Abstract
Emerging evidence has suggested that patients experiencing aneurysmal subarachnoid hemorrhage (aSAH) develop vascular dysregulation as a potential contributor to poor outcomes. Preclinical studies have implicated the novel microvascular constrictor, 20-hydroxyeicosatetraenoic acid (20-HETE) in aSAH pathogenesis, yet the translational relevance of 20-HETE in patients with aSAH is largely unknown. The goal of this research was to determine the relationship between 20-HETE cerebrospinal fluid (CSF) levels, gene variants in 20-HETE synthesis, and acute/long-term aSAH outcomes. In all, 363 adult patients (age 18 to 75) with aSAH were prospectively recruited from the University of Pittsburgh Medical Center neurovascular Intensive Care Unit. Patients were genotyped for polymorphic variants and cytochrome P450 (CYP)-eicosanoid CSF levels were measured over 14 days. Outcomes included delayed cerebral ischemia (DCI), clinical neurologic deterioration (CND), and modified Rankin Scores (MRS) at 3 and 12 months. Patients with CND and unfavorable 3-month MRS had 2.2- and 2.7-fold higher mean 20-HETE CSF levels, respectively. Patients in high/moderate 20-HETE trajectory groups (35.7%) were 2.5-, 2.1-, 3.1-, 3.3-, and 2.1-fold more likely to have unfavorable MRS at 3 months, unfavorable MRS at 12 months, mortality at 3 months, mortality at 12 months, and CND, respectively. These results showed that 20-HETE is associated with acute and long-term outcomes and suggest that 20-HETE may be a novel target in aSAH.
Keywords: arachidonic acid, cerebrospinal fluid, cerebrovascular disease, EET, HETE, subarachnoid hemorrhage
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) accounts for 5% of strokes, but 25% of stroke-related deaths due to its high mortality.1 aSAH survivors often experience functional and cognitive impairments. Complications after aSAH include clinical neurologic deterioration (CND) and delayed cerebral ischemia (DCI), which typically develop 3 to 14 days after the hemorrhage.1 Despite this time window for therapeutic intervention, strategies to improve aSAH outcomes have had limited success. Furthermore, variability in the assessment and definition of CND and DCI have limited their value as prognostic indicators of patient outcome, thereby, creating the need for clinically useful biomarkers of poor aSAH outcomes.
Eicosanoids, derived from the cytochrome P450 (CYP) pathway of arachidonic acid metabolism, regulate cerebrovascular tone and structure. Arachidonic acid is oxidized in multiple brain regions by CYP enzymes to form 20-hydroxyeicosatetraenoic acid (20-HETE) and epoxyeicosatrienoic acids (EETs).2 In humans, CYP4F2 and CYP4A11 are the primary enzymes that form 20-HETE. In the brain, 20-HETE promotes vasoconstriction, angiogenesis, inflammation, apoptosis, and platelet aggregation while EETs promote vasodilation and angiogenesis and inhibit inflammation, apoptosis, and platelet aggregation.2 Recent evidence suggests that 20-HETE mediates cerebral blood flow (CBF) regulation by pericytes and 20-HETE inhibition by nitric oxide (NO) facilitates prostaglandin E2-mediated dilation.3 These preclinical studies suggest that 20-HETE may be an important regulator of neurovascular unit function within the brain.
Recent evidence implicates 20-HETE in the pathophysiology of cerebral injury due to ischemic and hemorrhagic stroke. Inhibitors of 20-HETE synthesis improve CBF after aSAH and reduce infarct size after temporary focal ischemia while 20-HETE agonists reduce baseline CBF in rats.4, 5 Also, decreased NO has been suggested to play an important role in secondary insults after aSAH.2, 6 Since NO is known to directly inhibit 20-HETE formation, the aSAH-mediated decrease in NO would be expected to increase 20-HETE levels after insult. Currently, no studies have evaluated the role of 20-HETE in a large cohort of aSAH patients. Therefore, our primary hypothesis was that 20-HETE cerebrospinal fluid (CSF) levels over time will be associated with aSAH patient outcomes. We also hypothesized that single-nucleotide polymorphisms (SNPs) in 20-HETE biosynthesis genes will be associated with 20-HETE levels and aSAH patient outcomes.
Materials and methods
Design and Participants
Patients were prospectively recruited from the University of Pittsburgh Medical Center neurovascular intensive care unit. The study protocol was approved by the University of Pittsburgh Institutional Review Board and informed consent was obtained from the patient or their proxy. The study included 363 adult patients (age 18 to 75) with aSAH diagnosed via cerebral angiogram and classified as Fisher grade >1.7 Cerebrospinal fluid from 269 patients was available for CYP-eicosanoid analysis. All racial groups were included in the biomarker analysis. Genotype analysis was completed in 304 Caucasians to address population stratification.8 All patients received standard medical care.8
Biomarker Analysis
Cerebrospinal fluid samples were withdrawn from collection bags on external ventricular drains approximately every 12 hours for up to 14 days. We previously showed stability of 20-HETE assessment from sample collection bags.9 Samples were processed using solid phase extraction. Sample volumes of 2.0 to 3.0 mL were loaded onto 3cc Oasis HLB-SPE cartridges (Waters, Milford, MA, USA). Columns were washed and eluted with 3 mL of 5% MeOH and 100% MeOH, respectively. Samples were reconstituted in 50 μL of 80:20 MeOH:dH2O. Quantitation of CYP-eicosanoids was performed by UPLC-MS/MS with minor modifications.9 Long-term freezer stability and sample analysis reproducibility was <20% in reanalyzed samples (data not shown). Concentrations of 20-HETE, EETs, and dihydroxyeicosatrienoic acids were determined from the standard curve of the ratio of their peak areas to internal standard peak areas of 20-HETE-d6, 14,15-EET-d11, and 14,15-dihydroxyeicosatrienoic acid-d11, over a linear range of 0.014 to 8.88 ng/mL.
Genetic Analysis
Candidate genes associated with 20-HETE synthesis include CYP4A11 and CYP4F2. Tagging SNPs (tSNPs) were selected using the CEU population from Hapmap database (Release 27; www.hapmap.org) and criteria included r2>0.8 and minor allele frequency ⩾20% while functional SNPs (fSNPs) were defined as those previously reported to affect mRNA transcription, protein expression, or enzyme activity in vitro. Our genetic analysis excluded SNPs with variant genotype frequencies <1% in our aSAH population. Our genetic analysis included five tSNPs (CYP4A11-g.13414C>G(rs3890011), CYP4F2-g.4593T>C(rs3093089), CYP4F2-g.4211A>T(rs3093156), CYP4F2-g.8575T>C(rs3093168), CYP4F2-g.16162A>G(rs3093207)), six fSNPs (CYP4A11-g.4207A>G(rs9332978), CYP4A11-g.13661G>A(rs1126742), CYP4F2-g.7222002G>A(rs2189784), CYP4F2-g.5416G>C(rs3093100), CYP4F2-g.5373T>C(rs3093098), CYP4F2-g.5497T>C(rs3093105)), and one fSNP/tSNP (CYP4F2-g.14389C>T(rs2108622)).
Genotyping was performed using Taqman allele discrimination assay with ABI Prism 7000 Sequence Detection System (Applied Bioscience, Carlsbad, CA, USA) for rs3093105, sequencing with BigDye and ABI 3730xl (Applied Bioscience) for rs1126742, and iPLEX MassArray (Sequenom, San Diego, CA, USA) for all other SNPs as previously described.8 Consistency and integrity of genotyping data was checked by inclusion of duplicate Human Polymorphism Study Center controls on each plate for internal as well as plate-to-plate consistency, using genotype call rate criteria of >85%, comparing the observed and Hapmap Caucasian (CEU) frequencies, and performing checks for Hardy–Weinberg Equilibrium (HWE) consistency. Analyses of genetic data included both genotype groups (codominant) and the presence or absence of the variant allele (dominant) groups. SNPstats software was used for SNP association analysis.10
Outcomes Assessment
The primary outcomes in this study were the long-term outcomes determined by global functional recovery at 3 and 12 months using the Modified Rankin score (MRS) obtained during a face-to-face interview or phone call with the patient or their surrogate. The 3 and 12 months were assessed to represent periods of early and late recovery after hospitalization.11 The primary acute outcomes were the presence or absence of CND and/or DCI during the inpatient stay (up to 14 days).7 Changes in neurologic function were assessed twice daily during the acute stay and verified by chart review after discharge. Clinical neurologic deterioration was determined by a decline in neurologic exam for >1 hour evidenced by a documented global or focal deficit, increase (two or more points) in NIH Stroke Scale or decrease (two or more points) in Glasgow Coma Scale score that is not associated with concurrent medication administration that would inhibit a good exam. Delayed cerebral ischemia was defined as the presence of CND accompanied by evidence of impaired CBF (simultaneously or within 12 hours pre- or post-determination of CND). Patients are excluded from a DCI diagnosis if any other identifiable cause of CND is present including concurrent fever, seizure, re-bleed, increased intracranial pressure, hydrocephalus, or medication administration. Impaired CBF was determined using surrogate markers of blood flow including angiography (⩾25% cerebral vessel narrowing) and/or elevated transcranial Doppler flow velocities (⩾200 cm/s or Lindegaard ratio⩾3) or computed tomography/magnetic resonance perfusion scans (impaired perfusion or new cerebral infarction).
Statistical Analysis
Hunt and Hess (HH) scores were dichotomized into high (3 to 5) and low (1 to 2) groups and MRS was dichotomized into favorable (MRS 0 to 2) and unfavorable (MRS 3 to 6) groups. The CYP-eicosanoid concentrations below the LLQ (lower limit of quantitation) were reported as LLQ/2. Our original study effect size determination showed that the sample size of 304 has 80% power to detect a small effect size of 0.2 using a 1 degree of freedom with a significance level (alpha) of 0.0022. The mean and maximum CYP-eicosanoid levels for each patient were calculated and were used to compare the mean±standard error of the mean (s.e.m.) in the genotype and outcome groups using t-test (with Levene's test for equality of variances) or ANOVA. Homogeneous latent trajectory classes of 20-HETE CSF levels after aSAH were performed with the PROC TRAJ macro in SAS version 9.4 (SAS Institute Incorporated, Cary, NC, USA) as previously described.12 The time range of 2 to 11 days was selected to minimize the missing data and the time from hemorrhage was rounded up to the nearest day. Log transformation was applied to reduce sample variation/skewness and improve model fit. 20-Hydroxyeicosatetraenoic acid trajectory groups and genotype/allele frequencies were compared with acute outcomes, long-term outcomes, and covariate groups using chi-square analysis or t-test.
The relationship between genotype, CYP-eicosanoid concentrations/trajectory groups, and outcomes was determined using logistic regression after controlling for covariates such as age, sex, race (for comparison of CYP-eicosanoid levels and outcomes), and either Fisher grade (acute outcomes) or HH score (long-term outcomes) as previously described. The HH score was utilized for long-term outcomes because it has been shown to have the strongest predictive power for patient outcomes.13 The cumulative incidence of acute outcomes during the inpatient stay was compared in genotype/trajectory groups using Kaplan–Meier (log-rank) and Cox regression. Statistical significance was determined at *P values<0.05. Bonferroni's multiple comparison correction was used in analyses involving genetic and concentration data (corrected **P<0.0042 and **P<0.0167, respectively).
Results
Outcomes and Covariates
It was determined that 83% of patients had the first CSF sample obtained within the first 4 days after injury. Results comparing covariates with acute and long-term outcomes are shown in Table 1. The severity of injury measured by Fisher grade and HH score was associated with worse acute and long-term outcomes (P<0.001), respectively. Increased age was associated with the presence of CND (P=0.001) but not DCI and long-term outcomes. Race was associated with better outcomes at 3 months, but not at 12 months, in Caucasians compared with non-Caucasians (P=0.003). Sex was not associated with acute and long-term outcomes. The presence of CND and DCI was detected in 54.7% (n=192) and 36.2% (n=127), respectively.
Table 1. Outcomes in covariate groups.
| Covariate | Group | CND (+) | CND (−) | OR | P-value | DCI (+) | DCI (−) | OR | P-value |
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | (95% CI) | N (%) | N (%) | (95% CI) | ||||
| Race | Non-Caucasian | 21 (58.3) | 15 (41.7) | 1.16 (0.57–2.33) | 0.681 | 16 (44.4) | 20 (55.6) | 1.40 (0.70–2.82) | 0.344 |
| Caucasian | 162 (54.7) | 134 (45.3) | 108 (36.4) | 189 (63.6) | |||||
| Sex | Male | 53 (57) | 40 (43) | 1.11 (0.69–1.80) | 0.669 | 37 (39.8) | 56 (60.2) | 1.16 (0.71–1.90) | 0.549 |
| Female | 130 (54.4) | 109 (45.6) | 87 (36.3) | 153 (63.8) | |||||
| Age | Years | 54.4 (11.2) | 50.5 (10.2) | NA | 0.001a | 52.3 (10.9) | 53.3 (11.1) | NA | 0.434 |
| Fisher grade | 2 | 39 (36.4) | 68 (63.6) | NA | <0.001a | 26 (24.3) | 81 (75.7) | NA | <0.001a |
| 3 | 98 (58) | 71 (42) | 67 (39.6) | 102 (60.4) | |||||
| 4 | 46 (83.6) | 9 (16.4) | 31 (55.4) | 25 (44.6) |
| Covariate | Group | MRS3 (3–6) | MRS3 (0–2) | OR | P-value | MR12 (3–6) | MRS12 (0–2) | OR | P-value |
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | (95% CI) | N (%) | N (%) | (95% CI) | ||||
| Race | Non-Caucasian | 14 (58.3) | 10 (41.7) | 3.51 (1.48–8.30) | 0.003a | 8 (40) | 12 (60) | 1.83 (0.71–4.70) | 0.202 |
| Caucasian | 65 (28.5) | 163 (71.5) | 60 (26.7) | 165 (73.3) | |||||
| Sex | Male | 24 (33.8) | 47 (66.2) | 1.17 (0.65–2.10) | 0.599 | 16 (24.2) | 50 (75.8) | 0.78 (0.41–1.50) | 0.456 |
| Female | 55 (30.4) | 126 (69.6) | 52 (29.1) | 127 (70.9) | |||||
| Age | Years | 53.0 (10.9) | 55.0 (10.9) | NA | 0.175 | 53.9 (11.1) | 55.5 (11.6) | NA | 0.326 |
| Hunt and Hess | Good (0–2) | 17 (15.3) | 94 (84.7) | 4.34 (2.35–8.02) | <0.001a | 13 (11.9) | 96 (88.1) | 5.01 (2.56–9.83) | <0.001a |
| Poor (3–5) | 62 (44) | 79 (56) | 55 (40.4) | 81 (59.6) |
Abbreviations: CI, confidence interval; CND, clinical neurologic deterioration; DCI, delayed cerebral ischemia; MRS, Modified Rankin Scale score at 3 and 12 months: unfavorable (3 to 6), favorable (0 to 2); OR, odds ratio. This demographic table has been published in our recent paper by Donnelly et al.24
Statistical significance established at P<0.05.
Cytochrome P450-Eicosanoid Analysis
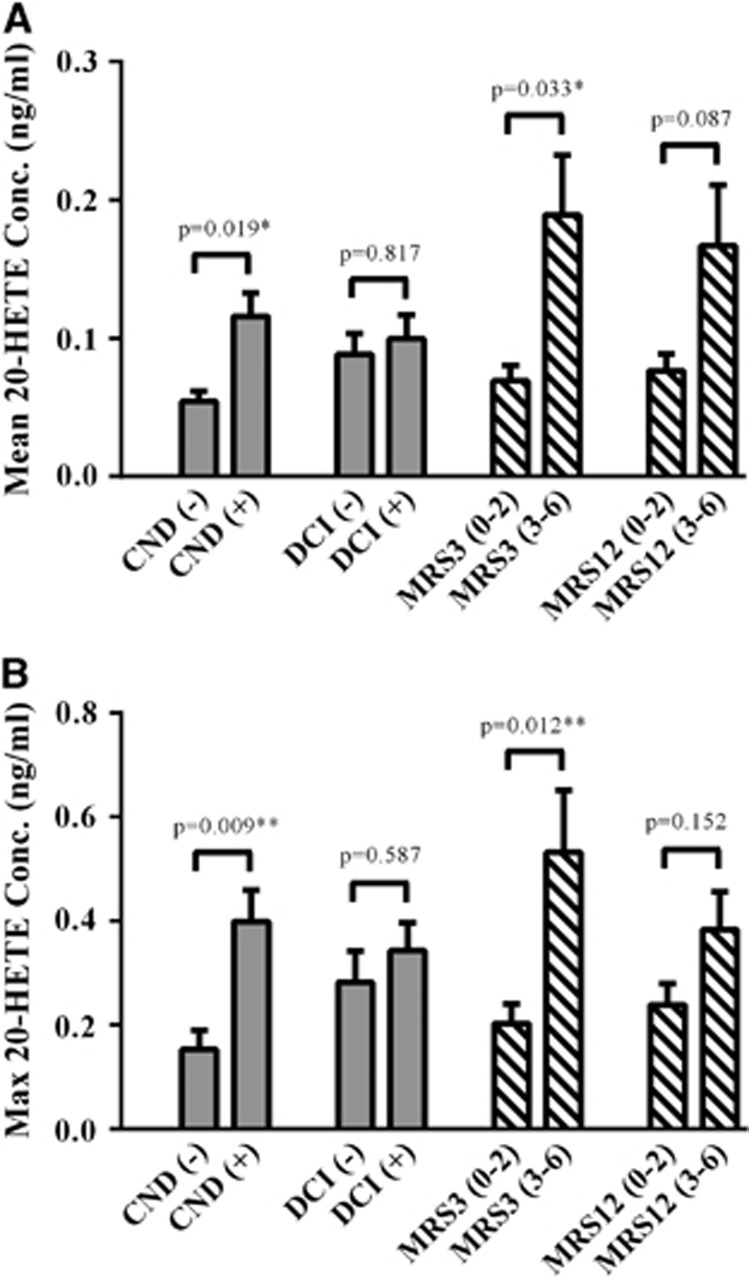
Patients with unfavorable 3-month outcomes had ~2.7- and 2.6-fold higher mean and maximum 20-HETE levels when compared with those with favorable outcomes (P=0.009 and P=0.010; Figure 1), respectively. Patients with unfavorable 12-month outcomes showed a trend for a 2.2- and 1.6-fold increase in mean and maximum 20-HETE levels when compared to those with favorable outcomes (P=0.052 and P=0.063; Figure 1), respectively. 20-Hydroxyeicosatetraenoic acid was higher in patients classified as Fisher grade 4 when compared with Fisher grade 2/3 (Supplementary Figure 1). Patients with CND had ~2.2- and 2.6-fold higher mean and maximum 20-HETE levels, respectively, when compared to those without CND (P=0.001; Figure 1, Supplementary Table 1). Detectable 20-HETE, EET, and DHET levels were found in 71.6%, 13.1%, and 97.9% of samples (n=3,151) and 98.5%, 64.3%, and 98.9% of patients (n=269), respectively.
Figure 1.
20-Hydroxyeicosatetraenoic acid (20-HETE) levels in outcome groups. Mean (A) and maximum (B) 20-HETE levels in cerebrospinal fluid (CSF) from patients with aneurysmal subarachnoid hemorrhage (aSAH) are compared in outcome groups. Acute outcomes (solid bars) included the presence or absence of clinical neurologic deterioration (CND) and/or delayed cerebral ischemia (DCI) up to 14 days after the hemorrhage. Long-term outcomes (stripped bars) were determined by global functional recovery at 3 and 12 months using the Modified Rankin Scale (MRS) and were dichotomized into favorable (MRS 0 to 2) and unfavorable (MRS 3 to 6) groups. The mean and maximum 20-HETE CSF levels for each patient (n=269) were calculated and were used to compare the mean±standard error of the mean of the cytochrome P450 (CYP)-eicosanoid levels in outcome groups using t-test (with Levene's test for equality of variances). Depicted P values are adjusted for age, sex, race, and either Fisher grade (for acute outcomes) or Hunt and Hess score (for long-term outcomes). Statistical significance established at *P<0.05 and **P<0.0167 (correction for multiple comparisons).
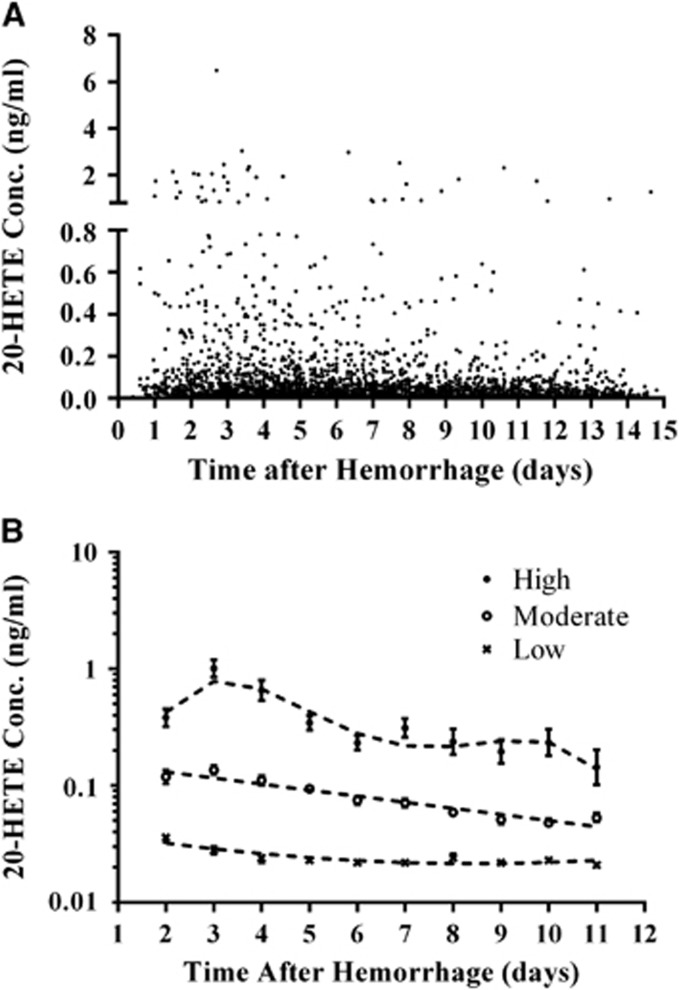
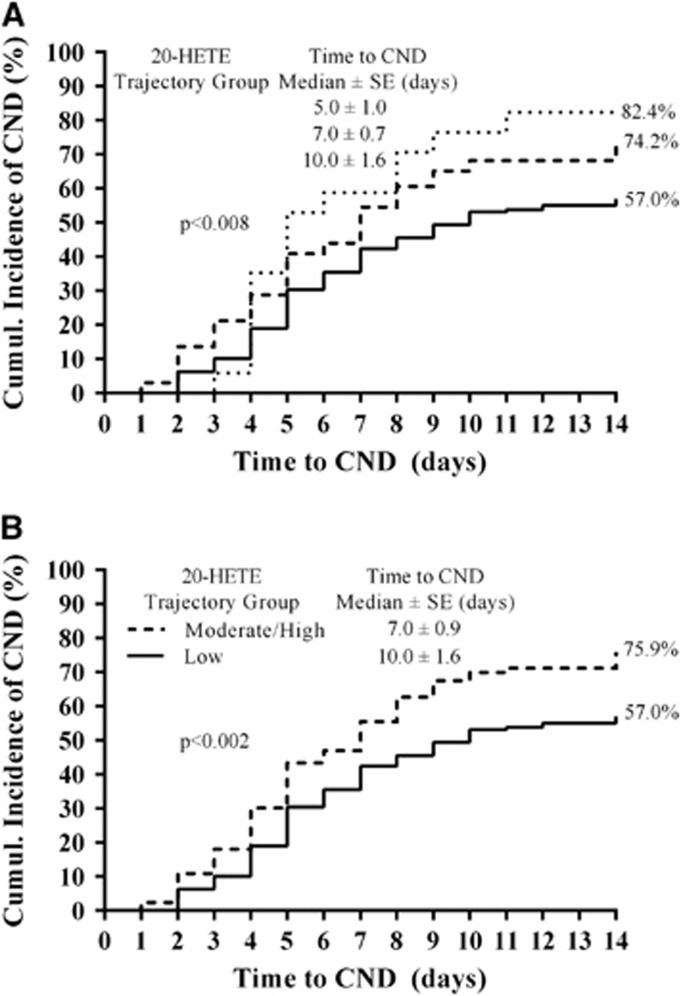
The trajectory model evaluating 20-HETE CSF levels identified three groups of patients with concentration profiles categorized as low, moderate, and high (P<0.001; Figure 2). 20-Hydroxyeicosatetraenoic acid trajectory groups showed an association with 3-month MRS, 12-month MRS, 3-month mortality, 12-month mortality, and CND (P=0.001, P=0.046, P=0.002, P=0.001, and P=0.012, respectively; Table 2). Patients in moderate/high 20-HETE trajectory groups were 2.5-, 2.1-, 3.1-, 3.3-, and 2.1-fold more likely to have unfavorable MRS at 3 and 12 months, mortality at 3 and 12 months, and CND (P=0.002, P=0.014, P=0.001, P=0.001, and P=0.004, respectively; Table 2) and had lower median time to develop CND (P=0.002; Figure 3). As depicted in Table 2, similar significant relationships between 20-HETE trajectory groups and outcomes were observed in the multivariate analysis, which included correction for age, sex, race, and either Fisher grade (acute outcomes) or HH score (long-term outcomes). No association was observed with 20-HETE trajectory groups and the presence of infarct in a subset of 163 patients (data not shown).
Figure 2.
20-Hydroxyeicosatetraenoic acid (20-HETE) temporal concentration profiles and trajectory patterns. (A) Raw population values of 20-HETE cerebrospinal fluid (CSF) concentrations from 269 patients up to 14 days after hemorrhage are shown. (B) 20-HETE CSF concentration versus time from hemorrhage (days) in high (filled circles, n=21 (7.8%)), moderate (open circles, n=75 (27.9%)), and low (X, n=173 (64.3%)) concentration groups as identified by trajectory analysis are shown. Concentration data are presented as geometric mean with 95% confidence interval (CI).
Table 2. Outcomes in 20-HETE Trajectory Groups.
| 20-HETE | CND (−) | CND (+) | Unadjusted |
Adjusteda |
|
|---|---|---|---|---|---|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.047* | ||||
| Low | 68 (43) | 90 (57) | 0.012** | Reference | — |
| Moderate | 17 (25.8) | 49 (74.2) | 1.98 (1.02–3.82) | 0.043* | |
| High | 3 (17.6) | 14 (82.4) | 3.07 (0.82–11.47) | 0.096 | |
| Moderate/High | 20 (24.1) | 63 (75.9) | 0.004** | 2.14 (1.16–3.96) | 0.015** |
| 20-HETE | DCI (−) | DCI (+) | Unadjusted |
Adjusteda |
|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.342 | ||||
| Low | 97 (61) | 62 (39) | 0.283 | Reference | — |
| Moderate | 34 (51.5) | 32 (48.5) | 1.46 (0.81–2.66) | 0.208 | |
| High | 8 (47.1) | 9 (52.9) | 1.66 (0.59–4.66) | 0.334 | |
| Moderate/High | 42 (50.6) | 41 (49.4) | 0.120 | 1.50 (0·87–2.61) | 0.148 |
| 20-HETE | MRS3 (0–2) | MRS3 (3–6) | Unadjusted |
Adjusteda |
|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.011** | ||||
| Low | 85 (71.4) | 34 (28.6) | 0.001** | Reference | — |
| Moderate | 25 (55.6) | 20 (44.4) | 1.88 (0.88–4.03) | 0.105 | |
| High | 5 (27.8) | 13 (72.2) | 5.33 (1.65–17.22) | 0.005** | |
| Moderate/High | 30 (47.6) | 33 (52.4) | 0.002** | 2.49 (1.16–4.93) | 0.009** |
| 20-HETE | MRS12 (0–2) | MRS12 (3–6) | Unadjusted |
Adjusteda |
|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.111 | ||||
| Low | 83 (72.2) | 32 (27.8) | 0.046* | Reference | — |
| Moderate | 26 (55.3) | 21 (44.7) | 2.08 (0.99–4.38) | 0.053 | |
| High | 8 (50) | 8 (50) | 2.00 (0.65–6.15) | 0.224 | |
| Moderate/High | 34 (54) | 29 (46) | 0.014** | 2.06 (1.05–4.06) | 0.036* |
| 20-HETE | Mortality3 (Survived) | Mortality3 (Died) | Unadjusted |
Adjusteda |
|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.016** | ||||
| Low | 102 (85.7) | 17 (14.3) | 0.002** | Reference | — |
| Moderate | 30 (66.7) | 15 (33.3) | 2.78 (1.16–6.67) | 0.022* | |
| High | 10 (55.6) | 8 (44.4) | 4.17 (1.28–13.61) | 0.018* | |
| Moderate/High | 40 (63.5) | 23 (36.5) | 0.001** | 3.13 (1.41–6.92) | 0.005** |
| 20-HETE | Mortality12 (Survived) | Mortality12 (Died) | Unadjusted |
Adjusteda |
|
| Trajectory | N (%) | N (%) | P-value | OR (95% CI) | P-value |
| Overall | 0.010** | ||||
| Low | 96 (83.5) | 19 (16.5) | 0.001** | Reference | — |
| Moderate | 30 (63.8) | 17 (36.2) | 3.00 (1.27–7.09) | 0.012** | |
| High | 8 (50.0) | 8 (50.0) | 4.29 (1.27–14.56) | 0.019* | |
| Moderate/High | 38 (60.3) | 25 (39.7) | 0.001** | 3.31 (1.51–7.26) | 0.003** |
Abbreviations: DCI, delayed cerebral ischemia; CND, clinical neurological deterioration; MRS, Modified Rankin Scale score at 3 and 12 months: Unfavorable (3–6), Favorable (0–2); Mortality3/12, Mortality at 3 and 12 months; 20-HETE, 20-hydroxyeicosatetraenoic acid.
Multivariate analysis included correction for age, sex, race, and either Fisher grade (for acute outcomes) or Hunt & Hess score (for long-term outcomes); *Statistical significance established at *P<0.05 or **P<0.0167 (for multiple comparison correction).
Figure 3.
Cumulative incidence of acute outcomes in trajectory groups. The cumulative incidence of clinical neurologic deterioration (CND) in 153 patients (A) and delayed cerebral ischemia (DCI) in 103 patients (B) was compared in trajectory groups using Kaplan–Meier log-rank analysis. For the analysis, the time to develop CND and DCI was rounded up to the nearest day and the trajectory groups were defined in Figure 2. Cox regression was used to control for age, sex, race, and Fisher grade or Hunt and Hess (HH) score. 20-HETE, 20-hydroxyeicosatetraenoic acid.
Genetic Analysis
Multiple CYP4F2 gene variants were not in HWE including g.5416G>C (P<0.001), g.5373 T>C (P=0.023), g.14389C>T(*3) (P<0.001), g.7222002G>A (P=0.001), g.4593 T>C (P=0.013), and g.16162 A>G (P=0.002; Supplementary Table 2). Also, our aSAH population showed different allele frequencies from those reported in the Hapmap CEU population for CYP4F2 gene variants including g.14389C>T(*3), g.7222002G>A, g.16162 A>G (P<0.001) and a trend for a difference for g.5416G>C (P=0.074) and g.5373 T>C (P=0.083).
CYP4A11-g.4207G allele carriers had 43% lower mean 20-HETE levels when compared with CYP4A11-g.4207 A/A carriers (n=185; Supplementary Table 3). 20-Hydroxyeicosatetraenoic acid levels were not associated with genotype in the codominant model.
In the multivariate analysis, patients with CYP4F2-g.4593C/C genotype were 2.3-fold less likely to develop CND compared with CYP4F2-g.4593 T/T carriers (P=0.047; Table 3). CYP4A11-g.13414C allele carriers were ~2-fold more likely to have unfavorable 3- and 12-month MRS (P=0.019 and P=0.042), respectively. Also, patients with the variant CYP4F2-g.4211 T allele or CYP4F2-g.8575 T/T genotype were ~2.4-fold less likely and ~3.3-fold more likely to have unfavorable outcomes at 3 months (P=0.016 and P=0.017), respectively.
Table 3. Outcomes in Genetic Groups.
| Gene | Genotype | CND (+) | CND (−) | Unadjusted |
Adjusteda |
|
|---|---|---|---|---|---|---|
| SNP (rs#) | N (%) | N (%) | P-value | OR (95% CI) | P-value | |
| Overall | 0.138 | |||||
| CYP4F2 | TT | 77 (60.6) | 50 (39.4) | 0.223 | Reference | — |
| g.4593T>C | TC | 59 (56.7) | 45 (43.3) | 0.82 (0.46–1.44) | 0.482 | |
| (rs3093089)b | CC | 16 (44.4) | 20 (55.6) | 0.44 (0.20–0.99) | 0.047* | |
| C-carriers | 75 (53.6) | 65 (46.4) | 0.245 | 0.70 (0.41–1.17) | 0.175 | |
| Gene | Genotype | MRS3 (3–6) | MRS3 (0–2) | Unadjusted |
Adjusteda |
|
| SNP (rs#) | N (%) | N (%) | P-value | OR (95% CI) | P-value | |
| Overall | 0.025* | |||||
| CYP4A11 | GG | 31 (23) | 106 (77) | 0.004* | Reference | — |
| g.13414G>C | GC | 31 (42) | 43 (58) | 2.43 (1.28–4.62) | 0.007* | |
| (rs3890011)b | CC | 0 (0) | 6 (100) | — | 0.999 | |
| C-carriers | 31 (39) | 49 (61) | 0.011* | 2.12 (1.13–3.99) | 0.019* | |
| Overall | 0.055 | |||||
| CYP4F2 | AA | 22 (40) | 33 (60) | 0.08 | Reference | — |
| g.4211A>T | TA | 25 (23) | 82 (77) | 0.42 (0.2–0.89) | 0.023* | |
| (rs3093156)b | TT | 15 (27) | 41 (73) | 0.44 (0.19–1.03) | 0.059 | |
| T-carriers | 40 (25) | 123 (75) | 0.028* | 0.43 (0.21–0.85) | 0.016* | |
| Overall | 0.055 | |||||
| CYP4F2 | CC | 23 (25) | 69 (75) | 0.088 | Reference | — |
| g.8575C>T | CT | 26 (26) | 74 (74) | 1.21 (0.61–2.41) | 0.579 | |
| (rs3093168)b | TT | 12 (46) | 14 (54) | 3.30 (1.23–8.83) | 0.017* | |
| T-carriers | 38 (30) | 88 (70) | 0.402 | 1.51 (0.8–2.88) | 0.206 | |
| Gene | Genotype | MRS12 (3–6) | MRS12 (0–2) | Unadjusted |
Adjusteda |
|
| SNP (rs#) | N (%) | N (%) | P-value | OR (95% CI) | P-value | |
| Overall | 0.067 | |||||
| CYP4A11 | GG | 29 (21) | 108 (79) | 0.007* | Reference | — |
| g.13414G>C | GC | 27 (40) | 41 (61) | 2.22 (1.13–4.34) | 0.020* | |
| (rs3890011)b | CC | 0 (0) | 5 (100) | — | 0.999 | |
| C-carriers | 27 (37) | 46 (63) | 0.014* | 1.99 (1.03–3.86) | 0.042* | |
Multivariate analysis included correction for age, sex, race, and either Fisher grade (for acute outcomes) or Hunt & Hess score (for long-term outcomes).
Tagging SNP, DCI, delayed cerebral ischemia; CND, clinical neurological deterioration; MRS, Modified Rankin Scale score at 3 and 12 months: Unfavorable (3–6), Favorable (0–2); *Statistical significance established at *P<0.05 or **P<0.0042 (for multiple comparison correction).
Discussion
This clinical study determined the relationship between SNPs in 20-HETE synthesis genes, 20-HETE CSF levels, and acute/long-term outcomes in a large aSAH cohort. We report that higher 20-HETE CSF levels and trajectory patterns were associated with unfavorable acute and long-term outcomes before and after controlling for age, sex, race, and Fisher grade or HH score. Also, SNPs in 20-HETE synthesis genes were associated with 20-HETE levels and outcomes and showed different frequencies compared with control populations in Hapmap. Collectively, these results support the translational importance of 20-HETE in pathophysiology of aSAH.
CYP-eicosanoids concentrations at levels known to affect cerebrovascular function.6 20-Hydroxyeicosatetraenoic acid constricts isolated cat and rat cerebral arteries at 0.1 nmol/L (0.032 ng/mL) and 10 nmol/L (3.2 ng/mL) concentrations, respectively.4, 14 A dog model of aSAH-induced DCI was associated with 20-HETE CSF levels of ~1.2 nmol/L.15 Our clinical study reports maximum 20-HETE CSF concentration of ~1.0 nmol/L. Moreover, it is expected that 20-HETE levels in the cerebrovasculature are higher than the CSF,2 suggesting the presence of physiologically relevant levels.
We observed that increased 20-HETE CSF levels were associated with unfavorable long-term outcomes and mortality, but not with infarct. The effects of 20-HETE on long-term outcomes may be mediated through inflammation, vascular remodeling, or other mechanisms.16 We and others have observed relationships between Fisher grade, HH score, and increasing age with acute and/or long-term outcomes.17, 18 Our multivariate analysis showed that 20-HETE CSF levels/trajectory groups remained strongly associated with acute and long-term outcomes and mortality after controlling for Fisher grade and HH score, respectively. These studies suggest that 20-HETE may serve as a potential biomarker of acute and long-term outcomes and mortality in patients with aSAH.
Elevated 20-HETE CSF levels and temporal concentration profiles were associated with CND, but not with DCI. Patients with high/moderate 20-HETE trajectory patterns developed CND earlier and to a greater extent than those in the low group, which remained significant after controlling for age, sex, race, and injury severity. Maximum 20-HETE levels occurred before the typical onset of DCI and CND19 in first few days after aSAH. Roman et al15 reported that nine aSAH patients with angiographic vasospasm and neurologic deficits had elevated 20-HETE CSF levels compared with controls.
Previously, our laboratory reported that 20-HETE is elevated early in the CSF and is associated with DCI.7, 20 Siler et al21 recently showed that both 20-HETE and 14,15-EET levels are elevated in the CSF after aSAH and was associated with DCI in these patients. This study also showed that these metabolites are elevated over age- and sex-matched control CSF samples. Previously, our laboratory reported that patients with the CYP2C8*4 variant allele have lower EET and DHET concentrations in the CSF and a greater likelihood of CND and DCI during their hospital stay.22 The results of our findings in this manuscript expand on these observations as the first evaluation of a large cohort of aSAH patients thereby allowing for multivariate analysis. Our observation that 20-HETE is associated with poor outcomes upon correction for age, sex, and injury severity further validates the role of 20-HETE in aSAH pathogenesis. Also, growing evidence suggests that cerebral vasospasm in large arteries has a limited role in CND after aSAH.23 20-HETE is a primary regulator of microvasculature tone2 and therefore may affect CND to a greater extent than large vessel vasospasm.24 In addition, our current study reports that 20-HETE trajectory groups were significantly associated with 3- and 12-month outcomes after multivariate logistic regression and that the relationship at 3 months survived correction for multiple testing. These results imply that CSF 20-HETE levels may serve as a putative biomarker for poor outcomes in aSAH patients.
The CYP4F2 putative genetic markers for aSAH identified in this study were not associated with altered 20-HETE CSF levels, but previous in vitro studies associated the CYP4F2 variants g.14389C>T [*3], g.5373T>C, and g.5416G>C with reduced enzymatic activity, reduced transcriptional activity, and increased transcriptional activity, respectively.25, 26 Interpretation of our results is complicated by the discrepant in vitro/in vivo findings on the effect of CYP4F3*3 on 20-HETE levels, which despite decreased activity in vitro have shown increased 20-HETE concentrations in the urine of patients.16, 27 With respect to CYP4A11, our genetic analysis showed that patients with the variant g.4207G-allele located in the promoter region of CYP4A11 showed 43% lower mean 20-HETE CSF levels. In silico models predicted that this SNP will lower CYP4A11 transcriptional activity.28 When the CYP4A11 g.4207G/G(c.-825G/G) mutant construct was expressed in vitro, CYP4A11 transcriptional activity was reduced by ~30% compared with the WT construct.29 Based on these studies, we expect that patients with the variant CYP4A11 g.4207G-allele would have decreased 20-HETE CSF levels, which is consistent with our results.
Multiple tSNPs in CYP4F2 and CYP4A11 were associated with acute and long-term outcomes. Patients with the variant CYP4F2g.4593C/C genotype were 2.3-fold less likely to develop CND compared to those with g.4593 T/T genotype in the multivariate analysis. In a study of Chinese hypertensive patients, no association was observed between the CYP4F2g.4593 T>C polymorphism and both urinary 20-HETE levels and hypertension.30 Thus, factors other than 20-HETE may mediate the lower risk to develop CND in patients with the variant CYP4F2g.4593C/C genotype. We report that CYP4A11g.13414C allele carriers were ~2-fold more likely to develop unfavorable outcomes at 3 and 12 months. Also, patients with the variant CYP4F2g.4211 T allele or CYP4F2g.8575 T/T genotype were ~2.4-fold less likely and ~3.3-fold more likely to have unfavorable outcomes at 3 months, respectively. These tSNPs may be in linkage disequilibrium with fSNPs or contribute to haplotype constructs and thus may serve as a surrogate for clinical phenotypes.
Our genetic integrity tests show that multiple gene variants on CYP4F2 violated HWE conditions possibly due to enrichment in the aSAH population. Many of the SNPs that violated HWE conditions also showed genotype frequencies in our aSAH population that were different from those in the Hapmap CEU population, suggesting putative genetic markers for the formation and rupture of intracranial aneurysms. Previous studies report a strong link between hypertension and vascular remodeling with the incidence of aSAH.31
Higher 20-HETE levels were observed in patients with worse Fisher grades, which is consistent with the possibility that neutrophils, macrophages, platelets, and activated microglia are sources of 20-HETE production32, 33, 34 in addition to brain production. The primary source of 20-HETE found in the CSF of aSAH patients is important and is currently unknown. It is important to note that the size of the bleed as noted by the Fisher grade did not entirely account for the association between 20-HETE in the CSF and CND and/or MRS. The relationship between 20-HETE in the CSF and CND and/or MRS was still significant when completing multivariate regression and including Fisher grade and/or HH as a covariate. If the 20-HETE association was largely/entirely explained by the blood load, then significance would have been lost with the inclusion of Fisher grade in the multivariate regression. So, while 20-HETE levels are associated with increased blood load, the relationship with CND and/or MRS is not entirely explained by the Fisher grade, thereby suggesting additional unknown mechanisms and/or sources of 20-HETE production for future study. Such mechanisms for future research include the evaluation of central 20-HETE production and the potential protection afforded by microvascular dysregulation.
We also note some limitations of our results. First, our biomarker assessment was performed only in patients with ventricular drains, which commonly have more significant hemorrhage than those without drains. 20-Hydroxyeicosatetraenoic acid CSF levels represent time-averaged values during the 12-hour collection period. Also, our findings implicate regions of association and not necessarily causative genetic variation. Finally, we determined that we had genotype designation accuracy across platforms, therefore it is unlikely that the differences that we observed with the Hapmap population are due to misspecification and are likely enrichment in the aSAH population. Future validation cohort studies will be needed to confirm these findings.
In summary, 20-HETE has been shown to have an important role in the regulation of cerebrovascular tone and vessel remodeling in vitro and significantly affect CBF and cerebral ischemic injury in vivo. Our study, involving one of the largest aSAH cohorts to date, implicates 20-HETE and CYP4F2 gene variants in the risk for aSAH and subsequent acute and long-term outcomes. These results are important to help elucidate the mechanisms involved in the pathophysiology of aSAH and possibly identify patients at high risk for unfavorable outcomes; thereby, lending insight into future interventional strategies. Furthermore, these results implicate an important shift toward microvascular and/or inflammatory fatty acid regulators as important factors in the development of neurologic deterioration and poor outcomes after aSAH. Alternatively, there are other reasons for neurologic deterioration, which include neurologic stress due to seizures, hydrocephalus, increased Intracranial pressure, or rebleed that could result in an increase in 20-HETE. Future studies are needed to determine the specific underlying mechanisms of this association to elucidate the role of 20-HETE in the pathogenesis of aSAH.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author Contributions
MKD was responsible for completing the biomarker analysis, completion of the analysis of all biomarker and genetic data with respect to outcomes, and preparation of the manuscript.
EAC was responsible for subject recruitment, collection of acute clinical outcomes data, clinical data interpretation, as well as review and partial preparation of the manuscript.
YPC was responsible for acquisition of the genetic data as well as provision of her expertise in the interpretation of the genetic data, as well as review of the prepared manuscript
. JRB was responsible for the interpretation of the clinical data with respect to acute patient outcomes as well as review of the prepared manuscript.
DR was responsible for the statistical approaches used, application, and interpretation of the final data as well as the review of the prepared manuscript
. AD was responsible for the neurosurgical expertise in the final data interpretation as well as significant contribution and revision of the final manuscript.
PMK was responsible for aid in the original study design for biomarker assessment as well as significant revision of the final prepared manuscript.
PRS is the multiple principal investigator on the parent grant that supported this research. She was also instrumental in the original experimental design and overall data interpretation. She also provided extensive expertise in patient outcome assessment, as well as final manuscript preparation.
SMP is the multiple principal investigator on the parent grant proposal that supported this research. He was involved in all aspects of this study from inception through final data analysis providing specific expertise in biomarker assessment. He also was highly involved in final manuscript preparation and revision.
This study was supported by National Institute of Nursing Research R01NR0044339-05 and F31NR012608-01 and National Center of Research Resources S10RR023461.
Supplementary Material
References
- 1Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals. Stroke 2012; 43: 1711–1737. [DOI] [PubMed] [Google Scholar]
- 2Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 2002; 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 3Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol 2004; 486: 297–306. [DOI] [PubMed] [Google Scholar]
- 5Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M et al. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N'-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 2005; 314: 77–85. [DOI] [PubMed] [Google Scholar]
- 6Rodriguez-Rodriguez A, Egea-Guerrero JJ, Ruiz de Azua-Lopez Z, Murillo-Cabezas F. Biomarkers of vasospasm development and outcome in aneurysmal subarachnoid hemorrhage. J Neurol Sci 2014; 341: 119–127. [DOI] [PubMed] [Google Scholar]
- 7Crago EA, Thampatty BP, Sherwood PR, Kuo CW, Bender C, Balzer J et al. Cerebrospinal fluid 20-HETE is associated with delayed cerebral ischemia and poor outcomes after aneurysmal subarachnoid hemorrhage. Stroke 2011; 42: 1872–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Gallek M, Alexander S, Crago E, Sherwood P, Horowitz M, Poloyac S et al. Endothelin-1 and endothelin receptor gene variants and their association with negative outcomes following aneurysmal subarachnoid hemorrhage. Biol Res Nurs 2013; 15: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Miller TM, Donnelly MK, Crago EA, Roman DM, Sherwood PR, Horowitz MB et al. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 3991–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22: 1928–1929. [DOI] [PubMed] [Google Scholar]
- 11Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999; 30: 1538–1541. [DOI] [PubMed] [Google Scholar]
- 12Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Meth 2001; 6: 18–34. [DOI] [PubMed] [Google Scholar]
- 13Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care 2005; 2: 110–118. [DOI] [PubMed] [Google Scholar]
- 14Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB et al. Formation and action of a P-450 4 A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol 1994; 266: H2098–H2107. [DOI] [PubMed] [Google Scholar]
- 15Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res 2006; 28: 738–749. [DOI] [PubMed] [Google Scholar]
- 16Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Exp Rev Mol Med 2011; 13: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Starke RM, Komotar RJ, Otten ML, Schmidt JM, Fernandez LD, Rincon F et al. Predicting long-term outcome in poor grade aneurysmal subarachnoid haemorrhage patients utilising the Glasgow Coma Scale. J Clin Neurosci 2009; 16: 26–31. [DOI] [PubMed] [Google Scholar]
- 18de Rooij NK, Greving JP, Rinkel GJ, Frijns CJ. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke 2013; 44: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 19Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–2395. [DOI] [PubMed] [Google Scholar]
- 20Poloyac SM, Reynolds RB, Yonas H, Kerr ME. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J Neurosci Methods 2005; 144: 257–263. [DOI] [PubMed] [Google Scholar]
- 21Siler DA, Martini RP, Ward JP, Nelson JW, Borkar RN, Zuloaga KL et al. Protective role of P450 epoxyeicosanoids in subarachnoid hemorrhage. Neurocrit Care 2015; 22: 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Donnelly MK, Conley YP, Crago EA, Ren D, Sherwood PR, Balzer JR et al. Genetic markers in the EET metabolic pathway are associated with outcomes in patients with aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2014; 35: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Brit J Anaesth 2012; 109: 315–329. [DOI] [PubMed] [Google Scholar]
- 24Naraoka M, Matsuda N, Shimamura N, Asano K, Ohkuma H. The role of arterioles and the microcirculation in the development of vasospasm after aneurysmal SAH. Biomed Res Int 2014; 2014: 253746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 2007; 30: 74–81. [DOI] [PubMed] [Google Scholar]
- 26Liu H, Zhao Y, Nie D, Shi J, Fu L, Li Y et al. Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J Am Soc Nephrol 2008; 19: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 2008; 51: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 28Lino Cardenas CL, Renault N, Farce A, Cauffiez C, Allorge D, Lo-Guidice JM et al. Genetic polymorphism of CYP4A11 and CYP4A22 genes and in silico insights from comparative 3D modelling in a French population. Gene 2011; 487: 10–20. [DOI] [PubMed] [Google Scholar]
- 29Sugimoto K, Akasaka H, Katsuya T, Node K, Fujisawa T, Shimaoka I et al. A polymorphism regulates CYP4A11 transcriptional activity and is associated with hypertension in a Japanese population. Hypertension 2008; 52: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 30Hu BC, Li Y, Li FH, Zhang Y, Sheng CS, Fan HQ et al. Peripheral and central augmentation indexes in relation to the CYP4F2 polymorphisms in Chinese. J Hypertens 2011; 29: 501–508. [DOI] [PubMed] [Google Scholar]
- 31Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36: 2773–2780. [DOI] [PubMed] [Google Scholar]
- 32Zhu D, Zhang C, Medhora M, Jacobs ER. CYP4A mRNA, protein, and product in rat lungs: novel localization in vascular endothelium. J Appl Physiol 2002; 93: 330–337. [DOI] [PubMed] [Google Scholar]
- 33Kawasaki T, Marumo T, Shirakami K, Mori T, Doi H, Suzuki M et al. Increase of 20-HETE synthase after brain ischemia in rats revealed by PET study with 11C-labeled 20-HETE synthase-specific inhibitor. J Cereb Blood Flow Metab 2012; 32: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol 2011; 300: H1194–H1200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.