Abstract
Purpose of Review:
This article reviews the evidence base for the preventive treatment of migraine.
Recent Findings:
Evidence-based guidelines for the preventive treatment of migraine have recently been published by the American Academy of Neurology (AAN) and the Canadian Headache Society (CHS), providing valuable guidance for clinicians. Strong evidence exists to support the use of metoprolol, timolol, propranolol, divalproex sodium, sodium valproate, and topiramate for migraine prevention, according to the AAN. Based on best available evidence, adverse event profile, and expert consensus, topiramate, propranolol, nadolol, metoprolol, amitriptyline, gabapentin, candesartan, Petasites (butterbur), riboflavin, coenzyme Q10, and magnesium citrate received a strong recommendation for use from the CHS.
Summary:
Migraine preventive drug treatments are underutilized in clinical practice. Principles of preventive treatment are important to improve compliance, minimize side effects, and improve patient outcomes. Choice of preventive treatment of migraine should be based on the presence of comorbid and coexistent illness, patient preference, reproductive potential and planning, and best available evidence.
INTRODUCTION
Migraine is a chronic neurologic disease that varies in its frequency, severity, and impact on patients’ quality of life. A treatment plan should consider not only the patient’s diagnosis, symptoms, and coexistent or comorbid conditions, but also the patient’s expectations, needs, and goals.1 Effective migraine treatment begins with making an accurate diagnosis, ruling out alternate causes, ordering appropriate studies, and addressing the headache’s impact on the patient2; educating the patient with regard to treatment options, side effect profile, duration of therapy, and expectations for improvement; and developing a treatment plan that considers coincidental and comorbid conditions.3 Comorbidity is the presence of two or more disorders, the association of which is more likely than would occur by chance. Conditions that occur in patients with migraine with a higher prevalence than coincidence include stroke, comorbid pain disorders, angina, patent foramen ovale (aura), epilepsy, and certain psychiatric disorders, which include depression, mania, anxiety, and panic disorder.
The pharmacologic treatment of migraine may be acute (abortive) or preventive (prophylactic), and patients with frequent severe headaches require both approaches. Preventive therapy is used to reduce the frequency, duration, or severity of attacks. Additional benefits may include enhancement of response to acute treatments, improvement of a patient’s ability to function, and reduction of disability.3 Preventive treatment may also result in reduction of health care costs.4
IMPACT OF PREVENTIVE TREATMENT
Silberstein and colleagues4 found that the addition of migraine preventive drug therapy to therapy that consisted of only an acute medication was effective in reducing resource consumption. During the second 6 months after the initial preventive medication, as compared with the 6 months preceding preventive therapy, migraine diagnosis–related office and other outpatient visits decreased by 51.1%, emergency department visits with a migraine diagnosis decreased 81.8%, CT scans decreased 75.0%, MRIs decreased 88.2%, and other migraine medication dispensements decreased 14.1%.4
The cost and consumption of triptan medications is also an important factor and has been evaluated after the addition of preventive medication. Silberstein and colleagues evaluated the medical resource utilization and overall cost of care among patients treated with topiramate for migraine prevention in a commercially insured population that included 2645 plan members. Topiramate utilization was associated with significantly less triptan utilization. In addition, in postindex period 1, results showed a 46% decrease in emergency department visits, a 39% decrease in diagnostic procedures (eg, CT scans and MRIs), and a 33% decrease in hospital admissions; physician office visits were unchanged. In postindex period 2, results showed a 46% decrease in emergency department visits, a 72% decrease in diagnostic procedures, a 61% decrease in hospital admissions, and a 35% decrease in physician office visits.5
Several studies have examined the impact of migraine prevention therapy on patients’ quality of life. These have included specific therapies, such as topiramate, as well as more far-reaching assessment. Using the SF-36 Health Survey6 to examine quality of life, studies showed highly statistically significant changes across the range of scores with as little as 6 months of treatment.7,8 With a migraine-specific quality-of-life assessment, broad improvements of at least moderate size and an effect that persisted over a prolonged period of observation were found across domains.9
PRINCIPLES OF PREVENTIVE TREATMENT
Preventive treatment can be preemptive, short term, or maintenance. Preemptive treatment is used when a known headache trigger exists, such as exercise or sexual activity. Patients can be instructed to pretreat prior to the exposure or activity. For example, a single dose of indomethacin can be used to prevent exercise-induced migraine. Short-term prevention is used when patients are undergoing a time-limited exposure to a provoking factor, such as ascent to a high altitude or menstruation. These patients can be treated with daily medication just before and during the exposure. For example, the perimenstrual use of a nonsteroidal anti-inflammatory drug (NSAID) or triptan for 3 to 5 days may prevent the emergence of menstrually related migraine. Maintenance prevention is used when patients need ongoing treatment.
Recent US, Canadian, and European guidelines10–15 have established the circumstances under which migraine preventive treatment should be considered. (Refer to Appendix A and Appendix B for summaries of the American Academy of Neurology’s evidence-based guidelines for clinicians). These guidelines include:
Recurring migraine attacks that significantly interfere with a patient’s quality of life and daily routine despite trigger management, appropriate use of acute medications, and lifestyle modification strategies
Frequent headaches (four or more attacks per month or eight or more headache days per month) because of the risk of chronic migraine
Failure of, contraindication to, overuse of, or troublesome side effects from acute medications
Patient preference, that is, the desire to have as few acute attacks as possible
Presence of certain migraine conditions: hemiplegic migraine; basilar migraine (now called migraine with brainstem aura); frequent, prolonged, or uncomfortable aura symptoms; or migrainous infarction
A preventive migraine drug is considered successful if it reduces migraine attack frequency or days by at least 50% within 3 months. As illustrated in Case 2-1, additional benefits include reduced attack duration or severity, enhanced response to acute treatments, improved ability to function, and reduced disability. According to the American Migraine Prevalence and Prevention (AMPP) Study, 38.8% of patients with migraine should be considered for (13.1%) or offered (25.7%) preventive migraine therapy.16 Unfortunately, the underutilization of migraine preventive medications is underscored by the fact that only 13% of all patients with migraine currently use preventive therapy to control their attacks.11
The following classes of medications are used for migraine prevention: antiepileptic drugs, antidepressants, beta-blockers, calcium channel antagonists, serotonin antagonists, botulinum neurotoxins, NSAIDs, and others (including riboflavin, magnesium, and Petasites). A drug is chosen based on its efficacy, its adverse event profile, the patient’s preference, and the presence of any coexistent or comorbid conditions. Preventive drugs with the best proven efficacy for migraine are certain beta-blockers, divalproex sodium, and topiramate. The chosen drug should have the best risk-to-benefit ratio for the individual patient and, where possible, take advantage of the drug’s side effect profile. An underweight patient would be a candidate for one of the medications that commonly produce weight gain, such as a tricyclic antidepressant; in contrast, one would try to avoid these drugs and consider topiramate when the patient is overweight. Tertiary tricyclic antidepressants that have a sedating effect would be useful at bedtime for patients with insomnia. Older patients with cardiac disease or patients with significant hypotension may not be able to use tricyclic antidepressants, calcium channel blockers, or beta-blockers, but could use divalproex sodium or topiramate.
Case 2-1
A 24-year-old university student presented with chronic daily headache. He had a history of migraine that began in high school while he was a varsity football player. The frequency of his attacks increased after starting college. He attributed the increase in frequency to the sleep deprivation and stress that came with taking 18 credits to graduate. Most of the headaches were moderate or severe, and while sumatriptan 100 mg helped, it took about 5 hours before he got significant relief and the headache often came back the next day. He was using sumatriptan 100 mg on about 12 days per month. He had no significant past medical history or family history. His neurologic examination was normal.
He was started on topiramate 15 mg at bedtime, and the dose was titrated to 50 mg 2 times a day over the course of 6 weeks. There was a reduction in headache days to about 18 days per month. Importantly, the duration of attacks was reduced to less than 60 minutes after taking sumatriptan 100 mg. Moreover, he had only required three tablets of sumatriptan per month as the severity of each attack was reduced. Other attacks were treated with over-the-counter ibuprofen 600 mg. After 3 months of treatment, he was experiencing only about one headache per week and using sumatriptan 2 times per month.
Comment. This case illustrates the potential for effective preventive medication to reduce headache frequency, acute medication consumption, and duration and severity of attacks. It also illustrates the importance of continuing treatment even if the initial response is considered “partial,” ie, absence of a 50% reduction in frequency of headache. Headache duration and severity are important considerations, and significant decrease in these end points can lead to a dramatic reduction in disability and improved ability to function.
General Principles for Instituting Preventive Therapy
The following principles will help increase the chances of successful preventive treatment:
Start the chosen drug at a low dose and increase it slowly until therapeutic effects develop, the ceiling dose is reached, or adverse events become intolerable.
Consider comorbidity and coexistent illnesses in drug choice. Conditions comorbid with migraine are shown in Table 2-1.
Avoid exacerbating, overused, and contraindicated drugs (because of coexistent or comorbid illnesses).
Give each treatment an adequate trial. A full therapeutic trial may take 2 to 6 months before the maximal response to a treatment is evident. Case 2-2 demonstrates the benefits of an adequate course of preventive treatment at an adequate dose.
Table 2-1.
Migraine Comorbid Disease

Set realistic goals. Success is defined as a 50% reduction in attack frequency or headache days, a significant decrease in attack duration, or an improved response to acute medication.
Reevaluate therapy; migraine may improve or remit independent of treatment.
Be sure that a woman of childbearing age is aware of any possible risks and avoid preventive drugs if at all possible in anticipation of and during pregnancy.
To maximize compliance, involve patients in their care. Discuss the rationale for a particular treatment, when and how to use it, and what adverse events are likely. Address patient expectations, and set realistic goals.
Set realistic expectations regarding adverse events. Most are self-limited and dose dependent, and patients should be encouraged to tolerate the early adverse events that may develop when a new medication is started.
While monotherapy is a treatment goal and taking advantage of comorbid or coexistent illness may facilitate treatment of both disorders with a single drug, limitations exist to using a single medication to treat two illnesses. Giving a single medication may not treat two different conditions optimally; although one of the conditions may be adequately treated, the second illness may require a higher or lower dose, and, therefore, a risk exists that the second illness is not being adequately treated. Therapeutic independence may be needed should monotherapy fail. Avoiding drug interactions or increased adverse events is a primary concern when using polypharmacy. Polytherapy may enable therapeutic adjustments based on the status of each illness. For example, tricyclic antidepressants are often recommended for patients with migraine and depression.20 However, appropriate management of depression often requires higher doses of tricyclic antidepressants, which may be associated with more adverse events. A better approach might be to treat the depression with a selective serotonin reuptake inhibitor (SSRI) or serotonin norepinephrine reuptake inhibitor (SNRI) and to treat the migraine with an antiepileptic drug. Migraine and epilepsy may both be controlled with an antiepileptic drug, such as topiramate or divalproex sodium, which are also the drugs of choice for the patient with migraine and bipolar illness. When individuals have more than one disease, certain categories of treatment may be relatively contraindicated. For example, beta-blockers should be used with caution for the patient with migraine and depression, while tricyclic antidepressants or neuroleptics may lower the seizure threshold and should be used with caution for the patient with migraine and epilepsy.
Although monotherapy is preferred, it often does not yield the desired therapeutic effect, and it may be necessary to combine preventive medications. The need for selective treatment of two disorders is illustrated in Case 2-2, in which a tricyclic antidepressant alone was inadequate to manage both migraine and major depression. Antidepressants are often used with beta-blockers or calcium channel blockers, and topiramate or divalproex sodium may be used in combination with any of these medications.
Case 2-2
A 35-year-old mother of two presented for an evaluation for migraine. Her migraine attacks began at the age of 13 but were infrequent until the birth of her second child 3 years ago. Attacks occurred about 2 times per week, but she was never without some type of headache every day. On average, her ability to function was impaired or impossible on 20 days per month. She also had a history of postpartum depression, which had not responded to fluoxetine 20 mg per day or bupropion 75 mg per day. Her Migraine Disability Assessment (MIDAS)17 score was 80, Patient Health Questionnaire (PHQ-9)18 was 13, and Generalized Anxiety Disorder 7-Item Scale (GAD-7)19 was 4. She was started on amitriptyline 25 mg nightly at bedtime, which was continued for 1 month but then discontinued because of sedation and constipation. She was switched to nortriptyline at a dose of 10 mg, and the dose was titrated to 75 mg at bedtime over the course of 2 months. After 3 months on 75 mg, she was tolerating the medication and experiencing only 10 headache days per month, her MIDAS score dropped to 18, and only half of the headaches were moderately severe, and those responded promptly with rizatriptan 10 mg. However, depression persisted, and her PHQ-9 score was still elevated (12). She was started on venlafaxine and titrated to a dose of 75 mg 2 times a day over 1 month. She reported a significant improvement in her mood.
Comment. This case illustrates several of the key principles in the preventive treatment of migraine. Switching from a tertiary to secondary amine may be better tolerated. Starting at a low dose and titrating the dose very slowly minimizes side effects and improves adherence, and continuing treatment for at least 2 to 3 months after the target dose is achieved is important to determine maximal efficacy. Moreover, a single drug (nortriptyline) failed to adequately treat both the depression and migraine. Therefore, the addition of an additional antidepressant, including one shown to be effective for migraine prevention (venlafaxine), may be necessary.
Guidelines for Stopping Preventive Therapy
Preventive migraine therapy should be stopped when:
The patient develops intolerable adverse events or a severe drug reaction.
The drug does not demonstrate even partial efficacy after 2 months of therapy and disorders such as acute medication overuse have been eliminated.
The patient has shown significant benefit. If the headaches are well controlled for at least 6 months, slowly taper and, if possible, discontinue the drug.
Preventive treatment is often recommended for 6 to 9 months, but until now, no randomized placebo-controlled trials have been performed to investigate migraine frequency after the preventive treatment has been discontinued. Diener and colleagues21 assessed 818 patients with migraine who were treated with topiramate for 6 months to see the effects of topiramate discontinuation. Patients received topiramate in a 26-week open-label phase. They were then randomly assigned to continue topiramate or switch to placebo for a 26-week double-blind phase. Of the 559 patients who completed the open-label phase, 514 entered the double-blind phase and were assigned to topiramate (n = 255) or placebo (n = 259). The mean increase in number of migraine days was greater in the placebo group (1.19 days in 4 weeks, 95% confidence interval 0.71 to 1.66; P<.0001) than in the topiramate group (0.10, confidence interval –0.36 to 0.56; P=.5756). Patients in the placebo group had a greater number of days on acute medication than did those in the topiramate group (mean difference between groups 0.95, confidence interval –1.49 to –0.41; P=.0007). Sustained benefit was reported after topiramate was discontinued, although the number of migraine days did increase. In a subsequent analysis of this study, no factors were identified that predicted consistent relapse after withdrawal of topiramate therapy.22 While the authors did find evidence that the likelihood of sustained relapse was higher when the initial response to migraine preventive treatment was more pronounced, the same effect was found in the placebo group, leading the authors to speculate that this observation likely reflected “regression to the mean.” Wöber and colleagues found that 75% of patients developed increased migraine frequency after flunarizine or beta-blockers were stopped.23 Relapse occurred on average 6 months after cessation of the medication. These findings suggest that patients may relapse after the discontinuation of preventive treatment; patients should be cautioned in this regard and followed carefully for escalating attack frequency. It is unclear which factors increase the risk of relapse or sustained remission.
SPECIFIC MIGRAINE PREVENTIVE AGENTS
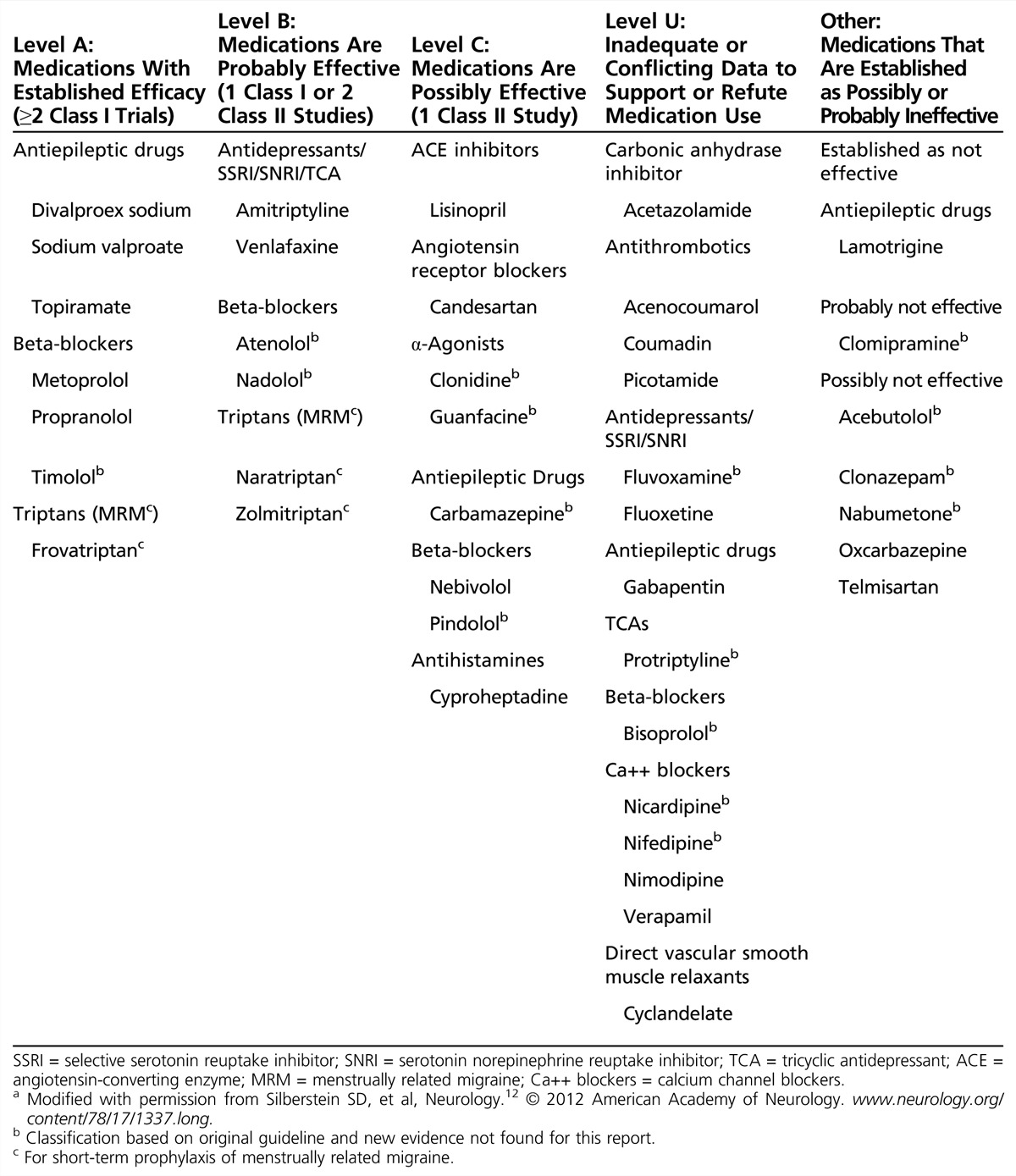
Specific migraine preventive agents and their classification per American Academy of Neurology (AAN) evidence guidelines are listed in Table 2-2. (Refer to Appendix A for a summary of the AAN’s evidence-based guideline for clinicians, Appendix C for the AAN classification of evidence for the rating of a therapeutic study, and Appendix D for the classification of recommendations.)
Table 2-2.
Classification of Migraine Preventive Therapies (Available in the United States)a
Beta-Blockers for the Prevention of Migraine
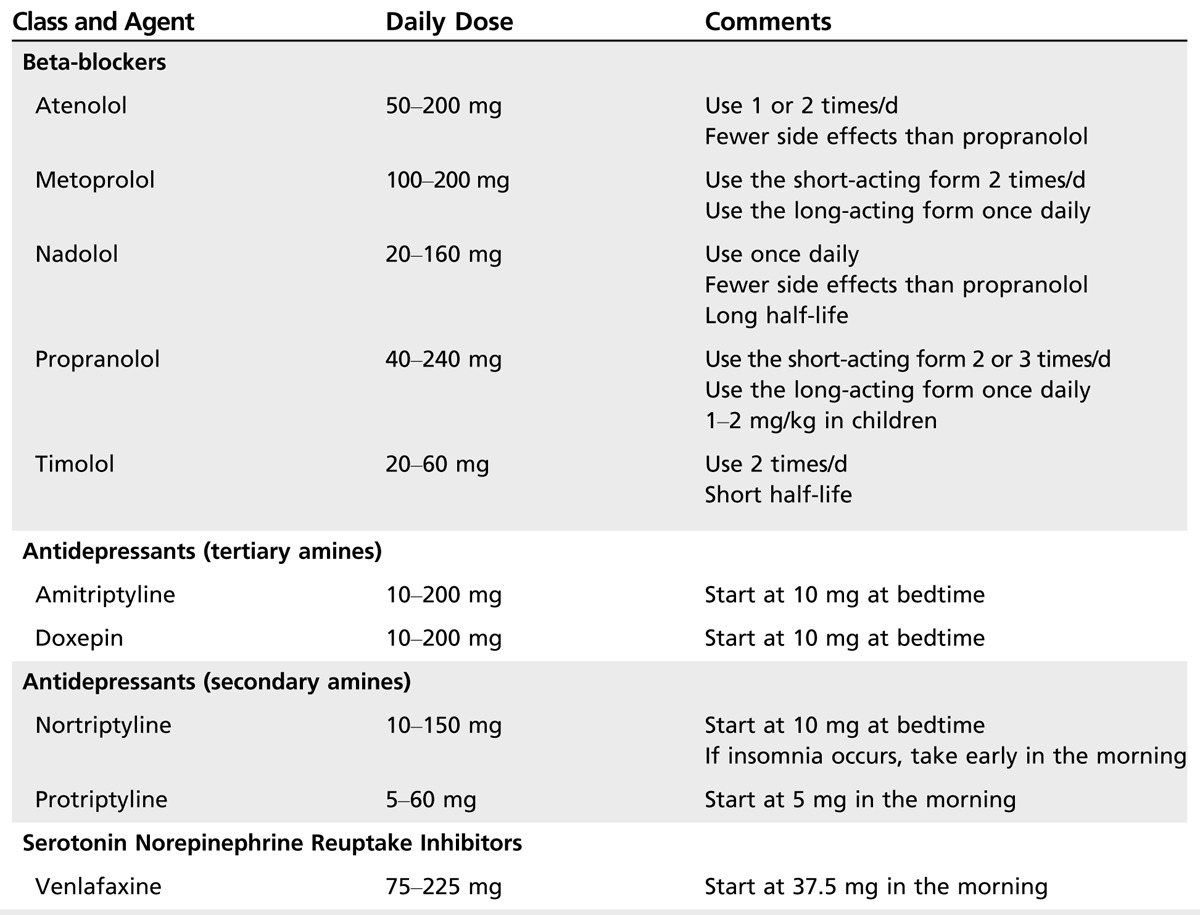
Beta‐blockers are the most widely used class of drugs in prophylactic migraine treatment and are about 50% effective in producing a greater than 50% reduction in attack frequency. Table 2-3 lists beta-blockers and dosages used for the prevention of migraine. Evidence has consistently demonstrated the efficacy of the nonselective beta-blocker propranolol and of the selective beta1-blocker metoprolol. Atenolol, bisoprolol, nadolol, and timolol are also likely to be effective. Beta-blockers with intrinsic sympathomimetic activity (eg, acebutolol, alprenolol, oxprenolol, pindolol) are not effective for migraine prevention.
Table 2-3.
Beta-Blockers and Antidepressants in the Preventive Treatment of Migraine
The combination of propranolol and topiramate versus topiramate alone was examined as a preventive treatment for chronic migraine in the National Institute of Neurological Diseases and Stroke (NINDS) Clinical Research Collaboration Chronic Migraine Treatment Trial (CMTT). This was a randomized double-blind placebo-controlled parallel study to examine the safety and efficacy of topiramate (up to 100 mg per day) and propranolol (up to 240 mg per day long-acting formulation) taken in combination, compared with treatment with topiramate (up to 100 mg per day) and placebo. The trial was terminated in September 2010, when an interim analysis determined that the combination of topiramate and propranolol offered no additional advantage over topiramate alone.24,25
Contraindications to the use of beta-blockers for the treatment of migraine include asthma and chronic obstructive lung disease, atrioventricular conduction defects, Raynaud disease, peripheral vascular disease, and severe diabetes mellitus. All beta-blockers can produce behavioral adverse events, such as drowsiness, fatigue, lethargy, sleep disorders, nightmares, depression, memory disturbance, and hallucinations.26 Other potential adverse events include gastrointestinal symptoms, decreased exercise tolerance, hypotension, bradycardia, and impotence. Although stroke has been reported to occur after patients with migraine with aura were started on beta-blockers, neither an absolute nor a relative contraindication to their use by patients with migraine, either with or without aura, exists.
Antidepressant Medication for Migraine Prevention
Antidepressants consist of a number of different drug classes with different mechanisms of action. Although the mechanism by which antidepressants work to prevent migraine headache is uncertain, it does not result from treating latent or undiagnosed depression. Antidepressants are useful in treating many chronic pain states, including headache, independent of the presence of depression, and the response occurs sooner and at lower dosages than that expected for an antidepressant effect. In animal pain models, antidepressants potentiate the effects of coadministered opioids. The antidepressants that are clinically effective in headache prevention either inhibit norepinephrine and 5-hydroxytryptamine (5-HT) reuptake or are antagonists at the 5-hydroxytryptamine 2 (5-HT2) receptors.
Tricyclic antidepressants. Tricyclic antidepressants are used for migraine prevention; however, only one tricyclic antidepressant (amitriptyline) has proven efficacy in migraine.
The dosage range for tricyclic antidepressants is wide and must be individualized. Amitriptyline and doxepin are sedating. Patients with coexistent depression may require higher doses of these drugs to treat underlying depression. Start with a low dose of the chosen tricyclic antidepressant at bedtime, except when using protriptyline, which should be administered in the morning (since protriptyline has alerting properties). If the tricyclic antidepressant is too sedating, switch from a tertiary tricyclic antidepressant (eg, amitriptyline, doxepin) to a secondary tricyclic antidepressant (eg, nortriptyline, protriptyline). Adverse events are common with tricyclic antidepressant use. Antimuscarinic adverse events include dry mouth, a metallic taste, epigastric distress, constipation, dizziness, mental confusion, tachycardia, palpitations, blurred vision, and urinary retention. Other adverse events include weight gain (rarely seen with protriptyline), orthostatic hypotension, reflex tachycardia, palpitations, QT interval prolongation, decreased seizure threshold, and sedation. Antidepressant treatment may change depression to hypomania or frank mania (particularly in bipolar patients). Older patients may develop confusion or delirium. The antimuscarinic and antiadrenergic effects of these agents may pose increased risks for cardiac conduction abnormalities, especially in the elderly, and these patients should be carefully monitored or other agents considered.
Selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors. Evidence for the use of SSRIs or other antidepressants for migraine prevention is mixed and overall poor. The efficacy analysis summarized in the Agency for Health Care Policy and Research (AHCPR) Evidence Report did not indicate a clear benefit of the racemic mixture of fluoxetine over placebo.26 One class II study showed fluoxetine (racemic) was significantly better than placebo for migraine prevention, but the results were not duplicated in a second study.
Other antidepressants not effective in placebo-controlled trials were clomipramine and sertraline; for other antidepressants, only open or non–placebo-controlled trials are available. Because their tolerability profile is superior to that of tricyclic antidepressants, SSRIs may be helpful for patients with comorbid depression. The most common adverse events include sexual dysfunction, anxiety, nervousness, insomnia, drowsiness, fatigue, tremor, sweating, anorexia, nausea, vomiting, and dizziness or lightheadedness. The combination of an SSRI and a tricyclic antidepressant can be beneficial in treating refractory depression and, in the author’s experience, resistant cases of migraine. Some combinations require the tricyclic antidepressant dose to be adjusted, because tricyclic antidepressant plasma levels may significantly increase.
Venlafaxine, a selective serotonin and norepinephrine reuptake inhibitor, has been shown to be effective.27 Adverse events include insomnia, nervousness, mydriasis, and seizures.
Calcium Channel Antagonists for the Prevention of Migraine
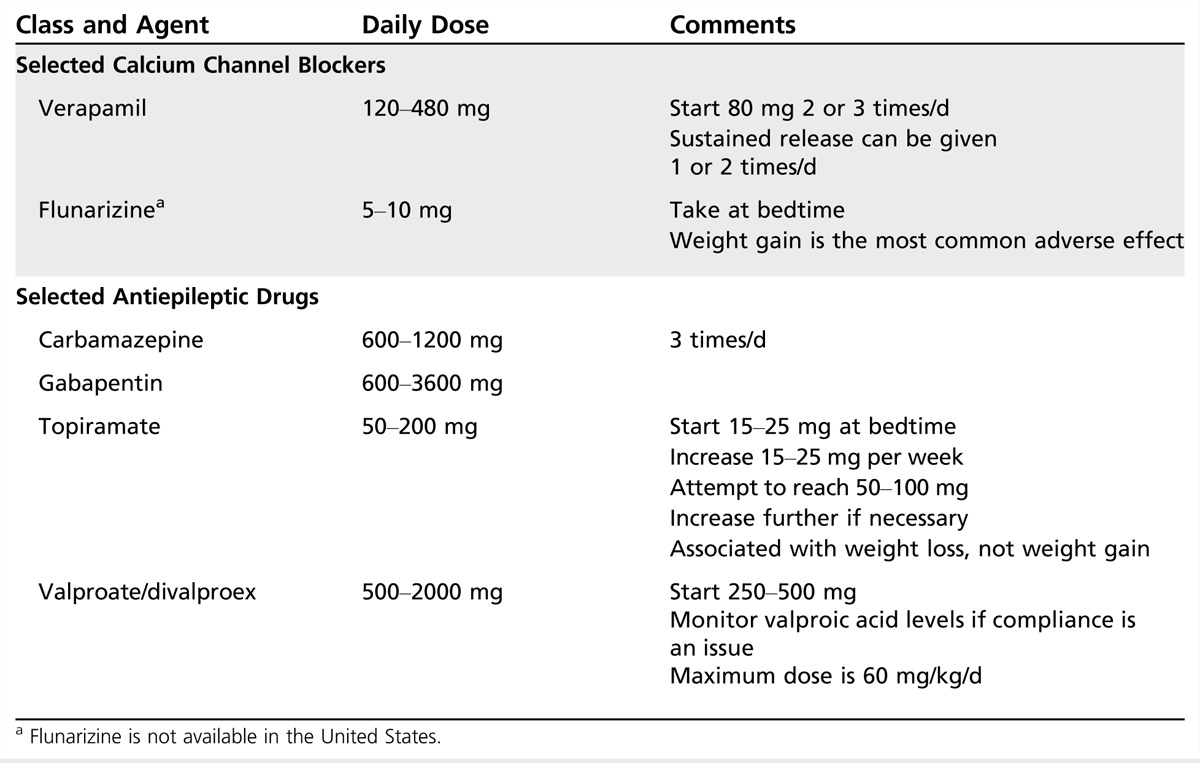
Table 2-4 lists selected calcium channel blockers used for the preventive treatment of migraine. Data from older studies regarding verapamil, nimodipine, nicardipine, diltiazem, cyclandelate, and other nonselective calcium channel antagonists have not shown superiority over placebo in well-designed clinical trials, and these medications cannot be recommended for migraine prophylaxis.
Table 2-4.
Selected Calcium Channel Blockers and Selected Antiepileptic Drugs in the Preventive Treatment of Migraine
Antiepileptic Drugs for the Prevention of Migraine
Antiepileptic drugs are increasingly recommended for migraine prevention because of well-conducted placebo-controlled trials (Table 2-4). It is important for the clinician to recognize that most antiepileptic drugs may substantially interfere with the efficacy of oral contraceptives, with the exception of valproic acid, topiramate (dose less than 200 mg per day), zonisamide, gabapentin, pregabalin, and levetiracetam, among others.
Gabapentin. Gabapentin (1800 mg per day to 2400 mg per day) showed efficacy in a placebo-controlled double-blind trial only when a modified intent-to-treat analysis was used. Another double-blind placebo-controlled trial showed positive results; however, the ability to draw conclusions from the placebo-controlled studies is limited because of their methodologic and analytical limitations. Recent reviews, including a Cochrane review,28 conclude that further evaluation of gabapentin in migraine prophylaxis is warranted in order to inform clinical practice.
Silberstein and colleagues29 conducted a randomized double-blind placebo-controlled trial of gabapentin enacarbil, a transported prodrug of gabapentin that provides sustained dose-proportional exposure to gabapentin. No statistically significant difference between active treatment and placebo was found.
Valproic acid. Valproic acid is a simple 8-carbon, 2-chain fatty acid. Divalproex sodium (approved by the US Food and Drug Administration [FDA] for the treatment of migraine) is a combination of valproic acid and sodium valproate. Several subsequent randomized placebo-controlled studies have confirmed its efficacy, with responder rates ranging between 43% and 48% with dosages ranging from 500 mg per day to 1500 mg per day. Extended-release (ER) divalproex sodium has also been shown to be effective for migraine prevention, and compliance and side effect profile may be more favorable with this formulation. Since the 2000 AAN guideline30 was published, one double-blind randomized class I placebo-controlled 12-week trial showed divalproex sodium ER was superior to placebo without significant differences between groups in the number of treatment-emergent adverse events.
Clinical context. In most headache trials, patients taking divalproex sodium or sodium valproate reported no more adverse events than those on placebo. However, weight gain has been clinically observed with the long-term use of divalproex sodium. Nausea, vomiting, and gastrointestinal distress are the most common adverse events; their incidence decreases, however, particularly after 6 months. Tremor and alopecia can, however, occur later. Treatment with these agents requires careful follow-up and testing because of the risk of pancreatitis, liver failure, teratogenicity, and thrombocytopenia and other blood dyscrasias. In addition to well-known teratogenic effects, including neural tube defects, the FDA recently issued an alert to health care providers and patients that medications including and related to valproate acid can cause decreased IQ scores in children whose mothers took the medication during pregnancy. These drugs are contraindicated and should never be used by pregnant women for the prevention of migraine headaches. In fact, in women of childbearing potential, sodium valproate and all related drugs should be used with extreme caution. Hyperandrogenism, polycystic ovary syndrome, and obesity are of concern in young women with epilepsy who use valproate. Absolute contraindications are pregnancy (valproic acid/divalproex sodium are pregnancy category X) and a history of a hepatic disorder or pancreatitis. Other contraindications are thrombocytopenia, pancytopenia, and bleeding disorders.
Topiramate. Topiramate and divalproex sodium are the only two antiepileptic drugs that have FDA approval for migraine prevention. Topiramate is not associated with significant reductions in estrogen exposure at doses below 200 mg per day. At doses above 200 mg per day, there may be a dose-related reduction in exposure to the estrogen component of oral contraceptives.
Four class I studies and seven class II studies report that topiramate (50 mg per day to 200 mg per day) is effective in migraine prevention. It is comparable to amitriptyline in efficacy.31 Topiramate’s most common adverse event is paresthesia; other common adverse events are difficulty with concentration and memory, language problems, fatigue, decreased appetite, nausea, diarrhea, kidney stones, weight decrease, taste perversion, hypoesthesia, and abdominal pain. In the migraine trials, body weight was reduced an average of 2.3% in the 50-mg group, 3.2% in the 100-mg group, and 3.8% in the 200-mg group. Renal calculi can occur with topiramate use. The reported incidence is about 1.5%, representing a twofold to fourfold increase over the estimated occurrence in the general population. A very rare adverse event is acute myopia associated with secondary angle-closure glaucoma. No cases of this condition were reported in the clinical studies. Oligohidrosis has been reported in association with an elevation in body temperature; most reports have involved children. A risk for hyperchloremic non-anion gap metabolic acidosis has also been described during topiramate treatment. In 2011, the FDA notified health care professionals and patients of an increased risk of development of cleft lip and cleft palate (oral clefts) in infants born to women who were treated with topiramate during pregnancy. Because of new human data that show an increased risk for oral clefts, topiramate is being placed in pregnancy category D, which means positive evidence of human fetal risk exists based on human data.
Lamotrigine. Although open-label studies have suggested that lamotrigine may have a select role in the treatment of patients with migraine with frequent or prolonged aura, results from a placebo-controlled study in migraine without aura were negative.32 Both lamotrigine and topiramate may have a special role in the treatment of migraine with aura.
Other Drugs for the Prevention of Migraine
Other drugs used for the prevention of migraine include angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, histamines, antihistamines, leukotriene receptor antagonists, aspirin and other NSAIDS, medicinal herbs, and vitamins (Table 2-5).
Table 2-5.
Miscellaneous Medications in the Preventive Treatment of Migraine
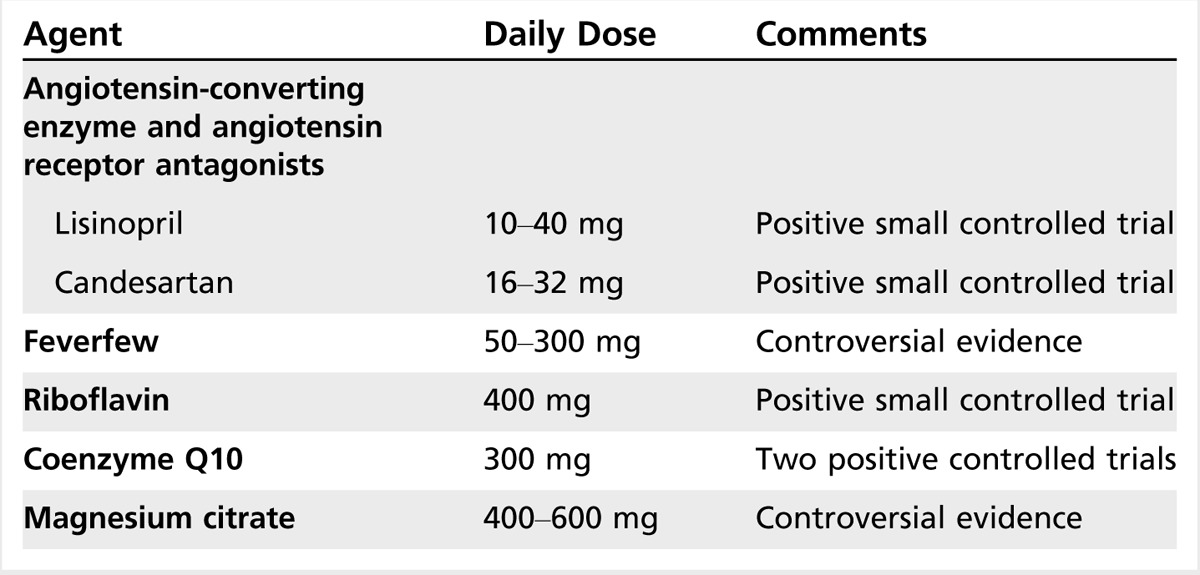
Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Schrader and colleagues33 conducted a double-blind placebo-controlled crossover study of lisinopril, an angiotensin-converting enzyme inhibitor, in migraine prophylaxis. Days with migraine were reduced by at least 50% in 14 participants for active treatment versus placebo and in 17 patients for active treatment versus run-in period. Days with migraine were fewer by at least 50% in 14 participants for active treatment versus placebo. Tronvik and colleagues34 performed a randomized double-blind placebo-controlled crossover study of candesartan (16 mg), an angiotensin II receptor blocker, in migraine prevention. In a 12-week period, the mean number of days with headache was 18.5 with placebo versus 13.6 with candesartan (P=.001) in the intention-to-treat analysis (n = 57). The number of candesartan responders (reduction of 50% or more compared with placebo) was 18 of 57 (31.6%) for days with headache and 23 of 57 (40.4%) for days with migraine. Adverse events were similar in the two periods. In this study, the angiotensin II receptor blocker candesartan was effective, with a tolerability profile comparable with that of placebo.
A second randomized triple-blind double-crossover placebo-controlled trial with propranolol as an active comparator confirmed the preventive efficacy of candesartan.35 Candesartan was noninferior to propranolol. The proportion of responders was significantly higher on candesartan (43%) and propranolol (40%) than on placebo (23%) (P<.025 and P<.050, respectively). The authors concluded that candesartan 16 mg per day is effective for migraine prevention, with an effect size similar to propranolol 160 mg per day.
In a single class II placebo-controlled trial, telmisartan 80 mg per day did not show a significant difference from placebo for reduction in migraine days (–1.65 versus –1.14).36
Histamines/antihistamines/leukotriene receptor antagonists. The 2012 AAN guideline includes studies of histamines, antihistamines, and leukotriene receptor antagonists for migraine prevention.13
Histamine. Three class II single-center studies (all from the same center) show the efficacy of histamine for migraine prevention. N-α-methyl histamine (1 ng to 10 ng 2 times a week) subcutaneous injections reduced attack frequency from baseline as compared with placebo.37 Histamine was statistically superior to placebo at all treatment visits through 12 weeks for reduction in migraine frequency, severity, and duration (P<.0001). Transient itching at the injection site was the only reported adverse effect, but it did not reach significance. In a second class II study, histamine was shown to be as effective as sodium valproate in reducing attack frequency and better than sodium valproate in reducing headache duration and intensity. A third study reported the efficacy of histamine in migraine prevention as compared with topiramate. Topiramate 100 mg per day was compared with histamine (1 ng to 10 ng 2 times a week subcutaneous), and both active treatments showed improvement over baseline measures for attack frequency, intensity, and use of rescue medication.38 Eleven percent of subjects (5 of 45) treated with histamine withdrew from the histamine group because they were not satisfied with the speed of results, although no adverse events were reported. A few subjects reported transitory burning and itching at the injection site. Similar adverse events and withdrawal rates (for slow reaction speed) were reported for the sodium valproate study. Histamine subcutaneous was associated with transitory burning and itching at the injection site.
Cyproheptadine. Cyproheptadine, an antagonist at the 5-HT2, histamine H1, and muscarinic cholinergic receptors, is widely used in the prophylactic treatment of migraine in children. Cyproheptadine is available as 4-mg tablets. The total dose ranges from 12 mg per day to 36 mg per day (given 2 to 3 times per day or at bedtime). Common adverse events are sedation and weight gain; dry mouth, nausea, lightheadedness, ankle edema, aching legs, and diarrhea are less common. Cyproheptadine may inhibit growth in children and reverse the effects of SSRIs. A single class II study showed cyproheptadine (4 mg per day) was as effective as propranolol (80 mg per day) in reducing migraine frequency and severity.39
Montelukast. One class I study of montelukast (20 mg per day) for migraine prevention reported no significant difference between treatments in the percentage of patients with a greater than 50% decrease in migraine attack frequency per month (15.4% for montelukast versus 10.3% for placebo).
Aspirin and other nonsteroidal anti-inflammatory drugs. The efficacy of NSAIDs for migraine prevention was reported in the original AAN guideline,30 including 23 controlled trials of 10 different NSAIDs that showed a modest but significant benefit for naproxen sodium, with similar trends for flurbiprofen, ketoprofen, and mefenamic acid. In the guideline, studies of aspirin had conflicting results. Since the original report, two additional class II studies have been reported. Aspirin was found to be as effective as metoprolol for migraine prevention. In a second study, aspirin 100 mg per day, in combination with vitamin E 600 IU every other day, was compared with placebo in combination with vitamin E.40 No differences were noted between aspirin and placebo treatments for migraine frequency or severity at 12 months or 36 months.
Medicinal herbs and vitamins. Medicinal herbs and vitamins used for migraine prevention are listed in Table 2-5 and discussed in the article “Nutraceutical and Other Modalities for the Treatment of Headache” by Stewart J. Tepper, MD, FAHS, in this issue of CONTINUUM.
CANADIAN HEADACHE SOCIETY GUIDELINES FOR MIGRAINE PREVENTION
A comprehensive series of guidelines for migraine prevention were also developed by the Canadian Headache Society. Randomized double-blind controlled trials and relevant Cochrane reviews were graded according to criteria developed by the US Preventive Services Task Force.14 The principles of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group Recommendations were used to develop expert consensus that incorporated the best available evidence, side effect profile, and migraine characteristics, and comorbid and coexisting disorders were used to develop final recommendations for drug selection. In this guideline, topiramate, propranolol, nadolol, metoprolol, amitriptyline, gabapentin, candesartan, butterbur, riboflavin, coenzyme Q10, and magnesium citrate received a strong recommendation for use, while divalproex sodium, flunarizine, pizotifen, venlafaxine, verapamil, and lisinopril received a weak recommendation. Clinicians should exercise caution when considering the use of butterbur given the recent concerns regarding the potential for hepatic toxicity (refer to the article “Nutraceutical and Other Modalities for the Treatment of Headache” by Stewart J. Tepper, MD, FAHS, in this issue of CONTINUUM).
TRENDS
Calcitonin Gene-Related Peptide Antibodies
Calcitonin gene-related peptide (CGRP) is an important emerging migraine target. CGRP is a potent vasodilator and important neurotransmitter in the trigeminovascular system. CGRP is released during migraine attacks and inhibited after pain relief occurs with triptans; when infused systemically, it triggers a migraine attack. Multiple human monoclonal antibodies that specifically target either human CGRP or its receptor have been generated, and the results of two randomized placebo-controlled phase 2 trials have been published. A single IV dose of ALD403 1000 mg per day, a genetically engineered humanized anti-CGRP IgG1 antibody, was effective for migraine prevention with no safety concerns noted.41 Similar efficacy and tolerability results were seen with LY2951742, a fully humanized monoclonal antibody to CGRP delivered subcutaneously every 2 weeks over a 3-month period.42 Both ALD403 and LY2951742 have long terminal half-lives (31 and 28 days, respectively), so dosing frequency should improve compliance; because of the high target specificity and metabolism to constitutive amino acids, off-target and systemic toxicity are not anticipated, making monoclonal CGRP antibodies a very attractive option should these preliminary efficacy and safety findings be duplicated in phase 3 trials.
CONCLUSION
Preventive therapy plays an important role in migraine management. When a preventive medication is added, attack frequency may be reduced and response to acute treatment improved, which can result in reduced health care resource utilization and improved quality of life. Despite research suggesting that a large percentage of patients with migraine are candidates for prevention, only a fraction of these patients are receiving or have ever received preventive migraine medication.
Many preventive medications are available, and guidelines for their selection and use have been established. Since comorbid medical and psychological illnesses are prevalent in patients with migraine, one must consider comorbidity when choosing preventive drugs. However, optimal treatment of migraine and a comorbid disorder may require the use of two different medications.
No biological markers or clinical characteristics are predictive of response to a particular migraine preventive medication. The impact of prevention on the natural history of migraine remains to be fully investigated.
KEY POINTS
A preventive migraine drug is considered successful if it reduces migraine attack frequency or days by at least 50% within 3 months.
Migraine preventive drugs with the best proven efficacy are certain beta-blockers, divalproex sodium, and topiramate.
A full therapeutic trial may take 2 to 6 months before the maximal response to a preventive migraine treatment is evident.
Although monotherapy for migraine prevention is preferred, it often does not yield the desired therapeutic effect, and it may be necessary to combine preventive medications.
Patients may relapse after the discontinuation of preventive migraine treatment; patients should be cautioned in this regard and followed carefully for escalating attack frequency. It is unclear which factors increase the risk of relapse or sustained remission.
Beta-blockers are the most widely used class of drugs in prophylactic migraine treatment and are about 50% effective in producing a greater than 50% reduction in attack frequency.
If the tricyclic antidepressant being used for migraine prevention is too sedating, switch from a tertiary tricyclic antidepressant (eg, amitriptyline, doxepin) to a secondary tricyclic antidepressant (eg, nortriptyline, protriptyline).
Evidence for the use of selective serotonin reuptake inhibitors or other antidepressants for migraine prevention is mixed and overall poor.
In addition to well-known teratogenic effects, including neural tube defects, the US Food and Drug Administration recently issued an alert to health care providers and patients that medications including and related to sodium valproate can cause decreased IQ scores in children whose mothers took the medication during pregnancy.
Topiramate and divalproex sodium are the only two antiepileptic drugs that have US Food and Drug Administration approval for migraine prevention.
Topiramate is being placed in pregnancy category D, which means positive evidence of human fetal risk exists based on human data.
REFERENCES
- 1. Silberstein SD. Migraine. Lancet 2004; 363 (9406): 381– 391. [DOI] [PubMed] [Google Scholar]
- 2. Silberstein SD, Saper JR, Freitag F. Migraine: diagnosis and treatment. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff’s headache and other head pain. 7th ed New York, NY: Oxford University Press, 2001: 121– 237. [Google Scholar]
- 3. Lipton RB, Silberstein SD. Why study the comorbidity of migraine? Neurology 1994; 44 (10 suppl 7): S4– S5. [PubMed] [Google Scholar]
- 4. Silberstein SD, Winner PK, Chmiel JJ. Migraine preventive medication reduces resource utilization. Headache 2003; 43 (3): 171– 178. doi:10.1046/j.1526-4610.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 5. Silberstein SD, Feliu AL, Rupnow MF, et al. Topiramate in migraine prophylaxis: long-term impact on resource utilization and cost. Headache 2007; 47 (4): 500– 510. doi:10.1111/j.1526-4610.2007.00754.x. [DOI] [PubMed] [Google Scholar]
- 6. Ware JE. SF-36 health survey update. www.sf-36.org/tools/SF36.shtml. Accessed June 11, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Bordini CA, Mariano da Silva H, Garbelini RP, et al. Effect of preventive treatment on health-related quality of life in episodic migraine. J Headache Pain 2005; 6 (5): 387– 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D’Amico D, Solari A, Usai S, et al. Improvement in quality of life and activity limitations in migraine patients after prophylaxis. A prospective longitudinal multicentre study. Cephalalgia 2006; 26 (6): 691– 696. [DOI] [PubMed] [Google Scholar]
- 9. Diamond M, Dahlof C, Papadopoulos G, et al. Topiramate improves health-related quality of life when used to prevent migraine. Headache 2005; 45 (8): 1023– 1030. [DOI] [PubMed] [Google Scholar]
- 10. Silberstein SD. Headaches in pregnancy. Neurol Clin 2004; 22 (4): 727– 756. doi:10.1007/s10194-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 11. Lipton RB, Diamond M, Freitag F, et al. Migraine prevention patterns in a community sample: results from the American migraine prevalence and prevention (AMPP) study. Headache 2005; 45: 792– 793. [Google Scholar]
- 12. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78 (17): 1337– 1345. doi:10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78 (17): 1346– 1353. doi:10.1212/WNL.0b013e3182535d0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pringsheim T, Davenport W, Mackie G, et al. Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci 2012; 39 (2 suppl 2): S1– S59. [PubMed] [Google Scholar]
- 15. Carville S, Padhi S, Reason T, Underwood M Guideline Development Group. Diagnosis and management of headaches in young people and adults: summary of NICE guidance. BMJ 2012; 345: e5765 doi:10.1136/bmj.e5765. [DOI] [PubMed] [Google Scholar]
- 16. Silberstein SD, Diamond S, Loder E, et al. Prevalence of migraine sufferers who are candidates for preventive therapy: results from the American migraine study (AMPP) study. Headache 2005; 45: 770– 771. [Google Scholar]
- 17. American Headache Society. MIDAS (The Migraine Disability Assessment Test). www.americanheadachesociety.org/midas/. Accessed June 11, 2015.
- 18. Patient Health Questionnaire PHQ-9 for Depression. biolincc.nhlbi.nih.gov/static/studies/masm/PHQ_9.pdf. Published April 2006. Accessed June 11, 2015.
- 19. Spitzer RL, Williams JB, Kroenke K, et al. GAD-7. www.phqscreeners.com/pdfs/03_GAD-7/English.pdf. Accessed June 11, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Silberstein SD, Lipton RB, Breslau N. Migraine: association with personality characteristics and psychopathology. Cephalalgia 1995; 15 (5): 358– 369. doi:10.1046/j.1468-2982.1995.1505358.x. [DOI] [PubMed] [Google Scholar]
- 21. Diener HC, Agosti R, Allais G, et al. Cessation versus continuation of 6-month migraine preventive therapy with topiramate (PROMPT): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2007; 6 (12): 1054– 1062. doi:10.1016/S1474-4422(07)70272-7. [DOI] [PubMed] [Google Scholar]
- 22. Schoenen J, Reuter U, Diener HC, et al. Factors predicting the probability of relapse after discontinuation of migraine preventive treatment with topiramate. Cephalalgia 2010; 30 (11): 1290– 1295. doi:10.1177/0333102409359709. [DOI] [PubMed] [Google Scholar]
- 23. Wöber C, Wöber-Bingöl C, Koch G, Wessely P. Long-term results of migraine prophylaxis with flunarizine and beta-blockers. Cephalalgia 1991; 11 (6): 251– 256. doi:10.1046/j.1468-2982.1991.1106251.x. [DOI] [PubMed] [Google Scholar]
- 24. Dodick D, Silberstein SD, Lindblad A, et al. Clinical trial design in chronic migraine: lessons learned from the NINDS CRC chronic migraine treatment trial (CMTT). Neurology 2011. [Google Scholar]
- 25. Silberstein SD, Dodick DW, Lindblad AS, et al. Randomized, placebo-controlled trial of propranolol added to topiramate in chronic migraine. Neurology 2012; 78 (13): 976– 984. doi:10.1212/WNL.0b013e31824d5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gray RN, Goslin RE, McCrory DC, et al. Drug treatments for the prevention of migraine headache. Rockville, MD: Agency for Health Care Policy and Research, 1999. [PubMed] [Google Scholar]
- 27. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache 2005; 45 (2): 144– 152. doi:10.1111/j.1526-4610.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 28. Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia 2008; 28 (6): 585– 597. doi:10.1111/j.1468-2982.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- 29. Silberstein S, Goode-Sellers S, Twomey C, et al. Randomized, double-blind, placebo-controlled, phase II trial of gabapentin enacarbil for migraine prophylaxis. Cephalalgia 2013; 33 (2): 101– 111. doi:10.1177/0333102412466968. [DOI] [PubMed] [Google Scholar]
- 30. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology for the United States Headache Consortium. Neurology 2000; 55 (6): 754– 762 [DOI] [PubMed] [Google Scholar]
- 31. Dodick DW, Freitag F, Banks J, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin Ther 2009; 31 (3): 542– 559. doi:10.1016/j.clinthera.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 32. Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia 1997; 17 (2): 109– 112. doi:10.1046/j.1468-2982.1997.1702109.x. [DOI] [PubMed] [Google Scholar]
- 33. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo-controlled, crossover study. BMJ 2001; 322 (7277): 19– 22. doi:10.1136/bmj.322.7277.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA 2003; 289 (1): 65– 69. doi:10.1001/jama.289.1.65. [DOI] [PubMed] [Google Scholar]
- 35. Stovner LJ, Linde M, Gravdahl GB, et al. A comparative study of candesartan versus propranolol for migraine prophylaxis: a randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia 2013; 34 (7): 523– 532. doi:10.1177/0333102413515348. [DOI] [PubMed] [Google Scholar]
- 36. Diener HC, Gendolla A, Feuersenger A, et al. Telmisartan in migraine prophylaxis: a randomized, placebo-controlled trial. Cephalalgia 2009; 29 (9): 921– 927. doi:10.1111/j.1468-2982.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 37. Millán-Guerrero RO, Isais-Millán R, Benjamin TH, Tene CE. Nalpha-methyl histamine safety and efficacy in migraine prophylaxis: phase III study. Can J Neurol Sci 2006; 33 (2): 195– 199. [DOI] [PubMed] [Google Scholar]
- 38. Millán-Guerrero RO, Isais-Millán R, Barreto-Vizcaíno S, et al. Subcutaneous histamine versus topiramate in migraine prophylaxis: a double-blind study. Eur Neurol 2008; 59 (5): 237– 242. doi:10.1159/000115637. [DOI] [PubMed] [Google Scholar]
- 39. Rao BS, Das DG, Taraknath VR, Sarma Y. A double blind controlled study of propranolol and cyproheptadine in migraine prophylaxis. Neurol India 2000; 48 (3): 223– 226. [PubMed] [Google Scholar]
- 40. Benseñor IM, Cook NR, Lee IM, et al. Low-dose aspirin for migraine prophylaxis in women. Cephalalgia 2001; 21 (3): 175– 183. doi:10.1046/j.0333-1024.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- 41. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13 (11): 1100– 1107. doi:10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- 42. Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014; 13 (9): 885– 892. doi:10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]