-derived gold standard CBF values was observed. Individual regression was performed for CBF in humans (bottom row) and yielded stronger linear relations between both measures. ADC, apparent diffusion coefficient; MRI, magnetic resonance imaging; PET, positron emission tomography; ROI, region of interest.
Supplementary Figures 2 and 3 provide an overview on the individual image data obtained in sheep and humans. In four human patients, arterial cannulation allowed to establish an arterial input function for full kinetic modeling of the dynamic PET data for absolute perfusion[PET] quantification. The CBF values in the unaffected cerebral hemispheres were 0.43±0.03 mL/g per minute for gray matter and 0.23±0.03 mL/g per minute for white matter. On a regional brain level, linear relation between pooled perfusion[PET] and perfusion[MRI] in stroke patients was weak to moderate for Tmax/TTPdelay/CBF (R=0.24/0.27/0.47, all P<0.001), however intra-subject correlations between perfusion[PET] and CBF from perfusion[MRI] were moderate to very strong (R=0.96, 0.95, 0.49, 0.78, all P<0.001). Moderate-to-strong linear relations were observed for pooled Tmax/TTPdelay and CBF in the animal data (R=0.49/0.31/0.58, P<0.05/P<0.001/P<0.01); see Figures 2B and 2C.
Discussion
We were able to demonstrate for the first time that simultaneous [15O]H2O PET/MRI is feasible in a large animal stroke model and in a clinical acute stroke setting without significantly delaying diagnostic MRI routines and without compromising standard MRI diagnosis. Compared with sequential studies, the comparability of perfusion[MRI] and perfusion[PET] is not only improved by simultaneous data acquisition, but also by the inherent spatial image data match. Gray matter and white matter CBF values as measured by the PET component of the integrated PET/MRI system were in good agreement with values reported in the literature.11, 12, 13 Furthermore, the diagnostic quality of the obtained MRI data was not affected by the concurrent PET data acquisition.
Notably, we observed limited correspondence between perfusion[PET] and perfusion[MRI] on an pooled basis with a higher variability of MRI-based blood flow estimates as compared with the gold standard PET-based CBF values in human patients and sheep. In pooled regression analyses of PWI CBF and PET CBF in prior sequential PET/MRI studies, R2 ranged from 0.9614 in pigs to 0.35–0.410 in human patients. Similarly, we observed a stronger correlation in sheep (R=0.58) than in humans (R=0.47), which can be most likely explained by the more homogeneous disease etiology in the animal model. Of note, intra-subject correlations between PWI CBF and PET CBF in the humans were much higher (mean R=0.8) but also showed substantial variability across patients, which has been observed before.10, 15 Moreover, we observed the same overestimation of PWI CBF as compared with PET CBF, which was more pronounced for higher flow values (see Figure 2C and Supplementary Figure 4) as in previous studies.10, 15 Likewise, there was a weak-to-moderate correlation between the pooled Tmax and PET CBF values in animals (R=0.49) and humans (R=0.24), which is also in line with previous studies, where the regression analyses of the pooled time-based variables and PET CBF values were considered weak.10, 16 Despite the low number of patients and animals examined in this pilot study and despite the inhomogeneous disease etiologies observed, quantitative results are therefore comparable with prior studies. It has to be mentioned, however, that unlike in prior studies,10, 15, 16 the quantity of data is not sufficient for the calibration of PWI parameters. Nonetheless, we observed a correct PW MR classification of non-critical hypoperfusion by the use of a Tmax thresholds of >6 seconds in two patients with MCA infarction.
The grouped quantitative differences observed in this study cannot anymore be related to a different disease state after an imaging delay ~1 hour between sequential PET and MRI perfusion measures.10, 14, 15, 16 These differences must solely be because of the different nature of the imaging modalities and are potentially related to the nonlinear relation between signal intensity and the concentration of the contrast agent in MRI and to the fact that gadolinium is an intravascular contrast agent, whereas [15O]H2O is freely diffusible. Both shortcomings can lead to an incorrect patient selection for late thrombolysis in PW MRI.8 Arterial spin labeling (ASL), a more recently developed MRI perfusion measurement technique as compared with PW MRI, better corresponds to [15O]H2O PET CBF and has recently been shown to correlate well with the PET CBF measure in healthy young humans.17 However, exact ASL measurements require prior knowledge about the degree of perfusion disturbance, which makes it hard to implement in the diseased brain. Future translational studies in larger cohorts will have to elucidate the exact relationship between perfusion[PET] and perfusion[MRI] and to possibly correct perfusion[MRI] by factors obtained from this head-to-head PET versus MRI comparison. In our view, simultaneous PET/MRI studies could possibly deliver a nonlinear correction function to derive a pseudo-CBF from time-based MR variables in the future.
As a limitation of this present study, the PET attenuation correction (see Supplementary Methods), as currently implemented in this hybrid PET/MR system, is still suboptimal and can lead to underestimations of the PET signal in the vicinity of cortical bone.18 Further, in the sheep data, spatial distortion of perfusion[MRI] and DWI was present but was not corrected in this pilot study. This may have affected region of interest overlap and quantitative results in the animal experiments. Moreover, we mainly examined mildly affected patients at relatively late time points after stroke onset at which penumbral tissue cannot necessarily be expected anymore. Moreover, the variety of disease etiologies, ranging from, e.g., lacunar infarctions over cerebral bleeding to typical MCA territory infarctions, poses another drawback. This may have contributed to the correlations found in this present study, which are not stronger between perfusion[PET] and perfusion[MRI] as compared with those reported in prior sequential studies with more homogenous patient collectives.10, 15 Further, it would be desirable to measure the oxygen extraction fraction (OEF) as the gold standard PET technique to delineate the ischemic penumbra by means of PET2 also in a simultaneous PET/MRI environment. Because of the fact that an MR-compatible multitracer administration equipment, which needs to be operated close to the patient to achieve OEF measurements, is currently not available, we focused our research on one—if not the (with regard to pathogenesis and therapy decisions) decisive aspect of ischemic stroke pathophysiology, i.e., measuring CBF. It will remain the task of future investigations with more advanced technical opportunities to relate the PW/DW mismatch MRI penumbra concept against simultaneous OEF CBF PET measurements. Those investigations will then, by providing the full picture, potentially offer the optimization of stroke MR imaging, for instance by the introduction of PW MR CBF correction factors.
Taken together, combined PET/MRI is a promising tool for validating MR-based stroke imaging concepts and provides excellent preclinical stroke imaging opportunities to improve the assessment of novel diagnostic and therapeutic concepts. As such, hybrid PET/MRI provides the opportunity to obtain synergistic PET data during standard stroke MRI, which then may guide individual treatment decisions, for instance within therapeutic trials, more precisely.
Acknowledgments
The authors thank Julian Klingbeil for patient recruitment. The authors are particularly grateful for the invaluable support of Torsten Böhm, Tanja Uhlisch, and Tanja Winkler in patient preparation and PET/MRI data acquisition. The cyclotron and radiopharmacy staff of the Department of Nuclear Medicine is greatly acknowledged for radiotracer production.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
The work was supported by the German Research Foundation that funded the PET/MRI system (grantcode: SA 669/9-1). The Max Planck Society co-funded the system.
HB and OS served as consultants and speakers for Bayer Healthcare and Piramal Imaging. OS served as primary investigator for Bayer Healthcare, Piramal Imaging, Siemens Healthcare, and GE Healthcare. HB and OS received speaker honoraria from Siemens Healthcare. K-TH served as speaker for Bayer Healthcare and Bracco.
Supplementary Material
References
- 1Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 2Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol 1996; 40: 216–226. [DOI] [PubMed] [Google Scholar]
- 3Merino JG, Warach S. Imaging of acute stroke. Nat Rev Neurol 2010; 6: 560–571. [DOI] [PubMed] [Google Scholar]
- 4Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol 2009; 8: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008; 7: 299–309. [DOI] [PubMed] [Google Scholar]
- 6Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Sobesky J. Refining the mismatch concept in acute stroke: lessons learned from PET and MRI. J Cereb Blood Flow Metab 2012; 32: 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Boltze J, Förschler A, Nitzsche B, Waldmin D, Hoffmann A, Boltze CM et al. Permanent middle cerebral artery occlusion in sheep: a novel large animal model of focal cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1951–1964. [DOI] [PubMed] [Google Scholar]
- 10Zaro-Weber O, Moeller-Hartmann W, Heiss W-D, Sobesky J. MRI perfusion maps in acute stroke validated with 15O-water positron emission tomography. Stroke J Cereb Circ 2010; 41: 443–449. [DOI] [PubMed] [Google Scholar]
- 11Huisman MC, van Golen LW, Hoetjes NJ, Greuter HN, Schober P, Ijzerman RG et al. Cerebral blood flow and glucose metabolism in healthy volunteers measured using a high-resolution PET scanner. EJNMMI Res 2012; 2: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Momjian S, Owler BK, Czosnyka Z, Czosnyka M, Pena A, Pickard JD. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain J Neurol 2004; 127: 965–972. [DOI] [PubMed] [Google Scholar]
- 13Frackowiak RS, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 1980; 4: 727–736. [DOI] [PubMed] [Google Scholar]
- 14Sakoh M, Røhl L, Gyldensted C, Gjedde A, Ostergaard L. Cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking after acute stroke in pigs: comparison with [(15)O]H(2)O positron emission tomography. Stroke J Cereb Circ 2000; 31: 1958–1964. [DOI] [PubMed] [Google Scholar]
- 15Takasawa M, Jones PS, Guadagno JV, Christensen S, Fryer TD, Harding S et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke J Cereb Circ 2008; 39: 870–877. [DOI] [PubMed] [Google Scholar]
- 16Zaro-Weber O, Moeller-Hartmann W, Heiss W-D, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke J Cereb Circ 2010; 41: 2817–2821. [DOI] [PubMed] [Google Scholar]
- 17Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER et al. Comparison of cerebral blood flow acquired by simultaneous [(15)O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab 2014; 34: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Izquierdo-Garcia D, Hansen AE, Förster S, Benoit D, Schachoff S, Fürst S et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med 2014; 55: 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.