Abstract
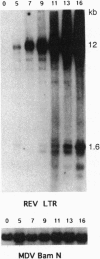
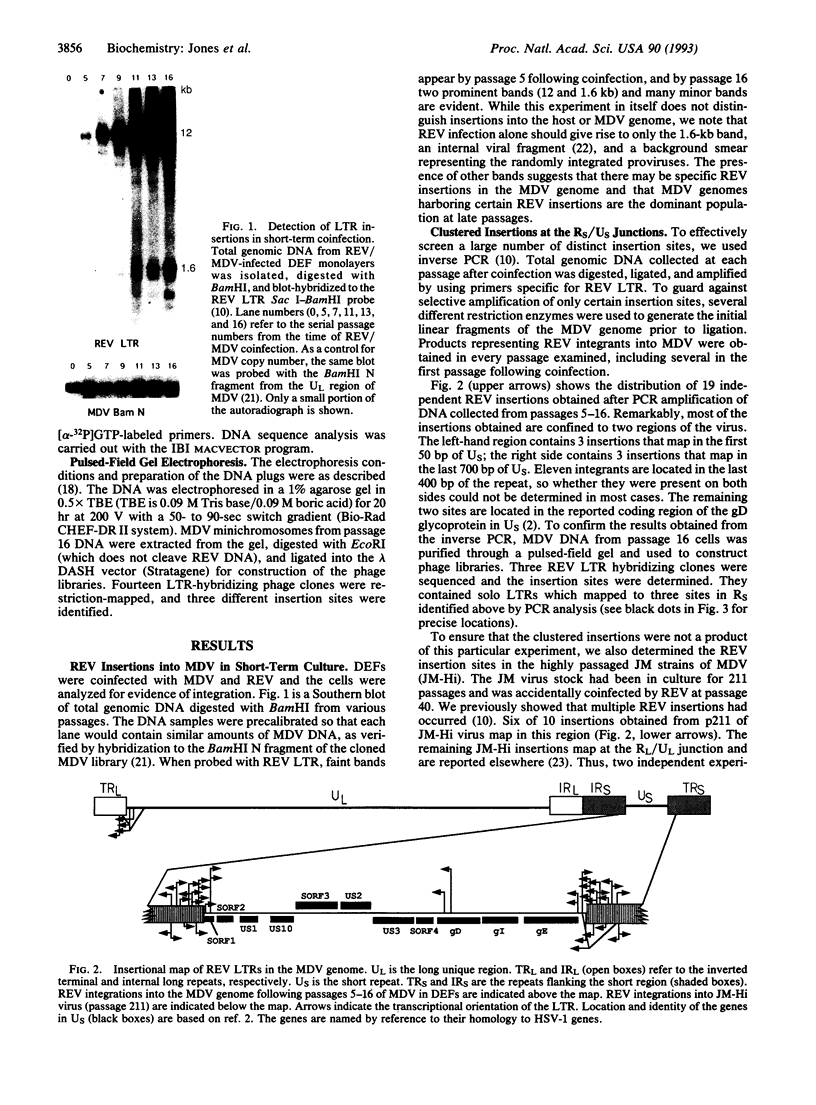
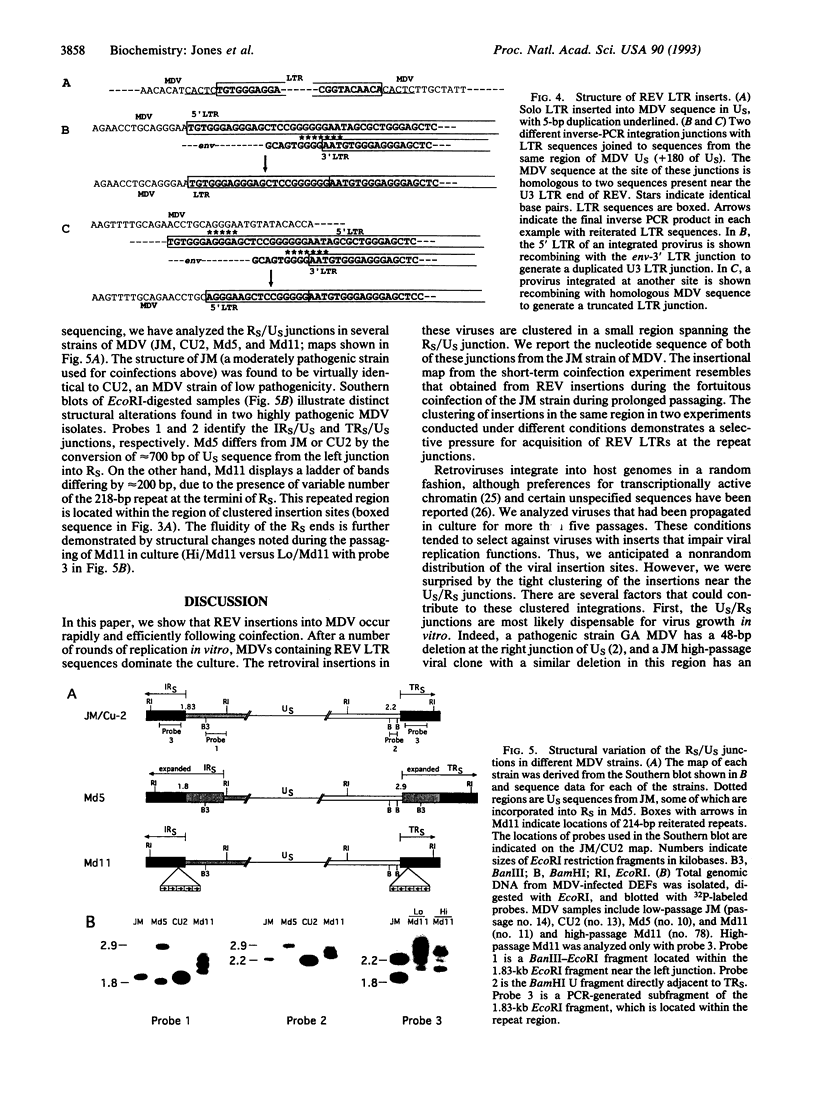
We previously described the integration of a nonacute retrovirus, reticuloendotheliosis virus (REV), into the genome of a herpesvirus, Marek disease virus (MDV), following both long-term and short-term coinfection in cultured fibroblasts. The long-term coinfection occurred in the course of attenuating the oncogenicity of the JM strain of MDV and was sustained for > 100 passages. The short-term coinfection, which spanned only 16 passages, was designed to recreate the insertion phenomenon under controlled conditions. We found that REV integrations into MDV were common and could occur within the first passage following coinfection. Now we have mapped the integration sites. After 5-16 passages in vitro, 17 out of 19 REV junction sites are clustered in two 1-kilobase regions at the junctions of the short unique and short repeat region of MDV. In the long-term cocultivation experiment, 6 out of 10 insertions also mapped in this region. In both cases, integrated proviruses are unstable and undergo subsequent recombinative deletion, often leaving a solitary long terminal repeat. The long terminal repeat sequences are, however, stably maintained for many rounds of passaging in vitro. This clustering of insertions presumably is influenced by selection for viable and fast-growing viruses, and occurs in a region of the MDV genome which shows significant size heterogeneity in several strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. M., Harland J. Three mutants of herpes simplex virus type 2: one lacking the genes US10, US11 and US12 and two in which Rs has been extended by 6 kb to 0.91 map units with loss of Us sequences between 0.94 and the Us/TRs junction. J Gen Virol. 1987 Jan;68(Pt 1):1–18. doi: 10.1099/0022-1317-68-1-1. [DOI] [PubMed] [Google Scholar]
- Buckmaster A. E., Scott S. D., Sanderson M. J., Boursnell M. E., Ross N. L., Binns M. M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988 Aug;69(Pt 8):2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- Cook M. K. Cultivation of a filterable agent associated with Marek's disease. J Natl Cancer Inst. 1969 Jul;43(1):203–212. [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Jessip J., Fukuchi K., Smith M., Tanaka A., Nonoyama M. The structure of Marek's disease virus DNA: amplification of repeat sequence in IRs and TRs. Microbiol Immunol. 1988;32(3):265–274. doi: 10.1111/j.1348-0421.1988.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Robinson D., Kung H. J. Purification of genomic sized herpesvirus DNA using pulse-field electrophoresis. J Virol Methods. 1990 Mar;27(3):311–317. doi: 10.1016/0166-0934(90)90099-2. [DOI] [PubMed] [Google Scholar]
- Isfort R., Jones D., Kost R., Witter R., Kung H. J. Retrovirus insertion into herpesvirus in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):991–995. doi: 10.1073/pnas.89.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost R., Jones D., Isfort R., Witter R., Kung H. J. Retrovirus insertion into herpesvirus: characterization of a Marek's disease virus harboring a solo LTR. Virology. 1993 Jan;192(1):161–169. doi: 10.1006/viro.1993.1018. [DOI] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner K., Karls U., Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990 Jun;64(6):3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall G. F., Kupershmidt S., Sugg N., Veach R. A., Ben-Porat T. Functions of the sequences at the ends of the inverted repeats of pseudorabies virus. J Virol. 1992 Mar;66(3):1506–1519. doi: 10.1128/jvi.66.3.1506-1519.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Gagnon G. C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986 Jan;57(1):28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Binns M. M., Pastorek J. DNA sequence and organization of genes in a 5.5 kbp EcoRI fragment mapping in the short unique segment of Marek's disease virus (strain RB1B). J Gen Virol. 1991 Apr;72(Pt 4):949–954. doi: 10.1099/0022-1317-72-4-949. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seperack P. K., Strobel M. C., Corrow D. J., Jenkins N. A., Copeland N. G. Somatic and germ-line reverse mutation rates of the retrovirus-induced dilute coat-color mutation of DBA mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):189–192. doi: 10.1073/pnas.85.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Calnek B. W. Effect of virus pathogenicity on antibody production in Marek's disease. Avian Dis. 1973 Oct-Dec;17(4):727–736. [PubMed] [Google Scholar]
- Stephens E. A., Witter R. L., Lee L. F., Sharma J. M., Nazerian K., Longenecker B. M. Characteristics of JMV Marek's disease tumor: a nonproductively infected transplantable cell lacking in rescuable Virus. J Natl Cancer Inst. 1976 Oct;57(4):865–874. doi: 10.1093/jnci/57.4.865. [DOI] [PubMed] [Google Scholar]
- Swift R. A., Boerkoel C., Ridgway A., Fujita D. J., Dodgson J. B., Kung H. J. B-lymphoma induction by reticuloendotheliosis virus: characterization of a mutated chicken syncytial virus provirus involved in c-myc activation. J Virol. 1987 Jul;61(7):2084–2090. doi: 10.1128/jvi.61.7.2084-2090.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene K. Conversion of a fraction of the unique sequence to part of the inverted repeats in the S component of the herpes simplex virus type 1 genome. J Gen Virol. 1986 Jun;67(Pt 6):1035–1048. doi: 10.1099/0022-1317-67-6-1035. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Challberg M. D., Nelson N. J., Levine M., Glorioso J. C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988 Jul 29;54(3):369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Nazerian K., Purchase H. G., Burgoyne G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970 Mar;31(3):525–538. [PubMed] [Google Scholar]
- Witter R. L., Offenbecker L. Nonprotective and temperature-sensitive variants of Marek's disease vaccine viruses. J Natl Cancer Inst. 1979 Jan;62(1):143–151. [PubMed] [Google Scholar]
- Witter R. L., Solomon J. J., Burgoyne G. H. Cell culture techniques for primary isolation of Marek's disease-associated herpesvirus. Avian Dis. 1969 Feb;13(1):101–118. [PubMed] [Google Scholar]
- Zhang G., Leader D. P. The structure of the pseudorabies virus genome at the end of the inverted repeat sequences proximal to the junction with the short unique region. J Gen Virol. 1990 Oct;71(Pt 10):2433–2441. doi: 10.1099/0022-1317-71-10-2433. [DOI] [PubMed] [Google Scholar]