Abstract
Introduction
Fetuin-A is a novel hepatokine, and there is preliminary evidence that it may contribute to the pathogenesis of type 2 diabetes. Exercise reduces fetuin-A, but the specific metabolic effects particularly as they relate to the regulation of insulin resistance are unknown. This led us to examine the effect of exercise training on circulating fetuin-A in relation to skeletal muscle and/or hepatic insulin resistance in obese adults.
Methods
Twenty older adults (66.3 ± 0.9 yr; body mass index, 34.1 ± 1.2 kg·m−2) participated in this prospective 12-wk study and underwent supervised exercise training (5 d·wk−1, 60 min·d−1 at approximately 85% HRmax). Insulin resistance was assessed using the euglycemic–hyperinsulinemic clamp (40 mU·m−2·min−1) with isotope dilution ([6,6-2H2]-glucose). Skeletal muscle insulin sensitivity (rate of glucose disposal), hepatic insulin resistance (rate of glucose appearance × fasting insulin), metabolic flexibility (respiratory quotientclamp – respiratory quotientfasting), fetuin-A, high-molecular weight adiponectin, high-sensitivity C-reactive protein, leptin, and body fat (dual energy x-ray absorptiometry) were measured before and after the intervention.
Results
Exercise reduced body fat, high-sensitivity C-reactive protein, leptin and hepatic as well as skeletal muscle insulin resistance (each, P < 0.05). Fetuin-A was decreased by approximately 8% (pre, 1.01 ± 0.08, vs post, 0.89 ± 0.06 g·L−1; P < 0.05) after the intervention, and lower fetuin-A after exercise correlated with lower hepatic insulin resistance (r = −0.46, P < 0.01), increased metabolic flexibility (r = −0.70, P < 0.01) and high-molecular weight adiponectin (r = −0.57, P < 0.01).
Conclusions
Fetuin-A may contribute to exercise training-induced improvements in hepatic insulin resistance, CHO utilization, and inflammation in older obese adults. Further work is required to determine the cellular mechanism(s) of action for fetuin-A because this hepatokine is related to type 2 diabetes risk
Keywords: Obesity, Inflammation, Exercise Training, Aging, Glucose Tolerance
Fetuin-A is a cytokine that is synthesized in the liver and is associated with the development of type 2 diabetes and nonalcoholic fatty liver disease (7,20,23). This association seems to be based on increased nonesterified free fatty acids (NEFA) and induction of pro- and anti-inflammatory cytokines (e.g., tumor necrosis factor (TNF)-α and high-molecular weight (HMW) adiponectin) that in turn contribute to insulin resistance and metabolic inflexibility (i.e., inability to switch from predominantly fat use in the fasted state to mainly insulin-stimulated CHO reliance) (5,7). We recently reported that 7 d of exercise decreased plasma fetuin-A in patients with nonalcoholic fatty liver disease, independent of changes in body weight or hepatic fat content (14). Moreover, we had suggested that fetuin-A impairs skeletal muscle insulin signaling and contributes to in vivo hyperglycemia (14). However, use of an oral glucose tolerance test to estimate skeletal muscle insulin resistance was a limiting factor in that study. Because previous in vitro studies have shown that fetuin-A inhibits insulin receptor tyrosine phosphorylation and Akt activity in the liver, fetuin-A may affect glycemia by influencing hepatic insulin resistance after exercise (1,15,16). To date, however, no study has examined the relation between fetuin-A and skeletal muscle and hepatic insulin resistance after lifestyle modification using the euglycemic clamp with glucose isotopes in humans. Therefore, we investigated the in vivo relation between exercise-induced reductions in circulating fetuin-A and improvements in skeletal muscle and/or hepatic insulin resistance. To gain additional mechanistic insight into how glucose was being used under these conditions, we also measured metabolic flexibility. This allowed us to hypothesize that lower fetuin-A after exercise would be linked to improved skeletal muscle and hepatic insulin resistance, metabolic flexibility, and inflammation.
Methods
Subjects
Twenty older obese adults (Table 1) volunteered for this study, and a subgroup had participated in a previous investigation (13). They were nonsmokers, weight stable (<2-kg weight loss during the previous 6 months), and sedentary (exercising <60 min·wk−1). Subjects were excluded if they had a known chronic disease (e.g., renal, liver, or cardiovascular diseases, type 2 diabetes, etc.) or took medications known to affect glucose metabolism. Before metabolic testing, subjects were fed isocaloric meals (resting metabolic rate × 1.2 activity factor; 55% CHO, 30% fat, 15% protein) and instructed to refrain from vigorous physical activity for 3 d. Subjects underwent 12 wk of supervised exercise, which consisted mainly of aerobic treadmill walking performed at 85% HRmax for 60 min·d−1, as previously described (13). Postintervention metabolic testing was conducted approximately 16–18 h after the last exercise bout. Subjects were instructed to maintain their preintervention macronutrient intake throughout the study. Three-day food records were collected before and after the intervention to assess macronutrient intake. All participants signed informed consent documents approved by our institutional review board.
Table 1.
Effects of exercise on anthropometrics, cardiometabolic risk, and glucose metabolism.
| Before Intervention | After Intervention | Delta (Δ) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Female/male (n) | 20 (11/9) | – | – | – |
| Age (yr) | 66.4 ± 0.9 | – | – | – |
| Weight (kg)a | 97.9 ± 3.2 | 91.5 ± 3.1 | −6.3 ± 0.7 | <0.001 |
| Body mass index (kg·m−2)a | 34.1 ± 1.1 | 31.9 ± 1.0 | −2.1 ± 0.7 | 0.001 |
| Body fat (kg) | 40.8 ± 2.2 | 36.5 ± 2.1 | −4.3 ± 2.2 | 0.001 |
| V̇O2max (mL·kg−1·min−1) | 20.8 ± 0.5 | 24.9 ± 0.8 | 4.0 ± 0.5 | <0.001 |
| Cardiometabolic | ||||
| Systolic blood pressure (mm Hg) | 134.3 ± 2.4 | 119.9 ± 2.4 | −14.4 ± 1.9 | <0.001 |
| Diastolic blood pressure (mm Hg) | 81.4 ± 2.4 | 71.9 ± 1.9 | 2.3 ± 2.4 | 0.001 |
| Total cholesterol (mg·dL−1) | 201.9 ± 7.7 | 173.3 ± 7.7 | −28.5 ± 5.1 | <0.001 |
| LDL cholesterol (mg·dL−1) | 130.1 ± 5.5 | 113.6 ± 6.5 | −16.4 ± 4.6 | 0.002 |
| HDL cholesterol (mg·dL−1)a | 43.6 ± 2.6 | 40.8 ± 2.4 | −3.0 ± 1.5 | 0.07 |
| Triglycerides (mg·dL−1) | 154.1 ± 16.8 | 107.5 ± 9.5 | −46.5 ± 10.9 | <0.001 |
| NEFA (mEq·L−1) | 0.55 ± 0.02 | 0.55 ± 0.02 | −0.004 ± 0.027 | 0.88 |
| Leptin (ng·mL−1) | 20.4 ± 3.0 | 15.0 ± 2.9 | −5.4 ±1.7 | 0.01 |
| hs-CRP (mg·L−1) | 3.8 ± 0.7 | 3.1 ± 0.69 | −0.68 ± 0.31 | 0.04 |
| HMW adiponectin (ng·mL−1) | 3803.6 ± 421.0 | 3380.6 ± 385.9 | −423.0 ± 257.9 | 0.12 |
| TNF-α (pg·mL−1) | 2.1 ± 0.2 | 2.0 ± 0.2 | −0.1 ± 0.2 | 0.63 |
| Glucose regulation | ||||
| Fasting glucose (mg·dL−1) | 102.3 ± 1.5 | 96.5 ± 1.6 | −5.7 ± 1.5 | 0.001 |
| Fasting insulin (μU·mL−1)a | 17.9 ± 2.3 | 11.5 ± 1.0 | −6.4 ± 1.6 | <0.001 |
| 2-h glucose (mg·dL−1) | 146.0 ± 5.6 | 143.9 ± 6.2 | −2.1 ± 6.2 | <0.001 |
| 2-h insulin (μU·mL−1) | 121.0 ± 21.5 | 82.3 ± 15.9 | −38.6 ± 14.4 | 0.02 |
| Skeletal muscle IS (mg·kg−1·min−1) | 3.3 ± 0.3 | 4.4 ± 0.4 | 1.0 ± 0.4 | <0.02 |
| Metabolic flexibility (au) | −0.030 ± 0.014 | 0.002 ± 0.080 | 0.032 ± 0.02 | 0.05 |
| Hepatic IR (mg·kg−1·min−1 × μU·mL−1) | 46.5 ± 6.9 | 27.3 ± 5.4 | −19.2 ± 4.3 | <0.001 |
| HGPfast (mg·kg−1·min−1) | 2.6 ± 0.2 | 2.2 ± 0.3 | −0.4 ± 0.2 | 0.09 |
| Adipose IR (mEq·L−1 × μU·mL−1) | 9.5 ± 1.0 | 6.2 ± 0.5 | −3.2 ± 0.7 | 0.001 |
Data are presented as mean ± SEM.
Nonnormally distributed data were analyzed by the Wilcoxon rank sum test.
Au, arbitrary units; delta (Δ) = after–before intervention; metabolic flexibility = RQclamp – RQfast; HGP, hepatic glucose production; IR, adipose insulin resistance = fasting NEFA × fasting insulin (mEq·L−1 × μU·mL−1); IR, hepatic insulin resistance = glucose rates of appearance × fasting insulin (mg·kg−1·min−1 × μU·mL−1); IS, skeletal muscle insulin sensitivity = Rd (mg·kg−1·min−1) during the final 30 min of the clamp.
Cardiometabolic risk
After a 10- to 12-h overnight fast, a catheter was inserted into the antecubital vein for collection of fasting triglyceride, cholesterol, high-sensitivity C-reactive protein (hs-CRP), HMW adiponectin, leptin, TNF-α, glucose, and insulin. A 75-g oral glucose load was administered to determine 2-h glucose concentrations approximately 24 h before determining insulin resistance. An incremental treadmill exercise test was used to determine maximal oxygen consumption (V̇O2max) (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA), and the HRmax obtained during this test was used to prescribe exercise intensity during training. Height and body weight were recorded using standard techniques, and dual-energy x-ray absorptiometry (Lunar Prodigy, Madison, WI) or hydrostatic weighing was used to quantify fat mass.
Insulin resistance
After an overnight fast, a euglycemic–hyperinsulinemic clamp was performed. Briefly, indirect calorimetry (Vmax Encore; Viasys, Yorba Linda, CA) was used to determine respiratory quotient (RQ) after approximately 30 min of rest in supine position. A retrograde hand catheter was placed, and the hand was warmed to 60°C for collection of arterialized blood samples (e.g., fasting glucose, insulin, and NEFA). A primed (3.28 mg·kg−1) continuous infusion (0.036 mg·kg−1·min−1) of [6,6-2H2]-glucose was then started at t =−120 min. At t = 0 min, a constant infusion (40 mU·m2·min−1) of insulin was administered via an indwelling catheter placed in the antecubital vein. Glucose was infused for 120 min at a variable rate to maintain plasma glucose at 90 mg·dL−1. Insulin-stimulated rates of glucose disposal (Rd) were averaged during the final 30 min of the clamp and used to characterize skeletal muscle insulin sensitivity. RQ was also determined during the final 30 min of the clamp, and metabolic flexibility was defined as RQclamp – RQfast. Nonoxidative glucose disposal, largely representing glycogen storage, was calculated as insulin-stimulated Rd – total CHO oxidation. Basal rates of endogenous glucose appearance were averaged during minute t = −30 to t = 0 and used to characterize hepatic glucose production (HGPfast). Hepatic insulin resistance was defined as HGPfast × insulinfast, and adipose insulin resistance was estimated as NEFAfast × insulinfast.
Plasma biochemical analysis
Fetuin-A (Quantikine; R&D Systems, Minneapolis, MN), leptin, HMW adiponectin, TNF-α (Millipore, Billerica, MA), and hs-CRP (Calbiotech, Spring Valley, CA) were assessed by enzyme-linked immunosorbent assay. Glucose kinetics, triglycerides, cholesterol, glucose, insulin, and NEFA were assessed by standard assays, as previously described (10,13).
Statistics
Data were analyzed using the GraphPad Prism 4 (San Diego, CA). Reductions of fetuin-A in men and women were similar after exercise (−0.08 ± 0.02 vs −0.12 ± 0.02 g·L−1, respectively, P = 0.64). Therefore, data were collapsed for statistical analysis. Normally and non-normally distributed variables were analyzed by paired two-tailed t-tests and Wilcoxon rank sum tests, respectively, to determine statistical differences before and after the intervention. Pearson product-moment or Spearman rank correlations were used, when appropriate, to determine bivariate associations. To assess the relation of fetuin-A on glucose regulation, independent of ambient inflammation, multivariate linear regression analysis was used to examine associations with leptin, hs-CRP, HMW adiponectin, and TNF-α used as covariates. Data are presented as mean ± SEM, and significance was accepted as P ≤ 0.05.
Results
Cardiometabolic risk
Exercise increased V̇O2max and reduced body fat (P < 0.05). Although caloric content (1879.2 ± 72.1 vs 1706.7 ± 99.8 kcal·d−1, P = 0.14) and CHO intake (55.2% ± 1.2% vs 55.0% ± 1.6%, P = 0.91) were not statistically different after the intervention, subjects reported lower dietary fat (30.8% ± 1.2% vs 28.0% ± 1.4%, P < 0.01) and increased protein consumption (16.2% ± 0.8% vs 17.6% ± 0.9%, P < 0.04). Exercise also lowered blood pressure, triglycerides, leptin, hs-CRP, and total cholesterol (P < 0.05) (Table 1) but had no effect on HDL or NEFA.
Insulin resistance and metabolic flexibility
Exercise reduced skeletal muscle, liver, and adipose insulin resistance by approximately 45%, 37%, and 26%, respectively (P < 0.02) (Table 1). The intervention had no effect on fasting RQ (i.e., 0.83 ± 0.01 vs 0.82 ± 0.01, P = 0.56) or nonoxidative glucose disposal (2.3 ± 0.5 vs 3.1 ± 0.5 mg·kg−1·min−1, P = 0.11), but metabolic flexibility was increased (P = 0.05) (Table 1).
Fetuin-A and correlation analysis
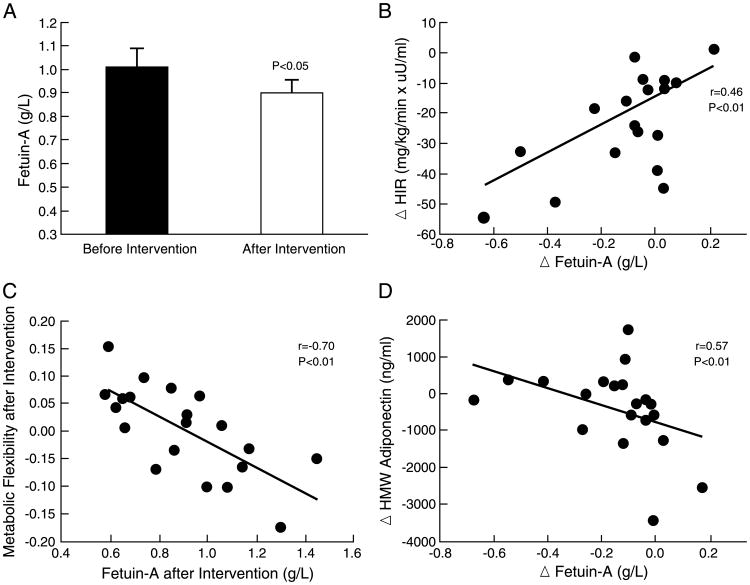
Exercise reduced fetuin-A by approximately 8% (P < 0.05) (Fig. 1A). The exercise-induced decrease in fetuin-A correlated with lower hepatic insulin resistance (r = −0.46, P < 0.01) (Fig. 1B) but not with enhanced insulin-stimulated skeletal muscle glucose uptake (r = 0.18, P = 0.43) or reduced adipose insulin resistance (r = 0.09, P = 0.67). Furthermore, reduced fetuin-A concentrations were significantly associated with higher metabolic flexibility at the 12-wk time point (r = −0.70, P < 0.01) (Fig. 1C). Exercise-induced changes in fetuin-A were not associated with body mass index reduction (r = 0.02, P = 0.90), fat loss (r = −0.41, P = 0.06), caloric restriction (r = 0.15, P = 0.54), dietary fat intake (r = 0.06, P = 0.79), fasting insulin (r = 0.20, P = 0.39), or V̇O2max (r = 0.22, P = 0.34), although reductions in fetuin-A were associated with increased HMW adiponectin (r = −0.57, P < 0.01) (Fig. 1D). Fetuin-A remained a significant predictor of hepatic insulin resistance and metabolic flexibility, independent of changes in hs-CRP, leptin, TNF-α, or HMW adiponectin (P < 0.03) (Table 2). The independent association between increased HMW adiponectin and reduced fetuin-A was attenuated after adjustment for changes in hs-CRP, leptin, and TNF-α (P = 0.07) (Table 2).
Figure 1.
Plasma fetuin-A before and after a 12-wk supervised exercise intervention (A) in relation to hepatic insulin resistance (B), metabolic flexibility (C), and HMW adiponectin (D) after the intervention. Data are expressed as mean ± SEM. Delta (Δ) = after – before intervention; HIR, hepatic insulin resistance; metabolic flexibility = RQclamp – RQfast.
Table 2.
Multivariate regression analysis between fetuin-A and glucose metabolism.
| Estimate | SE | t | P | |
|---|---|---|---|---|
| Δ Hepatic IR (mg·kg1·min−1 × μU·ml−1) | ||||
| Intercept | −1.56e1 | 8.9 | −1.75 | 0.10 |
| ΔFetuin-A (g·L−1) | 5.50e1 | 2.33e1 | 2.35 | 0.03 |
| ΔHMW-adiponectin (ng·mL−1) | 8.35e−4 | 4.00e−3 | 0.20 | 0.83 |
| Δhs-CRP (mg·L−1) | 3.54e−1 | 2.94e0 | 0.12 | 0.90 |
| ΔTNF-α (pg·mL−1) | 7.67e−1 | 5.51e0 | 0.13 | 0.89 |
| ΔLeptin (ng·mL−1) | −3.05e−1 | 5.51e−1 | −.46 | 0.65 |
| Metabolic flexibility after intervention (au) | ||||
| Intercept | 2.87e−1 | 6.52e−2 | 4.18 | 0.001 |
| Fetuin-A (g·L−1) after intervention | −2.76e−1 | 6.28e−2 | −4.40 | 0.001 |
| ΔHMW-adiponectin (ng·mL−1) | 1.29e−5 | 1.33e−5 | 0.97 | 0.35 |
| Δhs-CRP (mg·L−1) | 8.18e−3 | 1.10e−2 | 0.74 | 0.47 |
| ΔTNF-α (pg·mL−1) | 3.25e−2 | 2.11e−2 | 1.53 | 0.15 |
| ΔLeptin (ng·mL−1) | 3.63e−3 | 2.33e−3 | 1.55 | 0.14 |
| Δ HMW adiponectin (ng·mL−1) | ||||
| Intercept | −2.47e−1 | 8.33e−2 | −2.96 | 0.01 |
| ΔFetuin-A (g·L−1) | −8.43e−5 | 4.30e−5 | −1.95 | 0.07 |
| Δhs-CRP (mg·L−1) | −2.36e−2 | 3.56e−2 | −.66 | 0.52 |
| ΔTNF-α (pg·mL−1) | −1.01e−2 | 6.79e−2 | −.15 | 0.88 |
| ΔLeptin (ng·mL−1) | −1.10e−2 | 7.52e−3 | −1.42 | 0.16 |
Au, arbitrary units; delta (Δ) = after − before intervention; IR, hepatic insulin resistance = glucose rates of appearance × fasting insulin (mg·kg−1·min−1 × μU·mL−1); metabolic flexibility = RQclamp – RQfast (au).
Discussion
Skeletal muscle is responsible for the majority of insulin-mediated glucose disposal during euglycemic clamp conditions, and fetuin-A has been reported to inhibit insulin receptor tyrosine phosphorylation and tyrosine kinase activity in rodent skeletal muscle (15,16). We have previously reported that fetuin-A decreases insulin-stimulated glucose uptake in C2C12 skeletal muscle cells by decreasing AS160 phosphorylation (14). This observation led us to conclude that fetuin-A downregulates GLUT4 translocation and contributes to the improvement in skeletal muscle glucose disposal after exercise. However, it is important to recognize that hepatic glucose production also contributes to postprandial glucose levels, and exercise reduces hepatic insulin resistance (13,14). Most lifestyle interventions, but not all, have reported lower plasma fetuin-A levels in conjunction with reduced diabetes risk (8,17,20,21,23,26). To date, however, it remains unclear whether reductions in fetuin-A contribute to improved glycemic control after exercise training because of changes in skeletal muscle and/or hepatic insulin resistance. Herein, we detected a statistically significant correlation between fetuin-A and hepatic, but not skeletal muscle insulin resistance after the intervention. On the basis of this correlation and the lack of association with inflammatory markers, our data suggest that the exercise-induced lowering of fetuin-A may be predominantly linked to hepatic glucose production, independent of changes in systemic inflammation (1,7,9,15,16). We recognize that correlations do not prove causation, and our data do not exclude the possibility that fetuin-A acts on skeletal muscle glucose metabolism, independent of insulin signaling. Despite the absence of a significant correlation between changes in fetuin-A and skeletal muscle insulin resistance, we did find that the reduction in fetuin-A was linked to the increase in metabolic flexibility after the intervention. These data suggest that fetuin-A may act indirectly to modulate skeletal muscle glucose uptake by influencing postprandial CHO oxidation. The exact mechanism by which fetuin-A affects CHO oxidation (i.e., glycolysis, Krebs cycle, and/or oxidative phosphorylation) was not the objective of this study, but our observation is consistent with work in rodents wherein fetuin-A was related to decreased skeletal muscle glycogen content (16). Collectively, our study supports the hypothesis that fetuin-A is a circulating endocrine factor that contributes to glucose regulation through multiple target tissues.
It is now well established that inflammation is a contributing protagonist in the development of systemic insulin resistance (24). In vitro studies show that fetuin-A increases TNF-α expression and suppresses the synthesis of adiponectin from adipocytes, suggesting that fetuin-A may contribute to chronic inflammation (5). Exercise is a cornerstone therapy for reducing inflammation (27), and we show here that our intervention induced significant reductions in fetuin-A, hs-CRP, and leptin in these older pre-diabetic adults. Furthermore, we found that lower fetuin-A was inversely related to the rise in HMW adiponectin after exercise. This observation is consistent with in vitro evidence (5) and may be clinically relevant because elevated HMW adiponectin is reported to enhance metabolic flexibility and insulin sensitivity (6,7,10). In addition, recent animal work has demonstrated that increased adiponectin, in conjunction with lower fetuin-A, is important for preventing hepatic steatosis and improving hepatic insulin sensitivity (9). It is worth recognizing, however, that we did not detect a statistical rise in HMW adiponectin in this group of subjects. Variability in the HMW adiponectin multimer response to exercise training has been previously noted (4,22,25,27), including some of our own work (18). Indeed, we have found that it is not the increase in the HMW complex that correlates with improved insulin sensitivity after exercise training, but instead, adiponectin's role as an insulin-sensitizing agent is linked to a decrease in the middle-molecular weight complex, leading to a shift in the HMW to total ratio (i.e., the adiponectin SA ratio) (18). Although we did not measure the middle-molecular weight multimer in this study, our data suggest that there may be a role for HMW adiponectin as a regulator of insulin sensitivity through some fetuin-A–linked pathway yet to be discovered. Thus, the lowering of fetuin-A may play a role in communicating with adipose tissue to regulate adipokines, and by attenuating proinflammation, this may help regulate glucose homeostasis.
This study has limitations that warrant discussion. The sample size is modest and specific to older adults with pre-diabetes. Thus, the findings may not be applicable to different age groups or disease states (e.g., chronic kidney disease) (7). We also acknowledge that dietary intake was not strictly controlled and changes in total caloric content/dietary fat may affect fetuin-A (2,4,12,19). Although we observed no relation between body weight and caloric deficit with changes in fetuin-A, we recognize that the energy deficit observed in the current study may provide context to the time course improvement in skeletal muscle versus hepatic insulin resistance between our current data and past work (14). Previously, our 7-d exercise study suggested that the exercise-induced lowering of fetuin-A was not related to hepatic insulin resistance when body weight and hepatic triglyceride content were maintained (14). However, after 12 wk of exercise with approximately 6% weight loss, we did observe a significant relation between decreased plasma fetuin-A and lower hepatic insulin resistance. Collectively, these data are consistent with work reported in animals and humans and suggest that energy balance may modulate the interaction of fetuin-A on hepatic and skeletal muscle insulin resistance after exercise (3,4,11). Next, no control group was included in this current study. However, previous cross-sectional work reported that physical inactivity is linked to high fetuin-A and individuals randomized to a standard of care control group for 12 wk had unaltered fetuin-A levels (4,8). Lastly, although not an objective of this study, we did not perform biopsies of skeletal muscle and liver or adipose tissue to determine the molecular mechanism by which lower fetuin-A modifies glucose homeostasis, and this still awaits further investigation. However, we did use the gold standard euglycemic clamp insulin sensitivity test and studied a high-risk group of adults undergoing supervised exercise, thereby strengthening the conclusion that fetuin-A affects multitissue glucose homeostasis.
In conclusion, fetuin-A was directly linked to the reduction in hepatic insulin resistance, insulin-stimulated CHO use, and systemic inflammation after exercise training in older, obese, prediabetic adults. Given that overfeeding has been shown to increase fetuin-A and impair systemic insulin sensitivity (12,16), our data highlight lower fetuin-A as an important mechanism whereby exercise reduces type 2 diabetes risk. Future work is needed to provide mechanistic data on the role that exercise may play in regulating changes in fetuin-A and chronic metabolic disease.
Acknowledgments
We thank the participants for their efforts and Julianne Filion, R.N., B.S.N., for the exceptional organizational assistance.
This research was supported by National Institutes of Health (NIH) grants RO1 AG-12834 (J. P. K.) and NIH National Center for Research Resources, UL1RR024989, Cleveland, OH. Support for S. K. M. was provided by NIH grant T32 DK-007319, and J. P. D. R. was supported by R01DK089547-S1 and the Department of Medicine Physician Scientist Pathway at the MetroHealth Campus of Case Western Reserve University.
S. K. M. and J. P. D. R. shared first-author responsibilities. S. K. M. developed the study hypothesis. S. K. M. and J. P. D. R. shared responsibility for data analysis, statistical integrity, and writing of the manuscript. H. H. analyzed the plasma samples. J. P. K. collected the data and is the guarantor of this work.
The results of this study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
The authors report no conflict of interest.
References
- 1.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58(4):631–40. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 2.Blüher M, Rudich A, Klo¨ting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35(2):342–9. doi: 10.2337/dc11-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brons C, Jensen CB, Storgaard H, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young health men. J Physiol. 2009;15(587):2387–97. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi KM, Han KA, Ahn HJ, et al. The effects of caloric restriction on fetuin-A and cardiovascular risk factors in rats and humans: a randomized controlled trial. Clin Endocrinol (Oxf) 2013;29(3):356–63. doi: 10.1111/cen.12076. [DOI] [PubMed] [Google Scholar]
- 5.Hennige AM, Staiger H, Wicke C, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008;3(3):e1765. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21(3):406–12. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins NT, McKenzie JA, Hagberg JM, Witkowski S. Plasma fetuin-A concentrations in young and older high- and low-active men. Metabolism. 2011;60(2):265–71. doi: 10.1016/j.metabol.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung TW, Young BS, Choi HY, et al. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013;86(7):960–9. doi: 10.1016/j.bcp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Kelly KR, Blaszczak A, Haus JM, et al. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc. 2012;44(1):69–74. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 11.Kirk E, Reeds DN, Finck BN, Mayurranjan MS, Patterson BW, Klein S. Dietary fat and carbohydrate differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136(5):1552–60. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, Braymer HD, Bray GA, York DA. Differential expression of insulin receptor tyrosine kinase inhibitor (fetuin) gene in a model of diet-induced obesity. Life Sci. 1998;63(2):145–53. doi: 10.1016/s0024-3205(98)00250-1. [DOI] [PubMed] [Google Scholar]
- 13.Malin SK, Haus JM, Solomon TPJ, Blaszczak A, Kashyap SR, Kirwan JP. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin resistant phenotypes. Am J Physiol Endocrinol Metab. 2013;305(10):E1292–8. doi: 10.1152/ajpendo.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malin SK, Mulya A, Fealy CE, et al. Fetuin-A is linked to improved glucose tolerance after short-term exercise training in nonalcoholic fatty liver disease. J Appl Physiol (1985) 2013;115(7):988–94. doi: 10.1152/japplphysiol.00237.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews ST, Rakhade S, Zhou X, Parker GC, Coscina DV, Grunberger G. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun. 2006;350(2):437–43. doi: 10.1016/j.bbrc.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 16.Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51(8):2450–8. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Emoto M, Araki T, et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57(9):1248–52. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary VB, Jorett AE, Marchetti CM, et al. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistance adults. Am J Physiol Endocrinol Metab. 2007;293(1):E421–7. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 19.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–85. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 20.Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(11):4479–85. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 21.Schultes B, Frick J, Ernst B, Stefan N, Fritsche A. The effect of 6-weeks of aerobic exercise training on serum fetuin-A levels in non-diabetic obese women. Exp Clin Endocrinol Diabetes. 2010;118(10):754–6. doi: 10.1055/s-0030-1253418. [DOI] [PubMed] [Google Scholar]
- 22.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16(2):241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 23.Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29(4):853–7. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2(2):82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 25.Turner AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 26.Yang SJ, Hong HC, Choi HY, et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf) 2011;75(4):464–9. doi: 10.1111/j.1365-2265.2011.04078.x. [DOI] [PubMed] [Google Scholar]
- 27.You T, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43(4):243–56. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]