Abstract
We examined the effect of resuscitation with liposome-encapsulated hemoglobin (LEH) on cerebral bioenergetics in a rat model of 45% hypovolemia. The rats were resuscitated with isovolemic LEH or saline after 15 minutes of shock and followed up to 6 hours. Untreated hypovolemic rats received no fluid. The cerebral uptake of F-18-fluorodeoxyglucose (FDG) was measured by PET, and at 6 hours, the brain was collected for various assays. Hypovolemia decreased cellular adenosine triphosphate (ATP), phosphocreatine, nicotinamide adenine dinucleotide (NAD)/NADH ratio, citrate synthase activity, glucose-6-phosphate, and nerve growth factor (NGF), even when FDG uptake remained unchanged. The FDG uptake was reduced by saline, but not by LEH infusion. The reduced FDG uptake in saline group was associated with a decrease in hexokinase I expression. The LEH infusion effectively restored ATP content, NAD/NADH ratio, and NGF expression, and reduced the hypovolemia-induced accumulation of pyruvate and ubiquitinated proteins; in comparison, saline was significantly less effective. The LEH infusion was associated with low pH and high anion gap, indicating anionic gap acidosis. The results suggest that hypovolemic shock perturbs glucose metabolism at the level of pyruvate utilization, resulting in deranged cerebral energy stores. The correction of volume and oxygen deficits by LEH recovers the cerebral metabolism and creates a prosurvival phenotype.
Keywords: brain, glucose metabolism, hypovolemic shock, liposome-encapsulated hemoglobin, resuscitation
Introduction
Intensivists correct inadequate tissue perfusion and cellular hypoxia in the victims of hemorrhagic shock by resuscitation with fluids such as 0.9% saline, lactated Ringer's solution, Hextend, whole blood and packed red blood cells (pRBCs). Whole blood and pRBCs are the products of choice because of their oxygen carrying capacity, but clinically-matched blood products are not always available at the needed location in a timely manner. Crystalloids and colloids only correct salt imbalance and circulatory volume deficit in a transient manner; they increase the cardiac preload, but do not increase oxygen content in circulation. We have been investigating liposome-encapsulated hemoglobin (LEH) as a universal nanometric-sized oxygen carrier, the advantages of which have been elaborated elsewhere.1 Like RBCs, LEH spatially isolates hemoglobin, thus restraining the vasoactivity and toxicity of free hemoglobin. Moreover, the oxygen diffusivity of LEH mimics that from the RBCs, enabling the restoration of hemodynamics and microvascular circulation.2
An important issue in resuscitation with hemoglobin-based oxygen carriers, including whole blood and packed RBCs, is the relationship between the oxygen-carrying capacity in circulation and metabolic improvement in the perfused organ. The entire debate about the selection and use of resuscitation fluids in clinical practice is determined largely by clinician preference,3 and blood pressure and hemoglobin levels still guide resuscitation choices.4 Hence, most investigations of experimental therapeutics in hemorrhagic shock focus on the hemodynamic effects rather than the tissue markers of metabolic activity.5 It is not necessary that enhanced oxygen-carrying capacity in circulation afforded by hemoglobin-based oxygen carriers translates into metabolic restitution in a hypoxic organ. For instance, it has been observed that even after the increase in hematocrit and hemoglobin level by blood transfusion in pediatric septic shock patients, there was no increase in oxygen consumption.6 Only recently the appraisal of cerebral blood flow, metabolism, and oxygen delivery in preclinical investigations has been given due attention.7, 8, 9
The brain is a special organ because it is spared the initial hypoxic and mal-perfusion insults by an increase in tissue oxygen extraction ratio and diversion of cardiac output from splanchnic organs.9 However, when the blood pressure decreases below the autoregulatory level, cerebral perfusion starts to go into a decompensation phase which is characterized by decoupling of metabolic activities. Perturbed cellular metabolism and energy depletion precede neuronal cell death and mark the early signs of reaching a point of no return. Previously, we have reported the ability of LEH to improve cerebral oxygen metabolism and tissue energetics in hypovolemic shock (HS).8, 9 Recently, LEH infusion was found to be efficacious in the treatment of cerebral infarction by improving the oxygen delivery to ischemic cerebral tissues.10 The goal of this study was to evaluate the brain energetics in response to LEH resuscitation. We determined the brain regional glucose metabolism, the levels of energy metabolites, and the markers of cerebral homeostasis in a rat model of 45% HS. To our knowledge, this is the first study of its kind demonstrating the effect of HS and resuscitation with an artificial oxygen carrier on brain glucose and energy metabolism.
Materials and methods
Liposome-Encapsulated Hemoglobin Preparation
Hemoglobin was encapsulated inside the liposomes carrying anionic charge. The phospholipid dipalmitoylphosphatidylcholine was purchased from Lipoid (Ludwigshafen, Germany), and high-purity cholesterol (Cho) was obtained from Calbiochem (Gibbstown, NJ, USA). Anionic lipid hexadecylcarbamoylmethylhexadecanoate (HDAS) and its conjugate with poly(ethylene glycol)-2000 (HDAS-PEG2K) were synthesized in-house using methods described elsewhere.11 We employed a high pressure homogenization method using Emulsiflex-C3 (Avestin, Ottawa, ON, Canada) for the preparation of LEH composed of dipalmitoylphosphatidylcholine (~38 mol%), Cho (~38 mol%), HDAS (~20 mol%), HDAS-PEG2K (0.3 mol%), and vitamin E (~2.4 mol%). Stroma-free Hb was isolated from outdated RBC units and characterized by essentially following methods described previously.12 Outdated RBC units were sourced from Sylvan Goldman Center of Oklahoma Blood Institute (Oklahoma City, OK, USA). To enhance the circulation persistence of LEH, it was PEGylated with HDAS-PEG2K using post-insertion method.11 The final LEH preparation was made iso-oncotic by adding human serum albumin (Albutein, Alpha Therapeutics Corporation, Los Angeles, CA, UAS) to 5% w/v. The LEH preparation was characterized for Hb content, metHb, size, oxygen affinity (p50), and phospholipid concentration.12, 13 Oxygen affinity (p50) was measured by a Hemox-analyzer (TCS Scientific Corp., New Hope, PA, USA). The amount of encapsulated Hb was determined by monitoring absorbance of the LEH lysate at 540 nm and metHb content was measured in stroma-free Hb as well as in LEH. Particle size was determined by dynamic light scattering using a Brookhaven particle size analyzer equipped with Particle Solutions v.1.2 (Brookhaven Instruments Corp., Holtsville, NY, USA). These processing and characterization methods associated with LEH manufacturing are described in detail elsewhere.12, 13
Rat Model of Hypovolemic Shock
The animal experiments were performed according to the NIH Animal Use and Care Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Male Sprague Dawley rats (250 to 300 g, 9 to 10 months of age) were purchased from Harlan (Indianapolis, IN, USA), housed in regular light/dark cycles of 12/12 and allowed to acclimatize for at least 5 days. The method of femoral artery catheterization in rats has been described elsewhere.9 Briefly, the left femoral artery was cannulated with a Teflon-tipped catheter and the catheter was subcutaneously tunneled and secured to the nape; the rats were allowed at least 2 days to recover from surgery. On the day of the experiment, the rats were handled under isoflurane (2% to 3%) anesthesia in medical air stream (2 L/min) containing 21% oxygen and 78% nitrogen. The rats were heparinized with 100 units of heparin to prevent catheter blockade. Hypovolemic shock was induced by withdrawing ~45% of circulating blood at the rate of 1.0 mL/min. The total volume of blood was estimated ~6% of the total body weight. The rats were treated and recruited for imaging studies (described below), while allowing them to wake up and freely compensate. After 6 hours of hemorrhage, the surviving rats were euthanized with an overdose of SOMNASOL, Euthanasia-III Solution (Butler Schein Animal Health, Dublin, OH, USA). Brain was isolated, cleaned with ice-cold saline, and preserved in liquid nitrogen.
Resuscitation
The cannulated rats were clustered a priori in four groups (n=6/group): control (CTRL), HS, HS+Saline (SAL), and HS+LEH (LEH). No blinding of the investigators involved was performed. Isovolemic resuscitation with saline and LEH was administered after 15 minutes of the completion of blood withdrawal. We used isovolemic saline for comparison, and not three times the blood lost, to avoid the possibility of hyperchloremic metabolic acidosis and cerebral edema. Moreover, mildly hypotensive resuscitation has been reported to increase survival rate and time in rat model of hemorrhagic shock.14 The fluids were warmed to ~35°C and intravenously infused at 1 mL/min using a syringe pump.
Hemodynamics
Blood pressure was digitally monitored by instrumenting the rats to a data acquisition system consisting of IX118 and ETH-255 amplifier (iWorx Systems, Dover, NH, USA). The femoral artery catheter was connected to a BP-102 transducer system (iWorx Systems, Dover, NH, USA) via a 23G × 12” blunt-ended butterfly infusion set. For blood gas monitoring, ~200 μL whole blood sample was collected and introduced into a disposable blood gas cartridge placed in a VetStat Electrolyte and Blood Gas Analyzer (IDDEX Laboratories, Westbrook, ME, USA).
Imaging
Positron emission tomography (PET) for the measurement of standard uptake value (SUV) of F-18-labeled fluorodeoxyglucose (FDG) was performed as described in detail elsewhere.15 The rats (404.5±11.7 g bodyweight) were recruited for the imaging study in four groups: CTRL (n=6), HS (n=5), LEH (n=5), and Saline (n=4). F-18-fluorodeoxyglucose was synthesized in a Biomarker Generator BG75 (Advanced Biomarker Technologies, Knoxville, TN, USA). Briefly, ~100 μCi of FDG (101.1±0.9 μCi, 0.345±0.011 mL) was injected intravenously in the tail vein of anesthetized (2% isoflurane–air mixture) rats 2 hours before the scheduled euthanasia at 6 hours. After 45 minutes of injection, the rats were reanesthetized for imaging, a blood sample was collected (45 minutes blood sample), and the rats were positioned supine in a gantry of a PET-CT dual modality machine from Gamma Medica Ideas (Northridge, CA, USA). A fly-mode CT of brain was acquired before a 20-minute long list-mode PET acquisition. Through the imaging period, the rats were kept anesthetized by 2% isoflurane–air mixture. After imaging, the rats were allowed to wake up and kept in their cage until the time of euthanasia.
The acquired images were reconstructed by filtered back projection algorithm and fused with CT image to generate a composite PET-CT image. The composite image was used for segmentation-based drawing of a 3D-region of interest surrounding various regions of the brain. A 3D Sprague Dawley brain atlas (International Neuroinformatics Coordinating Facility, Stockholm, Sweden) was superimposed on the PET/CT images using Amira 5.6 (FEI Visualization Sciences Group, Burlington, MA, USA). This Waxolm Space Atlas (version 1.01) is based on high-resolution magnetic resonance imaging anatomic landmarks and diffusion-weighted image volumes of rat brains. No attempt was made to correct the images for attenuation. The radioactive counts in the region of interest were determined as Ct (counts per unit volume) and the amount of radioactivity in the 45-minute blood sample was measured in a well counter as CFDG. These values were used to compute SUV of FDG by the following equation:
 |
Glucose-6-Phosphate Concentration
The concentration of glucose-6-phosphate in brain tissue was determined by a colorimetric assay based on the reduction of the 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium in a reduced nicotinamide adenine dinucleotide phosphate-coupled enzymatic reaction. The assay kit was obtained from Biomedical Research Service (University of Buffalo, NY).
Citrate Synthase Assay
We measured citrate synthase activity in brain tissue homogenates by using a commercially available 96-well kit from Cayman Chemical (Ann Arbor, MI, USA). The enzyme activity was normalized to protein concentration and expressed as nmol/mg protein/min.
Adenosine Triphosphate and Phosphocreatine
We determined adenosine triphosphate (ATP) using a kit based on luciferin-luciferase system (Sigma-Aldrich, St Louis, MO, USA). The light output was converted into nmol/L ATP per mg of protein by comparing with an ATP standard curve. To determine phosphocreatine, the endogenous ATP levels are first destroyed in the extract, and the phosphocreatine is allowed to generate ATP from exogenously added ADP.16 Briefly, the extract sample (5 μL) was allowed to react with 5 μL of an ATP→ADP reagent containing 390 mmol/L 2-amino-2-methyl-a-propanol (pH 8.8), 20 mmol/L glucose, 4 mmol/L MgCl2, 40 μmol/L ATP-free ADP, 0.04% BSA, 24 mmol/L NaF, and 7 U/mL hexokinase. After allowing 15 minutes at room temperature, 5 μL of 5 mmol/L EDTA was added and the reaction mixture was immersed in boiling water path for 2 minutes. To the cooled reaction mixture, 5 μL of the phosphocreatine→ATP reagent (containing 715 mmol/L imidazole (pH 6.5), 15 mmol/L MgCl2, 0.07% BSA, 18 mmol/L NaF, and 500 U/mL creatine kinase) was added. A series of control reactions and standard ATP were also ran simultaneously. Control reagent solutions were prepared without hexokinase or creatine kinase enzymes. The reaction was allowed to proceed for 50 minutes, before being stopped by addition of 100 μL of 0.2 mol/L NaOH, 1.2 mmol/L EDTA, and immersion in boiling water bath for 2 minutes. Finally, 20 μL of reaction mixture was used for the determination of ATP as described above.
Nicotinamide Adenine Dinucleotide (NAD) and Reduced NAD (NADH)
The brain tissue nicotinamides NAD and NADH were determined in equal amounts of homogenate proteins by using a kit from Abcam (Cambridge, MA, USA). The values were expressed as NAD/NADH ratio.
Lactate and Pyruvate
The lactate and pyruvate content in brain tissue (nmol/L per mg) was estimated by using a commercially available kit from Cayman Chemical (Ann Arbor, MI, USA).
Thiobarbituric Acid Reactive Substances Assay
We quantified the formation of malondialdehyde (MDA) by monitoring its reaction with thiobarbituric acid in a colorimetric assay (Cayman Chemical, Ann Arbor, MI, USA). Thiobarbituric acid reactive substances (TBARS) were normalized to protein concentration (μmol/L per mg).
Nerve Growth Factor, Brain-Derived Neurotrophic Factor, and L-Glutamate
Commercially available enzyme-linked immunosorbent assays (ELISA, EMD Millipore, Billerica, MA, USA) were employed for the determination of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in brain tissue homogenate and expressed in pg/mL. L-Glutamate in brain tissue was measured by a kit from Biomedical Research Service (University of Buffalo, NY).
Immunoblotting
Fifty microgram of protein was separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), electro-transferred onto nitrocellulose membranes, and blotted with rat-specific antibodies. The rabbit primary antibodies against rat hexokinase I and ubiquitin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were used at a dilution of 1:500, whereas hexokinase II antibody (EMD Millipore) was used at 1:1,000 dilution. The primary antibody against actin and HRP-conjugated secondary antibody was purchased from Sigma-Aldrich and used at 1:5,000 dilution. The immunoreactive bands were detected by SuperSignal West Femto detection reagent (Thermo Fischer Scientific, Rockford, IL, USA) on an Ultraquant image acquisition machine (Claremont, CA, USA). The densitometry of immunoreactive bands was performed from three replicates using the Image J 1.46r freeware (NIH, USA).
Data Analysis
The data are reported as the mean±standard error of mean (s.e.m.). For comparison among four groups (Control, HS, SAL, and LEH) at the same time point, ordinary one-way analysis of variance (ANOVA) with Bonferroni correction (single-pooled variance) was employed using the Prism 6 software (GraphPad, San Diego, CA, USA). A P-value of <0.05 was considered as statistically significant. For blood gas and hemodynamic data, the mean values after 6 hours of shock, with or without LEH, were compared with the mean baseline values in the same animals. One-way ANOVA with Bonferroni correction was also used for these repeated-measures. To the best of knowledge, all sections of this report adhere to the ARRIVE Guidelines for reporting animal research.
Results
We created a nanoparticulate LEH preparation for evaluation as an oxygen-carrying resuscitation fluid in a rat model of 45% HS. The LEH nanoparticles were 215.6±2.16 nm in size, with a polydispersity of 0.187 and a zeta potential of −30.92 mV. It carried 7.2±0.2 g/dL of hemoglobin of which the methemoglobin content was <5%. The pH of the preparation was 7.2 and the osmolality was 331±3 mOsmoL/kg. The total phospholipid and cholesterol concentration of LEH preparation was 11.43 and 10.8 mg/mL, respectively. Below, we describe the effects of LEH resuscitation in a rat model of 45% HS. We observed a mortality rate of ~40% in this study lasting 6 hours.
Liposome-Encapsulated Hemoglobin Resuscitation Improves Hemodynamic Responses
The hematocrit, mean arterial pressure (MAP) values, heart rate, and blood gas values in various experimental groups are provided in Table 1. After 45% hemorrhage, the hematocrit decreased commensurate to the amount of blood lost, 40.6 at baseline versus 25.3 in HS group after 6 hours (P<0.05). Although not statistically significant, there was a further decrease in hematocrit in rats treated with LEH and saline, perhaps because of the dilution effect of the administered fluids. The blood withdrawal caused a massive decrease in MAP (mm Hg) from 108.6 to 48.6 mm Hg. The MAP significantly improved to 83.3 mm Hg immediately after LEH infusion, and this improvement was maintained through 6 hours data point (78.9 mm Hg). The improvement in MAP after saline infusion was also quantitatively similar to that after LEH infusion. However, the MAP in both LEH and saline groups remained significantly lower than the baseline MAP (P<0.05). Moreover, even the untreated rats in HS group showed spontaneous improvement in MAP (60.7 mm Hg, P<0.05 versus HS). The heart rate immediately after hemorrhage also decreased from the baseline levels of 317 to 192 b.p.m. All the groups, including the HS group, showed a significant increase in heart rate after 6 hours.
Table 1. Hemodynamics and arterial blood gases.
| Baseline | iHS | HS 6 hours | LEH 6 hours | SAL 6 hours | |
|---|---|---|---|---|---|
| Hematocrit | 40.57±1.02 | ND | 25.33±1.14* | 22.4±2.28* | 23.25±0.52* |
| Heart rate (beats/min) | 317±1.8 | 192±2.62* | 236±10.5*¥ | 257±26.9*¥ | 225±16.8*¥ |
| MAP (mm Hg) | 108.6±0.58 | 48.6±0.77* | 60.7±4.03*¥ | 78.9±3.05*¥$ | 77.3±3.18*¥$ |
| pH | 7.51±0.01 | ND | 7.49±0.01 | 7.26±0.08*$ | 7.48±0.01ϕ |
| HCO3 (mmol/L) | 29.67±1.65 | ND | 27.27±1.20 | 15.92±1.15*$ | 27.45±0.89 |
| Anion gap | 10.68±1.40 | ND | 14.95±3.19 | 18.73±1.98 | 9.15±0.57 |
| Total CO2 (mmol/L) | 29.17±0.58 | ND | 28.45±1.28 | 17.16±1.14*$ | 28.65±0.89ϕ |
| Base excess (mmol/L) | 5.53±0.29 | ND | 4.23±0.98 | -10.04±2.38*$ | 4.25±0.94ϕ |
| pO2 (mm Hg) | 85.67±1.71 | ND | 106.00±6.65 | 89.40±19.74 | 101.75±1.65 |
| Total Hb (g/dL) | 15.38±0.15 | ND | 6.45±0.29* | 8.64±0.41*$ | 7.25±0.22*ϕ |
| Na+ (mmol/L) | 140.67±0.76 | ND | 139.17±1.08 | 140.60±1.94 | 139.50±0.65 |
| K+ (mmol/L) | 4.20±0.07 | ND | 4.08±0.14 | 4.57±0.72 | 4.03±0.19 |
| Cl− (mmol/L) | 106.17±0.91 | ND | 104.33±1.17 | 109.00±0.95$ | 106.50±0.29 |
Abbreviations: LEH, liposome-encapsulated hemoglobin; ND, not determined; The data are provided as mean±s.e.m. The heart rate and arterial blood pressure (MAP) were measured at baseline (before induction of hypovolemic shock (HS)), immediately after hypovolemic shock (iHS) and at 6 hours. P-value <0.05 versus Baseline*, iHS¥, HS$, and LEHϕ.
Blood gas values were measured at baseline and at 6 hours after hemorrhage before inducing euthanasia (Table 1). In HS group, all the blood gas parameters (except for hemoglobin) were not significantly different from the baseline levels. However, in LEH-treated group, a significant decrease in pH, HCO3, total CO2, and base excess was observed as compared with the baseline levels (Table 1). Hemoglobin and Cl− content in LEH group were significantly increased as compared with the HS group. Rats infused with saline did not show any significant changes in blood gas as compared with the HS group. Despite infusion with LEH containing 7.2 g/dL hemoglobin, blood gas data only showed an incremental increase in total hemoglobin. We speculate that this discrepancy is due to the interference caused by the presence of large amounts of lipid in blood samples from LEH-infused rats.
Liposome-Encapsulated Hemoglobin Resuscitation Restores Cerebral Energetic
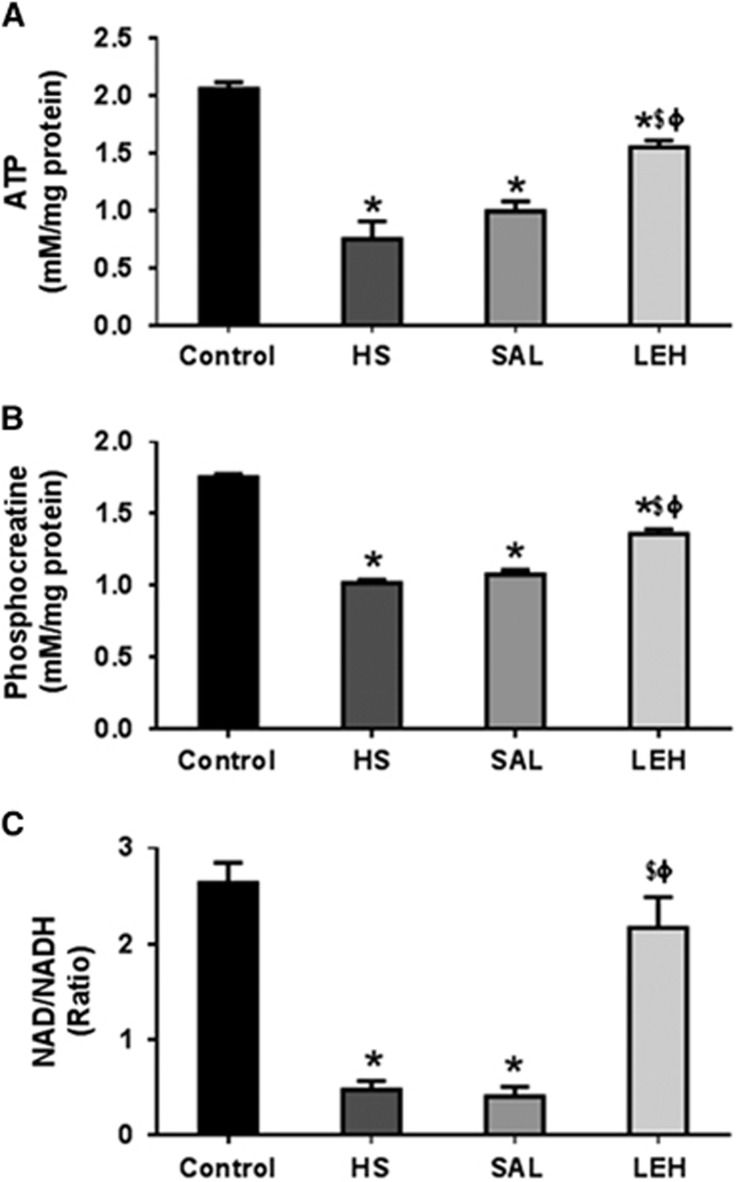
The assay for cerebral ATP and phosphocreatine revealed that HS led to a significant reduction in the levels of these metabolites in brain tissue. The ATP content reduced from control levels of 2.1 to 0.8 mmol/L in HS group (62% reduction, P<0.05 versus control, Figure 1A). Isovolemic saline infusion had no impact on HS-induced decrease in cellular ATP concentration. However, LEH infusion resulted in a recovery of ATP levels to 1.6 mmol/L (76% recovery, P<0.05 versus HS). Like the ATP content, the phosphocreatine concentration (Figure 1B) also decreased after HS (1.8 mmol/L in CTRL versus 1.0 mmol/L in HS, P<0.05). In LEH group, the phosphocreatine concentration was 1.4 nmol/L (78% of the control levels), which was statistically significant as compared with both HS and SAL groups (P<0.05). The cerebral concentrations of phosphocreatine were not improved by saline infusion after HS.
Figure 1.
The cerebral (A) adenosine triphosphate (ATP) (n=5 per group), (B) phosphocreatine (n=5 per group), and (C) nicotinamide adenine dinucleotide (NAD)/NADH ratio (n=4 per group) in brain tissue. P<0.05 versus Ctrl*, versus HS$, and versus SALϕ (ANOVA, Bonferroni correction). HS, hypovolemic shock.
Furthermore, HS caused an ~80% decrease in NAD/NADH ratio from 2.6 in CTRL group to 0.5 in HS group (P<0.05, Figure 1C). Treatment with LEH recovered NAD/NADH ratio to 2.2, which was 83% of the control level (P<0.05 versus HS). The NAD/NADH ratio in LEH group was not statistically different from that in the control group. However, saline infusion did not show any improvement in the depressed NAD/NADH ratio.
Effect of Liposome-Encapsulated Hemoglobin Resuscitation on Cerebral Glucose Metabolism
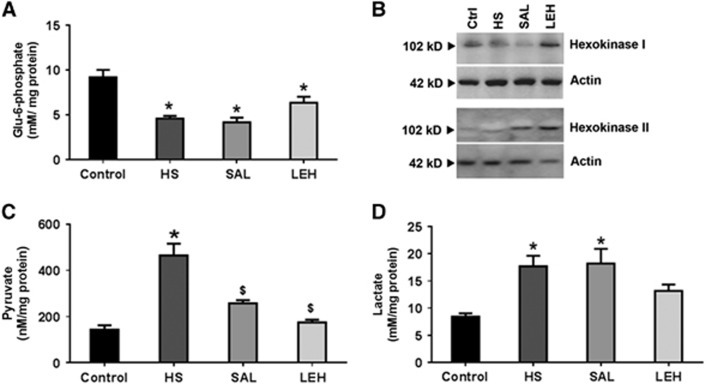
We examined the first step in cellular glucose utilization, namely the generation of glucose-6-phosphate in a reaction catalyzed by hexokinase. We found that HS caused a 50% decrease in the levels of Glu6P from 9.2 to 4.6 mmol/L (Figure 2A, P<0.05). The cellular Glu6P levels were partially recovered to 6.4 mmol/L in brain tissue of rats treated with LEH, but the recovery was not significant in comparison with the HS group (P>0.05 versus HS). Isovolemic saline infusion did not show any impact on the reduced Glu6P concentration as compared with the HS group. The conversion of glucose to glucose-6-phosphate is catalyzed by hexokinase. The representative expression of hexokinases I and II in brain tissue is shown in Figure 2B and the densitometric analyses of their expression are provided in Supplementary material (Supplementary Figures S1a and b). The expression of hexokinase I was not altered in the HS group, but it was significantly decreased in the saline group as compared with both CTRL and HS groups. The hexokinase I expression in LEH group was not significantly different from either CTRL or HS group, but was significantly more than that in SAL group. Like hexokinase I, the expression of hexokinase II was also not affected by hemorrhage in HS group. Saline treatment led to an increase in hexokinase II expression as compared with the HS group (P<0.05 versus HS), but LEH infusion did not result in any significant change in hexokinase II expression as compared with the HS group. Hexokinase II expression in both SAL and LEH groups was not significantly altered as compared with the control group.
Figure 2.
(A) Glucose-6-phosphate content (n⩾4 per group), (B) a representative immunoblot of hexokinases I and II expression from three different sets, (C) pyruvate (n⩾5 per group), and (D) lactate (n⩾5 per group) in brain tissue after hypovolemic shock (HS) and liposome-encapsulated hemoglobin (LEH) or saline resuscitation. P<0.05 versus Ctrl* and versus HS$ (ANOVA, Bonferroni correction).
The end product of glycolysis in mammalian cells is pyruvic acid. We found that HS significantly increased the level of cellular pyruvate in brain tissue from 143 nmol/L in control rats to 467 nmol/L in HS group (3.3-fold increase, Figure 2C). The LEH and saline treatment significantly reduced pyruvate concentration back to 175 and 258 nmol/L, respectively (P<0.05 versus HS). The corresponding lactate concentrations in brain tissue from control, HS, SAL, and LEH groups were 8.4, 17.7, 18.2, and 13.2 mmol/L, respectively (Figure 2D). The lactate levels in LEH group were not significantly different from those in control group; however, the reduced levels were also not significantly different as compared with the HS group. These results show that LEH reduced accumulation of the monocarboxylates, particularly pyruvate in brain tissue; isovolemic saline infusion was comparatively less effective.
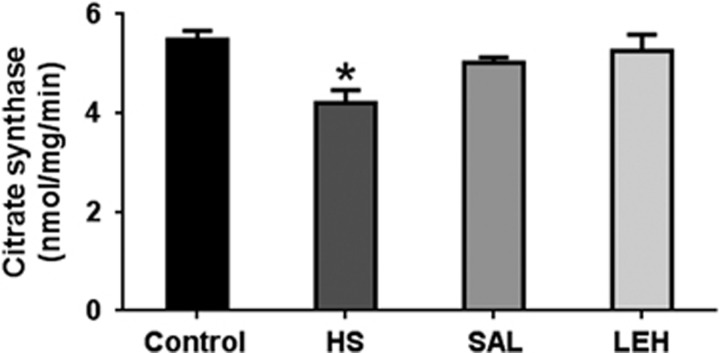
As an indicator of tricarboxylic acid (TCA) cycle, we determined the activity of citrate synthase in the tissue homogenate. Hypovolemic shock caused a significant reduction in the activity of citrate synthase from 5.5 to 4.2 mmoL/min (Figure 3). The LEH treatment of hemorrhaged rats increased the citrate synthase activity to ~5.3 mmoL/min. Isovolemic saline infusion also increased citrate synthase activity to 5.0 mmoL/min. However, the citrate synthase activity in LEH and SAL groups was not significantly different from that in the HS group.
Figure 3.
The citrate synthase activity (n=4 per group) in brain tissue after hypovolemic shock (HS) and liposome-encapsulated hemoglobin (LEH) or saline resuscitation. P<0.05 versus Ctrl* (ANOVA, Bonferroni correction).
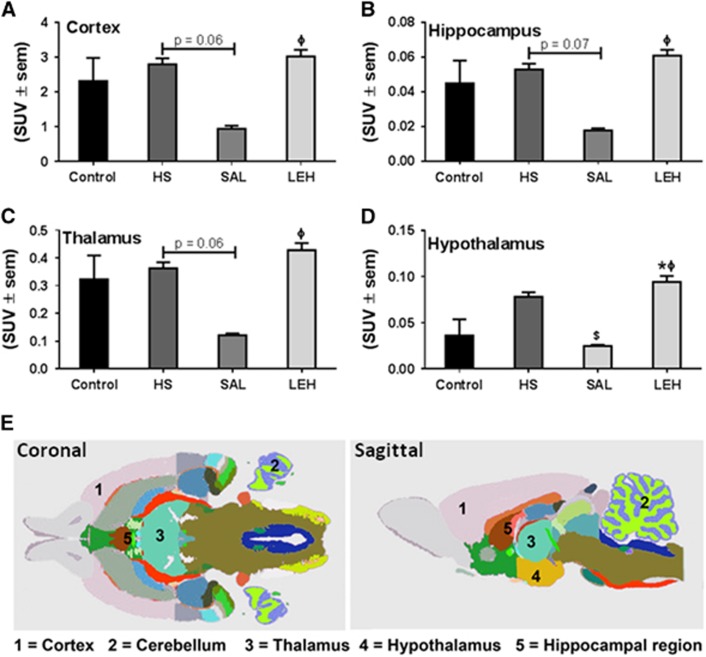
We further evaluated glucose metabolism by determining the SUVs of FDG in four regions of the brain from PET images (Figure 4). There was no change in FDG uptake in cortex, hippocampus, thalamus, and hypothalamus regions of HS group as compared with the control group (Figures 4A–4D, P>0.05 versus Control). Although saline infusion substantially reduced the regional FDG uptake as compared with the HS group, the differences were not statistically significant except for hypothalamic region. The LEH infusion did not change FDG uptake as compared with that in control group. The glucose levels in blood samples of various groups are provided in Supplementary material (Supplementary Figure S3). Here, it is important to note a caveat that SUV-based FDG uptake analysis is dependent on an assumption that the arterial input function with respect to glucose is homogenous among various groups.
Figure 4.
The uptake of F-18-fluorodeoxyglucose (FDG) as determined by positron emission tomography (PET) imaging of brain (n=5 per group). The uptake was quantitated in (A) cortex (B) hippocampus, (C) thalamus, and (D) hypothalamus. (E) Coronal and sagittal views of rat brain showing the selected regions from an MRI atlas (Courtesy: International Neuroinformatics Coordinating Facility, Stockholm, Sweden). P<0.05 versus Ctrl*, versus HS$, and versus SALϕ (ANOVA, Bonferroni correction). HS, hypovolemic shock; LEH, liposome-encapsulated hemoglobin; SUV, standard uptake value.
Effect of Liposome-Encapsulated Hemoglobin Resuscitation on Cerebral Oxidative Stress
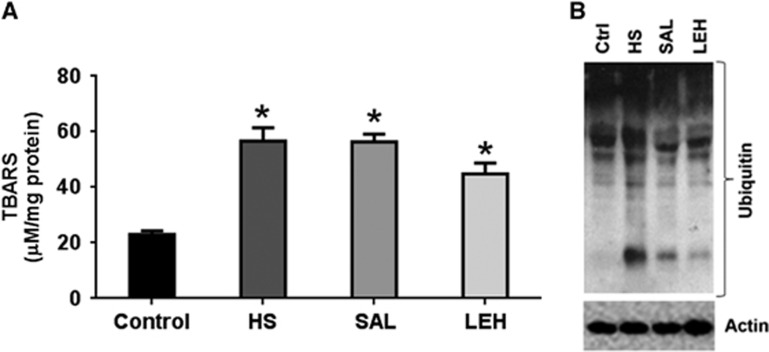
We assessed the level of TBARS in tissue homogenates as an indicator of brain oxidative stress (Figure 5A). As compared with the control group, TBARS more than doubled in HS group (23 and 57 μmol/L, respectively, P<0.05). In LEH-infused rats, there was an ~20% decrease in brain TBARS; however, TBARS in this group remained at a significantly higher level as compared with the control values (P<0.05). Saline infusion had no impact on TBARS levels, and the differences among HS, SAL, and LEH groups were not statistically significant.
Figure 5.
(A) The concentration of thiobarbituric acid reactive substances (TBARS) (n=4 per group) and (B) a representative immunoblot showing accumulation of ubiquitinated proteins in brain tissue after hypovolemic shock (HS) and liposome-encapsulated hemoglobin (LEH) or saline resuscitation. P<0.05 versus Ctrl* (ANOVA, Bonferroni correction).
The intracellular accumulation of oxidatively damaged proteins is alleviated by their ubiquitination and degradation by the ubiquitin proteasome system. We monitored the accumulation of ubiquitinated proteins (Ub-Pr) as an indicator of this quality control system. As compared with the control tissues, HS increased the presence Ub-Pr in the HS group. The LEH infusion reduced the hemorrhage-induced accumulation of Ub-Pr (Figure 5B). Saline infusion was marginally less effective than LEH in reducing the hemorrhage-induced accumulation of Ub-Pr. The densitometric values for Ub-Pr expression from four sets of experimental groups are provided in Supplementary material (Supplementary Figure S1c).
Liposome-Encapsulated Hemoglobin Resuscitation Recovers Cerebral Nerve Growth Factor
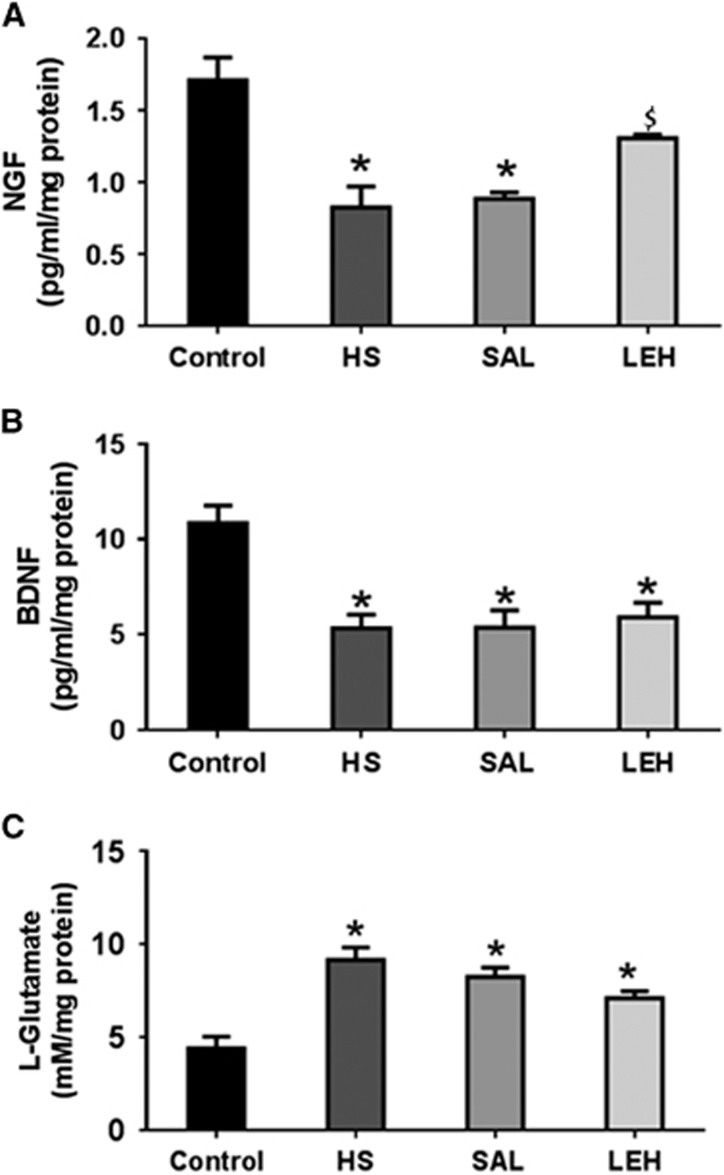
As a further measure of neuroprotection afforded by LEH administration, we measured the brain concentrations of NGF and BDNF (Figures 6A and 6B, respectively). Hypovolemic shock decreased the concentration of NGF by more than 50% (1.7 pg/mL in control versus 0.8 pg/mL in HS, P<0.05). In saline infused rats, the NGF concentration remained at low levels (0.9 pg/mL). However, LEH administration increased the NGF content to 1.3 pg/mL, which was not significantly different from the control levels. Like the NGF content, the concentration of BDNF also decreased by approximately half in HS group (10.8 pg/mL in control group versus 5.3 pg/mL, P<0.05, Figure 6B). Both saline and LEH infusions were ineffective in recovering the loss in BDNF concentration after hemorrhagic shock.
Figure 6.
The concentration of (A) nerve growth factor (NGF) (n=4 per group), (B) brain-derived neurotrophic factor (BDNF) (n=5 per group), and (C) L-glutamate (n=5 per group) in brain tissue. P<0.05 versus Ctrl*, versus HS$ (ANOVA, Bonferroni correction). HS, hypovolemic shock.
L-glutamate is an excitotoxic neurotransmitter in brain. Hypovolemia increased the concentration of L-glutamate in brain tissue (Figure 6C). The L-glutamate concentration in HS group was 9.2 mmol/L as compared with 4.4 mmol/L in control group (210% increase, P<0.05). The slight reduction in L-glutamate concentration after LEH infusion was not significant. In SAL group also, the L-glutamate concentration remained significantly high at 8.3 mmol/L (P>0.05 versus HS).
Discussion
The two most important goals of intensive care in HS are the correction of oxygen deficit and maintenance of perfusion pressure. Liposome-encapsulated hemoglobin is an oncotic oxygen carrier that could be made universally available without the need of blood typing.1 In this work, we determined the status of cerebral energetics in 45% hemorrhaged rats resuscitated with LEH. The physical characteristics of LEH used in this study are typical of an effective preparation (Supplementary Table S1, Supplementary material). The particle size of LEH (216 nm) was within the reported range of PEG-liposomes for enhanced circulation.17 However, the LEH formula is slightly different from the previously reported preparations, which were composed entirely of phospholipids and cholesterol.1 Here, the lipid composition contained nonphospholipid constituents HDAS and HDAS-PEG.11, 13 Hexadecylcarbamoylmethylhexadecanoate is an anionic nonphospholipid replacement of negatively-charged phospholipids in liposomes, and its use in the enhancement of hemoglobin encapsulation has been reported elsewhere.12 However, HDAS-PEG delays clearance of LEH by the monocyte phagocytic system and increases the mean residence time in circulation. Hexadecylcarbamoylmethylhexadecanoate and HDAS-PEG replace significant amounts of phospholipids, which can potentially cause toxic effects.18, 19, 20 Here, we show that resuscitation with LEH containing HDAS and HDAS-PEG reduces pyruvate accumulation, recovers the concentration of energy metabolites, and restores pro-survival neurotrophin NGF in brain of rats with 45% HS.
The hemodynamic effects of HS and single isovolemic infusion of LEH were on expected lines. Earlier, infusion of hemoglobin vesicles in a similar rat model was reported to recover MAP to ~89% of the baseline value after 6 hours of infusion.21 The blood gas data suggested the presence of anion gap metabolic acidosis in rats treated with LEH. Although the precise reasons for this phenomenon are unknown, we suspect albumin in our LEH preparation as a possible culprit. Hyperalbuminemia causes high anion gap because it is an anionic protein under physiologic conditions.22 Anion gap is affected by changes in unmeasured ions, such as albumin. In these conditions, bicarbonate concentrations are reduced because of their consumption as a buffer against the increased presence of acids. However, saline resuscitation has been known to cause hyperchloremic metabolic acidosis because of supraphysiologic concentrations of Cl−.23 However, we did not find any significant change in Cl− content after saline infusion, perhaps because of the low volume (isovolemic) resuscitation.
For cellular maintenance functions and biosynthetic reactions, ATP is the preferred energy source. Like other tissues with high and variable ATP turnover, cerebral tissue has high phosphocreatine concentration.24 Being excitable cells, the neurons need an immediate availability of vast amounts of energy that may be used in a pulsed manner. However, the neurons avoid building a large pool of ATP because adenylate pool and ATP:ADP ratios are key regulators of many fundamental processes of the cell. Instead, large quantities of metabolically inert phosphogens, such as phosphocreatine, are stored. Our results suggest that LEH infusion resulted in moderate recovery of ATP and phosphocreatine levels compared with HS; however, they were still significantly below the control levels. During energy crisis, phosphocreatine is consumed for replenishing the ATP reserves in a reaction catalyzed by creatine kinase. Cerebral phosphocreatine concentration stabilizes when the ATP depletion is arrested.
A major source of the cellular ATP in brain is glucose. We performed FDG-PET to study the uptake of glucose in brain of hemorrhaged rats. The FDG uptake was not affected by hemorrhage or by LEH infusion, but saline infusion substantially reduced regional FDG uptake in hemorrhaged rats, especially in the hypothalamus (Figure 4). The reduced FDG uptake in saline group was associated with a decrease in hexokinase I expression (Figure 2B). However, LEH infusion showed no effect on hexokinase I expression and FDG uptake. The expression of other isoform of hexokinase, the hexokinase II, was not affected in any of the groups as compared with the control group. Notwithstanding the dependence of glucose uptake on hexokinase I expression, the reduction in Glu6P levels despite normal FDG uptake in HS group is an intriguing observation because FDG is trapped intracellularly as FDG6P. We speculate that intracellular Glu6P content is also affected by several other factors, which in turn are variably influenced by hemorrhagic shock. The net intracellular levels of Glu6P are the balance between rates of production by glucose phosphorylation, glycogenolysis, and gluconeogenesis and its metabolic influx in several pathways such as glycogen synthesis, glycolysis, and the pentose phosphate pathway.25, 26 Furthermore, Glu6P concentration is also affected by the expression of Glu6P phosphatases.27, 28 What exact mechanism is responsible for the regulation of Glu6P content after hemorrhagic shock is a matter of further investigation.
The glycolysis product pyruvate is oxidatively metabolized in the TCA cycle to generate ATP. In this process, NAD and NADH act as an electron acceptor and donor, respectively. The decrease in NAD/NADH ratio after HS may be indicative of a metabolic shift in energy production from oxidative phosphorylation to glycolysis. Glycolytic shift is invoked during acute stress because of its ability to rapidly produce ATP in cytosol without the need of oxygen or mitochondrial function.29 However, the continuous consumption of NAD in the glycolytic process, during prolonged stress, leads to its exhaustion and the arrest of glycolytic process. Accordingly, we noted a sharp decrease in NAD/NADH ratio in brain tissue of hemorrhaged rats. The failure to regenerate NAD in HS could be directly linked to the inability of brain cells to metabolize pyruvate via TCA cycle, because TCA cycle is the major producer of NAD. The administration of LEH not only regenerated NAD, but also reduced the accumulation of pyruvate after hemorrhagic shock. On the basis of these results, it could be argued that the hypovolemia blocks the cerebral utilization of pyruvate in the TCA cycle, resulting in its accumulation and NAD depletion. The inability to correctly utilize pyruvate and its intracellular accumulation is considered damaging to the neuronal cell in HS.30, 31, 32 The accumulating pyruvate would also inhibit the entire upstream pathway of glycolysis by a feedback mechanism. Given the slight increase in citrate synthase activity in LEH group as compared with the HS group, there is some indication of improvement in mitochondrial metabolism by LEH infusion. However, more evidence is needed to definitively prove the beneficial effect of LEH on mitochondrial metabolism. It is notable that saline infusion had no effect on the levels of brain Glu6P, NAD/NADH ratio, and lactate, but had a modest effect on pyruvate concentration. These results are in agreement with the previous observations made by Hwabejire et al30 in swine polytrauma model infused with saline.
Oxidative stress in brain ischemia-reperfusion injury has been shown to impair proteasome activity,33 and the proteasome-mediated protein quality control is majorly dependent on the availability of ATP.34 In our model, the inability of brain to handle oxidized/damaged proteins by the proteasome system was manifested as an accumulation of Ub-Pr (Figure 5B). The LEH infusion resulted in a significant reduction in Ub-Pr expression, indicating the salutary effect of improved ATP content caused by LEH. Another symptom of deranged energy metabolism in HS was the reduced levels of neurotrophin, NGF. The NGF signaling is neuroprotective and regulates repair functions in brain.35 The ATP binding to NGF has been shown to be a prerequisite for its neuroprotective bioactivity.36 The LEH infusion increased NGF in hemorrhaged rats (Figure 6A), thus reducing the DNA fragmentation, a marker of apoptotic cell death (Supplementary Figure S2, Supplementary material). However, saline infusion was ineffective in recovering NGF levels and protecting against apoptosis.
Overall, these results suggest that HS considerably alters the cerebral glucose metabolism and reduces the energy stores in brain tissue. Administration of LEH resurrected the cerebral energy metabolism by correcting the defects in glycolytic pathway and improving the mitochondrial metabolism. Saline as a resuscitation fluid for restoration of metabolic activity was relatively ineffective. This is significant because isotonic crystalloids are the mainstay in resuscitative intervention. Here, it must be noted that instead of using three times the lost blood volume, we used isovolemic saline as a replacement fluid. This choice was deliberate to achieve euvolemia without aggressive fluid resuscitation, which might have resulted in cerebral edema and hyperchloremic metabolic acidosis.
Acknowledgments
The authors thank Dr Gary T Kinasewitz, Professor of Pulmonary Medicine at the University of Oklahoma Health Sciences Center, for kindly reviewing the manuscript. Help from Mr Chengwen Teng (Eric) in analysis of PET images is also acknowledged.
Author Contributions
GR acquired in vitro data on tissue samples, assisted in data interpretation, and participated in manuscript writing. AFH was responsible for imaging PET imaging studies and image analysis in rats. VY manufactured and characterized LEH used in this work. AH was integral in animal experiments and analysis of hemodynamics data. JX assisted in collection of data from biochemical assays and western blots. VA designed the experiments, participated in animal studies, wrote manuscript, and guided the overall research.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by a grant from National Heart, Lung & Blood Institute (R01HL104286).
Supplementary Material
References
- 1Awasthi V. Pharmaceutical aspects of hemoglobin-based oxygen carriers. Curr Drug Deliv 2005; 2: 133–142. [DOI] [PubMed] [Google Scholar]
- 2Sakai H, Takeoka S, Wettstein R, Tsai AG, Intaglietta M, Tsuchida E. Systemic and microvascular responses to hemorrhagic shock and resuscitation with Hb vesicles. Am J Physiol Heart Circ Physiol 2002; 283: H1191–H1199. [DOI] [PubMed] [Google Scholar]
- 3Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013; 369: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 4Bougle A, Harrois A, Duranteau J. Resuscitative strategies in traumatic hemorrhagic shock. Ann Intensive Care 2013; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T et al. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock 2009; 31: 64–79. [DOI] [PubMed] [Google Scholar]
- 6Mink RB, Pollack MM. Effect of blood transfusion on oxygen consumption in pediatric septic shock. Crit Care Med 1990; 18: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 7Foley LM, Iqbal O'Meara AM, Wisniewski SR, Hitchens TK, Melick JA, Ho C et al. MRI assessment of cerebral blood flow after experimental traumatic brain injury combined with hemorrhagic shock in mice. J Cereb Blood Flow Metab 2013; 33: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Awasthi V, Agashe H, Doblas S, Towner R. Magnetic resonance spectroscopy for evaluation of liposome-encapsulated hemoglobin as a resuscitation fluid. Artif Cells Blood Substit Immobil Biotechnol 2010; 38: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol 2007; 103: 28–38. [DOI] [PubMed] [Google Scholar]
- 10Kaneda S, Ishizuka T, Sekiguchi A, Morimoto K, Kasukawa H. Efficacy of liposome-encapsulated hemoglobin in a rat model of cerebral ischemia. Artif Organs 2014; 38: 650–655. [DOI] [PubMed] [Google Scholar]
- 11Nag OK, Yadav VR, Hedrick A, Awasthi V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int J Pharm 2013; 446: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces 2010; 75: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Yadav VR, Nag O, Awasthi V. Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif Organs 2014; 38: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Li T, Zhu Y, Fang Y, Liu L. Determination of the optimal mean arterial pressure for postbleeding resuscitation after hemorrhagic shock in rats. Anesthesiology 2012; 116: 103–112. [DOI] [PubMed] [Google Scholar]
- 15Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med 2007; 48: 277–287. [PubMed] [Google Scholar]
- 16Ronner P, Friel E, Czerniawski K, Frankle S. Luminometric assays of ATP, phosphocreatine, and creatine for estimation of free ADP and free AMP. Anal Biochem 1999; 275: 208–216. [DOI] [PubMed] [Google Scholar]
- 17Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm 2003; 253: 121–132. [DOI] [PubMed] [Google Scholar]
- 18Bastiat G, Oliger P, Karlsson G, Edwards K, Lafleur M. Development of non-phospholipid liposomes containing a high cholesterol concentration. Langmuir 2007; 23: 7695–7699. [DOI] [PubMed] [Google Scholar]
- 19Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J 2006; 20: 2591–2593. [DOI] [PubMed] [Google Scholar]
- 20Nag OK, Awasthi V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 2013; 5: 542–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Sakai H, Seishi Y, Obata Y, Takeoka S, Horinouichi H, Tsuchida E et al. Fluid resuscitation with artificial oxygen carriers in hemorrhaged rats: profiles of hemoglobin-vesicle degradation and hematopoiesis for 14 days. Shock 2009; 31: 192–200. [DOI] [PubMed] [Google Scholar]
- 22Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med 2005; 146: 317–320. [DOI] [PubMed] [Google Scholar]
- 23Kellum JA. Saline-induced hyperchloremic metabolic acidosis. Crit Care Med 2002; 30: 259–261. [DOI] [PubMed] [Google Scholar]
- 24Kekelidze T, Khait I, Togliatti A, Benzecry JM, Wieringa B, Holtzman D. Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J Neurosci Res 2001; 66: 866–872. [DOI] [PubMed] [Google Scholar]
- 25Agius L, Centelles J, Cascante M. Multiple glucose 6-phosphate pools or channelling of flux in diverse pathways? Biochem Soc Trans 2002; 30: 38–43. [DOI] [PubMed] [Google Scholar]
- 26Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 2003; 206: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 27Gautier-Stein A, Soty M, Chilloux J, Zitoun C, Rajas F, Mithieux G. Glucotoxicity induces glucose-6-phosphatase catalytic unit expression by acting on the interaction of HIF-1alpha with CREB-binding protein. Diabetes 2012; 61: 2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Marcolongo P, Fulceri R, Gamberucci A, Czegle I, Banhegyi G, Benedetti A. Multiple roles of glucose-6-phosphatases in pathophysiology: state of the art and future trends. Biochim Biophys Acta 2013; 1830: 2608–2618. [DOI] [PubMed] [Google Scholar]
- 29Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001; 292: 504–507. [DOI] [PubMed] [Google Scholar]
- 30Hwabejire JO, Jin G, Imam AM, Duggan M, Sillesen M, Deperalta D et al. Pharmacologic modulation of cerebral metabolic derangement and excitotoxicity in a porcine model of traumatic brain injury and hemorrhagic shock. Surgery 2013; 154: 234–243. [DOI] [PubMed] [Google Scholar]
- 31Lin T, Koustova E, Chen H, Rhee PM, Kirkpatrick J, Alam HB. Energy substrate-supplemented resuscitation affects brain monocarboxylate transporter levels and gliosis in a rat model of hemorrhagic shock. J Trauma 2005; 59: 1191–1202, discussion 1202. [DOI] [PubMed] [Google Scholar]
- 32Mongan PD, Capacchione J, Fontana JL, West S, Bunger R. Pyruvate improves cerebral metabolism during hemorrhagic shock. Am J Physiol Heart Circ Physiol 2001; 281: H854–H864. [DOI] [PubMed] [Google Scholar]
- 33Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab 2000; 20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 34Peth A, Nathan JA, Goldberg AL. The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J Biol Chem 2013; 288: 29215–29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001; 24: 1217–1281. [DOI] [PubMed] [Google Scholar]
- 36Ferenz KB, Gast RE, Rose K, Finger IE, Hasche A, Krieglstein J. Nerve growth factor and brain-derived neurotrophic factor but not granulocyte colony-stimulating factor, nimodipine and dizocilpine, require ATP for neuroprotective activity after oxygen-glucose deprivation of primary neurons. Brain Res 2012; 1448: 20–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.