Abstract
Background
Flavonoids and other polyphenols play a protective role in liver diseases and possess a high antioxidant capacity.
Objective
To compare and evaluate the antioxidant and hepatotoprotective activities of 4 deserts plants, Fagonia indica Burm. f., Calotropis procera R.Br., Zygophylum hamiense Schweinf. and Salsola imbricata Forssk. in correlation to their composition especially their phenolic content.
Methods
The influence of extracting solvent on total phenolic and flavonoidal contents was assessed spectrophotometrically. The flavonoid and other polyphenolic components of the methanol extracts were analyzed by RP-HPLC. DPPH radical scavenging potential of the different extracts was estimated. The hepatoprotective and antioxidant activities of the extracts against CCl4-induced hepatotoxicity in mice were evaluated.
Results
The flavonol quercitrin and rosmarinic acid were major in the F. indica, C. procera and S. imbricata samples, while rutin prevailed in that of Z. hamiense. The ethanolic and methanolic extracts showed noticeable DPPH radical-scavenging activity as compared to ascorbic acid. Assessment of liver enzymes revealed that oral administration of the extracts did not show any evidence of hepatotoxicity. Moreover, protection against CCl4-induced liver damage was evident upon administration of three plants extracts namely, F. indica, C. procera and S. imbricata.
Conclusion
Overall, hepatotoxicity induced by CCl4 was effectively prevented by the three plants extracts through scavenging of free radicals and by boosting the antioxidant capacity of the liver. The protective effect of the plants could be attributed to their high quercitrin and rosmarinic acid contents.
Keywords: Antioxidant, Flavonoids, Hepatoprotective, Phenolic acids
Background
Human beings are daily exposed to various compounds that can cause serious diseases either per se or through their metabolic activation to highly reactive substances such as reactive oxygen species (ROS). Free radical induced lipid peroxidation is regarded as one of the main causes of cell membrane damage leading to various pathological conditions [1, 2]. Liver disorders are considered among the major world health problems [3]. Despite their prevalence, morbidity and mortality rates, their current medical management is still considered inadequate. Until now, no therapy shows complete success in preventing the disease progression [4]. Besides, the newly developed drugs used in management of chronic liver diseases are usually associated with various, and sometime intolerable, side effects [5]. Consequently, medicinal plants, especially those with traditional use, have always been considered as a rich source of new effective drugs which could help in ameliorating liver conditions.
Among plant metabolites, phenolics are reputed to play a noticeable protective role against several health disorders [6]. Phenolics possess various biological activities, for instance, antiulcer, anti-inflammatory [7], antidiabetic [8], antioxidant, cytotoxic and antitumor [9, 10].
Fagonia indica Burm. f. (Mushikka or white spine) (Zygophyllaceae) is a widely distributed plant in the deserts of Asia and Africa. It has been reported as medicinal herb in the scientific literature. In an earlier study, the main author reported that the plant could be considered as safe and that it contained a variety of bioactive flavonoids, sterols and triterpenoids; its alcoholic extract was found to exhibit antitumor, antimicrobial and analgesic activities [11]. Furthermore, the methanolic extract of an Indian sample of the plant was proven to exert a hepatoprotective effect in rats; however, the mechanism of action has not yet been explored [12].
Calotropis procera R. Br. (Asclepiadaceae), known as Giant milkweed and locally called Al-ashkhar [13], has been used for treating various diseases like rheumatism, filariasis and skin disorders [14] and its leaf to treat jaundice [15]. The flowers extract have been used for treating spleen, liver and abdomen diseases [16]. Additionally, various extracts of its different parts showed antibacterial and in-vitro and in-vivo antioxidant activities [17–20]. Earlier phytochemical investigation of C. procera revealed the presence of cardenolides, flavonoids, steroids and saponins [21, 22]. The composition of the volatiles, lipoids and flavonoids of its flowers were previously investigated by the author [23].
Zygophyllum species (Family Zygophyllaceae) are used as anthelmintic and for management of diabetes mellitus [24, 25]. The aqueous extract of Zygophyllum album showed in vivo antihyperglycemic, antioxidant and antihyperlipidemic effects [26] as well in-vitro and in-vivo antioxidant properties and phenolic contents of Zygophyllum species were investigated [27–29]. Zygophyllum hamiense Schweinf. spreads largely along the Arabian Gulf area and grows on salt accumulated land. The dead trees are commonly used as firewood and the sprouts as camel food [30]. Yet, there are no available reports regarding either the composition or biological activities of the Zygophyllum hamiense Schweinf.
Genus Salsola (Family Chenopodiaceae or Amaranthaceae) exhibited significant in-vitro antioxidant activities [31–33]. Flavonoid and other phenolic compounds from different species of Salsola have been reported. In addition, triterpenes with significant antioxidant activity were isolated [34, 35]. Salsola imbricata Forssk. (Arabic names: Harm), is a shrub wild growing in Middle East deserts; it is distributed throughout Central and Southwest Asia, North Africa, and Mediterranean countries [36, 37]. Previous phytochemical investigations and biological study of the plant were limited. Two triterpenoidal saponin glycosides were isolated and identified from the roots of the Egyptian plant [38]. The phenolic profile of the alcoholic extract of the plant was analyzed and its contraceptive effect in male albino rats previously evaluated by the authors [39].
The selected plants are growing and existing in the deserts. The deserts plants almost contain variety of secondary metabolites like flavonoids and phenolic acids to protect themselves from herbivores. Thus it was valuable and interesting to perform a comparative study on some selected desert plants from different genus, Fagonia indica Burm. f., Calotropis procera R.Br., Zygophylum hamiense Schweinf. and Salsola imbricata Forssk., and to correlate their biological activities such antioxidant and hepatotoprotective to their phenolic composition.
Methods
Chemicals and drugs
Methanol, ethanol, acetone and ethyl acetate were purchased from Fisher Scientifics (UK) & Scharlam. Carbon tetrachloride (CCl4), 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium carboxymethylcellulose (CMC), Biochemical kits for determination of glutathione peroxidase (GPx), superoxide dismutase (SOD), Catalase (CAT), Thiobarbituric acid reactive substances (TBARS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Folin-Ciocalteu reagent was obtained from Merck (Darmstadt, Germany). All other chemicals were of analytical grade.
Plants material
Whole plants of Fagonia indica Burm. f., Zygophylum hamiense Schweinf., Salsola imbricata Forssk. and leaves of Calotropis procera R. Br. were collected during September 2012 from Muhaisnah desert, Dubai, UAE. The samples were kindly identified and authenticated by Prof. Hassnaa Ahmed Hosny, Department of Botany, Faculty of Science, Cairo University, Egypt. Voucher specimens were kept at the Herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Cairo University. Samples, air-dried in shade, were powdered and preserved for further study.
Experimental animals
Acute toxicity
The acute toxicity studies of both F. indica and S. imbricata have been previously reported [11, 39]. Male albino mice weighing 20–25 g (10 per group) were used to estimate the acute toxicity of the other two plants viz., Z. hamiense and C. procera. LD50 was estimated using 50 % death within 72 h following oral administration of the extracts at different doses (250, 500, 1000, 2500 and 5000 mg/kg). The number of animals, which died during this interval, was expressed as a percentile, and the LD50 determined by probit test using a death percent versus doses’ log [40].
Treatment protocol
Eighty four healthy male albino mice of weights ranging from 30-35 g were used. Animals were kept under the same standard hygienic conditions (temperature 22.0 ± 2.0 °C, relative humidity 50–60 %, with 12 h day/night lighting cycle), fed with well-balanced normal diet and water supplied ad libitum. They were left for a period of one week for accommodation before performing the experiments. All animals’ investigations were performed in accordance with the ethical standards for the proper care and use of laboratory animals and upon approval of the Research Ethical Committee of the Dubai Pharmacy College, Dubai, United Arab Emirates.
Plants extracts
The four air-dried powdered plants materials (500 g, each) were exhaustively extracted by cold maceration in 70 % ethanol (3 L X 2). The solvents were evaporated under reduced pressure at 50 °C. The residual weights for F. indica, C. procera,, Z. hamiense and S. imbricata amounted to 80.0, 44.5, 28.0 and 20.0 g, respectively. These dried extractives were saved and used for biological evaluation.
Standardization of the plants extracts
Colorimetric monitoring of phenolic content in different extracting solvents
Solvents of different polarities, namely: 70 % ethanol, methanol, acetone and ethyl acetate were individually used for extraction of the air-dried powdered plant materials (100 g, each).
The efficiency of the extracting solvent was monitored by colorimetric estimation of total phenolic and flavonoid contents using a spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan). All experiments were carried out in triplicate.
The total phenolic contents were determined by using Folin-Ciocalteu reagent as described by Singleton and Rossi [41] and modified by Oktay et al. [42]. Results were expressed as mg/g gallic acid equivalent, calculated on dry weight of plant material; serial dilutions of gallic acid (10, 20, 30, 40, and 50 g/mL) were used for establishment of the calibration curve. Aliquots (1 mL, each) of tested samples and standard were, separately, added to a volumetric flask containing 9 mL of water followed by addition of 1 mL of Folin-Ciocalteu reagent and the reaction mixture was carefully blended by vortex. After 5 min, 10 mL of 7 % sodium carbonate was added to the mixture which was further incubated for 90 min, at room temperature. Finally, the absorbance was determined at 750 nm against the reagent blank.
The total flavonoid content of the prepared extracts was measured, spectrophotometrically, by the aluminum chloride method, quercetin being used as standard, by adopting the procedure described by Dewanto et al. [43]. The plants extracts (0.1 mL each) were added to 0.3 mL distilled water followed by 5 % NaNO2 (0.03 mL) and the reaction mixture was left for 5 min, at 25 °C. Aluminium chloride (0.03 mL, 10 %) was then added and the mixture left for another 5 min, then treated with 0.2 mL of 1 mM NaOH, and finally diluted to 1 mL with water and the absorbance of the yellow colour produced read at 510 nm.
HPLC analysis of phenolics
The phenolic composition of the methanolic extract of S. imbricata was previously analysed by the authors [39]. Methanolic extracts of the other three plants (F. indica, C. procera and Z. hamiense) were investigated in aliquots of 1g each via RP-HPLC on a Hewlett Packard HPLC System (HP 1050HPLCDADw/Data System). Analyses were carried out at operating conditions suitable for detection of either phenolic acids or flavonoids [44, 45]. For determination of phenolic acids, the apparatus was equipped with an Alltima C18 column (particle size 5 mm, 150 × 4.6 mm) and Alltima C18 guard column (5 mm) (Alltech, USA), the UV detector being set at 280 nm. Meanwhile, the separation of flavonoids was carried out on a Hypersil-ODS C18 column (particle size 5 m, 4.6 × 250 mm) and the UV detector was set at 330 nm. All analyses were performed at 35∘C; gradient elution was employed using acetonitrile-acetic acid mixtures as mobile phase, at a flow rate of 1 mL/min, and the injected volume was 10 L for both standard and tested samples. Authentic reference samples were prepared by diluting stock solutions with methanol to afford a 50 g/mL final concentration. Identification of individual components was performed by comparing their retention times with those of the available standards similarly analyzed. Quantification was based on peak area computation using the external standard method. All analyses were carried out in triplicate. Samples were analyzed at 280 and 330 nm, respectively.
Antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The free radical-scavenging activities of the extracts of the four plants (prepared in the following solvents: 70 % ethanol, methanol, acetone, and ethyl acetate) were measured through the hydrogen donating or radical-scavenging ability using the stable DPPH radical. The assay was performed in a 96-well microtiter plate using the modified previously described method [46]. Hundred μl of each of the samples and the standard solutions were mixed with 100 μl of 0.1 mM ethanolic DPPH solution in the wells. The reaction mixtures were shaken vigorously and incubated in dark for 30 min at 37 °C. The absorbance was measured at 517 nm using UV–vis microplate reader. The percentage inhibition (%) of the DPPH radical by the samples was calculated using the following formula:
Where A0 is the absorbance of the control, A1 is the absorbance in the presence of the sample and A2 is the absorbance of the sample under identical conditions as A1 with ethanol instead of DPPH solution. Ascorbic acid (AA) was used as a reference compound. IC50 values were calculated. Samples were analyzed in triplicate.
Experimental design
The residues of the ethanolic extracts for the four plants were suspended in 1 % CMC. The animals were randomly assigned to 14 groups, of 6 animals each (n = 6). Table 1 describes the animal grouping with their corresponding treatment.
Table 1.
Animals groups and corresponding treatment with vehicle, tested samples and carbon tetrachloride
| Oral treatment | Frequency | i.p. CCl4 injection | |
|---|---|---|---|
| Control | Vehicle | daily | - |
| CCl4 control | Vehicle | daily | 1.0ml/kg |
| F. indica extract | 10 mg/kg | Twice daily | - |
| 5mg/kg | daily | 1.0 ml/kg | |
| 10 mg/kg | daily | 1.0 ml/kg | |
| Z. hamiense extract | 500 mg/kg | Twice daily | - |
| 250 mg/kg | daily | 1.0 ml/kg | |
| 500 mg/kg | daily | 1.0 ml/kg | |
| C. procera extract | 200 mg/kg | Twice daily | - |
| 100 mg/kg | daily | 1.0 ml/kg | |
| 200 mg/kg | daily | 1.0 ml/kg | |
| S. imbricata extract | 500 mg/kg | Twice daily | - |
| 250 mg/kg | Daily | 1.0 ml/kg | |
| 500 mg/kg | Daily | 1.0 ml/kg |
The first group served as normal control and during the experiment received vehicle only (1 % CMC). The second group was given 1 % CMC solution for 14 days before CCl4 intoxication and served as a hepatotoxicity control group. For each plant, three groups were devoted; the first was treated with the plant extract twice daily for 14 days while the second and the third groups were given the plant extract in two different doses for 14 days as shown in Table 1. After the 14-days treatment period, hepatic injury was induced by intraperitoneal injection of 1.0 ml/kg of CCl4 and the mice were sacrificed six hours after the last treatment.
Assay of liver enzyme
Blood samples were collected from the hearts with the use of 5 ml sterile syringe individually for each mouse and transferred into non-heparinized tubes immediately and used later for the analyses of liver enzymes: alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP).
Estimation of oxidative parameters
Liver samples were surgically removed from the mice immediately and stored in -80° for further antioxidant enzyme assay including activity of catalase, superoxide dismutase, glutathione peroxidase and TBARS as per the method described by Abu-Gharbieh et al. [47]. The levels of total protein were determined in the serum of experimental animals by using the Lowry method and the bromocresol green method, respectively [48, 49].
Histopathological study
Liver samples were suspended in 10 % formaldehyde for histological evaluation. These tissues were processed and embedded in paraffin wax. Sections of 5 μm in thickness were cut and stained with hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) stains.
Statically analysis
The results were reported as Mean ± Standard Deviation (S.D) from three repeated determination. The data obtained were statistically analyzed using One-way analysis of variance ANOVA, followed by Dunnett’s multiple comparison test (DMCT). P-value of < 0.05 was considered as statistically significant.
Results
Standardization of the plants extracts
Influence of extracting solvent on total phenolic and flavonoid contents
Different solvents were used to select the most efficient, safe and applicable solvent for phenolic compounds extraction as shown in Table 2.
Table 2.
Total Flavonoid and phenolic acid contents of the different extracts of Fagonia indica, Calotropis procera, Zygophyllum hamiense and S.imbricata
| Plant name | Total flavonoid content g quercetin/100 g | Total phenolic content mg GAE/g | ||||||
|---|---|---|---|---|---|---|---|---|
| Methanol extract | Ethanol extract | Acetone extract | Ethyl acetate extract | Methanol extract | Ethanol extract | Acetone extract | Ethyl acetate extract | |
| F. indica | 0.22 | 3.00 | 0.32 | 0.10 | 3.91 | 4.00 | 2.1 | 0.75 |
| C. procera | 0.12 | 0.3 | 0.90 | 0.10 | 3.13 | 4.00 | 4.00 | 0.92 |
| Z. hamiense | 0.38 | 0.14 | 1.48 | 0.11 | 2.60 | 3.61 | 1.20 | 4.00 |
| S. imbricata a | 0.571 | 0.217 | 0.374 | 0.11 | 2.60 | 0.64 | 4.00 | 0.93 |
aResults previously reported [39]
Spectrophotometric evaluation of the total phenolic content (expressed as mg gallic acid equivalent (mg GAE)/g dry plant material) and flavonoid content (as quercetin g/100 g dry plant material) in the extracts of F. indica, C. procera and Z. hamiense revealed variable efficiency. Ethanol was found to be the best solvent for extracting the F. indica sample with highest concentration of phenolics (4 mg GAE/g dry plant wt.) and flavonoids (3 g quercetin (Q) % w/dry plant wt.). Concerning C. procera, the highest flavonoid content was detected when using acetone (0.9 g Q % w/dry wt.), while both ethanol and acetone extracts were found the richest in total phenolics (4 mg GAE/g dry wt.). On the other hand, the maximum flavonoid amount (1.48 %) was extracted with acetone in Z. hamiense, meanwhile ethyl acetate and ethanol appeared of close efficiencies for solubilisation of total phenolics (4 and 3.61 mg GAE/g dry plant wt., respectively). Finally, among the tested plants and including S. imbricata, the most enriched sample in both total phenolics and flavonoids was F. indica (4 mg GAE/g of plant dry wt. and 3 g % w/dry wt., expressed as quercetin respectively).
RP-HPLC profiling of phenolics
RP-HPLC analysis and total phenolic and flavonoid contents of S. imbricata were previously determined [39]. RP-HPLC analyses of the methanolic extracts of the remaining three plants, F. indica, C. procera, and Z. hamiense, allowed the identification and quantitation of several phenolics. Total of 14 components were identified at 280 nm in both C. procera and F. indica (corresponding to 10.297 and 7.955 % of the total composition, respectively) and 13 components in Z. hamiense (corresponding to 19.52 %) as shown in Table 3. Among these, the identified phenolic acids were 10 in F. indica (representing 4.84 %), 9 in C. procera (6.274 %) and 8 in Z. hamiense (11.35 %). Ellagic and non-phenolic benzoic acids (1.2 and 1.02 %) were the prevalent in F. indica while in C. procera, benzoic and salicylic acids (1.59 and 1.53 %) were the major. On the other hand, chlorogenic and gallic acids were predominant in Z. hamiense (2.31 and 2.02 %). In contrast, 9 phenolic acids (9.734 %) with prevalence of coumaric acid (4.251 %) were detected in S. imbricata [39]. On the other hand, by setting the detector at = 330 nm, 8 components were identified in both F. indica and C. procera among which 7 were of flavonoidal nature with major quercitrin (5.29 and 4.16 % respectively) while 5 components only were determined in Z. hamiense among which 4 were of flavonoidal nature with major rutin (10.71 %) while quercitrin was the minor (0.77 %) as shown in Table 4. Alternatively, 7 flavonoidal components with major quercitrin (12.692 %) were previously detected in S. imbricata. Besides, rosmarinic acid was detected in all samples under investigation with relatively appreciable amounts (Table 4).
Table 3.
Phenolics identified by RP-HPLC analysis (at = 280 nm) of the methanolic extracts of Fagonia indica, Calotropis procera, Zygophyllum hamiense and S.imbricata
| Retention time | Identified constituent | Relative area % | |||
|---|---|---|---|---|---|
| F. indica | C. procera | Z. hamiense | S. imbricata a | ||
| 6.81 | Pyrogallol | 0.145 | 0.173 | 1.94 | - |
| 6.92 | Gallic acid | 0.05 | 0.27 | 2.02 | 0.145 |
| 8.235 | Protocatechuic acid | 0.12 | 0.504 | 0.97 | 0.068 |
| 8.444 | Catechin | - | 0.38 | 1.81 | 0.461 |
| 8.593 | Chlorogenic acid | 0.46 | 0.12 | 2.31 | 0.377 |
| 8.950 | Catechol | 0.16 | 0.44 | 2.57 | 0.329 |
| 10.040 | Caffeic acid | 0.25 | - | 1.54 | 1.759 |
| 11.073 | Vanillic acid | 0.37 | 0.82 | 1.37 | 0.286 |
| 11.620 | Ferulic acid | 0.34 | 0.36 | 0.84 | 1.323 |
| 12.466 | Salicylic acid | 0.73 | 1.53 | 1.1 | 1.154 |
| 12.943 | Ellagic acid | 1.20 | 1.20 | - | - |
| 13.127 | Benzoic acid | 1.02 | 1.59 | 1.51 | 2.306 |
| 13.789 | Coumaric acid | 0.51 | 0.52 | 1.2 | 4.251 |
| 14.980 | Cinnamic acid | 0.81 | 0.95 | - | 0.371 |
| 18.657 | Chrysin | 1.79 | 1.44 | 0.34 | 1.074 |
| Total identified constituents | 7.955 | 10.297 | 19.52 | 13.904 | |
aResults previously reported [39]
Table 4.
Phenolics identified by RP-HPLC analysis (at = 330 nm) of the methanolic extracts of Fagonia indica, Calotropis procera, Zygophyllum hamiense and S.imbricata
| Retention time | Identified constituent | Relative area % | |||
|---|---|---|---|---|---|
| F. indica | C. procera | Z. hamiense | S. imricata a | ||
| 3.83 | Quercetin | 0.034 | 0.08 | 0.04 | 0.031 |
| 11.78 | Rosmarinic acid | 2.83 | 2.68 | 2.33 | 2.734 |
| 12.06 | Hesperidin | 1.21 | 2.22 | - | 1.854 |
| 12.44 | Rutin | - | 1.72 | 10.71 | 2.101 |
| 13.267 | Quercitrin | 5.29 | 4.16 | 0.77 | 12.692 |
| 14.576 | Naringenin | 1.36 | 1.15 | 0.94 | 1.300 |
| 14.952 | Hesperitin | 1.41 | - | - | 0.730 |
| 15.147 | Kampferol | 1.61 | 1.5 | - | - |
| 16.167 | Apigenin | 1.00 | 0.21 | - | 0.474 |
| Total identified constituents | 14.744 | 13.72 | 14.79 | 21.916 | |
aResults previously reported [39]
DPPH free radical scavenging activity
DPPH is a stable free radical and its noticeable purple color shows absorption at 517 nm. Antioxidants scavenge the free radical by donating a hydrogen atom and the color of the DPPH assay solution becomes yellowish, resulting in a decrease of the absorbance. DPPH free radical scavenging activity is considered as in vitro screening for possible in vivo antioxidant potentialities.
The four plants were tested and the results are presented in Fig. 1. All extracts were found to be potent DPPH free radical scavengers and the highest activity among all investigated plants samples was observed for the ethanolic and methanolic extracts as shown in Fig. 1.
Fig. 1.
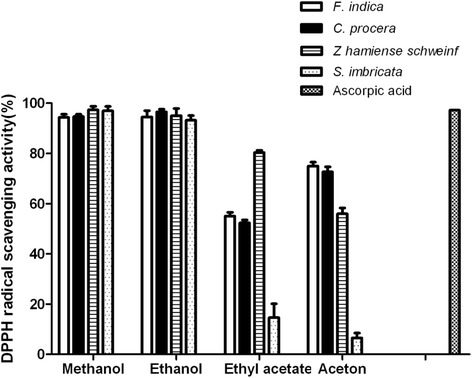
DPPH radical scavenging activity of several plants extracts
Acute toxicity study
The acute toxicity study was essential to evaluate the plants extracts safety and to determine the tested doses.
The LD50 of both F. indica and S. imbricata extracts had been previously reported and they were found to be safe up to 4 and 5 g/kg respectively [11, 39]. Z. hamiense was found to be safe up to dose 5 g/kg while C. procera leaves extract was safe up to 3 g/kg. No signs of morbidity or behavioral changes in any of the treated groups of animals during the period of observation. The safety margin of the ethanolic extracts of the plants under investigation is highly encouraging the biological evaluation.
Effects on liver enzymes and histological findings
The plants extracts were given twice daily over two weeks in order to evaluate any possible hepatotoxicity caused by the plants extracts themselves. Moreover, this was useful to evaluate the effect of the extracts on the antioxidant enzyme system apart from the CCl4 challenge.
Treating the animals twice daily with the plants extracts alone over two weeks, did not cause any significant elevation on both the ALT and AST as shown in Table 5. On the other hand, significant reduction in ALP levels was observed by administration of the ethanolic extracts of the four plants. This indicates that no possible cholestasis occurred at the dose levels tested since a rise in plasma ALP level is usually a characteristic feature in cholestatic liver disease [50]. Moreover, histological assessment revealed that the hepatocytes maintained its architecture with normal glycogen storage. This gave evidence that the four plants did not produce any harmful on the hepatocytes as shown in Fig. 2a. Beside this, treating the animals with the plants extracts did not show any significant enhancement of the antioxidant enzyme system.
Table 5.
Evaluation of the hepatotoxic effect of the plant extracts on the biochemical parameters with twice daily oral administration in mice
| Control | F. indica | Z hamiense | C. procera | S. imbricata | |
|---|---|---|---|---|---|
| ALT | 290.1 ± 54.0 | 350.2 ± 113.6 | 155.3 ± 65.5 | 211.0 ± 35.8 | 253.3 ± 24.0 |
| AST | 850.0 ± 63.2 | 800.0 ± 235.8 | 520.3 ± 220.0 | 404.2 ± 37.2b | 653.3 ± 78.8 |
| ALP | 180.0 ± 20.3 | 113.3 ± 16.7a | 105.6 ± 15.4a | 98.2 ± 9.3b | 60.0 ± 23.1b |
| CAT kU/g protein | 462.0 ± 21.1 | 461.6 ± 14.9 | 428.750 ± 10.3 | 413.950 ± 11.4 | 486.3 ± 18.0 |
| GSH-Px U/g protein | 1.3 ± 0.1 | 1.3 ± 0.05 | 1.3 ± 0.07 | 1.6 ± 0.06 | 1.31 ± 0.14 |
| SOD μg/g protein | 34.6 ± 2.9 | 35.5 ± 2.7 | 32.8 ± 1.1 | 35.5 ± 1.4 | 32.1 ± 1.4 |
| TBARS (nmol/g prot.) | 0.49 ± 0.02 | 0.39 ± .03 | 0.046 ± 0.03 | 0.038 ± 0.02 | 0.035 ± 0.4 |
aSignificant difference (p < 0.05) compared to control group; bSignificant difference (p < 0.01) compared to control group
Fig. 2.
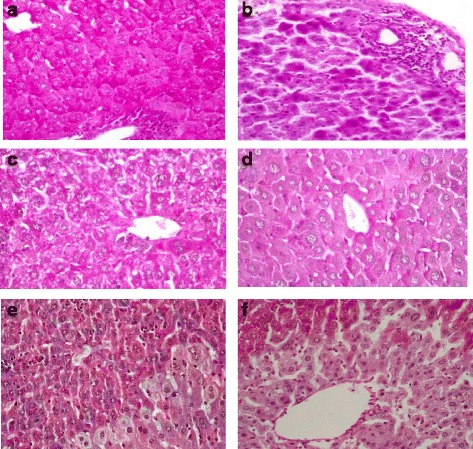
Histopathological findings of mice liver sections (PAS x400); (a) treated with 10 mg/kg F. indica twice daily showing excess glycogen synthesis; (b) negative control group treated with CCl4 alone showing dense periportal and lobular lymphocytic infiltrate with pyknotic nuclei within necrotic hepatocytes in periportal areas and some other cells show degenerative changes; (c) treated with 10mg/kg F. indica and CCl4 showing normal regenerating hepatocytes sometimes binucleated; (d) treated with 200mg/kg C. procera and CCl4 showing normal glycogen storage, stimulates regeneration and renewal of hepatocytes; (e) treated with 500mg/kg S. imbricata and CCl4 showing slight glycogen depletion and (f) treated with Z. hamiense Schweinf and CCl4 showing glycogen depletion in centrizonal area and more glycogen and ballooning degeneration on peripheral zone
Results in Table 6, showed that CCl4 caused sharp and significant elevation in liver enzymes, ALT and AST by 598 % and 204 % respectively compared to control group (p < 0.01), while ALP was not significantly affected. Histological data revealed a dense periportal and lobular lymphocytic infiltrate with diffused pyknotic nuclei within necrotic hepatocytes in periportal areas. Fig. 2b.
Table 6.
Evaluation of the protective effect of the plants extracts on the biochemical parameters of liver in CCl4-induced hepatic damage in mice
| Control | CCl4 control | F. indica | Z hamiense | C. procera | S. imbricata | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg/kg | 10 mg/kg | 250 mg/kg | 500 mg/kg | 100 mg/kg | 200 mg/kg | 250mg/kg | 500mg/kg | |||
| ALT (U/L) | 290.1 ± 54.0 | 1736.7 ± 161.2a | 2166.7 ± 157.8b | 1064.0 ± 264.9c | 1416.7 ± 105.8 | 950.3 ± 84.4 | 1054.3 ± 117.5c | 801.7 ± 76.0c | 1135.0 ± 127.9c | 476.7 ± 151.0c |
| AST(U/L) | 850.0 ± 63.2 | 1736.7 ± 215.1a | 1310.0 ± 181.5b | 926.2 ± 258.6c | 756.7 ± 128.6c | 783.3 ± 80.1c | 787.8 ± 69.6c | 922.7 ± 65.4c | 647.5 ± 62.8c | 530.0 ± 120.8c |
| ALP(U/L) | 180.0 ± 20.3 | 150.2 ± 55.7 | 125.0 ± 22.5 | 124.3 ± 17.4 | 106.7 ± 24.0 | 113.3 ± 29.1 | 51.8 ± 4.7 | 59.5 ± 6.1 c | 110.0 ± 14.7 | 63.3 ± 8.8 |
| CAT kU/g protein | 462.0 ± 21.1 | 241.3 ± 11.4a | 307.2 ± 9.2c | 392.200 ± 9.4c | 225.1 ± 10.5 | 259.1 ± 5.1 | 324.7 ± 25.8b | 335.0 ± 25.0c | 356.2 ± 24.1c | 374.9 ± 10.7c |
| GSH-Px U/g protein | 1.3 ± 0.1 | 0.85 ± 0.06a | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.72 ± 0.1 | 0.94 ± 0.2 | 1.4 ± 0.06 | 1.4 ± 0.06 | 0.91 ± 0.06 | 1.39 ± 0.19b |
| SOD μg/g protein | 34.6 ± 2.9 | 22.0 ± 2.4a | 26.7 ± 1.8 | 32.9 ± 1.6c | 21.1 ± 1.7 | 23.6 ± 1.5 | 22.7 ± 1.2 | 31.5 ± 1.1b | 24.8 ± 0.5 | 26.4 ± 1.3 |
| TBARS (nmol/g prot.) | 0.049 ± 0.02 | 0.54 ± 0.20a | 0.12 ± 0.09c | 0.085 ± 0.05c | 0.42 ± 0.2 | 0.36 ± 0.4 | 0.14 ± 0.07c | 0.06 ± 0.01c | 0.11 ± 0.05c | 0.071 ± 0.02c |
aSignificant difference (p < 0.01) compared to control group; bSignificant difference (p < 0.05) compared to CCl4 treated group; cSignificant difference (p < 0.01) compared to CCl4 treated group
Pre-treating the mice with F. indica, C. procera and S. imricata extracts at the highest and lowest doses for 14 days prior CCl4 administration showed significant reduction in serum levels of ALT, AST but not ALP enzymes in a dose response manner (p-value less than 0.05 and 0.01, respectively). Those findings are supported by the histological features; hepatocytes renewal and regeneration with mild glycogen depletion were observed with F. indica, C. procera and S. imbricata only as shown in Figs. 2c, d and e. On the other hand, pretreating the animals with Z. hamiense extract at both doses showed significant reduction in AST level (p < 0.01) though ALT and ALP levels were not changed (p > 0.05). Furthermore, histological study showed centralized gross glycogen depletion (Fig. 2f).
Effects on antioxidant enzymes and TBARS contents
Hepatotoxicity induced by CCL4 is characterized by suppression of the antioxidant defense system [51–53] and increased lipid peroxidation [51].
Administration of CCl4 markedly depleted the antioxidant enzymes (CAT, GSH-Px and SOD) in the mice livers (Table 6). Nevertheless, CCl4 increased significantly (p < 0.01) the hepatic lipid peroxidation that is expressed by high TBARS content. Whereas the administration of plants extracts twice daily for two weeks did not result in significant enhancement of the antioxidant enzymes nor reduction in the TBARS content as shown in Table 5.
Pretreating the animals with the plants extracts at different doses opposed significantly the reduction in the antioxidant enzymes and reduced markedly the TBARS content induced by CCl4 except for Z. hamiense extract that has no potential effects on the antioxidant enzymes as well as the TBARS content.
Discussion
Liver diseases are one of the major causes of morbidity and mortality and affects people of all ages throughout the world especially in the Arab countries. The drugs that are currently available to treat this condition pose serious drawbacks [5], which justifies the search for new hepatoprotective agents. In this context, the use of plants extracts and isolates therefrom with hepatoprotective properties can provide beneficial means for prevention and treatment of liver conditions.
Alanine aminotransferase (ALT), aspartate aminotransferase (ASL) are present mostly in the hepatic and biliary cells [54]. These enzymes are usually released from the hepatocytes and leak into circulation causing increase in their serum levels under hepatocellular injury or inflammation of the biliary tract cells resulting predominantly in an elevation of the alkaline phosphatase levels. On the other hand, elevation in ALP is usually indicates a cholestatic liver diseases.
Chronic administration of the four plants extracts for two weeks resulted in significant reduction in alkaline phosphatase levels. This shows that no possible cholestasis occurred at the dose levels tested since a rise in plasma ALP level is usually a characteristic finding in cholestatic liver diseases [50]. This was further confirmed by the fact that there were no significant changes in ALT and AST. Accordingly the plants extracts did not exhibit any signs of hepatotoxicity on chronic administration.
In this study, we were focusing on the liver injury that always accompanied by elevated levels of serum hepatic enzymes that are indicative of cellular leakage [55]. The hepatotoxic effects induced by CCl4 are related to its active metabolite trichloromethyl radical, •CCl3, this is manifested by marked elevation in the serum liver enzymes namely AST, ALT, ALP [52]. Antioxidant enzymes, particularly, CAT, GSH-Px and SOD play a vital role in protecting cells against oxidative damage. Administration of CCl4 to the animals leads to induction of hepatic oxidative stress that is characterized by significant decrease in CAT, GSH-Px and SOD activities and increased TBARS content in liver tissue [52].
It was found that phenolic, especially polyphenolic, compounds such as flavonoids are very efficient scavengers of free radicals [56] because of their molecular structures, which include an aromatic ring with hydroxyl groups containing mobile hydrogen. Actually, it is known that the 3',4'-ortho-dihydroxy group in the B-ring and the 5-OH group in the A-ring with a 4-carbonyl group are required for the high antioxidant activity of flavonoids. In addition, the presence of the o-catechol group (3',4' -OH) in the flavonoid-B ring is a determinant for high antioxidant capacities in flavonoids (Fig. 3), [57]. The flavonoid content of Fagonia indica exceeded those of the other plants by 10 to 30 folds. The phenolic content of Salsola imbricata extract was previously reported to be 0.571 % [39]; while Calotropis procera extract showed high phenolic acids content. The frequently used DPPH assay was applied as a first in vitro approach to assess the free radical-scavenging activity of the plants extracts prepared in the different solvents. This evaluation revealed that the methanolic and ethanolic extracts of the four plants exhibited the highest DPPH radical scavenger potential with activity comparable to those of ascorbic acid, a well-known antioxidant. However, for safety and economic considerations, the ethanol (70 %) was selected for further biological study.
Fig. 3.
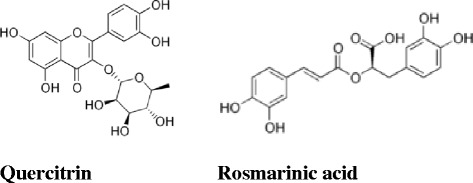
Chemical structures of quercitrin and rosmarinic acid
Dose selection for the subsequent biological study for the three plants (Zygophyllum hamiense, Calotropis procera and Salsola imbricata) was based on their LD50 values (less than 1/10 of LD50). Moreover, the major influencing factors to select smaller doses for Fagonia indica extract (5 and 10 mg/kg) was the extremely high contents of flavonoid and phenolic acid compared to the other plants extracts. Additionally, similar doses of Fagonia indica were tested previously for its analgesic effect [11].
The three plants, Fagonia indica, Calotropis procera and Salsola imbricata possessed high antioxidant and hepatoprotective activities. These effects may be attributed to the presence of high content of different groups of phenolic compounds including flavonoids aglycone and/or glycosides and phenolic acids especially quercetrin glycoside and rosmarinic acid (Fig. 3) that have been earlier reported to exhibit strong antioxidant and hepatoprotective effects [58, 59]. Moreover, hesperidin and its aglycone hesperitin, apigenin and cinnamic acid that exhibited strong antioxidant and hepatoprotective activities [60–64] were detected in all plants extracts except Zygophyllum hamiense of which rutin was the predominant flavonoid (10.71 %). The presence of high rutin concentration in Zygophyllum hamiense extract explains its in vitro free radical scavenging activity and possibly it’s in vivo effect on AST level. Anyway, the plant could not be considered as hepatoprotective since ALT level, that is thought to be more specific for hepatic injury [54], was not significantly improved. Accordingly, the lack of other flavonoids especially quercetrin that presents in the other three plants explains the weak in vivo antioxidant and hepatoprotective activities of Zygophyllum hamiense against CCl4 intoxication.
Interestingly, it was found that although S. imbricata contains the lowest amount of phenolic contents, its efficacy in reducing ALT level in CCL4 treated mice still high. This can be explained by the presence of triterpenoid saponin. Two triterpenoidal saponin glycosides were isolated and identified from the S. imbricata, namely, salisomide and salisoflavan [38], and it is reported in literature that saponins interact with and increase permeability of the mucosal cells in the gut and enhance the absorption of various nutrient [65, 66]. Therefore, saponin enhanced the absorption of phenolic compounds. Additionally, saponins themselves possess antioxidant activity that contributes to efficacy of the phenolic compounds to protect against liver injury induced by CCL4 [67, 68].
Based on that, it was found that F. indica, C. procera and S. imbricata possess hepatoprotective effects by preventing the induction of oxidative stress and enhancing the hepatic antioxidant defences involving CAT, GSH-PX and SOD enzymes and were highly efficient in reducing the TBARS content in liver tissue. Accordingly, the three plants could be added to the growing list of medicinal plants. Further clinical studies are needed to evaluate their clinical significance.
Conclusion
In conclusion, this study evidences the efficiency of the ethanolic extracts of F. indica, C. procera and S. imbricata in preventing the CCl4-induced hepatotoxicity in mice through scavenging of free radicals and by boosting the antioxidant capacity of the liver. The bioactive antioxidant principles detected in the extracts are probably responsible for this hepatoprotective effect. Therefore the parent plants could be considered as a potential source of safe protective from liver diseases or for reduction of undesirable hepatotoxic side effects of some drugs.
Acknowledgments
The authors want to thank Professor Saeed Ahmed Khan - Dean of Dubai Pharmacy College for his support in all the research steps.
Footnotes
Competing interests
The authors declare that there is no conflict of interests.
Authors’ contributions
Naglaa Gamil and Eman Abu-Gharbieh were responsible for designing the experimental work and manuscript writing and publication. Specifically, Naglaa Gamil was responsible for the idea, plants extraction, interpreted the HPLC results and carried the quantitative analysis of total phenolic and flavonoid. Eman Abu-Gharbieh designed and carried out the the in-vitro, in-vivo experiments, statistical data and results analysis. Fatehia A.Bayoumi carried out the histological study. All authors approved the final article.
References
- 1.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 2.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 4.Bruck R, Hershkoviz R, Lider O, Aeed H, Zaidel L, Matas Z, et al. Inhibition of experimentally-induced liver cirrhosis in rats by a nonpeptidic mimetic of the extracellular matrix-associated Arg-Gly-Asp epitope. J Hepatol. 1996;24:731–738. doi: 10.1016/S0168-8278(96)80270-4. [DOI] [PubMed] [Google Scholar]
- 5.Fujisawa K, Tandon BN. Therapeutic Approach to the Chronic Active Liver Disease: Summary of a Satellite Symposium. In: Nishioka K, Suzuki H, Mishiro S, Oda T (eds). Viral Hepatitis and Liver Disease. Tokyo: Springer Japan; 1994;662–5.
- 6.Hung TM, Na M, Thuong PT, Su ND, Sok D, Song KS, et al. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J Ethnopharmacol. 2006;108:188–192. doi: 10.1016/j.jep.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Son K, Chang H, Do J, Jung K, Kang S, et al. Antiinflammatory activity of naturally occurring flavone and flavonol glycosides. Arch Pharm Res. 1993;16:25–28. doi: 10.1007/BF02974123. [DOI] [Google Scholar]
- 8.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–364. doi: 10.1016/S1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 9.Vivian Cody, Elliott Middleton, Jeffrey B.Harborne. Plant flavonoids in biology and medicine. Biochemical, pharmacological, and structure-activity relationships. Proceedings of a symposium. Buffalo, New York, July 22–26, 1985. 1985. Ref Type: Conference Proceeding [PubMed]
- 10.Shaikh R, Pund M, Dawane A, Iliyas S. Evaluation of Anticancer, Antioxidant, and Possible Anti-inflammatory Properties of Selected Medicinal Plants Used in Indian Traditional Medication. J Tradit Complement Med. 2014;4:253–257. doi: 10.4103/2225-4110.128904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naglaa GS, Amina M. Constituents and analgesic activity of the alcoholic extract of Fagonia indica Burm F. New Egypt J Med. 2009;40:357–371. [Google Scholar]
- 12.Bagban IM, Roy SP, Chaudhary A, Das SK, Gohil KJ, Bhandari KK. Hepatoprotective activity of the methanolic extract of Fagonia indica Burm in carbon tetra chloride induced hepatotoxicity in albino rats. Asian Pac J Trop Biomed. 2012;2:S1457–60.
- 13.Rameshkumar S, Eswaran K. Ecology, Utilization and Coastal Management of Salt Tolerant Plants (Halophytes And Mangroves) of Mypad Coastal Regions, Andhra Pradesh, India. Int J Environ Biol. 2013;3:1–8. [Google Scholar]
- 14.Agharkar SP. Medicinal plant of Bombay presidency. India: Scientific publication; 1991. [Google Scholar]
- 15.Murti Y, Yogi B, Pathak D. Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br. (Asclepiadaceae) Int J Ayurveda Res. 2010;1:14–17. doi: 10.4103/0974-7788.59938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandra Setty S, Quereshi AA, Viswanath Swamy AHM, Patil T, Prakash T, Prabhu K, et al. Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia. 2007;78:451–454. doi: 10.1016/j.fitote.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Gupta A, Pandey AK. Calotropis procera Root Extract Has the Capability to Combat Free Radical Mediated Damage. ISRN Pharmacol. 2013;2013:691372. doi: 10.1155/2013/691372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary P, de Araújo Viana C, Ramos MV, Kumar VL. Antiedematogenic and antioxidant properties of high molecular weight protein sub-fraction of Calotropis procera latex in rat. J Basic Clin Pharm. 2015;6:69–73. doi: 10.4103/0976-0105.152098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar VL, Padhy BM. Protective effect of aqueous suspension of dried latex of Calotropis procera against oxidative stress and renal damage in diabetic rats. Biocell. 2011;35:63–69. [PubMed] [Google Scholar]
- 20.Mohamed MA, Hamed MM, Ahmed WS, Abdou AM. Antioxidant and cytotoxic flavonols from Calotropis procera. Z Naturforsch C. 2011;66:547–554. doi: 10.5560/ZNC.2011.66c0547. [DOI] [PubMed] [Google Scholar]
- 21.Moustafa AM, Ahmed SH, Nabil ZI, Hussein AA, Omran MA. Extraction and phytochemical investigation of Calotropis procera: effect of plant extracts on the activity of diverse muscles. Pharm Biol. 2010;48:1080–1190. doi: 10.3109/13880200903490513. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar A, Verma DR, Suryavanshi S, Dubey P. Characterization of chemical constituents of Calotropis procera. Chem Nat Compd. 2012;48:155–157. doi: 10.1007/s10600-012-0189-1. [DOI] [Google Scholar]
- 23.Shehab N. Volatiles, Lipoids and Flavonoids of the Flowers of Calotropis procera R.Br.: Composition and Bioactivity. Bull Fac Pharm Cairo Univ. 2008;46:139–149. [Google Scholar]
- 24.Elgamal MH, Shaker KH, Pollmann K, Seifert K. Triterpenoid saponins from Zygophyllum species. Phytochemistry. 1995;40:1233–1236. doi: 10.1016/0031-9422(95)00436-B. [DOI] [PubMed] [Google Scholar]
- 25.Jaouhari JT, Lazrek HB, Jana M. The hypoglycemic activity of Zygophyllum gaetulum extracts in alloxan-induced hyperglycemic rats. J Ethnopharmacol. 2000;69:17–20. doi: 10.1016/S0378-8741(99)00064-1. [DOI] [PubMed] [Google Scholar]
- 26.Ghoul JE, Boughattas NA, Ben-Attia M. Antihyperglycemic and antihyperlipidemic activities of ethanolic extract of Zygophyllum album in streptozotocin-induced diabetic mice. Toxicol Ind Health. 2013;29:43–51. doi: 10.1177/0748233712442706. [DOI] [PubMed] [Google Scholar]
- 27.Belguidoum M, Dendougui H, Kendour Z. In vitro antioxidant properties and phenolic contents of Zygophyllum album L. from Algeria. J Chem Pharm Res. 2015;7:510–514. [Google Scholar]
- 28.Bayarmaa J, Antbagu M. Effect Zygophylllm potaninii Maxim on histopathologicaland enzymatic chances in experimental liver Injury of rats. Mongolian J Health Sci. 2006;3(1):53–58.
- 29.Yildiztugay E, Ozfidan-Konakci C. Profiling of rutin-mediated alleviation of cadmium-induced oxidative stress in Zygophyllum fabago. Environ Toxicol. 2015;30:816–835. doi: 10.1002/tox.21960. [DOI] [PubMed] [Google Scholar]
- 30.Appropriate Agriculture International Co. L. 2015. http://www.koushu.co.jp/AAI_E/NewsE/News05-4-E.pdf. Accessed 10 June 2015.
- 31.Beyaoui A, Chaari A, Ghouila H, Ali HM, Ben JH. New antioxidant bibenzyl derivative and isoflavonoid from the Tunisian Salsola tetrandra Folsk. Nat Prod Res. 2012;26:235–242. doi: 10.1080/14786419.2010.536950. [DOI] [PubMed] [Google Scholar]
- 32.Khan KM, Maharvi GM, Abbaskhan A, Hayat S, Khan MTH, Makhmoor T, et al. Three Tyrosinase Inhibitors and Antioxidant Compounds from Salsola foetida. HCA. 2003;86:457–464. doi: 10.1002/hlca.200390045. [DOI] [Google Scholar]
- 33.Meltem Asan O, Mahmut E, Derya Onal D, Seher Karaman E, Mehtap T. Antimicrobial and antioxidant activity of various solvent extracts of Salsola stenoptera Wagenitz and Petrosimonia nigdeensis Aellen (Chenopodiaceae) plants. Chiang Mai J Sci. 2015;42(1):156–172. [Google Scholar]
- 34.Tundis R, Loizzo MR, Statti GA, Menichini F. Inhibitory effects on the digestive enzyme alpha-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharmazie. 2007;62:473–475. [PubMed] [Google Scholar]
- 35.Xiang Y, Li YB, Zhang J, Li P, Yao YZ. Studies on chemical constituents of Salsola collina. Zhongguo Zhong Yao Za Zhi. 2007;32:409–413. [PubMed] [Google Scholar]
- 36.Tackholm V: Students' Flora of Egypt. Egypt: Cairo University, Cairo; 1974.
- 37.Boulos L. The Identity, Typification and Distribution of Salsola imbricata Forsskal: Studies in the Chenopodiaceae of Arabia. Kew Bull. 1991;46:137–140. doi: 10.2307/4110753. [DOI] [Google Scholar]
- 38.Hamed AI, Masullo M, Sheded MG, Mahalel UA, Tawfik MM, Perrone A, et al. Triterpene saponins from Salsola imbricata. Phytochem Lett. 2011;4:353–356. [Google Scholar]
- 39.Shehab NG, Abu-Gharbieh E. Phenolic Profiling and Evaluation of Contraceptive Effect of the Ethanolic Extract of Salsola imbricata Forssk. in Male Albino Rats. Evid Based Complement Alternat Med. 2014;2014:695291. doi: 10.1155/2014/695291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 41.Singleton VL, Rossi JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 42.Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci Technol. 2003;36:263–271. doi: 10.1016/S0023-6438(02)00226-8. [DOI] [Google Scholar]
- 43.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 44.Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79:1625–1634. doi: 10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8. [DOI] [Google Scholar]
- 45.Mattila P, Astola J, Kumpulainen J. Determination of Flavonoids in Plant Material by HPLC with Diode-Array and Electro-Array Detections. J Agric Food Chem. 2000;48:5834–5841. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 47.Abu-Gharbieh E, Bayoumi FA, Ahmed NG. Alleviation of antioxidant defense system by ozonized olive oil in DNBS-induced colitis in rats. Mediators Inflamm. 2014;2014:967205. doi: 10.1155/2014/967205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 49.Webster D, Bignell AH, Attwood EC. An assessment of the suitability of bromocresol green for the determination of serum albumin. Clin Chim Acta. 1974;53:101–108. doi: 10.1016/0009-8981(74)90357-X. [DOI] [PubMed] [Google Scholar]
- 50.Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- 51.Brattin WJ, Glende EA, Jr, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 52.Muriel P, Escobar Y. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J Appl Toxicol. 2003;23:103–108. doi: 10.1002/jat.892. [DOI] [PubMed] [Google Scholar]
- 53.Ohta Y, Kongo-Nishimura M, Matsura T, Yamada K, Kitagawa A, Kishikawa T. Melatonin prevents disruption of hepatic reactive oxygen species metabolism in rats treated with carbon tetrachloride. J Pineal Res. 2004;36:10–17. doi: 10.1046/j.1600-079X.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 54.Jensen JE, Stainberg SE, Freese P, Marino E. Liver function tests. J Digest Disord. 2004;6:1–3. [Google Scholar]
- 55.Ryan CJ, Aslam M, Courtney JM. Transference of hepatic coma to normal rats from galactosamine treated donors by reverse plasma exchange. Biomater Artif Cells Artif Organs. 1990;18:477–482. doi: 10.3109/10731199009119621. [DOI] [PubMed] [Google Scholar]
- 56.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 57.Sekher PA, Chan TS, O'Brien PJ, Rice-Evans CA. Flavonoid B-ring chemistry and antioxidant activity: fast reaction kinetics. Biochem Biophys Res Commun. 2001;282:1161–1168. doi: 10.1006/bbrc.2001.4705. [DOI] [PubMed] [Google Scholar]
- 58.Kim JW, Yang H, Cho N, Kim B, Kim YC, Sung SH. Hepatoprotective constituents of Firmiana simplex stem bark against ethanol insult to primary rat hepatocytes. Pharmacogn Mag. 2015;11:55–60. doi: 10.4103/0973-1296.149704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucarini R, Bernardes WA, Tozatti MG, Filho A, Silva M, Momo C, et al. Hepatoprotective effect of Rosmarinus officinalis and rosmarinic acid on acetaminophen-induced liver damage. Emir J Food Agric. 2014;26:878–884. [Google Scholar]
- 60.Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, et al. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208:149–161. doi: 10.1016/j.toxlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 61.Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–4761. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 62.Pradeep K, Park SH, Ko KC. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague–Dawley rats. Eur J Pharmacol. 2008;587:273–280. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 63.Zheng QS, Sun XL, Xu B, Li G, Song M. Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed Environ Sci. 2005;18:65–70. [PubMed] [Google Scholar]
- 64.Sal'nikova SI, Dorogovoz SM, Slyshkov VV, Guzhva NN. Hepatoprotective activity of analogs of cinnamic acid. Farmakol Toksikol. 1989;52:77–80. [PubMed] [Google Scholar]
- 65.Johnson IT, Gee JM, Price K, Curl C, Fenwick GR. Influence of saponins on gut permeability and active nutrient transport in vitro. J Nutr. 1986;116:2270–2277. doi: 10.1093/jn/116.11.2270. [DOI] [PubMed] [Google Scholar]
- 66.Cheeke PR. Biological effects of feed and forage saponins and their impacts on animal production. Adv Exp Med Biol. 1996;405:377–385. doi: 10.1007/978-1-4613-0413-5_32. [DOI] [PubMed] [Google Scholar]
- 67.Xi M, Hai C, Tang H, Wen A, Chen H, Liu R, et al. Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep. 2010;15:20–28. doi: 10.1179/174329210X12650506623041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponou BK, Teponno RB, Ricciutelli M, Quassinti L, Bramucci M, Lupidi G, et al. Dimeric antioxidant and cytotoxic triterpenoid saponins from Terminalia ivorensis A. Chev Phytochemistry. 2010;71:2108–2115. doi: 10.1016/j.phytochem.2010.08.020. [DOI] [PubMed] [Google Scholar]


