Abstract
Purpose
Cystinosis is caused by a deficiency in the lysosomal cystine transporter, cystinosin (CTNS gene), resulting in cystine crystal accumulation in tissues. In eyes, crystals accumulate in the cornea causing photophobia and eventually blindness. Hematopoietic stem progenitor cells (HSPCs) rescue the kidney in a mouse model of cystinosis. We investigated the potential for HSPC transplantation to treat corneal defects in cystinosis.
Methods
We isolated HSPCs from transgenic DsRed mice and systemically transplanted irradiated Ctns−/− mice. A year posttransplantation, we investigated the fate and function of HSPCs by in vivo confocal and fluorescence microscopy (IVCM), quantitative RT-PCR (RT-qPCR), mass spectrometry, histology, and by measuring the IOP. To determine the mechanism by which HSPCs may rescue disease cells, we transplanted Ctns−/− mice with Ctns−/− DsRed HSPCs virally transduced to express functional CTNS-eGFP fusion protein.
Results
We found that a single systemic transplantation of wild-type HSPCs prevented ocular pathology in the Ctns−/− mice. Engraftment-derived HSPCs were detected within the cornea, and also in the sclera, ciliary body, retina, choroid, and lens. Transplantation of HSPC led to substantial decreases in corneal cystine crystals, restoration of normal corneal thickness, and lowered IOP in mice with high levels of donor-derived cell engraftment. Finally, we found that HSPC-derived progeny differentiated into macrophages, which displayed tunneling nanotubes capable of transferring cystinosin-bearing lysosomes to diseased cells.
Conclusions
To our knowledge, this is the first demonstration that HSPCs can rescue hereditary corneal defects, and supports a new potential therapeutic strategy for treating ocular pathologies.
Keywords: cystinosis, hematopoietic stem cell, corneal cystine crystals, tunneling-nanotubes
Corneal disease is a significant cause of blindness worldwide,1 and corneal transplant for hereditary or acquired diseases is limited by low availability of donor corneas and risks associated with transplantation, such as immune rejection, cataract, and glaucoma.2 Stem cell transplantation represents an attractive alternative for focal corneal repair, for example with limbal epithelial stem cells to repair corneal epithelium,3 and with mesenchymal stem cells (MSCs) that have been shown to migrate and promote corneal healing, secrete soluble factors, and suppress inflammation and angiogenesis.4 However, repairing hereditary eye deficiencies with stem cells has remained relatively unexplored. We have been investigating the therapeutic potential of hematopoietic stem and progenitor cells in a mouse model of an inheritable lysosomal storage disease, cystinosis.
Cystinosis is an autosomal recessive lysosomal storage disorder in which cystine accumulates within lysosomes due to impairment of the transmembrane lysosomal cystine transporter, cystinosin, encoded by the ubiquitously expressed gene CTNS.5,6 Cystine accumulation leads to the formation of cystine crystals within tissues, causing cell death and eventually organ degeneration.7–10 Kidneys and eyes are the primary tissues impacted by the disease in mice and humans11,12; Ctns−/− mice develop ocular pathology similar to humans.11,13,14 The main ocular manifestation is crystal deposition in the cornea, which begins in infancy, increases with age, and gradually leads to photophobia, blepharospasm, keratopathy, and recurrent corneal erosions.15 In older patients, filamentous keratopathy, band keratopathy, and peripheral corneal neovascularization also are observed.15–17 A similar spectrum is seen in the mouse model, with central corneal opacification and loss of the corneal cellular architecture, and eventually phthisis bulbi.11,13,14,18 Crystals also accumulate in conjunctiva, lens, sclera, ciliary body, iris, trabecular meshwork, choroid, retinal pigment epithelium, and retina, and can cause retinopathy.19,20 The current treatment for cystinosis is the drug cysteamine, but taken orally it does not improve ocular manifestations of the disease, presumably due to the lack of bioavailability in the cornea.21 Topical treatment with cysteamine hydrochloride eye drops or a more recent gel formulation is effective in reducing corneal crystal density and alleviating symptoms,15–17,22–24 but patients still exhibit significant crystal accumulation and associated ocular morbidity, suggesting the need for more potent and durable therapeutics.24
Hematopoietic stem and progenitor cells (HSPCs) are multipotent cells capable of self-renewal and are easily mobilized from bone marrow into the circulatory system. These cells enhance reepithelialization of corneal wounds after alkali injuries in mice,25 but beyond this finding limited data exist to indicate the potential of HSPCs for reversing ocular pathology.
We demonstrated previously that systemic transplantation of wild-type HSPCs resulted in a significant decrease of cystine levels in tissues and the long-term preservation of the kidney in Ctns−/− mice.26–28 The objective of the present study was to test whether systemic HSPC transplantation also could treat eye pathology in Ctns−/− mice. We showed that HSPCs differentiate into macrophage progeny into ocular tissues, deliver cystinosin product locally, and improve corneal defects in Ctns−/− mice.
Methods
Mice
The C57BL/6 Ctns−/− mice were provided by C. Antignac (Inserm U1163, Paris, France) and bred continuously at University of California, San Diego (UCSD). Transgenic mice constitutively expressing GFP (C57BL/6-Tg[ACTB-EGFP]1Osb/J) or DsRed (B6.Cg-Tg[CAG-DsRed*MST]1Nagy/J) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and both were cross-bred with the C57BL/6 Ctns−/− mice to produce transgenic GFP Ctns−/− and DsRed Ctns−/− mice. All protocols were approved by the UCSD Institutional Animal Care and Use Committee (IACUC) and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Bone Marrow Cell (BMC) Isolation, Transduction, and Transplantation
Bone marrow cells were flushed from the long bones of 6- to 80-week-old GFP-transgenic mice or DsRed Ctns−/− mice. ScaI+ HSCs were isolated as described previously.29 One million GFP cells were directly transplanted in lethally irradiated (8 Gy) DsRed Ctns−/− mice by tail vein injection while DsRed Ctns−/− cells were first transduced with SIN lentivirus expressing the fused protein CTNS-GFP before transplantation in lethally irradiated (8 Gy) Ctns−/− mice as described previously.29 To analyze bone marrow engraftment of the transplanted cells, fresh blood was treated with red blood cell lysis buffer (eBioscience, San Diego, CA, USA) and subsequently analyzed by flow cytometry to the GFP-expressing cells.
Fluorescence Microscopy
Eyes were fixed in 5% formaldehyde for 2 to 3 hours and equilibrated in 20% sucrose overnight. Eyes were frozen in Tissue-Tek Optimal Cutting Temperature buffer at −80°C (Sakura Finetek USA, Inc., Torrance, CA, USA) and 20-μm sections were cut. Sections were stained with 4′,6-diamidino-2-phenylendole (DAPI). The Keyence BZ-X700 microscope (Keyence, Osaka, Japan) was used to capture the pictures and the Keyence software was used to perform image stitching and 3D reconstitution.
In Vivo Confocal Microscopy (IVCM) of the Cornea and Cystine Crystal Quantification
Images of corneal nerves were collected using the Heidelberg Retina Tomograph (HRT) 3 with Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany) and a custom built small animal platform as described previously.30 Briefly, general anesthesia was induced using 2.5% isoflurane in oxygen and GenTeal gel (Novartis Pharmaceuticals Corp., East Hanover, NJ, USA) was placed on both eyes for laser light coupling and to prevent the eye from drying. To evaluate the effectiveness of the HSC transplantation, the microscope objective was positioned at the center apex of the cornea, the depth adjusted to 25 μm and one volume stack of 40 images (384 × 384 pixels, 1 μm lateral resolution) collected. To evaluate formation of cystine crystals, the microscope operator identified easily recognizable physical attributes located as near to the apex as possible. The operator then used these attributes as landmarks to collect identical volume scan images in the same area of the cornea each week for 6 weeks. Images were imported into Matlab (MathWorks, Natick, MA, USA) and cystine crystals in each layer picture was quantified using ImageJ software (available in the public domain at http://imagej.nih.gov/ij/; National Institutes of Health [NIH], Bethesda, MD, USA). The threshold was adjusted to recognize specifically the cystine crystals and pixels were quantified.
IOP Measurement
Intraocular pressure was measured using the Icare TONOLAB tonometer (Icare, Vanda, Finland), as described by the manufacturer. Briefly, mice were placed on a stand with eyes positioned approximately 1 to 4 mm away from the TONOLAB tonometer, which was securely fixed to a ring stand. Corneas were aligned centrally with the TONOLAB tonometer probe and five measurements were collected and averaged from each mouse eye. Before IOP measurements, eyelashes were trimmed carefully and eyes were lightly moistened with balanced salt solution to avoid IOP measurement obstruction and errors, sometimes caused by corneal drying and tonometer probe-corneal sticking. In addition, to maintain steady readings during this procedure, mice were placed under anesthesia using an isoflurane-oxygen mixture.
Cystine Content Measurement
Explanted eyes were ground in 500 μL of N-ethylmaleimide (Fluka Biochemika, Bushs, Switzerland) at 650 μg/mL using the Precellys 24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). Proteins were precipitated using 15% 5-sulfosalicylic acid dihydrate (Fluka Biochemika), resuspended in NaOH 0.1 N and measured using the Pierce BCA protein assay kit (Pierce, Rockford, IL, USA). The cystine-containing supernatants were sent to the UCSD Biochemical Genetics laboratory for measurement by mass spectrometry as described previously.28
Reverse Transcription and Quantitative PCR (RT-qPCR)
The RNA from explanted eyes was isolated using the RNeasy kit (Qiagen, Venlo, The Netherlands) according to manufacturer's instructions. One microgram of RNA was reverse transcribed using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The Ctns-specific qPCR was performed using 2 μL of cDNA, 2X SsoAdvanced Universal Probes Supermix (Bio-Rad Laboratories, Inc.), and primers and probe were obtained from Applied Biosystems (Carlsbad, CA, USA): Ctns oligo 1: 5′-TTGTGGTGCAGTCGGTATC-3′, Ctns oligo 2: 5′-AGCTTGATGTAGGAGAAGCAGAAGA-3′, and Ctns probe: 5′-CACATGGCTCCAGTTC-3′, and 18S primer mix on a CFX96 (Bio-Rad Laboratories, Inc.). The expression level of Ctns gene, expressed as fold change, was calculated using the ΔΔCt method between the target gene and an endogenous control (18S).
Histology and Central Cornea Thickness (CCT) Measurement
Explanted eyes were fixed in formalin and embedded in paraffin. Sections (10 μm) were stained with hematoxylin and eosin. Using a light microscope (Keyence), pictures of the central cornea were acquired (×20) and CCT as well as the epithelial layer thickness were measured using Keyence software at 3 different parts of the cornea and averaged.
Immunofluorescence Analysis
Eyes were fixed in 5% formaldehyde for 2 to 3 hours and equilibrated in 20% sucrose overnight. Eyes were frozen in Tissue-Tek Optimal Cutting Temperature buffer at −80°C (Sakura Finetek USA) and 20-μm sections were cut. Sections were stained with DAPI and with Rat-anti-F4/80 (14-4801-82, eBioscience), Rat-anti-MHC Class II [NIMR-4] (ab25333, Abcam) and Mouse-anti-CD11c [3.9] (ab11029; Abcam, Cambridge, UK) antibodies. To quantify HSPCs-derived cells (DsRed) and F4/80+ cells, three sections per animal were used and cells were counted manually in the entire cornea. To visualize the nanotubes, all pictures were acquired using a ×63 objective on an Olympus FluoView1000 (Olympus, Center Valley, PA, USA) or Leica TCS SP5 II confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA). Images were processed with ImageJ or Volocity software (Perkin Elmer, Waltham, MA, available in the public domain at www.perkinelmer.com) to generate 3D reconstructions from optical slices (Z-stacks).
Statistics
We summarized continuous data as arithmetic means ± SEM. Group comparisons were made using t-test. Associations between blood engraftment and Ctns expression or cystine content was investigated with linear regressions. The χ2 test was used to identify whether IOP above or below 20 mm Hg was dependent on cell engraftment. All analyses were performed using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 was considered as statistically significant.
Results
HSPCs Migrate to Different Regions of the Eye in Ctns−/− Mice
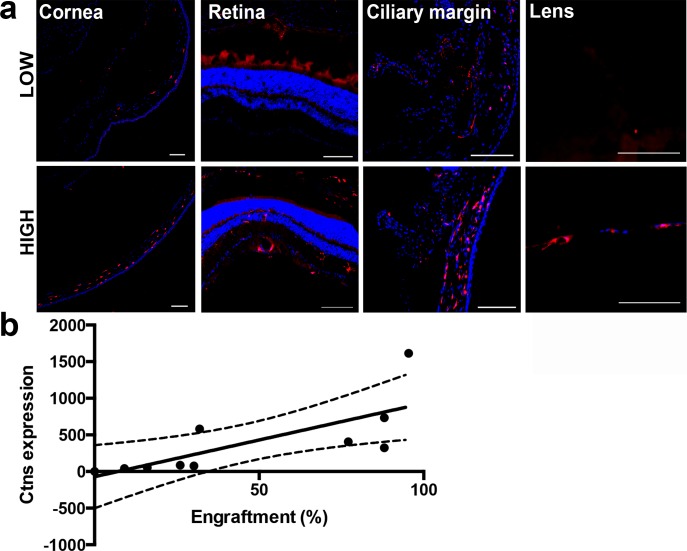
Lethally irradiated Ctns−/− mice between 1.5 and 3.5 months of age were transplanted with wild-type DsRed-expressing bone marrow stem cells to study the fate and impact of HSPCs on the corneal defects in cystinosis. Nontreated Ctns−/− mice and lethally irradiated Ctns−/− irradiated mice transplanted with DsRed Ctns−/− HSPCs were used as controls. At 1 year after transplantation, bone marrow–derived cells were present through various parts of the eyes of Ctns−/− mice, including the cornea, retina, lens, sclera, and ciliary margin (Fig. 1a), demonstrating that systemically injected HSPCs migrate to and integrate in the diseased eyes.
Figure 1.
Ocular engraftment of transplanted HSPCs depends on donor-derived blood cell engraftment. (a) Systemic DsRed-expressing HSPC transplantation resulted in the presence of bone marrow–derived cells (seen in red) in the whole eye as shown in sagittal sections of the eyes of mice with donor-derived blood cell engraftment <50% (Low) compared to >50% (High). More abundant HSPC-derived cells were seen when donor-derived blood cell engraftment in High than in Low mice in the different eye compartments. Scale bars: 100 μm. (b) Linear regression analysis of Ctns expression in the whole eye showing that Ctns expression increases with the level of transplanted DsRed-HSPCs. The linear regression interrupted lines correspond to 95% confidence.
To determine the level of donor bone marrow stem cell engraftment, DsRed-positive cells were quantified in peripheral blood samples by flow cytometry; engraftment ranged from 16 to 95.4% (Low, n = 7, mean = 27.91% ± 12.24%; High, n = 7, mean = 85.46% ± 6.09%). Mice with high levels of engraftment (>50%; High) exhibited more abundant bone marrow–derived cells within the eyes compared to mice with a low level of engraftment (<50%; Low; Fig. 1a). This histologic observation was confirmed by RT-qPCR quantitative analysis of Ctns expression (derived from the donor cells), demonstrating that Ctns expression in the eyes of the treated Ctns−/− mice increased with increasing engraftment levels (F1,9 = 9.635, P = 0.0146; Fig. 1b). Since we previously showed that a correlation exists between level of donor-derived blood cell engraftment and long-term preservation of the kidney,28 we assessed the subsequent structural, biochemical, and functional effect of HSPCs on corneas in the Ctns−/− mice by differentiating between the High and Low engraftment groups, compared to age-matched wild-type and nongrafted Ctns−/− controls.
Prevention of Corneal Cystine Crystal Accumulation in Ctns−/− Mice With High HPSC Engraftment
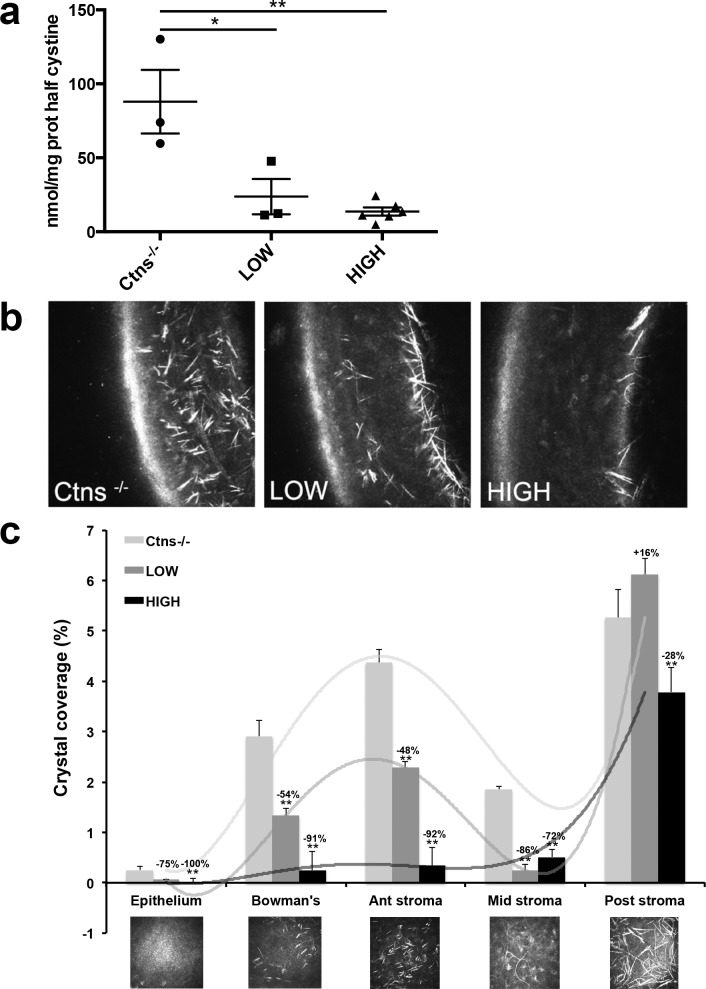
First, tissue cystine content was measured by mass spectrometry in whole enucleated eye. A significant decrease in cystine levels was observed in high (n = 6) and low (n = 3) treated mice compared to Ctns−/− controls (n = 3, reductions of 85% and 73%, respectively; Fig. 2a). To evaluate corneal cystine crystals, we performed IVCM in live mice, scanning through the entire cornea, differentiating the epithelium, Bowman's membrane, stroma, Descemet's membrane, and endothelium (Fig. 2b). Crystals in each layer then were quantified using ImageJ software (Fig. 2c). Because there is asymmetry of crystal deposition between the two eyes of the same animal,18 we analyzed each eye individually. At 1 year of age, all Ctns−/− mouse controls (nontreated or transplanted with Ctns−/− HSPCs, n = 4) exhibited abundant cystine crystals within all layers of the cornea, with the majority being present in the anterior stroma and endothelium layers. The Ctns−/− mice with low level of engraftment (Low; n = 5) showed a partial reduction of crystal counts, and mice with high engraftment levels (High; n = 5) exhibited almost a complete resolution of crystals from the epithelial layer to the mid-stroma (100% and 72% clearance, respectively; Fig. 2c). These data showed, for the first time to our knowledge, that systemic HSPC transplantation has a significant beneficial impact on corneal cystine crystals and also suggested that some minimum level of Ctns-expressing bone marrow–derived cells must be present in the cornea to resolve the corneal cystine crystals in the stroma and epithelium.
Figure 2.
Transplantation of HSPC leads to ocular cystine and corneal cystine crystal clearance. (a) Cystine levels measured in the whole eye were significantly reduced in the HSPC-transplanted mice, Low and High, compared to the Ctns−/− controls. Error bars: SEM (*P < 0.05, **P < 0.005). (b) Lateral cornea IVCM representations of Ctns−/− controls and Low and High HSPC-transplanted mice. Abundant cystine crystals were observed in the whole cornea in Ctns−/− mice in contrast to Ctns−/− with high level of engraftment where residual crystals can be seen only in the posterior stroma. (c) Surface crystal quantification within each layer of the full IVCM cornea scans from both eyes of Ctns−/− controls and transplanted (Low and High) mice. Transplantation of HSPC with high level of engraftment prevents almost completely cystine crystal formation within the superior layers of the cornea (epithelium to middle stroma). The mice with low level of engraftment exhibit significant but more modest crystal reduction. Error bars: SEM (*P < 0.05, **P < 0.005).
Cystine crystal levels in the posterior stroma also were significantly reduced in the High engraftment mice, by 28% (Fig. 2c), a less dramatic reduction than with the anterior cornea. No reduction in the posterior stroma was found in Low engraftment animals. These data might be explained by the kinetics of the formation of the corneal cystine crystals that were shown to first appear in the peripheral posterior stroma/corneal endothelium.18 Thus, crystal formation may occur within the endothelium before HSPC transplantation, and our data suggested that, although HSPCs prevent progression to stromal layers, they do not remove crystals already present in the endothelium at 2 months.
Rescue of Corneal Atrophy in HSPC-Treated Ctns−/− Eyes
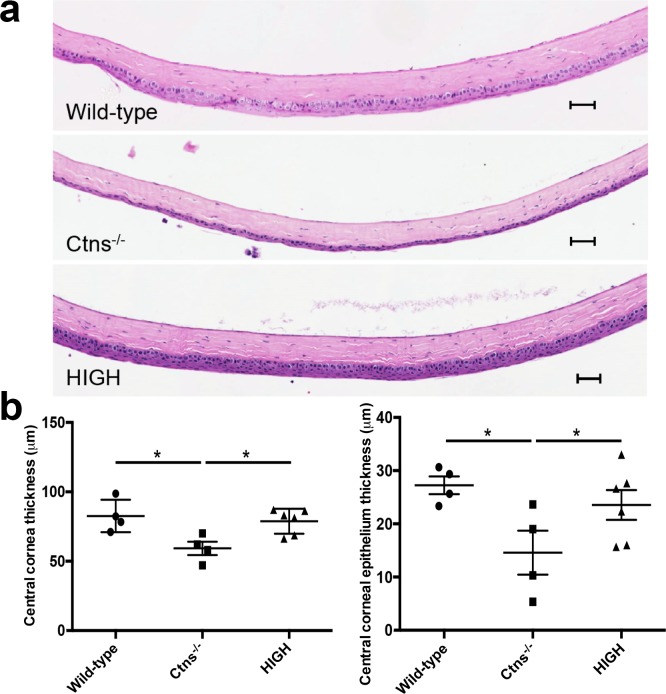
Corneas, and in some cases the entire eye, degenerate over time in Ctns−/− mice.11,13,14,18 To evaluate whether HSPCs prevent corneal atrophy in Ctns−/− mice, we measured the CCT in hematoxylin-stained eye sections, as described previously.31 We found that the cornea of old Ctns−/− mice (approximately 13 months of age; n = 4) was significantly thinner than in wild-type animals (n = 4), an effect most apparent in the epithelial layer (Fig. 3a). Interestingly, HSPC transplantation (n = 6) prevented the reduction of CCT and allowed maintenance of normal epithelial structure in mice with a high level of engraftment (Fig. 3b). Data from Ctns−/− mice with low levels of engraftment also showed an improvement of corneal structure, but histological sections were only available from two animals (data not shown). Thus HSPC engraftment prevents late corneal atrophy in cystinosis.
Figure 3.
Corneal structure is preserved in HSPC-transplanted Ctns−/− mice. (a) Representative sagittal hematoxylin and eosin sections of the central cornea of wild-type mice, nontreated Ctns−/−, and highly engrafted Ctns−/− (High) mice. The Ctns−/− mice exhibit markedly reduced CCT compared to wild-type animal, while treated Ctns−/− mice presented with CCT comparable wild-type. Scale bars: 200 μm. (b) Thickness measurement of the central cornea and central cornea epithelium of wild-type, Ctns−/−, and transplanted (High) Ctns−/− mice showing that HSPC transplantation prevents corneal reduction. Error bars: SEM (*P < 0.05).
Rescue of Elevated IOP in HSPC-Treated Ctns−/− Eyes
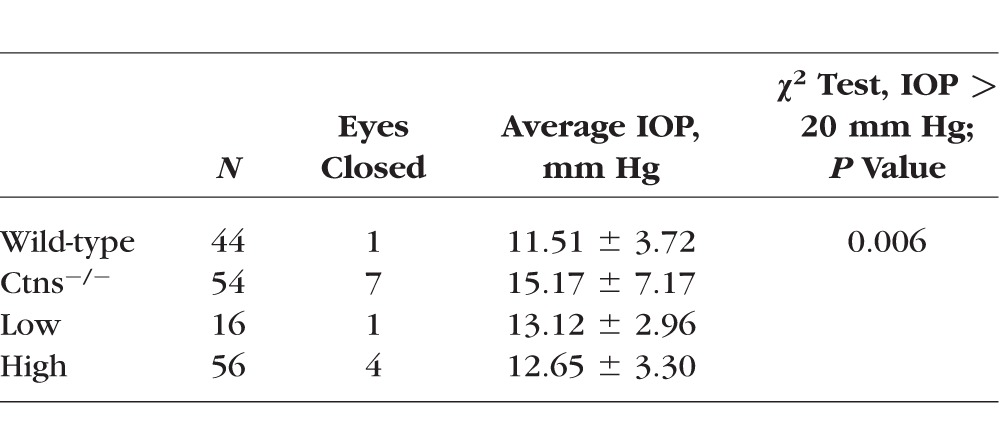
Cystinosis leads to IOP fluctuations and elevation in mice and humans.13,24 IOP measures in Ctns−/− mice were significantly higher compared to wild-type mice (see Table), reaching greater than 20 mm Hg in 5 of 54 eyes. In contrast, high- and low-HSPC engraftment in HPSC-treated Ctns−/− mice demonstrated decreased IOP compared to Ctns−/− mice, with no observed measures above 20 mm Hg (P = 0.006, χ2 test).
Table.
IOP in Control and HSPC-Transplanted Mice
Mechanism of Corneal Rescue by HSPC Transplantation in Ctns−/− Mice
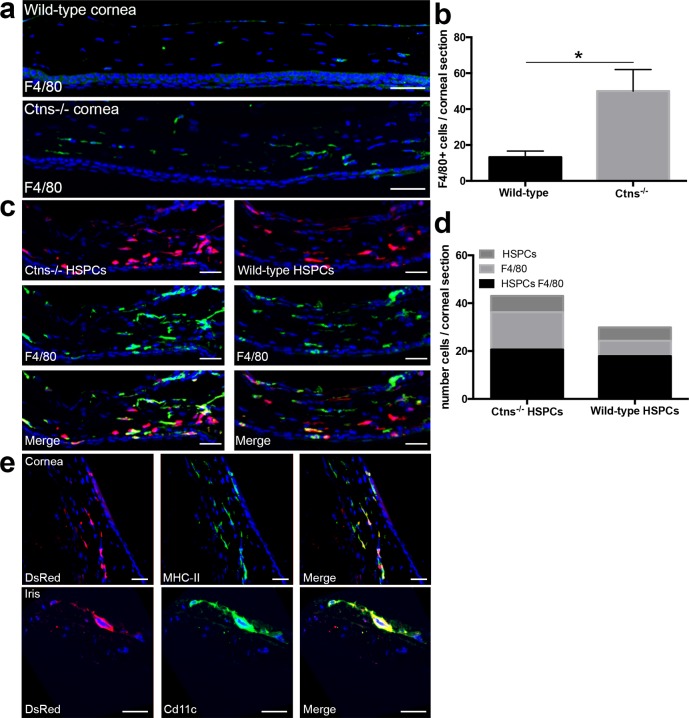
We recently showed that the cellular mechanism of kidney repair in HSPC-transplanted cystinosis mice involved lysosomal cross-correction via tunneling nanotubes (TNTs) after HSPC differentiation into macrophages.29 Here, we asked whether the same mechanism is observed in eyes. Confocal microscopy showed that most HSPC progeny in the cornea differentiated into mature macrophages, as confirmed by the colocalization of all bone marrow–derived DsRed-positive cells with F4/80 (Fig. 4c). Quantification of F4/80-positive cells within corneas of nontransplanted animals showed that Ctns−/− mice exhibit approximately 75% more macrophages in the corneas compared to wild-type mice, presumably due to the underlying tissue injury and degeneration associated with cystinosis (Figs. 4a, 4b). We then compared the level of tissue engraftment and differentiation potential of wild-type HSPCs and Ctns−/− HSPCs by quantifying and characterizing the DsRed-positive cells in the cornea of Ctns−/− mice transplanted with either DsRed wild-type HSPCs and DsRed Ctns−/− HSPCs. We found that the level of engraftment and phenotype of transplanted wild-type and Ctns−/− bone marrow–derived cells within the cornea was equivalent in the two groups (Figs. 4c, 4d). These data suggested that wild-type HSPCs show no advantage in migration, differentiation, or integration in ocular tissues compared to Ctns−/− donor cells. However, more endogenous, DsRed-negative F4/80-positive cells were found in corneas from animals transplanted with Ctns−/− HSPCs compared to wild-type HSPCs-transplanted mice (Fig. 4d). These results suggested that tissue injury in Ctns-deficient mice leads to chronic inflammation, which is reduced when the macrophages express a functional Ctns gene associated with tissue rescue. Further characterization of the macrophages showed that most of the macrophages expressed MHC-II (Fig. 4e). We also observed that few HSPCs (∼10%) colocalized with CD11c suggesting that some of the HSPCs also differentiated into dendritic cells (Fig. 4e).
Figure 4.
Hematopoietic stem and progenitor cells differentiate into phagocytic cells within the cornea. (a) Representative confocal pictures of corneal sections from wild-type and Ctns−/− mice. Nuclei were stained with DAPI are seen in blue and F4/80+ cells are seen in green. (b) Quantification of F4/80-positive cells within corneas of wild-type (n = 4) and Ctns−/− (n = 4) mice. (c) Representative confocal pictures of corneal sections from Ctns−/− mice transplanted with either DsRed Ctns−/− HSPCs or DsRed wild-type HSPCs. Nuclei were stained with DAPI are seen in blue, HSPCs-derived cells are seen in red, and F4/80-positive cells are seen in green. (d) Quantification of DsRed-positive HSPC-derived cells (HSPCs), host F4/80-positive cells (F4/80), and F4/80-positive and DsRed-positive HSPC-derived cells (HSPCs F4/80) within the corneas of Ctns−/− mice transplanted with Ctns−/− DsRed HSPCs (n = 5) or wild-type DsRed HSPCs (n = 7) mice. (e) All the DsRed-expressing bone marrow–derived colocalize with the antigen presenting cells (APC) marker MHC-II class II (seen in green, upper). Few of them expressed CD11c (seen in green, lower) indicating that some of them differentiated into dendritic cells. Error bars: SEM (*P < 0.05). Scale bars: (a, c) 50 μm and (e) 30 μm.
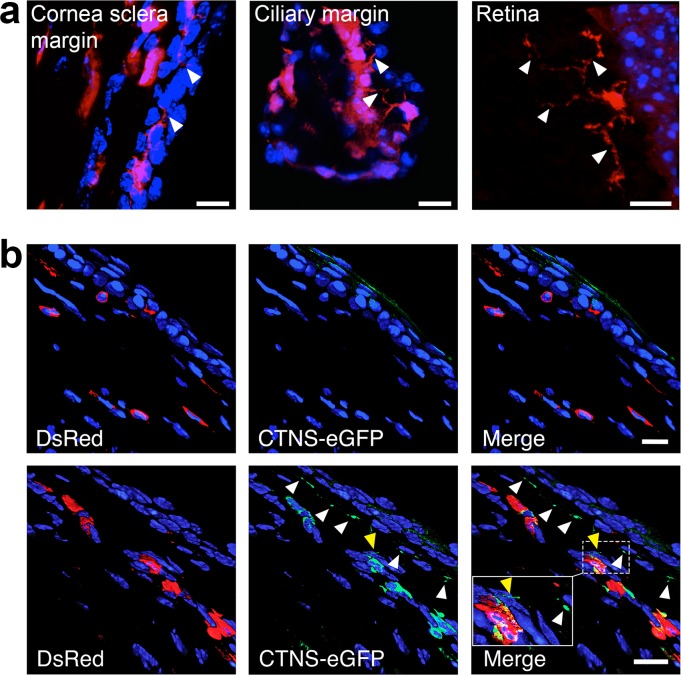
Higher magnification confocal images showed that bone marrow–derived macrophages form TNT structures. We identified TNTs generated from bone marrow–derived cells in the cornea sclera margin, ciliary margin and retina (Fig. 5a). To test if lysosomal cross-correction occurs in the cornea, we transplanted normal Ctns−/− mice with DsRed Ctns−/− HSPCs stably expressing cystinosin-eGFP fusion protein or with DsRed Ctns−/− HSPCs as control. Eyes were analyzed 7 months posttransplantation by confocal microscopy. No GFP-positive lysosomes were identified in Ctns−/− mice transplanted with DsRed Ctns−/− HSPCs alone (Fig. 5b, upper). In contrast, we observed cystinosin-eGFP-bearing lysosomes within the DsRed-positive bone marrow–derived cells as expected in mice transplanted with cystinosin–eGFP–HSPCs and also within host corneal cells, directly demonstrating lysosomal transfer from the HSPC-derived cells to the adjacent diseased cells (Fig. 5b, lower). Thus protein transfer from donor to host cells is detected in the corneas of HSPC-treated Ctns−/− mice, providing evidence for lysosomal cross correction in the eye.
Figure 5.
HSPCs-derived macrophages transfer cystinosin-bearing lysosomes via TNTs to the adjacent disease cells. (a) Tunneling nanotubes generated from the DsRed bone marrow–derived cells were observed in the cornea (sclera margin and ciliary margin) and retina (arrows). (b) Representative confocal pictures of the cornea of Ctns−/− mice transplanted with Ctns−/− DsRed HSPCs as control (upper) or with Ctns−/− DsRed HSPCs stably expressing the fusion protein CTNS-eGFP (lower). Green vesicles are observed within the DsRed-bone marrow–derived cells in the mice treated with CTNS-eGFP-HSPCs but also in the no color host cells (white arrowheads) showing that lysosomes containing CTNS-eGFP could be transferred. Inset shows a TNT containing cystinosin-bearing vesicles (yellow arrowhead). Scale bars: (a) 10 μm, (b) 15 μm.
Discussion
Here we report for the first time that systemic HSPC transplantation can treat corneal defects in the context of a degenerative, hereditary metabolic disorder, cystinosis. This treatment led to a long-term prevention of corneal cystine crystal deposition, preservation of corneal structure, and reduction in IOP in the Ctns−/− mice. These results demonstrate that HSPCs have the capacity to produce bone marrow–derived cells that home to and integrate into injured/diseased eyes, to actively participate in the preservation of eye function and structure over prolonged periods of time. These results are particularly important considering that they demonstrate this correction in the avascular cornea tissue after systemic transplantation. We present in this study 1-year posttransplantation data, but there is no indication that results would not extend to the entire life span of the study animals, as previously shown for the kidney.28 Therefore, transplantation of normal, CTNS-expressing HSPCs in patients affected with cystinosis may represent a long-term treatment that can preserve disease-affected tissues, including the kidneys and the eyes. Based on these data, ocular exams will be included as important efficacy endpoints in an upcoming hematopoietic stem cell-based human clinical trial for cystinosis. We do not know whether transplanting HSPCs into aged Ctns−/− mice with advanced ocular deficits will rescue established tissue damage, an important future study when considering the potential for therapeutic impact when treating adults with cystinosis.
We found that long-term preservation of ocular function and structure in the Ctns−/− mice were dependent on achieving a relatively high level of donor-derived blood cell engraftment of Ctns-expressing cells (>50%), similar to our prior studies of kidney function.32 Improvement in some measures was noted in treated mice with a low level of engraftment (<50%) compared to Ctns−/− controls, but for corneal crystal accumulation, an outcome comparable to wild-type was achieved only following high levels of engraftment. Thus, it seems that a critical load of properly engrafted HSPCs is required to achieve a meaningful integration of disease-correcting bone marrow–derived cells within the eyes. Thus, maximizing engraftment will have to be considered for the clinical application of this strategy. As a major complication associated with allogeneic HSPC transplantation is graft-versus-host disease, which also can lead to severe ocular damage,33 a clinical trial currently being developed by our group for cystinosis will use autologous transplantation of ex vivo gene-modified HSPCs using a lentiviral vector containing CTNS cDNA.26
The major pathological ocular symptom described in cystinosis patients is the presence of corneal cystine crystals that result in photophobia, keratopathy, and recurrent corneal erosions.15 In vivo confocal analysis revealed abundant cystine crystals first in the posterior stromal-endothelium layers and later progressing anteriorly, similar to previous descriptions.13,14 Interestingly, we observed that the crystal accumulation in the middle stroma of Ctns−/− mice was lower than the anterior and posterior stroma. This differed from one study of 8 patients where the highest amount of cystine crystals was found in the anterior stroma and less in the mid- and posterior stroma,24 although this could be explained by breakdown of crystals and/or scarring.18 Studying the time course of corneal engraftment of the HSPC-derived cells will be important to determine differences in tissue response between the anterior and posterior stroma.
Our data revealed significantly lower amounts of crystals within the anterior region of the cornea (epithelium and upper stroma) in high HSPC engrafted Ctns−/− mice. A previous study performed on Ctns−/− mice treated with topical cysteamine applied 4 times daily for 4 weeks beginning at 5 months showed that the drug only delays the formation of cystine crystals; the cysteamine-treated group still showed a 15% increase in the crystal volume index.18 Similar results were observed in patients using a newer gel formulation of topical cysteamine.24 Our data suggested that HSPC transplantation could be a more efficient alternative, almost totally preventing crystal formation in the cystinotic eyes. However, the prevention of cystine crystal accumulation was observed to a lesser extent in the posterior than in the anterior cornea. This may be explained by prior findings that engrafted HSPC-derived cells localize initially in the anterior stroma and later migrate to the posterior stromal layers.34 Thus, we hypothesize that by the time the HSPCs reach the posterior stroma, crystal formation and accumulation has occurred and cannot be reversed. Note that HSPC-derived cells also were present in the sclera, ciliary margin, iris, retina, choroid, and to lesser degree the lens, all previously identified sites of crystal accumulation.14 Cystine clearance in these regions also may have increased in HPSC-engrafted animals, as suggested by the significant decrease of global ocular cystine content in the HSPC treated mice (Fig. 2a).
Central corneal thickness was thinner in the Ctns−/− mice, and restoration of physiological corneal thickness was found in the treated mice with high level of engraftment. Thin corneas may lead to an underestimation of IOP,35,36 but increased IOP in these nontreated Ctns−/− mice may have been caused by corneal sclerosis or scarring.37 In contrast, physiological IOPs were observed in the HPSC-engrafted Ctns−/− mice. It also should be noted that pupillary block may elevate IOP in cystinosis patients and in the mouse model.13,38 This is caused by a deposition of cystine crystals in the iris, which can lead to a seal between the iris and lens, bulging the iris forward, and closing the flow of aqueous fluid out the trabecular meshwork.13,39 Thus, in addition to corneal-related IOP lowering, it also is possible that lower IOP we observed in HSPC engrafted Ctns−/− mice was due to lower crystal accumulation in the iris, where HSPC-derived cells were found (Fig. 4e). We also found HSPC-derived cells in the ciliary margin (Fig. 5a) and at locations in or around the trabecular meshwork, but it is not clear whether these cells have a role in lowering IOP since most documented cystinosis cases conclude that IOP increases as a result of crystal accumulation in the iris and not decreased trabecular meshwork outflow.39 These data emphasized the powerful impact of HSPC transplantation on the long-term structural and functional preservation of the eye.
We showed that most of the HSPC-derived bone marrow–derived cells that migrated to the eye were F4/80+ MHC class II+ macrophages, with a few dendritic cells. Interestingly, we observed that these macrophages generated TNTs as observed previously within the kidney.29 These membrane extensions are heterogeneous cell-connecting tubules that have been involved in pathogen spreading and intercellular communication.40,41 In vivo existence of TNT was reported initially in MHC II-positive cells present in mouse corneal stroma.42 The function of these nanotubes has been attributed to the transfer of small molecules, such as MHC and/or MHC-antigen complexes, to help the propagation of immune responses.42–44 We showed that TNTs are likely to provide lysosomal cross-correction by delivering cystinosin-bearing lysosomes to the adjacent diseased corneal cells, thereby providing functional cystine clearance mechanisms for Ctns−/− cells, similar to the TNT-mediated crossing of the tubular membrane that halts kidney degeneration.29 Therefore, TNTs generated by MHC class II+ bone marrow–derived cells, which penetrate corneal layers, are likely leading to the our presented findings of structural and functional preservation in corneas. Cystinosin-containing microvesicle transfer from the bone marrow–derived macrophages to the host cells is an alternative route for cystinosin cross-correction. Other groups have reported that microvesicles/exosomes shed by CTNS-expressing cells could lead to significant cystine decrease in cystinotic cells in vitro.45,46 This mechanism also has been described in co-culture assays of mucopolysaccharidosis VII fibroblasts with umbilical MSCs and as the mechanism responsible for the rescue of the corneal defects in mice after direct corneal transplantation of the MSCs.47 However, we previously demonstrated using coculture assays of wild-type MSCs or macrophages with Ctns-deficient fibroblasts that, while microvesicle transfer could lead to a substantial decrease of cystine in the fibroblasts (approximately 19% clearance), TNT-mediated lysosomal transfer was a more efficient route (approximately 75% clearance).29
Taken together, these results suggested that, rather than replacing defective corneal tissues with transplanted or stem cell-derived tissues, the transfer mode of action between gene-corrected, HSPC-derived donor cells and diseased cells and tissues may be an efficient alternative to correct genetic deficiencies and stabilize ocular pathology. Future directions should include determining if there is a therapeutic window for disease correction, if HSPCs delivered directly within the eyes have the same therapeutic potential as systemic delivery, and if similar therapeutic efficacy can be detected in human patients.
Acknowledgments
The authors thank Corinne Antignac (Inserm U983, Paris, France) for providing the original Ctns−/− mice.
Supported by the Cystinosis Research Foundation and NIH Grants RO1-DK090058, RO1-DK099338, and R21-NS090066 (SC) and P30-EY022589 (JLG). All microcopy work at UCSD was supported by UCSD Neuroscience Microscopy Shared Facility Grant P30-NS047101. Corneal confocal microscopy was supported by DK076169-23789-5 and NS081082 awards (NAC). The authors alone are responsible for the content and writing of this paper.
Disclosure: C.J. Rocca, None; A. Kreymerman, None; S.N. Ur, None; K.E. Frizzi, None; S. Naphade, None; A. Lau, None; T. Tran, None; N.A. Calcutt, None; J.L. Goldberg, None; S. Cherqui, None
References
- 1. Oliva MS,, Schottman T,, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012; 60: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haddadin RI,, Chodosh J. Corneal transplantation and glaucoma. Semin Ophthalmol. 2014; 29: 380–396. [DOI] [PubMed] [Google Scholar]
- 3. He H,, Yiu SC. Stem cell-based therapy for treating limbal stem cells deficiency: a review of different strategies. Saudi J Ophthalmol. 2014; 28: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li F,, Zhao SZ. Mesenchymal stem cells: potential role in corneal wound repair and transplantation. World J Stem Cells. 2014; 6: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Town M,, Jean G,, Cherqui S,, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998; 18: 319–324. [DOI] [PubMed] [Google Scholar]
- 6. Kalatzis V,, Cherqui S,, Antignac C,, Gasnier B. Cystinosin the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001; 20: 5940–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneider JA,, Schulman JD. Cystinosis: a review. Metabolism. 1977; 26: 817–839. [DOI] [PubMed] [Google Scholar]
- 8. Schneider JA,, Katz B,, Melles RB. Update on nephropathic cystinosis. Ped Nephrol. 1990; 4: 645–653. [DOI] [PubMed] [Google Scholar]
- 9. Gahl WA,, Thoene JG,, Schneider JA. Cystinosis. N Engl J Med. 2002; 347: 111–121. [DOI] [PubMed] [Google Scholar]
- 10. Kleta R,, Kaskel F,, Dohil R,, et al. First NIH/Office of Rare Diseases Conference on Cystinosis: past, present, and future. Ped Nephrol. 2005; 20: 452–454. [DOI] [PubMed] [Google Scholar]
- 11. Cherqui S,, Sevin C,, Hamard G,, et al. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol. 2002; 22: 7622–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz B,, Melles RB,, Schneider JA. Corneal sensitivity in nephropathic cystinosis. Am J Ophthalmol. 1987; 104: 413–416. [DOI] [PubMed] [Google Scholar]
- 13. Kalatzis V,, Serratrice N,, Hippert C,, et al. The ocular anomalies in a cystinosis animal model mimic disease pathogenesis. Ped Res. 2007; 62: 156–162. [DOI] [PubMed] [Google Scholar]
- 14. Simpson J,, Nien CJ,, Flynn K,, Jester B,, Cherqui S,, Jester J. Quantitative in vivo and ex vivo confocal microscopy analysis of corneal cystine crystals in the Ctns knockout mouse. Mol Vis. 2011; 17: 2212–2220. [PMC free article] [PubMed] [Google Scholar]
- 15. Gahl WA,, Kuehl EM,, Iwata F,, Lindblad A,, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000; 71: 100–120. [DOI] [PubMed] [Google Scholar]
- 16. Kaiser-Kupfer MI,, Caruso RC,, Minkler DS,, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986; 104: 706–711. [DOI] [PubMed] [Google Scholar]
- 17. Tsilou ET,, Rubin BI,, Reed GF,, Iwata F,, Gahl W,, Kaiser-Kupfer MI. Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea. 2002; 21: 173–176. [DOI] [PubMed] [Google Scholar]
- 18. Simpson JL,, Nien CJ,, Flynn KJ,, Jester JV. Evaluation of topical cysteamine therapy in the CTNS(-/-) knockout mouse using in vivo confocal microscopy. Mol Vis. 2011; 17: 2649–2654. [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto GK,, Schulman JD,, Schneider JA,, Wong VG. Long-term ocular changes in cystinosis: observations in renal transplant recipients. J Pediatr Ophthalmol Strabismus. 1979; 16: 21–25. [DOI] [PubMed] [Google Scholar]
- 20. Richler M,, Milot J,, Quigley M,, O'Regan S. Ocular manifestations of nephropathic cystinosis. The French-Canadian experience in a genetically homogeneous population. Arch Ophthalmol. 1991; 109: 359–362. [DOI] [PubMed] [Google Scholar]
- 21. Cantani A,, Giardini O. Ciarnella Cantani A. Nephropathic cystinosis: ineffectiveness of cysteamine therapy for ocular changes. Am J Ophthalmol. 1983; 95: 713–714. [DOI] [PubMed] [Google Scholar]
- 22. Dufier JL,, Dhermy P,, Gubler MC,, Gagnadoux MF,, Broyer M. Ocular changes in long-term evolution of infantile cystinosis. Ophthalmic Paediatr Genet. 1987; 8: 131–137. [DOI] [PubMed] [Google Scholar]
- 23. Kaiser-Kupfer MI,, Gazzo MA,, Datiles MB,, Caruso RC,, Kuehl EM,, Gahl WA. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol. 1990; 108: 689–693. [DOI] [PubMed] [Google Scholar]
- 24. Labbe A,, Baudouin C,, Deschenes G,, et al. A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: the Cystadrops OCT-1 study. Mol Genet Metab. 2014; 111: 314–320. [DOI] [PubMed] [Google Scholar]
- 25. Sel S,, Schilling UM,, Nass N,, et al. Bone marrow cells and CD117-positive haematopoietic stem cells promote corneal wound healing. Acta Ophthalmol. 2012; 90: e367–373. [DOI] [PubMed] [Google Scholar]
- 26. Harrison F,, Yeagy BA,, Rocca CJ,, Kohn DB,, Salomon DR,, Cherqui S. Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol Ther. 2013; 21: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Syres K,, Harrison F,, Tadlock M,, et al. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009; 114: 2542–2552. [DOI] [PubMed] [Google Scholar]
- 28. Yeagy BA,, Harrison F,, Gubler MC,, Koziol JA,, Salomon DR,, Cherqui S. Kidney preservation by bone marrow cell transplantation in hereditary nephropathy. Kidney Int. 2011; 79: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 29. Naphade S,, Sharma J, GaideChevronnay HP,, et al. Brief reports: lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells. 2015; 33: 301–309. [DOI] [PMC free article] [PubMed]
- 30. Chen DK,, Frizzi KE,, Guernsey LS,, Ladt K,, Mizisin AP,, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Periph Nerv Syst. 2013; 18: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lively GD,, Jiang B,, Hedberg-Buenz A,, et al. Genetic dependence of central corneal thickness among inbred strains of mice. Invest Ophthalmol Vis Sci. 2010; 51: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherqui S. Is genetic rescue of cystinosis an achievable treatment goal? Nephrol Dial Transplant. 2014; 29: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shikari H,, Amparo F,, Saboo U,, Dana R. Onset of ocular graft-versus-host disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea. 2015; 34: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakamura T,, Ishikawa F,, Sonoda KH,, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005; 46: 497–503. [DOI] [PubMed] [Google Scholar]
- 35. Shah S,, Chatterjee A,, Mathai M,, et al. Relationship between corneal thickness and measured intraocular pressure in a general ophthalmology clinic. Ophthalmology. 1999; 106: 2154–2160. [DOI] [PubMed] [Google Scholar]
- 36. Wei W,, Fan Z,, Wang L,, Li Z,, Jiao W,, Li Y. Correlation analysis between central corneal thickness and intraocular pressure in juveniles in Northern China: the Jinan city eye study. PLoS One. 2014; 9: e104842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Emre S,, Kayikcioglu O,, Ates H,, et al. Corneal hysteresis, corneal resistance factor, and intraocular pressure measurement in patients with scleroderma using the reichert ocular response analyzer. Cornea. 2010; 29: 628–631. [DOI] [PubMed] [Google Scholar]
- 38. Sonmez K,, Ozcan PY. Angle-closure glaucoma in a patient with the nanophthalmos-ocular cystinosis-foveoschisis-pigmentary retinal dystrophy complex. BMC Ophthalmology. 2012; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wan WL,, Minckler DS,, Rao NA. Pupillary-block glaucoma associated with childhood cystinosis. Am J Ophthalmol. 1986; 101: 700–705. [DOI] [PubMed] [Google Scholar]
- 40. Marzo L,, Gousset K,, Zurzolo C. Multifaceted roles of tunneling nanotubes in intercellular communication. Front Physiol. 2012; 3: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rustom A,, Saffrich R,, Markovic I,, Walther P,, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004; 303: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 42. Chinnery HR,, Pearlman E,, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008; 180: 5779–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seyed-Razavi Y,, Hickey MJ,, Kuffova L,, McMenamin PG,, Chinnery HR. Membrane nanotubes in myeloid cells in the adult mouse cornea represent a novel mode of immune cell interaction. Immunol Cell Biol. 2013; 91: 89–95. [DOI] [PubMed] [Google Scholar]
- 44. Brown K,, Fidanboylu M,, Wong W. Intercellular exchange of surface molecules and its physiological relevance. Arch Immunol Ther Exp (Warsz). 2010; 58: 263–272. [DOI] [PubMed] [Google Scholar]
- 45. Iglesias DM,, El-Kares R,, Taranta A,, et al. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PLoS One. 2012; 7: e42840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thoene J,, Goss T,, Witcher M,, et al. In vitro correction of disorders of lysosomal transport by microvesicles derived from baculovirus-infected Spodoptera cells. Mol Genet Metab. 2013; 109: 77–85. [DOI] [PubMed] [Google Scholar]
- 47. Coulson-Thomas VJ,, Caterson B,, Kao WW. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells. 2013; 31: 2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]