Abstract
Postsynaptic density-95 (PSD95) is a scaffolding protein in cerebral vascular smooth muscle cells (cVSMCs), which binds to Shaker-type K+ (KV1) channels and facilitates channel opening through phosphorylation by protein kinase A. β1-Adrenergic receptors (β1ARs) also have a binding motif for PSD95. Functional association of β1AR with KV1 channels through PSD95 may represent a novel vasodilator complex in cerebral arteries (CA). We explored whether a β1AR-PSD95-KV1 complex is a determinant of rat CA dilation. RT-PCR and western blots revealed expression of β1AR in CA. Isoproterenol induced a concentration-dependent dilation of isolated, pressurized rat CA that was blocked by the β1AR blocker CGP20712. Cranial window imaging of middle cerebral arterioles in situ showed isoproterenol- and norepinephrine-induced dilation that was blunted by β1AR blockade. Isoproterenol-induced hyperpolarization of cVSMCs in pressurized CA was blocked by CGP20712. Confocal images of cVSMCs immunostained with antibodies against β1AR and PSD95 indicated strong colocalization, and PSD95 co-immunoprecipitated with β1AR in CA lysate. Blockade of KV1 channels, β1AR or disruption of PSD95-KV1 interaction produced similar blunting of isoproterenol-induced dilation in pressurized CA. These findings suggest that PSD95 mediates a vasodilator complex with β1AR and KV1 channels in cVSMCs. This complex may be critical for proper vasodilation in rat CA.
Keywords: beta1-adrenergic receptor, cerebral circulation, potassium channel, scaffolding protein, vascular smooth muscle
Introduction
The diameter of cerebral arteries (CA) is dynamically regulated in response to local metabolic demands and hemodynamic changes.1 The relaxation of cerebral vascular smooth muscle cells (cVSMCs) may be triggered by several different stimuli, which include β-adrenergic receptor (AR) agonists.2 Although previous studies have reported βAR-mediated vasodilation in CA,3, 4, 5 the downstream signaling complex and effectors remain largely unknown. In this study, we explored the function of a novel adrenergic vasodilator complex in CA mediated by postsynaptic density-95 (PSD95).
Postsynaptic density-95 belongs to a family of scaffolding proteins known as MAGUKs (Membrane-Associated Guanylate Kinase homologs).6 Membrane-Associated Guanylate Kinase homologs associate with membrane-bound proteins to form clusters of multimeric structure near the plasma membrane and modulate the expression and function of ion channels and receptors.7, 8 First identified and characterized in neurons, PSD95 is reported to associate with over 50 proteins in neurons and expression systems.9 The interaction of PSD95 with signaling proteins is facilitated by three PDZ binding domains (postsynaptic density-95, disc large, zonal occludens-1), which exhibit preferential association with distinct binding partners.9 Included among these binding partners are voltage-gated ion channels, G protein-coupled receptors, and a variety of cytoplasmic proteins involved in signal transduction.9, 10
Previously, we reported that PSD95 associates with Shaker-type voltage-gated K+ (KV1) channels in rat cVSMCs to mediate vasodilation.11 In that study, PSD95 and KV1.2α subunits were shown to colocalize in cVSMCs and coimmunoprecipitate in rat CA lysate while antisense knockdown of PSD95 in rat CA resulted in a concomitant loss of KV1 channel protein and reduced basal CA diameter. In addition, we recently reported that PSD95 scaffolding is required for proper protein kinase A (PKA) phosphorylation and vasodilatory function of KV1 channels in cVSMCs.12 Disruption of PSD95-KV1 binding by a competing peptide reduced basal levels of KV1 channel phosphorylation and K+ current without changing surface protein expression and caused a significant constriction of CA both ex vivo and in vivo. The functional association of an effector (KV1 channels) and a signaling molecule (PKA) with PSD95 in maintaining basal levels of vasodilation, considered together with the many potential binding partners of PSD95, suggests that PSD95 may function as the scaffolding backbone of a complete receptor–effector signalosome in cVSMCs.
Several features of the β1AR make it a prime candidate to complete the PSD95-KV1 signalosome. β1-Adrenergic receptors have a distal carboxyl terminus that contains a PDZ binding motif (-ESKV), are expressed in VSMCs,13 and may signal through PKA to open vascular KV channels.14 The PDZ-binding carboxyl terminus is not conserved among βARs and confers selective binding of PSD95 to β1AR but not to β2AR.10, 15, 16 Therefore, a β1AR-PSD95-KV1 complex signaling through PKA may represent a critical determinant of CA dilation. CA of several species including human and rat express both αAR and βAR.17, 18, 19 The distribution of adrenergic receptor subtypes varies between species and vascular beds.13, 20 However, β1AR-mediated dilation in CA has been observed in rats and other mammals.4, 5 In the present study, we evaluated whether β1AR-mediated dilation of rat CA requires PSD95 scaffolding of KV1 channels in cVSMCs.
Materials and methods
Animals
All experiments in this study were in compliance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. In all experiments, 10- to 14-week-old male Sprague-Dawley (SD) rats (Harlan Labs, Indianapolis, IN, USA) were used. Tissue from βAR-knockout (Adrb1tm1BkkAdrb2tm1Bkk/J) mice and C57BL/6J wild-type mice (Jackson Labs, Bar Harbor, ME, USA) were used as negative and positive controls for β1AR western blots. The reporting of experiments conforms with ARRIVE guidelines.
Dissection of Cerebral Arteries
Rats were anesthetized with inhaled isoflurane (3.5%) before decapitation for removal of the brain. The brain was placed in chilled Hank's balanced salt solution for dissection. The superior cerebellar arteries (SCA) were isolated for pressurized artery studies to measure diameter and membrane potential (Em). The entire circle of Willis and its branches were isolated to evaluate mRNA levels by real-time PCR and protein expression by western blot analysis. We showed previously that CA preparations have minimal contamination from brain tissue.11
Real-Time RT-PCR
Real-time PCR was used to detect β1AR mRNA in rat CA as described before.21 cDNA was generated from 500 ng of RNA by reverse transcription and amplified by real-time PCR with β1AR-specific primers (forward: 5′-AGACGCTCACCAACCTCTTCATCA-3′, reverse: 5′-ACATAGCACGTCTACCGAAGTC-3′, Integrated DNA Technologies, Coralville, IA, USA) The threshold cycles (Ct) for β1AR and α-actin were determined from the amplification curves. The resulting amplified DNA was analyzed on an agarose gel to ensure a single product (155 bp) and confirmed to be β1AR fragment by DNA sequencing. Negative control reactions contained all components except cDNA template.
Protein Homogenization and Western Blot
Cerebral arteries were homogenized in lysis buffer using a Bullet Blender (Next Advance, Averill Park, NY, USA). Large tissue debris and nuclear fragments were removed by centrifugation. Proteins were analyzed by western blot. Membranes were incubated with β1AR polyclonal antibodies (1:200 dilution, lot# C1313, Santa Cruz, Dallas, TX, USA) or PSD95 monoclonal antibody (1:100 dilution, Neuromab, Davis, CA, USA) and immunoreactive bands were identified using enhanced chemiluminescence. Antibodies against smooth muscle α-actin (1:5,000 dilution, Sigma-Aldrich, St Louis, MO, USA) were used to confirm comparable lane loading.
Pressurized Cerebral Arteries Diameter and Membrane Potential Recording
Measurements of diameter and membrane potential from isolated CA were performed as previously described.11, 12 Briefly, SCA were isolated, cannulated and pressurized to 80 mmHg and equilibrated at 37°C for 1 hour in physiologic salt solution (PSS) bubbled with a 7% CO2/93% O2 gas mixture to pH 7.4. Any SCA that did not develop spontaneous tone after equilibration were not used for study. External diameters were automatically measured and recorded by DMTVAS software (Danish Myo Technology, Aarhus, Denmark). Drugs were added directly to the superfusate bath away from the artery and allowed to diffuse. At the end of the study, the arteries were superfused with a Ca2+-free PSS to induce maximal dilation. All isolated CA dilation data are expressed as a percentage of the maximal Ca2+-free dilation.
Membrane potential (Em) was measured using glass microelectrodes as previously described.12 A successful measurement of Em was defined as an abrupt decrease in voltage upon impalement, maintenance of a stable value for a minimum of 20 seconds and an immediate return to baseline upon withdrawal of the microelectrode. Three such measurements were recorded before the application of isoproterenol and the reading was maintained through the application of isoproterenol to the tissue bath. At least two additional measurements were made after the addition of isoproterenol and averaged to yield the final Em value for each artery.
Immunocytochemistry and Confocal Imaging
Cerebral vascular smooth muscle cells were enzymatically isolated as described previously.11, 22 The isolated cVSMCs were fixed, permeabilized, and blocked with donkey serum followed by an overnight incubation in β1AR (Santa-Cruz, 1:200 dilution) and PSD95 (Abcam, Cambridge, MA, USA, 1:200 dilution) antibody at 4°C. After several washes, cVSMCs were incubated in Cy2 anti-rabbit and Cy3 anti-mouse secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA, 1:500 dilution). Confocal images were taken using a Zeiss Axio Observer Z.1 microscope equipped with a spinning disk confocal using a × 63/1.40 NA oil-immersion objective (Gottingen, Germany) and a Hamamatsu Orca ER camera (Hamamatsu City, Japan). Pearson's colocalization coefficient was calculated using ImageJ (NIH, Bethesda, MD, USA)23 and images were processed by Zeiss Axiovision 3D deconvolution for clarity. The specificity of the β1AR antibody was determined by colocalization of β1AR and Flag immunostaining in HEK cells (Supplementary Methods, Supplementary Figure S1). The specificity of PSD95 antibody had been shown in cVSMCs by antisense-mediated knockdown of PSD95 in our earlier publication.11
Coimmunoprecipitation
Coimmunoprecipitation (coIP) was performed with Novex Dynabeads Protein G and a DynaMag-2 separation magnet (Life Technologies, Oslo, Norway). According to the manufacturer's instructions, 75 μL of bead slurry was prepared and 50 μL (10 μg) of β1AR antibody was added to the beads. The antibody–bead mixture was incubated at room temperature for 90 minutes. A separate tube of beads was prepared without antibody as a negative control. Each tube of beads was loaded with equal amounts of CA lysate (115 μg) and incubated overnight at 4°C. Unbound protein ‘flow-through' was removed and the beads were washed. Antibody-bound proteins were eluted by denaturing elution. Both elute and flow-through portions were analyzed by western blot. To preserve protein–protein interactions, the centrifugation speed for lysate preparation was reduced to 3,000 g for coIP studies.12
Craniectomy and In Situ Imaging
Under isoflurane anesthesia (2% at 1.0 L/min O2), rats were mounted in a stereotaxic frame (KOPF, Tujunga, CA, USA) and positioned under a dissecting microscope as previously described.12 In some rats, femoral arteries were catheterized and connected to a pressure transducer (ADInstruments, Colorado Springs, CO, USA), through which blood pressure and heart rate were recorded. A section of bone was removed from the right parietal plate and the dura mater retracted. A custom-made ported cranial window was fitted. The tissue underneath the cranial window was suffused with 37°C PSS bubbled (7% CO2, 93% O2) to physiologic pH. Middle cerebral artery (MCA) branches were imaged using an HDR-PJ580 camera (Sony, Tokyo, Japan) and analyzed using an automated script in IPLab (Scanalytics, Milwaukee, WI, USA). All drugs were suffused through the window ports. KCl (60 mmol/L) and sodium nitroprusside (10 and 100 μmol/L) were given at the end of concentration–response treatments to confirm artery integrity and ability to constrict and dilate. The diameter data are expressed as the %change from baseline diameter.
Drugs
All drugs and chemicals were obtained from Sigma-Aldrich unless otherwise indicated. The following drugs were prepared as stock solutions in DMSO: 5-(4-phenylalkoxypsoralen) (Psora-4), 100 μmol/L; and indomethacin, 100 mmol/L. Stock solutions for other drugs were prepared in water: N-ω-nitro-L-arginine methyl ester (L-NAME), 100 mmol/L; ICI118551, 10 mmol/L (Tocris, Ellisville, MO, USA); CGP20712, 10 mmol/L (Tocris); isoproterenol (ISO) 20 mmol/L; norepinephrine (NE) 20 mmol/L; dobutamine, 20 mmol/L; KV1-C and Scm peptides, 1 mmol/L (21st Century Biochemicals, Marlborough, MA, USA); myristoylated PKA inhibitor peptide 14-22 (PKI), 1 mmol/L (Tocris); bradykinin, 1 μmol/L. For pressurized vessel experiments, drugs were diluted first in PSS when appropriate and added directly to the tissue bath at either × 100 or × 1,000 dilution for the final bath concentrations referred to in the text. For cranial window experiments, drugs were diluted in PSS to their final concentration and directly suffused into the cranial window chamber. DMSO (1 μL in 10 mL of PSS) was used as a negative control.
Statistical Analysis
Data are expressed as mean±standard error of the mean (s.e.m.). Data were analyzed using unpaired t tests or when appropriate with one-way ANOVA or two-way ANOVA with Bonferroni post hoc for cranial window measurements. Sample sizes were chosen based on our previous experience to yield power of 0.8 to 0.9 and P<0.05 was considered as statistically significant. Experiment number (N) represents the number of arteries in pressurized CA ex vivo studies and the number of animals in cranial window in vivo studies.
Results
Expression of β1-Adrenergic Receptor in Cerebral Arteries and Selective Inhibition of Isoproterenol-Induced Dilation of Cerebral Arteries by β1-Adrenergic Receptor Blockade
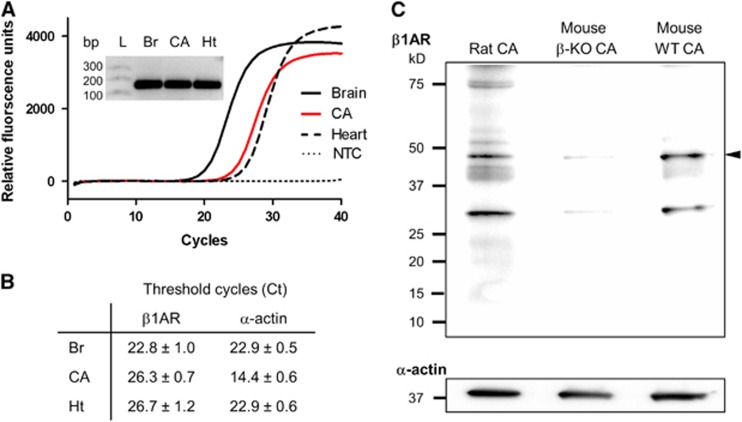
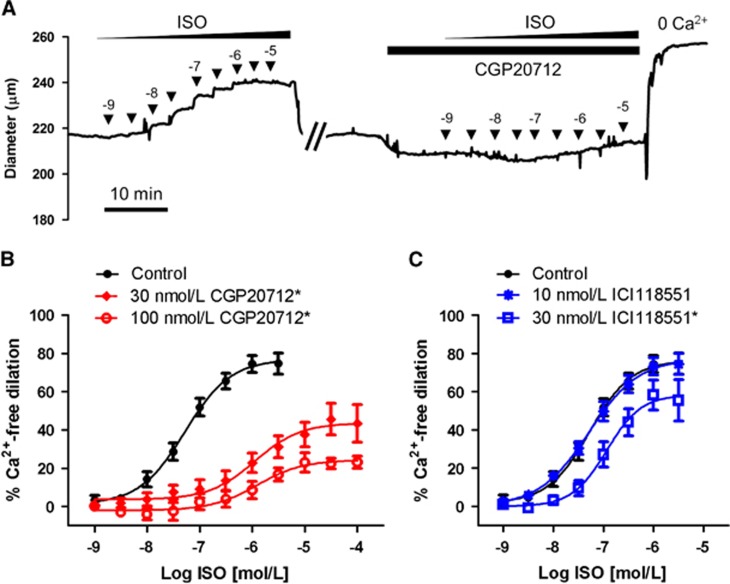
The expression of β1AR and their functional contribution to the artery diameter were determined in isolated CA. β1-Adrenergic receptor mRNA was detected in CA using real-time RT-PCR (Figures 1A and 1B). Rat brain (Br) and heart (Ht) cDNA were used as positive controls. Single PCR products of β1AR amplification were of the predicted size (155 bp). Comparison of CA branches, circle of Willis, and basilar artery separately revealed a nearly identical ratio of β1AR to α-actin for each as well as the whole CA (Supplementary Figure S2). β1-Adrenergic receptor protein expression was detected by western blot of CA lysates (30 μg/each) probed with an anti-β1AR antibody (Figure 1C and Supplementary Figure S3). The CA lysate from β-AR knockout mice (β-KO CA) and wild-type mice (WT CA) were used as negative and positive controls, respectively. Diameter recordings of isolated, pressurized (80 mm Hg) rat SCA in response to increasing half-log concentrations of isoproterenol (1 nmol/L to 10 μmol/L) resulted in concentration-dependent increases in artery diameter, achieving near-maximum dilation (Emax) at 1 μmol/L isoproterenol (Figure 2). Repeated application of isoproterenol itself did not significantly alter vasodilation (Supplementary Figure S4). A significant loss of dilation was observed in the presence of the β1AR selective blocker CGP20712 (Figures 2A and 2B),24, 25 while the β2AR selective blocker ICI118551 had a much smaller, but significant effect at the higher, less selective concentration (Figure 2C). Existence of β1AR-mediated dilation in SCA was also validated by dilation to β1AR-selective agonist dobutamine and near-complete blockade by 30 nmol/L CGP20712 (Supplementary Figure S4C). Isoproterenol-induced dilation was not altered by the presence of 100 μmol/L L-NAME and 10 μmol/L indomethacin, blocking nitric oxide synthase and cyclooxygenase, respectively, nor did the physical denudation of endothelial cells affect isoproterenol-induced dilation (Supplementary Figure S5). Isoproterenol-induced dilation and its blockade by the presence of 30 nmol/L CGP20712 were similar in SCA and MCA (Supplementary Figure S6A). The change in baseline diameter induced by 30 nmol/L CGP20712 (Figure 2A) was only −1.5±0.7% and was not statistically significant from the time control or all concentrations of CGP20712 and ICI118551 used (Supplementary Figure S6B). CGP20712 is 500-fold more selective for β1AR over other βAR subtypes while ICI118551 is 550-fold more selective for β2AR. Based on their relative binding affinities, 30 nmol/L of CGP20712 and 10 nmol/L of ICI118551 were chosen to preferentially block β1AR and β2AR.24, 25, 26 A higher, less selective concentration of 100 nmol/L of CGP20712 or 30 nmol/L of ICI118551 was also tested in our initial experiments. Concentrations of 30 and 100 nmol/L of CGP20712 reduced isoproterenol-induced maximal dilation by 42% and 69%, respectively. Both concentrations right-shifted the half-maximal effective concentration (EC50) similarly, more than one log unit (logEC50: ISO, −7.3±0.2; 30 nmol/L CGP, −6.0±0.2; 100 nmol/L CGP, −6.0±0.2). ICI11855125, 26 had no effect on isoproterenol-induced dilation at 10 nmol/L and blunted dilation by 26% at 30 nmol/L with little change in EC50 (LogEC50: ISO, −7.3±0.1; 10 nmol/L ICI, -7.3±0.1; 30 nmol/L ICI, −6.9±0.2).
Figure 1.
Expression of β1-adrenergic receptor (β1AR) in cerebral arteries. (A) Real-time RT-PCR amplification of β1AR cDNA from rat brain (Br), cerebral arteries (CA) and heart (Ht). Inset: Single PCR products of β1AR amplification are of predicted size (155 bp). (B) Threshold cycles (Ct) for β1AR and α-actin from three real-time PCR experiments (mean±s.e.m.). (C) Western blot immunoreactive bands corresponding to β1AR (arrowhead) in protein lysates from rat and mouse CA (30 μg/each). CA from βAR-knockout mouse (β-KO CA) and C57BL/6 J wild-type mouse (WT CA) represent negative and positive controls. Representative blot from three experiments from three separate sets of animals. L, Ladder; NTC, no template control.
Figure 2.
Selective blockade of isoproterenol-induced dilation in superior cerebellar arteries (SCA). (A) Representative recording of outer diameter of an isolated, pressurized (80 mm Hg) rat SCA. The artery was initially exposed to increasing half-log concentrations (▾) of isoproterenol (ISO). After repeated washes, the artery was incubated with 30 nmol/L CGP20712 for 10 minutes before repeating the ISO concentration response. (B) ISO concentration response in the presence of the β1AR-selective blocker CGP20712 (30 nmol/L, 100 nmol/L; n=5 to 8). (C) ISO concentration response in the presence of the β2AR selective blocker ICI118551 (10 nmol/L, 30 nmol/L; n=6 to 8). *Significant difference from Control, P<0.05.
Isoproterenol-Induced Dilation of Middle Cerebral Artery Is Blocked by Topical β1-Adrenergic Receptor Blocker In Vivo
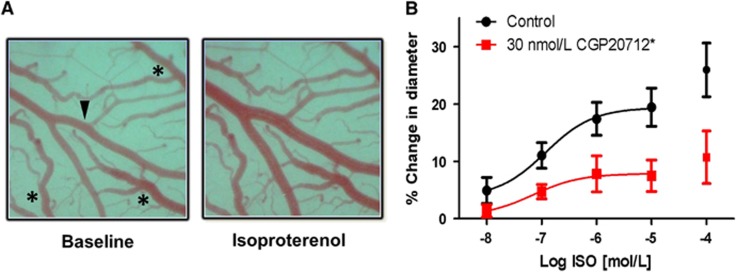
After establishing the effect of β1AR blockade on SCA response to isoproterenol in isolated arteries, we evaluated the effect of topical blockade of β1ARs on isoproterenol-induced dilation of MCA in vivo. In vivo experiments were performed to control for potential artifacts introduced by ex vivo experiments and to confirm the physiologic contribution of β1AR-mediated dilation in CA with intact physiology. Branches of the MCA were exposed via partial craniectomy of the right parietal plate of anesthetized rats immobilized in a stereotaxic frame, followed by mounting of a cranial window as previously described.12 The ported cranial window allowed for topical drug treatment and imaging of the artery. Suffusion of the pial tissue beneath the cranial window with increasing log concentrations of isoproterenol (10 nmol/L to 100 μmol/L) resulted in dilation of the artery from baseline (19.4±3.3% Figure 3). Five-minute pretreatment with topical 30 nmol/L CGP20712 followed by combined administration of isoproterenol and 30 nmol/L CGP20712 resulted in a dilation from baseline of 7.5±2.7%, an ~60% loss of isoproterenol dilation (Figure 3B). EC50 was not significantly affected (logEC50: ISO, −6.9±0.4; CGP+ISO, −7.2±0.8). 30 nmol/L CGP20712 alone did not significantly affect the basal arteriole diameter (−0.9±0.7%, n=6). Maximum in vivo dilation was about a third smaller compared with ex vivo responses expressed as a % change in diameter (Supplementary Figure S7A; 29.9±4.8% ex vivo, 19.4±3.3% in vivo). These results confirm the presence of the β1AR-mediated vasodilator complex in rat MCA in vivo.
Figure 3.
Isoproterenol-induced dilation of middle cerebral arteries (MCA) in vivo. (A) Representative images of an MCA branch (arrowhead) in cranial window at baseline and in response to 10 μmol/L isoproterenol (ISO). Veins (asterisks) are unresponsive. (B) Percent change from baseline diameter of MCA branch in cranial window in response to increasing concentrations of topically suffused ISO alone and after a 5-minute pretreatment and coadministration of 30 nmol/L CGP20712, n=5 to 6. The data points for the highest concentration, 100 μmol/L ISO, were excluded from the analysis as systemic changes in heart rate and blood pressure were observed at that concentration. *Significant difference from Control, P<0.05.
The approach of topically suffusing CA in vivo through a cranial window instead of a systemic dosing was taken to keep effects local and avoid confounding global cardiovascular effects such as peripheral vasodilation or increase in cardiac output. In our cranial window preparation, concentrations up to 10 μmol/L isoproterenol had no effect on blood pressure and heart rate (Supplementary Figure S8). A higher concentration of isoproterenol (0.1 mmol/L) generated a decrease in diastolic blood pressure (−8.8±3.6%) and an increase in heart rate (14±3%, n=5; Supplementary Figure S8). This systemic hemodynamic change may have contributed to the additional increase in MCA diameter observed at the concentration of 0.1 mmol/L isoproterenol (Figure 3B), which was consequently left out of the analysis.
Effect of β1-Adrenergic Receptor Blocker on Norepinephrine-Induced Dilation of Cerebral Arteries Ex Vivo and In Vivo
Isoproterenol is a synthetic nonselective β-AR agonist. To examine the physiologic consequences of a β1AR-mediated dilation pathway in CA, we evaluated the effect of the endogenous catecholamine norepinephrine in vitro and in vivo (Figure 4). In isolated, pressurized SCA, increasing concentrations of norepinephrine (10 nmol/L to 10 μmol/L) dilated arteries in a concentration-dependent manner (Emax: 43.7±7.5%) (Figure 4A). The presence of a β2AR blocker, 10 nmol/L ICI118551, did not affect norepinephrine-induced dilation significantly (49.5±4.6%). The β1AR blockade by 30 nmol/L CGP20712 resulted in reversal of norepinephrine-induced dilation to constriction (−11.2±4.6%). The greatest dilation (82.3±5.8%) produced in response to norepinephrine was in the presence of an αAR blocker, 1 μmol/L phentolamine, revealing the presence of αAR-dependent tone (Figure 4A). Middle cerebral artery response in vivo to suffused norepinephrine and concurrent β1AR blockade was evaluated in the cranial window preparation (Figure 4B). Log concentrations of norepinephrine (10 nmol/L to 100 μmol/L) resulted in MCA dilation (6.8±0.8%) from basal diameter that was reversed to constriction in the presence of 30 nmol/L CGP20712 (−3.8±1.8%). Maximum in vivo dilation was 57% smaller compared to ex vivo responses expressed as % change in diameter (Supplementary Figure S7B). However, the reversal of dilation to constriction by β1AR blockade was the same (ISO: 15.7±2.6% ex vivo versus 6.8±0.8% in vivo; ISO+CGP: −3.8±1.8% ex vivo versus −3.8±1.8% in vivo).
Figure 4.
Norepinephrine-induced dilation of superior cerebellar arteries (SCA) ex vivo and middle cerebral arteries (MCA) in vivo. (A) Outer diameter measurements of isolated, pressurized (80 mm Hg) SCA in response to increasing concentrations of norepinephrine (NE) in the presence of β2-adrenergic receptor (AR) blocker ICI118551 (10 nmol/L), β1AR blocker CGP20712 (30 nmol/L), and the αAR blocker phentolamine (1 μmol/L), n=5 each. (B) Concentration response of MCA branches to norepinephrine (NE) alone and with 5-minute pretreatment and coadministration of 30 μmol/L CGP20712 in cranial window, n=7 each. *Significant difference from control, P<0.05.
Effect of β-Adrenergic Stimulation and β-Adrenergic Receptor Blockade on Cerebral Vascular Smooth Muscle Cells Membrane Potential
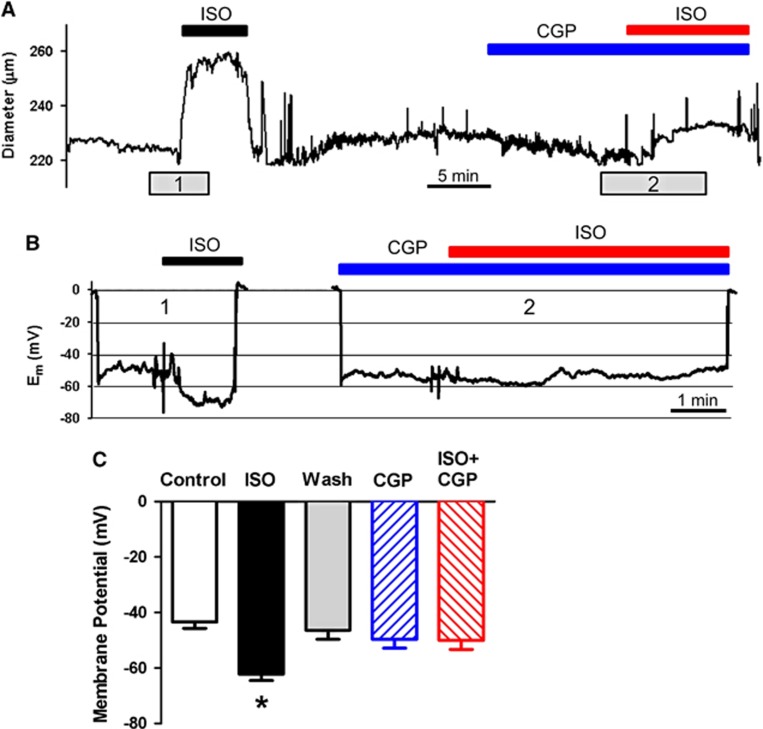
Microelectrode recordings of cVSMCs in pressurized SCA indicated a significant hyperpolarization of the membrane potential (Em) from −43.4±2.4 mV to −62.2±2.3 mV in response to 1 μmol/L of isoproterenol (Figures 5B and 5C), corresponding to vessel dilation (Figures 5A and 5B) and consistent with the activation of hyperpolarizing K+ current. Superior cerebellar arteries returned to baseline diameter and membrane potential after washout (−46.3±3.3 mV). Pretreatment with 30 nmol/L CGP20712 followed by addition of 1 μmol/L isoproterenol showed no change in membrane potential from resting baseline (−49.6±3.2 and −50.0±3.3 mV, respectively) and a suppressed dilation (Figure 5). These findings indicate isoproterenol-stimulated vasodilation of pressurized SCA is β1AR-mediated and leads to an increase in hyperpolarization and dilation from resting tone.
Figure 5.
Effect of β-adrenergic stimulation and β1 blockade on cerebral vascular smooth muscle cell membrane potential. (A and B) Recordings of artery diameter and membrane potential (Em) in rat superior cerebellar artery (SCA) exposed to 1 μmol/L isoproterenol (ISO), 30 nmol/L CGP20712 (CGP) and 1 μmol/L isoproterenol added to CGP. After recording baseline Em, ISO was added to the bath (1). After washout, CGP was added to the bath followed by ISO (2). (C) Average Em values before any addition of drugs (Control), after ISO alone, multiple washouts (Wash), CGP, and ISO in the presence of CGP (ISO+CGP), n=5 arteries each. *Significant difference from Control, P<0.05.
β1-Adrenergic Receptor and Postsynaptic Density-95 Colocalization in Cerebral Vascular Smooth Muscle Cells and Co-Immunoprecipitation in Cerebral Arteries
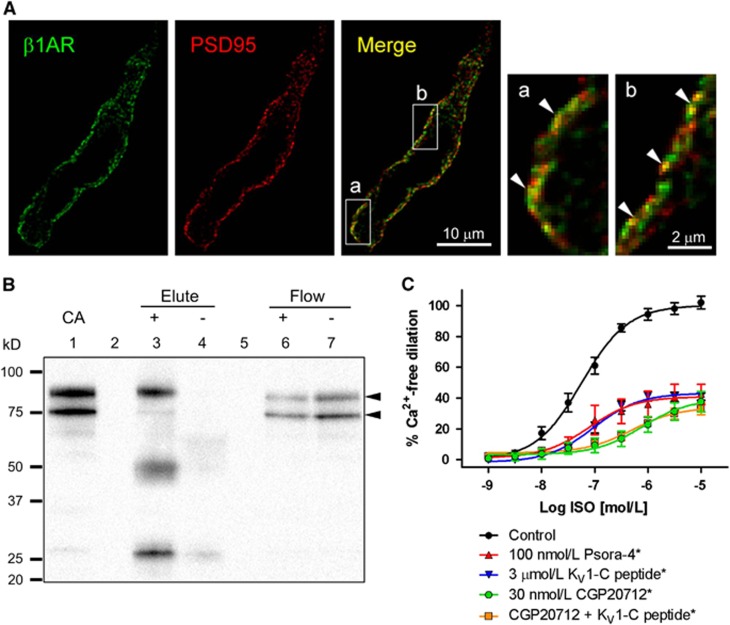
The association of β1AR with PSD95 is suggested by the existence of C-terminus PDZ binding motif of β1AR.9, 10, 15 To confirm surface expression of β1AR and its association with PSD95, isolated cVSMCs were simultaneously labeled with an anti-β1AR antibody and an anti-PSD95 antibody. β1-Adrenergic receptor was detected near the surface of cVSMCs by confocal microscopy (Figure 6A, β1AR). Deconvoluted confocal images of cVSMCs indicated distinct but overlapping distribution of β1AR and PSD95. A scatterplot of individual pixel intensities of the confocal stack stained for PSD95 and β1AR revealed clustering of points along a single straight line (Supplementary Figure S9). Images of five cells from three separate experiments yielded an average Pearson's colocalization coefficient of 0.96±0.01 indicating a very strong correlation between β1AR and PSD95 signal. Direct interaction of β1AR with PSD95 was tested by coIP of PSD95 by β1AR antibody-conjugated beads. The β1AR immunoprecipitation resulted in an immunoreactive signal for the full form of PSD95 (upper band at 95 kDa)11 and a corresponding decrease in the band in the flow-through fraction (Figure 6B) indicating a direct protein–protein interaction. Both the full form (~95 kD) and the proteolytic fragment (~75 kD) of PSD95 are detected in the control CA, but primarily only the full form of PSD95 coimmunoprecipitated with β1AR (Figure 6B and Supplementary Figure S10), similar to KV1.2-PSD95 coIP reported previously.11 Antibody heavy chain (~50 kD) and light chain (~25 kD) co-elution bands were present in the coIP blot (Supplementary Figure S10). Detection of β1AR after pulldown with PSD95 antibody was attempted but confounded by the presence of the overlapping antibody heavy chain band at ~50 kD (data not shown).
Figure 6.
Physical and functional association of β1-adrenergic receptor (β1AR) and postsynaptic density-95 (PSD95) in cerebral arteries (CA). (A) A representative confocal image of a cerebral vascular smooth muscle cell immunostained for β1AR (green) and PSD95 (red). The yellow pixels in the merged image represent colocalization. Magnified images of boxed regions (a, b) illustrate clusters of pixels with strong colocalization (arrowheads). (B) Immunoprecipitation of cerebral artery lysate using anti-β1AR antibody-conjugated bead pull-down (+). β1AR immunoprecipitate (Elute) and flow through (Flow) (lanes 3 and 6) and non-conjugated bead controls (−) (lanes 4 and 7) were probed for PSD95 (arrowheads) on a western blot. Depicted is a representative scan from three similar experiments. Lane 1: untreated cerebral artery lysate (CA). Lanes 2 and 5: empty lanes. (C) Isoproterenol (ISO) response of isolated superior cerebellar arteries (SCA) pretreated with Psora-4 (100 nmol/L), KV1-C peptide (3 μmol/L) and CGP20712 (30 nmol/L), n=5 to 6. *Significant difference from Control, P<0.05.
Effect of KV1 Channel Blockade and Disruption of PSD95-KV1 Binding on Isoproterenol-induced Dilation of Isolated Superior Cerebellar Arteries
To establish a functional role of β1AR-PSD95-KV1 association in isoproterenol-induced CA dilation, isolated, pressurized rat SCA were treated with a selective KV1 channel blocker or a dominant-negative peptide (KV1-C) that disrupts PSD95-KV1 interaction (Figure 6C). KV1-C peptide consists of the final 10 amino acids of the C-terminus of the KV1.2α channel subunit conjugated to an N-terminus HIV-tat sequence, which confers membrane permeability.12 The peptide is designed to compete with KV1 channels for the PDZ-1 binding domain on PSD95. Increasing concentrations of isoproterenol (1 nmol/L to 10 μmol/L) resulted in near-maximal dilation at 1 μmol/L isoproterenol. Application of the KV1 channel blocker Psora-4 (100 nmol/L)11, 21, 27 or PSD95-KV1 disruptive peptide KV1-C (3 μmol/L)12 to the bath produced nearly identical blunting of isoproterenol dilation (42.4±6.6% and 40.9±2.4% Figure 6C). There was no significant shift in EC50 as expected for blockade of a downstream effector (Log EC50: ISO, −7.2±0.1; Psora-4, −7.1±0.3; KV1-C, −7.0±0.1). The β1AR blockade with 30 nmol/L CGP20712 blunted maximal isoproterenol dilation to 37.6±6.5%, while KV1-C peptide plus 30 nmol/L CGP20712 decreased maximal dilation to 33.0±3.9%, a loss of dilation by 64% and 69%. The curves for CGP20712 and CGP+KV1-C were nearly identical at all concentrations and the 5% divergence at the highest concentration was not statistically significant. The EC50 was similarly right-shifted a whole log unit for both treatments (LogEC50: ISO, −7.2±0.1; CGP, −6.1±0.2; CGP+KV1-C, −6.3±0.1). This could reflect a small degree of inverse agonism activity of CGP20712.28 In comparison, 30 μmol/L BaCl2, a blocker of KIR channels, or 3 μmol/L of a negative control peptide (Scm) containing a random order carboxyl terminus12 had no effect on CA dilation to isoproterenol (Supplementary Figure S11A). Reduction of isoproterenol-induced dilation by KV1-C and CGP20712 was not significantly different from the effect produced by 1 μmol/L PKA inhibitor peptide (PKI), nor were there any additive effects of PKI when combined with CGP20712 or KV1-C (Supplementary Figures S12 and S13). CGP20712 or Scm peptide alone did not have a significant effect on baseline CA diameter. However, KV1-C and PKI (alone or in combination with CGP20712) were statistically equivalent in significantly reducing baseline CA diameter (Supplementary Figure S11B). All of the baseline diameter changes by KV1-C and PKI combinations were statistically equivalent to the constriction caused by the selective KV1 channel blocker Psora-4 (−21.8±1.5%) reported previously.12 The PKI results strongly suggest a central role of PKA activation in βAR-mediated CA vasodilation.
Discussion
The ability of scaffolding proteins to interact with G protein-coupled receptors and modulate receptor function and signal transduction has been widely acknowledged and is summarized in a number of review articles.29, 30 The purpose of this study was to explore whether β1ARs express in small CA in rats and function in a signalosome with PSD95 and KV1 channels. Postsynaptic density-95 is abundant in neurons but it has been shown to express in nonneural tissues such as the lung, kidney, liver, colon, skeletal muscle, and heart.31 In vascular smooth muscle, we have recently shown the presence and functional importance of PSD95 in rat cVSMCs where it associates with KV1 channels and regulates the basal tone of CA.11, 12
This association of PSD95 with KV1 channels in cVSMCs enables PKA-mediated phosphorylation and opening of KV1 channels, which maintains the basal tone of CA. Disruption of PSD95-KV1 interaction by a dominant-negative KV1-C peptide resulted in constriction of CA ex vivo and in vivo.12 The linkage of a secondary signaling molecule (PKA) to an effector (KV1 channels) by PSD95 suggests that this may be one part of a larger signal transduction pathway mediated by PSD95. The PKA signaling may be enabled by PSD95-AKAP150 binding that anchor PKA32 for an efficient phosphorylation of KV1 channels. AKAP150 in vascular smooth muscle cells forms punctate structures at the cell surface suggestive of scaffolding clusters.33 β1-Adrenergic receptor have been shown to bind to PSD95 in neurons10 and may signal through PKA to open vascular KV1 channels.14 While the distribution and pharmacological responsiveness of β-AR in CA has been studied for a long time3, 4, 5, 17, 18, 19, 20 and β1AR-mediated dilation has been observed in several mammal species,5, 34, 35 the distribution and physiologic contribution of β1AR throughout the cerebral vasculature remains controversial. Also, how the receptors are coupled to downstream signaling molecules and effectors is poorly understood. Our study provides initial evidence that β1AR-mediated dilation of rat CA requires KV1 channels on PSD95 scaffolding and that a β1AR-PSD95-KV1 complex may represent a critical determinant of CA dilation in response to adrenergic stimulation.
Our initial experiments determined the presence of β1AR in rat CA and the relative contribution of β1AR and β2AR to isoproterenol-stimulated dilation. β1-Adrenergic receptor mRNA and protein was detected in rat CA lysates. In isolated, pressurized CA, isoproterenol produced a concentration-dependent increase in artery diameter. The response of MCA to isoproterenol was statistically equivalent to SCA dilation (Supplementary Figure S6A) suggesting the results from SCA could be more generally applied to other artery branches. The response was not altered by pharmacological blockade or mechanical removal of the endothelium (Supplementary Figure S5). The β1AR selective blocker CGP20712 significantly blunted isoproterenol dilation, while the β2AR selective blocker ICI118551 had minimal effect suggesting that β1AR is primarily responsible for isoproterenol dilation of rat CA. In vivo responses to isoproterenol and blockade with topical CGP20712 were similar to isolated arteries, although maximum dilation to topical isoproterenol was one-third smaller (Figure 3 and Supplementary Figure S7A). There was also an absence of shift in EC50 in response to CGP20712 in the in vivo preparations while CGP20712 caused one log unit right-shift in EC50 in ex vivo preparations. The smaller in vivo response to isoproterenol may be due to the difference in the density of AR subtypes in different size arteries. For example, pial arteries have a significant αAR population compared with smaller penetrating arterioles.18, 20, 36 Additionally, compensatory autoregulation of CA, distribution of drugs to adjacent tissues, and metabolism of drugs may all contribute to a smaller dilation response in vivo that are not factors in isolated arteries.
In the present study, we also examined the functional effect of β1AR stimulation by norepinephrine, an endogenous mixed adrenergic agonist. Norepinephrine in isolated CA produced a concentration-dependent dilation similar to isoproterenol but with only about half the maximum dilation. The β2AR blocker ICI1118551 had no effect on norepinephrine-induced vasodilation, while the β1AR blocker CGP20712 reversed norepinephrine dilation to constriction. The presence of αAR influence in rat CA was revealed in our experiments by a doubling of norepinephrine-induced dilation in the presence of the αAR blocker phentolamine. Responses to topical norepinephrine and β1AR blockade in vivo (Figure 4B) were only one-third of the dilation induced by isoproterenol (Figure 3B), presumably due to the concurrent activation of αAR. The in vivo results highlight the potentially-important implication of beta blockade by reversing norepinephrine-induced dilation to constriction in small branches of the MCA, which may exacerbate disease states associated with impaired cerebral blood flow.
The βAR activation by isoproterenol has been shown to cause membrane hyperpolarization in vascular smooth muscle.14, 37, 38 In our experiments, application of isoproterenol to isolated, pressurized SCA resulted in vasodilation and concurrent membrane hyperpolarization of cVSMCs. Pretreatment with CGP20712 significantly blunted isoproterenol-induced vasodilation and ablated membrane hyperpolarization suggesting that both effects are mediated by β1AR. A small fraction of isoproterenol-induced dilation remained in the presence of CGP20712 although the membrane potential was unchanged (Figures 2A and 5). This residual vasodilation likely reflects the effects of global cAMP elevation at high isoproterenol concentrations and/or a contribution from β2AR. A high concentration of isoproterenol (1 μmol/L) can propagate adenylyl cyclase-produced cAMP beyond the vicinity of the receptor and act on distal subcellular compartments. Additionally, although β2AR does not directly form complexes with PSD95, our results cannot completely rule out the expression and contribution of β2ARs in cVSMCs.
The isoproterenol-mediated vasodilation was significantly blunted by a highly-selective KV1 channel blocker, Psroa4, and the dominant-negative KV1-C peptide,12 suggesting that an increase in KV1 channel activity and K+ efflux constitutes a major part of the β1AR-mediated vasodilatory pathway. The idea of KV1 channels being the selective target of β1AR activation in CA is consistent with the results of Hong et al.39 In that study, isoproterenol-induced vasodilation and increases in cAMP production in rat pial arterioles were not affected by glibenclamide, a KATP channel blocker, or charybdotoxin, a KCA channel blocker.39 In addition, 30 μmol/L BaCl2, a blocker of KIR channels, did not alter isoproterenol-induced dilation (Supplementary Figure S11A). These results, however, do not exclude potential contributions by other K+ channels to isoproterenol-induced vasodilation.
To determine whether β1ARs colocalize with PSD95 at the plasma membrane and are functionally coupled to KV1 channels, the spatial association of β1ARs and PSD95 was explored with confocal microscopy and coIP. Their functional coupling to KV1 channels was examined by pharmacological characterization of the response of SCA to isoproterenol. β1-Adrenergic receptor was detected near the surface of cVSMCs by confocal microscopy, which indicated strong colocalization with PSD95 (Pearson's coefficient 0.96±0.01, range −1 to +1). However, colocalization of two proteins using immunocytochemistry cannot unequivocally prove the interaction of two proteins as the resolution limit of confocal microscopy (300 to 400 nm) far exceeds the interaction distance of proteins (~5 nm). Coimmunoprecipitation of PSD95 by anti-β1AR antibody revealed a direct physical interaction of β1AR with PSD95 (Figure 6B and Supplementary Figure S10). Isoproterenol-induced vasodilation was severely blunted by Psora4, a highly selective KV1 channel blocker that does not block other KV channels or large-conductance KCa channels.11, 21, 27 The disruption of PSD95-KV1 channel binding by the competing dominant-negative peptide KV1-C produced a nearly identical isoproterenol concentration-response curve as Psora-4. KV1-C peptide is a membrane-permeable peptide that contains the same carboxyl terminus PDZ motif as the KV1.2 channel α-subunit.12 Blockade of β1AR with CGP20712 produced a similar maximal reduction of isoproterenol-induced dilation, although the concentration-response curve was slightly shifted right. This could reflect a small degree of inverse agonism activity of CGP20712 as previously reported by others.28 The effect of CGP20712 alone or in combination with KV1-C peptide was the same and not additive suggesting that the PSD95-KV1 interaction may exist downstream of β1AR activation. Additionally, treatment of SCA with PKA inhibitor peptide (PKI) reduced basal diameter and isoproterenol-induced dilation in an equivalent and nonadditive manner as direct β1AR blockade with CGP20712 or disruption of PSD95-KV1 channel binding by KV1-C peptide (Supplementary Figures S11–S13). This is consistent with the proposition that PKA signaling is responsible for β1AR-induced vasodilation of CA through activation of KV1 channels. Aiello et al37 also showed the involvement of β1AR, PKA, and K+ channels in isoproterenol-induced dilation in rabbit portal veins. Their study was the first to show that delayed rectifier K+ current was enhanced by a signal transduction mechanism involving β1AR and PKA. In that study, however, whether the target of PKA phosphorylation was on the ion channel or an associated regulator protein was not determined.37 In this context, our previous work examining the interaction of KV1.2α channel subunits and PSD95 found that PSD95 facilitated basal PKA-phosphorylation of KV1 channels in rat cVSMCs.12
There are several limitations of the present study that should be acknowledged. First, although we have provided evidence of functional coupling of β1AR-KV1 channels via PSD95 and the pairwise interactions of β1AR-PSD95 and PSD95-KV1 channels,11, 12 whether β1AR and KV1 channels can bind to PSD95 simultaneously remains to be directly determined. Second, our demonstration of a PSD95-mediated signaling pathway was limited to a pharmacological approach focusing on KV1 and β1AR function rather than PSD95. This limitation stems from the inherent nature of scaffolding proteins. Since scaffolding proteins have no intrinsic activity of their own, measurements of scaffolding function are indirect and focus on functional changes in the associated proteins.29 Third, signaling pathways on scaffolding proteins can be very complex29 and drug treatments may trigger unknown nonspecific effects. For example, there are approximately 440 PDZ domains in the human proteasome40 and PSD95 alone is known to associate with at least 50 different proteins.9 Although individual PDZ binding interactions are highly selective to particular PDZ motifs, high concentrations of KV1-C peptide may directly target other PDZ domains. However, in this study and the previous study of PSD95 mediated PKA-phosphorylation of KV1 channels,11 the effects of KV1-C in cVSMCs correlate very closely to the effects of specific blockers of KV1 and β1AR alone.12 Fourth, we did not directly test whether ~20% baseline constriction caused by Psora4 or KV1-C peptide per se attenuates isoproterenol-induced dilation, as we could not locate an agent that constricts CA to a similar degree without affecting KV1 channels. Finally, we did not determine whether β1AR is expressed in the endothelial cells of CA. While all three AR subtypes have been found in mesenteric resistance arteries and linked to the endothelium NO/cGMP pathway,41, 42 endothelium dependence of βAR-mediated dilation in CA is less clear. For example, Hempelmann and Ziegler43 observed endothelium-dependent norepinephrine-induced relaxation of isolated rat basilar arteries in tension recording. However, Kitazono et al44 reported norepinephrine-induced dilation of rat basilar arteries in vivo was not altered by L-NAME and indomethacin. In our hands, both the presence of L-NAME and indomethacin, and the physical removal of endothelial cells did not alter β1AR-mediatated dilation of CA (Supplementary Figure S5).
While the present study has a number of limitations due to its scope and the nature of scaffolding proteins, it does have the advantage of being conducted in endogenous tissue ex vivo and in vivo. Most studies of PDZ scaffolding protein interactions with G protein-coupled receptors are performed in heterologous overexpression systems. The lack of physiologic context inherent in heterologous expression systems makes determining interaction specificity between scaffolding proteins and target proteins difficult. For example, studies in cardiomyocyte-like H9c2 cells showed that overexpression of β1AR and β2AR saturates the binding capacity of scaffolding proteins that locally confine the receptors leading to an increase in membrane diffusion of unbound receptors. Similarly, overexpression of AKAPs disrupted PDZ-receptor complexes resulting in increased membrane mobility of βARs. These results suggest that overexpression of proteins in heterologous expression systems may not fully reproduce physiologic interaction of receptors and scaffolding proteins.45 The in vivo studies confirmed the physiologic relevance of β1AR-mediated dilation in intact small CA branches, while ex vivo studies allowed for the dissection of the signaling pathway without the confounding effects of other tissues and feedback mechanisms.
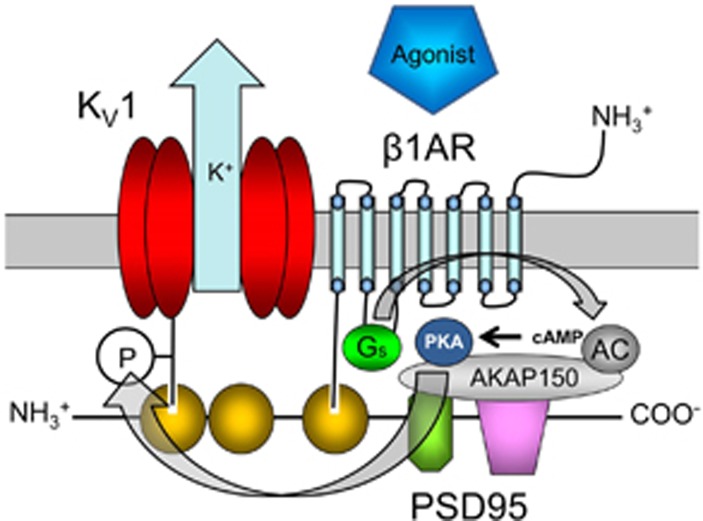
In conclusion, we propose that β1AR-mediated dilation of rat CA requires Shaker-type voltage-gated K+ (KV1) channels on a PSD95 scaffold (Figure 7). β1-Adrenergic receptor and PSD95 colocalize at the plasma membrane of cVSMCs and coimmunoprecipitate in CA. Isoproterenol-induced dilation of CA is predominantly mediated by β1AR and acts by hyperpolarizing cVSMCs. Disruption of PSD95-KV1 binding by a dominant-negative peptide, blockade of KV1 channels and β1AR similarly blunt isoproterenol-induced dilation of SCA. The effect of β1AR blockade on isoproterenol- and norepinephrine-induced dilation of CA is consistent between ex vivo and physiologically intact in vivo preparations. Our results suggest that the β1AR-PSD95-KV1 signalosome may be a critical contributor to the regulation of membrane potential and contractility of CA by endogenous catecholamines or exogenous agonists. Dysfunction of the β1AR-PSD95-KV1 complex in CA could potentially contribute to abnormal cerebral blood flow in conditions such as stroke and vascular dementia, which may be exacerbated by common drug therapies with βAR blockers.
Figure 7.
Proposed β1 adrenergic receptor (β1AR)-PSD95-KV1 vasodilator complex in cerebral vascular smooth muscle cells. Carboxyl terminus binding of β1AR and KV1 channels to PDZ domains on PSD95 scaffolding protein creates a receptor-effector signaling complex. Agonist binding to β1AR initiates the GS, adenylyl cyclase (AC), protein kinase A (PKA) cascade. Activation of this second-messenger cascade leads to PKA phosphorylation of KV1 channels (P), enhancing their open probability and vasodilatory effect. PDZ domains (yellow), SH3 domain (green octagon), guanylate kinase domain (purple), A-kinase anchoring protein (AKAP150), cyclic adenosine monophosphate (cAMP). Involvement of gray-colored proteins has not been tested directly in this manuscript but is speculated based on reported associations in other cell types. For simplicity, PSD95 is depicted linearly to illustrate proposed association of the receptor, signaling proteins, and ion channels. This two-dimensional schematic may not represent the actual relative positions of components in three-dimensional space.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author Contributions
Design (CLM, BKJ, SWR), data acquisition and analysis (CLM, SJM, HMH, PLN, DJ, BKJ, SWR; all), data interpretation (CLM, SJM, BKJ, SWR), drafting and revision (all), final approval (all).
This study was supported by American Heart Association grant 13PRE17070035 (CLM), National Institutes of Health grant R01-HL097107 (SWR), and Institutional Hornick Award (SWR).
Supplementary Material
References
- 1Cipolla MJ. The cerebral circulation. Morgan & Claypool Life Sciences: San Rafael, CA. 2009. [PubMed] [Google Scholar]
- 2Toda N, Okamura T. Cerebral vasodilators. Jpn J Pharmacol 1998; 76: 349–367. [DOI] [PubMed] [Google Scholar]
- 3Gorshkova OP, Shuvaeva VN, Kostylev AV, Dvoretsky DP. Adrenoreactivity of rat pial arteries under conditions of stabilized systemic blood pressure. Bull Exp Biol Med 2011; 151: 553–555. [DOI] [PubMed] [Google Scholar]
- 4Winquist RJ, Bohr DF. Characterization of the rat basilar artery in vitro. Experientia 1982; 38: 1187–1188. [DOI] [PubMed] [Google Scholar]
- 5Winquist RJ, Webb RC, Bohr DF. Relaxation to transmural nerve stimulation and exogenously added norepinephrine in porcine cerebral vessels. A study utilizing cerebrovascular intrinsic tone. Circ Res 1982; 51: 769–776. [DOI] [PubMed] [Google Scholar]
- 6Anderson JM. Cell signalling: MAGUK magic. Curr Biol 1996; 6: 382–384. [DOI] [PubMed] [Google Scholar]
- 7Christopherson KS, Sweeney NT, Craven SE, Kang R, El-Husseini Ael D, Bredt DS. Lipid- and protein-mediated multimerization of PSD-95: implications for receptor clustering and assembly of synaptic protein networks. J Cell Sci 2003; 116 (Pt 15): 3213–3219. [DOI] [PubMed] [Google Scholar]
- 8Hsueh YP, Sheng M. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel KV1.4. J Biol Chem 1999; 274: 532–536. [DOI] [PubMed] [Google Scholar]
- 9Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 2001; 24: 1–29. [DOI] [PubMed] [Google Scholar]
- 10Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ et al. Beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem 2000; 275: 38659–38666. [DOI] [PubMed] [Google Scholar]
- 11Joseph BK, Thakali KM, Pathan AR, Kang E, Rusch NJ, Rhee SW. Postsynaptic density-95 scaffolding of Shaker-type K(+) channels in smooth muscle cells regulates the diameter of cerebral arteries. J Physiol 2011; 589 (Pt 21): 5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Moore CL, Nelson PL, Parelkar NK, Rusch NJ, Rhee SW. Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries. Circ Res 2014; 114: 1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of b-adrenergic receptor subtypes in blood vessels of knockout mice lacking b1- or b2-adrenergic receptors. Mol Pharmacol 2001; 60: 955–962. [DOI] [PubMed] [Google Scholar]
- 14Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol 1998; 275 (2 Pt 2): H448–H459. [DOI] [PubMed] [Google Scholar]
- 15Hall RA. Beta-adrenergic receptors and their interacting proteins. Semin Cell Dev Biol 2004; 15: 281–288. [DOI] [PubMed] [Google Scholar]
- 16He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X et al. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem 2006; 281: 2820–2827. [DOI] [PubMed] [Google Scholar]
- 17Alexander E, 3rd, Friedman AH. The identification of adrenergic receptors in human pial membranes. Neurosurgery 1990; 27: 52–59. [DOI] [PubMed] [Google Scholar]
- 18Mayhan WG. Responses of cerebral arterioles to activation of beta-adrenergic receptors during diabetes mellitus. Stroke 1994; 25: 141–146. [DOI] [PubMed] [Google Scholar]
- 19Tsukahara T, Taniguchi T, Shimohama S, Fujiwara M, Handa H. Characterization of beta adrenergic receptors in human cerebral arteries and alteration of the receptors after subarachnoid hemorrhage. Stroke 1986; 17: 202–207. [DOI] [PubMed] [Google Scholar]
- 20Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 1995; 68: 473–501. [DOI] [PubMed] [Google Scholar]
- 21Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT et al. Loss of cerebrovascular Shaker-type K(+) channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol 2009; 297: H293–H303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation 1997; 4: 35–50. [DOI] [PubMed] [Google Scholar]
- 23Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 2006; 224 (Pt 3): 213–232. [DOI] [PubMed] [Google Scholar]
- 24Dooley DJ, Bittiger H. Quantitative assessment of central beta 1- and beta 2-adrenoceptor regulation using CGP 20712A. J Pharmacol Methods 1987; 18: 131–136. [DOI] [PubMed] [Google Scholar]
- 25Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther 1999; 13: 123–126. [DOI] [PubMed] [Google Scholar]
- 27Marzian S, Stansfeld PJ, Rapedius M, Rinne S, Nematian-Ardestani E, Abbruzzese JL et al. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat Chem Biol 2013; 9: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Janssens K, Boussemaere M, Wagner S, Kopka K, Denef C. Beta1-adrenoceptors in rat anterior pituitary may be constitutively active. Inverse agonism of CGP 20712A on basal 3',5'-cyclic adenosine 5'-monophosphate levels Endocrinology 2008; 149: 2391–2402. [DOI] [PubMed] [Google Scholar]
- 29Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 2002; 91: 672–680. [DOI] [PubMed] [Google Scholar]
- 30Perino A, Ghigo A, Scott JD, Hirsch E. Anchoring proteins as regulators of signaling pathways. Circ Res 2012; 111: 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Stathakis DG, Hoover KB, You Z, Bryant PJ. Human postsynaptic density-95 (PSD95): location of the gene (DLG4) and possible function in nonneural as well as in neural tissues. Genomics 1997; 44: 71–82. [DOI] [PubMed] [Google Scholar]
- 32Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 2000; 27: 107–119. [DOI] [PubMed] [Google Scholar]
- 33Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 2008; 102: e1–e11. [DOI] [PubMed] [Google Scholar]
- 34Ayajiki K, Toda N. Regional difference in the response mediated by beta 1-adrenoceptor subtype in bovine cerebral arteries. J Cereb Blood Flow Metab 1992; 12: 507–513. [DOI] [PubMed] [Google Scholar]
- 35Lagaud GJ, Skarsgard PL, Laher I, van Breemen C. Heterogeneity of endothelium-dependent vasodilation in pressurized cerebral and small mesenteric resistance arteries of the rat. J Pharmacol Exp Ther 1999; 290: 832–839. [PubMed] [Google Scholar]
- 36Sercombe R, Hardebo JE, Kahrstrom J, Seylaz J. Amine-induced responses of pial and penetrating cerebral arteries: evidence for heterogeneous responses. J Cereb Blood Flow Metab 1990; 10: 808–818. [DOI] [PubMed] [Google Scholar]
- 37Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol 1995; 268 (2 Pt 2): H926–H934. [DOI] [PubMed] [Google Scholar]
- 38Fujioka M, Suzuki H. Effects of amosulalol on the electrical responses of guinea-pig vascular smooth muscle to adrenoceptor activation. Br J Pharmacol 1985; 84: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Hong KW, Yoo SE, Yu SS, Lee JY, Rhim BY. Pharmacological coupling and functional role for CGRP receptors in the vasodilation of rat pial arterioles. Am J Physiol 1996; 270 (1 Pt 2): H317–H323. [DOI] [PubMed] [Google Scholar]
- 40Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem 2002; 277: 5699–5702. [DOI] [PubMed] [Google Scholar]
- 41Figueroa XF, Poblete I, Fernandez R, Pedemonte C, Cortes V, Huidobro-Toro JP. NO production and eNOS phosphorylation induced by epinephrine through the activation of beta-adrenoceptors. Am J Physiol Heart Circ Physiol 2009; 297: H134–H143. [DOI] [PubMed] [Google Scholar]
- 42Flacco N, Segura V, Perez-Aso M, Estrada S, Seller JF, Jimenez-Altayo F et al. Different beta-adrenoceptor subtypes coupling to cAMP or NO/cGMP pathways: implications in the relaxant response of rat conductance and resistance vessels. Br J Pharmacol 2013; 169: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Hempelmann RG, Ziegler A. Endothelium-dependent noradrenaline-induced relaxation of rat isolated cerebral arteries: pharmacological characterization of receptor subtypes involved. Br J Pharmacol 1993; 110: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. Am J Physiol 1993; 264 (1 Pt 2): H178–H182. [DOI] [PubMed] [Google Scholar]
- 45Valentine CD, Haggie PM. Confinement of beta(1)- and beta(2)-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell 2011; 22: 2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.