Abstract
Background
IgA nephropathy, a frequent cause of end-stage renal disease, is an autoimmune disease wherein immune complexes consisting of IgA1 with galactose-deficient O-glycans (autoantigen) and anti-glycan autoantibodies deposit in glomeruli and induce renal injury. Multiple genetic loci associated with disease risk have been identified. The prevalence of risk alleles varies geographically: it is the highest in eastern Asia and northern Europe, lower in other parts of Europe and North America, and the lowest in Africa. IgA nephropathy is diagnosed by the pathological assessment of a renal biopsy specimen. Currently, therapy is not disease targeted but rather focused on maintaining control of blood pressure and proteinuria, ideally with suppression of angiotensin II. Possible additional approaches differ between countries. Disease-specific therapy as well as new tools for the diagnosis, prognosis, and assessment of responses to therapy are needed.
Summary
Glycosylation pathways associated with aberrant O-glycosylation of IgA1 and, thus, production of autoantigen, have been identified. Furthermore, unique characteristics of the autoantibodies in IgA nephropathy have been uncovered. Many of these biochemical features are shared by patients with IgA nephropathy and Henoch-Schönlein purpura nephritis, suggesting that the two diseases may represent opposite ends of a spectrum of a disease process. Understanding the molecular mechanisms involved in the formation of pathogenic IgA1-containing immune complexes will enable the development of disease-specific therapies as well as diagnostic and prognostic biomarkers.
Key Message
IgA nephropathy is an autoimmune disease caused by the glomerular deposition of nephritogenic circulating immune complexes consisting of galactose-deficient IgA1 (autoantigen) bound by anti-glycan autoantibodies. A better understanding of the multi-step process of the pathogenesis of IgA nephropathy and the genetic and environmental contributing factors will lead to the development of biomarkers to identify patients with progressive disease who would benefit from a future disease-specific therapy.
Key Words: Autoantibodies, Galactose deficiency, IgA nephropathy, Immune complexes, <italic>O</italic>-glycans
Introduction
IgA nephropathy is the most common primary glomerulonephritis in the world [1,2,3]. This disease is characterized by IgA-containing immune deposits in the glomerular mesangium and was initially described by Berger and Hinglais in 1968 [4] as a new entity based on the observation of ‘intercapillary deposits of IgA-IgG’ in renal biopsy specimens from patients presenting with microscopic hematuria and proteinuria. Subsequently, it was established that the IgA in the deposits is exclusively of the IgA1 subclass [5].
IgA nephropathy is diagnosed by the examination of renal cortical tissue based on the detection of IgA as the dominant or co-dominant immunoglobulin; the deposits are predominantly in the mesangium. Complement component C3 is usually found in the same distribution and is commonly accompanied by IgG, IgM, or both, although often with a lesser intensity (for reviews, see [3,6]). Up to 60% of renal biopsy specimens from patients with IgA nephropathy have IgG co-deposits. In a minority of patients, IgA is the sole immunoglobulin detected. Light microscopy typically shows mesangial proliferation and expansion of the extracellular matrix [6]. Glomerular sclerosis and interstitial fibrosis are associated with progressive disease that leads to renal insufficiency.
The initial signs of IgA nephropathy frequently manifest in adolescence and young adulthood. Asymptomatic proteinuria and hematuria are common clinical presentations [3]. Painless macroscopic hematuria is frequent in children and adolescents and often coincides with mucosal infections, particularly those of the upper respiratory tract and/or digestive system. Disease incidence varies greatly by geographical location [3]. IgA nephropathy is found in up to 40% of native kidney biopsies in eastern Asia, but in less than 5% of such biopsies in central Africa [3,7]. Notably, in some regions of the southeastern United States, African-Americans may be affected at the same rate as Caucasians [8,9]. IgA nephropathy more often affects male than female Caucasians (ratio: 2-3:1) but affects both genders equally in eastern Asia. Although some of this geographic variability in disease incidence may be due to differences in thresholds or criteria for performing the diagnostic renal biopsy, genetically determined influences on the pathogenesis of the disease are thought to also play a significant role [10].
In most western countries there is no nationwide screening program for kidney disease. Thus, the discovery of kidney disease that merits a kidney biopsy depends on several factors that do not apply uniformly to all segments of the populations, most importantly the availability and cost of health insurance. Furthermore, some patients with mild disease and their physicians elect not to proceed with an invasive procedure with risks or prefer not to establish a diagnosis of kidney disease that may raise the cost of health, disability, or life insurance. By contrast, in Japan an annual medical checkup system in schools and workplaces was established by law. Individuals found to have proteinuria, with or without microscopic hematuria, are encouraged to consult a nephrologist. This practice undoubtedly contributes to the 10,000 native kidney biopsies performed in Japan each year. About 30-40% of these patients have IgA nephropathy. Thus, many Japanese patients are diagnosed at early stages of the disease that might not be discovered in western countries.
The immunohistological features of IgA nephropathy are similar to those of Henoch-Schönlein purpura nephritis [11,12]. IgA nephropathy likely represents one end of a spectrum of an IgA1-induced disease process that leads to organ damage due to deposition (or in situ formation) of immune deposits generated by the binding of anti-glycan antibodies to galactose-deficient IgA1 (Gd-IgA1). The manifestation of the disease in patients with IgA nephropathy is generally restricted to the kidneys (rarely, patients also have IgA deposits in the walls of dermal capillaries). In contrast, Henoch-Schönlein purpura is an acute multi-organ disorder characterized by IgA1-mediated vasculitis involving the small vessels of the skin, gastrointestinal tract, kidneys, joints, and, rarely, lungs and central nervous system. Injury from such immune complexes can cause pneumonitis, bleeding from the gastrointestinal tract, arthritis/arthralgia, and purpura. Many patients have contracted a mucosal infection, especially in the upper respiratory tract, about 1-2 weeks before the appearance of the purpura. There is no laboratory test unique for Henoch-Schönlein purpura. Rather, the diagnosis is based on the combination of signs and symptoms, as very few other diseases cause the same syndrome. The disease typically affects children for whom it often exhibits a self-limited and benign course. In contrast, Henoch-Schönlein purpura in adults is associated with worse clinical features and outcome because of a higher prevalence of renal involvement.
Pathogenesis
Hypothesis on the Pathogenesis of IgA Nephropathy
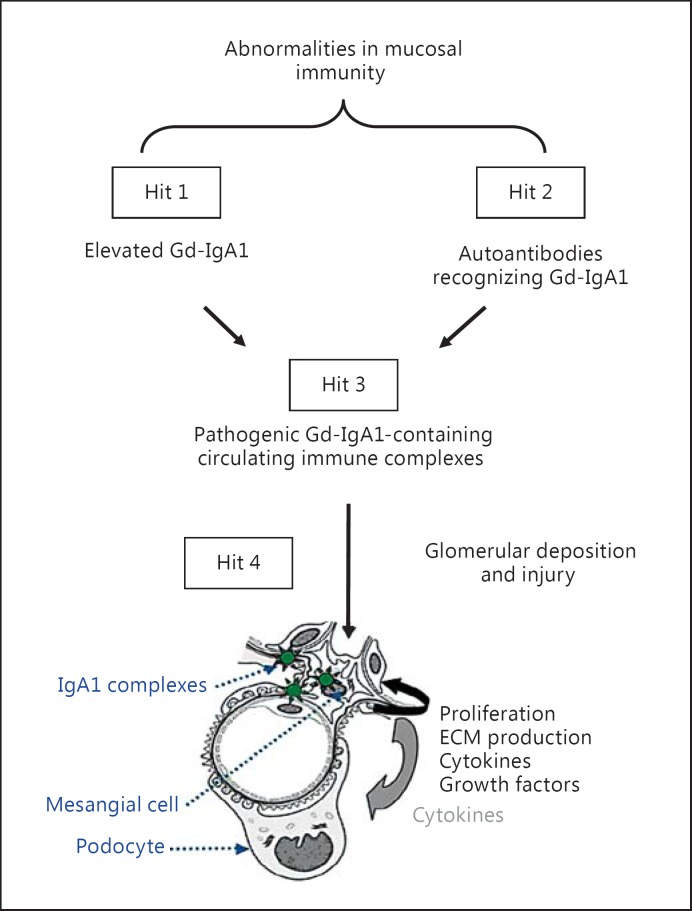
The understanding of the pathogenesis of IgA nephropathy has evolved since 1968, when IgA nephropathy was described as an IgA-IgG immune complex disease. Progress in technology and the development of new tools led to the definition of IgA nephropathy as an autoimmune disease with a multi-hit pathogenetic process (fig. 1). Specifically, Gd-IgA1 produced in elevated amounts in patients with IgA nephropathy (hit 1) is recognized in the circulation by unique autoantibodies (hit 2). The result is the formation of pathogenic immune complexes (hit 3), some of which ultimately deposit in the glomerular mesangium and induce renal injury (hit 4) [13]. Notably, serum levels of Gd-IgA1 as well as IgG and/or IgA autoantibodies specific for Gd-IgA1 correlate with disease severity and may predict disease progression, lending further support to this hypothesis [14,15,16]. In the absence of either hit 1 or hit 2, no pathogenic complexes can be formed (i.e., no hit 3 ensues). Thus, elucidating both hits is critical for understanding the disease processes that result in the formation of pathogenic immune complexes containing Gd-IgA1 as the key autoantigen.
Fig. 1.
Hypothesis on the pathogenesis of IgA nephropathy. Synthesis of IgA1 with some O-glycans deficient in galactose (autoantigen) is elevated. Gd-IgA1 is present in the circulation at increased levels (hit 1). This immunoglobulin is recognized by unique circulating anti-glycan autoantibodies (hit 2) [13,14,20]. This process results in the formation of pathogenic IgA1-containing circulating immune complexes (hit 3), some of which deposit in the glomeruli and induce renal injury (hit 4) [20]. Upstream factors are likely involved in abnormal mucosal immune responses characteristic for patients with IgA nephropathy [3]. An alternative hypothesis has been proposed to suggest that aberrantly glycosylated IgA1 accumulates in the mesangium as lanthanic deposits that are later bound by newly appearing autoantibodies, resulting in the in situ formation of immune complexes [92]. All hits and additional factors may be genetically regulated; 15 distinct genetic loci have been identified by genome-wide association studies to be associated with the risk of IgA nephropathy [7,38]. It is certainly possible that other loci may influence the mechanisms of disease or the clinical expression of IgA nephropathy. ECM = Extracellular matrix.
Structure of IgA1 and Synthesis of IgA1 O-Glycans
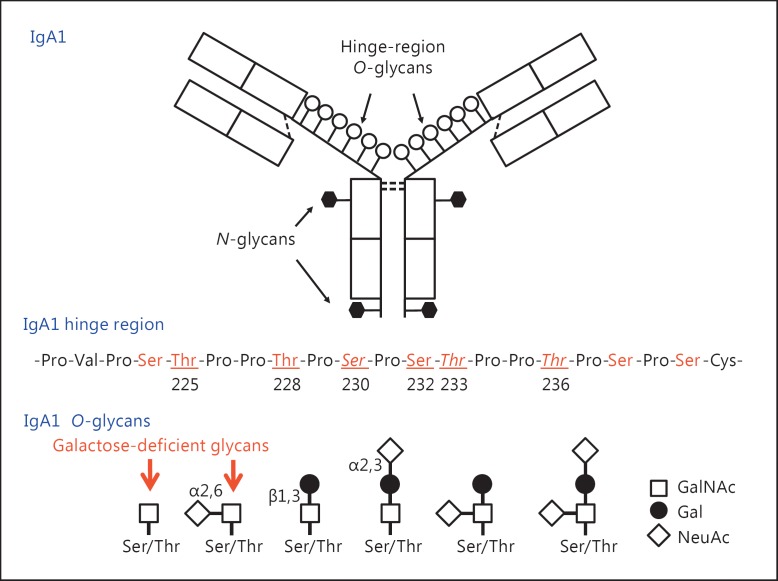
IgA can form polymers, similarly as IgM, by binding the penultimate cysteine residue in a C-terminal ‘tail’ to a joining chain (J-chain) [17]. These J-chain-containing polymeric forms of IgA bind to the polymeric immunoglobulin receptor (pIgR) that mediates the transepithelial transport of external secretions. A part of pIgR, termed secretory component, remains attached to the secreted immunoglobulins. In humans, IgA is present in the circulation and external secretions in two subclasses, IgA1 and IgA2. Human circulatory IgA is mostly of the IgA1 subclass (∼85% of total serum IgA) in its monomeric form [18]. The heavy chains of IgA1 and IgA2 exhibit a high degree of identity in the primary structure, but only IgA1 has O-glycans located in the unique hinge region between constant-region domains 1 and 2 of the heavy chain (CH1 and CH2) (fig. 2). The hinge region of IgA1 contains clustered O-glycans and is also a target of IgA1-specific proteases produced by several bacterial species, including human pathogens such as Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Neisseria gonorrhoeae[19]. IgA1 proteases cleave the IgA1 hinge region, usually at specific sites, and generate Fc and Fab fragments. These IgA1-specific proteases are an important virulence factor of pathogenic bacteria [19], and offer a unique tool for analyzing the site-specific heterogeneity of IgA1 O-glycans (for a review, see [20]).
Fig. 2.
Structure and O-glycosylation of human circulatory IgA1: monomeric IgA1 and hinge-region amino acid sequence with the sites of O-glycan attachment (top and middle panels). There are nine threonine and serine amino acids in the hinge region of monomeric IgA1 that can serve as the site for O-glycosylation (in red). Of these, usually up to six have an attached O-linked glycan (underlined and numbered in the amino acid sequence in the middle panel) in serum IgA1 [30]. The most common core 1 glycan consists of a disaccharide of GalNAc and galactose, with or without sialic acid. Serum levels of IgA1 with galactose-deficient glycans (red arrows in the bottom panel) are elevated in patients with IgA nephropathy. The sites most commonly found to have galactose-deficient glycans are shown in italics (S230, T233, T236 in the middle panel) [30]. See table 1 for details on glycoforms of normal serum IgA1.
Of the nine potential O-glycosylation sites in the hinge region of IgA1, usually three to six are glycosylated (fig. 2). In normal human serum IgA1, hinge-region glycoforms with four and five glycans are the most common (for a review, see [20]). Each heavy chain of IgA1 also contains two N-glycans, one in the CH2 domain (Asn263) and the second in the tailpiece portion (Asn459). The CH2 site contains a biantennary glycan that is usually not fucosylated, whereas the tailpiece site contains a fucosylated glycan [21,22]. Normal human IgA1 in the circulation has core 1 O-glycans consisting of N-acetylgalactosamine (GalNAc) with β1,3-linked galactose. One or both saccharides can be sialylated: galactose with α2,3-linked and GalNAc with α2,6-linked sialic acid. The carbohydrate composition of the O-linked glycans on normal serum IgA1 is variable and the prevailing forms include the GalNAc-galactose disaccharide and its mono- and disialylated forms (fig. 2) [23,24,25,26]. Normal serum IgA1 had been thought to contain little or no galactose-deficient O-glycans [26], but recent findings indicate that some terminal or sialylated GalNAc is likely present (table 1).
Table 1.
Heterogeneity of desialylated O-glycans in normal human serum IgA1
 |
The initiation of O-glycosylation of the hinge region of IgA1 had been originally attributed to the ubiquitously expressed GalNAc transferase T2 (GalNAc-T2), but more recently it has been shown that other GalNAc-Ts, including GalNAc-T1, GalNAc-T11, and GalNAc-T14, can also initiate O-glycosylation of the IgA1 hinge-region peptide (for a review, see [27]). After GalNAc attachment, the biosynthesis of O-glycans continues in a stepwise manner. The processing starts with the formation of the core 1 disaccharide structure, GalNAc-β1,3-galactose. This reaction is catalyzed by a β1,3-galactosyltransferase (C1GalT1). The expression of active C1GalT1 in the Golgi apparatus depends on a specific chaperone (Cosmc). Core 1 structures are modified by attaching sialic acid from CMP-N-acetylneuraminic acid (CMP-NeuAc) to galactose residues in a reaction catalyzed by Galβ1,3GalNAc α2,3-sialyltransferase (ST3Gal) and/or by attaching sialic acid to GalNAc residues catalyzed by an α2,6-sialyltransferase-II (ST6GalNAc-II). Notably, the premature sialylation of terminal GalNAc of IgA1 by ST6GalNAc-II would block other modifications, including galactosylation [28,29].
Aberrant Glycosylation of IgA1 in IgA Nephropathy
Some galactose-deficient O-glycans may be present in normal IgA1 [30]. Extensive glycosylation analyses revealed aberrancies of IgA1 in patients with IgA nephropathy: most patients have elevated serum levels of Gd-IgA1, i.e., IgA1, with some O-glycans deficient in galactose [31,32,33,34,35,36]. Specifically, a fraction of circulatory IgA1 molecules has some hinge-region O-glycans without galactose, i.e., consisting of terminal or sialylated GalNAc (fig. 2). This galactosylation defect appears to be specific for IgA1, as other O-glycosylated serum proteins such as C1 inhibitor and IgD do not exhibit galactose deficiency [33,37]. Genetic influences on the development and expression of IgA nephropathy have been recognized, and risk alleles of multiple genomic loci have been recently identified (for reviews, see [7,38]). Notably, serum levels of Gd-IgA1 are genetically determined [8,39,40].
Humans produce ∼70 mg of IgA per day per kg of body weight [18,41]. Most IgA is produced in mucosal tissues, e.g., the gut, as polymeric IgA that is then selectively transported by a pIgR-mediated pathway into external secretions; only a small fraction of polymeric IgA enters the circulation [41]. IgA in the circulation, mostly IgA1, originates from the bone marrow and, to a lesser degree, from the spleen and lymph nodes. IgA-producing tonsillar cells may contribute to serum IgA and have been considered to play a role in the pathogenesis of IgA nephropathy [42,43,44,45,46]. IgA is catabolized predominantly in the liver by hepatocytes; the half-life of IgA in the circulation is about 5 days [47,48,49,50,51].
Analytical Approaches to IgA1 O-Glycosylation
Assessment of IgA1 O-glycosylation in IgA nephropathy initially utilized monosaccharide compositional analysis and O-glycan-specific lectins [32,35,52] and later developed into a quantitative lectin ELISA [34,53,54]. To characterize O-glycosylation of IgA1 at a molecular level, mass spectrometric analyses have been applied [55,56,57,58,59,60,61]. Direct localization of the attachment sites of O-glycan on IgA1 is allowed by combining a gentle fragmentation of hinge-region glycopeptides (electron-capture or electron-transfer dissociation) with high-resolution mass spectrometry [30,62,63,64,65]. Individual glycoforms have been identified by their molecular masses and sites of O-glycan attachment. There are multiple O-glycoform isomers, i.e., hinge-region glycopeptides with the same number of glycans but with some attached at different sites [30]. These techniques also revealed significant heterogeneity of O-glycans of IgA1 in the circulation of healthy controls (table 1), but it remains to be determined precisely which IgA1 glycoform(s) in patients with IgA nephropathy is associated with disease expression and/or prognosis.
Autoantibodies Specific for Gd-IgA1 in IgA Nephropathy
Circulatory autoantibodies (IgG and/or IgA) bind to Gd-IgA1 and drive the formation of large-molecular-mass pathogenic immune complexes [66]. Better understanding of the nature of these autoantibodies came from studies of EBV-immortalized lymphocytes from patients with IgA nephropathy that were used for cloning cell lines producing IgG specific for Gd-IgA1 [14]. IgG secreted by these cells bound Gd-IgA1 in a glycan-dependent manner. Experiments using enzymatically modified Gd-IgA1 revealed that terminal GalNAc is required for autoantibody binding, whereas the addition of galactose or sialic acid blocked binding. Subsequent analyses of the cloned heavy- and light-chain antigen-binding domains of these IgG autoantibodies specific for Gd-IgA1 identified unique features in complementarity-determining region (CDR) 3 of the heavy chains. Specifically, the amino acid in the third position in CDR3 was S in 6 of 7 patients with IgA nephropathy (first four amino acids: YCSR/K), whereas healthy controls had A in that position (first four amino acids: YCAR/K) [14]. Additional experiments with recombinant IgG from these autoantibodies revealed that S in the third position in CDR3 of the heavy chains is necessary for efficient binding of IgG to Gd-IgA1. Currently, it is unknown whether this alteration (S vs. A in CDR3) originates from a genetic variation or a somatic mutation during an active immune response. Several observations suggest that the serum level of these autoantibodies represents a marker of disease activity as well as disease progression [14,15]. If this finding is confirmed, this biomarker would be useful in assessing the response to treatment or selecting patients who would be at risk of progressive IgA nephropathy.
Formation of Pathogenic Immune Complexes and Activation of Mesangial Cells
Gd-IgA1 is recognized by unique circulating anti-glycan autoantibodies, thus driving a process leading to the formation of pathogenic immune complexes. These complexes activate mesangial cells in vitro, inducing cellular proliferation and overproduction of extracellular matrix components and cytokines/chemokines. IgA1 must be within an immune complex to activate mesangial cell proliferation; uncomplexed Gd-IgA1 does not stimulate the proliferation of mesangial cells [67,68,69]. Additional components from serum need to be present to form stimulatory complexes [67]. Complement likely plays a role in the formation and activities of these complexes (for a review, see [70]). The receptors engaged by pathogenic IgA1-containing immune complexes on mesangial cells are not well understood. Several studies identified transferrin receptor (CD71) as a key receptor for binding Gd-IgA1 (for reviews, see [71,72,73]).
An alternative hypothesis for the formation of IgA-containing complexes has been proposed, outlining a role of the soluble form of Fcα receptor (sCD89) that can generate complexes with Gd-IgA1 (for a review, see [71]). Notably, an association between the levels of sCD89-IgA complexes in serum and the severity of IgA nephropathy has been observed [74]: patients with IgA nephropathy without a progressive clinical course had high levels of sCD89 in contrast to low levels of sCD89 in the disease progression group, suggesting that sCD89-pIgA complexes may be protective. In contrast, an animal model suggested that the interaction between Gd-IgA1, sCD89, transferrin receptor, and transglutaminase 2 in mesangial cells is needed for disease development [75]. These aspects require further studies to clarify all of the processes occurring in patients with IgA nephropathy and identify the major mechanisms of disease pathogenesis and progression.
Among the growth factors playing a role in mesangial cell proliferation, platelet-derived growth factors (PDGFs) are considered leading contenders for the pathogenesis of IgA nephropathy (for a review, see [76]). PDGFs are potent mitogens and chemoattractants for mesenchymal cells and play important roles in mesangioproliferative disease, particularly in IgA nephropathy. Once Gd-IgA1-containing immune complexes are deposited or formed in the mesangium, PDGFs may be synthesized and released by resident cells or infiltrating inflammatory cells. Of the five PDGF dimers (PDGF-AA, -AB, -BB, -CC, and -DD) and three dimeric PDGF receptors (PDGFRs) with tyrosine-kinase activity (PDGFR-αα, -αβ, and -ββ), PDGFR-β and its ligands PDGF-BB (and possibly -AB) and -DD are crucial mediators of mesangial cell proliferation. PDGF-BB and PDFGR-β are overexpressed in experimental mesangioproliferative nephritis and human IgA nephropathy and are related to the degree of glomerular proliferation and extent of interstitial fibrosis. The systemic administration of PDGF-BB or overexpression of PDGF-DD in podocytes of mice induces mesangioproliferative glomerulonephritis. Specific anti-PDGF therapy to neutralize PDGF-B or -D or blocking the PDGFR-β prevented the development of mesangial proliferation in this animal model (for a review, see [76]).
Pathogenesis of IgA Nephropathy and Henoch-Schönlein Purpura Nephritis
Multiple lines of evidence, including shared glycosylation abnormalities of IgA1, anti-Gd-IgA1 autoantibodies, and presence of IgA1-containing immune complexes in the circulation, have led to the postulate that the two diseases, IgA nephropathy and Henoch-Schönlein purpura nephritis, represent the opposite ends of a spectrum of a disease process (for a comprehensive comparison of all aspects of the two disorders, see table 1 in [3]). Multiple genetic loci with their risk alleles for the development IgA nephropathy have been identified that fit the multi-hit pathogenesis scheme (fig. 1). There has not been a similar genetic study for patients with Henoch-Schönlein purpura nephritis.
Implications for Diagnosis, Prognosis, and Treatment of IgA Nephropathy
Diagnosis and Prognosis
An elevated serum level of Gd-IgA1 is not sufficient to induce disease [13] and, thus, is unlikely to be suitable as a stand-alone biomarker for diagnosis. However, a combination of markers, such as Gd-IgA1 and anti-glycan autoantibodies, shows more promise [77]. Also, urinary peptides may be of future interest for developing diagnostic and prognostic biomarkers relevant to IgA nephropathy. Such markers may be developed, for example, using urinary peptidomics [78,79,80,81,82].
The prognostic value of serum levels of Gd-IgA1, anti-glycan autoantibodies, or their combination needs to be assessed in future studies. Moreover, the heterogeneity of IgA1 galactosylation and/or the fine specificity of the autoantibodies have yet to be tested for an association with the clinical expression of disease or prognosis.
Treatment
There is no therapy that is based on disrupting or dampening disease-specific mechanisms in the pathogenesis of IgA nephropathy. The current approaches to treatment vary substantially between the western and eastern hemispheres. Recommendations and suggestions for the treatment in the western approach have been summarized in the KDIGO guidelines [83]. The primary emphasis entails efforts to control blood pressure and proteinuria (to <0.5 g/day) with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. For patients with proteinuria persistently greater than 1 g/day despite maximum tolerated doses of such agents and well-controlled blood pressure, two options have been suggested: (1) fish oil and (2) a 6-month course of glucocorticoids for the subset of patients with eGFR >50 ml/min/1.73 m2. For patients with crescentic IgA nephropathy (defined as crescents in >50% of glomeruli) and a rapid deterioration in renal clearance function, treatment with glucocorticoids and cyclosporine (or azathioprine) is suggested. The use of immunosuppressive agents in patients with stable eGFR <30 ml/min/1.73 m2, or mycophenolate, anti-platelet agents, or tonsillectomy in any setting is not supported.
The approach to therapy in the eastern hemisphere includes the suppression of angiotensin II for the treatment of proteinuria and hypertension. The use of tonsillectomy in the treatment armamentarium is favorably viewed in Japan, and the procedure is often combined with glucocorticoid therapy. Since tonsillectomy with pulse glucocorticoids was first reported in 2001 [84], numerous reports of uncontrolled studies in Japan have described benefits of such a therapy for preserving renal function and improving proteinuria and hematuria [85,86]. The benefit may be limited to patients with preserved eGFR [45,87]. A recent multicenter randomized controlled trial of tonsillectomy with pulse glucocorticoids found a greater effect to attenuate proteinuria compared to glucocorticoid pulse therapy alone, but there was no benefit for eGFR [88]. In China, the clinical remission rate was higher in patients with tonsillectomy than in those without [89]. The mechanism of benefit of tonsillectomy may include a reduction of serum levels of Gd-IgA1 and/or autoantibodies specific for Gd-IgA1.
Traditional Chinese medicine has been used for many years to treat patients with chronic kidney diseases, including IgA nephropathy, to decrease proteinuria and improve or stabilize renal clearance function. In the 1980s, kidney biopsy was widely accepted by many traditional Chinese medical facilities. New diagnostic criteria for several kidney diseases were developed by combining kidney biopsy results and traditional Chinese medical theory. The treatment of IgA nephropathy using traditional Chinese medicine has been applied under these guidelines, although variations of diagnostic criteria exist due to differences in interpretation. Tripterygium wilfordii Hook F (thunder god vine), recognized recently as an immunosuppressive treatment, was widely used for patients with chronic kidney diseases to decrease proteinuria. This therapy has been associated with fewer side effects than the treatment with glucocorticoids and/or cytotoxic agents. Unfortunately, only a few of the results have been analyzed, summarized, and published. In 2010, one study examined the findings of four randomized clinical trials with a total of 188 IgA nephropathy patients [90]. Treatment with the herb significantly decreased nonnephrotic proteinuria and stabilized renal function [90]. For the current treatment of patients with IgA nephropathy, most regimens include a mixture of drugs. Some prescriptions are prepared by traditional Chinese pharmaceutical companies in the form of ready-to-use capsules or decoctions. One preparation, Shenle capsules with hirudo and other herbs, was tested in 36 non-nephrotic patients with IgA nephropathy; the therapeutic benefit for decreasing proteinuria was similar to that of fosinopril, an angiotensin-converting enzyme inhibitor [89]. The major active chemical components of the traditional medicine prescriptions and their pharmacological mechanisms remain unclear. In contrast, the biological effects of nephrokeli, a decoction with several herbal mixtures that has been used successfully for decades in China to treat IgA nephropathy patients, have been examined. Using the mixture in a rat model reduced the expression of sphingosine-1-phosphate receptor 2 or 3 in the kidney, and that effect correlated with less proliferation of mesangial cells [91].
While waiting for the clarification of the mechanisms responsible for the therapeutic benefit of tonsillectomy and traditional Chinese medications, it is now possible to envision novel approaches to disease-specific treatment based on the 4-hit hypothesis for the pathogenesis of IgA nephropathy (table 2). Disrupting the cascade of events at hit 1 and hit 2 (fig. 1) would prevent the formation of nephritogenic immune complexes [3,13,92] and will likely require the development of parenteral formulations of small biological molecules. Dampening the involvement of complement would attenuate the effects of hit 3 and hit 4. Repurposing some agents that interfere with cytokine pathways may reduce renal injury after nephritogenic immune complexes bind to mesangial cells (hit 4). The clinical development of disease-specific therapy is at least several years in the future.
Table 2.
Potential biomarkers and disease-specific approaches for the treatment of IgA nephropathy
| Hit | Pathogenic process | Potential biomarkers | Disease-specific approaches to therapy |
|---|---|---|---|
| 1 | Elevated synthesis of Gd-IgA1 | Serum level of Gd-IgA1 by lectin ELISA and/or mass spectrometric profiling | Reduce Gd-IgA1 production
|
| 2 | Production of autoantibodies binding to Gd-IgA1 | Serum anti-glycan antibodies | Reduce production of Gd-IgA1-specific autoantibodies
|
| 3 | Formation of pathogenic Gd-IgA1-containing immune complexes | Circulating immune complexes and their specific components | Blockade of immune-complex formation
|
| 4 | Glomerular deposition and injury | Urinary immune complexes or complement degradation products, or novel markers of glomerular injury | Blockade mesangial cell activation
|
Disclosure Statement
The authors have nothing to disclose.
Acknowledgements
The authors have been supported in part by grants DK078244, DK082753, DK099228, and GM098539 from the National Institutes of Health and a gift from the IGA Nephropathy Foundation of America. The authors also thank all co-workers and collaborators who have participated in various aspects of these studies as well as the many patients and their family members who volunteered their time and provided biological specimens.
References
- 1.D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Quart J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 2.Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988;84:129–132. doi: 10.1016/0002-9343(88)90019-8. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Hinglais N. Les dépôts intercapillaires d'IgA-IgG (Intercapillary deposits of IgA-IgG) J Urol Nephrol. 1968;74:694–695. [PubMed] [Google Scholar]
- 5.Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980;66:1432–1436. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennette JC. The immunohistology of IgA nephropathy. Am J Kidney Dis. 1988;12:348–352. doi: 10.1016/s0272-6386(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 7.Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124:2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings MC, Moldoveanu Z, Julian BA, Novak J, Sanders JT, McGlothan KR, Gharavi AG, Wyatt RJ. Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability. Clin J Am Soc Nephrol. 2010;5:2069–2074. doi: 10.2215/CJN.03270410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehic AM, Gaber LW, Roy Sr, Miller PM, Kritchevsky SB, Wyatt RJ. Increased recognition of IgA nephropathy in African-American children. Pediat Nephrol. 1997;11:435–437. doi: 10.1007/s004670050311. [DOI] [PubMed] [Google Scholar]
- 10.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davin JC. Henoch-Schönlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol. 2011;6:679–689. doi: 10.2215/CJN.06710810. [DOI] [PubMed] [Google Scholar]
- 12.Davin JC, Coppo R. Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563–573. doi: 10.1038/nrneph.2014.126. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H, Fun R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23:1579–1587. doi: 10.1681/ASN.2012010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790–796. doi: 10.1038/ki.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestecky J, Schrohenloher RE, Kulhavy R, Wright GP, Tomana M. Site of J chain attachment to human polymeric IgA. Proc Natl Acad Sci USA. 1974;71:544–548. doi: 10.1073/pnas.71.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, Novak L, Matousovic K, Novak J. IgA nephropathy: molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 19.Kilian M, Russell MW. Microbial evasion of IgA functions. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology. ed 3. Vol. 1. Amsterdam: Elsevier Academic Press; 2005. pp. 291–303. [Google Scholar]
- 20.Novak J, Julian BA, Mestecky J, Renfrow MB. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol. 2012;34:365–382. doi: 10.1007/s00281-012-0306-z. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka A, Iwase H, Hiki Y, Kokubo T, Ishii-Karakasa I, Toma K, Kobayashi Y, Hotta K. Evidence for a site-specific fucosylation of N-linked oligosaccharide of immunoglobulin A1 from normal human serum. Glycoconj J. 1998;15:995–1000. doi: 10.1023/a:1006989910120. [DOI] [PubMed] [Google Scholar]
- 22.Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB. Analysis of IgA1 N-glycosylation and its contribution to FcαRI binding. Biochemistry. 2008;47:11285–11299. doi: 10.1021/bi801185b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 24.Field MC, Dwek RA, Edge CJ, Rademacher TW. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989;17:1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- 25.Tomana M, Niedermeier W, Mestecky J, Hammack WJ. The carbohydrate composition of human myeloma IgA. Immunochemistry. 1972;9:933–940. doi: 10.1016/0019-2791(72)90166-8. [DOI] [PubMed] [Google Scholar]
- 26.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 27.Stuchlova Horynova M, Raska M, Clausen H, Novak J. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell Mol Life Sci. 2013;70:829–839. doi: 10.1007/s00018-012-1082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak J, Julian BA, Tomana M, Mestecky J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289:5330–5339. doi: 10.1074/jbc.M113.512277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K, Smith AD, Poulsen K, Kilian M, Julian BA, Mestecky J, Novak J, Renfrow MB. Identification of structural isomers in IgA1 hinge-region O-glycosylation using high-resolution mass spectrometry. J Proteome Res. 2012;11:692–702. doi: 10.1021/pr200608q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andre PM, Le Pogamp P, Chevet D. Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal. 1990;4:115–119. doi: 10.1002/jcla.1860040208. [DOI] [PubMed] [Google Scholar]
- 32.Mestecky J, Tomana M, Crowley-Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 33.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moldoveanu Z, Wyatt RJ, Lee J, Tomana M, Julian BA, Mestecky J, Huang W-Q, Anreddy S, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 35.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 36.Shimozato S, Hiki Y, Odani H, Takahashi K, Yamamoto K, Sugiyama S. Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant. 2008;23:1931–1939. doi: 10.1093/ndt/gfm913. [DOI] [PubMed] [Google Scholar]
- 37.Smith AC, de Wolff JF, Molyneux K, Feehally J, Barratt J. O-Glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol. 2006;17:1192–1199. doi: 10.1681/ASN.2005101115. [DOI] [PubMed] [Google Scholar]
- 38.Kiryluk K, Novak J, Gharavi AG. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013;64:339–356. doi: 10.1146/annurev-med-041811-142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2011;80:79–87. doi: 10.1038/ki.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mestecky J, Moro I, Kerr MA, Woof JM. Mucosal immunoglobulins. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology. ed 3. Vol. 1. Amsterdam: Elsevier Academic Press; 2005. pp. 153–181. [Google Scholar]
- 42.Bene MC, Faure GC. Mesangial IgA in IgA nephropathy arises from the mucosa. Am J Kidney Dis. 1988;12:406–409. doi: 10.1016/s0272-6386(88)80035-0. [DOI] [PubMed] [Google Scholar]
- 43.Harper SJ, Allen AC, Bene MC, Pringle JH, Faure G, Lauder I, Feehally J. Increased dimeric IgA-producing B cells in tonsils in IgA nephropathy determined by in situ hybridization for J chain mRNA. Clin Exp Immunol. 1995;101:442–448. doi: 10.1111/j.1365-2249.1995.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh A, Iwase H, Takatani T, Nakamura I, Hayashi M, Oba K, Hiki Y, Kobayashi Y, Okamoto M. Tonsillar IgA1 as a possible source of hypoglycosylated IgA1 in the serum of IgA nephropathy patients. Nephrol Dial Transplant. 2003;18:1108–1114. doi: 10.1093/ndt/gfg108. [DOI] [PubMed] [Google Scholar]
- 45.Miura N, Imai H, Kikuchi S, Hayashi S, Endoh M, Kawamura T, Tomino Y, Moriwaki K, Kiyomoto H, Kohagura K, Nakazawa E, Kusano E, Mochizuki T, Nomura S, Sasaki T, Kashihara N, Soma J, Tomo T, Nakabayashi I, Yoshida M, Watanabe T. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460–466. doi: 10.1007/s10157-009-0179-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Yanagawa H, Matsuzaki K, Horikoshi S, Novak J, Tomino Y. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;9:e89707. doi: 10.1371/journal.pone.0089707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baenziger JU, Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980;22:611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- 48.Moldoveanu Z, Epps JM, Thorpe SR, Mestecky J. The sites of catabolism of murine monomeric IgA. J Immunol. 1988;141:208–213. [PubMed] [Google Scholar]
- 49.Moldoveanu Z, Moro I, Radl J, Thorpe SR, Komiyama K, Mestecky J. Site of catabolism of autologous and heterologous IgA in non-human primates. Scand J Immunol. 1990;32:577–583. doi: 10.1111/j.1365-3083.1990.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 50.Tomana M, Phillips JO, Kulhavy R, Mestecky J. Carbohydrate-mediated clearance of secretory IgA from the circulation. Mol Immunol. 1985;22:887–892. doi: 10.1016/0161-5890(85)90074-4. [DOI] [PubMed] [Google Scholar]
- 51.Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology. 1988;94:887–892. doi: 10.1016/0016-5085(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 52.Allen AC, Bailey EM, Brenchley PEC, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 53.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JE, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiki Y, Horii A, Iwase H, Tanaka A, Toda Y, Hotta K, Kobayashi Y. O-linked oligosaccharide on IgA1 hinge region in IgA nephropathy. Fundamental study for precise structure and possible role. Contrib Nephrol. 1995;111:73–84. [PubMed] [Google Scholar]
- 56.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 57.Iwase H, Tanaka A, Hiki Y, Kokubo T, Ishii-Karakasa I, Nishikido J, Kobayashi Y, Hotta K. Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry to the analysis of glycopeptide-containing multiple O-linked oligosaccharides. J Chromatogr Biomed Sci Appl. 1998;709:145–149. doi: 10.1016/s0378-4347(98)00050-4. [DOI] [PubMed] [Google Scholar]
- 58.Iwase H, Tanaka A, Hiki Y, Kokubo T, Karakasa-Ishii I, Kobayashi Y, Hotta K. Estimation of the number of O-linked oligosaccharides per heavy chain of human IgA1 by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) analysis of the hinge glycopeptide. J Biochem. 1996;120:393–397. doi: 10.1093/oxfordjournals.jbchem.a021425. [DOI] [PubMed] [Google Scholar]
- 59.Odani H, Yamamoto K, Iwayama S, Iwase H, Takasaki A, Takahashi K, Fujita Y, Sugiyama S, Hiki Y. Evaluation of the specific structures of IgA1 hinge glycopeptide in 30 IgA nephropathy patients by mass spectrometry. J Nephrol. 2010;23:70–76. [PubMed] [Google Scholar]
- 60.Takahashi K, Hiki Y, Odani H, Shimozato S, Iwase H, Sugiyama S, Usuda N. Structural analyses of O-glycan sugar chains on IgA1 hinge region using SELDI-TOFMS with various lectins. Biochem Biophys Res Commun. 2006;350:580–587. doi: 10.1016/j.bbrc.2006.09.075. [DOI] [PubMed] [Google Scholar]
- 61.Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 62.Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. [DOI] [PubMed] [Google Scholar]
- 63.Renfrow MB, MacKay CL, Chalmers MJ, Julian BA, Mestecky J, Kilian M, Poulsen K, Emmett MR, Marshall AG, Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: Implications for IgA nephropathy. Anal Bioanal Chem. 2007;389:1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi K, Wall SB, Suzuki H, Smith AD, Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, Novak J, Renfrow MB. Clustered O-glycans of IgA1:Defining macro- and micro-heterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wada Y, Dell A, Haslam SM, et al. Comparison of methods for profiling O-glycosylation: Human Proteome Organisation Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1. Mol Cell Proteomics. 2010;9:719–727. doi: 10.1074/mcp.M900450-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanagihara T, Brown R, Hall S, Moldoveanu Z, Goepfert A, Julian BA, Tomana M, Mestecky J, Novak J. In vitro-formed immune complexes containing galactose-deficient IgA1 stimulate proliferation of mesangial cells. Results Immunol. 2012;2:166–172. doi: 10.1016/j.rinim.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, Novak L, Novak Z, Mayne R, Julian BA, Mestecky J, Wyatt RJ. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured mesangial cells. Nephrol Dial Transplant. 2011;26:3451–3457. doi: 10.1093/ndt/gfr448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 70.Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, Novak J. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014101000. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monteiro RC. Pathogenic role of IgA receptors in IgA nephropathy. Contrib Nephrol. 2007;157:64–69. doi: 10.1159/000102306. [DOI] [PubMed] [Google Scholar]
- 72.Mkaddem SB, Christou I, Rossato E, Berthelot L, Lehuen A, Monteiro RC. IgA, IgA receptors, and their anti-inflammatory properties. Curr Top Microbiol Immunol. 2014;382:221–235. doi: 10.1007/978-3-319-07911-0_10. [DOI] [PubMed] [Google Scholar]
- 73.Tamouza H, Chemouny J, Raskova Kafkova L, Berthelot L, Flamant M, Demion M, Mesnard L, Walker F, Julian BA, Tissandié E, Tiwari MK, Camara NOS, Vrtovsnik F, Benhamou M, Novak J, Monteiro RC, Moura IC. IgA1 immune complex-mediated activation of MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 2012;82:1284–1296. doi: 10.1038/ki.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vuong MT, Hahn-Zoric M, Lundberg S, Gunnarsson I, van Kooten C, Wramner L, Seddighzadeh M, Fernstrom A, Hanson LA, Do LT, Jacobson SH, Padyukov L. Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int. 2010;78:1281–1287. doi: 10.1038/ki.2010.314. [DOI] [PubMed] [Google Scholar]
- 75.Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogne M, Moura IC, Monteiro RC. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793–806. doi: 10.1084/jem.20112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant. 2014;29(suppl 1):i45–i54. doi: 10.1093/ndt/gft273. [DOI] [PubMed] [Google Scholar]
- 77.Yanagawa H, Suzuki H, Suzuki Y, Kiryluk K, Gharavi AG, Matsuoka K, Makita Y, Julian BA, Novak J, Tomino Y. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 2014;9:e98081. doi: 10.1371/journal.pone.0098081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Julian BA, Suzuki H, Spasovski G, Suzuki Y, Tomino Y, Novak J. Application of proteomic analysis to renal disease in the clinic. Proteomics Clin Appl. 2009;3:1023–1028. doi: 10.1002/prca.200800244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Julian BA, Suzuki H, Suzuki Y, Tomino Y, Spasovski G, Novak J. Sources of urinary proteins and their analysis by urinary proteomics for the detection of biomarkers of disease. Proteomics Clin Appl. 2009;3:1029–1043. doi: 10.1002/prca.200800243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Good DM, Zurbig P, Argiles A, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mischak H, Allmaier G, Apweiler R, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001249. 46ps42. [DOI] [PubMed] [Google Scholar]
- 82.Mischak H, Ioannidis JP, Argiles A, et al. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest. 2012;42:1027–1036. doi: 10.1111/j.1365-2362.2012.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines - application to the individual patient. Kidney Int. 2012;82:840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 84.Hotta O, Miyazaki M, Furuta T, Tomioka S, Chiba S, Horigome I, Abe K, Taguma Y. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- 85.Miura N, Imai H, Kikuchi S, Hayashi S, Endoh M, Kawamura T, Tomino Y, Moriwaki K, Kiyomoto H, Kohagura K, Nakazawa E, Kusano E, Mochizuki T, Nomura S, Sasaki T, Kashihara N, Soma J, Tomo T, Nakabayashi I, Yoshida M, Watanabe T. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460–466. doi: 10.1007/s10157-009-0179-1. [DOI] [PubMed] [Google Scholar]
- 86.Xie Y, Chen X, Nishi S, Narita I, Gejyo F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004;65:1135–1144. doi: 10.1111/j.1523-1755.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 87.Sato M, Hotta O, Tomioka S, Horigome I, Chiba S, Miyazaki M, Noshiro H, Taguma Y. Cohort study of advanced IgA nephropathy: efficacy and limitations of corticosteroids with tonsillectomy. Nephron Clin Pract. 2003;93:c137–c145. doi: 10.1159/000070233. [DOI] [PubMed] [Google Scholar]
- 88.Kawamura T, Yoshimura M, Miyazaki Y, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29:1546–1553. doi: 10.1093/ndt/gfu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen XM, Chen YP, Zhou ZL. Prospective, multi-centered, randomized and controlled trial on effect of Shenle Capsule in treating patients with IgA nephropathy of Fei-Pi qi-deficiency syndrome (in Chinese) Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:1061–1065. [PubMed] [Google Scholar]
- 90.Chen YZ, Gao Q, Zhao XZ, Chen XM, Zhang F, Chen J, Xu CG, Sun LL, Mei CL. Meta-analysis of Tripterygium wilfordii Hook F in the immunosuppressive treatment of IgA nephropathy. Intern Med. 2010;49:2049–2055. doi: 10.2169/internalmedicine.49.3704. [DOI] [PubMed] [Google Scholar]
- 91.Zhong Y, Wang K, Zhang X, Cai X, Chen Y, Deng Y. Nephrokeli, a Chinese herbal formula, may improve IgA Nephropathy through regulation of the sphingosine-1-phosphate pathway. PLoS One. 2015;10:e0116873. doi: 10.1371/journal.pone.0116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glassock RJ. The pathogenesis of IgA nephropathy. Curr Opin Nephrol Hypertens. 2011;20:153–160. doi: 10.1097/MNH.0b013e3283436f5c. [DOI] [PubMed] [Google Scholar]