Abstract
T helper 17 (TH17) cells are CD4+ T cells that secrete the proinflammatory cytokine interleukin-17 (IL-17) and that play a key pathogenic role in autoimmune diseases. Through inducible and tissue-specific deletion systems, we described the temporal and cell type–specific roles of the mitogen-activated protein kinase (MAPK) p38α in mediating TH17 cell–induced tissue inflammation. Inducible deletion of Mapk14 (which encodes p38α) after the onset of experimental autoimmune encephalomyelitis (EAE), a murine model for human multiple sclerosis, protected mice from inflammation. Furthermore, the severity of EAE was markedly reduced in mice with specific loss of p38α in neuroectoderm-derived cells, including astrocytes, an effect that was associated with defective production of chemokines and decreased infiltration of the target tissue by immune cells. p38α linked IL-17 receptor (IL-17R) signaling to the expression of genes encoding proinflammatory chemokines and cytokines. Mice that lacked MAPK phosphatase 1 (MKP-1), an inhibitor of p38α, had exacerbated EAE and enhanced expression of IL-17R–dependent genes. Our results suggest that the p38α–MKP-1 signaling axis links IL-17R signaling in tissue-resident cells to autoimmune inflammation dependent on infiltrating TH17 cells.
Introduction
Immune dysregulation is the cause of human autoimmune disorders. Multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS), results from uncontrolled autoreactive T cells that infiltrate the CNS and attack the myelin sheath (1, 2). In particular, T helper 17 (TH17) cells, a subset of CD4+ effector T cells that secrete the proinflammatory cytokine interleukin-17 (IL-17), play a key pathogenic role in MS (3). In experimental autoimmune encephalomyelitis (EAE), the principal animal model used to study the neuroinflammatory events associated with MS (4), autoimmune inflammation occurs in a multi-step process, which includes both the induction and effector phases. Myelin-reactive CD4+ T cells are activated in the periphery and then enter the perivascular space of the CNS, in which they re-encounter myelin antigens presented by local antigen-presenting cells (APCs). After reactivation, these T cells invade the CNS parenchyma, recognize antigens on myelinated axons, and release IL-17, granulocyte macrophage colony-stimulating factor (GM-CSF), and other proinflammatory molecules, which in turn stimulate CNS-resident cells to activate an inflammatory cascade. Therefore, a complex inflammatory reaction involving both the adaptive and innate immune systems governs disease progression.
The differentiation of TH17 cells is orchestrated by polarizing cytokines, including IL-1, IL-6, transforming growth factor-β (TGF-β), and IL-23, which are mainly produced by APCs, especially dendritic cells (DCs) (3). Studies have revealed an intricate network of molecular pathways that program the differentiation of TH17 cells. At the transcriptional level, TH17 cell differentiation requires the function of a set of transcription factors, including RAR-related orphan receptor α (RORα), RORγt, interferon regulatory factor 4 (IRF4), signal transducer and activator of transcription 3 (STAT3), and runt-related transcription factor 1 (RUNX1) (3). In contrast to our extensive knowledge of the T cell–intrinsic pathways that are required for IL-17 production, there has been far less emphasis on how TH17 cells mediate the inflammation of target tissues at the molecular level. Available evidence suggests that both IL-17 and GM-CSF produced by TH17 cells contribute to the encephalitogenic program (5-8). IL-17 and IL-17 receptors (IL-17Rs) are the founding members of a particular subclass of cytokines and receptors that exhibit signaling properties distinct from those of the better-defined cytokines and their receptors, such as the tumor necrosis factor receptor (TNFR), IL-1R, and IL-12R families (9). Although studies have identified the pathogenic roles of IL-17Rs (10) and the IL-17R signaling adaptor molecule Act1 (11, 12), how TH17 cells participate in EAE pathogenesis and, in particular, how IL-17R signals are transduced by an intracellular signaling network in target cells, remain unresolved.
Mitogen-activated protein kinases (MAPKs), which include extracellular signal–regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, constitute fundamental pathways for cellular responses to a wide range of inflammatory signals (13). In particular, p38 MAPK is by far the most extensively investigated protein kinase target for the development of anti-inflammatory drugs in the pharmaceutical industry, because of its potent role in inflammation and the availability of a large array of pharmacological inhibitors (14); however, the nonspecific effects inherent in pharmacological approaches make them unlikely to provide definitive mechanistic insights (15, 16). Of note, p38 inhibitors interfere with the functions of important immune regulators, such as receptor-interacting protein 2 (RIP2) (17) and the Akt–mechanistic target of rapamycin (mTOR) pathway (18). From this perspective, genetic dissection of signaling pathways has been highly instrumental in our understanding of the specific roles of MAPKs in immune cells (13, 19). For example, we and others established a key role for p38α in DCs in mediating the crosstalk between innate and adaptive immunity for the induction of TH17 cell differentiation, as well as for the pathogenesis of autoimmune disorders (20, 21). Conversely, MAPK phosphatase 1 (MKP-1), a potent inhibitor of p38 and other MAPKs (22), suppresses the DC–dependent differentiation of TH17 cells (23). Moreover, p38α exerts both pro- and anti-inflammatory activities in a cell context–dependent manner (24, 25). These effects contribute to the variable effects observed in the preclinical and clinical targeting of p38α, and reinforce the importance of genetic dissection of p38α functions in vivo.
Despite these advances in our understanding of the physiological roles of p38α in immune cells, little evidence exists about the functional importance of p38α in tissue-resident cells for mediating autoimmune inflammation. Another open question is the specific stages regulated by p38α in disease pathogenesis. Here, we report the temporal and tissue-specific effects of p38α signaling in CNS-resident cells in mediating TH17 cell–dependent inflammation of the CNS. Temporally, p38α played an important role in the effector phase of EAE; anatomically, p38α acted in CNS-resident cells, especially astrocytes, in propagating a TH17 cell–mediated inflammatory cascade. These effects were associated with the role of p38α in promoting the production of the chemokines required for the recruitment of peripheral inflammatory cells that infiltrated the CNS and promoted inflammation. Furthermore, we found that loss of p38α in astrocytes and mouse embryonic fibroblasts (MEFs) diminished IL-17–induced proinflammatory gene expression, which was associated with the defective activation of MAPK-activated protein kinase 2 (MK2), a downstream target of p38α. Finally, we found that MKP-1 functioned in a reciprocal manner to p38α in regulating CNS pathogenesis as well as IL-17 and IL-17R signaling; however, a closely related MAPK, JNK, was dispensable for IL-17R signaling. Collectively, our studies identify a key mechanism of CNS inflammation mediated by the p38α– MKP-1 axis that links IL-17 and IL-17R signaling to the expression of genes encoding proinflammatory factors in CNS-resident cells.
Results
p38α is required for the effector phase of EAE
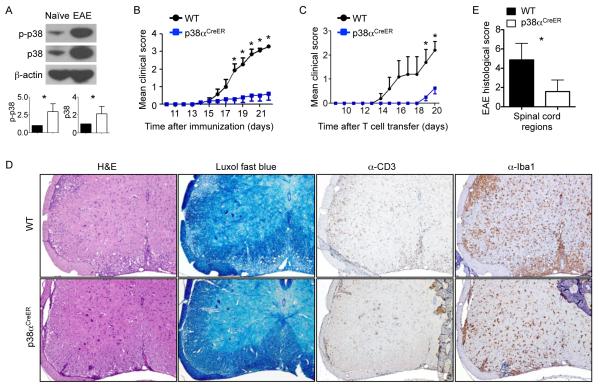
The p38 MAPK pathway is an important regulator and therapeutic target of inflammation (13, 14). Microarray analysis has identified the increased expression of the gene encoding p38α in MS lesions in the CNS (26). To investigate the role of p38 in the murine model of neuroinflammation, we immunized wild-type mice with myelin oligodendrocyte glycoprotein (MOG) peptide and complete Freund’s adjuvant (CFA), and then injected them with pertussis toxin to induce EAE. We found that both the abundance and phosphorylation of p38α were markedly increased in spinal cord cells of mice with active EAE compared to those in the spinal cord cells of mice without immune challenge (Fig. 1A). For functional analysis of p38 signaling, most studies to date have focused on its roles in immune cells with the use of pharmacological inhibitors that have limited specificities (15, 16). The temporal and cell type–specific roles of p38 signaling in mediating the inflammatory responses of target tissues remain poorly understood. To determine the temporal requirement of p38α in autoimmune inflammation in vivo, we first used an inducible deletion system by crossing mice containing floxed p38α alleles (p38αfl/fl) with Rosa26-Cre-ERT2 mice [in which a Cre-ER fusion gene, composed of the Cre recombinase and the estrogen receptor (ER) moiety was recombined into the ubiquitously expressed Rosa26 locus], which we termed as p38αCreER mice (20). After immunization of wild-type and p38αCreER mice with MOG peptide and CFA, we started treating them with tamoxifen at the onset of clinical disease (at day 12 or 13 after immunization), which corresponded to the initiation of the effector phase of EAE, and continued treatment for a total of 3 days. Treatment with tamoxifen resulted in a substantial reduction in p38 abundance and activity in the spinal cords and brains of p38αCreER mice (fig. S1), indicating a predominant contribution of p38α, but not other p38 family members, to the total activity of p38 in inflammation of the CNS. In contrast, the activities of other signaling pathways, including those mediated by ERK, JNK and NF-κB, were largely comparable between tamoxifen-treated wild-type and p38αCreER mice, except for a slight reduction in the abundance of phosphorylated ERK (pERK) in the spinal cords of the p38αCreER mice (fig. S1). In this treatment regimen, tamoxifen markedly delayed disease progression in the p38αCreER mice and ameliorated clinical scores (Fig. 1B). Furthermore, treatment of p38αCreER mice with tamoxifen during EAE disease remission (days 21 to 23 after immunization) resulted in a modestly reduced disease score (fig. S2A). These results are suggestive of an important role for p38α in the effector phase of CNS inflammation.
Fig. 1. p38α is required for the effector stage of EAE.
(A) Spinal cord cells from unimmunized mice (naïve) and from mice immunized to develop EAE (day 15) were analyzed by Western blotting to determine the relative abundances of total and phosphorylated p38 proteins. β-actin was used as a loading control. Bottom: Relative band intensities for the indicated proteins from three independent experiments were determined by densitometry and are shown as means ± SEM. Samples from naïve mice are in filled bars, whereas those from EAE mice are in empty bars. (B) EAE disease course of MOG-immunized wild-type (WT) and p38αCreER mice that were treated with tamoxifen at the onset of disease (day 12 or 13). Data are means ± SEM of five to seven mice per group. (C to E) WT and p38αCreER mice treated with tamoxifen before they were injected with in vitro–derived TH17 cells were (C) monitored to determine EAE disease course, (D) subjected to histopathological analysis of spinal cords, and (E) given histology scores. Data in (C) and (E) are means ± SEM of four to five mice per group. (D) Images are 10× original magnification. Data are representative of two to three independent experiments. *P < 0.05.
Despite the heterogeneity of immune responses in EAE, TH17 cells are crucial for disease pathogenesis (3). Consistent with this notion, neutralization of IL-17 signaling with an anti-IL-17 antibody substantially ameliorated EAE pathogenesis in wild-type mice, although the effect was less profound than that caused by loss of p38α (fig. S2B). Furthermore, neutralization of IL-17 reduced the phenotypic difference in EAE between wild-type and p38α-deficient mice (fig. S2B). To further investigate the direct contribution of p38α to the effector phase of CNS inflammation, we used a transfer model of EAE by injecting TH17-polarized cells into recipient mice (20, 27). Briefly, we isolated draining lymph node (DLN) cells from MOG-immunized wild-type mice, cultured them with MOG and IL-23 in vitro, and transferred the resulting cells into tamoxifen-treated wild-type and p38αCreER mice. Whereas wild-type mice rapidly developed EAE, the p38α-deficient mice exhibited delayed disease development (Fig. 1C). Histological analysis of spinal cords revealed that, compared to the wild-type recipient mice, the p38αCreER recipient mice had decreased inflammation and demyelination, as revealed by staining with H&E and Luxol fast blue (a copper phthalocyanine dye that stains the myelin sheath), respectively (Fig. 1, D and E). Immunostaining further showed that there were reduced numbers of T cells (CD3+). macrophages, and microglia (Iba1+ cells) in p38αCreER mice (Fig. 1D). Results from these complementary systems suggested an important role for p38α in the effector phase of EAE.
p38α function in CNS-resident cells is required for EAE
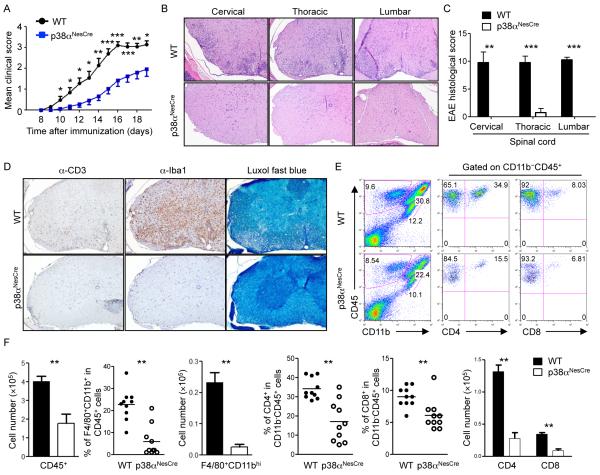
We next determined the cell type–specific requirement for p38α in mediating the inflammatory responses in the CNS by focusing on non-immune cells in the target tissue. To this end, we crossed p38αfl/fl mice with Nestin-Cre mice (to generate “p38αNesCre mice”); the Nestin-Cre transgene mediates efficient gene inactivation in neuroectoderm-derived CNS-resident cells, namely neurons, astrocytes, and oligodendrocytes (28, 29). The gene encoding p38α was efficiently deleted in the brain and spinal cord, but not the spleen or lymph nodes, of p38αNesCre mice (fig. S3). After immunization with MOG and CFA, p38αNesCre mice exhibited delayed onset and diminished severity of EAE compared to wild-type mice (Fig. 2A). The immunized p38αNesCre mice also exhibited decreased CNS inflammation and demyelination compared to the immunized wild-type mice, and showed less infiltration of T cells, macrophages, and microglia (Fig. 2, B to D). We next used flow cytometry to examine the immune cells that had infiltrated the spinal cords of wild-type and p38αNesCre mice at the peak stage of EAE. The p38αNesCre mice had fewer numbers of total leukocytes (CD45+), and both the percentages and numbers of infiltrating macrophages (F4/80+CD11bhi cells), CD4+ T cells, and CD8+ T cells were all substantially reduced compared to those in wild-type mice (Fig. 2, E and F). These data suggest that p38α acts in CNS-resident cells to promote EAE and infiltration of the CNS by immune cells.
Fig 2. Loss of p38α in CNS-resident cells delays EAE progression and impairs the infiltration of inflammatory cells into the CNS.
(A) EAE disease course of MOG-immunized WT and p38αNesCre mice. Data are means ± SEM of 8 to 11 mice per group. (B to D) WT and p38αNesCre EAE mice were analyzed on day 16 after immunization. (B) H&E staining of the indicated regions of the spinal cord. (C) Histology scores of the indicated regions of the spinal cord. (D) Staining of thoracic spinal cords with anti-CD3 antibody (α-CD3), α-Iba1, and Luxol fast blue. Images are 10× original magnification. Data are from five mice per group. (E) Flow cytometric analysis of cells from the spinal cords of WT and p38αNesCre EAE mice on day 16 after immunization. Data are representative of 10 mice per group. (F) Analysis of the relative numbers and percentages of the indicated cell types in the spinal cords of the mice analyzed in (E). *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of two to three independent experiments.
p38α promotes the production of chemokines and cytokines, but not the generation of TH17 or TH1 cells
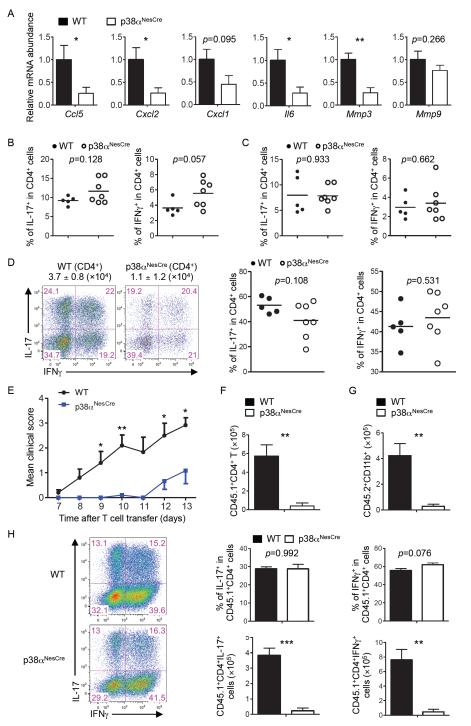
Chemokines play an essential role in guiding the infiltration of inflammatory cells into tissues. To determine whether the CNS-restricted loss of p38α affected chemokine production, we measured the abundances of messenger RNAs (mRNAs) of several chemokines that have been linked to EAE. Multiple mRNAs, including Ccl5, Cxcl2, and to a lesser extent, Cxcl1 mRNA were decreased in abundance in the CNS of p38αNesCre mice compared to those in the CNS of wild-type mice (Fig. 3A). We also examined the expression of genes encoding cytokines and matrix metalloproteases that are involved in EAE pathogenesis. The abundances of Il6 and Mmp3 mRNAs, but not Mmp9 mRNA, were reduced in p38αNesCre mice compared to those in wild-type mice (Fig. 3A). These data suggest that p38α activity in CNS-resident cells mediates chemokine gene expression, which likely facilitates the infiltration of inflammatory cells into the CNS during EAE.
Fig. 3. Ablation of p38α in CNS-resident cells inhibits expression of genes encoding chemokines and inflammatory factors and represses TH17 cell–induced EAE.
(A) Spinal cord cells from WT and p38αNesCre EAE mice on day 11 after immunization were subjected to real-time PCR analysis to determine the relative abundances of the indicated mRNAs. Data are means ± SEM of five to seven mice per group. (B to D) Cells were isolated from (B) the spleens, (C) DLNs, and (D) spinal cords of MOG-immunized WT and p38αNesCre mice (on day 11), stimulated with PMA and ionomycin in vitro, and then analyzed by flow cytometry to determine the percentages of cells positive for IL-17 and IFN-γ among CD4+ T cells. Data are means ± SEM of five to seven mice per group. (D) The total numbers of CD4+ T cells that infiltrated the spinal cords are indicated above the flow cytometry plots. (E) EAE disease course of WT and p38αNesCre mice (CD45.2+) that received in vitro–derived TH17 cells from CD45.1 mice. Data are means ± SEM of 10 mice per group. (F and G) Analysis of the numbers of (F) donor-derived CD4+ (CD45.1+) T cells and (G) host-derived (CD45.2+CD11b+) myeloid cells isolated from the spinal cords of WT and p38αNesCre mice on day 11 after adoptive transfer of cells. Data are means ± SEM of four mice per group. (H) Left: Cells were isolated from the spinal cords of recipient WT and p38αNesCre mice on day 11 after transfer, stimulated with PMA and ionomycin in vitro, and then analyzed by flow cytometry to determine the percentages of cells positive for IL-17 and IFN-γ among donor-derived CD4+ (CD45.1+) T cells. Right: Data are means ± SEM of four mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of two to three independent experiments.
The efficient activation of autoreactive T cells is a prerequisite for the induction of CNS inflammation and pathology in EAE. Because we used CNS-specific ablation of p38α in our studies, peripheral immune functions should not be affected in these mice. To directly address this point, we examined the generation of TH17 and TH1 cells in MOG-immunized wild-type and p38αNesCre mice. CD4+ T cells from the spleens and DLNs of p38αNesCre had normal amounts of IL-17 and interferon-γ (IFN-γ) (Fig. 3, B and C). Furthermore, T cells from the immunized wild-type and p38αNesCre mice produced comparable amounts of Il17 and Ifng mRNAs upon ex vivo stimulation with MOG peptide (fig. S4). Moreover, wild-type and p38αNesCre mice contained similar percentages of IL-17– and IFN-γ–expressing cells among CD4+ T cells in the spinal cord, although the total numbers of CD4+ T cells were decreased in the p38αNesCre mice (Fig. 3D). These results suggest that the ameliorated EAE in p38αNesCre mice was not caused by impaired differentiation of TH17 or TH1 cells.
Infiltration of the CNS with TH17 cells is attenuated in p38α NesCre mice
The requirement of p38α for increased chemokine mRNA abundance and T cell infiltration in the CNS, but not for TH17 or TH1 cell differentiation, prompted us to explore the role of p38α in the recruitment of MOG-specific T cells into CNS. MOG-specific T cells were isolated from DLN cells of CD45.1+ mice after they were immunized with MOG peptide, polarized in vitro to become TH17 cells, and transferred into wild-type or p38αNesCre (CD45.2+) mice. The use of congenic markers enabled us to specifically determine the trafficking and maintenance of donor TH17 cells. We detected comparable percentages of CD45.1+ donor CD4+ T cells in the CNS and spleens of recipient WT or p38αNesCre mice at 4 days after adoptive transfer (fig. S5, A and B). In addition, donor CD4+ T cells that were transferred into different hosts exhibited similar proliferation and survival, as determined by BrdU, Ki-67, and caspase staining (fig. S5, A and B). However, as disease progressed rapidly in the wild-type, but not p38αNesCre, mice (Fig. 3E), there was less infiltration of donor CD4+ T cells into the CNS of p38αNesCre mice at 11 days after adoptive transfer compared to that of the CNS of wild-type recipients (Fig. 3F). In addition, host-derived CD11b+ myeloid cells were markedly reduced in numbers in the spinal cords of p38αNesCre mice compared to those in the spinal cords of wild-type mice (Fig. 3G), consistent with the role of IL-17 production in the CNS in mediating the subsequent recruitment of myeloid cells (3). Furthermore, the amounts of Il6 and Mmp3 mRNAs were substantially reduced in the spinal cord cells of p38αNesCre recipient mice compared to those of wild-type recipients (Fig. S5C). A similar trend was observed for Cxcl1 and Cxcl2 mRNAs, but this did not reach statistical significance in the limited number of mice analyzed (fig. S5C). Despite the considerable reduction in the number of donor CD4+ T cells in the CNS of p38αNesCre mice, those that infiltrated the tissue had comparable amounts of IL-17 and IFN-γ to those of cells that infiltrated the CNS of wild-type mice (Fig. 3H). Therefore, consistent with the requirement for p38α for chemokine expression in the CNS (Fig. 3A), p38α acts in CNS-resident cells to mediate the recruitment of TH17 cells into the CNS to sustain TH17 cell–mediated inflammation.
p38α mediates IL-17–induced proinflammatory gene expression and MK2 activation
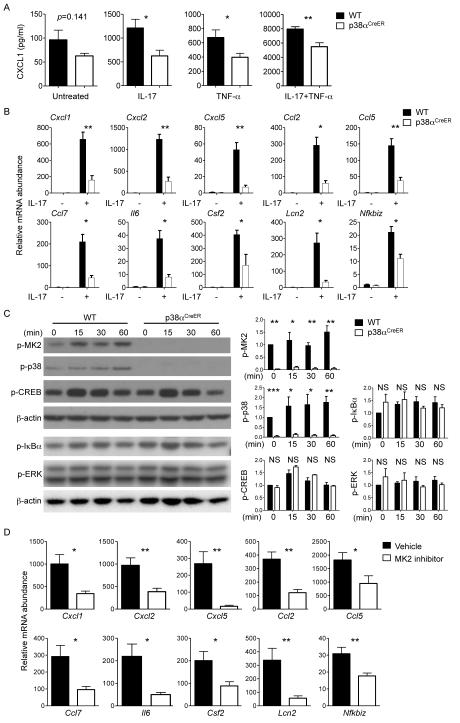
IL-17, the signature cytokine for TH17 cells, stimulates inflammation in autoimmune diseases (9, 30, 31). We hypothesized that p38α mediated IL-17–signaling to induce expression of genes encoding inflammatory factors. To test this, we isolated mouse embryonic fibroblasts (MEFs) from wild-type and p38αCreER mice, treated the cells with 4-hydroxytamoxifen (4-OHT), and stimulated them with IL-17, tumor necrosis factor α (TNF-α), or both. The amount of CXCL1 protein in the culture medium of IL-17–treated p38αCreER MEFs was substantially decreased compared to that in the culture medium of IL-17–treated wild-type cells (Fig. 4A). To a lesser extent, p38α deficiency also resulted in a reduction in the amount of CXCL1 produced in response to TNF-α, as well as in response to both IL-17 and TNF-α. These results suggest that p38α is required for IL-17R signaling in MEFs.
Fig. 4. Analysis of IL-17–induced proinflammatory gene expression and MK2 activation in p38α-deficient MEFs and astrocytes.
(A) MEFs isolated from WT or p38αCreER mice were treated with 4-OHT, and were left untreated or treated with IL-17 or TNF-α alone or in combination for 24 hours. Cell culture medium was then analyzed to determine the amounts of CXCL1 protein secreted by the cells. Data are means ± SEM of six mice per group. (B) Astrocytes isolated from WT or p38αCreER mice were treated with 4-OHT, and were then incubated in the absence or presence of IL-17 for 5 hours. Cells were subjected to real-time PCR analysis to determine the relative abundances of the indicated mRNAs. Data are means ± SEM of three to seven mice per group. (C) Left: Astrocytes isolated from WT or p38αCreER mice were treated with 4-OHT, and were then incubated in the absence or presence of IL-17 for the indicated times. Samples were then analyzed by Western blotting with antibodies specific for the indicated proteins. Blots are representative of five mice per group from two independent experiments. Right: Relative band intensities were determined by densitometry and are shown as means ± SEM of five mice per group. (D) Astrocytes from WT mice were treated with vehicle or an MK2 inhibitor before being stimulated with IL-17 for 5 hours. Cells were analyzed by real-time PCR to determine the relative abundances of the indicated mRNAs. Data are means ± SEM of seven mice per group. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of two to three independent experiments.
Results from a previous study highlighted the importance of IL-17R signaling in astrocytes for EAE (12). To determine the role of p38α in astrocytes, we isolated astrocytes from wild-type and p38αCreER mice and treated them with 4-OHT to deplete p38α in vitro, thereby circumventing any potential secondary effects that might occur as a result of the chronic loss of p38α in vivo. Depletion of p38α did not affect the relative expression of either of the genes encoding the IL-17R subunits IL-17RA and IL-17RC (fig. S6A), but it substantially reduced the expression of a number of IL-17–responsive genes in astrocytes. These include the chemokine-encoding genes Cxcl1, Cxcl2, Cxcl5, Ccl2, Ccl5, and Ccl7, as well as genes encoding other inflammatory factors, including Il6, Csf2, Lcn2, and Nfkbiz (Fig. 4B). To determine the mechanisms underlying the p38α–mediated regulation of target gene expression, we examined the relative abundances of the primary transcripts of Cxcl1 and Cxcl2 and their decay rates in IL-17–stimulated astrocytes to measure transcription initiation frequency (32) and mRNA stability (33, 34), respectively. We found that p38α deficiency mainly affected the expression of Cxcl1 and Cxcl2 rather than the stability of Cxcl1 and Cxcl2 mRNAs (fig. S6, B and C). Together, our findings from experiments with MEFs and astrocytes support a crucial role for p38α in mediating IL-17R signaling required for inflammatory gene expression.
We next examined signaling pathways activated by p38α in IL-17–stimulated astrocytes. Phosphorylation of MK2 and p38 was lost in p38α-deficient cells (Fig. 4C). In contrast, phosphorylation of cAMP response element–binding protein (CREB), another p38α target, was not affected by p38α deletion in IL-17–stimulated astrocytes. In addition, the activities of ERK and NF-κB, were comparable between wild-type and p38α-deficient cells (Fig. 4C). MK2 is implicated in TH17 cell–dependent diseases such as arthritis (35), although the physiological role of MK2 and its interaction with p38α in EAE are not defined. We hypothesized that an important pathway to transduce p38α signals in IL-17–stimulated astrocytes might be mediated by MK2. Indeed, treatment of astrocytes with an MK2 inhibitor reduced the expression of multiple inflammatory genes (Cxcl1, Cxcl2, Cxcl5, Ccl2, Ccl5, Ccl7, Il6, Csf2, Lcn2, and Nfkbiz) in response to IL-17 (Fig. 4D). These data suggest that p38α links IL-17R signaling to the activation of MK2 to mediate proinflammatory gene expression.
Role of MKP-1 in CNS-resident cells in EAE pathogenesis and IL-17R signaling
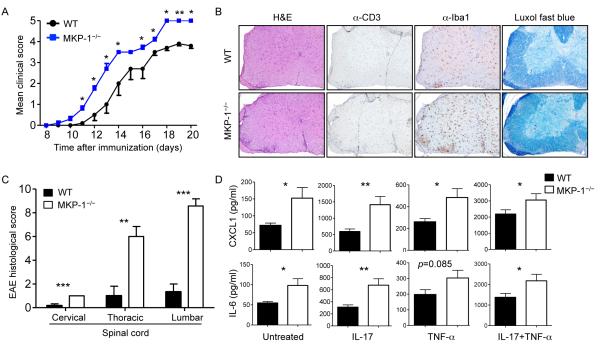
Given the marked effects of p38α in CNS inflammation, it was critical to delineate the pathways that suppress its activity. MKP-1 is a potent inhibitor of the activities of p38 and other MAPKs (22, 36-38). We therefore investigated the role of MKP-1 in CNS-resident cells in EAE. To exclude any potential roles of MKP-1 in DCs (23) or T cells (39) that might contribute to EAE, we generated bone marrow chimeric mice in which MKP-1 deficiency was restricted to the radio-resistant non-hematopoietic cell compartment, including CNS-resident cells, by transferring MKP-1+/+ bone marrow cells into lethally irradiated MKP-1+/+ and MKP-1−/− recipient mice. Lack of MKP-1 in the radio-resistant cells rendered the mice more susceptible to EAE, which was associated with more severity and faster kinetics, as compared with the disease course in MKP-1+/+ bone marrow chimeric mice (Fig. 5A). Histological analysis revealed that there was more severe inflammation and increased infiltration of T cells as well as of macrophages or microglia in the spinal cords of MKP-1−/− bone marrow chimeric mice at the peak of disease (Fig. 5, B and C). Increased leukocyte infiltration was also observed in the cerebellum of MKP-1−/− bone marrow chimeric mice (fig. S7A). These data suggest that lack of MKP-1 in radio-resistant cells exacerbates EAE.
Fig 5. Loss of MKP-1 in nonhematopoietic cells exacerbates the development of EAE.
(A to C) Bone marrow cells isolated from CD45.1+ mice were transferred into lethally irradiated WT or MKP-1−/− mice to generate bone marrow chimeric mice. After reconstitution, the WT and MKP-1−/− bone marrow chimeric mice were immunized with MOG and CFA before being (A) monitored to determine EAE disease course (data are means ± SEM of five mice per group), (B) subjected to histopathological analysis of spinal cords, and (C) given histology scores (data are means ± SEM of six or seven mice per group). Images are 10× original magnification. (D) MEFs from WT or MKP-1−/− mice were left untreated or were treated with IL-17 or TNF-α alone or in combination for 24 hours. Cell culture medium was then analyzed to determine the amounts of CXCL1 and IL-6 proteins secreted by the cells. Data are means ± SEM of 10 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. Data are representative of two to three independent experiments.
To test the role of MKP-1 in IL-17 signaling, we stimulated wild-type and MKP-1−/− MEFs with IL-17 alone or in combination with TNF-α. Deficiency in MKP-1 is associated with enhanced p38 activity in MEFs (40). MKP-1−/− MEFs produced increased amounts of CXCL1 and IL-6 compared with wild-type MEFs (Fig. 5D). JNK is another important target of MKP-1 (22). However, inducible deletion of JNK did not alter the IL-17–stimulated expression of Cxcl1 or Il6 (fig. S7B). These results suggest that the p38α–MKP-1 signaling axis, but not JNK signaling, regulates IL-17R signaling.
Discussion
TH17 cells function as a principal cell type in neuroinflammation, but the effector mechanisms mediating their pathogenicity in the CNS remain under debate. In addition to IL-17, TH17 cells produce a number of other proinflammatory cytokines, including IL-17F, IL-21, IL-22, and GMCSF (3). In particular, GM-CSF produced by TH17 cells is a key stimulator of the encephalitogenic program (5, 6). Nonetheless, loss of IL-17, IL-17R, or Act1 or neutralization of IL-17 signaling substantially ameliorates EAE (7, 8, 10-12), highlighting the important role of IL-17R signaling in inflammation of the CNS. Here, we showed that p38α mediates IL-17 signaling in CNS-resident cells, such as astrocytes, to stimulate the production of chemokines and proinflammatory factors, thereby sustaining neuroinflammation in the effector phase of EAE. In the absence of p38α signaling in CNS-resident cells, TH17 cells failed to efficiently infiltrate into the CNS or recruit myeloid cells to propagate the inflammatory cascade; however, the generation of TH17 cells per se was not affected. At the molecular level, we found that p38α linked IL-17R signaling to MK2 activation as an important mechanism for proinflammatory gene expression. Furthermore, we found that p38α and MKP-1 constituted a signaling axis to positively and negatively regulate IL-17R signaling and neuroinflammation, respectively, whereas the closely related kinase JNK was dispensable for IL-17R signaling. Together, these data suggest that the p38α–MKP-1 axis in CNS-resident cells orchestrates the effector phase of EAE by mediating the IL-17R–dependent activation of MK2 and the expression of genes that encode proinflammatory factors.
Among the target genes downstream of IL-17 signaling are those encoding a distinct subset of chemokines and cytokines that are implicated in the pathogenesis of EAE (12, 41). Consistent with the reduced amounts of these proinflammatory factors in the CNS of p38αNesCre mice after induction of EAE, loss of p38α attenuated the expression of these genes in IL-17–stimulated astrocytes in vitro. This effect was likely mediated by the p38α downstream target MK2, because activation of MK2 was impaired in the absence of p38α, and direct inhibition of MK2 recapitulated the defects in gene expression observed in p38α-deficient astrocytes. In addition, p38α-deficient MEFs showed reduced expression of Cxcl1 in response to IL-17. In a reciprocal manner, MKP-1-deficient cells overexpressed Cxcl1 in response to IL-17. However, loss of JNK1 and JNK2 did not affect the expression of IL-17–responsive genes. Furthermore, although the pathogenic role of NF-κB signaling in EAE models has been extensively studied (42, 43), whether it is involved in IL-17R signaling is unknown. Our results therefore indicate a previously unrecognized and selective role of p38α and its negative regulator MKP-1 in mediating IL-17R signaling.
The effector phase of TH17–mediated EAE is characterized by a persistent inflammatory cascade that can be described by a two-wave hypothesis (44). After they are primed in the periphery, MOG-specific TH17 cells infiltrate the CNS, where they re-encounter myelin antigens presented by local APCs, including microglia, macrophages, and myeloid DCs (45). The proinflammatory cytokines released from reactivated TH17 cells act on CNS-resident cells, such as astrocytes, to produce leukocyte-attracting chemokines, which then mediate a second wave of recruitment of peripheral inflammatory cells. Our results from experiments with the TH17-transfer model of EAE indicate that p38α activity in CNS-resident cells is intricately involved in amplifying and propagating this inflammatory vicious cycle. Although the initial infiltration of TH17 cells occurred normally in p38αNesCre mice, the impaired expression of chemokines and proinflammatory molecules rendered these mice unable to recruit additional TH17 cells and myeloid cells as EAE progressed. However, loss of p38α in CNS-resident cells did not affect the differentiation of TH17 or TH1 cells in the CNS or the periphery. These results suggest that p38α acts in the effector phase of EAE, likely at the transition from wave 1 to wave 2 of the inflammatory cascade.
The precise cellular basis by which IL-17 contributes to EAE pathogenesis remains unresolved. Multiple CNS-resident cells have been implicated in this process, including neurons (46), astrocytes (12), NG2+ glial cells (41), and possibly microglia (47). Our in vitro models with astrocytes and MEFs provide some of the earliest genetic evidence for the involvement of the p38α–MKP-1 axis in IL-17R signaling, although the specific types of neuroectoderm-derived cells involved in vivo remain to be established. In addition, p38α likely mediates proinflammatory signals in addition to those stimulated by IL-17, because deficiencies in p38α and MKP-1 also affected, albeit to a lesser extent, gene expression in response to TNF-α. Nevertheless, the crucial role of the p38α–MKP-1 signaling axis in mediating the effector responses of neuroinflammation in vivo makes it a legitimate therapeutic target. Furthermore, inhibition of p38α would be expected to halt the progression of CNS inflammation even when autoreactive T cell responses are underway, which can be explored for therapeutic intervention of MS and possibly other TH17 cell–mediated inflammatory disorders.
Materials and Methods
Mice and bone marrow chimeras
C57BL/6, CD45.1, and Nestin-Cre mice were purchased from the Jackson Laboratory. Rosa26-Cre-ER, p38αfl, JNKfl, and MKP-1−/− mice were described previously (20, 22, 48, 49). All mice were backcrossed to the C57BL/6 background for at least eight generations, and mice were used for experiments at 6 to 12 weeks of age. Wild-type (WT) littermate controls were in the same genetic background, and where relevant, included Cre+ mice to account for the effects of Cre. For complete bone marrow experiments, bone marrow cells from WT CD45.1+ mice were transferred into lethally irradiated (11 Gy) WT or MKP-1−/− mice. All mice were kept in specific pathogen-free conditions in the Animal Resource Center at St. Jude Children’s Research Hospital. Animal protocols were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital.
Induction of EAE
Mice were immunized subcutaneously (s.c.) with 100 μl of emulsified incomplete Freund’s adjuvant (IFA) supplemented with 500 μg of Mycobacterium tuberculosis H37Ra (DIFCO) and 100 μg of MOG35-55, and received an intraperitoneal (i.p.) injection of 200 ng of pertussis toxin (List Biological Laboratories) at the time of immunization and 48 hours later. The mice were observed daily for clinical signs and scored as described previously (20, 27). For inducible deletion of floxed alleles in vivo, mice were treated with 2 mg of tamoxifen (Sigma) daily for 3 continuous days. To induce EAE by adoptive transfer of TH17-polarized cells, WT (C57BL/6 or CD45.1) mice were immunized as described earlier, and after 9 days, DLN cells were isolated and cultured in vitro with MOG35-55 peptide and IL-23 for 5 days. Live cells were harvested at the end of the culture period, and 1 to 2 × 107 cells were transferred into recipient mice, supplemented by i.p. injection of 200 ng pertussis toxin on the day of transfer and 48 hours later. Neutralization of IL-17 signaling was performed with a monoclonal antibody MAB421 specific for IL-17 and IL-17F, or an isotype control antibody (MAB006). The mice were injected i.p. with 100 μg of neutralizing antibody or isotype control at days 6, 10, and 14 after immunization.
CNS leukocyte isolation
Sacrificed mice were perfused with phosphate-buffered saline (PBS) containing 2 mM ethylene diamine tetraacetic acide (EDTA) to remove blood from internal organs. The spinal columns were dissected, cut open, and the intact spinal cord was separated carefully from the vertebrae. The spinal cord was cut into small pieces and placed in digestion solution containing collagenase D (10 mg/ml, Roche) in Hank’s balanced salt solution (HBSS). Digestion was performed for 45 min at 37°C with brief mixing by vortex every 15 min. At the end of the digestion, the solution was mixed thoroughly and passed through a 40-μm cell strainer. The cells were washed once in PBS, placed in 6 ml of 38% Percoll solution, and centrifuged for 20 min at 860g. Cell pellets were resuspended in buffer or medium for subsequent analysis.
Histopathology and immunohistochemistry
Mice were euthanized, and brains and spinal cords were removed and fixed by immersion with a 10% neutral-buffered formalin solution and decalcified. Fixed tissues were embedded in paraffin, sectioned, and stained with H&E, with serial histological sections were stained immunohistochemically to determine the distribution and types of inflammatory cells in the brain and spinal cord. Spinal cord pathology was assigned scores by an experienced pathologist (P. Vogel) as described previously (27).
Cell culture and stimulation
Primary astrocyte cultures were prepared from neonatal mice (2 to 5 days old). In brief, the mice had their cerebral cortices aseptically dissected and the meninges removed. The tissues were then dissociated with 1-ml pipettes, passed through 40-μm cell strainers and centrifuged. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum (FBS, vol/vol) supplemented with penicillin and streptomycin. Primary MEFs were isolated from embryos at day 14.5 of gestation (E14.5) and maintained in DMEM, 10% FBS, supplemented with penicillin and streptomycin. The cells were left untreated or were stimulated with IL-17 (200 ng/ml, R&D) or TNF-α (20 ng/ml, R&D) alone or in combination. For treatment with inhibitors, cells were incubated with vehicle or 10 μM MK2 inhibitor (EMD) for 30 min to 1 hour before they were stimulated.
Flow cytometric analysis
To analyze cell-surface markers, cells were stained in PBS containing 2% (wt/vol) bovine serum albumin (BSA), with anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-TCRβ (H57-597), anti-CD11b (M1/70), anti-F4/80 (8M8), anti-CD45 (30-F11), anti-CD45.1 (A20), anti-CD45.2 (104) antibodies, all of which were obtained from eBioscience. For intracellular staining, T cells were stimulated for 5 hours with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma) with monensin (BD Biosciences) before they were stained with antibodies specific for IFN-γ (XMG1.2) and IL-17 (17B7), both of which were obtained from eBioscience, according to the manufacturer’s instructions (BD Biosciences). To detect caspase activity, cells were stained with fluorescein isothiocyanate (FITC)-conjugated VAD-FMK (Promega) according to the manufacturer’s instructions. BrdU and Ki-67 labeling were performed according to the manufacturer’s instructions (BD Biosciences). Flow cytometry data were acquired on an upgraded 5-color FACScan or LSRII flow cytometers (BD) and were analyzed with FlowJo software (Tree star).
RNA and protein analyses
Real-time polymerase chain reaction (PCR) analysis was performed with primer and probe sets obtained from Applied Biosystems, and the abundances of complementary DNAs (cDNAs) of genes of interest were normalized to that of the endogenous control gene Hprt, as described previously (48). To analyze primary transcripts, RNAs treated with RNase-free DNase I were used for reverse transcription to generate cDNAs. cDNAs were subjected to PCR analysis with the Power SYBR Green Master Mix (Life Technologies) with the following primers. Cxcl1: 5′-GTTAGCTTCTGCCACTTCCAG-3′ (intron 2), and 5′-AGCTTCAGGGTCAAGGCAAG-3′ (exon 3); Cxcl2: 5′-TGAGTGTCTCCCTGGATAGC-3′ (intron 2), and 5′-TTTTGACCGCCCTTGAGAG-3′ (exon 3); and actin: 5′-CCTTGCATGTCTCAGATCTATC-3′ (intron 3), and 5′-GAGTCCATCACAATGCCTG-3′ (exon 4). To measure mRNA stability, 4-OHT-treated astrocytes from WT and p38αCreER mice were stimulated with TNF-α (10 ng/ml) for 2 hours, and then were treated with actinomycin D (5 mg/ml) and IL-17 (50 ng/ml) for the times indicated in the figure legends. For bio-plex assays, the amounts of cytokines in cell culture medium was measured with MILLIPLEX kits (Millipore) for mouse cytokines and chemokines according to the manufacturer’s instructions. Western blotting analysis was performed as described previously (50) with antibodies specific for phosphorylated p38 (p-p38) at Thr180/Tyr182 (9211), total p38 (9212), p-MK2 at Thr334 (27B7), p-CREB at Ser133 (87G3), p-IκBα (inhibitor of κB α) at Ser32 (2859), p-ERK at Thr202/Tyr204 (9101), p-JNK at Thr183/Tyr185 (9251, all from Cell Signaling Technology), and β-actin (AC-15, Sigma).
Statistical analysis
P values were calculated with the Mann Whitney U test (for EAE clinical scores) or the student’s t-test (for other analyses). P < 0.05 was considered to be statistically significant and P values are designated as follows: *P < 0.05; **P < 0.01; ***P < 0.001. All error bars in graphs represent the standard error of the mean (SEM) calculated from at least three replicates.
Supplementary Material
Acknowledgments
We thank C. Cloer and B. Rohde for help with animal colony management; K. Yang, J. Wei, and X. Du for help with cellular and molecular analysis; K. Otsu for the p38α floxed mice; and the St. Jude Immunology FACS core facility for cell sorting.
Funding: This work was supported by NIH R01 grants NS064599, AI105887, and AI101407 and National Multiple Sclerosis Society grant RG4691-B-2 (to H.C.).
Footnotes
Fig. S1. Effects of p38α deficiency on immune signaling pathways in CNS inflammation.
Fig. S2. Effects of late p38α deletion and IL-17 neutralization on EAE disease course.
Fig. S3. p38α is specifically deleted in the CNS of p38αNesCre mice.
Fig. S4. The activity of p38α in CNS-resident cells is not required for peripheral TH17 and TH1 cell responses.
Fig. S5. The activity of p38α in CNS-resident cells is dispensable for the initial infiltration of transferred TH17 cells into the CNS, but contributes to proinflammatory gene expression.
Fig. S6. Expression of IL-17R components and mechanisms of chemokine gene regulation in astrocytes.
Fig. S7. Analysis of MKP-1 and JNK functions.
Author contributions: G.H. and Y.W. designed and performed cellular and molecular experiments and in vivo models; P.V. contributed to histopathology analysis; and H.C. designed experiments, wrote the manuscript, and provided overall direction.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci. 2012;15:1074–1077. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 7.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 8.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Garcia I, Zhao Y, Ju S, Gu Q, Liu L, Kolls JK, Lu B. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 12.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Bain J, Plater L, Elliott M, Shpiro N, Hastie J, McLauchlan H, Klevernic I, Arthur S, Alessi D, Cohen P. The selectivity of protein kinase inhibitors; a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godl K, Wissing J, Kurtenbach A, Habenberger P, Blencke S, Gutbrod H, Salassidis K, Stein-Gerlach M, Missio A, Cotten M, et al. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci U S A. 2003;100:15434–15439. doi: 10.1073/pnas.2535024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argast GM, Fausto N, Campbell JS. Inhibition of RIP2/RIck/CARDIAK activity by pyridinyl imidazole inhibitors of p38 MAPK. Mol Cell Biochem. 2005;268:129–140. doi: 10.1007/s11010-005-3701-0. [DOI] [PubMed] [Google Scholar]
- 18.Menon MB, Kotlyarov A, Gaestel M. SB202190-induced cell type-specific vacuole formation and defective autophagy do not depend on p38 MAP kinase inhibition. PLoS One. 2011;6:e23054. doi: 10.1371/journal.pone.0023054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krementsov DN, Thornton TM, Teuscher C, Rincon M. The emerging role of p38 mitogen-activated protein kinase in multiple sclerosis and its models. Mol Cell Biol. 2013;33:3728–3734. doi: 10.1128/MCB.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G, Wang Y, Vogel P, Kanneganti TD, Otsu K, Chi H. Signaling via the kinase p38alpha programs dendritic cells to drive T(H)17 differentiation and autoimmune inflammation. Nat Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krementsov DN, Noubade R, Dragon JA, Otsu K, Rincon M, Teuscher C. Sex-specific control of CNS autoimmunity by p38 MAPK signaling in myeloid cells. Ann Neurol. 2014;75:50–66. doi: 10.1002/ana.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–1036. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 26.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 27.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1a-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 29.Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 30.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 2011;32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol. 2013;191:640–649. doi: 10.4049/jimmunol.1203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177:1913–1917. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Reynolds JM, Chang SH, Martin-Orozco N, Chung Y, Nurieva RI, Dong C. MKP-1 is necessary for T cell activation and function. J Biol Chem. 2009;284:30815–30824. doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 41.Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013;16:1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7:954–961. doi: 10.1038/ni1372. [DOI] [PubMed] [Google Scholar]
- 43.Raasch J, Zeller N, van Loo G, Merkler D, Mildner A, Erny D, Knobeloch KP, Bethea JR, Waisman A, Knust M, et al. IkappaB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-kappaB in the central nervous system. Brain. 2011;134:1184–1198. doi: 10.1093/brain/awq359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodgers JM, Zhou L, Miller SD. Act1, scene brain: astrocytes play a lead role. Immunity. 2010;32:302–304. doi: 10.1016/j.immuni.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 46.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.