Summary
Background
Elderly persons have the highest rates of tuberculosis (TB) in the United States compared to all other age groups. A systematic literature review was conducted to determine if older age was a risk factor for hepatotoxicity resulting from treatment with first-line drugs used to treat active (TB) and latent tuberculosis (LTBI).
Methods
A systematic review of MEDLINE, Cochrane Controlled Trial Registry, CINAHL®, and Science Citation Index Expanded (from 1970 to 2011) was performed to determine the risk of hepatotoxicity, comparing those over 60 with those under 60. A meta-analysis was performed using a random effects model along with log odds ratios and the chi-square test.
Findings
Thirty-eight studies (40,034 participants; 1208 cases of hepatotoxicity) met the selection criteria. For active TB, an overall mean effect of 0.277 (p = 0.024, 95% CI: 0.037–0.517) was observed, which is equivalent to an odds ratio of 1.32 (95% CI: 1.04–1.68). For LTBI, an overall mean effect of 1.42 (p < 0.001, 95% CI: 0.794–2.05) was observed, which translates to an odds ratio of 4.14 (95% CI: 2.21 –7.74).
Interpretation
Our analysis revealed that patients older than 60 had significantly more risk of hepatotoxicity. These studies suggest that a gentler regimen of treatment for older individuals could benefit health outcomes in this population of TB patients and minimize risks to the public's health.
Keywords: Tuberculosis, Latent tuberculosis, Therapy, Treatment, Elderly, Hepatotoxicity, Aging, Systematic review, Toxicity, Antituberculous
1. Introduction
Elderly persons (65 years of age and older) have the highest rates of tuberculosis (TB) in the United States compared to all other age groups [1]. Additionally, anti-tuberculosis treatment regimens are considered the leading cause of drug induced liver injury (DILI) and drug-induced acute liver failure in much of the developing world [2]. Most guidelines have considered hepatotoxicity from TB treatment as a function of age over 35 years old and have not discussed issues related to treating more aged individuals [3]. The current TB treatment guidelines in the United States, only mention dosage adjustments in the elderly for the aminoglycosides. Otherwise the dosing and the frequency of drugs for both active disease and latent infection is the same for elderly as for younger patients [4]. In contrast, special problems of drug tolerance in the elderly are considered in guidelines for the treatment of nontuberculous mycobacterial diseases, including initiation with a single drug and then gradual addition of other drugs [5]. Such an approach is not usually considered in managing tuberculosis due to the perceived urgency to reduce infectiousness to protect the publics' health. However after observing cases of severe drug-induced hepatotoxicity in elderly patients, we questioned whether the approach to initiation of TB treatment in the elderly should be re-evaluated. A systematic literature review was conducted to determine if older age was a risk factor for hepatotoxicity resulting from treatment with first-line drugs used to treat active and latent tuberculosis. We hypothesized that older patients have higher rates of hepatotoxicity.
2. Methods
2.1. Data sources
Studies were identified from an electronic search of MEDLINE (1970–2011), Cochrane Controlled Trial Registry (1970–2011), CINAHL® (1970–2011), and Science Citation Index Expanded (1970–2011). Variants of key words such as “tuberculosis,” “first-line drugs,” “antitubercular agents”, “hepatotoxicity,” “aged,” and “adverse effects” were used. References from articles we identified were also searched for relevant publications. Only manuscripts in English and Spanish were considered. An elderly person was defined as being an individual who was 60 years of age or older.
2.2. Study selection
Two independent reviewers (J.H. and M.V.) screened titles and abstracts for relevant content using broad criteria, yielding 321 full text selections. Information on study characteristics, overall quality, and relevant results were extracted from each article using a well-established form based off of the PRISMA model [6]. Reviewers compared notes and article selection instruments on the texts and operated by consensus. These full texts were further scrutinized and reduced to 38 eligible publications using the following study selection criteria: 1) infection with active TB or LTBI; 2) treatment with INH, RMP, PZA, streptomycin (STM) or ethambutol (EMB) in combination or given as single drugs; 3) information on age-related rate of hepatotoxicity, defined as clinically confirmed elevation in LFTS >2–5 times the upper reference level reported by the laboratory, equivalent elevated liver enzymes, and/or symptoms of hepatitis; 4) contained participants above the age of 60. Single patient case reports, news articles and editorials were not considered for review. The main outcome of interest was TB or LTBI drug-induced hepatotoxicity leading to: mortality, change in drug/treatment regimen, hospitalization, or liver transplantation. All authors whose articles were published after 1990 were contacted and asked to provide full datasets from their published study of interest.
2.3. Data analysis
A log odds ratio was used as the effect size statistic, with estimates of odds ratios amendedbyadding0.5toeach cell frequency.A positive value of log odds ratio indicates a positive association between age >60 and hepatotoxicity, while a negative value indicates a negative association. Each effect size was weighted by its inverse variance in calculating mean effect sizes. Heterogeneity was examined using an I-square statistic, which represents the approximate proportion of total variability (0%–100%) in the association between age >60 and hepato toxicity that can be attributed to systematic difference across studies (larger percentages reflects greater heterogeneity). Heterogeneity was evaluated by chi-square test. The overall effect sizes reported are based on the random effect model since these estimates are more conservative than a fixed effect model. SAS software (SAS institute, Cary, North Carolina), version 9.2 was used for all analysis.
3. Results
There were a total of 1852 citations obtained through electronic searches and an additional 290 were obtained through reference checking. After duplications were removed, 1567 published abstracts remained, spanning from 1970 until 2011 for review. Three-hundred and twenty-one full text selections were further reviewed. However, many did not meet the study criteria due to the absence of measurable data on hepatotoxicity or failure to specify age-specific groups/events under comparison, leaving 38 studies for which we were able to obtain full datasets from the authors or article [7–44]. These included 40,034 participants with 1208 cases of hepatotoxicity, of which 339 occurred in those over the age of 60. See Figure 1. There was 95.7% agreement between the two independent reviewers based on agreement between study selection instruments. See appendix.
Figure 1.
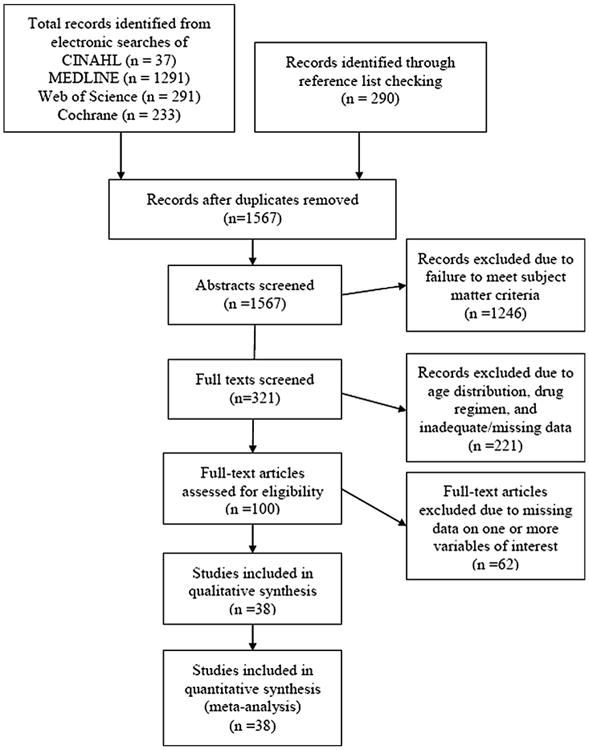
Study selection process for systematic review of studies comparing age-related rates of hepatotoxicity in treatment for tuberculosis infection.
Twelve out of the 38 studies evaluated age-specific risk of hepatotoxicity in persons using a first-line agent for treatment of LTBI. The other 26 studies looked at treatment of active TB infection. Six of these studies focused on treatment in patients who were undergoing organ transplant or dialysis. Selected articles are listed in Table 1. All articles were in English.
Table 1.
Characteristics of studies included in a systematic review of age-related risk of hepatotoxicity in the treatment of LTBI and Active Tuberculosis.
| Studies assessing hepatotoxicity reference, country, year | Study period | Study design | Study population number of subjects | Definition of hepatotoxicity | Drugs administered and length of treatment | LTBI/Active TB disease | Transplant/Dialysis |
|---|---|---|---|---|---|---|---|
| Baghaei et al., Iran, 2010 | 2006–2008 | Prospective | Patients treated for pulmonary tuberculosis, regardless of age N = 662 | Elevated AST/ALT; clinically evident hepatotoxicity | Standard 6 month regimen | Active TB | No |
| Bartacek et al., Multinational, 2009 | 2003–2004 | Open label RCT | Patients aged >15 years who were AFB positive and no hx of drug-induced hepatitis N = 1142 | Elevated SGOT, as defined by guidelines; evidence of clinical hepatitis | HRZE 75/150/400/275 mg per tablet daily for 2 months followed by H/R 75/150 mg daily for 4 months | Active TB | No |
| Byrd et al., US, 1979 | Not reported | Prospective | Patients with an intermediate PPD reaction given INH chemoprophylaxis, regardless of age N = 1000 | SGOT >5× normal level with or without symptoms | Standard INH regimen for 9 months | LTBI | No |
| Chien et al., Taiwan, 2010 | 2004–2008 | Retrospective | Patients of all ages with active TB disease with normal AST/ ALT levels of <40 IU/I at baseline N = 295 | Increase in ALT and/or AST >3× ULN with symptoms, or 5× without symptoms | INH (5 mg/kg), RMP (10 mg/kg), EMB (15 mg/kg), PZA (20 mg/kg) for 2 months, followed by 4 months of INH, RMP, EMB | Active TB | No |
| Chong, Brunei, 2008 | 1995–2005 | Retrospective | Patients with clinically diagnosed hepatobilliary TB, regardless of age N = 14 | ALT >2–3× ULN | STP (15 mg/kg daily), RIF (10 mg/kg daily), INH (5 mg/kg daily), PZA (30 mg/kg daily) before 1997 and RIF, INH, PZA, and EMB (20 mg/kg day) after 1997, length of treatment-unknown | Active TB | No |
| Chou et al., Taiwan, 2004 | 1989–2003 | Retrospective | Patients undergoing IST following HTx, with AFB confirmed TB, regardless of age N=5 | Clinical hepatitis as defined by guidelines | Standard WHO regimen, adjusted for concentration interactions with transplant drugs. Twenty-four months for extrapulmonry, and at least twelve months for pulmonary TB | Active TB | Yes |
| Cook et al., US, 2006 | 2000–2006 | Prospective | Patients, of all ages, treated for LTBI N = 291 | 2–5× normal ALT/AST levels | PZA (15 mg/kg daily), RIF (10 mg/kg daily); PZA (50 mg/kg), RIF (10 mg/kg) two times/week for 2 months; RIF (10 mg/kg daily) for 4 months (6 months for children <15 years old); INH (5 mg/kg daily) for adults, INH (10 mg/kg daily) for children | LTBI | No |
| Ekochin et al., US, 2009 | 2001–2008 | Retrospective | Patients, of all ages, treated for LTBI with concommittant MTX treatment N = 40 | LFT elevation >3× ULN | INH (5 mg/kg per day for adults, and 10 mg/ kg per day for children [maximum, 300 mg per day]) length of treatment-unknown | LTBI | No |
| Haley et al., US, 2008 | 2000–2004 | Retrospective | Patients >18 years of age treated for LTBI, with no previous treatment history N = 749 | Serum ALT ≥ 120 U/l with GI symptoms or ≥200 regardless of symptoms | RMP (10 mg/kg daily) for 4 months | LTBI | No |
| Jahng et al., US, 2007 | 2003–2006 | Prospective | Patients with end-stage liver disease > 18 years of age treated for LTBI N = 14 | 2× baseline LFT; clinical presentation of Hepatoxicity | INH (600 mg daily) for 4 months; RIF (300 mg daily) for 9 months | LTBI | Yes |
| Khan et al., Malaysia, 2010 | 2006–2008 | Retrospective cohort | Patients of all ages with active TB disease with normal AST/ ALT levels of <40 IU/I at baseline N = 1542 | Increase to 5× the ULN ALT/AST; Bilirubin >2 mg/dl; clinical jaundice | INH (5 mg/kg), RMP (10 mg/kg), EMB (15 mg/kg), PZA (25 mg/kg), STP (15 mg/kg) daily until end of treatment | Active TB | No |
| Kwon et al., South Korea, 2007 | 1994–2005 | Case-control | Patients of all ages with newly diagnosed active TB disease with normal AST/ALT levels of <40 IU/I at baseline, positive for HCV antibody and negative for hepatitis B surface antigen N = 151 | AST/ALT > 120IU/L AST/ ALT < 200 IU/L, defined as mild AST/ALT ≥ 200–500 IU/L defined as moderate hepatotoxicity AST/ALT levels ≥ 500 IU/L defined as severe hepatotoxicity | RIF (450–600 mgdaily), INH (300 mg daily), PZA (1500 mg daily), EMB(800 to 1200 mg daily) for 2 months followed by 4 months of INH, RIF, EMB | Active TB | No |
| Lee et al., South Korea, 2005 | 1994–2000 | Prospective | Patients of all ages with active Pulmonary TB disease N = 232 | AST/ALT increase >3 times ULN; any elevation of transaminase above basal levels in the presence of icteric hepatitis | INH (300–400 mg daily), RIF (450–600 mg daily), EMB (600–800 mg daily), PZA (1000 –1500 mg daily) for at least 6 months | Active TB | No |
| Lorent et al., Rawanda, 2011 | 2008–2010 | Prospective | Patients aged ≥21 years, both inpatient and outpatient – with newly diagnosed active TB N = 245 | Elevated liver enzymes and/or serum creatinin per guidelines | RIF (450–600 mg daily), INH (300 mg daily), PZA (1500 mg daily), EMB (800–1200 mg daily) for 6 months followed by 4 months of INH, RMP, EMB | Active TB | No |
| Menzies et al., Canada, Brazil, and Saudi Arabia, 2008 | 2004–2007 | Open label RCT | Patients >18 years of age treated for LTBI N = 847 | ALT 3–10 or 5–10× ULN w/ symptoms met criteria for grade 3 hepatotoxicity ALT >10× ULN met criteria for grade 4 toxicity | RIF (600 mg daily for 4 months) INH (300 mg daily for 9 months) | LTBI | No |
| Meyers et al., US, 2000 | 1988–1996 | Retrospective | Patients undergoing therapy for active TB following OLTx, regardless of age N = 8 | Elevation in AST/ALT per guidelines; histological features consistent with toxicity | RIF (450–600 mg daily), INH (300 mg daily), PZA (1500 mg daily), EMB (15 mg/kg/day) for 6 months, followed by 6–12 months of INH, RIF or OFL (800 mg daily) and EMB (15 mg/kg/day) | Active TB | Yes |
| Nader et al., Brazil, 2010 | 1998–2006 | Retrospective | Patients ≥18 years of age treated for active TB infection N = 534 | Increase in ALT >3× ULN; total bilirubin >2× ULN | Patients 20–40 kg: RMP (300 mg), INH (200 mg), PZA (1000 mg); Patients 40 –60 kg: RMP (450 mg), INH (300), PZA (1500 mg); Patients > 60 kg: RMP (600 mg), INH (400 mg), PZA (2000 mg); Standard RHZ length of treatment | Active TB | No |
| Nolan et al., US, 1999 | 1989–1995 | Prospective | Patients, of all ages, treated for LTBI N = 11,141 | AST 5× ULN without symptoms, clinical symptoms of hepatitis; resolution of signs and symptoms after withdrawl of INH | INH preventative therapy, length of treatment-6–12 months | LTBI | No |
| Ormerod et al., UK, 1996 | 1978–1992 | Retrospective cohort | Patients, of all ages, treated for active TB disease N = 1317 | Jaundice and/or elevation of ALT 5× pretreatment level | Adults: INH (300 mg), RMP (450–600 mg), PZA (1500–2000 mg), EMB (15 mg/kg), STM (1.0 gm 6 d/w) Children: INH (10 mg/kg), RMP (10 mg/kg), PZA (30 mg/kg), 2–4 months of RMP, INH, EMB, with continuation of INH/EMB for up to 15 mos/ RMP/INH for up to 12 months | Active TB | No |
| Possuelo et al., Brazil, 2008 | 2005–2007 | Prospective | Patient ≥ 18 years of age with newly diagnosed active TB disease N = 253 | AST/ALT > 3× ULN; and/or total bilirubin >2.0 mg/dL | <45 kg: RIF (300 mg), INH (200 mg), PZA (1000 mg); 45–55 kg: RIF (450 mg), INH (300 mg), PZA (1500 mg); >55 kg: RIF (600 mg), INH (400 mg), PZA (2000 mg); 2 months of daily INH, RIF, PZA, EMB followed by 4 months INH, RMP daily | Active TB | No |
| Quantrill et al., UK, 2003 | 1986–1999 | Retrospective | Patients of all ages with active TB disease and CRF N = 24 | Clinical Hepatitis as defined by BTS guidelines | Standard combination and doses of RMP, INH and PZA, per the BTS Guidelines for 6 –18 months | Active TB | Yes |
| Samandari et al., Botswana, 2011 | 2004–2006 | RCT | Patient ≥18 years of age or older with HIV infection and no symptoms or previous treatment of active TB N = 1995 | AST/ALT > 5× ULN, irrespective of symptoms | INH (300 mg per day) for individuals weighing 30–49 kg; INH (400 mg per day) for those weighing ≥50 kg; 6 months vs 36 months INH prophylaxis | LTBI | No |
| Schaberg et al., Germany, 1996 | 1990–1994 | Retrospective | Patients of all ages with active TB disease N = 519 | AST/ALT 3× the ULM; SGOT>54 U/L; SGPT>60 U/L | INH (5 m/kg daily), RIF (10 mg/kg daily), and PZA (25–30 mg/kg daily), with or without EMB and/or STP; length of treatment not reported | Active TB | No |
| Schluger et al., US, 1996 | 1988–1995 | Retrospective | Patients undergoing therapy for LTBI or active TB following OLTx, regardless of age N = 13 | Abnormal LFTs; exclusion of other causes of hepatitis | INH (300 mg daily); INH (300 mg daily), RIF (600 mg daily), EMB (15 mg/kg daily), PZA (20 mg/kg daily) or HRZE + OFL (800 mg daily) for up to 1 year | Both | Yes |
| Sen et al., Turkey, 2008 | 2002–2007 | Retrospective | Patients of all ages undergoing treatment for active TB with ESRD N = 18 | Elevated AST/ALT; jaundice | INH (300 mg daily), RMP (600 mg daily), MPZ (35–40 mg/kg 3d/w), EMB (20 mg/kg 3 d/w) for 2 months followed by INH and RMP for 4–6 months | Active TB | Yes |
| Shang et al., China, 2011 | 2007–2008 | Prospective | Patients of all ages undergoing treatment for active TB N = 4304 | ALT/AST > three times ULN; >2× ULN, when other causes were excluded | HRZE 75/150/400/275 mg per tablet daily for 2 months followed by H/R 75/150 mg daily for 4 months with or without STP | Active TB | No |
| Sharifzadeh et al., Iran, 2005 | 1999–2002 | Prospective | Patients of all ages undergoing treatment for active TB N = 112 | AST/ALT > three times ULN with any clinical signs/ symptoms or AST/ALT > five times ULN with no symptoms | RIF (10 mg/kg daily), INH (5 mg/kg daily), PZA (25 mg/kg daily), EMB (15 mg/kg daily) for standard treatment length | Active TB | No |
| Sistanizad et al., Iran, 2011 | 2006–2008 | Prospective | Patients of all ages undergoing treatment for newly diagnosed active TB disease N = 50 | ALT or AST > 2–3× ULN; clinical symptoms of liver disease i.e., jaundice and ascites | INH (5 mg/kg), RIF (10 mg/kg), PZA (25 –30 mg/kg), EMB (15 mg/kg daily) for 2 months, followed by 4 months of INH, RIF | Active TB | No |
| Smith et al., Canada, 2011 | 1998–2003 | Cross-sectional | Patients of all ages undergoing treatment for LTBI = 9145 | Toxic hepatitis as defined by guidelines | INH (5 mg/kg daily) for 6 months; RIF (10 mg/kg daily) for 4 months | LTBI | No |
| Sotsuka et al., Japan, 2011 | Not reported | Prospective | Patients of all ages undergoing treatment for active TB = 144 | AST/ALT > 5× ULN, irrespective of symptoms; AST/ALT > 3× ULN in the presence of symptoms; Bilirubin >3 mg/dL | INH (5 mg/kg), RIF (10 mg/kg), PZA (25 –30 mg/kg), EMB (15 mg/kg daily) for 2 months, followed by 4 months of INH, RIF; HER or HRZ were alternative regimens | Active TB | No |
| Stout et al., US, 2003 | 1999–2002 | Retrospective | Patients of all ages undergoing treatment for LTBI N = 119 | AST/ALT >5× ULN with one or more accompanying clinical symptoms/signs (nausea, vomiting, abdominal pain, or jaundice) | 60 doses of daily RIF (10 mg/kg, maximum daily dose of 600 mg) plus PZA (20 mg/kg, maximum daily dose of 2000 mg) or 16 doses of twice-weekly RIF (10 mg/kg, maximum dose of 600 mg) plus PZA (50 mg/kg, maximum dose of 4000 mg) for two months | LTBI | No |
| Sun et al., Taiwan, 2009 | 2000–2001 | Prospective | Patients of all ages undergoing treatment for active TB N = 261 | AST/ALT > 5× ULN, irrespective of symptoms; AST/ALT > 3× ULN in the presence of symptoms; Bilirubin >3 mg/dL | HERZ; HRZ; Combinations of: RIF (mg/kg/ day) INH (mg/kg/day) EMB (mg/kg/day) PZA (mg/kg/day) | Active TB | No |
| Tariq et al., Pakistan, 2009 | Not reported | Descriptive | Patients of all ages undergoing treatment for active TB N = 500 | ALT > 5× ULN; clinically evident hepatitis | Standard first-line regimen of HRZE | Active TB | No |
| Teleman et al., Singapore, 2002 | 1998 | Retrospective | Patients >16 years of age undergoing treatment for active TB disease N = 1036 | ALT/AST > 3× ULN; Bilirubin > ULN and ALT and/or AST >2 × ULN | INH, RMP, PZA with or without EMB or STP for 6 months; RMP, EMB, INH for 9 months | Active TB | No |
| van den Brande et al., Belgium, 1995 | 1980–1985 | Retrospective | Patients of all ages undergoing treatment for active TB N = 131 | ALT > 5× ULN with or without symptoms | RIF (10 mg/kg daily), INH (5 mg/kg daily), EMB (25 mg/kg for 6 wk and 15 mg/kg thereafter) for 9 months | Active TB | No |
| Wang et al., Taiwan, 2011 | 2007–2008 | Prospective | Patients >16 years of age undergoing treatment for active TB disease N = 360 | AST/ALT > 5× ULN, irrespective of symptoms; AST/ALT > 3× ULN in the presence of symptoms | Standard daily INH, RMP, EMB, PZA for 2 months followed by daily INH and RMP | Active TB | No |
| Young et al., US, 2009 | 2003–2007 | Retrospective | Patients > 18 years of age treated for LTBI N = 777 | ALT > 2.5–5× ULN with or without symptoms | INH (5 mg/kg daily) for 9 months; RIF (10 mg/kg daily) for 4 months | LTBI | No |
| Zabana et al., Spain, 2008 | 2003–2006 | Retrospective | Patients of all ages with LTBI and concomitant anti-TNF therapy N = 83 | AST/ALT elevation by standard definition | INH (5 mg/kg daily) for 6 months; INH (15 mg/kg 2× weekly) for 6 months; INH (5 mg/kg daily) for 9 months; INH (15 mg/ kg 2× weekly) for 9 months; INH (5 mg/kg daily) for 12 months; RMP (15 mg/kg daily), INH (10 mg/kg daily) for 6 months | LTBI | No |
3.1. Meta-analysis
The effect sizes (log odds ratios with 95% CI) from all studies (N = 38) included in the meta-analysis are listed in Table 2. Among the 38 studies, 25 (66%) reported a positive association (log odds ratio > 0), and 13 (34%) reported a negative association (log odds ratio < 0). An I-square value of 71% was calculated, indicated that most of the variation in the association between age >60 and hepatotoxicity was due heterogeneity across studies. This was confirmed by the Chi-square test (p < 0.001). Therefore, a random effect model was used to estimate the overall effect size. Based on the random effect model, the overall mean effect size was 0.534 (95% CI: 0.215–0.853), which is equivalent to an odds ratio of 1.71 (95% CI: 1.24–2.35). This analysis reveals that patients older than 60 had significantly higher risk of hepatotoxicity than patients 60 years or younger (p < 0.001, chi-square test).
Table 2.
Characteristics and Log Odds Ratios of studies included in a Meta-Analysis.
| Study | ID | Study N | # ≥60 | #with Event | Event ≥60 | LTBI/Active TB | Transplant-dialysis | Log OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartacek | 1 | 1142 | 89 | 30 | 7 | Active TB | No | 1.383 | 0.531 | 2.235 |
| Possuelo | 2 | 253 | 10 | 14 | 1 | Active TB | No | 0.992 | −0.816 | 2.799 |
| Quantrill | 3 | 24 | 6 | 4 | 1 | Active TB | Yes | 0.189 | −1.957 | 2.335 |
| Sen | 4 | 18 | 2 | 3 | 0 | Active TB | Yes | −0.260 | −3.516 | 2.997 |
| Shang | 5 | 4304 | 754 | 106 | 21 | Active TB | No | 0.172 | −0.307 | 0.652 |
| Sistanizad | 6 | 50 | 18 | 14 | 5 | Active TB | No | 0.008 | −1.238 | 1.253 |
| Chong | 10 | 14 | 5 | 3 | 2 | Active TB | No | 1.398 | −0.978 | 3.775 |
| Khan | 12 | 1548 | 256 | 126 | 13 | Active TB | No | −0.551 | −1.132 | 0.030 |
| Kwon | 13 | 151 | 48 | 42 | 11 | Active TB | No | −0.348 | −1.130 | 0.434 |
| Nader | 14 | 534 | 40 | 47 | 3 | Active TB | No | −0.057 | −1.195 | 1.081 |
| Sotsuka | 15 | 144 | 54 | 52 | 23 | Active TB | No | 0.442 | −0.250 | 1.133 |
| Baghaei | 17 | 662 | 246 | 99 | 49 | Active TB | No | 0.598 | 0.170 | 1.027 |
| Chou | 19 | 5 | 2 | 3 | 1 | Active TB | Yes | −0.511 | −3.547 | 2.526 |
| Wang | 20 | 360 | 182 | 60 | 25 | Active TB | No | −0.424 | −0.981 | 0.133 |
| van de Brande | 21 | 131 | 64 | 22 | 14 | Active TB | No | 0.698 | −0.228 | 1.624 |
| Teleman | 22 | 1036 | 397 | 55 | 29 | Active TB | No | 0.617 | 0.077 | 1.157 |
| Tariq | 23 | 500 | 20 | 40 | 4 | Active TB | No | 1.200 | 0.105 | 2.296 |
| Sun | 24 | 261 | 94 | 42 | 14 | Active TB | No | −0.126 | −0.815 | 0.563 |
| Sharifzadeh | 26 | 112 | 24 | 31 | 5 | Active TB | No | −0.408 | −1.457 | 0.642 |
| Lee JH | 28 | 232 | 119 | 32 | 16 | Active TB | No | −0.060 | −0.796 | 0.677 |
| Chien | 29 | 295 | 208 | 25 | 20 | Active TB | No | 0.489 | −0.487 | 1.465 |
| Meyers | 32 | 8 | 2 | 5 | 0 | Active TB | Yes | −2.909 | −6.441 | 0.624 |
| Ormerod | 34 | 1317 | 203 | 30 | 8 | Active TB | No | 0.747 | −0.056 | 1.551 |
| Schaberg | 36 | 519 | 102 | 55 | 17 | Both | No | 0.702 | 0.090 | 1.314 |
| Schluger | 37 | 13 | 2 | 5 | 0 | Active TB | Yes | −1.442 | −4.684 | 1.799 |
| Lorent | 38 | 245 | 9 | 23 | 2 | Active TB | No | 1.206 | −0.292 | 2.705 |
| Stout | 7 | 119 | 10 | 4 | 1 | LTBI | No | 1.570 | −0.455 | 3.594 |
| Zabana | 8 | 83 | 6 | 10 | 1 | LTBI | No | 0.676 | −1.252 | 2.605 |
| Samandari | 9 | 1995 | 7 | 19 | 0 | LTBI | No | 1.907 | −0.990 | 4.804 |
| Cook | 11 | 291 | 71 | 57 | 38 | LTBI | No | 2.474 | 1.818 | 3.131 |
| Young | 16 | 777 | 33 | 12 | 0 | LTBI | No | −0.134 | −2.982 | 2.714 |
| Byrd | 18 | 1000 | 15 | 64 | 1 | LTBI | No | 0.407 | −1.293 | 2.108 |
| Smith | 25 | 9145 | 857 | 45 | 22 | LTBI | No | 2.248 | 1.666 | 2.831 |
| Jahng | 27 | 14 | 4 | 4 | 1 | LTBI | Yes | −0.085 | −2.380 | 2.210 |
| Ekochin | 30 | 40 | 9 | 0 | 0 | LTBI | No | 1.199 | −2.788 | 5.185 |
| Menzies | 31 | 802 | 34 | 11 | 0 | LTBI | No | −0.046 | −2.898 | 2.806 |
| Nolan | 33 | 11,141 | 359 | 11 | 1 | LTBI | No | 1.457 | −0.257 | 3.171 |
| Haley | 35 | 749 | 9 | 3 | 0 | LTBI | No | 2.406 | −0.626 | 5.438 |
| Mean effect | 0.534 | 0.215 | 0.853 |
Data was analyzed separately analyzed between active TB studies (Table 3) and LTBI studies (Table 4). Among the 26 studies for active TB, 16 (61%) reported a positive association (log odds ratio > 0), and 10 (39%) reported a negative association (log odds ratio <0) between age > 60 and hepatotoxicity. An I-square value of 45% (p = 0.01), indicates substantial variation in association due to heterogeneity across studies. Based on a random effect model, an overall mean effect of 0.277 was (p = 0.024, 95% CI: 0.037–0.517) is equivalent to an odds ratio of 1.32 (95% CI: 1.04–1.68), as presented in Figure 2. Among the 13 studies for LTBI, 10 (77%) reported a positive association, and 3 (23%) reported a negative association. An I-square value of 40% (p = 0.09) was observed. Based on the random effect model, an overall mean effect of 1.42 was observed in the LTBI studies (p < 0.001, 95% CI: 0.794–2.05), as seen in Figure 3, which is equivalent to an odds ratio of 4.14 (95% CI: 2.21–7.74). This analysis reveals that patients older than 60 had significantly higher risk of hepatotoxicity than patients 60 years or younger, for both active TB and LTBI treatment groups.
Table 3.
Characteristics and Log Odds Ratios of studies included in a Meta-Analysis assessing age-related risk of hepatotoxicity in those with Active Tuberculosis only.
| Study | ID | Study N | # ≥60 | # with Event | Event & ≥ 60 | Transplant-dialysis (Yes/No) | Log OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Bartacek | 1 | 1142 | 89 | 30 | 7 | No | 1.383 | 0.531 | 2.235 |
| Possuelo | 2 | 253 | 10 | 14 | 1 | No | 0.992 | −0.816 | 2.799 |
| Quantrill | 3 | 24 | 6 | 4 | 1 | Yes | 0.189 | −1.957 | 2.335 |
| Sen | 4 | 18 | 2 | 3 | 0 | Yes | −0.260 | −3.516 | 2.997 |
| Shang | 5 | 4304 | 754 | 106 | 21 | No | 0.172 | −0.307 | 0.652 |
| Sistanizad | 6 | 50 | 18 | 14 | 5 | No | 0.008 | −1.238 | 1.253 |
| Chong | 10 | 14 | 5 | 3 | 2 | No | 1.398 | −0.978 | 3.775 |
| Khan | 12 | 1548 | 256 | 126 | 13 | No | −0.551 | −1.132 | 0.030 |
| Kwon | 13 | 151 | 48 | 42 | 11 | No | −0.348 | −1.130 | 0.434 |
| Nader | 14 | 534 | 40 | 47 | 3 | No | −0.057 | −1.195 | 1.081 |
| Sotsuka | 15 | 144 | 54 | 52 | 23 | No | 0.442 | −0.250 | 1.133 |
| Baghaei | 17 | 662 | 246 | 99 | 49 | No | 0.598 | 0.170 | 1.027 |
| Chou | 19 | 5 | 2 | 3 | 1 | Yes | −0.511 | −3.547 | 2.526 |
| Wang | 20 | 360 | 182 | 60 | 25 | No | −0.424 | −0.981 | 0.133 |
| van de Brande | 21 | 131 | 64 | 22 | 14 | No | 0.698 | −0.228 | 1.624 |
| Teleman | 22 | 1036 | 397 | 55 | 29 | No | 0.617 | 0.077 | 1.157 |
| Tariq | 23 | 500 | 20 | 40 | 4 | No | 1.200 | 0.105 | 2.296 |
| Sun | 24 | 261 | 94 | 42 | 14 | No | −0.126 | −0.815 | 0.563 |
| Sharifzadeh | 26 | 112 | 24 | 31 | 5 | No | −0.408 | −1.457 | 0.642 |
| Lee JH | 28 | 232 | 119 | 32 | 16 | No | −0.060 | −0.796 | 0.677 |
| Chien | 29 | 295 | 208 | 25 | 20 | No | 0.489 | −0.487 | 1.465 |
| Meyers | 32 | 8 | 2 | 5 | 0 | Yes | −2.909 | −6.441 | 0.624 |
| Ormerod | 34 | 1317 | 203 | 30 | 8 | No | 0.747 | −0.056 | 1.551 |
| Schaberg | 36 | 519 | 102 | 55 | 17 | No | 0.702 | 0.090 | 1.314 |
| Schluger | 37 | 13 | 2 | 5 | 0 | Yes | −1.442 | −4.684 | 1.799 |
| Lorent | 38 | 245 | 9 | 23 | 2 | No | 1.206 | −0.292 | 2.705 |
| Mean effect | 0.277 | 0.037 | 0.517 |
Table 4.
Characteristics and Log Odds Ratios of studies included in a Meta-Analysis assessing age-related risk of hepatotoxicity in those with LTBI only.
| Study | ID | Study N | # ≥60 | # with Event | Event & ≥60 | Transplant-dialysis (Yes/No) | Log OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Stout | 7 | 119 | 10 | 4 | 1 | No | 1.570 | −0.455 | 3.594 |
| Zabana | 8 | 83 | 6 | 10 | 1 | No | 0.676 | −1.252 | 2.605 |
| Samandari | 9 | 1995 | 7 | 19 | 0 | No | 1.907 | −0.990 | 4.804 |
| Cook | 11 | 291 | 71 | 57 | 38 | No | 2.474 | 1.818 | 3.131 |
| Young | 16 | 777 | 33 | 12 | 0 | No | −0.134 | −2.982 | 2.714 |
| Byrd | 18 | 1000 | 15 | 64 | 1 | No | 0.407 | −1.293 | 2.108 |
| Schaberg | 36 | 519 | 102 | 55 | 17 | No | 0.702 | 0.090 | 1.314 |
| Smith | 25 | 9145 | 857 | 45 | 22 | No | 2.248 | 1.666 | 2.831 |
| Jahng | 27 | 14 | 4 | 4 | 1 | Yes | −0.085 | −2.380 | 2.210 |
| Ekochin | 30 | 40 | 9 | 0 | 0 | No | 1.199 | −2.788 | 5.185 |
| Menzies | 31 | 802 | 34 | 11 | 0 | No | −0.046 | −2.898 | 2.806 |
| Nolan | 33 | 11,141 | 359 | 11 | 1 | No | 1.457 | −0.257 | 3.171 |
| Haley | 35 | 749 | 9 | 3 | 0 | No | 2.406 | −0.626 | 5.438 |
| Mean effect | 1.420 | 0.794 | 2.050 |
Figure 2.

Log Odds Ratios and Associated 95% Confidence Intervals of studies included in a Meta-Analysis assessing age-related risk of hepatotoxicity in those with Active Tuberculosis.
Figure 3.

Log Odds Ratios and Associated 95% Confidence Intervals of studies included in a Meta-Analysis assessing age-related risk of hepatotoxicity in those with LTBI Tuberculosis.
4. Discussion
As the population in industrially developed countries ages and the incidence of TB in these same countries recedes into more well-defined risk groups, TB among the elderly will become an increasingly important problem. Relatively little information exists in the literature that is specific to TB in the elderly and the unique challenges faced by older people with TB. The findings from this study provide evidence of the independent association of older age and the incidence of TB drug associated hepatic events. An odds ratio of 1.71 (95% CI 1.24–2.35), based on a random effects model, suggests a significant increase in hepatotoxic events in those over 60 years of age when compared to those younger than 60. The higher odds ratio observed in LTBI studies, 4.14 (95% CI 2.21–7.74) could be attributed to toxicity associated with standard INH monotherapy, or to less stringent patient monitoring in this group compared to active TB infections. Furthermore, there has never been a study looking at the rate of hepatotoxic events in older LTBI patients and measuring these events against a gain in quality-adjusted life years (QALYs). Should we be treating this older group of patients for LTBI if the risk of developing an active infection is smaller than the risk of developing a life-threatening adverse event? We believe that further studies are warranted to determine if policy changes are necessary to address how we treat latent infection in older populations.
But is the increased risk of hepatotoxicity in older patients undergoing therapy for either latent infection or active disease really something new? Remarkably the earliest clinical trials of isoniazid did not report on the risk of hepatotoxicity in any age group. It was not until an outbreak of TB at the US Capitol in the early 1970s that the risk of hepatotoxicity from isoniazid became well known and it was from that event that the guidelines regarding age and risk were first established [45]. However, the age cut-off was above 35 years of age and co-morbidities that could contribute to hepatotoxicity were not controlled. Thus in 2003, CDC guidelines for the treatment of TB infection in the United States do not mention age at all and recommend monitoring for toxicity based on risk factors other than age [46]. Treatment guidelines for active TB disease in the US and elsewhere make few if any specific recommendations regarding age and risk of toxicity.
In a landmark observational study published in 1999, it was found that the rate of hepatotoxicity in persons receiving preventive therapy increased with increasing age (X2 for linear trend = 5.22, p = 0.02) [24]. More recently, investigators in India explored the risk of hepatotoxicity in all patients undergoing therapy for active TB [47]. In their prospective case-control study they found that age over 35 years was associated with increased risk of hepatotoxicity (Adjusted OR 1.61, p ≤ 0.01). Furthermore, in another systematic review [48], which evaluated the age-related risk of hepatotoxicity in those undergoing treatment of latent TB infection (this study included 18,610 participants, including 115 cases of hepatotoxicity) it was found that the median rate of hepatotoxicity was 1.8% (range 0.07–11.9). On average, rates were higher among those aged ≥35 years (1.7%, 95%CI 1.4–2.2) than those aged <35 years (0.2%, 95%CI 0.1–0.3).
A study by Borgdorff et al. [49] suggested that transmission of pulmonary tuberculosis is associated with the age and sex of source cases. This study found that the number of secondary cases of tuberculosis generated per source case decreased with increasing age. An analysis of a contact investigation in Rotterdam in similarly found that older individuals were less likely to transmit TB [50]. Implications to be drawn from these findings include the potential mismanagement of tuberculosis and LTBI infections in elderly patients. Further research examining alternative TB treatment dosing schedules and regimens for the 60+ population are necessary to ensure patients receive the proper quality of care. These studies suggest that a gentler regimen of treatment for older individuals, who do not have additional risk factors which could impact transmission of TB infection, could benefit health outcomes in this population of TB patients and minimize risks to the publics' health. Providers and policy makers should have a serious discussion on developing stricter standards for hepatic event monitoring and grounds for treatment discontinuation in the elderly. Current treatment guidelines for those over the age of 60 should be reevaluated, as this age group has been proven to be less likely to spread disease and more likely to need treatment modification due to other underlying conditions.
These findings add to our knowledge in the sense that although numerous studies have used 35 years of age as a cut-off, no studies to our knowledge have looked at older age, 60 and above, nor have previous studies looked systematically at the risk of older patients undergoing therapy for active or latent TB therapy. Efforts to increase the sensitivity of drug-induced adverse event monitoring, should investigate novel biomarkers, e.g., keratin M65 and micro-RNA expression, as early predictors of liver injury, that could be incorporated into current treatment practices [51,52]. The strengths of the study are the robust methodological standards used for study inclusion, the novelty of examining hepatotoxicity by the age cutoff of 60 opposed to previous standards of 35 and the biological plausibility between treatment tolerance and age. We were able to examine datasets from 38 studies, giving a final sample size of 40,034 participants with 1208 cases of hepatotoxicity, 339 of which occurred in those over the age of 60.
4.1. Limitations
The limitations include those common to most systematic reviews, being variability of subjects within the studies, limited information on other potential risk factors for hepatotoxicity, and variable definitions of hepatotoxicity. However, the purpose of this review was to determine if elderly individuals over the age of 60 on anti-tuberculosis drugs were more likely to experience hepatic events than those 60 years and younger. Only one study focused on a population of HIV infected individuals, making it difficult to extend our conclusions to this population in particular [28]. Future studies are needed to determine the driving factors behind this phenomenon, including gender, concomitant medications, alcohol use, and other co-morbidities.
The evidence provided by this systematic review and the work of others is sufficient to warrant a re-examination of current TB treatment policies in the United States and recommend enhanced monitoring for hepatotoxicity in patients 60 and older who are undergoing treatment for either latent or active TB. Moreover, it is possible we need to re-evaluate treating older LTBI patients with INH monotherapy as the four-fold risk in experiencing a hepatotoxic event might obviate the benefit of treatment. We propose for patients 60 and over, careful review of the medical record to minimize other co-morbidities, choose effective, yet potentially more “liver sparing” drug regimens to reduce toxicity as much as possible, and more frequent symptom and biochemical monitoring (e.g., every two weeks symptom and liver function monitoring) in older patients.
Supplementary Material
Acknowledgments
The authors would like to thank the participating researchers who provided the raw data which was analyzed in this study.
Funding: This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and by the University of Florida Emerging Pathogens Institute.
Footnotes
Conflicts of interest: The authors have no conflicts of interest with the findings from this study.
Ethical approval: The research protocol was submitted to the University of Florida Institutional Review Board and granted exempt status.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.tube.2014.10.006.
References
- 1.Centers for Disease Control and Prevention (CDC) Reported tuberculosis in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 2.Devarbhavi H. Antituberculous drug-induced liver injury: current perspective. Trop Gastroenterol. 2011;32(3):167–74. [PubMed] [Google Scholar]
- 3.Saukkonen JJ. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HM. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baghaei P, et al. Incidence, clinical and epidemiological risk factors, and outcome of drug-induced hepatitis due to antituberculous agents in new tuberculosis cases. Am J Ther. 2010;17(1):17–22. doi: 10.1097/MJT.0b013e31818f9eae. [DOI] [PubMed] [Google Scholar]
- 8.Bartacek A, et al. Comparison of a four-drug fixed-dose combination regimen with a single tablet regimen in smear-positive pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(6):760–6. [PubMed] [Google Scholar]
- 9.Byrd RB, et al. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. J Am Med Assoc. 1979;241(12):1239–41. [PubMed] [Google Scholar]
- 10.Chien JY, et al. Hepatitis C virus infection increases hepatitis risk during anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2010;14(5):616–21. [PubMed] [Google Scholar]
- 11.Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcomes. South Med J. 2008;101(4):356–61. doi: 10.1097/SMJ.0b013e318164ddbb. [DOI] [PubMed] [Google Scholar]
- 12.Chou NK, et al. Various clinical presentations of tuberculosis in heart transplant recipients. Transplant Proc. 2004;36(8):2396–8. doi: 10.1016/j.transproceed.2004.08.114. [DOI] [PubMed] [Google Scholar]
- 13.Cook PP, et al. Safety and completion rate of short-course therapy for treatment of latent tuberculosis infection. Clin Infect Dis. 2006;43(3):271–5. doi: 10.1086/505398. [DOI] [PubMed] [Google Scholar]
- 14.Ekochin LH, et al. Absence of transaminase elevation during concomitant methotrexate and isoniazid therapy. J Rheumatol. 2009;36(9):2127. doi: 10.3899/jrheum.090149. [DOI] [PubMed] [Google Scholar]
- 15.Haley CA, et al. Successful use of rifampicin for Hispanic foreign-born patients with latent tuberculosis infection. Int J Tuberc Lung Dis. 2008;12(2):160–7. [PubMed] [Google Scholar]
- 16.Jahng AW, et al. Safety of treatment of latent tuberculosis infection in compensated cirrhotic patients during transplant candidacy period. Transplantation. 2007;83(12):1557–62. doi: 10.1097/01.tp.0000266578.45634.4f. [DOI] [PubMed] [Google Scholar]
- 17.Khan AH, et al. Anti-tubercular chemotherapy and drug induced hepatitis. Healthmed. 2010;4(3):536–44. [Google Scholar]
- 18.Kwon YS, et al. Hepatitis C virus infection and hepatotoxicity during antituberculosis chemotherapy. Chest. 2007;131(3):803–8. doi: 10.1378/chest.06-2042. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, et al. Diagnostic and therapeutic problems of pulmonary tuberculosis in elderly patients. J Korean Med Sci. 2005;20(5):784–9. doi: 10.3346/jkms.2005.20.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorent N, et al. Incidence and risk factors of serious adverse events during antituberculous treatment in Rwanda: a prospective Cohort study. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menzies D, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149(10):689–97. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 22.Meyers BR, et al. Tuberculosis in orthotopic liver transplant patients: increased toxicity of recommended agents; cure of disseminated infection with nonconventional regimens. Transplantation. 2000;69(1):64–9. doi: 10.1097/00007890-200001150-00013. [DOI] [PubMed] [Google Scholar]
- 23.Nader LA, et al. Hepatotoxicity due to rifampicin, isoniazid and pyrazinamide in patients with tuberculosis: is anti-HCV a risk factor? Ann Hepatol. 2010;9(1):70–4. [PubMed] [Google Scholar]
- 24.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. J Am Med Assoc. 1999;281(11):1014–8. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- 25.Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis. 1996;77(1):37–42. doi: 10.1016/s0962-8479(96)90073-8. [DOI] [PubMed] [Google Scholar]
- 26.Possuelo LG, et al. Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from Southern Brazil. Eur J Clin Pharmacol. 2008;64(7):673–81. doi: 10.1007/s00228-008-0484-8. [DOI] [PubMed] [Google Scholar]
- 27.Quantrill SJ, et al. Side-effects of antituberculosis drug treatment in patients with chronic renal failure. Eur Respir J. 2002;20(2):440–3. doi: 10.1183/09031936.02.00298002. [DOI] [PubMed] [Google Scholar]
- 28.Samandari T, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 29.Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996;9(10):2026–30. doi: 10.1183/09031936.96.09102026. [DOI] [PubMed] [Google Scholar]
- 30.Schluger LK, et al. Isoniazid hepatotoxicity after orthotopic liver transplantation. Mt Sinai J Med. 1996;63(5–6):364–9. [PubMed] [Google Scholar]
- 31.Sen N, et al. Tuberculosis in patients with end-stage renal disease undergoing dialysis in an endemic region of Turkey. Transplant Proc. 2008;40(1):81–4. doi: 10.1016/j.transproceed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Shang PH, et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifzadeh M, et al. Evaluation of patient-related factors associated with causality, preventability, predictability and severity of hepatotoxicity during antituberclosis treatment. Pharm Res. 2005;51(4):353–8. doi: 10.1016/j.phrs.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Sistanizad M, et al. Antituberculosis drug-induced hepatotoxicity in Iranian tuberculosis patients: role of isoniazid metabolic polymorphism. Iran J Pharm Res. 2011;10(3):633–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Smith BM, et al. Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ. 2011;183(3):E173–9. doi: 10.1503/cmaj.091824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotsuka T, et al. Association of isoniazid-metabolizing enzyme genotypes and isoniazid-induced hepatotoxicity in tuberculosis patients. In Vivo. 2011;25(5):803–12. [PubMed] [Google Scholar]
- 37.Stout JE, et al. Safety of 2 months of rifampin and pyrazinamide for treatment of latent tuberculosis. Am J Respir Crit Care Med. 2003;167(6):824–7. doi: 10.1164/rccm.200209-998OC. [DOI] [PubMed] [Google Scholar]
- 38.Sun HY, et al. A prospective study of hepatitis during antituberculous treatment in Taiwanese patients and a review of the literature. J Formos Med Assoc. 2009;108(2):102–11. doi: 10.1016/s0929-6646(09)60040-1. [DOI] [PubMed] [Google Scholar]
- 39.Tariq S, et al. Frequency of anti-tuberculous therapy-induced hepatotoxicity in patients and their outcome. J Ayub Med Coll Abbottabad. 2009;21(4):50–2. [PubMed] [Google Scholar]
- 40.Teleman MD, et al. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int J Tuberc Lung Dis. 2002;6(8):699–705. [PubMed] [Google Scholar]
- 41.van den Brande P, et al. Aging and hepatotoxicity of isoniazid and rifampin in pulmonary tuberculosis. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1705–8. doi: 10.1164/ajrccm.152.5.7582317. [DOI] [PubMed] [Google Scholar]
- 42.Wang JY, et al. Risk factors of hepatitis during antituberculous treatment and implications of hepatitis virus load. J Infect. 2011;62(6):448–55. doi: 10.1016/j.jinf.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Young H, et al. A retrospective evaluation of completion rates, total cost, and adverse effects for treatment of latent tuberculosis infection in a public health clinic in central massachusetts. Clin Infect Dis. 2009;49(3):424–7. doi: 10.1086/600394. [DOI] [PubMed] [Google Scholar]
- 44.Zabana Y, et al. Tuberculous chemoprophylaxis requirements and safety in inflammatory bowel disease patients prior to anti-TNF therapy. Inflamm Bowel Dis. 2008;14(10):1387–91. doi: 10.1002/ibd.20496. [DOI] [PubMed] [Google Scholar]
- 45.Garibaldi R, et al. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972;106(3):357. doi: 10.1164/arrd.1972.106.3.357. [DOI] [PubMed] [Google Scholar]
- 46.CDC. Treatment of tuberculosis, American Thoracic Society, CDC, and Infectious Diseases Society of America. MMWR. 2003;52:RR-11. [PubMed] [Google Scholar]
- 47.Singla R, et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132(1):81–6. [PubMed] [Google Scholar]
- 48.Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review [Review article] Int J Tuberc Lung Dis. 2010;14(11):1374–81. [PubMed] [Google Scholar]
- 49.Borgdorff MW, et al. Transmission of Mycobacterium tuberculosis depending on the age and sex of source cases. Am J Epidemiol. 2001;154(10):934–43. doi: 10.1093/aje/154.10.934. [DOI] [PubMed] [Google Scholar]
- 50.Van Geuns H, Meijer J, Styblo K. Results of contact examination in Rotterdam, 1967–1969. Bull Int Union Tuberc. 1975;50(1):107. [PubMed] [Google Scholar]
- 51.Thulin P, et al. Keratin-18 and microRNA-122 complement alanine amino-transferase as novel safety biiomarkers for drug-induced liver injury in two human cohorts. Liver Int. 2013;34(3):367–78. doi: 10.1111/liv.12322. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, et al. Identification of serum microRNA biomarkers for tuberculosis using RNA-seq. PLoS One. 2014;9(2):e88909. doi: 10.1371/journal.pone.0088909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


