Abstract
The porphyria diseases are a group of metabolic disorders caused by abnormal functioning of heme biosynthesis enzymes and characterized by the excessive accumulation and excretion of porphyrins and their precursors. Precisely which of these chemicals builds up depends on the type of porphyria. Porphyria is not a single disease but a group of nine disorders: acute intermittent porphyria (AIP), hereditary coproporphyria (HCP), variegate porphyria (VP), delta-aminolevelunic acid dehyratase deficiency porphyria (ADP), porphyria cutanea tarda (PCT), hepatoerythropoetic porphyria (HEP), congenital erythropoetic porphyria (CEP), erythropoetic protoporphyria (EPP), and X-linked protoporphyria (XLP). Each porphyria results from overproduction of heme precursors secondary to partial deficiency or, in XLP, increased activity of one of the enzymes of heme biosynthesis. Taken together, all forms of porphyria afflict fewer than 200,000 people in the United States. Based on European studies, the prevalence of the most common porphyria, PCT, is 1 in 10,000, the most common acute porphyria, AlP, is about 1 in 20,000, and the most common erythropoietic porphyria, EPP, is estimated at 1 in 50,000 to 75,000. CEP is extremely rare with prevalence estimates of 1 in 1,000,000 or less. Only 6 cases of ADP are documented.
The current porphyria literature is very exhaustive and a brief overview of porphyria diseases is essential in order for the reader to better appreciate the relevance of this area of research prior to undertaking biochemical diagnostics procedures. This unit summarizes the current knowledge on the classification, clinical features, etiology, pathogenesis, and genetics of these porphyria diseases.
INTRODUCTION
This overview of the clinical features, genetics, classification and pathogenesis of the porphyrias provides the background needed for understanding the importance of biochemical procedures for the diagnosis of these diseases.
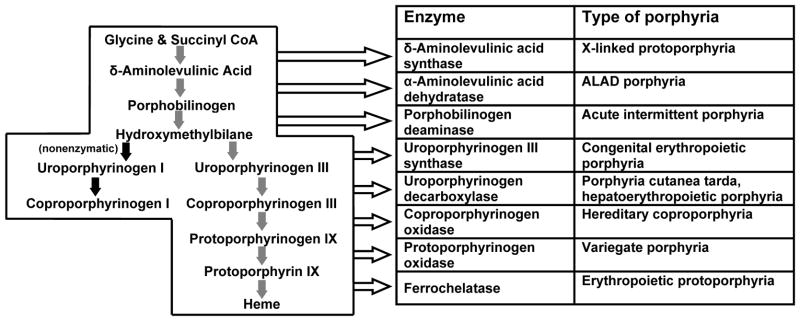
The porphyrias are a group of at least eight metabolic disorders caused by alterations in enzymes involved in heme biosynthesis (Figure 1). The terms porphyrin and porphyria are derived from the Greek word porphyrus, meaning purple. Urine from porphyria patients may be dark or reddish in color due to the presence of excess porphyrins and related substances, and may darken further after exposure to light. Porphyrias differ considerably from each other. A common feature in all porphyrias is the accumulation in the body of porphyrins or porphyrin precursors. Although these are normal body chemicals, they normally do not accumulate. Precisely which of these chemicals builds up depends on the type of porphyria (Anderson, 2003 and 2014; Desnick, Balwani and Anderson, 2013).
Figure 1.
The heme biosynthetic pathway showing intermediates, enzymes and types of porphyria associated with each enzyme.
Each porphyria results from altered activity of a specific enzyme of the heme biosynthetic pathway, leading to overproduction of heme precursors (Bonkovsky, Healey, and Pohl, 1990; Hahn and Bonkovsky, 1998; Anderson et al., 2011). Enzyme activity is diminished in all but one of the porphyrias; in X-linked protoporphyria (XLP), increased activity is a consequence of gain of function mutations of the first enzyme of the pathway (Ducamp et al., 2013).
1. Classification, inheritance and prevalence of the porphyrias
Heme precursors in the various types of porphyria initially accumulate in either the liver or bone marrow, which are the tissues most active in heme biosynthesis. This is the basis for classification of porphyrias as either hepatic or erythropoietic. The major clinical manifestations of the porphyrias are either neurologic, usually in the form of acute attacks, or cutaneous, resulting from phototoxicity. Based on these differences, porphyrias are classified as acute or cutaneous, with two types falling into both categories. The classifications and the major clinical features of the various porphyrias are summarized in Table 1.
Table 1.
Classifications and principal clinical features of each type of porphyria disease. Standard abbreviations are also shown.
| Porphyria | Classification | Principal Clinical Features |
|---|---|---|
| Acute intermittent porphyria (AIP) | Hepatic; Acute | Neurological |
| Hereditary coproporphyria (HCP) | Hepatic; Acute & Cutaneous | Neurological; blistering photosensitivity (uncommon) |
| Variegate porphyria (VP) | Hepatic; Acute & Cutaneous | Neurological; blistering photosensitivity (common) |
| δ-Aminolevulinic acid dehydratase porphyria (ADP) | Hepatic; Acute | Neurological |
| Porphyria cutanea tarda (PCT) | Hepatic: Cutaneous | Blistering photosensitivity |
| Hepatoerythropoietic porphyria (HEP) | Hepatic: Cutaneous | Blistering photosensitivity |
| Congenital erythropoietic porphyria (CEP) | Erythropoietic; Cutaneous | Neurological |
| Erythropoietic Protoporphyria (EPP) | Erythropoietic; Cutaneous | Nonblistering photosensitivity |
| X-linked protoporphyria (XLP) | Erythropoietic; Cutaneous | Nonblistering photosensitivity |
Mutations in the genes that encode enzymes of the heme biosynthetic pathway cause all porphyrias except for PCT, in which mutations for the affected enzyme is found in a minority of cases; one case of CEP was caused by a mutation of the transcription factor GATA-1 (Phillips et al., 2007b and 2007c; Puy, Gouya and Deybach, 2010). Autosomal dominant inheritance with low penetrance is seen in AIP, HCP, VP, EPP, and the familial form of PCT. Homozygous cases of these disorders are described, including HEP, which is the homozygous form of familial PCT. Autosomal recessive inheritance is seen in ADP, CEP and EPP, and X-linked inheritance in XLP.
Porphyrias are considered rare diseases in the United States because they afflict fewer than 200,000 people. However, their prevalence is difficult to estimate. In Europe, the prevalence of the three most common porphyrias, PCT, AIP and EPP is estimated to be 1 in 10,000, 1 in 20,000, and 1 in 50,000 to 75,000, respectively. CEP is extremely rare with prevalence estimates of 1 in 1,000,000 or less. Only 6 cases of ADP are documented (Deybach et al., 2006; European Porphyria Network, 2012; Elder et al., 2013). Hepatic porphyrias rarely cause clinical manifestations before puberty. Because erythropoietic porphyrias are generally symptomatic early in life, EPP is the most common porphyria in childhood, and is also the one associated with the longest delays in diagnosis.
The European Porphyria Network estimated prevalences based on newly diagnosed cases collected prospectively over a 3 year period (335 patients from 11 countries) (Deybach et al., 2006; European Porphyria Network, 2012; Elder et al., 2013). Prevalence was calculated from the incidence and mean disease duration. The incidence of hepato-cellular carcinoma (HCC) in acute hepatic porphyria and the prevalence of patients with recurrent acute attacks of porphyria were also investigated (Innala and Andersson, 2011). The incidence of symptomatic AIP was similar in all countries (0.13 per million per year; 95 % CI: 0.10 – 0.14) except Sweden (0.51; 95 % CI: 0.28–0.86). The incidence ratio for symptomatic AIP: VP: HCP was 1.00:0.62: 0.15. The prevalence of AIP (5.4 per million; 95 % CI: 4.5–6.3) was about half that previously reported. The prevalence of EPP was less uniform between countries and, in some countries, exceeded previous estimates. Fourteen new cases of HCC (11 from Sweden) were reported in patients with acute porphyria. Sixty seven patients (3 VP; 64 AIP: 53 females, 11 males) with recurrent attacks of acute porphyria were identified. The estimated percentage of patients with AIP that will develop recurrent acute attacks was 3–5 %. The data suggest that the prevalence of symptomatic acute porphyria may be decreasing, possibly due to improved management or less exposure to harmful drugs, whereas the prevalence of EPP may be increasing due to improved diagnosis and greater recognition.
Phototoxicity due to excess porphyrins in the skin or dermal blood vessels may occur in all porphyrias except ADP and AIP. This is understandable because the enzyme deficiencies in these two porphyrias precede porphyrin formation. However, photosensitivity develops even in AIP if complicated by advanced renal disease, which precludes urinary excretion of excess porphyrins. In most cutaneous porphyrias, porphyrin-induced photosensitivity causes chronic skin fragility and formation of vesicles or bullae on light-exposed skin. In contrast, a painful, acute and non-blistering type of photosensitivity is seen in EPP and XLP or acute erythema with burning and itching but with less chronic injury. Light that corresponds to the Soret band that maximally photoactivates porphyrins passes through window glass; therefore porphyric patients are not fully protected from skin photosensitivity by remaining indoors or in automobiles. In contrast, sunburn reactions result from light of shorter wavelength (290 to 320 nm), which is absorbed by window glass. Release of mediators and enzymes from mast cells and polymorphonuclear leukocytes and activation of complement contribute to inflammatory responses in patients with cutaneous porphyrias after light exposure (Bonkovsky, 1982; Bloomer and Bonkovsky, 1989; Anderson, 2003; Anderson and Lee, 2010).
2. Acute porphyrias
As shown in Table 1, effects on the nervous system occur in the four acute porphyrias, namely ADP, AIP, HCP and VP. Diagnosis is often delayed because the symptoms are nonspecific. AIP, HCP and VP are autosomal dominant inherited diseases whereas ADP is autosomal recessive. At the molecular level, all show extensive allelic heterogeneity with most mutations restricted to one or a few families. In some locations, founder effects have led to more common mutations; most notable are high prevalences of AIP in northern Sweden (Lee and Anvert, 1991), VP in South Africans of Dutch descent, and type-2 familial PCT in Norway (Meissner et al., 1996; Aarsand, Boman and Sandberg, 2009). Enzyme activities in AIP, HCP and VP are reduced to about 50% of normal, indicating near or complete haplodeficiency, and are much lower in ADP, in which both alleles are affected (Nordmann et al, 1997; Badminton and Elder, 2005). Many individuals who inherit AIP, HCP and VP never have clinical manifestations. However, porphyric attacks can be severe and life threatening, so the early diagnosis of carriers and affected individuals is important, so they can be educated to avoid factors that can trigger acute attacks, such as certain drugs, alcohol and fasting (Anderson, 2003 and 2014; Anderson and Lee, 2010).
AIP, HCP and VP are characterized by low clinical penetrance and environmental factors and probably genes at other loci are important in increasing susceptibility to the development of clinical disease. Family studies in France (Puy, Gouya and Deybach, 2010) and the UK suggest that about 10–20% of affected individuals develop symptoms but figures as high as 50% have been reported for AIP from Sweden (Bylesjo, Wikberg and Andersson, 2009). When minor skin lesions are taken into account, symptoms may develop in up to 40% of heterozygotes for VP mutations in South Africa (von und zu Fraunberg et al., 2002; Hift et al., 2004). Ascertainment bias may inflate these figures, since symptomatic individuals are more likely to be included in such analyses. No clear genotype–phenotype correlation has yet been established in any autosomal dominant porphyria, although there are reports that some mutations may be associated with higher penetrance (von und zu Fraunberg et al., 2002; Bylesjo, Wikberg, and Andersson, 2009).
AIP, HCP and VP are characterized by the episodic occurrence of life-threatening acute neurovisceral attacks which are identical in all three conditions. In VP and HCP, bullous skin lesions may occur with or apart from acute attacks. Skin lesions are much more common in VP than HCP. They occur in 10–50% of patients with VP, and may be the only clinical manifestation (Puy, Gouya and Deybach, 2010). In the UK, about 60% of patients with VP present only with skin lesions that are identical to those of PCT (Whatley et al., 1999). Acute attacks are rare before puberty and often provoked by identifiable precipitants, notably certain drugs, endocrine factors and alcohol. The management and prevention of porphyrias have recently been reviewed (Puy, Gouya, Deybach, 2010; Balwani and Desnick, 2012). Preventing acute attacks by advising patients to avoid porphyrogenic drugs (European Porphyria Network, 2012) and other potential precipitants is an essential part of the management of families with AIP, HCP or VP (Anderson et al., 2005).
2.1. Acute Intermittent Porphyria (AIP)
AIP (sometimes known as Swedish porphyria, pyrroloporphyria or intermittent acute porphyria) is the most common of the acute porphyrias worldwide, with an estimated prevalence of approximately 5 per 100,000 in the United States, including individuals with latent porphyria. The disease was first described by Stokvis, a Dutch physician, in 1889, who recognized that symptoms in his patient was precipitated by the barbiturate-related drug sulfonal (Wilson, 1990). Waldenström noted a high prevalence of AIP in an area of northern Sweden, and was inherited as an autosomal dominant trait (Waldenstrom, 1957; Tschudy, Valsamis, Magnussen, 1975).
AIP occurs in all races, but may be most common in northern European countries. In Europe (excluding Sweden), the incidence of newly diagnosed symptomatic individuals with AIP has been reported as 0.13 per million per year, with a calculated prevalence of 5.4 per one million (Elder et al., 2012 and 2013). In Sweden, the incidence and prevalence of AIP were about four times higher than in other parts of Europe due to a founder effect centered in Lappland (Floderus et al., 2002). The United Kingdom may be another high incidence region, with a prevalence of HMBS mutations as high as 1 per 500, with incomplete penetrance and an estimated prevalence of symptomatic disease of only 1–2 per 100,000 (Badminton and Elder, 2002). The estimated minimum prevalence of disease-specific hydroxymethylbilane synthase (HMBS) mutations in France was 597 per million (Nordmann et al, 1997; Nordmann and Puy, 2002). The penetrance of overt AIP was recently reported in Europe as 5.4 per million (Whatley and Badminton, 1993–2014; Badminton and Elder, 2002; Elder et al, 2012 and 2013; Whatley and Badminton, 2013).
2.1.1. Clinical features of AIP
Most heterozygotes remain asymptomatic throughout life. Symptoms are very rare before puberty, and are more common in women than men (Whatley and Badminton, 2013). Symptoms in AIP are due to effects on the visceral, peripheral, autonomic, and central nervous systems. They usually occur as intermittent attacks that are sometimes life-threatening (Herrick and McColl, 2005; Anderson et al., 2001 and 2005). The most common presentation is an attack of severe abdominal pain without peritoneal signs, often accompanied by nausea, vomiting, tachycardia, hypertension, anxiety and agitation (Herrick and McColl, 2005; Pischik and Kauppinen, 2009). Dark or reddish urine may be observed. Central nervous system manifestations may progress to confusion, hallucinations and seizures. The latter may be due to hyponatremia, resulting from hypothalamic involvement and inappropriately high levels of antidiuretic hormone. Peripheral neuropathy causing pain and weakness can progress to quadraparesis and respiratory embarrassment. Ordinarily the skin is not affected in AIP. However, concurrent advanced renal disease can limit porphyrin excretion and raise plasma porphyrin levels sufficiently to cause blistering lesions on sun-exposed skin. Early diagnosis is essential to avoid progression of attacks and to institute effective therapy (Herrick and McColl, 2005; Whatley and Badminton, 2013).
Factors that precipitate attacks include certain drugs, such as barbiturates, hydantoins, rifampicin and sulfonamide antibiotics, most of which are inducers of hepatic ALAS1 and cytochrome P450 enzymes (CYPs). Some women have attacks during the luteal phase of the cycle when levels of progesterone are increased. The reproductive steroids progesterone, medroxyprogesterone and testasterone are particularly potent inducers of hepatic ALAS1 (Kappas, Sassa and Anderson, 1983; Thunell, Pomp and Brun, 2007). Fasting and low calorie diets can cause attacks due to ALAS induction, and this effect is mediated by PGC-1α (Phillips and Kushner, 2005). Increased serum T4 and thyroxine binding globulin in the absence of hyperthyroidism, and elevated serum cholesterol and low-density lipoprotein (LDL), are sometimes seen.
Most people do not show symptoms of AIP attacks. Symptomatic AIP attacks, which are very rare before puberty, are more common in women than men (Whatley and Badminton, 2013). AIP manifests after puberty in women due to hormonal influences. Typically women develop symptoms after puberty in their twenties; men develop symptoms in their thirties. Attacks often result from a precipitating factor, which most likely induces ALAS1, such as drugs (especially barbiturates, sulfonamides and hydantoins), hormonal changes, or fasting. Some women have cyclic menstrual attacks, progesterone increases heme catabolism, and synthetic estrogens and progesterones induce porphyria. Severe abdominal pain is the most common symptom and other symptoms may include: nausea, vomiting, constipation, muscle weakness, urinary retention, palpitation (due to a rapid heart rate and often accompanied by hypertension), confusion, hallucinations, and seizures pain in the back, arms and legs. Symptoms develop over several hours or days. The skin is not affected (Chemmanur and Bonkovsky, 2004; Whatley and Badminton, 2013).
The most frequent presenting signs in patients with AIP are tachycardia, dark urine, confusion, and a peripheral motor deficit. The white blood cell count may be elevated because of stress or infection. Other abnormalities include increased serum T4, thyroxine binding globulin (but AIP patients are usually euthyroid, only rarely frankly thyrotoxic), and elevated cholesterol and low-density lipoprotein (LDL), simulating an exaggerated estrogen effect.
Attacks may be complicated by neurologic findings (mental changes, convulsions, and peripheral neuropathy that may progress to respiratory paralysis), and hyponatremia. Acute attacks, which may be provoked by certain drugs, alcoholic beverages, endocrine factors, calorie restriction, stress, and infections, usually resolve within two weeks. Most individuals with AIP have one or a few attacks; about 5% (mainly women) have recurrent attacks (defined as >4 attacks/year) that may persist for years. All individuals with a genetic change in the HMBS gene that predisposes to AIP are at risk of developing acute attacks; however, most never have symptoms and are said to have latent (or presymptomatic) AIP (Chemmanur and Bonkovsky, 2004; Badminton et al., 2012).
The major pathologic finding in AIP is muscle denervation. In patients with neuropathy, electromyographic (EMG) studies show a reduced motor nerve conduction velocity, and histopathology is consistent with axonal neuropathy and secondary demyelinization. Brain histopathology at autopsy may show vacuolization of neurons and focal demyelinization (Albers, Robertson and Daube, 1978; Kuo et al., 2011).
AIP is associated with increased risk for hepatocellular carcinoma, chronic hypertension and end stage renal disease (Chemmanur and Bonkovsky, 2004; Badminton et al., 2012).
2.1.2. Etiology and pathogenesis of AIP
AIP develops in some individuals who are heterozygous for mutations of porphobilinogen deaminase (PBGD; also known as hydroxymethylbilane synthase (HMBS), and formerly as uroporphyrinogen I synthase, the third enzyme in the heme biosynthetic pathway (Figure 1). The half-normal enzyme activity in all tissues results from the unaffected allele. PBGD catalyzes the assembly of four molecules of PBG to form HMB, a linear tetrapyrrole. PBGD has a unique cofactor, a dipyrrolomethane (i.e., a dipyrrole) that binds the pyrrole intermediates at the catalytic site until four additional pyrroles are assembled, forming a linear hexapyrrole, after which HMB is released (Jordan, 1990; Anderson et al., 2001). It is possible that high concentrations of PBG during exacerbations of acute porphyria may inhibit formation of the holo-deaminase and further reduce hepatic enzyme activity.
A 50% deficiency of PBGD is not sufficient by itself to cause symptoms of AIP. Other activating factors that must also be present which include, certain steroid hormones, drugs and dietary changes. During an attack of AIP, the regulatory heme pool in the liver is depleted and there is marked induction of the housekeeping form of δ-aminolevulinic acid synthase (ALAS1), the rate-limiting enzyme for heme synthesis in the liver, leading to accumulation of ALA and PBG, the two intermediates proximal to the deficient enzyme.
All individuals with a genetic change in the HMBS gene that predisposes to AIP are at risk of developing acute attacks; however, most never have symptoms and are said to have latent AIP. At least 342 different HMBS mutations have been identified in AIP (Grandchamp et al., 1989; Di Pierro et al., 2006; Whatley et al., 2009; Barbaro et al., 2012; HGMD 2014).
2.2. Hereditary Coproporphyria (HCP)
HCP is an inherited autosomal dominant and a rare recessive disorder form of hepatic porphyria and less common than AIP, though latent HCP and HCP-carriers are being increasingly recognized. The prevalence of HCP in Denmark is approximately 2 per million. HCP is the least prevalent of the three principal types of acute porphyria: AIP, VP, and HCP. However, symptoms in HCP may be less frequent than in AIP or VP. Environmental or physiologic factors play a role in the pathogenesis of acute attack (Badminton and Elder, 2005).
2.2.1. Clinical features of HCP
HCP is an acute hepatic porphyria with neurovisceral symptoms, usually occurring as attacks that are indistinguishable from those in AIP (Bissell et al., 2012). Attacks typically start with low-grade abdominal pain that slowly increases over a period of hours or days, often with nausea and vomiting. Sometimes pain is predominantly in the back or extremities. A motor neuropathy may develop over a period of days or weeks if not treated. The neuropathy and weakness is often first noted proximally in the arms and legs, and may then progress distally. Respiratory insufficiency may result from impaired innervation of the diaphragm and respiratory muscles. Acute attacks are often associated with use of certain medications, caloric deprivation, and luteal phase increases in progesterone. About 20% of those with an acute attack report photosensitivity although bullae and skin fragility are much less common than in VP (Anderson et al., 2005 and 2014; Anderson and Lee, 2010). Of 46 individuals tested in Germany with acute HCP, 90% had abdominal pain; only 13% had cutaneous findings despite substantial overproduction of coproporphyrin (Kühnel et al 2000). An earlier British study of 111 individuals with HCP reported similar findings (Brodie et al., 1977).
Although disease-causing CPOX mutations occur equally in males and females, acute attacks are much more frequent in women, mainly between ages 16 and 45 years (the years of active ovulation). Chronic cutaneous HCP is suspected in individuals with bullae and fragility of light-exposed skin which result in depigmented scars; however, the cutaneous signs occur in only a minority of heterozygotes, even during an acute attack. In general, HCP is milder than AIP and is associated with fewer attacks. For example, in 32 members of an Australian family, 14 (including 10 adults) were determined to have HCP on the basis of a high fecal coproporphyrin III/I ratio and/or low lymphocyte CPOX enzyme activity, but only one had clinical symptoms of porphyria (Blake et al., 1992).
In rare homozygous cases of HCP, a CPOX mutation is inherited from each parent, with at least one of the mutant alleles expressing some enzyme activity. Characteristically, photosensitivity and chronic neurological manifestations begin in childhood, but acute attacks are seldom described (Schmitt et al., 2005).
2.2.2. Etiology and Pathogenesis of HCP
Like AIP and VP, HCP is inherited in an autosomal dominant manner with low penetrance. Heme production in most heterozygotes appears to be adequate. HCP results from coproporphyrinogen oxidase (CPOX) deficiency. At least 64 CPOX gene mutations involving all seven exons have been identified in different HCP families, including missense and nonsense mutations, large and small deletions and insertions, and splice site mutations (Rosipal et al., 1999; Schmitt et al., 2005; HGMD, 2014), as well as a few large deletions (Whatley et al., 2009; Barbaro et al., 2012). The enzyme functions as a homodimer, and some CPOX mutations may disrupt dimer formation (Lee et al., 2005). Homozygous cases of HCP have more severe CPOX deficiency, with onset of the disease in childhood.
CPOX catalyzes the 2-step decarboxylation of coproporphyrinogen III to protoporphyrinogen IX. Certain CPOX mutations release the substrate after one decarboxylation as harderoporphyrinogen, a tricarboxylate porphyrinogen that is then oxidized to the corresponding porphyrin. Homozygotes for these mutations have some distinct features, such as hemolysis early in life, whereas heterozygotes are similar to other HCP patients.
Because of reduced penetrance, many individuals with a CPOX mutation have no signs or symptoms of HCP. Given the rarity of acute attacks of HCP relative to AIP, it is suspected that only a small minority of CPOX heterozygotes express the clinical disease.
2.3. Variegate Porphyria (VP)
This disease is termed “variegate” because it can cause severe neurovisceral symptoms or chronic blistering skin lesions that are identical to those seen in PCT (Eales, Day and Blekkenhorst, 1980; Kirsch, Meissner and Hift, 1998; Meissner, Hift and Corrigall, 2003). VP has also been referred to as porphyria variegata, South African genetic porphyria, mixed porphyria, protocoproporphyria, and porphyria cutanea tarda hereditaria (Kirsch, Meissner and Hift, 1998; Meissner, Hift and Corrigall, 2003).
VP is an autosomal dominant genetic disorder due to partially deficient activity of the mitochondrial enzyme protoporphyrinogen oxidase (PPOX) (Brenner and Bloomer, 1980; Singal and Anderson, 2013). Enzyme activity is approximately 50 percent of normal in all tissues and mitochondria-containing cells that have been examined, including lymphocytes and cultured fibroblasts (Meissner, Hift and Corrigall, 2003). Speculation that King George III of England and others in the Royal Family had VP (Macalpine and Hunter, 1966) has been discounted (Peters, 2009).
VP occurs worldwide, but its prevalence is not accurately known because many carriers of PPOX mutations remain asymptomatic. The prevalence of VP is 1 in 300 among the white population of Dutch descent in South African population, due to a founder effect (Eales, Day, and Blekkenhorst, 1980; Meissner et al., 1996; Hift et al., 1997; Meissner, Corrigall and Hift, 2012). A particular PPOX mutation (R59W) is highly prevalent in this population (Meissner et al., 1996). Lower prevalences of VP have been estimated in Finland (1.3 per 100,000) (Mustajoki, 1980) and Europe in general (0.3 per 100,000) (Elder, 1997).
2.3.1. Clinical manifestations of VP
The clinical presentation of VP is variable in nature and hence its name. Clinical manifestations are more common in women than men. VP can cause acute attacks identical to those in AIP, with abdominal pain, vomiting, constipation, hypertension, tachycardia, and neuromuscular weakness that can progress to quadriplegia and respiratory weakness (Chemmanur and Bonkovsky, 2004). Phototoxic manifestations may include bullae, erosions or ulcers after mild trauma, as in PCT.
The most common manifestation of VP is adult-onset cutaneous blistering lesions (subepidermal vesicles, bullae, and erosions that crust over and heal slowly) of sun-exposed skin, especially the hands and face. Other chronic skin findings include milia, scarring, thickening, and areas of decreased and increased skin pigmentation. Facial hyperpigmentation and hypertrichosis may occur.
Acute neurovisceral symptoms can occur any time after puberty, are highly variable, but may be similar from episode to episode in a patient with recurrent attacks. Symptoms may become chronic. The most common manifestations are abdominal pain, constipation, tachycardia, hypertension, pain in the back, chest, and extremities, anxiety, agitation, seizures, and neuromuscular weakness that may progress to quadriparesis and respiratory paralysis. (Singhal and Anderson, 2013).
Very rare homozygous cases of VP, in which a PPO mutation is inherited from each parent, manifest cutaneous photosensitivity and severe neurological manifestations beginning in childhood. At least one of the mutant alleles in the homozygous disease must express some enzyme activity. Interestingly, acute attacks are seldom described in homozygous cases (Frank et al, 1998; Kauppinen et al., 2001).
The diagnosis of VP is established by biochemical testing and confirmed by identification of a heterozygous mutation in PPOX (Singal and Anderson, 2013). Acute attacks are managed and prevented as in AIP (Anderson et al, 2005; Anderson and Lee, 2010). Treatment of acute attacks is the same as in AIP. No effective treatment for the skin is available, other than avoiding sunlight. It is important to distinguish VP or HCP from PCT, because treatment that is successful in PCT (phlebotomy or low-dose hydroxychloroquine) is not helpful in VP and HCP.
2.3.2. Etiology and pathogenesis of VP
The PPOX gene contains 13 exons and is localized to chromosome1 (Roberts et al., 1995). VP is genetically heterogeneous, with more than 140 different mutations identified (Whatley et al., 1999; Meissner, Hift and Corrigoll, 2003). In South Africa, about 95 percent of the cases are due to the R59W missense mutation, due to a well described founder effect (Meissner et al., 1996; Warnich et al., 1996). PPOX mutations in VP generally express little or no enzymatic activity. Accordingly, residual enzyme activity is due to the normal allele that is in trans to the mutant allele. Although some mutations are reported to cause a milder phenotype (von und zu Fraunberg et al., 2002), genotype-phenotype correlations are less evident in larger studies (Whatley et al., 1999).
The combined use of restriction enzyme and single-strand conformation polymorphism analysis allows a rapid and accurate diagnosis of VP in in South Africa. The mutation R59W was shown to be in association with one of four potential halotypes defined by two polymorphisms in exon 1 of of the PPOX gene which supports the founder hypothesis for VP in South Africa (Warnich et al., 1996. The diagnosis of VP is established by biochemical testing and confirmed by identification of a heterozygous mutation encoding the mitochondrial enzyme PPOX (Singal and Anderson, 2013).
2.4. δ-Aminolevulinic acid dehydratase deficiency porphyria (ADP)
ADP, also referred to as “Doss porphyria” after the investigator who described the first 2 cases, and subsequently a third, is due to a profound deficiency of ALAD (also known as PBG synthase) [EC4.2.1.24], the second enzyme in the heme biosynthetic pathway (Doss and Schmidt, 1972). ADP is the rarest type of porphyria, with only six documented cases reported worldwide (Sassa, 1998; Akagi et al., 2006; Balwani and Desnick, 2012). It is one of the acute porphyrias, and is an autosomal recessive disorder. Patients are usually compound heterozygotes for ALAD mutations. Heterozygotes may be more susceptible to toxicity from lead, which inhibits this enzyme by displacing zinc (Scinicariello et al., 2007).
2.4.1. Clinical manifestations ADP
Patients with ADP have neurovisceral symptoms, as in other acute porphyrias, and no cutaneous manifestations. Heterozygous subjects have approximately 50 percent of normal ALAD activity and are clinically asymptomatic (Bird et al., 1979). All 6 cases documented to date have been males, 4 of whom experienced onset of symptoms at about 14 years of age. Another patient was a child with neurological impairment from birth, and the sixth was an adult and disease onset associated with a myeloproliferative disorder (Sassa, 1998; Maruno et al., 2001; Doss et al., 2004). Treatment with Hemin was effective in the 4 young males with ADP, but glucose loading was not. ADP is assumed to be a hepatic porphyria, although liver transplantation was not beneficial in a child with severe disease.
In addition to decreased activity due to an inherited genetic defect, ALAD can be inhibited under a number of circumstances. The differential diagnosis of ADP should include other hepatic porphyrias (AIP, HCP and VP) and toxic states in which ALAD enzyme activity is suppressed, which include lead poisoning, hereditary tyrosinemia, zinc deficiency, smoking, alcoholism, diabetes mellitus, and chronic renal insufficiency. The ALAD enzyme activity is reduced in diabetes mellitus (Fernandez-Cuartero et al., 1999) patients, in dialysis patients with chronic liver failure (Guolo et al., 1996), and in smokers and alcoholics (McColl et al., 1981). ADP must also be differentiated from other causes of ALAD deficiency such as lead poisoning and hereditary tyrosinemia type 1, which can cause increases in ALA, coproporphyrin III and erythrocyte protoporphyrin and symptoms very much like those in acute porphyrias. Lead metal inhibits ALAD activity by displacing zinc from the enzyme (Bergdahl et al., 1997; Granick, Sassa and Kappas, 1978). Individuals with heterozygous ALAD deficiency may be more susceptible to lead poisoning (Doss, Laubenthal and Stoppler, 1984). Succinylacetone, a structural analogue of ALA and a potent inhibitor of ALAD (Sassa and Kappas, 1983), is increased in plasma and urine of patients with hereditary tyrosinemia type 1 (Lindblad, Lindstedt and Steen, 1977).
It has been estimated that approximately forty percent of children with hereditary tyrosinemia type 1 develop the signs and symptoms of ADP (Rank et al., 1987). The ALAD enzyme activity has also been shown to be reduced when rats were exposed to trichloroethylene (Fujita et al., 1984), styrene (Fujita et al., 1986), lead (Sassa, Granick, Kappas, 1975), and iron (Bonkovsky et al., 1987).
2.4.2. Etiology and pathogenesis of ADP
ALAD (ALA dehydratase; PBG synthase [EC 4.2.1.24]) catalyzes the formation of the monopyrrole PBG from two molecules of ALA, a precursor committed to synthesis of porphyrins, heme, and other tetrapyrroles. Human ALAD is a homo-octomer with a subunit size of 36,274 daltons (Wetmur et al., 1986), and is encoded by the ALAD gene localized at chromosome 9q34 (Potluri et al., 1987). The enzyme requires an intact sulfhydryl group and one zinc atom (Zn2+) per subunit for full activity (Sassa, 1982). ALAD is present in vast abundance in mammalian cells; for example in liver its activity is 80 to 100-fold greater than that of ALAS, the first and rate-limiting enzyme for hepatic heme biosynthesis (Kappas et al., 1995). The normal abundance of this enzyme explains why heterozygotes, with ~50 percent of normal ALAD activity, are asymptomatic.
On the basis of an analysis of a small population, it has been estimated that the prevalence of individuals with 50% of normal PBGS activity, putatively caused by one aberrant ALAD allele, is 2% in the normal asymptomatic population (Thunell, Holmberg, Lundgren, 1987). This study suggested that most instances of compound heterozygosity in ALAD result in spontaneous abortions. The frequency of genetic carriers for ALAD deficiency (i.e., heterozygotes) in the normal population in Sweden was estimated to be approximately 2 percent. Although subjects with heterozygosity for ALAD deficiency are asymptomatic, they may be considered to be at risk for developing ADP when exposed to environmental chemicals or toxins, which further inhibit ALAD activity (Thunell, Holmberg, Lundgren, 1987).
ALAD is markedly deficient and ALA substantially elevated in plasma and urine in patients with ADP. ALA is further metabolized to other heme precursors, and as a result coproporphyrin III is excreted in urine in large amounts and protoporphyrin IX is increased in erythrocytes. As in other acute porphyrias, the mechanism of neurological damage is not well understood.
3. Porphyria cutanea tarda (PCT)
PCT is a hepatic porphyria that causes chronic blistering skin phototoxicity, but has no neurological manifestations. Therefore, this disease is distinct from the acute hepatic porphyrias, discussed above. However, two of the acute porphyrias, HCP and VP, as well as mild cases of CEP and HEP (both discussed below), can present with skin lesions identical to those of PCT. Because PCT is the most common porphyria, it is important to differentiate it from these less common porphyrias, so appropriate management can be offered.
Both PCT and HEP (described in the next section) are due to deficient activity of hepatic uroporphyrinogen decarboxylase, the fifth enzyme in the heme biosynthetic pathway. HEP is usually a more severe disease, and is the homozygous form of familial PCT.
PCT is the most common of the porphyrias with an estimated incidence of 1 in 10,000 or 2–5 per million population in the United Kingdom (Elder, 1998; Bleasel and Varigos, 2000; Sarkany, 2001). The disease occurs in both sexes but is somewhat more common in men, with onset usually during the fifth and sixth decades.
3.1. Clinical features of PCT
Patients usually present with skin fragility and bullae on sun-exposed skin, most commonly the backs of the hands (Frank and Poblete-Gutierrez, 2010). Evidence of liver dysfunction is common, and amounts of iron in the liver are normal or increased. Risk of developing hepatocellular carcinoma is increased in PCT, and is often related in part to one or more liver-damaging susceptibility factors being greater than normal. (Bonkovsky et al., 1998; Frank and Poblete-Gutierrez, 2010).
PCT is the only porphyria that can develop in the absence of an inherited mutation of the affected enzyme. Indeed, only ~20% of patients with this condition have a heterozygous UROD mutation, which lowers UROD activity in all tissues to ~50% of normal. These mutations increase susceptibility to develop PCT, but acquisition of additional susceptibility factors are needed to reduce the hepatic enzyme activity to less than ~20% or normal and cause porphyrin accumulation. Therefore, PCT is primarily an acquired disorder in which behavioral, environmental, infectious and genetic factors interact to cause a substantial deficiency of hepatic UROD activity. PCT without UROD mutations is referred to as inherited, or type-2. However, the family history is usually negative for the disease in such patients. PCT with heterozygous UROD mutation is termed type-1. A type-3, which is rare, refers to patients with a clear family history of PCT but without UROD mutations.
PCT is also an iron-related disorder – another feature that is different from other porphyrias. The disease always occurs with a normal or increased amount of hepatic iron, and iron deficiency is protective, and the prevalence of HFE (hemochromatosis) mutations is increased. Repeated phlebotomy to reduce hepatic iron is effective treatment, and iron administration can lead to recurrence of the disease.
Multiple readily identified susceptibility factors are found in individual patients, but none is found in all (i.e. none is required for PCT to develop. These factors include the following (with their frequencies in one large US series in parentheses): alcohol (87%), smoking (81%), hepatitis C (69%), HIV infection (13%), estrogen (66% in females), HFE mutations (53%) and UROD mutations (17%) (Jalil, Grady, Lee, and Anderson, 2010). HFE mutations, alcohol and hepatitis C are known to decrease hepcidin levels and thereby increase iron absorption from the intestine. A number of these factors also increase oxidative stress and cytochrome P450 enzymes (CYPs) in hepatocytes.
PCT can develop and be reproduced in laboratory animals from exposure to certain halogenated chemicals. In the late 1950s, over 3000 people in Turkey developed toxic PCT from ingestion of the fungicide hexachlorobenzene used for preserving wheat that was intended for planting (Schmid, 1960; Cam and Nigogosyan G, 1963; Cripps et al., 1984). Smaller outbreaks of PCT from industrial exposures of other polyhalogenated hydrocarbons such as dioxins and triazine herbicides have been observed (Bleiberg et al., 1964, Oliver, 1975). Such exposures are rarely evident in sporadically occurring cases of PCT seen in clinical practice.
3.2. Etiology and pathogenesis of PCT
PCT results from inhibition of UROD in the liver. UROD is a cytosolic enzyme that catalyzes the sequential decarboxylation of the four acetic acid substituents of uroporphyrinogen to the four methyl groups of coproporphyrinogen. A single gene, mapped to chromosome 1p34, encodes UROD (Verneuil et al., 1984b and 1986). UROD is a 41-kilodalton polypeptide cytosolic enzyme that has no tissue-specific isoenzymes (Romeo et al., 1986). A porphomethene inhibitor of UROD has been identified in genetically engineered mice with homozygous HFE mutations and a heterozygous UROD mutation. The inhibitor is the product of oxidation of one of the four methene bridges of uroporphyrinogen. The oxidative process involves CYPs and requires ferrous iron (Phillips et al., 2007a).
Decreased UROD leads to increased production of 8- to 5-carboxymethyl substituted porphyrinogens (De Matteis, 1998). These compounds auto-oxidize to porphyrins, which accumulate in the liver in large amounts and are transported in plasma to the skin where they act as photosensitizers, and are excreted in urine and bile (De Matteis, 1998). Excess porphyrins in the skin interact with light of approximately 400 nm-wavelength and are activated and generate reactive oxygen species that cause skin damage and increased fragility (Grossman and Poh-Fitzpatrick, 1980; Lim, 1989).
The livers of patients with PCT contain high concentrations of uroporphyrins and hepta-carboxyl porphyrins. Most of the PCT patients have increased ferritin, serum iron, and iron-binding saturation suggesting that iron plays an important role in the pathogenesis of PCT. Liver function abnormalities are common but are usually mild, although they sometimes progress to cirrhosis and even liver cancer (Frank et al., 2001). The incidence of hepatocellular carcinoma in PCT is greater than normal. PCT is often associated with Hepatitis C infection, which can also cause these liver complications. However, liver tests are generally abnormal even in PCT patients without Hepatitis C infection (Bonkovsky et al., 1998; Frank and Poblete-Gutierrez, 2010).
Iron plays a role in the pathogenesis of PCT, although the exact mechanism is unclear. Mild to moderate systemic or liver iron overload is present in the majority of patients (Sampietro, Fiorelli, and Fargion, 1999). Over 80% of PCT patients have hepatic siderosis and elevated plasma ferritin (Grossman et al., 1979; Rocchi et al., 1986; Elder and Roberts, 1995; Siersema et al., 1995). Iron removal returns UROD activity to normal in sporadic PCT (Sweeney, 1986; Elder, Roberts, and de Salamanca, 1989). Administration of iron accelerates the onset of PCT and produces relapse in phlebotomy-induced remissions (Lundvall, 1971; Felsher, Jones, and Redeker, 1973). However, since PCT is uncommon in hereditary hemochromatosis, iron is not thought to be the sole etiological agent. Iron may increase production of peroxides and free radicals, leading to oxidation of uroporphyrinogen in the cell, direct liver damage, production of an inhibitor of UROD or direct modification of the enzyme itself (Mukerji, Pimstone, and Burns, 1984; De Matteis, 1998; Bonkovsky, 1989).
An association has been found between PCT and the hemochromatosis gene mutation C282Y (cysteine to tyrosine substitution at amino acid 282) (Fodinger and Sunder-Plassmann, 1999; Mehrany et al., 2004; Bovenschen and Vissers, 2009). The C282Y mutation has been found in approximately 44% of patients with s-PCT in the United Kingdom (Roberts et al., 1997), the Netherlands (Santos, Clevers, and Marx, 1997) and Australia (Stuart et al., 1998). An increase in the H63D mutation was found in Italy but not the C282Y mutation (Sampietro et al., 1998). In this population, the frequency of C282Y mutation was lower, and the incidence of HCV was higher. These observations have led to the hypothesis that C282Y mutation may trigger PCT independently of viral disease by promoting the accumulation of iron, whereas HCV and H63D may synergise to produce clinically manifest PCT (Sampietro et al., 1998; Elder and Worwood, 1998). Hence, the inheritance of C282Y mutation may contribute to the genetic susceptibility for PCT, precipitating PCT in predisposed individuals (Fodinger and Sunder-Plassmann, 1999).
Over 108 UROD mutations have been identified in patients with familial type-2 PCT and in HEP (Elder et al, 1981; Moran-Jimenez et al., 1996; Elder, 1998; Mendez et al., 1998). Erythrocyte UROD activity is ~50% of normal in type 2 PCT, but other factors such as increased erythropoiesis can increase UROD activity. Therefore, UROD gene sequencing has greater diagnostic accuracy for identifying those with UROD mutations (Aarsand, Boman, and Sandberg, 2009; Badenas et al., 2009). Identifying a UROD mutation is part of the complete evaluation of patients for susceptibility factors, and may account for earlier than usual onset of the disease.
Mutational analysis of UROD may not be needed for the diagnosis of PCT except in the rare instances when HEP is suspected (Moran-Jimenez et al., 1996). There is a debate about whether all patients with PCT should be offered testing to identify those with type-2 PCT (Sarkany, 2008; Aarsand, Boman, and Sandberg, 2009; Puy, Gouya, and Deybach J-C, 2010). But mutational analysis of UROD has greater diagnostic accuracy than measurement of erythrocyte UROD activity for this purpose (Aarsand, Boman, and Sandberg, 2009; Badenas et al., 2009).
Differentiation of type-2 PCT from type-1 PCT has no benefit for the individual patient since the response to treatment and prognosis of the two forms is similar. DNA results do not influence treatment, since all PCT types respond to phlebotomy or low-dose hydroxychloroquine. Screening of type-2 PCT families allow mutation carriers to be counselled about the need to avoid other susceptibility factors (Sarkany, 2008; Whatley and Badminton, 2013).
PCT is the most readily treated form of porphyria. Phlebotomies to deplete iron guided by estimating the serum ferritin level can be used to treat PCT. Low-dose hydroxychloroquine, which removes excess porphyrins from the liver, is also highly effective. Hydroxychloroquine (and chloroquine) in the amounts used for other diseases cause hepatotoxicity in PCT, and this adverse effect is largely avoided with the low-dose regimen. Time to remission is approximately the same with these treatments (Singal, Kormos-Hallberg, Lee, Sadagoparamanujam, Grady, Freeman, and Anderson, 2012)
3.3. Risk and other associated precipitating factors for PCT
The major risk factors of PCT are excess alcohol intake, cigarette smoking, increased iron (iron overload or hemochromatosis), certain chronic viral infections, especially hepatitis C virus (HCV) and human immunodeficiency virus (HIV), and estrogens. One or more of these precipitating factors can be found in over 80% of patients with PCT (Grossman et al., 1979; Elder, 1998; Egger et al., 2002; Gisbert et al., 2003). Genetic predisposition seems to an important factor since only a small proportion of people exposed to alcohol and estrogens develop PCT (De Matteis, 1998). About 20 to 25% of persons with PCT also have a genetic predisposition in the form of an inherited partial deficiency of UROD enzyme. However, such a deficiency in itself is not sufficient to produce symptomatic PCT; other factors are also needed (Anderson, 2003; Anderson and Lee, 2010).
There are several suggested mechanisms for the role of alcohol in PCT. Alcohol increases iron absorption resulting in iron accumulation in the liver, stimulates hepatic ALAS enzyme and free radical production and is independently hepatotoxic (De Matteis, 1998; Grossman and Poh-Fitzpatrick, 1980). Alcohol induces hepatic ALAS-1 in PCT patients and erythrocyte UROD activity. Alcohol also inhibits other enzymes in the heme pathway, and chronic alcoholism suppresses erythropoiesis and increases dietary iron absorption. Large amounts of porphyrins build up in the liver when the disease is becoming active. The disease becomes active when acquired factors, such as iron, alcohol, Hepatitis C Virus (HCV), HIV, estrogens (used, for example, in oral contraceptives and prostate cancer treatment) and possibly smoking, combine to cause a deficiency of UROD in the liver. Hemochromatosis, an iron overload disorder, also can predispose individuals to PCT ((De Matteis, 1998; Egger et al., 2002; Anderson and Lee, 2010).
3.4. PCT, Hepatitis C and Human Immunodeficiency Virus
HCV and HIV infections have been shown to be associated with PCT (Tsukazaki, Watanabe and Irifune, 1998; Gisbert et al., 2003). The role of HCV in PCT is not well understood, and several studies have indicated that HCV alone is insufficient to cause PCT (O’Reilly et al, 1996; Cribier et al., 1996; Gomi et al., 1997; Bonkovsky et al., 1998). A possible hypothesis was that HCV triggers symptomatic PCT in genetically predisposed individuals (Pawlotsky, Dhumeaux, and Bagot, 1995; Dabrowska et al., 1998; Lamoril et al., 1998; Moran et al., 1998; Chuang, Brashear, and Lewis, 1999; Chiaverini et al., 2003).
The HIV infection is associated with altered porphyrin metabolism and appears to be an independent risk factor for PCT. Several mechanisms have been proposed for the role of HIV in the development of PCT, including direct hepatic damage by HIV (Mansourati, Stone, and Mayer, 1999), altered porphyrin biosynthesis (Boisseau et al., 1991; Sepkowitz et al., 1995) and impairment of the cytochrome P-450 mixed function oxidase system (Wissel et al., 1987; McAlister et al., 1995). The combination of HCV and HIV seems to be more potent than either virus alone in causing abnormalities in porphyrin metabolism (O’Connor et al., 1996; Blauvelt, 1996).
4. Hepatoerythropoietic Porphyria (HEP)
HEP is a rare form of cutaneous porphyria (Romeo and Levin, 1969), with approximately 40 cases were reported up to 2010 (Cantatore-Francis et al., 2010). HEP is a homozygous or compound heterozygous form of type-2 PCT, and results from a severe UROD deficiency (Elder et al., 1981; Deybach et al., 1981; Verneuil et al., 1984b and 1986; Smith, 1986; Meguro et al., 1994; Elder, 1997; Fritsch et al., 1997; Cantatore-Francis et al., 2010). The disease occurs worldwide and in both males and females and is inherited as an autosomal recessive trait.
4.1. Clinical features of HEP
Clinical manifestations usually develop during infancy or early childhood and include extreme photosensitivity, skin fragility (bullae, erosions, and scarring) in sun-exposed areas, hypertrichosis, erythrodontia, and pink-to-red colored urine. Sclerodermoid skin changes often become evident over time. Compared to PCT, the cutaneous features of HEP typically have earlier onset, increased severity leading to disfigurement, and closer resemblance to those in CEP (Günther disease). However, extracutaneous findings including hemolytic anemia are more frequent and severe in CEP than HEP.
Cutaneous photosensitivity usually begins in early childhood and is usually more severe than in PCT, and resembles CEP (Elder et al., 1981; Lim and Poh-Fitzpatrick, 1984). Bacterial infection, scarring and photomutilation can occur. Hemolytic anemia is less frequent and severe than in CEP, but anemia due at least in part to hemolysis is common, and may be accompanied by hepatosplenomegaly ((Meguro et al., 1994; Elder, 2003). Mild anemia due at least in part to hemolysis is common, and may be accompanied by hepatosplenomegaly ((Meguro et al., 1994; Elder, 2003).
Anemia was present in >50% of HEP patients for whom hematologic status was reported (15/27), but severe anemia requiring transfusions or administration of Epoetin-α has only been observed in a few individuals (Horina and Wolf, 2000; Armstrong et al., 2004; Phillips et al., 2007c; Remenyik et al., 2008). Some patients present with hemolytic anemia and splenomegaly Hematological abnormalities may include mild, normocytic and normochromic anemia due in part to hemolysis. Mild and nonspecific abnormalities in liver function and histology are common, usually without siderosis (Desnick and Astrin, 2002; Anderson 2003; Anderson and Lee, 2010). The molecular mutational analysis is also indicated for the diagnosis of HEP (Phillips et al., 2007c). Developmental delay and seizures have been reported in HEP. A 4-year-old boy had delayed speech and language skills then presented with focal seizures and acute left hemiparesis (Parsons et al., 1994).
Onset in adulthood has been reported (Moran-Jimenez MJ, Ged C, Romana M, et al., 1996; Horina and Wolf, 2000), and such cases may be misdiagnosed as PCT. Two young adults, ages 21 and 23 years, with severe HEP developed generalized seizures and had neuroimaging evidence of cerebral cortical atrophy and calcifications in the frontal lobes, presumably related to hypoxic injury as in other porphyrias (Berenguer et al., 1997).
HEP is clinically manifest by the onset of photosensitivity within the first few years of life, scarring of sun-exposed skin and hypertrichosis (Elder et al., 1981; Lim and Poh-Fitzpatrick, 1984). Clinical features in milder and later onset cases may be indistinguishable from PCT. Most patients with HEP develop photosensitive eruptions during infancy or early childhood. Spontaneous improvement of acute photosensitivity during later childhood with persistent skin fragility has been described. Some patients have presented in the second or third decade of life with mild skin fragility or photodistributed annular plaques. Photomutilation can result in considerable morbidity in patients with HEP via impaired function of the hands and facial disfigurement, making photoprotection essential. Sclerodactyly, osteolysis and shortening of the phalanges, and progressive joint deformities can occur as components of acral photomutilation in patients with HEP, CEP, and homozygous VP. Bacterial infections and scarring with disfiguring can occur. Sun-induced erythema and blistering occurred by age 2 years in 75% of reported cases of patients. Sclerodermoid skin changes may be prominent.
Biochemical abnormalities resemble PCT, but with substantial elevations of erythrocyte protoporphyrin (predominantly zinc protoporphyrin) level. Molecular studies to identify both mutations are diagnostically important.
4.2. Etiology and pathogenesis of HEP
UROD activity is usually 10% or less than normal when measured in erythrocytes of patients with HEP (Gunther, 1967; Elder et al, 1981; Day and Strauss, 1982; Lazaro et al., 1984; de Verneuil et al, 1984; Fujita et al., 1987; Koszo et al., 1990; Meguro et al., 1994; Elder, 2003; Sassa, 2006; Cantatore-Francis et al., 2010). HEP is primarily hepatic, although a marked increase in erythrocyte zinc protoporphyrin indicates that the heme biosynthetic pathway in the bone marrow is also affected. The excess zinc protoporphyrin in erythrocytes is probably formed from pathway intermediates that accumulate during hemoglobin synthesis and are later metabolized to protoporphyrin, and then complexed with zinc.
Patients are either homozygous or compound heterozygous for UROD mutations, at least one of which must express some UROD enzymatic activity, since a null mutation would be lethal in the homozygous state (Elder et al., 1981; Verneuil et al., 1984a). Thus, null mutations are much less common in HEP than in heterozygotes with familial (type 2) PCT (Phillips et al., 2007c). Fifteen missense mutations, 2 deletions, and 1 nonsense mutation have been reported in HEP. The hepatic UROD inhibitor and susceptibility factors that are important in PCT generally do not have a significant role in HEP (Phillips et al., 2007c; Granata et al., 2009).
Since the UROD enzyme activity is markedly reduced in HEP, it is sufficient to lead to accumulation of highly carboxylated porphyrinogens and porphyrins in liver and possibly other tissues. This UROD deficiency is sufficient to lead to accumulation of highly carboxylated porphyrinogens and porphyrins in liver and possibly other tissues. HEP is the recessive form of type-2 PCT, an autosomal dominant condition in which heterozygous UROD mutations predispose carriers to clinical manifestations (Verneuil et al., 1984a).
HEP is primarily hepatic, although a marked increase in erythrocyte zinc protoporphyrin indicates that the heme biosynthetic pathway in the bone marrow is also affected. The excess zinc protoporphyrin in erythrocytes is probably formed from pathway intermediates that accumulate during hemoglobin synthesis and are later metabolized to protoporphyrin, and then complexed with zinc (Phillips et al., 2007c).
Over 40 UROD mutations have been described with some occurring in both HEP and familial PCT. Fifteen missense mutations, 2 deletions, and 1 nonsense mutation have been reported in HEP (Phillips et al., 2007c; Granata et al., 2009). Severe cutaneous findings, a HFE H63D mutation, and elevated ferritin levels were described in a 2-year-old boy with HEP (Phillips et al., 2007c). While HEP is considered as a homozygous form of familial PCT, only one of the 10 mutations identified in HEP has been shown to be associated with PCT (Garey et al., 1990; Meguro et al, 1994; Roberts et al., 1995; Moran-Jimenez et al, 1996; McManus et al., 1996).
5. Erythropoietic porphyrias
There are three erythropoietic types of porphyria. CEP is rare with skin manifestations resembling PCT, but usually much more severe. EPP is the most common porphyria in children, and on average is perhaps associated with the longest delay in diagnosis. XLP is less common and has the same phenotype as EPP, and therefore these are described together.
5.1. Congenital Erythropoietic Porphyria (CEP)
Congenital erythropoietic porphyria (CEP, Günther’s disease) is a very rare autosomal recessive disease, characterized by blistering cutaneous photosensitivity (Gunther, 1967). CEP is estimated to affect about three per ten million of the population in the UK (European Porphyria Network, 2012). Affected individuals are homozygous or compound heterozygous for UROS mutations. In one case the disease was due to mutations in the gene encoding the transcriptional regulator GATA1 (Phillips et al., 2007b and 2007c; Puy, Gouya and Deybach, 2010; Campbell et al, 2013; Whatley and Badminton, 2013).
5.1.1. Clinical features of CEP
The phenotype can vary depending on the severity of the inherited UROS mutations and levels of porphyrins in plasma and erythrocytes. Severe disease can present in utero with hydrops fetalis, or shortly after birth with red urine, severe blistering photosensitivity, hemolysis, splenomegaly and transfusion dependence. Repeated light exposure can lead to scarring, infection and loss of facial features and digits. Skeletal changes may result from bone marrow expansion and vitamin D deficiency (Fritsch et al., 1997). Milder cases and later onset forms, sometimes associated with a myeloproliferative disorder, can resemble PCT clinically (Deybach et al., 1981; Verstraeten et al., 1993; Murphy et al., 1995). It usually presents with red urine, severe blistering and haemolytic anaemia in infancy. Patients may develop photomutilation and become transfusion dependent.
In a study using 130 cases of CEP, dermal deposits of uroporphyrin frequently induced dramatic phototoxic oxygen-dependent skin damage with extensive ulcerations and mutilations. Splenomegaly and hemolytic anemia were typical internal symptoms. Skeletal changes such as osteolysis and calcifications were frequent (Fritsch et al., 1997). The disease severity in unrelated patients is markedly heterogeneous, ranging from fetal demise or severe transfusion dependency throughout life to milder adult cases with only cutaneous photosensitivity. CEP should be suspected when severe photosensitivity begins in infancy or childhood, and porphyrins are markedly increased in both erythrocytes and urine. Pink to dark reddish urine or staining of the diaper noted shortly after birth may be the first clue to the diagnosis. Clinical severity of CEP is highly variable, ranging from non-immune hydrops fetalis due to severe hemolytic anemia in utero to milder, later-onset forms, which may have only cutaneous lesions in adult life. Mild to severe hemolysis is a feature of CEP and is accompanied by anisocytosis, poikilocytosis, polychromasia, basophilic stippling, reticulocytosis, increased circulating nucleated red cells, and absence of haptoglobin. Severe cutaneous photosensitivity usually begins in early infancy and is manifested by increased friability and blistering of the epidermis on the hands and face and other sun-exposed areas. Pink or reddish-brown staining of diapers may be the first clue to the presence of this disorder. Porphyrins deposited in the teeth produce a reddish brown color in natural light, termed erythrodontia. The teeth may fluoresce on exposure to long wavelength ultraviolet light (Deybach et al., 1980; Anderson, 2003; Anderson and Lee, 2010).
5.1.2. Etiology and pathogenesis of CEP
CEP is an autosomal recessive genetic disorder charcterized by markedly reduced enzyme activity of uroporphyrinogen III synthase (UROIII-S), the fourth enzyme in the heme biosynthetic pathway. The mutations of UROIII-S gene were isolated as a full-length cDNA sequence (Desnick et al. 1998; Desnick and Astrin, 2002). CEP is characterized by severe over-production of uroporphyrin I, which manifests at birth and in the neonatal period. UROS catalyzes the conversion of hydroxymethylbilane, a linear tetrapyrrole, to the cyclic tetrapyrrole uroporphyrinogen III. In the absence of UROS, HMB undergoes spontaneous ring closure to uroporphyrinogen I, which can be further metabolized only to coproporphyrinogen-I. Therefore, the isomer-I porphyrinogens are not heme precursors. They are spontaneously oxidized to uroporphyrin-I and coproporphyrin- I, which are photosensitizing porphyrins, and cause the cutaneous manifestations of CEP (Romeo and Levin, 1969 and 1970; Deybach et al., 1981; Tanigawa, Takamura, Yamashita, 1995; Fritsch et al., 1997).
Multiple UROS mutations causing CEP have been described in different families with CEP, including single base substitutions, insertions, deletions, and splicing defects (Warner et al., 1992; Xu, Warner, Desnick, 1995; Ged et al., 1996; Desnick et al., 1998; Shady et al., 2002; Ged et al., 2004 and 2009; Berry et al., 2005). Most mutations were identified in one or a few unrelated families with the exception of C73R, L4F, and T228M which occurred in about 33%, 8%, and 7% of the mutant alleles studied, respectively. The mutation Cys73Arg, especially in homozygotes, is often associated with early onset of severe disease and transfusion-dependent hemolytic anemia (Desnick and Astrin, 2002). Prokaryotic expression of the mutant UROS alleles identified significant variation in residual activity, and elucidated genotype-phenotype correlation. In the largest series of biochemically proven CEP patients, UROS mutations were identified on 25 (74%) of 34 alleles; no mutation being identified on either allele in three patients. Low sensitivity of mutation detection in this and other case series of CEP is unexplained (Whatley and Badminton, 2013). Gain of function mutations of 5-aminolevulinate synthase 2 (ALAS2, the first enzyme of erythroid heme synthesis) can also influence the severity of CEP (To-Figueras et al., 2011; Bunn HF, 2011).
5.2. Erythropoietic Protoporphyria (EPP) and X-linked protoporphyria (XLP)
EPP, the third most common porphyria (10–20 per 100,000 individuals) and the most common in children, was first clearly described by Magnus and co-workers in 1961 (Magnus et al., 1961). The disease results from a deficiency of ferrochelatase enzyme (FECH), leading to accumulation of protoporphyrin and a painful, nonblistering form of photosensitivity. XLP is less common but has the same phenotype as EPP. It is the most recently described porphyria, and results from gain of function mutations of the erythroid-specific form on δ-aminolevulinic acid synthase (ALAS2), which catalyzes the first step in heme biosynthesis in marrow erythroid cells. EPP affects all ethnic groups without any sex predominance, but is more common in Orientals than Caucasians and much less common in Africans. Ethnic and geographic distribution of XLP has been little studied, but may be more common in the US than in Europe (Whatley and Badminton, 2013). Many persons with EPP have a mild degree of anemia suggesting iron deficiency (Goerz et al., 1996).
5.2.1. Clinical features of EPP and XLP
Cutaneous symptoms of EPP (and XLP) are usually noted in early childhood and are life long. Sunlight and to some extent other sources of visible light cause very painful skin photosensitivity. It causes very painful photosensitivity and can greatly impair quality of life. The clinical expression is highly variable. The symptoms can vary even among patients within the same family. Swelling, burning, itching, and redness of the skin may appear during or after exposure to sunlight. This can cause mild to severe burning pain on sun-exposed areas of the skin. Usually, these symptoms subside in 12 to 24 hours and heal without significant scarring. Blistering and scarring are characteristic of other types of cutaneous porphyria but are unusual in EPP. Photosensitivity is the major clinical manifestation of EPP. Skin manifestations generally begin early childhood and are more severe in the summer (Anderson, 2003; Chemmanur, Herbert and Bonkovsky, 2004; Anderson and Lee, 2010)
The need for affected individuals to avoid sunlight greatly impairs their quality of life. With sunlight exposure, the first symptom is often pain, burning or itching. Continued light exposure can cause swelling, and redness, and systemic symptoms that can last for several days. Patients are sensitive to sunlight that passes through window glass. There are usually no lasting skin manifestations such as blistering or scarring, although with repeated sun exposure the skin may become thickened especially on the backs of the hands and the face (Anderson, 2003; Chemmanur, Herbert and Bonkovsky, 2004; Anderson and Lee, 2010). Mild iron deficiency anemia is a common feature of protoporphyria, and is poorly explained (Goerz et al., 1996). At least in the UK, delay in diagnosis of EPP and XLP is greater than with any other type of porphyria (Whatley and Badminton, 2013). EPP rarely develops in adults in the presence of a myeloproliferative disorder, due to expansion of a clone of red blood cell precursors in the marrow that is FECH deficient.
The accumulation of protoporphyrin can cause liver damage when the hepatic load exceeds the canalicular excretion capacity. Approximately 5% of patients with protoporphyria develop protoporphyric hepatopathy, a severe liver disease that can progress rapidly and require liver transplantation (Sarkany and Cox, 1995; McGuire et al., 2005; Dowman et al., 2011).
Delay in diagnosis is greater than with any other type of porphyria. It affects all ethnic groups without any sex predominance. EPP is a disorder in which accumulation of protoporphyrin in erythrocytes, skin, liver and other tissues leads to lifelong, acute painful photosensitivity and, in about 2% of patients, severe liver disease. Chronic skin lesions are minor and skin fragility is absent (Todd, 1994; Cox, 2003). The most effective treatment is the prevention of the photosensitive reaction by avoiding sunlight, skin protection with clothing and occlusive sunscreen preparations. Increasing skin pigmentation with afamelanotide, an α-melanocyte stimulating hormone analogue, can significantly increase sunlight tolerance (Holme et al., 2006; Minder, 2010; Langendonk, Balwani, Anderson, et al., 2015)
5.2.2. Etiology and pathogenesis of EPP and XLP
EPP is caused by mutations in the gene encoding FECH, a mitochondrial enzyme that catalyzes the insertion of ferrous iron (Fe2+) into protoporphyrin to form heme (Todd 1994; Poh-Fitzpatrick, 1995; Cox, 1997; Elder, 1999). FECH also catalyzes the insertion of zinc, to form zinc protoporphyrin from any protoporphyrin that remains after completion of heme synthesis. In EPP, metal-free protoporphyrin accumulates in bone marrow reticulocytes, since formation of both heme and zinc protoporphyrin is impaired. Metal-free protoporphyrin enters the plasma from the bone marrow and circulating erythrocytes and is then transported to the skin, causing photosensitivity, and to the liver for excretion into bile (Bloomer et al., 1991) and hepatocytes (Bonkowsky et al., 1975). (Samuel et al., 1988; Bloomer et al., 1991). Some protoporphyrin may subsequently be reabsorbed and undergo enterohepatic circulation (Ibrahim and Watson, 1968). Because impaired FECH activity reduces formation of zinc protoporphyrin, more than ~85% of protoporphyrin measured in erythrocytes is metal-free.
More than 130 FECH mutations have been identified in EPP (HGMD, 2014). Less than half these mutations are missense; the rest are nonsense, frameshifts, large deletions or affect RNA splicing. In the UK, one particular mutation was identified in 24% of EPP families (Whatley et al., 2010). These are disabling mutations that reduce FECH activity by ~50%, which is not a sufficient reduction to cause EPP. It was discovered quite recently that most individuals with EPP have inherited a disabling FECH mutation from one parent and a low-expression (hypomorphic) FECH allele from the other (Gouya et al., 1996). The low expression intronic variant (IVS3-48C/T) produces a truncated unstable mRNA, reducing enzyme activity by 20–30% (Gouya et al., 2002); it is common in the population but by itself does not cause disease. The combination of two allelic variants reduces overall FECH activity to 35% of normal or less, causing accumulation of protoporphyrin during hemoglobin synthesis (Gouya et al., 2006). The low expression FECH allele is found in ~10% of Caucasians, is more common in Asian populations and very rare in Africa, thus accounting for the rarity of EPP among those of African descent. Rarely, two disabling mutations are present, with at least one expressing enough FECH protein to complete heme synthesis, since complete absence of heme synthesis would be lethal. It is not known why in families with two disabling FECH mutations, EPP is associated with palmar keratoderma.
EPP rarely develops in adults in the presence of a bone marrow disorder such as polycythemia vera, and is due to expansion of a clone of red blood cell precursors in the marrow that is deficient in FECH enzyme. In PCT, a number of different liver diseases may precipitate into the liver disease, whereas, in EPP, it is the porphyria itself which leads to liver disease, due to progressive deposition and accumulation of insoluble protoporphyrin-IX in hepatocytes and bile canaliculi. Excess accumulation of free protoporphyrin-IX results from partial deficiency of FECH enzyme activity. EPP is an inherited disorder with both recessive and dominant patterns of inheritance (Went and Klasen, 1984; Lamoril et al., 1991; Norris et al., 1991; Sarkany, Alexander and Cox, 1994)
XLP results from gain of function mutations of ALAS2, the only enzyme in the heme biosynthetic pathway encoded by a gene on the X-chromosome. These small, C-terminal deletion mutations lead to synthesis of ALA and protoporphyrin in amounts that exceed that needed for heme synthesis in the bone marrow. Because FECH is not deficient in XLP, some of the excess protopophryin measured in erythrocytes is zinc-chelated and only 50~85% is metal-free. On average, patients with XLP have more severe disease, with higher protoporphyrin levels and perhaps a greater risk of liver disease than do those with EPP. Heterozygous females may have normal or elevated levels of protoporphyrin depending on the degree of inactivation of the X chromosome that carries the ALAS2 mutation (Whatley et al., 2008).
Acknowledgments
Supports from NIH/FDA (5 R01 FD003720-02) and NIH (2 U54 DK083909-06) grants are gratefully acknowledged.
Literature Cited
- Aarsand AK, Boman H, Sandberg S. Familial and sporadic porphyria cutanea tarda: characterization and diagnostic strategies. Clin Chem. 2009;55:795–803. doi: 10.1373/clinchem.2008.117432. [DOI] [PubMed] [Google Scholar]
- Akagi R, Kato N, Inoue R, Anderson KE, Jaffe EK, Sassa S. Delta-Aminolevulinate dehydratase (ALAD) porphyria: the first case in North America with two novel ALAD mutations. Mol Genet Metab. 2006;87:329–36. doi: 10.1016/j.ymgme.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Albers JW, Robertson WC, Jr, Daube JR. Electrodiagnostic findings in acute porphyric neuropathy. Muscle Nerve. 1978;1(4):292–6. doi: 10.1002/mus.880010405. [DOI] [PubMed] [Google Scholar]
- Anderson KE. Approaches to treatment and prevention of human porphyrias. In: Kadish KK, Smith KM, Guilard R, editors. The Porphyrin Handbook, Vol. 14: Medical Aspects of Porphyrins. Academic Press; San Diego, CA: 2003. pp. 247–283. [Google Scholar]
- Anderson KE. Chapter 152. Clinical and laboratory diagnosis of the porphyrias. In: Ferreira GC, Kadish KM, Smith KM, Guilard R, editors. Handbook of porphyrin science with applications in chemistry, physics, materials science, engineering, biology, and medicine, Vol. 29: Porphyrias and sideroblastic anemias. World Scientific Publishing Co., Private Limited; Hackensack, NJ 07601, USA: 2014. pp. 370–406. [Google Scholar]
- Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Drummond GS, Freddara U, Sardana MK, Sassa S. Porphyrogenic effects and induction of heme oxygenase in vivo by delta-aminolevulinic acid. Biochim Biophys Acta. 1981;676:289–299. doi: 10.1016/0304-4165(81)90162-8. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Lee C. Testing for Porphyria. The American Porphyria Foundation; 2010. website ( http://www.porphyriafoundation.com/testing-for-porphyria) [Google Scholar]
- Anderson KE, Sassa S, Bishop DF, Desnick RJ. Disorders of heme biosynthesis: X-linked sideroblastic anemias and the porphyrias. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. 8. II. McGraw-Hill; New York: 2001. pp. 2991–3062. [Google Scholar]
- Anderson KE, Sassa S, Bishop DF, Desnick RJ. In: The Online Metabolic & Molecular Basis of Inherited Disease. Bishop DF, editor. McGraw-Hill; New York, NY: 2011. pp. 1–153. [Google Scholar]
- Armstrong DK, Sharpe PC, Chambers CR, Whatley SD, Roberts AG, Elder GH. Hepatoerythropoietic porphyria: a missense mutation in the UROD gene is associated with mild disease and an unusual porphyrin excretion pattern. Br J Dermatol. 2004;151(4):920–923. doi: 10.1111/j.1365-2133.2004.06101.x. [DOI] [PubMed] [Google Scholar]
- Badenas C, To-Figueras J, Phillips JD, Warby CA, Munoz C, Herrero C. Identification and characterization of novel uroporphyrinogen decarboxylase gene mutations in a large series of porphyria cutanea tarda patients and relatives. Clin Genet. 2009;75:346–53. doi: 10.1111/j.1399-0004.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badminton MN, Elder GH. Management of acute and cutaneous porphyrias. Int J Clin Pract. 2002;56:272–278. [PubMed] [Google Scholar]
- Badminton MN, Elder GH. Molecular mechanisms of dominant expression in porphyria. J Inherit Metab Dis. 2005;28:277–286. doi: 10.1007/s10545-005-8050-3. [DOI] [PubMed] [Google Scholar]
- Badminton MN, Wheatley SD, Deacon AC, Elder GH. The porphyrias and other disorders of porphyrin metabolism. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5. Elsevier Saunders; St Louis, MO: 2012. pp. 1031–1055. [Google Scholar]
- Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Blood. 2012;120(23):4496–4504. doi: 10.1182/blood-2012-05-423186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro M, Kotajärvi M, Harper P, Floderus Y. Identification of an AluY-mediated deletion of exon 5 in the CPOX gene by MLPA analysis in patients with hereditary coproporphyria. Clin Genet. 2012;81:249–256. doi: 10.1111/j.1399-0004.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- Berenguer J, Blasco J, Cardenal C, Pujol T, Cruces Prado MJ, Herrero C, Mascaró JM, de la Torre C, Mercader JM. Hepatoerythropoietic porphyria: neuroimaging findings. AJNR Am J Neuroradiol. 1997;18(8):1557–1560. [PMC free article] [PubMed] [Google Scholar]
- Bergdahl IA, Grubb A, Schütz A, Schutz A, Desnick RJ, Wetmur JG, Sassa S, Skerfving S. Lead binding to delta-aminolevulinic acid dehydratase (ALAD) in human erythrocytes. Pharmacol Toxicol. 1997;81(4):153–158. doi: 10.1111/j.1600-0773.1997.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Berry AA, Desnick RJ, Astrin KH, Shabeer J, Lucky AW, Lim HW. Two brothers with mild congenital erythropoietic porphyria due to a novel genotype. Arch Dermatol. 2005;141(12):1575–1579. doi: 10.1001/archderm.141.12.1575. [DOI] [PubMed] [Google Scholar]
- Bird TD, Hamernyik P, Nutter JY, Labbe RF. Inherited deficiency of delta-aminolevulinic acid dehydratase. Am J Hum Genet. 1979;31(6):662–668. [PMC free article] [PubMed] [Google Scholar]
- Bissell DM, Wang B, Cimino T, Lai J. Hereditary Coproporphyria. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews (Internet) University of Washington; Seattle, Washington: 2012. http://www.ncbi.nlm.nih.gov/books/NBK114807/ [Google Scholar]
- Blake D, McManus J, Cronin V, Ratnaike S. Fecal coproporphyrin isomers in hereditary coproporphyria. Clin Chem. 1992;38:96–100. [PubMed] [Google Scholar]
- Blauvelt A. Hepatitis C virus and human immunodeficiency virus infection can alter porphyrin metabolism and lead to porphyria cutanea tarda. Arch Dermatol. 1996;132:1503–1504. [PubMed] [Google Scholar]
- Bleasel NR, Varigos GA. Porphyria cutanea tarda. Aust J Dermatol. 2000;41(4):197–208. doi: 10.1046/j.1440-0960.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- Bleiberg J, Wallen M, Brodkin R, Applebaum IL. Industrially acquired porphyria. Arch Dermatol. 1964;80:793–797. doi: 10.1001/archderm.1964.01590300021006. [DOI] [PubMed] [Google Scholar]
- Bloomer JR, Bonkovsky HL. The pophyrias. Dis Mon. 1989;35:5–54. doi: 10.1016/0011-5029(89)90003-5. [DOI] [PubMed] [Google Scholar]
- Bloomer JR, Hill HD, Kools AM, Straka JG. Heme synthesis in protoporphyria. Curr Probl Dermatol. 1991;20:135–47. [PubMed] [Google Scholar]
- Bloomer J, Wang Y, Singhal A, Risheg H. Molecular studies of liver disease in erythropoietic protoporphyria. J Clin Gastroenterol. 2005;39:S167–S175. doi: 10.1097/01.mcg.0000155518.96629.ea. [DOI] [PubMed] [Google Scholar]
- Boisseau AM, Couzigou P, Forestier JF, Legrain V, Aubertin J, Doutre MS, Maleville J, Geniaux M, Beylot C. Porphyria cutanea tarda associated with human immunodeficiency virus infection. A study of four cases and review of the literature. Dermatologica. 1991;182:155–159. doi: 10.1159/000247768. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL. Porphyrin and heme metabolism and the porphyries. In: Zakim D, Boyer T, editors. Hepatology: a textbook of liver disease. Saunders; Philadelphia, PA: 1982. pp. 351–393. [Google Scholar]
- Bonkovsky HL. Mechanism of iron potentiation of hepatic uroporphyria: Studies in cultured chick embryo liver cells. Hepatology. 1989;10:354–364. doi: 10.1002/hep.1840100319. [DOI] [PubMed] [Google Scholar]
- Bonkowsky HL, Bloomer JR, Ebert PS, Mahoney MJ. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975;56:1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkovsky HL, Healey JF, Lincoln B, Bacon BR, Bishop DF, Elder GH. Hepatic heme synthesis in a new model of experimental hemochromatosis: studies in rats fed finely divided elemental iron. Hepatology. 1987;7(6):1195–1203. doi: 10.1002/hep.1840070605. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, Healey JF, Pohl J. Purification and characterization of heme oxygenase from chick liver: comparison of the avian and mammalian enzymes. Eur J Biochem. 1990;189:155–166. doi: 10.1111/j.1432-1033.1990.tb15472.x. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, Obando J, Di Bisceglie A, Tattrie C, Tortorelli K, LeClair P, Mercurio MG, Lambrecht RW. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27(6):1661–1669. doi: 10.1002/hep.510270627. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, Vissers WHPM. Primary hemochromatosis presented by porphyria cutanea tarda: a case report. Cases J. 2009;2:7246. doi: 10.4076/1757-1626-2-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DA, Bloomer JR. The enzymatic defect in variegate porphyria. Studies with human cultured skin fibroblasts. N Engl J Med. 1980;302:765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Thompson GG, Moore MR, Beattie AD, Goldberg A. Hereditary coproporphyria. Demonstration of the abnormalities in haem biosynthesis in peripheral blood. Q J Med. 1977;46:229–241. [PubMed] [Google Scholar]
- Bunn HF. UROS and ALAS-2: erstwhile partners in crime. Blood. 2011;118(6):1435–1437. doi: 10.1182/blood-2011-06-361311. [DOI] [PubMed] [Google Scholar]
- Bylesjo I, Wikberg A, Andersson C. Clinical aspects of acute intermittent porphyria in northern Sweden: a population-based study. Scand J Clin Lab Invest. 2009;69:612–618. doi: 10.1080/00365510902935979. [DOI] [PubMed] [Google Scholar]
- Cam C, Nigogosyan G. Aquired toxic porphyria cutanea tarda due to hexachlorobenzene. JAMA. 1963;183:88–91. [PubMed] [Google Scholar]
- Campbell AE, Wilkinson-White L, Mackay JP, Mathews JM, Blobel GA. Analysis of disease-causing GATA1 mutations in murine gene complementation systems. Blood. 2013;121(26):5218–5227. doi: 10.1182/blood-2013-03-488080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore-Francis JL, Cohel J, Balwani M, Khan P, Lazarus HM, Desnick RJ, Schaffer JV. Hepatoerythropoietic porphyria misdiagnosed as child abuse: cutaneous, arthritic and hematologic manifestations in siblings with a novel UROD mutation. Arch Dermatol. 2010;146(5):529–533. doi: 10.1001/archdermatol.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemmanur AT, Bonkovsky HL. Hepatic porphyrias: diagnosis and management. Clin Liver Dis. 2004;8:807–838. doi: 10.1016/j.cld.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Chiaverini C, Halimi G, Ouzan D, Halfon P, Ortonne JP, Lacour JP. Porphyria cutanea tarda, C282Y, H63D and S65C HFE genemutations and hepatitis C infection: a study from southern France. Dermatology. 2003;206(3):212–216. doi: 10.1159/000068895. [DOI] [PubMed] [Google Scholar]
- Chuang TY, Brashear R, Lewis C. Porphyria cutanea tarda and hepatitis C virus: A case-control study and meta-analysis of the literature. J Am Acad Dermatol. 1999;41:31–6. doi: 10.1016/s0190-9622(99)70402-0. [DOI] [PubMed] [Google Scholar]
- Cox TM. Erythropoietic protoporphyria. J Inherit Metab Dis. 1997;20:258–269. doi: 10.1023/a:1005317124985. [DOI] [PubMed] [Google Scholar]
- Cox T. Protoporphyria. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. Academic Press; Amsterdam: 2003. pp. 121–150. [Google Scholar]
- Cribier B, Chiaverini C, Dali-Youcef N, Schmitt M, Grima M, Hirth C, Lacour JP, Chosidow O. Porphyria cutanea tarda, hepatitis C, uroporphyrinogen decarboxylase and mutations of HFE gene. A case-control study. Dermatology. 2009;218(1):15–21. doi: 10.1159/000173696. [DOI] [PubMed] [Google Scholar]
- Cribier B, Rey D, Uhl G, Le Coz C, Hirth C, Libbrecht E, Vetter D, Lang JM, Stoll-Keller F, Grosshans E. Abnormal urinary coproporphyrin levels in patients infected by hepatitis C virus with or without human immunodeficiency virus. A study of 177 patients. Arch Dermatol. 1996;132:1448–1452. [PubMed] [Google Scholar]
- Cripps DJ, Peters HA, Gocmen A, Dogramici I. Porphyria turcica due to hexachlorobenzene: A 20–30 year follow-up study on 204 patients. Br J Dermatol. 1984;111:413–22. doi: 10.1111/j.1365-2133.1984.tb06603.x. [DOI] [PubMed] [Google Scholar]
- Dabrowska E, Jablonska-Kaszewska I, Falkiewicz B. High prevalence of hepatitis C virus infection in patients with porphyria cutanea tarda in Poland. Clin Exp Dermatol. 1998;23:95–96. doi: 10.1046/j.1365-2230.1998.00313.x. [DOI] [PubMed] [Google Scholar]
- Day RS, Strauss PC. Severe cutaneous porphyria in a 12-year-old boy: hepatoerythropoietic or symptomatic porphyria? Arch Dermatol. 1982;118:663–667. [PubMed] [Google Scholar]
- De Matteis F. Porphyria cutanea tarda of the toxic and sporadic varieties. Clin Dermatol. 1998;16:265–75. doi: 10.1016/s0738-081x(97)00206-x. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Astrin KH. Congenital erythropoietic porphyria: advances in pathogenesis and treatment. Br J Haematol. 2002;117(4):779–795. doi: 10.1046/j.1365-2141.2002.03557.x. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Balwani M, Anderson KE. Chapter 98 – Inherited Porphyrias. In: Rimoin DL, Pyeritz, Korf, editors. Emory and Rimoin’s Essential Medical Genetics. Academic Press; Kidlington, Oxford, U.K: 2013. [Google Scholar]
- Desnick RJ, Glass IA, Xu W, Solis C, Astrin KH. Molecular genetics of congenital erythropoietic porphyria. Semin Liver Dis. 1998;18(1):77–84. doi: 10.1055/s-2007-1007143. [DOI] [PubMed] [Google Scholar]
- Deybach JC, Badminton M, Puy H, Sandberg S, Frank J, Harper P, Martasek P, Minder E, Parker S, Thunell S, Elder G. European porphyria initiative (EPI): a platform to develop a common approach to the management of porphyrias and to promote research in the field. Physiol Res. 2006;55(Suppl 2):S67–S73. doi: 10.33549/physiolres.930000.55.S2.67. [DOI] [PubMed] [Google Scholar]
- Deybach JC, Grandchamp B, Grelier M, Nordmann Y, Boué J, Boué A, de Berrianger P. Prenatal exclusion of congenital erythropoietic porphyria (Gunther’s disease) in a fetus at risk. Hum Genet. 1980;53:217–21. doi: 10.1007/BF00273499. [DOI] [PubMed] [Google Scholar]
- Deybach JC, de Verneuil H, Phung N, Nordmann Y, Puissant A, Boffety B. Congenital erythropoietic porphyria (Gunther’s disease): enzymatic studies on two cases of late onset. J Lab Clin Med. 1981;97:551–558. [PubMed] [Google Scholar]
- Di Pierro E, Besana V, Moriondo V, Brancaleoni V, Tavazzi D, Casalgrandi G, Ventura P, Rocchi E, Cappellini MD. A large deletion on chromosome 11 in acute intermittent porphyria. Blood Cells Mol Dis. 2006;37:50–54. doi: 10.1016/j.bcmd.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Doss M, Laubenthal F, Stoeppler M. Lead poisoning in inherited delta-aminolevulinic acid dehydratase deficiency. Int Arch Occup Environ Health. 1984;54(1):55–63. doi: 10.1007/BF00378728. [DOI] [PubMed] [Google Scholar]
- Doss M, Schmidt A. Zwei Suchteste fur Porphyrien. Z Klin Chem Klin Biochem. 1972;10:230–231. [PubMed] [Google Scholar]
- Doss MO, Stauch T, Gross U, Renz M, Akagi R, Doss-Frank M, Seelig HP, Sassa S. The third case of Doss porphyria (delta-amino-levulinic acid dehydratase deficiency) in Germany. J Inherit Metab Dis. 2004;27(4):529–536. doi: 10.1023/B:BOLI.0000037341.21975.9d. [DOI] [PubMed] [Google Scholar]
- Dowman JK, Gunson BK, Bramhall S, Badminton MN, Newsome PN. Liver transplantation from donors with acute intermittent porphyria. Ann Intern Med. 2011;154:571–572. doi: 10.7326/0003-4819-154-8-201104190-00015. [DOI] [PubMed] [Google Scholar]
- Ducamp S, Kannengiesser C, Touati M, Garçon L, Guerci-Bresler A, Guichard JF, Vermylen C, Dochir J, Poirel HA, Fouyssac F, Mansuy L, Leroux G, Tertian G, Girot R, Heimpel H, Matthes T, Talbi N, Deybach JC, Beaumont C, Puy H, Grandchamp B. Sideroblastic anemia: molecular analysis of the ALAS2 gene in a series of 29 probands and functional studies of 10 missense mutations. Hum Mutat. 2011;32:590–597. doi: 10.1002/humu.21455. [DOI] [PubMed] [Google Scholar]
- Ducamp S, Schneider-Yin X, de Rooij F, Clayton J, Fratz EJ, Rudd A, Ostapowicz G, Varigos G, Lefebvre T, Deybach JC, Gouya L, Wilson P, Ferreira GC, Minder EI, Puy H. Molecular and functional analysis of the C-terminal region of human erythroid-specific 5-aminolevulinic synthase associated with X-linked dominant protoporphyria (XLDPP) Hum Mol Genet. 2013;22(7):1280–1288. doi: 10.1093/hmg/dds531. [DOI] [PubMed] [Google Scholar]
- Eales L, Day RS, Blekkenhorst GH. The clinical and biochemical features of variegate porphyria: an analysis of 300 cases studied at Groote Schuur Hospital, Vape Town. Int J Biochem. 1980;12:837–853. doi: 10.1016/0020-711x(80)90173-1. [DOI] [PubMed] [Google Scholar]
- Egger NG, Goeger DE, Payne DA, Miskovsy EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, Hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Digest Dis Sci. 2002;47(2):419–426. doi: 10.1023/a:1013746828074. [DOI] [PubMed] [Google Scholar]
- Elder GH. Porphyria cutanea tarda: A multifactorial disease. Recent Adv Dermatol. 1990;8:55–69. [Google Scholar]
- Elder GH. Hepatic porphyrias in children. J Inherit Metab Dis. 1997;20(2):237–246. doi: 10.1023/a:1005313024076. [DOI] [PubMed] [Google Scholar]
- Elder GH. Porphyria cutanea tarda. Semin Liver Dis. 1998;18:67–75. doi: 10.1055/s-2007-1007142. [DOI] [PubMed] [Google Scholar]
- Elder GH. The cutaneous porphyrias. In: Hawk JLM, editor. Photodermatology. Chapman & Hall; London: 1999. pp. 171–197. [Google Scholar]
- Elder GH. Porphyria cutanea tarda and related disorders. In: Kadish K, Smith K, Guilard R, editors. The Porphyrin Handbook. Vol. 14. Elsevier Science; San Diego: 2003. p. 67. [Google Scholar]
- Elder GH, Gouya L, Whatley SD, Puy H, Badminton MN, Deybach JC. The molecular genetics of erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand) 2009;55:118–126. [PubMed] [Google Scholar]
- Elder GH, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis. 2013;36:849–857. doi: 10.1007/s10545-012-9544-4. [DOI] [PubMed] [Google Scholar]
- Elder GH, Roberts AG. Uroporphyrinogen decarboxylase. J Bioenerg Biomembr. 1995;27:207–214. doi: 10.1007/BF02110035. [DOI] [PubMed] [Google Scholar]
- Elder GH, Roberts AG, de Salamanca RE. Genetics and pathogenesis of human uroporphyrinogen decarboxylase defects. Clin Biochem. 1989;22:163–168. doi: 10.1016/s0009-9120(89)80072-4. [DOI] [PubMed] [Google Scholar]
- Elder GH, Smith SG, Herrero C, Lecha M, Mascaro JM, Muniesa AM, Czarnecki DB, Brenan J, Poulos V, de Salamanca RE. Hepatoerythropoietic porphyria: a new uroporphyrinogen decarboxylase defect or homozygous porphyria cutanea tarda? Lancet. 1981;1(8226):916–919. doi: 10.1016/s0140-6736(81)91615-9. [DOI] [PubMed] [Google Scholar]
- Elder GH, Worwood M. Mutations in the hemochromatosis gene, porphyria cutanea tarda, and iron overload. Hepatology 1998. 1998;27:289–91. doi: 10.1002/hep.510270142. [DOI] [PubMed] [Google Scholar]
- European Porphyria Network. 2012 www.porphyria-europe.org.
- Felsher BF, Jones ML, Redeker AG. Iron and hepatic uroporphyrin synthesis. Relation in porphyria cutanea tarda. JAMA. 1973;226:663–665. [PubMed] [Google Scholar]
- Fernández-Cuartero B, Rebollar JL, Batlle A, Enriquez de Salamanca R. Delta aminolevulinate dehydratase (ALA-D) activity in human and experimental diabetes mellitus. Int J Biochem Cell Biol. 1999;31(3–4):479–488. doi: 10.1016/s1357-2725(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Floderus Y, Shoolingin-Hordan PM, Harper P. Acute intermittent porphyria in Sweden. Molecular, functional and clinical consequences of some new mutations found in the porphobilinogen deaminase gene. Clin Genet. 2002;62:288–297. doi: 10.1034/j.1399-0004.2002.620406.x. [DOI] [PubMed] [Google Scholar]
- Fodinger M, Sunder-Plassmann G. Inherited disorders of iron metabolism. Kidney Int Suppl. 1999;69:S22–S34. [PubMed] [Google Scholar]
- Francanzani AL, Taioli E, Sampietro M, Fatta E, Bertelli C, Fiorelli G, Fargion S. Liver cancer risk is increased in patients with porphyria cutanea tarda in comparison to matched control patients with chronic liver disease. J Hepatol. 2001;35:498–503. doi: 10.1016/s0168-8278(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Frank J, McGrath J, Lam H, Graham RM, Hawk JL, Christiano AM. Homozygous variegate porphyria: identification of both alleles of the protoporphyrinogen oxidase gene in a severely affected proband. J Invest Dermatol. 1998;110:452–455. doi: 10.1046/j.1523-1747.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- Frank J, Poblete-Gutierrez P. Porphyria cutanea tarda - when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735–745. doi: 10.1016/j.bpg.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Bolsen K, Ruzicka T, Goerz G. Congenital erythropoietic porphyria. J Am Acad Dermatol. 1997;36(4):594–610. doi: 10.1016/s0190-9622(97)70249-4. [DOI] [PubMed] [Google Scholar]
- Fujita H, Koizumi A, Hayashi N, Ikeda M. Reduced synthesis of 5-aminolevulinate dehydratase in styrene-treated rats. Biochim Biophys Acta. 1986;867(3):89–96. doi: 10.1016/0167-4781(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Fujita H, Koizumi A, Yamamoto M, Kumai M, Sadamoto T, Ikeda M. Inhibition of delta-aminolevulinate dehydratase in trichloroethylene-exposed rats, and the effects on heme regulation. Biochim Biophys Acta. 1984;800(1):1–10. doi: 10.1016/0304-4165(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Fujita H, Sassa S, Toback AC, Kappas A. Immunochemical study of uroporphyrinogen decarboxylase in a patient with mild hepatoerythropoietic porphyria. J Clin Invest. 1987;79(5):1533–1537. doi: 10.1172/JCI112985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey JR, Harrison LM, Franklin KF, Metcalf KM, Radisky ES, Kushner JP. Uroporphyrinogen decarboxylase: A splice site mutation causes the deletion of exon 6 in multiple families with porphyria cutanea tarda. J Clin Invest. 1990;86:1416–1422. doi: 10.1172/JCI114856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ged C, Megarbane H, Chouery E, Lalanne M, Megarbane A, de Verneuil H. Congenital erythropoietic porphyria: report of a novel mutation with absence of clinical manifestations in a homozygous mutant sibling. J Invest Dermatol. 2004;123:589–91. doi: 10.1111/j.0022-202X.2004.23401.x. [DOI] [PubMed] [Google Scholar]
- Ged C, Moreau-Godry F, Richard E, Robert-Richard E, de Verneuil H. Congenital erythropoietic porphyria: mutation update and correlations between genotype and phenotype. Cell Mol Biol (Noisy-le-grand) 2009;55(1):53–60. [PubMed] [Google Scholar]
- Ged C, Moreau-Gaudry F, Taine L, Hombrados I, Calvas P, Colombies P, De Verneuil H. Prenatal diagnosis in congenital erythropoietic porphyria by metabolic measurement and DNA mutation analysis. Prenatal Diagn. 1996;16:83–86. doi: 10.1002/(SICI)1097-0223(199601)16:1<83::AID-PD812>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39(4):620–627. doi: 10.1016/s0168-8278(03)00346-5. [DOI] [PubMed] [Google Scholar]
- Goerz G, Bunselmeyer S, Bolsen K, Schürer NY. Ferrochelatase activities in patients with erythropoietic protoporphyria and their families. Br J Dermatol. 1996;134(5):880–885. [PubMed] [Google Scholar]
- Gomi H, Hatanaka K, Miura T, Matsuo I. Type of impaired porphyrin metabolism caused by hepatitis C virus is not porphyria cutanea tarda but chronic hepatic porphyria. Arch Dermatol. 1997;133:1170–1171. [PubMed] [Google Scholar]
- Gouya L, Deybach JC, Lamoril J, Da Silva V, Beaumont C, Grandchamp B, Nordmann Y. Modulation of the phenotype in dominant erythropoietic protoporphyria by a low expression of the normal ferrochelatase allele. Am J Hum Genet. 1996;58:292–299. [PMC free article] [PubMed] [Google Scholar]
- Gouya L, Martin-Schmitt C, Robreau AM, Austerlitz F, Da Silva V, Brun P, Simonin S, Lyoumi S, Grandchamp B, Beaumont C, Puy H, Deybach JC. Contribution of a common single-nucleotide polymorphism to the genetic predisposition for erythropoietic protoporphyria. Am J Hum Genet. 2006;78(1):2–14. doi: 10.1086/498620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouya L, Puy H, Robreau AM, Bourgeois M, Lamoril J, Da Silva V, Grandchamp B, Deybach JC. The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet. 2002;30:27–28. doi: 10.1038/ng809. [DOI] [PubMed] [Google Scholar]
- Granata BX, Parera VE, Melito VA, Teijo MJ, Batlle AM, Rossetti MV. The very first description of a patient with hepatoerythropoietic porphyria in Argentina: biochemical and molecular studies. Cell Mol Biol (Noisy-le-grand) 2009;55(1):61–65. [PubMed] [Google Scholar]
- Grandchamp B, Picat C, Mignotte V, Wilson JH, Te Velde K, Sandkuyl L, Roméo PH, Goossens M, Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci USA. 1989;86:661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick JL, Sassa S, Kappas A. Some biochemical and clinical aspects of lead intoxication. In: Bodansky O, AL, editors. Advances in clinical chemistry. Academic Press; New York: 1978. p. 287. [DOI] [PubMed] [Google Scholar]
- Grossman ME, Bickers DR, Poh-Fitzpatrick MB, Deleo VA, Harber LC. Porphyria cutanea tarda. Clinical features and laboratory findings in 40 patients. Am J Med. 1979;67:277–86. doi: 10.1016/0002-9343(79)90403-0. [DOI] [PubMed] [Google Scholar]
- Grossman ME, Poh-Fitzpatrick MB. Porphyria cutanea tarda. Diagnosis and management. Med Clin North Am. 1980;64:807–827. doi: 10.1016/s0025-7125(16)31568-1. [DOI] [PubMed] [Google Scholar]
- Gunther WW. The porphyrias and erythropoietic porphyria: an unusual case. Australas J Dermatol. 1967;9(1):23–30. doi: 10.1111/j.1440-0960.1967.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Guolo M, Stella AM, Melito V, Parera V, Del C, Batlle AM. Altered 5-aminolevulinic acid metabolism leading to pseudoporphyria in hemodialysed patients. Int J Biochem Cell Biol. 1996;28:311–317. doi: 10.1016/1357-2725(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Hahn M, Bonkovsky HL. Disorders of porphyrin metabolism. In: Wu G, Israel J, editors. Diseases of the liver and bile ducts: a practical guide to diagnosis and treatment. Humana Press; Totowa, NJ: 1998. pp. 249–272. [Google Scholar]
- Herrick AL, McColl KEL. Acute intermittent porphyria. Best Practice Research Clin Gastroenterol. 2005;19(2):235–249. doi: 10.1016/j.bpg.2004.10.006. [DOI] [PubMed] [Google Scholar]
- HGMD. Human Gene Mutation Database. 2014 ( www.biobase-international.comproduct/hgmd)
- Hift RJ, Meissner PN, Corrigall AV, Ziman MR, Petersen LA, Meissner DM, Davidson BP, Sutherland J, Dalley HA, Kirsch RE. Variegate porphyria in South Africa, 1688–1996 – new developments in an old disease. S Afr Med J. 1997;87:722–731. [PubMed] [Google Scholar]
- Hift RJ, Meissner D, Meissner PN. A systematic study of the clinical and biochemical expression of variegate porphyria in a large South African family. Brit J Dermatol. 2004;151:465–471. doi: 10.1111/j.1365-2133.2004.06120.x. [DOI] [PubMed] [Google Scholar]
- Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol. 2006;155:574–581. doi: 10.1111/j.1365-2133.2006.07472.x. [DOI] [PubMed] [Google Scholar]
- Horina JH, Wolf P. Epoetin for severe anemia in hepatoerythropoietic porphyria. N Engl J Med. 2000;342:1294–1295. doi: 10.1056/NEJM200004273421717. [DOI] [PubMed] [Google Scholar]
- Ibrahim GW, Watson CJ. Enterohepatic circulation and conversion of protoporphyrin to bile pigment in man. Proc Soc Exp Biol Med. 1968;127:890–895. doi: 10.3181/00379727-127-32829. [DOI] [PubMed] [Google Scholar]
- Innala E, Andersson C. Screening for hepatocellular carcinoma in acute intermittent porphyria: a 15-year follow-up in northern Sweden. J Intern Med. 2011;269:538–545. doi: 10.1111/j.1365-2796.2010.02335.x. [DOI] [PubMed] [Google Scholar]
- Jaffe EK, Stith L. ALAD porphyria is a conformational disease. Am J Hum Genet. 2007;80(2):329–337. doi: 10.1086/511444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil S, Grady JJ, Lee C, Anderson KE. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8(3):297–302. doi: 10.1016/j.cgh.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM. The biosynthesis of 5-aminolevulinic acid and its transformation into coproporphyrinogen in animals and bacteria. In: Dailey HA, editor. Biosynthesis of heme and chlorophylls. McGraw-Hill; New York: 1990. p. 55. [Google Scholar]
- Kappas A, Sassa S, Anderson KE. The porphyrias. In: Stanbury JB, Wyyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The Metabolic Basis of Inherited Disease. 5. McGraw-Hill; New York: 1983. pp. 1301–1384. [Google Scholar]
- Kappas A, Sassa S, Galbraith RA, Nordmann Y. The porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. 7. Vol. 2. McGraw-Hill; New York: 1995. pp. 2103–2159. [Google Scholar]
- Kauppinen R, Timonen K, von und zu Fraunberg M, Laitinen E, Ahola H, Tenhunen R, Taketani S, Mustajoki P. Homozygous variegate porphyria: 20 y follow-up and charaterization of molecular defect. J Invest Dermatol. 2001;116(4):610–613. doi: 10.1046/j.1523-1747.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- Kirsch RE, Meissner PN, Hift RJ. Variegate porphyria. Semin Liver Dis. 1998;18:33–41. doi: 10.1055/s-2007-1007138. [DOI] [PubMed] [Google Scholar]
- Koszo F, Elder GH, Roberts A, Simon N. Uroporphyrinogen decarboxylase deficiency in hepatoerythropoietic porphyria: further evidence of genetic heterogeneity. Br J Dermatol. 1990;122(3):365–370. doi: 10.1111/j.1365-2133.1990.tb08285.x. [DOI] [PubMed] [Google Scholar]
- Kühnel A, Gross U, Doss MO. Hereditary coproporphyria in Germany: clinical-biochemical studies in 53 patients. Clin Biochem. 2000;33:465–473. doi: 10.1016/s0009-9120(00)00159-4. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Huang CC, Chu CC, Lee MJ, Chuang WL, Wu CL, Wu T, Ning HC, Liu CY. Neurological complications of acute intermittent porphyria. Eur Neurol. 2011;66(5):247–52. doi: 10.1159/000330683. [DOI] [PubMed] [Google Scholar]
- Lamoril J, Andant C, Bogard C, Puy H, Gouya L, Pawlotsky JM, Da Silva V, Soulé JC, Deybach JC, Nordmann Y. Epidemiology of hepatitis C and G in sporadic and familial porphyria cutanea tarda. Hepatology. 1998;27:848–852. doi: 10.1002/hep.510270329. [DOI] [PubMed] [Google Scholar]
- Lamoril J, Boulechfar S, de Verneuil H, Grandchamp B, Nordmann Y, Deybach JC. Human erythropoietic protoporphyria: two point mutations in the ferrochelatase gene. Biochem Biophys Res Commun. 1991;181(2):594–599. doi: 10.1016/0006-291x(91)91231-z. [DOI] [PubMed] [Google Scholar]
- Langendonk JG, Balwani M, Anderson KE, Bonkovsky HL, Anstey AV, Bissell DM, Bloomer JR, Edwards C, Neumann NJ, Parker C, Phillips J, Lim HW, Hamzavi I, Deybach J-C, Kauppinen R, Rhodes LE, Frank J, Murphy GM, Karstens FPJ, Sijbrands EJG, de Rooij FWM, Lebwohl M, Naik H, Goding CR, Wilson JHP, Desnick RJ. Afamelanotide for erythropoietic protoporphyria. New England Journal of Medicine. 2005 doi: 10.1056/NEJMoa1411481. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro P, de Slamanca RE, Elder GH, Villaseca ML, Chinarro S, Jaqueti G. Is hepatoerythropoietic porphyria a homozygous form of porphyria cutanea tarda? Inheritance of uroporphyrinogen decarboxylase deficiency in a Spanish family. Br J Dermatol. 1984;110(5):613–617. doi: 10.1111/j.1365-2133.1984.tb04687.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Anvret M. Identification of the most common mutation within the porphobilinogen deaminase gene in Swedish patients with acute intermittent porphyria. Proc Natl Acad Sci USA. 1991;8:10912–10915. doi: 10.1073/pnas.88.23.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Flachsová E, Bodnárová M, Demeler B, Martásek P, Raman CS. Structural basis of hereditary coproporphyria. Proc Natl Acad Sci U S A. 2005;102:14232–14237. doi: 10.1073/pnas.0506557102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW. Pathophysiology of cutaneous lesions in porphyrias. Semin Hematol. 1989;26:114–119. [PubMed] [Google Scholar]
- Lim HW, Poh-Fitzpatrick MB. Hepatoerythropoietic porphyria: A variant of childhood-onset porphyria cutanea tarda. Porphyrin profiles and enzymatic studies of two cases in a family. J Am Acad Dermatol. 1984;11:1103–11. doi: 10.1016/s0190-9622(84)70267-2. [DOI] [PubMed] [Google Scholar]
- Lindblad B, Lindstedt S, Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci USA. 1977;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundvall O. The effect of replenishment of iron stores after phlebotomy therapy in porphyria cutanea tarda. Acta Med Scand. 1971;189:51–63. doi: 10.1111/j.0954-6820.1971.tb04338.x. [DOI] [PubMed] [Google Scholar]
- Macalpine I, Hunter R. The “insanity” of King George 3rd: a classic case of porphyria. Br Med J. 1966;1:65–71. doi: 10.1136/bmj.1.5479.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus IA, Jarrett A, Prankerd TAJ, Rimington C. Erythropoietic protoporphyria: A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961;2:448–451. doi: 10.1016/s0140-6736(61)92427-8. [DOI] [PubMed] [Google Scholar]
- Mansourati FF, Stone VE, Mayer KH. Porphyria cutanea tarda and HIV/AIDS. A review of pathogenesis, clinical manifestations and management. Int J STD AIDS. 1999;10:51–56. doi: 10.1258/0956462991912944. [DOI] [PubMed] [Google Scholar]
- Maruno M, Furuyama K, Akagi R, Horie Y, Meguro K, Garbaczewski L, Chiorazzi N, Doss MO, Hassoun A, Mercelis R, Verstraeten L, Harper P, Floderus Y, Thunell S, Sassa S. Highly heterogeneous nature of d-aminolevulinate dehydratase (ALAD) deficiencies in ALAD porphyria. Blood. 2001;97:2972–2978. doi: 10.1182/blood.v97.10.2972. [DOI] [PubMed] [Google Scholar]
- McAlister F, McClean K, Hamilton PG, Houston S. Human immunodeficiency virus infection and porphyria cutanea tarda: Coexistence of risk factors or causative association? Clin Infect Dis. 1995;20:348–51. doi: 10.1093/clinids/20.2.348. [DOI] [PubMed] [Google Scholar]
- McColl KE, Moore MR, Thompson GG, Goldberg A. Abnormal haem biosynthesis in chronic alcoholics. Eur J Clin Invest. 1981;11:461–468. doi: 10.1111/j.1365-2362.1981.tb02014.x. [DOI] [PubMed] [Google Scholar]
- McGuire BM, Bonkovsky HL, Carithers RL, Jr, Chung RT, Goldstein LI, Lake JR, Lok AS, Potter CJ, Rand E, Voigt MD, Davis PR, Bloomer JR. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transplant 2005. 2005;11:1590–1596. doi: 10.1002/lt.20620. [DOI] [PubMed] [Google Scholar]
- McManus JF, Begley CG, Sassa S, Ratnaike S. Five new mutations in the uroporphyrinogen decarboxylase gene identified in families with cutaneous porphyria. Blood. 1996;88:3589–600. [PubMed] [Google Scholar]
- Meguro K, Fujita H, Ishida N, Akagi R, Kurihara T, Galbraith RA, Kappas A, Zabriskie JB, Toback AC, Harber LC, Sassa S. Molecular defects of uroporphyrinogen decarboxylase in a patient with mild hepatoerythropoietic porphyria. J Invest Dermatol. 1994;102:681–685. doi: 10.1111/1523-1747.ep12374134. [DOI] [PubMed] [Google Scholar]
- Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205–211. doi: 10.1016/j.jaad.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Meissner PN, Corrigall AV, Hift RJ. Fifty years of porphyria at the University of Cape Town. S Afr Med J. 2012;102:422–426. doi: 10.7196/samj.5710. [DOI] [PubMed] [Google Scholar]
- Meissner PN, Dailey TA, Hift RJ, Ziman M, Corrigall AV, Roberts AG, Meissner DM, Kirsch RE, Dailey HA. A 59W mutation in human protoporphyrinogen oxidase results in decreased enzyme activity and is prevalent in South Africans with variegate porphyria. Nat Genet. 1996;13:95–97. doi: 10.1038/ng0596-95. [DOI] [PubMed] [Google Scholar]
- Meissner P, Hift RJ, Corrigall A. Variegate porphyria. In: Kadish KM, Smith K, Guilard R, editors. Phorphyrin Handbook, Part II. Academic Press; San Diego: 2003. p. 93. [Google Scholar]
- Mendez M, Sorkin L, Rossetti MV, Astrin KH, del C Batlle AM, Parera VE, Aizencang G, Desnick RJ. Familial porphyria cutanea tarda: characterization of seven novel uroporphyrinogen decarboxylase mutations and frequency of hemochromatosis alleles. Am J Hum Genet. 1998;63:1363–1375. doi: 10.1086/302119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minder EI. Afamelanotide, an agonistic analog of alpha-melanocyte-stimulating hormone, in dermal phototoxicity of erythropoietic protoporphyria. Expert Opin Invest Drugs. 2010;19:1591–1602. doi: 10.1517/13543784.2010.535515. [DOI] [PubMed] [Google Scholar]
- Moran-Jimenez MJ, Ged C, Romana M, Enriquez de Salamanca R, Taieb A, Topi G, Alessandro LD, de Verneuil H. Uroporphyrinogen decarboxylase: complete human gene sequence and molecular study of three families with hepatoerythropoietic porphyria. Am J Hum Genet. 1996;58:712–721. [PMC free article] [PubMed] [Google Scholar]
- Moran MJ, Fontanellas A, Brudieux E, Hombrados I, de Ledinghen V, Couzigou P, de Verneuil H, De Salamanca RE. Hepatic uroporphyrinogen decarboxylase activity in porphyria cutanea tarda patients: the influence of virus C infection. Hepatology. 1998;27(2):584–589. doi: 10.1002/hep.510270237. [DOI] [PubMed] [Google Scholar]
- Mukerji SK, Pimstone NR, Burns M. Dual mechanism of inhibition of rat liver uroporphyrinogen decarboxylase activity by ferrous iron: Its potential role in the genesis of porphyria cutanea tarda. Gastroenterology. 1984;87:1248–1254. [PubMed] [Google Scholar]
- Murphy A, Gibson G, Elder GH, Otridge BA, Murphy GM. Adult-onset congenital erythropoietic porphyria (Gunther’s disease) presenting with thrombocytopenia. J R Soc Med. 1995;88:357P–358P. [PMC free article] [PubMed] [Google Scholar]
- Mustajoki P. Variegate porphyria. Twelve years’ experience in Finland. Q J Med. 1980;49(194):191–203. [PubMed] [Google Scholar]
- Nordmann Y, Puy H. Human hereditary porphyrias. Clin Chim Acta. 2002;325:17–37. doi: 10.1016/s0009-8981(02)00276-0. [DOI] [PubMed] [Google Scholar]
- Nordmann Y, Puy H, Da Silva V, Simonin S, Robreau AM, Bonaiti C, Phung LN, Deybach JC. Acute intermittent porphyria: prevalence of mutations in the porphobilinogen deaminase gene in blood donors in France. J Intern Med. 1997;242:213–217. doi: 10.1046/j.1365-2796.1997.00189.x. [DOI] [PubMed] [Google Scholar]
- Norris PG, Nunn AV, Hawk JLM, Cox TM. Genetic heterogeneity in erythropoietic protoporphyria: a study of the enzyme defect in nine affected families. J Invest Dermatol. 1991;95:260–263. doi: 10.1111/1523-1747.ep12484876. [DOI] [PubMed] [Google Scholar]
- O’Connor WJ, Murphy GM, Darby C, Fogarty J, Mulcahy F, O’Moore R, Barnes L. Porphyrin abnormalities in acquired immunodeficiency syndrome. Arch Dermatol. 1996;132:1443–1447. [PubMed] [Google Scholar]
- Oliver RM. Toxic effects of 2,3,7,8 tetrachlorodibenzo-1, 4- dioxin in laboratory workers. British J Indust Med. 1975;32:49–53. doi: 10.1136/oem.32.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly FM, Darby C, Fogarty J, O’Moore R, Courtney MG, O’Connor J, Kay EW, Leader M, Fielding JF, Murphy GM. Porphyrin metabolism in hepatitis C infection. Photodermatol Photoimmunol Photomed. 1996;12:31–33. doi: 10.1111/j.1600-0781.1996.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Parsons JL, Sahn EE, Holden KR, Pai GS. Neurologic disease in a child with hepatoerythropoietic porphyria. Pediatr Dermatol. 1994;11(3):216–221. doi: 10.1111/j.1525-1470.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Dhumeaux D, Bagot M. Hepatitis C virus in dermatology. A review. Arch Dermatol. 1995;131:1185–93. [PubMed] [Google Scholar]
- Peters T. Thomas Wakley, King George III and acute porphyria. J R Soc Med. 2009;102:506. doi: 10.1258/jrsm.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci (US A) 2007a;104:5079–84. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JD, Kushner JP. Fast track to the porphyrias. Nature Medicine. 2005;11(10):1049–1050. doi: 10.1038/nm1005-1049. [DOI] [PubMed] [Google Scholar]
- Phillips JD, Steensma DP, Pulsipher MA, Spangrude GJ, Kushner JP. Congenital erythropoietic porphyria due to a mutation in GATA1: the first transacting mutation causative for a human porphyria. Blood. 2007b;109:2618–2621. doi: 10.1182/blood-2006-06-022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JD, Whitby FG, Stadtmueller BM, Edwards CQ, Hill CP, Kushner JP. Two novel uroporphyrinogen decarboxylase (URO-D) mutations causing hepatoerythropoietic porphyria (HEP) Transl Res. 2007c;149(2):85–91. doi: 10.1016/j.trsl.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Pischik E, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol (Noise-le-grand) 2009;55(1):72–83. [PubMed] [Google Scholar]
- Poh-Fitzpatrick MB. Porphyrias. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, editors. Cutaneous medicine and surgery:an integrated program in dermatology. W.B. Saunders; Philadelphia: 1995. pp. 1753–1762. [Google Scholar]
- Potluri VR, Astrin KH, Wetmur JG, Bishop DF, Desnick RJ. Human delta-aminolevulinate dehydratase: chromosomal localization to 9q34 by in situ hybridization. Hum Genet. 1987;6(3):236–239. doi: 10.1007/BF00283614. [DOI] [PubMed] [Google Scholar]
- Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- Rank JM, Pascual-Leone A, Payne W, Glock M, Freese D, Sharp H, Bloomer JR. Hematin therapy for the neurologic crisis of tyrosinemia. J Pediatr. 1991;118(1):136–139. doi: 10.1016/s0022-3476(05)81867-0. [DOI] [PubMed] [Google Scholar]
- Remenyik E, Lecha M, Badenas C, Kószó F, Vass V, Herrero C, Varga V, Emri G, Balogh A, Horkay I. Childhood-onset mild cutaneous porphyria with compound heterozygotic mutations in the uroporphyrinogen decarboxylase gene. Clin Exp Dermatol. 2008;33(5):602–605. doi: 10.1111/j.1365-2230.2008.02734.x. [DOI] [PubMed] [Google Scholar]
- Roberts AG, Elder GH, de Salamanca RE, Herrero C, Lecha M, Mascaro JM. A mutation (G281E) of the human uroporphyrinogen decarboxylase gene causes both hepatoerythropoietic porphyria and overt familial porphyria cutanea tarda: Biochemical and genetic studies on Spanish patients. J Invest Dermatol. 1995;104:500–502. doi: 10.1111/1523-1747.ep12605953. [DOI] [PubMed] [Google Scholar]
- Roberts AG, Whatley SD, Morgan RR, Worwood M, Elder GH. Increased frequency of the haemochromatosis Cys282Tyr mutation in sporadic porphyria cutanea tarda. Lancet. 1997;349:321–323. doi: 10.1016/S0140-6736(96)09436-6. [DOI] [PubMed] [Google Scholar]
- Rocchi E, Gibertini P, Cassanelli M, Pietrangelo A, Jensen J, Ventura E. Hepatitis B virus infection in porphyria cutanea tarda. Liver. 1986;6:153–157. doi: 10.1111/j.1600-0676.1986.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Roméo PH, Raich N, Dubart A, Beaupain D, Pryor M, Kushner J, Cohen-Solal M, Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA. J Biol Chem. 1986;261:9825–9831. [PubMed] [Google Scholar]
- Romeo G, Levin EY. Uroporphyrinogen 3 cosynthetase in human congenital erythropoietic porphyria. Proc Natl Acad Sci USA. 1969;63:856–863. doi: 10.1073/pnas.63.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G, Kaback MM, Levin EY. Uroporphyrinogen 3 cosynthetase activity in fibroblasts from patients with congenital erythropoietic porphyria. Biochem Genet. 1970;4(6):659–664. doi: 10.1007/BF00486379. [DOI] [PubMed] [Google Scholar]
- Rosipal R, Lamoril J, Puy H, Da Silva V, Gouya L, De Rooij FW, Te Velde K, Nordmann Y, Martàsek P, Deybach JC. Systematic analysis of coproporphyrinogen oxidase gene defects in hereditary coproporphyria and mutation update. Hum Mutat. 1999;13:44–53. doi: 10.1002/(SICI)1098-1004(1999)13:1<44::AID-HUMU5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248–253. [PubMed] [Google Scholar]
- Sampietro M, Piperno A, Lupica L, Arosio C, Vergani A, Corbetta N, Malosio I, Mattioli M, Fracanzani AL, Cappellini MD, Fiorelli G, Fargion S. High prevalence of the His63Asp HFE mutation in Italian patients with porphyria cutanea tarda. Hepatology. 1998;27:181–184. doi: 10.1002/hep.510270128. [DOI] [PubMed] [Google Scholar]
- Samuel D, Boboc B, Bernuau J, Bismuth H, Benhamou JP. Liver transplantation for protoporphyria. Evidence for the predominant role of the erythropoietic tissue in protoporphyrin overproduction. Gastroenterology. 1988;95:816–819. [PubMed] [Google Scholar]
- Santos M, Clevers HC, Marx JJ. Mutations of the hereditary hemochromatosis candidate gene HLA-H in porphyria cutanea tarda. N Engl J Med. 1997;336:1327–1328. doi: 10.1056/NEJM199705013361817. [DOI] [PubMed] [Google Scholar]
- Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225–232. doi: 10.1046/j.1365-2230.2001.00825.x. [DOI] [PubMed] [Google Scholar]
- Sarkany RPE. Making sense of the porphyrias. Photodermatol Photoimmunol Photomed. 2008;24:102–108. doi: 10.1111/j.1600-0781.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Sarkany RPE, Alexander GJMA, Cox TM. Recessive inheritance of erythropoietic protoporphyria with liver failure. Lancet. 1994;343:1394–1395. doi: 10.1016/s0140-6736(94)92525-9. [DOI] [PubMed] [Google Scholar]
- Sarkany RP, Cox TM. Aurosomal recessive erythropoietic protoporphyria: a syndrome of severe photosensitivity and liver failure. QJM. 1995;88(8):541–549. [PubMed] [Google Scholar]
- Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28(2–3):133–145. doi: 10.1159/000459097. [DOI] [PubMed] [Google Scholar]
- Sassa S. ALAD porphyria. Semin Liver Dis. 1998;18:95–101. doi: 10.1055/s-2007-1007145. [DOI] [PubMed] [Google Scholar]
- Sassa S. Modern diagnosis and management of the porphyrias. Br J Haematol. 2006;135:281–292. doi: 10.1111/j.1365-2141.2006.06289.x. [DOI] [PubMed] [Google Scholar]
- Sassa S, Granick S, Kappas A. Effect of lead and genetic factors on heme biosynthesis in the human red cell. Ann N Y Acad Sci. 1975;244:419–440. doi: 10.1111/j.1749-6632.1975.tb41546.x. [DOI] [PubMed] [Google Scholar]
- Sassa S, Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway: Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983;71(3):625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. Cutaneous porphyria in Turkey. N Engl J Med. 1960;263:397–398. doi: 10.1056/NEJM196008252630807. [DOI] [PubMed] [Google Scholar]
- Schmitt C, Gouya L, Malonova E, Lamoril J, Camadro JM, Flamme M, Rose C, Lyoumi S, Da Silva V, Boileau C, Grandchamp B, Beaumont C, Deybach JC, Puy H. Mutations in human CPO gene predict clinical expression of either hepatic hereditary coproporphyria or erythropoietic harderoporphyria. Hum Mol Genet. 2005;4:3089–3098. doi: 10.1093/hmg/ddi342. [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Murray HE, Moffett DB, Abadin HG, Sexton MJ, Fowler BA. Lead and delta-aminolevulinic acid dehydratase polymorphism: where does it lead? A meta-analysis. Env Health Perspect. 2007;115:35–41. doi: 10.1289/ehp.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkowitz DV, Hennessey P, Torno RB, Lampert MA. Hepatic manifestations of porphyria cutanea tarda in a patient with AIDS. Am J Gastroenterol. 1995;90:658–659. [PubMed] [Google Scholar]
- Shady AA, Colby BR, Cunha LF, Astrin KH, Bishop DF, Desnick RJ. Congenital erythropoietic porphyria: identification and expression of eight novel mutations in the uroporphyrinogen III synthase gene. Br J Haematol. 2002;117:980–998. doi: 10.1046/j.1365-2141.2002.03558.x. [DOI] [PubMed] [Google Scholar]
- Siersema PD, Rademakers LH, Cleton MI, ten Kate FJ, de Bruijn WC, Marx JJ, Wilson JH. The difference in liver pathology between sporadic and familial forms of porphyria cutanea tarda: The role of iron. J Hepatol. 1995;23:259–267. [PubMed] [Google Scholar]
- Singal AK, Kormos-Hallberg C, Lee C, Sadagoparamanujam VM, Grady JJ, Freeman DJ, Anderson KE. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clinical Gastroenterology and Hepatology. 2012;10:1402–1409. doi: 10.1016/j.cgh.2012.08.038. NIHMS 417780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AK, Anderson KE. Variegate Porphyria – GeneReviews. 2013 http://www.ncbi.nlm.nih.gov/books/NBK121283/
- Smith SG. Hepatoerythropoietic porphyria. Semin Dermatol. 1986;5(2):125–137. [Google Scholar]
- Stuart KA, Busfield F, Jazwinska EC, Gibson P, Butterworth LA, Cooksley WG, Powell LW, Crawford DH. The C282Y mutation in the haemochromatosis gene (HFE) and hepatitis C virus infection are independent cofactors for porphyria cutanea tarda in Australian patients. J Hepatol. 1998;28:404–409. doi: 10.1016/s0168-8278(98)80313-9. [DOI] [PubMed] [Google Scholar]
- Sweeney GD. Porphyria cutanea tarda, or the uroporphyrinogen decarboxylase deficiency diseases. Clin Biochem. 1986;19:3–15. doi: 10.1016/s0009-9120(86)80064-9. [DOI] [PubMed] [Google Scholar]
- Tanigawa K, Takamura N, Yamashita S. Congenital erythropoietic porphyria. Nihon Rinsho. 1995;53(6):1422–1426. [PubMed] [Google Scholar]
- The drug database for acute porphyria. 2007 www.drugs-porphyria.org.
- Thunell S, Holmberg L, Lundgren J. Aminolaevulinate dehydratase porphyria in infancy. A clinical and biochemical study. J Clin Chem Clin Biochem. 1987;25(1):5–14. doi: 10.1515/cclm.1987.25.1.5. [DOI] [PubMed] [Google Scholar]
- Thunell S, Pomp, Brun A. Guide to drug porphyrogenicity prediction and drug prescription in the acute porphyrias. Br J Clin Pharm. 2007;64(5):668–679. doi: 10.1111/j.0306-5251.2007.02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ. Review: erythropoietic protoporphyria. Br J Dermatol. 1994;131:751–766. doi: 10.1111/j.1365-2133.1994.tb08577.x. [DOI] [PubMed] [Google Scholar]
- To-Figueras J, Ducamp S, Clayton J, Badenas C, Delaby C, Ged C, Lyoumi S, Gouya L, de Verneuil H, Beaumont C, Ferreira GC, Deybach JC, Herrero C, Puy H. ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood. 2011;118:1443–1451. doi: 10.1182/blood-2011-03-342873. [DOI] [PubMed] [Google Scholar]
- Touart DM, Sau P. Cutaneous deposition diseases. Part I. J Am Acad Dermatol. 1998;39:149–171. doi: 10.1016/s0190-9622(98)70069-6. [DOI] [PubMed] [Google Scholar]
- Tschudy DP, Valsamis M, Magnussen CR. Acute intermittent porphyria: clinical and sected research aspects. Ann Intern Med. 1975;83:851–864. doi: 10.7326/0003-4819-83-6-851. [DOI] [PubMed] [Google Scholar]
- Tsukazaki N, Watanabe M, Irifune H. Porphyria cutanea tarda and hepatitis C virus infection. Br J Dermatol. 1998;138:1015–1017. doi: 10.1046/j.1365-2133.1998.02269.x. [DOI] [PubMed] [Google Scholar]
- de Verneuil H, Beaumont C, Deybach JC, Nordmann Y, Sfar Z, Kastally R. Enzymatic and immunological studies of uroporphyrinogen decarboxylase in familial porphyria cutanea tarda and hepatoerythropoietic porphyria. Am J Hum Genet. 1984a;36(3):613–622. [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H, Grandchamp B, Beaumont C, Picat C, Nordmann Y. Uroporphyrinogen decarboxylase structural mutant (Gly281—Glu) in a case of porphyria. Science. 1986;234(4777):732–734. doi: 10.1126/science.3775362. [DOI] [PubMed] [Google Scholar]
- de Verneuil H, Grandchamp B, Foubert C, Weil D, N’Guyen VC, Gross MS, Sassa S, Nordmann Y. Assignment of the gene for uroporphyrinogen decarboxylase to human chromosome 1 by somatic cell hybridization and specific enzyme immunoassay. Hum Genet. 1984b;66:202–205. doi: 10.1007/BF00286601. [DOI] [PubMed] [Google Scholar]
- Verstraeten L, Van Regemorter N, Pardou A, de Verneuil H, Da Silva V, Rodesch F, Vermeylen D, Donner C, Noël JC, Nordmann Y, et al. Biochemical diagnosis of a fatal case of Gunther’s disease in a newborn with hydrops foetalis. Eur J Clin Chem Clin Biochem. 1993;31:121–128. doi: 10.1515/cclm.1993.31.3.121. [DOI] [PubMed] [Google Scholar]
- von und zu Fraunberg M, Timonen K, Mustajoki P, Kauppinen R. Clinical and biochemical characteristics and genotype-phenotype correlation in Finnish variegate porphyria patients. Eur J Hum Genet. 2002;10:649–657. doi: 10.1038/sj.ejhg.5200860. [DOI] [PubMed] [Google Scholar]
- Waldenstrom J. The porphyrias as inborn errors of metabolism. Am J Med. 1957;22(5):758–773. doi: 10.1016/0002-9343(57)90126-2. [DOI] [PubMed] [Google Scholar]
- Warner CA, Poh-Fitzpatrick MB, Zaider EF, Tsai SF, Desnick RJ. Congenital erythropoietic porphyria. A mild variant with low uroporphyrin I levels due to a missense mutation (A66V) encoding residual uroporphyrinogen III synthase activity. Arch Dermatol. 1992;128:1243–1248. doi: 10.1001/archderm.128.9.1243. [DOI] [PubMed] [Google Scholar]
- Warnich L, Kotze MJ, Groenewald IM, Groenewald JZ, van Brakel MG, van Heerden CJ, de Villiers JNP, van der Ven WJM, Schoenmakers EFPM, Taketani S, Retief A. Identification of Three Mutations and Associated Haplotypes in the Protoporphyrinogen Oxidase Gene in South African Families with Variegate Porphyria. Hum Mol Genet. 1996;5:981–984. doi: 10.1093/hmg/5.7.981. [DOI] [PubMed] [Google Scholar]
- Went LN, Klasen EC. Genetic aspects of erythropoietic protoporphyria 1984. Ann Hum Genet. 1984;48(Pt 2):105–17. doi: 10.1111/j.1469-1809.1984.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Wetmur JG, Bishop DF, Ostasiewicz L, Desnick RJ. Molecular cloning of a cDNA for human delta-aminolevulinate dehydratase. Gene. 1986;43(1–2):123–130. doi: 10.1016/0378-1119(86)90015-6. [DOI] [PubMed] [Google Scholar]
- Wetmur JG, Kaya AH, Plewinska M, Desnick RJ. Molecular characterization of the human d-aminolevulinate dehydratase 2 (ALAD2) allele: implications for molecular screening of individuals for genetic susceptibility to lead poisoning. Am J Hum Genet. 1991;49:757–763. [PMC free article] [PubMed] [Google Scholar]
- Whatley SD, Badminton MN. Acute intermittent porphyria. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Gong CT, Smith RJH, Stephens K, editors. GeneRiviews [Internet] University of Washington; Seattle: 1993–2014. [Google Scholar]
- Whatley SD, Badminton MN. Role of genetic testing in the management of patients with inherited porphyria and their families. Annals Clinical Biochem. 2013;50:204–216. doi: 10.1177/0004563212473278. [DOI] [PubMed] [Google Scholar]
- Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC, Puy H. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet. 2008;83:408–414. doi: 10.1016/j.ajhg.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley SD, Mason NG, Holme SA, Anstey AV, Elder GH, Badminton MN. Molecular epidemiology of erythropoietic protoporphyria in the U.K. Br J Dermatol. 2010;162:642–646. doi: 10.1111/j.1365-2133.2010.09631.x. [DOI] [PubMed] [Google Scholar]
- Whatley SD, Mason NG, Woolf JR, Newcombe RG, Elder GH, Badminton MN. Diagnostic strategies for autosomal dominant acute porphyrias: retrospective analysis of 467 unrelated patients referred for mutational analysis of the HMBS, CPOX, or PPOX gene. Clin Chem. 2009;55:1406–1414. doi: 10.1373/clinchem.2008.122564. [DOI] [PubMed] [Google Scholar]
- Whatley SD, Puy H, Morgan RR, Robreau AM, Roberts AG, Nordmann Y, Elder GH, Deyback JC. Variegate porphyria in Western Europe: identification of PPOX gene mutations in 104 families, extent of allelic heterogeneity, and absence of correlation between phenotype and type of mutation. Am J Hum Genet. 1999;65:984–994. doi: 10.1086/302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel PS, Sordillo P, Anderson KE, Sassa S, Savillo RL, Kappas A. Porphyria cutanea tarda associated with the acquired immune deficiency syndrome. Am J Hematol. 1987;25:107–13. doi: 10.1002/ajh.2830250112. [DOI] [PubMed] [Google Scholar]
- Xu W, Warner CA, Desnick RJ. Congenital erythropoietic porphyria: identification and expression of 10 mutations in the uroporphyrinogen III synthase gene. J Clin Invest. 1995;95:905–912. doi: 10.1172/JCI117742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J. Dominant versus recessive: molecular mechanisms in metabolic disease. J Inherit Metab Dis. 2008;31:599–618. doi: 10.1007/s10545-008-1016-5. [DOI] [PubMed] [Google Scholar]