Abstract
The molecular mechanisms underlying neuronal death are poorly understood. One of the most widely used models to study neuronal death are cultured cerebellar granule neurons (CGNs) which undergo apoptosis when switched from a medium containing depolarizing levels of potassium (HK) to a medium with low non-depolarizing levels of potassium (LK). Previously, other labs have used DNA microarray analysis to characterize gene expression changes in LK-treated CGNs. However, microarray analysis is only capable of measuring the status of known transcripts, and expression of low-abundance mRNAs is often not detected by the hybridization-based approach. We have used RNA-sequencing to conduct a more detailed and comprehensive analysis of gene expression changes in CGNs induced to die by LK treatment. RNA-seq investigates the status of both known transcripts as well as exploring new ones and is substantially more sensitive than the microarray approach. We have found that the expression of 4334 genes is significantly altered in LK-treated CGNs with 2199 being up-regulated while 2135 are down-regulated. Genes functioning in cell death and survival regulation, cell growth and proliferation and molecular transport were most affected by LK treatment. Further, a large number of genes involved in nervous system development and function were also deregulated. Analysis of signaling pathways that were affected in LK-induced death included but were not limited to mitochondrial dysfunction and oxidative phosphorylation, consistent with a number of studies showing perturbations of these pathways in neurodegenerative disorders. Thus, our study identifies a large number of new genes that are affected during the process of neuronal death. While a majority of these changes may reflect consequences of the induction of neuronal death, many of the genes that we have identified are likely to be critical and potentially novel mediators of neuronal death, including death associated with neurodegenerative disease.
Keywords: Cerebellar granule neurons, apoptosis, potassium withdrawal, RNA-sequencing
Introduction
During development of the vertebrate nervous system, about half of all the neurons that are produced are eliminated. This large scale death of neurons serves to ensure proper formation of the nervous system by matching neurons to the target that they innervate and to match innervation to target size.1 While necessary during nervous system development, abnormal loss of neurons is a characterizing feature of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis.2 Although the molecular events that lead to neuronal death in each of these neurodegenerative diseases is distinct, the downstream apoptotic process through which neurons die in these disorders is believed to share commonalities to each other as well as to developmentally-regulated neuronal death.3 Definitely, the mechanisms underlying neuronal death will therefore not only help to understand how neurons die during development, but also the mechanisms regulating neuronal death in various degenerative brain disorders.
Cultures of cerebellar granule neurons (CGNs) have been widely used to study the molecular mechanisms underlying neuronal viability. These neurons are kept healthy and alive by the addition of depolarizing levels of potassium (HK).4–7 A switch of the medium to low, non-depolarizing levels of potassium (LK) induces apoptosis. Survival by elevated potassium, or HK, mimics the survival-promoting effect of neuronal activity during normal neurodevelopment.8 As in other model systems of neuronal apoptosis,9,10 cell death in this model is believed to require new gene induction because treatment with transcriptional and translational inhibitors can protect neurons from LK-induced death.11 Other investigators have used DNA microarray analysis with the CGN model to identify transcriptional alterations that are associated with neuronal death,12,13 however, this method is only capable of measuring the status of known transcripts and does not allow for studying changes in global gene expression. Moreover, expression of mRNAs that are expressed at low levels is often not detected by the hybridization-based microarray analysis. To conduct a more detailed and comprehensive analysis of transcriptome alterations during neuronal death, we have used RNA-Seq analysis, a technique that investigates the status of both known transcripts and allows for exploring new ones.14 Because of low background signal, RNA-Seq can detect expression and alterations of expression of low abundance transcripts.14–17 Consistent with these advantages, using RNA-Seq we report that the extent of transcriptional changes during neuronal apoptosis is much larger than previously described using microarray technology.12,13 We have also looked at the signaling network and pathways that are affected during neuronal death as well as upstream regulators that are likely to be involved. We believe that this study identifies molecules that have thus far not been described as regulators of neuronal death, and may play a key role during developmentally-regulated neuronal death or in the pathogenesis of neurodegenerative disease.
Materials and methods
Primary neuronal culture, treatments and viability assay
Cultures enriched in CGNs were prepared from cerebella of seven-day-old rat pups and were plated in basal Eagle's minimal medium supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 0.2% gentamycin, and 25 mmol/L KCl as described previously.5 To prevent proliferation of non-neuronal cells, 10 µmol/L cytosine arabinoforanoside was added to the culture medium 18–22 h after plating. At seven days in vitro, CGN cultures were washed and incubated in the serum-free basal Eagle’s minimal medium supplemented with either 25 mmol/L KCl (high potassium, HK) or without KCl (low potassium, LK) for 6 h, a time, when almost half of the neurons are committed to die and can no longer be rescued. For viability assay, cells were fixed, immunocytochemistry was performed, and viability was quantified by cell morphology using 4′6-diamidino-2-phenylindole hydrochloride (DAPI) staining. Cells with condensed or fragmented nuclei were scored as dead. Unless mentioned otherwise, all viability experiments were performed in duplicate and each experiment was repeated three times. For each experiment, ≥200 cells were counted.
RNA preparation and RT-PCR
Total RNA was isolated from the cultured neurons treated with either HK or LK using Trizol reagent (Life Technologies, CA) according to the manufacturer’s guidelines. For reverse transcription, 3 µg of total RNA was used and cDNA was prepared using Superscript cDNA synthesis Kit (Life Technologies, CA). Resulting cDNA was used as a template for PCR to validate the expression data obtained from RNA-sequencing analysis. All the primer pairs were designed using primer blast and sequences are mentioned in Supplementary Table S1.
RNA-sequencing
For sequencing, total RNA was used from either HK- or LK-treated primary neurons. After an initial quality check on Bioanalyzer (Agilent Technologies, CA), 1 µg of total RNA was used and library was constructed using the Trueseq RNA sample preparation kit from Illumina according to the manufacturer’s protocol. Triplicate samples of each condition (HK and LK; S1, S2 and S3) were amplified and profile checked for size distribution and peak concentration on bioanalyzer. Amplified cDNA fragment libraries were sequenced on an Illumina HiSeq2000 at the University of Texas Southwestern Medical Center sequencing facility using default parameters (single end, forward sequencing).
Differential gene expression analysis
Quality assessment of the raw sequencing reads was done using NGS-QC-Toolkit.18 Sequencing reads with quality score under Phred Score < 20 were discarded. The quality filtered reads were then aligned to rat reference genome RGSC_v3.4 (rn4) using Bowtie2 (v 2.0.6) aligner.19 Differential gene expression analysis was done using DESeq (v 1.10.1)20 following the protocols outlined in Anders et al.21 Read counts are normalized by taking the median of each gene count across samples and dividing each sample gene count by the relative ratio of library sizes between the calculated median and sample size. Gene counts are scaled to counts per million. Fold change values, raw P values, and P values adjusted for multiple testing using Benjamini-Hochberg are calculated for each gene. Genes were considered significantly differentially-expressed if they had a false discovery rate (FDR) of less than 5%.
Significant gene ontology (GO) and pathway enrichment analysis
DAVID as well as ingenuity pathways analysis (IPA; Ingenuity Systems, CA) gene functional annotation and classification tool was used to annotate the list of differentially-expressed genes with respective GO terms and GO enrichment analysis was performed for molecular and biological functional categories.22 A Rattus norvegicus specific set of 3797 genes was used as background to calculate the enriched GO functional categories for the differentially-expressed genes identified through the RNA-Seq experiment. Functional GO groups were selected for significance by using a FDR cutoff of 5%.
Significant pathway enrichment analysis was performed using IPA. Differentially-expressed genes from the RNA expression data are associated with a biological function supported by at least one publication in the Ingenuity Pathways Knowledge Base. Fisher’s exact test is then used to calculate the P value and determine the probability that each biological function is enriched in the data set due to chance alone. Statistically significant biological pathways were then identified by selection for pathways with Benjamini-Hochberg adjusted P values < 0.05.
Upstream regulator analysis was done to identify common transcriptional regulators that can explain the observed gene expression data. The analysis was based on using prior knowledge of expected effects between transcriptional regulators and their target genes stored in the ingenuity knowledge base. The analysis examines how many known targets of each transcriptional regulator are present in the RNA expression data and also compares the direction of expression as expected from literature of the relevant transcriptional regulator. Observed gene expression changes can be consistent with the expected direction of expression of which can be in one of two transcriptional regulator activity states: activated or inhibited. Molecules classified as upstream transcriptional regulators include transcription factors, microRNA, kinases, and drugs. Functional significance of upstream transcriptional regulators is determined by using a z-score value that measures how well the observed gene expression data matches the literature-derived regulation direction. The absolute value of the z-score determines the likelihood that the regulator predicts the observed expression data was not by chance with positive z-score values indicating regulatory activation and negative z-score values for regulatory inhibition. Fisher’s exact test is used in a similar manner as the biological pathway analysis to calculate the P value and identify statistically significant upstream regulators (P value < 0.05).
Results
RNA-Seq analysis and global gene expression profiles in neurons primed to die
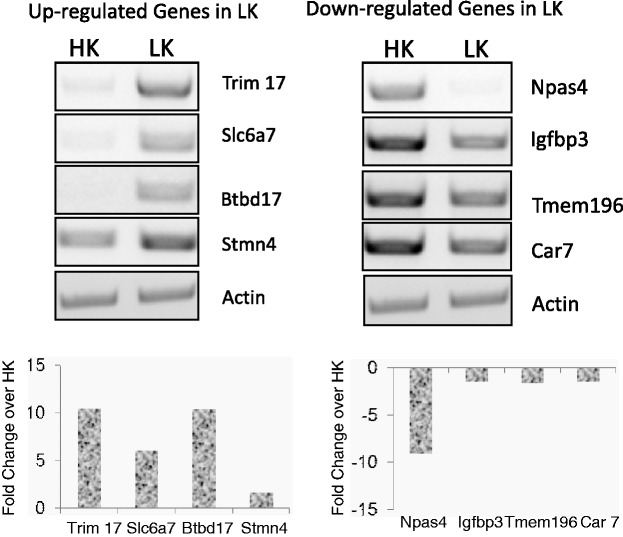
Switching cultured CGNs from HK medium to LK medium induces neuronal apoptosis. Although cell death is not observed until 12 h, the neurons are committed to death between 4 and 6 h of LK-treatment.23–25 We have therefore conducted our RNA-Seq analysis using RNA from HK- and LK-treated cultures prepared at 6 h after treatment. Three RNA samples from three separate CGN cultures were used. We confirmed by RT-PCR analysis that expression of c-jun, a widely recognized marker of neuronal death, was robustly induced in the three RNA samples before RNA-Seq was conducted (data not shown). The three sets of HK and LK samples were sequenced on one lane of the Illumina HiSeq 2000 platform (Illumina, San Diego, CA). Raw reads were mapped to the reference rat genome (rn4). Around 70% of total reads were successfully mapped to the reference genome. Detailed mapping statistics are listed in Table 1. First, clustering analysis of the expression data shows the samples are grouped according to their HK or LK classification. Correlation matrix of the six samples from the three sets of CGN cultures are given in supplementary data (Figure S1). Using criteria of FDR adjusted P value of less than 0.05, a total number of 4334 genes were identified to be differentially-expressed in the two conditions (Table 2). Out of these differentially-expressed genes, 2199 genes were up-regulated and 2135 genes were down-regulated under LK condition. The 10 most up- and down-regulated genes in LK are listed in Tables 3 and 4. A complete list of genes that are significantly regulated is provided in supplementary data (Tables S2–S4). To validate our sequencing data, we performed RT-PCR analysis using RNA from HK and LK-treated CGN cultures for four genes which, based on our RNA-Seq results, were up-regulated in LK (Trim 17, Slc6a7, Btbd17, Stmn4), and four more that were found to be down-regulated (Npas4, Igfbp3, Tmem 196, Car7) (Figure 1). These genes were chosen because they are potentially relevant to neuronal death but had not been previously focused as neuronal death-causing molecule. For example, Slc6a7 (solute carrier family 6 [neurotransmitter transporter], member 7), is a member of gamma-aminobutyric acid (GABA) neurotransmitter gene family and functions as a proline transporter. Stmn4 (Stathmin like 4) exhibits microtubule-destabilizing activity. The insulin-like growth factor binding proteins (IGFBP) family represents a family of conserved proteins that share the ability to bind IGF-I and IGF-II and are known to either inhibit or potentiate the action of IGF.26 Npas4 is an activity-dependent neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. Although the extent of alteration was not as much as indicated by RNA-Seq, we confirmed using RT-PCR that the expressions of these genes were either up- or down-regulated in accordance with the RNA-Seq data. We further validated our RNA-Seq data by examining whether our list of differentially-expressed genes included those already known in the literature to be deregulated during neuronal death. For example, c-Jun, Igfbp1, Egr-1, Caspase-3, Caspase-6 and p73, which are known to be up-regulated27–31 showed a significantly increased expression in our RNA seq data (Table S3). On the other hand, expression of Igfbp5, c-Fos and Npas4, previously reported to be down-regulated,32,33 was in the list significantly down-regulated genes (Table S4). These results provide confidence that the RNA-Seq analysis was successful in identifying genes that are differentially-expressed in CGNs during LK-induced death.
Table 1.
Detailed mapping statistics
| High potassium (HK) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mapped | Uniquely mapped | % of total mapped | |||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| Exon–exon | 4,757,117 | 4,495,316 | 3,736,405 | 4,313,864 | 4,079,542 | 3,385,667 | 16.91 | 16.87 | 16.01 |
| Exon–intron | 226,170 | 199,771 | 201,238 | 215,838 | 190,137 | 192,172 | 0.8 | 0.75 | 0.86 |
| Total exon | 23,908,062 | 22,694,951 | 19,824,076 | 22,084,046 | 20,997,447 | 18,393,002 | 84.96 | 85.19 | 84.97 |
| Total intron | 4,231,747 | 3,944,936 | 3,507,883 | 3,460,041 | 3,206,800 | 2,897,428 | 15.04 | 14.81 | 15.03 |
| Total gene | 28139,809 | 26,639,887 | 23,331,959 | 25,544,087 | 24,204,247 | 21,290,430 | 100 | 100 | 100 |
| Low potassium (LK) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mapped | Uniquely mapped | % of total mapped | |||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| Exon–exon | 4,328,289 | 4,105,310 | 3,853,533 | 3,924,946 | 3,738,989 | 3,493,850 | 16.27 | 16.11 | 15.29 |
| Exon–intron | 210,037 | 199,432 | 203,481 | 199,715 | 189,776 | 193,824 | 0.79 | 0.78 | 0.81 |
| Total exon | 22,578,563 | 21,682,543 | 21,465,130 | 20,960,096 | 20,195,084 | 20,021,671 | 84.89 | 85.08 | 85.2 |
| Total intron | 4,018,031 | 3,802,487 | 3,729,792 | 3,184,725 | 3,000,245 | 2,983,964 | 15.11 | 14.92 | 14.8 |
| Total gene | 26,596,594 | 25,485,030 | 25,194,922 | 24,144,821 | 23,195,329 | 23,005,635 | 100 | 100 | 100 |
Table 2.
Differentially-expressed gene list.a
| Differential expression | Gene count |
|---|---|
| Up-regulated genes | 2199 |
| Down-regulated genes | 2135 |
| Total genes | 4334 |
FDR cutoff of adjusted P value < 0.05.
Table 3.
Top 10 up-regulated genes in LK.a
| Genes | Fold change | FDR |
|---|---|---|
| Klrd1 | 82.4881 | 1.94E−07 |
| Slc17a1 | 79.85099 | 2.33E−07 |
| Ccin | 74.27893 | 3.21E−06 |
| Calhm1 | 71.5474 | 1.92E−07 |
| Sohlh1 | 50.58228 | 3.44E−04 |
| Stra6 | 47.56484 | 1.14E−07 |
| Slc17a3 | 46.26347 | 4.76E−54 |
| Dusp9 | 41.64245 | 6.65E−08 |
| Kcnj15 | 38.81296 | 7.71E−04 |
| Gpr113 | 36.52718 | 5.43E−38 |
Fold change values, raw P values and P values adjusted for multiple testing were calculated using Benjamini-Hochberg test. Genes were considered significantly differentially expressed if they had a false discovery rate of less than 5%.
Table 4.
Top 10 down-regulated genes in LK.a
| Genes | Fold change | FDR |
|---|---|---|
| Npas4 | 50.83 | 5.02E−04 |
| Pthlh | 27.06 | 3.47E−74 |
| Txlnb | 19.86 | 3.87E−07 |
| Bag2 | 15.25 | 1.37E−90 |
| Car7 | 14.61 | 7.46E−14 |
| Cacng6 | 13.82 | 7.95E−29 |
| Cytip | 13.25 | 5.32E−29 |
| Igfbp3 | 13.2 | 7.25E−35 |
| Irx3 | 12.43 | 4.21E−16 |
| RGD1304931 | 11.61 | 1.04E−69 |
Fold change values, raw P values and P values adjusted for multiple testing were calculated using Benjamini-Hochberg test. Genes were considered significantly differentially expressed if they had a false discovery rate of less than 5%.
Figure 1.
RT-PCR validation of up-regulated and down-regulated genes after LK treatment. Primary cultures of CGN were treated with HK or LK for 6 h on day 7 in vitro and RNA was used for RT-PCR. Actin was used as a normalization control. Primer pairs used to amplify cDNA are given in supplementary data (Table S1). Graphs represent densitometric analysis of the expression changes (average of two independent samples) in LK after normalizing with actin.
Functional annotation of the global gene expression profile and pathway analysis
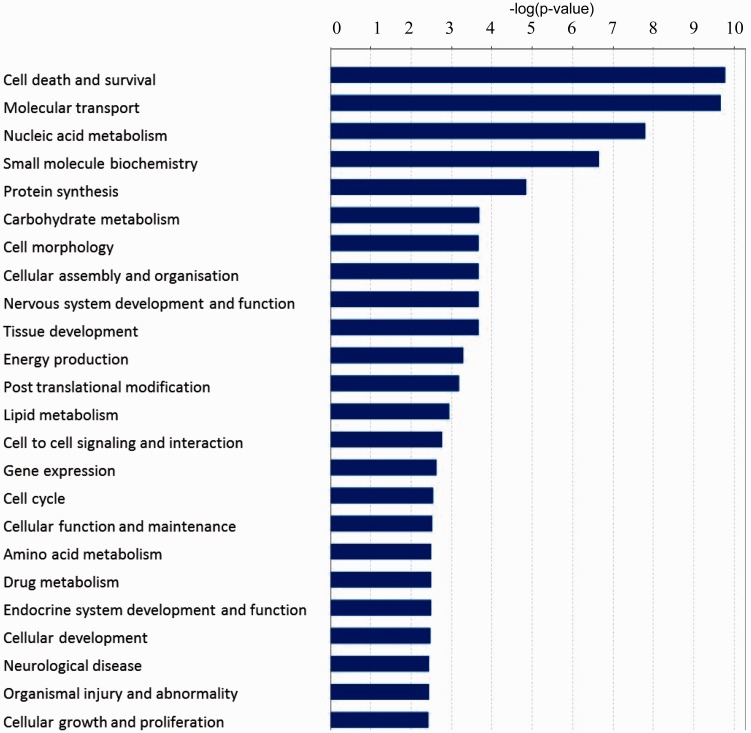
To investigate the physiological processes targeted by the differentially-expressed genes in response to LK-treatment, we carried out a GO classification analysis of the differentially-expressed genes using IPA. Figure 2 lists the functional categories with significant P value <0.01. Based on the GO analysis, a particularly large number of differentially-expressed genes are found to be associated with ‘cell death and survival’ (154 genes up-regulated and 125 genes down-regulated). The second highest group of significant functional categories is related to ion transport and molecular secretion. When looked at the list of up- and down-regulated genes in LK (Table 5), highly significant annotations were ‘Molecular Transport’, and ‘Cellular Growth and Proliferation’, where 147 and 132 genes, respectively, were found to be up-regulated in LK. On the other hand, functional annotations enriched in down-regulated genes category were ‘Nucleic Acid Metabolism’, ‘Small Molecule Biochemistry’, and ‘Energy Production’. Categories such as ‘Cell Death and Survival’ were more or less equally represented in both up-regulated and down-regulated genes (Table 5). When we looked at the physiological system development and functions, most significant annotations were ‘Behavior’ and ‘Nervous System Development and Function’ in the up-regulated and down-regulated gene categories, respectively (Table 5). A complete list of all the GO term enrichments is provided as supplementary data (Tables S5–S7).
Figure 2.
Graphical representation of biological functions derived from GO enrichment of differentially-expressed genes in CGNs treated with HK and LK. DAVID and IPA gene functional annotation and classification tool was used to annotate the list of differentially-expressed genes with respective GO terms. Bars represent −log (P value) in each functional category. Functional GO enrichments were selected for significance by using a false discovery rate (FDR) cutoff of 5%. (A color version of this figure is available in the online journal.)
Table 5.
Highly significant GO annotations in up-regulated and down-regulated genes after potassium withdrawal
| Up-regulated in LK | ||
|---|---|---|
| Molecular and cellular functions | P value | # molecules |
| Molecular transport | 7.52E−09–4.87E−02 | 147 |
| Cellular growth and proliferation | 2.04E−07–3.86E−02 | 132 |
| Cell death and survival | 4.28E−07–4.64E−02 | 154 |
| Gene expression | 4.25E−05–3.49E−02 | 83 |
| Amino acid metabolism | 1.24E−04–4.20E−02 | 29 |
| Physiological system development and function | ||
| Behavior | 1.71E−05–4.20E−02 | 45 |
| Endocrine system development and function | 1.24E−04–2.62E−02 | 22 |
| Cardiovascular system development and function | 1.32E−03–3.86E−02 | 34 |
| Organismal development | 1.32E−03–4.41E−02 | 45 |
| Tissue morphology | 1.32E−03–3.86E−02 | 13 |
| Down-regulated in LK | ||
|---|---|---|
| Molecular and cellular functions | ||
| Nucleic acid metabolism | 2.97E−09–1.27E−02 | 55 |
| Small molecule biochemistry | 2.97E−09–4.44E−02 | 91 |
| Energy production | 2.96E−07–1.27E−02 | 20 |
| Cell death and survival | 1.40E−05–4.76E−02 | 125 |
| Cellular function and maintenance | 5.01E−05–3.80E−02 | 95 |
| Physiological system development and function | ||
| Nervous system development and function | 2.56E−03–4.25E−02 | 87 |
| Tissue development | 2.56E−03–4.25E−02 | 67 |
| Endocrine system development and function | 5.25E−03–5.25E−03 | 3 |
| Embryonic development | 6.25E−03–3.54E−02 | 23 |
| Organ morphology | 1.20E−02–1.20E−02 | 3 |
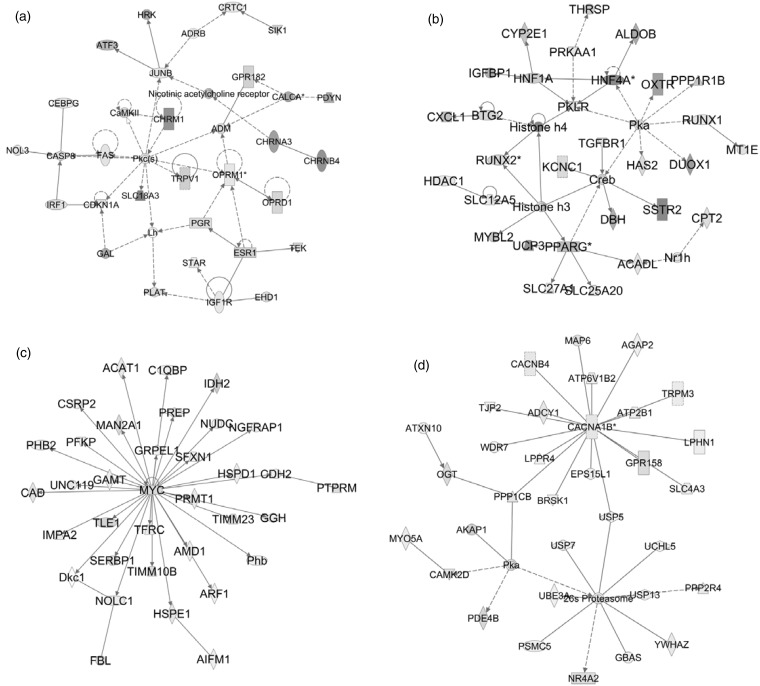
In order to investigate the pathways involved in neuronal death after potassium deprivation, the differentially-expressed gene data set was processed through IPA. Table 6 lists the top five significantly dysregulated pathways under LK condition. The two most significantly dysregulated canonical pathways in the down-regulated genes category include mitochondrial dysfunction and oxidative phosphorylation. Contrary to this, ‘Protein Kinase A Signaling’ was the most perturbed pathway in the up-regulated genes category (Table 6). One interesting finding was that the ‘Ubiquitination Pathway’ also appeared to be significantly dysregulated where 47 out of 232 molecules were down-regulated after LK-treatment (Table 6). We also performed network analysis using IPA to identify significantly enriched networks based on the RNA-Seq data. The two most significant networks are shown in Figure 3 for genes that are up-regulated (a and b) and down-regulated (c and d) in LK. The up-regulated genes category was largely associated with ‘nervous system development and function’ and ‘energy production’. On the other hand, networks like ‘cell death and survival’, ‘tumor morphology’, ‘neurological diseases’, ‘molecular transport’, and ‘cell signaling’ was associated with the category of genes down-regulated after LK treatment. Detailed lists of all the pathways and networks are given in supplementary data (Table S8–S13).
Table 6.
Top canonical pathways derived from IPA.a
| Top canonical pathways | P value | Ratio |
|---|---|---|
| Mitochondrial dysfunction | 1.30E−11 | 74/156 |
| Oxidative phosphorylation | 4.74E−11 | 53/100 |
| TCA cycle II (eukaryotic) | 2.59E−05 | 14/21 |
| Protein kinase A signaling | 3.86E−05 | 113/354 |
| Dopamine-DARP32 feedback in cAMP signaling | 5.27E−05 | 57/156 |
| Pathways formed under up-regulated genes in LK | ||
| Protein kinase A signaling | 5.40E−06 | 70/354 |
| Tweak signaling | 5.85E−06 | 14/35 |
| Role of PKR in interferon induction and antiviral response | 1.81E−05 | 15/43 |
| Axonal guidance signaling | 2.25E−05 | 74/406 |
| Hepatic cholestasis | 7.80E−05 | 31/133 |
| Pathways formed under down-regulated genes in LK | ||
| Mitochondrial dysfunction | 5.18E−25 | 68/156 |
| Oxidative phosphorylation | 6.11E−25 | 53/100 |
| TCA cycle II (eukaryotic) | 2.77E−09 | 14/21 |
| Protein ubiquitination pathway | 3.60E−05 | 47/232 |
| Glutaryl-CoA degradation | 5.02E−05 | 7/11 |
Fisher's exact test was used to calculate the P-value and determine the probability that each biological function is enriched in the dataset due to chance alone. Statistically significant biological pathways were then identified by selection for pathways with Benjamini-Hochberg adjusted P values <0.05.
Figure 3.
Top two significant networks developed from the differentially-expressed up-regulated (a and b) and down-regulated (c and d) genes in primary CGN cultures after LK treatment. (a) Network associated with ‘organismal injury and abnormalities’, ‘nervous system development and function’, and ‘inflammatory response’. (b) Network associated with ‘energy production’, ‘lipid metabolism’, and ‘small molecule biochemistry’. (c) Network associated with ‘cell death and survival’, ‘tumor morphology’, and ‘energy production’. (d) Network associated with ‘neurological disease’, ‘molecular transport’, and ‘cell signaling’
Identification of upstream regulators involved in gene regulation
To identify the different molecules that mediate the observed transcriptional changes, we performed an upstream regulator analysis with IPA. Factors that can be identified as potential upstream regulators include kinases, nuclear receptors, other transcription factors, and some micro RNAs. A list of top eight upstream molecules in up- and down-regulated category are listed in Table 7. Most notable upstream regulator in the up-regulated gene category was found to be protein kinase A (PKA). Other important molecules include ephrin receptor B1 (EPHB1) and upstream transcription factor 1 (USF1). Under down-regulated genes, the most important upstream molecule was predicted to be Myc and adrenergic receptor beta. Upstream regulator analysis also revealed micro RNA 221 (miR-221) as a regulatory molecule targeting cyclin-dependent kinase inhibitor1 (Cdkn1). A complete list of all the upstream regulators is provided in the supplementary data (Table S14–S16).
Table 7.
Top upstream regulatory molecules in LK derived from IPA.a
| Upstream regulators | P value |
|---|---|
| Under up-regulated genes | |
| PKA | 9.54E−07 |
| EPHB1 | 1.31E−04 |
| USF1 | 7.51E−04 |
| SP3 | 7.51E−04 |
| HNF1A | 7.51E−04 |
| P38 MAPK | 9.68E−04 |
| PRKAA2 | 1.46E−03 |
| AGTR2 | 2.05E−03 |
| Under down-regulated genes | |
| Myc | 1.28E−04 |
| ADRB | 1.10E−02 |
| KAT5 | 3.26E−02 |
| Hsp90 | 3.33E−02 |
| NRF1 | 3.33E−02 |
| PPP1CC | 3.33E−02 |
| Setd3 | 3.33E−02 |
| FSHR | 4.91E−02 |
Functional significance of upstream transcriptional regulators is determined by using a Z score value. Fisher’s exact test was used to calculate the P value and identify statistically significant upstream regulators.
Discussion
We have used RNA-Seq to obtain a global overview of changes in gene expression that occur during neuronal apoptosis. The paradigm we used, LK-treated CGNs, is one of the most commonly used experimental systems to study neuronal death and significant insight into the mechanism of neuronal death, including death associated with neurodegenerative diseases, has come from this model. We find that a total of 4334 genes are differentially-expressed after LK-treatment, with 2199 genes being up-regulated and 2135 genes being down-regulated. These numbers of genes showing up- or down regulation during neuronal apoptosis is much more than what others have found earlier.12,13 For example, Desagher et al.13 using microarray could find only 368 genes to be differentially-expressed. Out of these, 278 genes were significantly upregulated and 90 genes were significantly down-regulated in apoptotic neurons. Similarly, Chiang et al.12 again using a cDNA array analysis could pick only 790 genes showing down regulation. Similarly, using other paradigms of neuronal death such as nerve growth factor (NGF)-deprived sympathetic neurons34 a relatively smaller number of genes were identified as differentially-expressed. For example, Kristiansen et al.34 described using NGF-deprived sympathetic neurons and the same FDR value as we did, that 813 genes were down-regulated by NGF-deprivation compared with 415 that were up-regulated. However, again these results were obtained using microarray technology. Although we could not get the detailed data of microarray results by Desagher et al.13 or Chiang et al.,12 we compared the information that they did include in their publications to our results. A comparison of the 368 differentially-expressed genes identified in the Desagher et al. publication, only 36 overlapped with our 4334 differentially-expressed genes. Likewise, of the 128 differentially-expressed genes identified by Chiang et al., only14 overlapped with those identified by us. Bag1, Caspase-3, and Caspase-6 were the only genes identified in all three studies. The reason for the modest overlap is unclear but could be related to differences in culture conditions and time points chosen for analysis.
Although both microarray analyses and RNA-Seq can pull up false hits, based on our analysis we are confident that our screen has identified genes that are actually differentially regulated. Before our RNA samples were used for RNA-Seq, we made sure that expression of c-jun was up-regulated in all three sets of RNA from LK-treated samples. Once RNA-Seq data was obtained, we confirmed that genes previously shown to be regulated by LK-treatment were in the list of genes identified in our RNA-Seq experiment. Moreover, we validated by RT-PCR analyses that other genes not previously documented to display altered expression in paradigms of neuronal death but identified in the RNA-Seq analyses we conducted, were in fact differentially-expressed. Not surprisingly, alterations were most abundantly observed among genes that function in the regulation of cell death and survival. Genes functioning in the regulation of molecular transport or cell proliferation also showed extensive deregulation. Our analysis also reveals that expression of genes connected to signaling pathways mediating mitochondrial dysfunction and oxidative phosphorylation are also affected. This is consistent with numerous reports showing that key roles for mitochondrial dysfunction and oxidative stress in experimental models neuronal death and in neurodegenerative disorders.35–40
One very interesting finding from our RNA-seq analysis was dysregulation of the protein ubiquitination pathway following LK-treatment of CGNs. Involvement of ubiquitin/proteasome system (UPS) impairment has been known to be of much significance in neurodegenerative disorders.41 Even in non-neuronal systems, a large amount of data has been published showing a link between the UPS and cell death (see Zhang et al.42 for review). A large number of molecules involved in apoptosis are known to be regulated by the ubiquitin–proteasome.43–46 Analysis of the present data also revealed that a number of cell cycle related genes were deregulated following potassium withdrawal. This was not surprising considering the fact that deregulation of cell cycle control leads to an abortive reentry of mature differentiated post mitotic neurons and results in apoptosis rather than neuronal proliferation.47–51
Previously, studies related to neuronal death involved in most of the neurodegenerative disorders have largely focused on the roles of protein-coding genes; however, further studies have shown that disease pathogenesis can also be modulated through regulatory mechanisms mediated by small non-coding RNAs (reviewed in Abe and Bonini52). Our study further supports the idea that micro RNAs can also be a therapeutic targets in neuronal death as we found micro RNA as an upstream regulatory molecule affecting changes in the expression of genes downstream. Specifically miR-221 was found to be a regulatory molecule targeting cyclin-dependent kinase inhibitor1 (Cdkn1). Selective Cdk inhibitors have earlier been shown to limit neuroinflammation and progressive neurodegeneration after brain injury.53 In addition to miR-221, miR-183 was also found as a regulatory molecule targeting early growth response 1 (EGR1) and insulin receptor substrate 1 (IRS1). EGR1 belongs to the EGR family of proteins and functions as a transcriptional regulator, playing an important role in mitogenesis and differentiation. On the other hand, IRS1 is associated with the AKT signaling pathway.
To summarize, our study identifies a large number of genes previously unconnected to the regulation of neuronal death. While a majority of these changes may reflect consequences of the induction of neuronal death, many of the genes that we have identified are likely to be critical mediators of neuronal death. It is tempting to speculate that these genes may contribute to the pathogenesis of neurodegenerative diseases and could represent molecules that can be targeted in the development of therapies for these fatal brain disorders.
Acknowledgments
This research was supported by a grant from the NIH (R01 NS040408) to SRD. We thank Dr Michael Zhang and Dr Yunfei Wang from the University of Texas at Dallas for their suggestions and help in the discussion of our data.
Author contributions
Experiment was conceived and designed by SRD, DS. Experiments were performed by DS. Data was analyzed by MSK, DS. Manuscript was written by DS, MSK, and SRD.
References
- 1.Pettmann B, Henderson CE. Neuronal cell death. Neuron 1998; 20: 633–47. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 2000; 1: 120–29. [DOI] [PubMed] [Google Scholar]
- 3.Johnson K, Liu L, Majdzadeh N, Chavez C, Chin PC, Morrison B, Wang L, Park J, Chugh P, Chen HM, D'Mello SR. Inhibition of neuronal apoptosis by the cyclin-dependent kinase inhibitor GW8510: identification of 3' substituted indolones as a scaffold for the development of neuroprotective drugs. J Neurochem 2005; 93: 538–48. [DOI] [PubMed] [Google Scholar]
- 4.Gallo V, Giovannini C, Levi G. Modulation of non-N-methyl-D-aspartate receptors in cultured cerebellar granule cells. J Neurochem 1990; 54: 1619–25. [DOI] [PubMed] [Google Scholar]
- 5.D'Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A 1993; 90: 10989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller TM, Johnson EM., Jr Metabolic and genetic analyses of apoptosis in potassium/serum-deprived rat cerebellar granule cells. J Neurosci 1996; 16: 7487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma P, Pfister JA, Mallick S, D'Mello SR. HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J Neurosci 2014; 34: 1599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283: 70–74. [DOI] [PubMed] [Google Scholar]
- 9.Martin DP, Schmidt RE, DiStefano PS, Lowry OH, Carter JG, Johnson EM., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol 1988; 106: 829–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci 1996; 16: 4696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Mello SR. Molecular regulation of neuronal apoptosis. Curr Top Dev Biol 1998; 39: 187–213. [DOI] [PubMed] [Google Scholar]
- 12.Chiang LW, Grenier JM, Ettwiller L, Jenkins LP, Ficenec D, Martin J, Jin F, DiStefano PS, Wood A. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc Natl Acad Sci U S A 2001; 98: 2814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desagher S, Severac D, Lipkin A, Bernis C, Ritchie W, Le Digarcher A, Journot L. Genes regulated in neurons undergoing transcription-dependent apoptosis belong to signaling pathways rather than the apoptotic machinery. J Biol Chem 2005; 280: 5693–702. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One 2014; 9: e78644–e78644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Wang X, Wang X, Liang Y, Zhang X. Observations on novel splice junctions from RNA sequencing data. Biochem Biophys Res Commun 2011; 409: 299–303. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009; 10: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooney M, Bond J, Monks N, Eugster E, Cherba D, Berlinski P, Kamerling S, Marotti K, Simpson H, Rusk T, Tembe W, Legendre C, Benson H, Liang W, Webb CP. Comparative RNA-Seq and microarray analysis of gene expression changes in B-cell lymphomas of Canis familiaris. PLoS One 2013; 8: e61088–e61088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 2012; 7: e30619–e30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010; 11: R106–2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD. Count-based differential expression analysis of RNA sequencing data using R and bioconductor. Nat Protoc 2013; 8: 1765–86. [DOI] [PubMed] [Google Scholar]
- 22.Huang da W, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 2007; 35: W169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borodezt K, D'Mello SR. Decreased expression of the metabotropic glutamate receptor-4 gene is associated with neuronal apoptosis. J Neurosci Res 1998; 53: 531–41. [DOI] [PubMed] [Google Scholar]
- 24.Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci 1995; 15: 1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardi N, Avidan G, Daily D, Zilkha-Falb R, Barzilai A. Biochemical and temporal analysis of events associated with apoptosis induced by lowering the extracellular potassium concentration in mouse cerebellar granule neurons. J Neurochem 1997; 68: 750–59. [DOI] [PubMed] [Google Scholar]
- 26.Murphy LJ. Insulin-like growth factor-binding proteins: functional diversity or redundancy? J Mol Endocrinol 1998; 21: 97–107. [DOI] [PubMed] [Google Scholar]
- 27.Harris CA, Johnson EM., Jr BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J Biol Chem 2001; 276: 37754–60. [DOI] [PubMed] [Google Scholar]
- 28.Ginham R, Harrison DC, Facci L, Skaper S, Philpott KL. Upregulation of death pathway molecules in rat cerebellar granule neurons undergoing apoptosis. Neurosci Lett 2001; 302: 113–6. [DOI] [PubMed] [Google Scholar]
- 29.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol 1999; 19: 751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Mello SR, Kuan CY, Flavell RA, Rakic P. Caspase-3 is required for apoptosis-associated DNA fragmentation but not for cell death in neurons deprived of potassium. J Neurosci Res 2000; 59: 24–31. [PubMed] [Google Scholar]
- 31.Catania MV, Copani A, Calogero A, Ragonese GI, Condorelli DF, Nicoletti F. An enhanced expression of the immediate early gene, Egr-1, is associated with neuronal apoptosis in culture. Neuroscience 1999; 91: 1529–38. [DOI] [PubMed] [Google Scholar]
- 32.Roschier M, Kuusisto E, Suuronen T, Korhonen P, Kyrylenko S, Salminen A. Insulin-like growth factor binding protein 5 and type-1 insulin-like growth factor receptor are differentially regulated during apoptosis in cerebellar granule cells. J Neurochem 2001; 76: 11–20. [DOI] [PubMed] [Google Scholar]
- 33.Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 2013; 503: 121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristiansen M, Menghi F, Hughes R, Hubank M, Ham J. Global analysis of gene expression in NGF-deprived sympathetic neurons identifies molecular pathways associated with cell death. BMC Genomics 2011; 12: 551–2164-12-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson G, Maes M. Neurodegeneration in Parkinson's disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol 2014; 49: 771–83. [DOI] [PubMed] [Google Scholar]
- 36.Bonda DJ, Wang X, Lee HG, Smith MA, Perry G, Zhu X. Neuronal failure in Alzheimer's disease: a view through the oxidative stress looking-glass. Neurosci Bull 2014; 30: 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camilleri A, Vassallo N. The centrality of mitochondria in the pathogenesis and treatment of Parkinson's disease. CNS Neurosci Ther. Epub ahead of print 7 April 2014. [DOI] [PMC free article] [PubMed]
- 38.Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci 2008; 31: 251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 2013; 62: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witte ME, Mahad DJ, Lassmann H, van Horssen J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol Med 2014; 20: 179–87. [DOI] [PubMed] [Google Scholar]
- 41.Huang Q, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis 2010; 15: 1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang HG, Wang J, Yang X, Hsu HC, Mountz JD. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene 2004; 23: 2009–15. [DOI] [PubMed] [Google Scholar]
- 43.Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem 2000; 275: 21648–52. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A 2001; 98: 8662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 2000; 288: 874–7. [DOI] [PubMed] [Google Scholar]
- 46.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 2003; 424: 801–5. [DOI] [PubMed] [Google Scholar]
- 47.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 2007; 8: 368–78. [DOI] [PubMed] [Google Scholar]
- 48.Kranenburg O, van der Eb AJ, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J 1996; 15: 46–54. [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez-Ortega K, Quiroz-Baez R, Arias C. Cell cycle reactivation in mature neurons: a link with brain plasticity, neuronal injury and neurodegenerative diseases? Neurosci Bull 2011; 27: 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer's disease. Neurochem Int 2009; 54: 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Herrup K. Cell division in the CNS: protective response or lethal event in post-mitotic neurons? Biochim Biophys Acta 2007; 1772: 457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abe M, Bonini NM. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol 2013; 23: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabadi SV, Stoica BA, Byrnes KR, Hanscom M, Loane DJ, Faden AI. Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J Cereb Blood Flow Metab 2012; 32: 137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]