Abstract
Peptide-based vaccines are attractive approaches for cancer immunotherapy; but the success of these vaccines in clinical trials have been limited. Our goal is to improve immune responses and anti-tumor effects against a synthetic, multi-epitope, long peptide from rat Her2/neu (rHer2/neu) using the help of CD4+ T cells and appropriate adjuvant in a mouse tumor model. Female BALB/c mice were vaccinated with P5+435 multi-epitope long peptide that presents epitopes for cytotoxic T lymphocytes (CTL) in combination with a universal Pan DR epitope (PADRE) or CpG-oligodeoxynucleotides (CpG-ODNs) as a Toll-like receptor agonist adjuvant. The results show that vaccination with the multi-epitope long peptide in combination with the PADRE peptide and CpG-ODN induced expansion of subpopulations of CD4+ and CD8+ cells producing IFN-γ, the average tumor size in the vaccinated mice was less than that of the other groups, and tumor growth was inhibited in 40% of the mice in the vaccinated group. The mean survival time was 82.6 ± 1.25 days in mice vaccinated with P5+435 + CpG+ PADRE. Our results demonstrate that inclusion of PADRE and CpG with the peptide vaccine enhanced significant tumor specific-immune responses in vaccinated mice.
Introduction
Breast cancer is one of the most common malignancies in women and the second leading cause of cancer deaths among women worldwide [1]. Amplification and/or overexpression of the Her2/neu protein has been reported in 25–30% of human breast cancers [2]. Her2/neu is a member of the epidermal growth factor receptor family with tyrosine kinase activity [3] and is known as a tumor-associated antigen (TAA) [4]. Overexpression of Her2/neu is associated with aggressive disease and poor prognosis [5]. Although Her2/neu is a self-antigen, antibody and cytotoxic T lymphocyte (CTL) -specific responses against Her2/neu have been detected in some patients with Her2/neu overexpression in breast and ovarian cancers [6, 7];thus, immunological tolerance to Her2 is not absolute and can be overcome [5]. Therefore, Her2/neu is an attractive target for immunotherapeutic approaches [8]. Monoclonal antibodies have demonstrated considerable effects in HER2-positive breast cancer patients. Despite these successes, most metastatic tumors will progress. Therefore a other immunotherapy strategies are needed [9]. Use of Her2-specific peptide-based vaccines is an effective strategy for generating active immune responses to Her2 [10]. Peptide-based vaccines are easily produced, chemically stable, cost effective, non-toxic, and safe [11, 12]. Because CTLs play an important role in the prevention of tumor growth [13], many minimal CTL epitopes derived from TAAs have been identified [14], and numerous peptide-based vaccine investigations have used minimal sequences of MHC class I binding CD8+ Tcell epitopes [15]. Studies have shown peptide-based vaccine induction of CTL responses and anti-tumor protection [16]. In contrast, less encouraging results have been obtained in cancer patients in the clinic [12, 17]. Therefore, it is necessary to improve the effectiveness and potency of peptide vaccines. Multiple approaches have been used to augment the potency of peptide vaccines [18]. Multiepitope long vaccines as one choice are being developed to improve the efficacy of peptide-based vaccines [10, 19]. Several preclinical and clinical models demonstrate that vaccinations with long peptides result in more robust protective immunity capable of improving specific CTL responses than the minimal CTL epitope peptide-based vaccines [20–24]. Vaccination of HPV16-positive advanced or recurrent carcinoma patients with a mix of thirteen E6 and E7 overlapping 25–30 amino acids in an HPV-derived long peptide revealed that it was safe and able to induce HPV-specific responses in 11 of 13 patients [25].
It is well documented that CD4+ T cells play a central role in orchestrating anti-tumor immunity and in priming and maintenance of CD8+ Tcell effector functions. Immune responses have been enhanced by including CD4+ T cell epitopes in peptide vaccines [26, 27]. The presence of a universal T helper epitope such as the pan DR-biding epitope (PADRE) greatly improved antibody immune responses induced by a malaria recombinant antigen vaccine [28]. PADRE is a universal synthetic 13 amino acid peptide that activates CD4+ T cells [29]. Because PADRE binds with high affinity to 15 of the 16 most common human HLA-DR types, and with moderate-to-high affinity to mouse I-Ab/d and I-Eb/d MHC haplotypes, it provides effective CD4+ T cell responses [30, 31], and likely, PADRE can overcome the problems caused by polymorphism of HLA-DR molecules in the population [32]. A proliferation assay showed PADRE to be 100-fold more potent than other universal T helper epitopes such as the tetanus toxin-derived universal epitope [33]. In addition, human clinical studies have shown PADRE to be safe and well tolerated [34]. A study in a murine model using an E7 peptide-based vaccine in combination with PADRE and a Toll-like receptor 3 (TLR3) agonist indicated better CTL responses and anti-tumor protection than the vaccine without the T helper epitope [35]. To enhance immune responses by peptide-based vaccines and induce CD4+ T cell help, an appropriate adjuvant will be required. Bacterial DNA often have strong immunostimulatory effects that can be mimicked by synthetic oligodeoxynucleotides with unmethylated CpG motifs (CpG-ODNs) [36]. CpG-ODNs interact with TLR-9 and induce Th1-based immune responses [37, 38]. These can also stimulate monocytes, macrophages, and B cells, and induce activation and maturation of dendritic cells (DCs) [39]. Studies have shown that the addition of CpG as a vaccine adjuvant can enhance immune responses [40–42].
Our aim in this study was to improve CTL immune responses using a multi-epitope long peptide combined with PADRE as a universal T-helper cell epitope and CpG-ODN as an adjuvant.
Materials and Methods
Mice
Female six-to-eight-week-old BALB/c mice were purchased from the Pasteur Institute (Tehran, Iran). All mice were maintained under pathogen-free conditions and allowed to acclimate to the animal facility of the BuAli Research Institute (Mashhad, Iran) for one week before beginning the experiments. Experiments were conducted in accordance with the proposal and were approved by the Institute Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences.
Cell line
The TUBO cloned cell line is a Her2/neu-overexpressing murine tumor model derived from a lobular carcinoma that arose spontaneously from BALB/c mice transgenic for the rat Her2/neu (BALB/neuT) oncogene [4]. TUBO cells grow progressively in normal BALB/c mice and give rise to lobular carcinoma, which is histologically similar to that seen in BALB/neuT mice [43]. TUBO cells were kindly provided by Dr. Pier-Luigi Lollini (Department of Clinical and Biological Sciences, University of Turin, Orbassano, Italy). The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 100U/ml penicillin, 100 μg/ml streptomycin, and 20% heat-inactivated fetal bovine serum (FBS), at 37°C with 5% CO2.
Peptides
We used the P5+ 435 multiepitope peptide consisting of two CTL epitopes, P5 and P435, each with a length of 21 amino acids, which in a previous study were designed by in silico analysis and able to induce rat Her2/neu (rHer2/neu) -specific CTL immune responses. These peptides contain several motifs restricted by MHC class I molecules of BALB/c (H2-Dd, H2-Kd, and H2-Ld) mice [44]. In this study, the P5+435 multiepitope long peptide was prepared with a two-arginine residue sequence (RR) as linker between the P5 and P435 peptides. The R residues were introduced as a protease-sensitive linker, such that once the vaccine was internalized by DCs, intracellular proteases would cleave at the RR bipeptide and separate P5 from P435, thus enhancing processing and presentation of the epitopes [45, 46]. We also reasoned that many high-affinity CTL epitopes are hydrophobic, making them difficult to dissolve in the aqueous buffers necessary for immunization and biological testing. Hence, polar side chains, such as those of arginine, would increase the solubility of these peptides [47]. The 44 amino acid P5+435 synthetic peptide (ELAAWCRWGFLLALLPPGIAGRRIRGRILHDGAYSLTLQGLGIH) and PADRE (AKFVAAWTLKAAA) were synthesized and characterized by analytical high-performance liquid chromatography (HPLC) and mass spectrometry by Sbs Bio Inc (Beijing, China) with purity > 95%. Lyophilized peptides were dissolved in dimethylsulfoxide (DMSO) and stored at -20°C until further use. The immunostimulatory synthetic CpG-ODN 1826 (5-TCCATGACGTTCCTGACGTT-3), optimized for stimulation of the mouse immune system and containing two CpG motifs, was used in this work [42, 44]. Synthetic ODNs were prepared with a nuclease-restricted phosphorothioate backbone by Microsynth (Balgach, Switzerland).
Immunization
Six groups of female BALB/c mice (n = 9/group) were immunized by subcutaneous (s.c.) injections to the flank with 100 μg of the P5+435 peptide alone or combined with 25 μg of CpG-ODN 1826 or 25μg of CpG-ODN 1826 plus 50 μg of PADRE in a total volume of 100 μl/mouse, three times at 14 day intervals. Mice similarly vaccinated with phosphate-buffered saline (PBS), PADRE alone, and mixed CpG + PADRE were used as controls. Two weeks after the last immunization, three mice per group were euthanized and their spleens were collected to evaluate cellular immune responses.
Enzyme-linked immunospot (ELISpot) assay
Two weeks after the last immunization, single-cell splenocyte suspensions were prepared. Interferon-gamma (IFN-γ) production was assessed by a mouse IFN-γ ELISpot assay kit (U-Cy Tech, Utrecht, The Netherlands) according to the manufacturer’s protocol. Flat-bottom, 96-well ELISPOT plates were coated with 50 μl/well of anti-mouse IFN-γ capture antibody, and incubated overnight at 4°C. The plates were washed with PBS/0.05% Tween 20 and blocked with 200 μl/well of blocking buffer for 1 h at 37°C. After removal of the blocking solution, splenocytes were added to wells at concentrations of 105, 3× 105, and 3× 104 cells/well in final volumes of 200μl with RPMI 1640 containing 10 μg/ml of each peptide or a 0.3 μl/ml mixture of 50 ng/ml phorbol 12-myristate13-acetate (PMA) and 500 ng/ml ionomycin (IO) as positive controls, and media alone was used as a negative control. After 24 h at 37°C with 5% CO2, plates were washed with PBS/0.05% Tween 20 and then incubated for 24 h at 37°C with 5% CO2 with 100 μl/well of biotinylated antibody. After washing, 100 μl of anti-biotin antibody were added to the wells and incubated for 1 h at 37°C with 5% CO2. Plates were washed and spots were developed by incubation for 10–30 min with 35 μl/well of substrate at room temperature in the dark. Spots were counted with the Kodak 1D software package (Version 3.5, Eastman Kodak, Rochester, New York). The mean number of spots ± SD in triplicate wells was calculated and expressed as spot-forming units (SFU) per 106 splenocytes.
Surface and intracellular staining
For flow cytometry, splenocytes were stimulated with 0.7 μl/ml of 50 ng/ml PMA and 500 ng/ml IO, and then 1μg/ml of berefeldinA was added to retain the cytokine in the cytoplasm. After incubation for 4 h at 37°C with 5% CO2, these cells were stained for CD4 or CD8 surface markers using anti-CD4-PEcy5 or anti-CD8-PEcy5 antibodies (all from BD Biosciences) in separate tubes and incubated for 30 min at 4°C in the dark,. For IFN-γ and IL-4 intracellular staining after fixation and permeabilization using a Cytofix/Cytoperm™ Plus Fixation/Permeabilization Kit with BD Golgi PlugTM (cat no.555028), cells were stained with anti-IFN-γ-FITC and anti-IL-4-PE antibodies and incubated for 30 min at 4°C in the dark. Stained cells were analyzed using FACSCalibur™ (BD Biosciences).
Prophylactic model of TUBO challenge
Fourteen days after the last immunization, six mice per group were challenged by s.c. injections on the shaved right flank with 50 μl of a single-cell suspension of 5× 105 TUBO cells. Mice were observed weekly for 80 days to monitor tumor growth and tumors were measured with calipers. Tumor masses were measured in three orthogonal diameters, and volumes were determined. Mice with no evidence of tumors during the observation period were classed as tumor-free, mice with tumor masses with mean diameters > 3 mm were classed as tumor bearers. The mice were monitored for up to 80 days unless one of the following conditions for euthanization was met: (a) body weight dropped below 15% of their initial mass, (b) the tumor masses were greater than 20 mm in any dimension, (c) the mice became lethargic or sick and unable to feed. To reduce suffering during euthanizations, each mouse was anesthetized by an i.p. injection of 100 μl/10 g mouse body weight of a ketamine/xylazine mixture containing1 ml of 100mg/ml ketamine and 0.5 ml of 20mg/ml xylazine diluted in 8.5 ml of sterile injectable saline. Mice were euthanized by cervical dislocation. All surviving mice were euthanized on day 80. Five mice in the control group were euthanized by day 80 because of tumor progression.
Statistical Analysis
The significance of the differences between groups was determined with the One-way ANOVA statistical test using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). Tumor sizes data were analyzed by the repeated measures ANOVA test. The tukey test was used to compare the means of different groups. The log-rank test using GraphPad Software and the Fleming-Harrington test using STATA Software were used to analyze survival between groups. P < 0.05 was considered significant.
Results
Mice vaccinated with the P5+435 long peptide combined with the PADRE peptide and CpG produced the greatest amount of IFN-γ
To determine the immune response in mice vaccinated with P5+435 in combination with the PADRE peptide and CpG, we performed IFN-γ ELISpot assays. Mice were vaccinated with the P5+435 long peptide, the long peptide + CpG, or the long peptide + CpG + PADRE. Control groups were vaccinated with PADRE alone, PADRE + CpG, or PBS. Fourteen days after the last vaccination, three mice from each group were euthanized and splenocyte IFN-γ production was measured by the IFN-γ ELISpot assay. As shown in Fig 1, the mice vaccinated with the P5+435 long peptide + PADRE + CpG produced significantly more IFN-γ than the mice vaccinated with P5+435 alone, P5+435 + CpG, or controls (P <0.001).
Fig 1. Evaluation of the amount of IFN-γ produced in vaccinated mice.
Nine BALB/c female mice per group were vaccinated three times subcutaneously with 100 μg/mouse of P5+435 long peptide, P5+435 in combination with CpG, or in combination with both 50 μg/mouse of PADRE and 25 μg/mouse of CpG. Two weeks after the last vaccination, splenocytes from three mice from each group were collected and activated with the long peptide. Immune responses were then determined using an IFN-γ ELISpot assay. The data indicate the mean ± SD. (n = 3). * denotes significant difference from all other groups (P < 0.001).
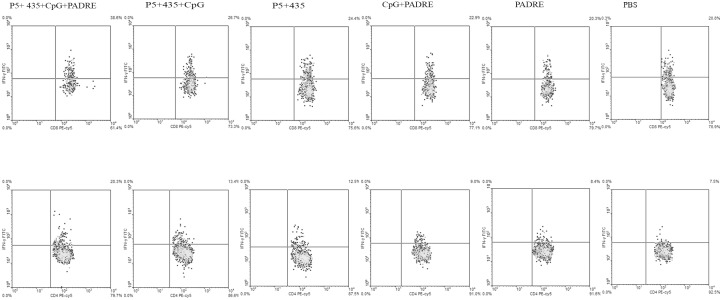
Immunization with the P5+435 long peptide in combination with PADRE and CpG increased the percentage of CD8 T cells that produce IFN-γ
To evaluate subpopulations of CD4+ and CD8+ T cells induced in vaccinated mice, two weeks after the last immunization, splenocytes from three mice from each group were harvested and characterized by flow cytometry. The cells were stained for CD4, CD8, IFN-γ, and IL-4. The percentage of cytokine-producing cells were obtained for each group. As shown in Fig 2, both CD4+ and CD8+ T cells from mice that received P5+435 + PADRE + CpG produced significantly greater amounts of IFN-γ than the control groups. We also found that CD4+ T cells from mice vaccinated with P5+435 alone produced significantly more IL-4 than the other groups (P = 0.009) (Fig 3).
Fig 2. The percentages of IFN-γ producing CD4+ and CD8+ T cells.
Flow cytometry data were also analyzed according to the percentage of cytokine-producing cells and dot plots were drawn for each vaccinated group. Quadrants showing dot plot of CD8 and CD4 cells producing IFN-γ percentage. Spleen cells were analyzed using a gating strategy to exclude debris and identify CD4+ and CD8+ T cells. The subsequent analysis was on CD8+ or CD4+ gates to describe IFN-γ-producing T cells.
Fig 3. The percentages of IL-4-producing CD4+ T cells.
Fourteen days after the last vaccination three mice per group were euthanized, and splenocytes were collected and characterized for CD4+ T cells using intracellular IL-4 staining followed by flowcytometry analysis. Data represent mean ± SEM (n = 3). ** denote significant differences from controls and all other groups, respectively.
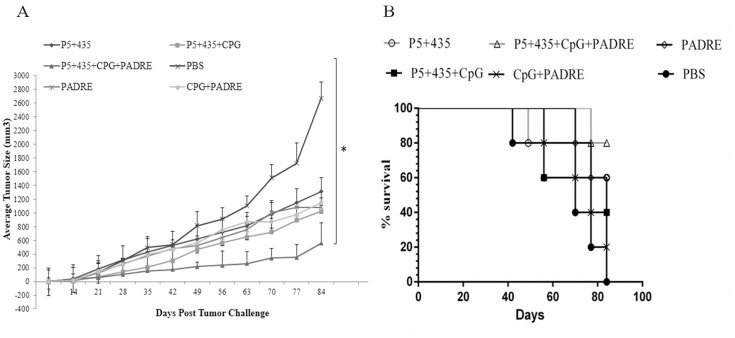
Vaccination with the P5+435 long peptide + PADRE peptide + CpG inhibited tumor growth in BALB/c mice challenged with live TUBO tumor cells
To determine whether the immune responses induced by vaccination were potent enough to generate antitumor protection, we used BALB/c mice in the prophylactic model. Fourteen days after the last vaccination, six mice from each group were challenged subcutaneously with 5×105 live TUBO cells. Fig 4A shows that tumor growth in the mice that received the P5+435 long peptide in + PADRE + CpG was slower than that of the other groups (P = 0.019). Although the tumors grew for a few weeks, the tumors growth in 33 percent of the mice vaccinated with P5+435 long peptide + PADRE peptide + CpG were completely prevented, and these mice remained tumor-free until the end of the 80-day experimental period. Survival was higher in this group than in the other groups but not statistically significant (P = 0.11) (Fig 4B).
Fig 4. In vivo antitumor effects experiments.
Six mice/group were immunized three times with P5+435 long peptide alone, long peptide + CpG, or long peptide + PADRE + CpG. Control mice were immunized with PADRE, PADRE + CpG, or PBS. After 14 days the mice were challenged subcutaneously with 5× 105 live TUBO cells. (A) Tumors were measured weekly and sizes were recorded. The values are means of tumor size and error bars indicate SD. (B) The survival times of the mice were analyzed by log-rank (P = 0.117) and Fleming-Harrington tests (P = 0.058) for 80 days. * denotes significant difference from PBS (P < 0.05).
Discussion
Our study had two goals, first to design a multi-epitope long peptide, and second, to increase immune responses generated by the long peptide with the addition of PADRE and a CpG adjuvant.
Various methods have been used to design multi-epitope long peptides. A typical approach consists of linking minimal epitopes together. Some studies showed that spacers or linkers must be used between epitopes to achieve optimal processing while others did not [48]. Another approach for multi-epitope long peptide design consists of applying “natural” peptides of more than 17 amino acids, because many previous studies reported that peptides with more than 17 amino acids require proteasome processing and presentation of the epitopes to CTLs [49].
Here, we combined both methods and used two 21 amino acid “natural” peptides from the CTL epitope of rHer2/neu protein described in a previous study [44, 50]. The two peptides were linked by an arginine linker. Thus the 44 amino acid peptide can be internalized, processed, and presented by antigen-presenting cells (APCs) and simultaneously stimulate different T cell clones. Given that both peptides are from CTL epitopes of the rHer2/neu protein, we decide to apply a universal T helper epitope to recruit CD4+ T cell help in immune responses induced by the long peptide. PADRE was used to induce CD4+ T cell help and augment the immune responses generated by the long peptide.
In the present study, We compared the effectiveness of different vaccines on immune responses. Effective immune responses were obtained with peptide alone, peptide combined with CpG, and peptide combined with CpG and PADRE. The mice immunized with the P5+435 multi-epitope long peptide vaccine, in combination with PADRE and the CpG adjuvant, showed the strongest specific CTL immune responses of all the immunized groups, had the longest survival times, and were the most tumor-resistant against the Her2/neu-expressing TUBO cell line.
We showed that CD4+ and CD8+ T cells from mice immunized with the P5+435 multi-epitope long peptide-based vaccine combined with PADRE and CpG produced more IFN-γ than T cells from mice vaccinated with the peptide alone or the peptide vaccine + CpG. CD4+ T cells play a central role in initiation and regulation of different aspects of immune responses. In addition, CD4+ T cells are essential for the priming of tumor-specific CTLs and maintaining anti-tumor responses [26]. We believe the CD4+ T cells were induced due to the presence of the PADRE peptide and likely contributed to the increased expression of IFN-γ. Our results are consistent with other studies that used peptide- and DNA-based vaccines with PADRE [35, 51].
Another important reason for the enhanced immune response and anti-tumor effects in mice vaccinated with the multi-epitope long peptide + PADRE + CpG is likely related to the use of CpG as an adjuvant. As previously mentioned, synthetic ODNs that contain immunostimulatory CpG motifs trigger an immunomodulatory cascade that involves B and T cells, natural killer cells, and professional APCs [52]. CpG-ODNs used as vaccine adjuvants are rapidly internalized by immune cells and interact with TLR9 in endocytic vesicles. CpG-ODNs can improve APC function and promote the induction of antigen-specific adaptive immune responses by supporting the development of Th1- rather than Th2-type immune responses. In the mice were vaccinated with long peptide + CpG tumors were smaller than those in the other groups but these not statistically significant. The CpG positive effects in improving the potency of immune response were shown in different studies when used as a cancer vaccine adjuvant. The CpG effects in our study were not statistically significant but this was more than long peptide alone and PBS groups.
We also found that CD4+ T cells from mice immunized with the long peptide + CpG or CpG + PADRE produced less IL-4 than mice immunized with the long peptide alone. Although tumors in mice immunized with the long peptide alone were smaller than those in mice immunized with PBS, but long peptide antitumor effects were weak in comparison with the mice that received the long peptide + CpG + PADRE. Almost 33 percent of the mice immunized with the long peptide + PADRE + CpG had no tumor growth throughout the experiment. Our results showed that use of the long peptide alone increases subpopulations of IFN-γ-producing CD4+ and CD8+ T cells, as well as a subpopulation of IL-4-producing CD4+ cells. Given that we identified CD4+ and CD8+ T cells producing IFN-γ and CD4+ T cells producing IL-4, we detected both Th1 and Th2 profiles in the spleens. In fact, when we used the long peptide without CpG and PADRE both Th1 and Th2 immune responses were induced. A likely reason appropriate antitumor responses were not achieved in the mice vaccinated with the long peptide alone is because in these mice the immune response was directed toward Th2. The induced Th2 response may be due to decreased potency of an induced Th1 response with weak antitumor activity generated by the long peptide alone.
In summary, our results demonstrate that the long peptide alone stimulated the immune system, but was not completely effective. Addition of the PADRE peptide and CpG adjuvant enhanced the immune response and antitumor effects of the rHer2 multi-epitope long peptide vaccine in immunized mice. PADRE and other strategies that induce CD4+ T cells can be used to improve the efficacy and potency of peptide vaccines.
Acknowledgments
We thank Dr. Javad Akhtari and Mr. Mohammad Ali Khodadoust for technical assistance. This study was a part of H. Ghaffari-Nazari’s MSc dissertation.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was financially supported by a grant (No. 910444) of the research council of Mashhad University of Medical Sciences in Iran.
References
- 1. Peoples GE, Holmes JP, Hueman MT, Mittendorf EA, Amin A, Khoo S, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14(3):797–803. Epub 2008/02/05. doi: 14/3/797 [pii] 10.1158/1078-0432.CCR-07-1448 . [DOI] [PubMed] [Google Scholar]
- 2. Tsang R, Finn R. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. British journal of cancer. 2012;106(1):6–13. 10.1038/bjc.2011.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC, et al. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. The Journal of Immunology. 2005;174(7):4228–36. [DOI] [PubMed] [Google Scholar]
- 4. Pakravan N, Langroudi L, Hajimoradi M, Hassan ZM. Co-administration of GP96 and Her2/neu DNA vaccine in a Her2 breast cancer model. Cell Stress and Chaperones. 2010;15(6):977–84. 10.1007/s12192-010-0208-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renard V, Sonderbye L, Ebbehøj K, Rasmussen PB, Gregorius K, Gottschalk T, et al. HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. The Journal of Immunology. 2003;171(3):1588–95. [DOI] [PubMed] [Google Scholar]
- 6. Baxevanis CN, Sotiriadou NN, Gritzapis AD, Sotiropoulou PA, Perez SA, Cacoullos NT, et al. Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunology, Immunotherapy. 2006;55(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riemer AB, Klinger M, Wagner S, Bernhaus A, Mazzucchelli L, Pehamberger H, et al. Generation of Peptide mimics of the epitope recognized by trastuzumab on the oncogenic protein Her-2/neu. The Journal of Immunology. 2004;173(1):394–401. [DOI] [PubMed] [Google Scholar]
- 8. Dakappagari NK, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer research. 2000;60(14):3782–9. [PubMed] [Google Scholar]
- 9. Morse MA, Wei J, Hartman Z, Xia W, Ren XR, Lei G, et al. Synergism from combined immunologic and pharmacologic inhibition of HER2 in vivo. International Journal of Cancer. 2010;126(12):2893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clinical cancer research. 2003;9(15):5559–65. [PubMed] [Google Scholar]
- 11. van der Burg SH, Bijker MS, Welters MJ, Offringa R, Melief CJ. Improved peptide vaccine strategies, creating synthetic artificial infections to maximize immune efficacy. Advanced drug delivery reviews. 2006;58(8):916–30. [DOI] [PubMed] [Google Scholar]
- 12. Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67(3):1326–34. Epub 2007/02/07. doi: 67/3/1326 [pii] 10.1158/0008-5472.CAN-06-3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sotiriadou NN, Kallinteris NL, Gritzapis AD, Voutsas IF, Papamichail M, von Hofe E, et al. Ii-Key/HER-2/neu (776–90) hybrid peptides induce more effective immunological responses over the native peptide in lymphocyte cultures from patients with HER-2/neu+ tumors. Cancer Immunology, Immunotherapy. 2007;56(5):601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. Epub 2002/11/26. doi: imr18806 [pii]. . [DOI] [PubMed] [Google Scholar]
- 15. Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci U S A. 2000;97(22):12198–203. Epub 2000/10/12. 10.1073/pnas.220413497 220413497 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gritzapis AD, Mahaira LG, Perez SA, Cacoullos NT, Papamichail M, Baxevanis CN. Vaccination with human HER-2/neu (435–443) CTL peptide induces effective antitumor immunity against HER-2/neu-expressing tumor cells in vivo. Cancer research. 2006;66(10):5452–60. [DOI] [PubMed] [Google Scholar]
- 17. Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer research. 1998;58(21):4902–8. [PubMed] [Google Scholar]
- 18. Perez SA, von Hofe E, Kallinteris NL, Gritzapis AD, Peoples GE, Papamichail M, et al. A new era in anticancer peptide vaccines. Cancer. 2010;116(9):2071–80. 10.1002/cncr.24988 [DOI] [PubMed] [Google Scholar]
- 19. Amir Jalali S, Parmiani G. Pre-Clinical and Clinical Aspects of peptide-based vaccine against human solid tumors. Recent patents on biotechnology. 2011;5(2):108–17. [DOI] [PubMed] [Google Scholar]
- 20. Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. The Journal of Immunology. 2007;179(8):5033–40. [DOI] [PubMed] [Google Scholar]
- 21. Vambutas A, DeVoti J, Nouri M, Drijfhout J, Lipford G, Bonagura V, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23(45):5271–80. [DOI] [PubMed] [Google Scholar]
- 22. Zwaveling S, Mota SCF, Nouta J, Johnson M, Lipford GB, Offringa R, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. The Journal of Immunology. 2002;169(1):350–8. [DOI] [PubMed] [Google Scholar]
- 23. Kenter GG, Welters MJ, Valentijn ARP, Löwik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clinical cancer research. 2008;14(1):169–77. 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 24. Mansourian M, Badiee A, Jalali SA, Shariat S, Yazdani M, Amin M, et al. Effective induction of anti-tumor immunity using p5 HER-2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co-administrated with CpG-ODN. Immunol Lett. 2014;162(1 Pt A):87–93. Epub 2014/08/03. 10.1016/j.imlet.2014.07.008 . [DOI] [PubMed] [Google Scholar]
- 25. van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, van Meerten ELvP, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. Journal of translational medicine. 2013;11(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4+ T cells. Current opinion in immunology. 2009;21(2):200–8. 10.1016/j.coi.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yo-Ping L, Chung-Jiuan J, Shu-Ching C. The Roles of CD4+ T Cells in Tumor Immunity. ISRN Immunology. 2011;2011. [Google Scholar]
- 28. Rosa DS, Tzelepis F, Cunha MG, Soares IS, Rodrigues MM. The pan HLA DR-binding epitope improves adjuvant-assisted immunization with a recombinant protein containing a malaria vaccine candidate. Immunology letters. 2004;92(3):259–68. [DOI] [PubMed] [Google Scholar]
- 29. Alexander J, Guercio M-Fd, Frame B, Maewal A, Sette A, Nahm MH, et al. Development of experimental carbohydrate-conjugate vaccines composed of< i> Streptococcus pneumoniae capsular polysaccharides and the universal helper T-lymphocyte epitope (PADRE®). Vaccine. 2004;22(19):2362–7. [DOI] [PubMed] [Google Scholar]
- 30. Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. The Journal of Immunology. 2005;174(3):1580–6. [DOI] [PubMed] [Google Scholar]
- 31. Alexander J, Fikes J, Hoffman S, Franke E, Sacci J, Appella E, et al. The optimization of helper T lymphocyte (HTL) function in vaccine development. Immunologic research. 1998;18(2):79–92. [DOI] [PubMed] [Google Scholar]
- 32. Ghochikyan A. Rationale for peptide and DNA based epitope vaccines for Alzheimer’s disease immunotherapy. CNS & neurological disorders drug targets. 2009;8(2):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alexander J, del Guercio M-F, Maewal A, Qiao L, Fikes J, Chesnut RW, et al. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. The Journal of Immunology. 2000;164(3):1625–33. [DOI] [PubMed] [Google Scholar]
- 34. Weber JS, Hua FL, Spears L, Marty V, Kuniyoshi C, Celis E. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund's adjuvant in patients with resected high-risk melanoma. J Immunother. 1999;22(5):431–40. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 35. Wu C-Y, Monie A, Pang X, Hung C-F, Wu T. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci. 2010;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adamsson J, Lindblad M, Lundqvist A, Kelly D, Holmgren J, Harandi AM. Novel immunostimulatory agent based on CpG oligodeoxynucleotide linked to the nontoxic B subunit of cholera toxin. The Journal of Immunology. 2006;176(8):4902–13. [DOI] [PubMed] [Google Scholar]
- 37. Meng W, Yamazaki T, Nishida Y, Hanagata N. Nuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonists. BMC biotechnology. 2011;11(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shargh VH, Jaafari MR, Khamesipour A, Jaafari I, Jalali SA, Abbasi A, et al. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine. 2012;30(26):3957–64. 10.1016/j.vaccine.2012.03.040 [DOI] [PubMed] [Google Scholar]
- 39. Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert review of vaccines. 2011;10(4):499–511. 10.1586/erv.10.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kochenderfer JN, Chien CD, Simpson JL, Gress RE. Maximizing CD8< sup>+ T cell responses elicited by peptide vaccines containing CpG oligodeoxynucleotides. Clinical Immunology. 2007;124(2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiner GJ, Liu H-M, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proceedings of the National Academy of Sciences. 1997;94(20):10833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miconnet I, Koenig S, Speiser D, Krieg A, Guillaume P, Cerottini J-C, et al. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. The Journal of Immunology. 2002;168(3):1212–8. [DOI] [PubMed] [Google Scholar]
- 43. Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. The Journal of Immunology. 2000;165(9):5133–42. [DOI] [PubMed] [Google Scholar]
- 44. Jalali SA, Sankian M, Tavakkol-Afshari J, Jaafari MR. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2012;8(5):692–701. [DOI] [PubMed] [Google Scholar]
- 45. Ulrich JT, Cieplak W, Paczkowski NJ, Taylor SM, Sanderson SD. Induction of an antigen-specific CTL response by a conformationally biased agonist of human C5a anaphylatoxin as a molecular adjuvant. The Journal of Immunology. 2000;164(10):5492–8. [DOI] [PubMed] [Google Scholar]
- 46. Barr PJ. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991;66(1):1–3. [DOI] [PubMed] [Google Scholar]
- 47. Sundaram R, Sun Y, Walker CM, Lemonnier FA, Jacobson S, Kaumaya PT. A novel multivalent human CTL peptide construct elicits robust cellular immune responses in HLA-A< sup>* 0201 transgenic mice: implications for HTLV-1 vaccine design. Vaccine. 2003;21(21):2767–81. [DOI] [PubMed] [Google Scholar]
- 48. Velders MP, Weijzen S, Eiben GL, Elmishad AG, Kloetzel P-M, Higgins T, et al. Defined flanking spacers and enhanced proteolysis is essential for eradication of established tumors by an epitope string DNA vaccine. The Journal of Immunology. 2001;166(9):5366–73. [DOI] [PubMed] [Google Scholar]
- 49. Yang B, Hahn YS, Hahn CS, Braciale TJ. The requirement for proteasome activity class I major histocompatibility complex antigen presentation is dictated by the length of preprocessed antigen. The Journal of experimental medicine. 1996;183(4):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shariat S, Badiee A, Jalali SA, Mansourian M, Yazdani M, Mortazavi SA, et al. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer letters. 2014;355(1):54–60. 10.1016/j.canlet.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 51. Hung C-F, Tsai Y-C, He L, T Wu. DNA Vaccines Encoding Ii-PADRE Generates Potent PADRE-specific CD4+ T-Cell Immune Responses and Enhances Vaccine Potency. Molecular Therapy. 2007;15(6):1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Reviews Immunology. 2004;4(4):249–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.